Abstract
As noroviruses are transmitted through the fecal–oral route, we investigated humoral and mucosal (salivary immunoglobulin A [IgA]) immune responses in a phase 2 trial of Takeda’s bivalent norovirus virus-like particle (VLP) vaccine candidate in 50 healthy 18- to 49-year-olds. