Abstract
Background
The development of an in vitro cultivation system for human noroviruses allows the measurement of neutralizing antibody levels.
Methods
Serum neutralizing antibody levels were determined using a GII.4/Sydney/2012-like virus in human intestinal enteroids in samples collected before and 4 weeks after administration of an investigational norovirus vaccine and were compared with those measured in histo-blood group antigen (HBGA)–blocking assays.
Results
Neutralizing antibody seroresponses were observed in 71% of 24 vaccinated adults, and antibody levels were highly correlated (r = 0.82, P < .001) with those measured by HBGA blocking.
Conclusions
HBGA-blocking antibodies are a surrogate for neutralization in human noroviruses.
Clinical Trials Registration
Keywords: human norovirus, vaccine, neutralization, antibody, immunogenicity, histo-blood group antigen, human intestinal enteroids
Norovirus antibody levels measured by microneutralization are highly correlated with those measured by histo-blood group antigen–blocking assays.
Human noroviruses cause acute gastroenteritis and are a significant healthcare and economic burden in the United States and worldwide [1]. This has led to efforts to develop vaccines that can prevent norovirus-associated disease [2]. Previous studies have identified antibodies that block binding of norovirus virus-like particles (VLPs) to histo-blood group antigens (HBGAs), putative attachment factors on gut epithelium, as a correlate of protection against norovirus-associated illness and infection [3–5]. The recent development of a cultivation system for human noroviruses using human intestinal enteroids (HIEs) has allowed an assessment of the neutralizing activity of serum and monoclonal antibodies [6, 7]. The purpose of this study was to assess neutralizing antibody responses to a GII.4 norovirus strain after vaccination and to correlate the neutralizing antibody levels with those that block HBGA-binding to GII.4 VLPs.
METHODS
Virus and VLPs
The virus used in these studies, BCM16-1/2016/USA, is a GII.P31/GII.4-Sydney/2012 strain and its titer was determined to be 1.8 × 109 genomic equivalents/mL by reverse transcription quantitative polymerase chain reaction (RT-qPCR) and 3.6 × 105 tissue culture infectious dose 50% (TCID50)/mL in J2 HIEs using previously described methods [6]. VLPs consisting of the major viral capsid protein, VP1, were produced and used as described [8], with sequences representing several GII.4 variants including the Sydney/NSW0514/2012/AU (Sydney/2012; GenBank JX459908), New Orleans 1805/2009/US (New Orleans/2009; GenBank GU445325), Yerseke 38/2006/NL (2006a; GenBank EF126963), Den Haag 89/2006/NL (2006b; GenBank EF126965), a Lanzou/2002-like strain (Houston/TCH186/2002/US, GenBank EU310927), and a consensus GII.4 strain (GII.4c; a consensus sequence from 3 different GII.4 variants [Houston/2002, Yerseke/2006 and Den Haag/2006]) used in the vaccine [9].
Study Design
The NOR-210 study design has been previously reported [10]; in brief, healthy men and women 18–49 years of age who were eligible and provided informed consent received a 0.5-mL dose of a candidate bivalent norovirus vaccine (lot number 3-FIN-1897) that contained 15 µg GI.1 genotype VLP, 50 µg GII.4c VLP, and 0.5 mg aluminium hydroxide (Brenntag Biosector A/S, Denmark). Serum was collected prior to vaccination and at 4 weeks postvaccination. The primary objective of the clinical study was to use these serum samples to establish proficiency panels and to evaluate serological assays, including an assessment of responses to vaccination. The study was approved by the study center’s institutional review board and was performed according to the prevailing Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was registered on ClinicalTrials.gov (NCT02475278).
HBGA-Blocking Antibody Assay
Serum HBGA blocking antibodies to different GII.4 variant VLPs were assayed as previously described [11]. Pig gastric mucin (Sigma-Aldrich) was the HBGA ligand used in the assay. The HBGA blocking antibody results have been reported previously with the GII.4c VLPs [10] and the other GII.4 variants [8].
Neutralizing Antibody Assay
Six sera purchased from BioIVT with HBGA-blocking titers ranging from 15 to 1562 were used in the initial assay development studies. The neutralizing antibody assay was performed as previously described [7] with the following modifications used in the developed assay. A jejunal HIE line (J2 HIE) was used for all the experiments described in this work. J2 HIEs were propagated as 3-dimensional cultures in complete media with growth factors (CMGF+) [6, 12]. HIE monolayers were prepared from trypsinized 3D cultures where cell pellets were suspended in commercial proliferation Intesticult (INT) human organoid growth medium (Stem Cell Technologies), prepared by mixing equal volume of components A and B, and supplemented with 10 μM ROCK inhibitor Y-27632. The dispersed cells were plated into 96-well plates precoated with collagen IV for 2 hours (Sigma-Aldrich). After 1 day of cell growth as a monolayer, the proliferation INT medium was changed to differentiation INT medium, which consisted of an equal volume of component A and complete media without growth factors (CMGF–). After 5 days of differentiation, the cell monolayers were used for neutralization assays. Serum samples were heat inactivated for 30 minutes at 56°C and, starting at a 1:100 dilution, were serially diluted 5-fold in CMGF– containing 500 μM glycochenodeoxycholic acid (GCDCA; Sigma, G0759). BCM16-1/2016/USA was diluted in the same medium. Serum dilutions or the medium control were mixed 1:1 with 10, 30, or 100 TCID50 of the virus in developmental studies; the 100 TCID50 inoculum subsequently was selected as the dose to be used in further studies (see Results). The serum:virus mixtures were incubated for 1 hour at 37°C and then were inoculated onto duplicate wells of the differentiated J2 HIE monolayers and incubated for an additional 1 hour at 37°C. After the incubation period (1 hour postinfection [hpi]), the monolayers were washed twice with CMGF– and incubated with differentiation INT medium supplemented with 500 μM GCDCA. Cells and media then were collected immediately (1 hpi) and at 24 hpi, and total RNA was extracted using KingFisher Flex Purification System and MagMax-96 Viral RNA Isolation Kit. RNA extracted at 1 hpi was used as a baseline to determine the amount of input virus that remained associated with cells after washing the infected cultures. Virus replication was determined by quantifying RNA levels from samples extracted at 24 hpi in comparison to the 1 hpi time point. RT-qPCR was performed with qScript XLT One-Step RT-qPCR ToughMix reagent with ROX reference dye (Quanta Biosciences) using the primer pair COG2R/QNIF2d and probe QNIFS and a standard curve as described previously [6].
Statistical Methods
The neutralizing antibody titer was determined by interpolation as the reciprocal of the dilution that resulted in a 1 log10 reduction in virus yield at 24 hpi compared with a no-added serum (medium alone) control. As an alternative approach, the Spearman–Karber method [13] was also used to calculate the reciprocal of the dilution resulting in the 1 log10 reduction in virus yield at 24 hpi. The reciprocal of half of the starting dilution was assigned to samples with no detectable functional antibody or activity (15 for HBGA blocking and 50 for neutralizing antibody). Geometric mean titers, geometric mean fold rises, and seroresponse frequencies with 95% confidence intervals were calculated using GraphPad Prism version 6.05 for Windows (GraphPad Software). The 6 reference sera with a range of HBGA-blocking titers were tested in triplicate to determine a between-assay coefficient of variations. Pearson correlation coefficients were also determined.
RESULTS
Six reference sera with HBGA-blocking titers were used to assess different concentrations (approximately 10, 30, and 100 TCID50) of virus in initial studies to select a working concentration of virus for the neutralization assays. The 30 and 100 TCID50 concentrations had less variability in endpoint determination, and the 100 TCID50 concentration was selected for further studies (data not shown). In addition, different statistical methods for assessing the neutralizing antibody titer were assessed, including 50% (0.3 log10) and 90% (1.0 log10) reductions, with titers being determined by either the Spearman–Karber method or interpolation at the reduction level. An endpoint titer could not be identified by interpolation for some samples when the 50% reduction method was used, so the 90% reduction method was chosen for use. The titers determined by the Spearman–Karber and interpolation methods were highly correlated (r = 0.97). The assay was performed in triplicate, and the between-assay coefficient of variation for each method was <14%.
Next, paired serum samples from the clinical study NOR-210 were assessed. Samples were available for analysis in 24 study participants. Ten participants had no detectable neutralizing anti-Sydney/2012 antibody on day 1 (Supplementary Table); 8 of these individuals also had no anti-Sydney/2012 HBGA-blocking antibody, and 3 had no HBGA-blocking antibody to any of the GII.4 VLPs tested. All but 2 of the seronegative subjects had at least a 4-fold increase in serum neutralizing antibody. Overall, 71% of study subjects had a ≥4-fold increase in antibody level after vaccination using the interpolation method (Table 1). The geometric mean fold-rise in neutralizing antibodies after vaccination was 7.8 (95% confidence interval, 3.7–16.6). Results determined by the Spearman–Karber method were very similar. One additional seroresponse was identified (Table 1), and the results obtained with the 2 different calculation methods were highly correlated (R = 0.98; Supplementary Figure 1A).
Table 1.
GII.4 Neutralization and Histo-Blood Group Antigen–Blocking Antibody Responses Among 24 Healthy Participants Who Received a Bivalent GI.1/GII.4c Norovirus Vaccine
| Antigen | Prevaccination GMT (95% CI) | Postvaccination GMT (95% CI) | GMFR (95% CI) |
Seroresponse Frequency, % (95% CI) |
|---|---|---|---|---|
| Neutralization | ||||
| Sydney/2012-like (interpolation) | 182 (105–315) | 1422 (699–2890) | 7.8 (3.7–16.6) | 71 (49–87) |
| Sydney/2012-like (Spearman–Karber) | 184 (105–321) | 1269 (630–2559) | 6.9 (3.3–14.3) | 75 (53–90) |
| HBGA-blockinga | ||||
| GII.4cb | 83 (47–148) | 1609 (1065–2433) | 19.4 (10.1–37.2) | 92 (73–99) |
| Houston/2002 | 225 (120–424) | 2896 (1673–5014) | 12.9 (7.7–21.6) | 96 (79–100) |
| Yerseke/2006a | 317 (158–636) | 4079 (2296–7245) | 12.9 (7.5–22.1) | 79 (58–93) |
| Den Haag/2006b | 99 (51–192) | 755 (436–1308) | 7.6 (4.5–13.1) | 67 (45–84) |
| New Orleans/2009 | 64 (36–117) | 378 (210–679) | 5.5 (3.0–10.0) | 52 (31–73) |
| Sydney/2012 | 62 (36–108) | 543 (323–914) | 8.7 (5.4–14.2) | 67 (45–84) |
Abbreviations: CI, confidence interval; GMFR, geometric mean fold rise; GMT, geometric mean titer; HBGA, histo-blood group antigen.
aGMTs reported previously in [8].
bGII.4c is a consensus sequence between Houston/2002 (Lanzou/2002 variant), Yerseke/2006 (2006a variant), and Den Haag 89/2006 (2006b variant).
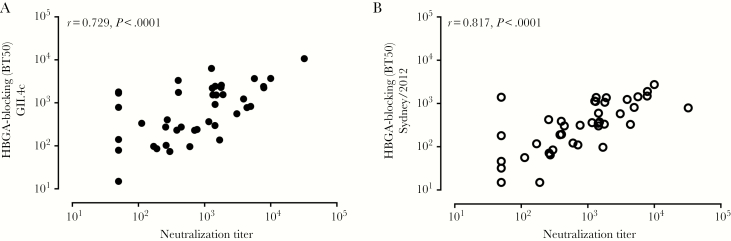
HBGA-blocking antibody titers had been determined and reported previously against several different GII.4 variants (Table 1). Seroresponse frequency was greatest for the Houston/2002 strain followed by that for the vaccine consensus antigen (GII.4c). The HBGA-blocking antibody titers to the Sydney/2012 variant were most highly correlated with the neutralizing antibody response measured using a strain that is also a Sydney/2012 variant (Figure 1, interpolation method; Supplementary Figure 1B), although neutralizing antibody titers were higher than those measured in the HBGA-blocking assay (Table 1). The HBGA-blocking antibody titers against the other GII.4 variants were also correlated significantly with the antibody levels measured by neutralization, although the correlation coefficients were lower than that against the homologous variant (Figure 1 and Supplementary Figure 2).
Figure 1.
Correlation of Sydney/2012-like virus neutralizing antibody titers determined by interpolation with histo-blood group antigen–blocking antibody titers measured against consensus GII.4 (GII.4c) (A) and Sydney/2012 (B) strains. Dots represent test results for the serum samples (n = 48) from the vaccine study, and some values are overlapping. Abbreviations: BT50, blocking titer 50%; HBGA, histo-blood group antigen.
DISCUSSION
The recognition that HBGA expression is important as a host susceptibility factor, likely as a viral attachment factor, led to the concept that antibodies that block the norovirus VLP binding to HBGAs could have neutralizing activity and that HBGA-blocking antibody levels could serve as a surrogate for neutralizing antibody levels. However, direct evaluation of virus neutralization with antibody and potential correlation with HBGA-blocking activity was not feasible until recently, when the first intestinal replication system for human noroviruses was established using human intestinal enteroids [6]. Human monoclonal antibodies that have HBGA-blocking activity also have been reported to neutralize virus infectivity [7]. To develop a standard neutralization assay that can be used in norovirus serological studies and vaccine trials, we modified the assay used in the current study from the one we originally described in which a 50% reduction in virus yield was used to assess neutralization. We noted variability in the measurement of viral RNA levels that interfered with use of a 50% reduction threshold, which represents only a 2-fold change from baseline. In contrast, we observed less variability between results when we used a 90% reduction in virus yield for the determination of serum virus neutralization antibody level. We also chose a standard virus inoculum (100 TCID50). Antibody levels were similar when measured by interpolation or by the Spearman–Karber method. This study now demonstrates that HBGA-blocking antibody titers induced following vaccination are highly correlated with neutralizing antibody responses. Additionally, this work confirms that a VLP-based vaccine can boost antibody responses that block the binding of VLPs to glycans and that recognize native norovirus and neutralize infectivity.
These observations led to the question of whether all neutralization epitopes are involved in HBGA binding. The finding that neutralizing antibody titers were higher than those measured by HBGA-blocking may reflect the existence of neutralizing epitopes that are distinct from those measured in HBGA-blocking assays. Previous studies have shown that human monoclonal antibodies generated after infection or vaccination recognize sites on the shell domain and on the protruding domain that are not involved in blocking binding of HBGAs to the virus [7, 14]; it remains to be determined whether any of these antibodies have neutralization activity. On the other hand, these observed differences may also reflect differences in the amount of viral antigen used in the 2 assays. Less antigen used in the infectivity assays as compared to VLPs in the blocking assay may lead to higher measured antibody levels. Future studies will assess whether other epitopes apart from those involved in HBGA blocking are associated with virus neutralization. Similarly, HBGA-blocking antibodies were observed in some sera in which neutralization was not detected. Additional studies are needed to determine whether some antibodies with HBGA-blocking activity are nonneutralizing.
The highest correlation between HBGA-blocking and neutralization activity was observed with the homologous Sydney/2012 variant, although good correlations also were observed with other GII.4 variants. Future studies will address whether similar findings will be observed with other GII.4 variants and other virus genotypes. The results of this study suggest that HBGA-blocking antibody levels for the homologous Sydney/2012 variant are a good surrogate for neutralization antibody levels and can be used until cultivation systems become easier and cheaper to perform.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors are grateful to all the volunteers who participated in the study.
Financial support. This work was entirely supported by Takeda Pharmaceuticals International AG.
Potential conflicts of interest. R. P. B. is a full-time employee of the study sponsor. R. L. A. and M. K. E. have received grant support from Takeda Vaccines Business Unit. The Baylor College of Medicine (R. L. A., K. E., M. K. E. as inventors) has a pending patent for norovirus growth in human intestinal enteroids, and M. K. E. has a patent on methods and reagents to detect and characterize Norwalk virus and related viruses. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Seventh International Calicivirus Conference, Sydney, Australia, 13–17 October 2019.
References
- 1.Shah MP, Hall AJ. Norovirus illnesses in children and adolescents. Infect Dis Clin North Am 2018; 32:103–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortes-Penfield NW, Ramani S, Estes MK, Atmar RL. Prospects and challenges in the development of a norovirus vaccine. Clin Ther 2017; 39:1537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeck A, Kavanagh O, Estes MK, et al. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis 2010; 202:1212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atmar RL, Bernstein DI, Harro CD, et al. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med 2011; 365:2178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atmar RL, Bernstein DI, Lyon GM, et al. Serological correlates of protection against a GII.4 norovirus. Clin Vaccine Immunol 2015; 22:923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ettayebi K, Crawford SE, Murakami K, et al. Replication of human noroviruses in stem cell-derived human enteroids. Science 2016; 353:1387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarado G, Ettayebi K, Atmar RL, et al. Human monoclonal antibodies that neutralize pandemic GII.4 noroviruses. Gastroenterology 2018; 155:1898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haynes J, Perry V, Benson E, et al. In depth breadth analyses of human blockade responses to norovirus and response to vaccination. Viruses 2019; 11. doi: 10.3390/v11050392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parra GI, Bok K, Taylor R, et al. Immunogenicity and specificity of norovirus consensus GII.4 virus-like particles in monovalent and bivalent vaccine formulations. Vaccine 2012; 30:3580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atmar RL, Cramer JP, Baehner F, Han C, Borkowski A, Mendelman PM. An exploratory study of the salivary immunoglobulin a responses to 1 dose of a norovirus virus-like particle candidate vaccine in healthy adults. J Infect Dis 2019; 219:410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atmar RL, Baehner F, Cramer JP, Song E, Borkowski A, Mendelman PM; NOR-201 Study Group . Rapid responses to 2 virus-like particle norovirus vaccine candidate formulations in healthy adults: a randomized controlled trial. J Infect Dis 2016; 214:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou WY, Blutt SE, Crawford SE, et al. Human intestinal enteroids: new models to study gastrointestinal virus infections. Methods Mol Biol 2019; 1576:229–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramakrishnan MA. Determination of 50% endpoint titer using a simple formula. World J Virol 2016; 5:85–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindesmith LC, McDaniel JR, Changela A, et al. Sera antibody repertoire analyses reveal mechanisms of broad and pandemic strain neutralizing responses after human norovirus vaccination. Immunity 2019; 50: 1530–41.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.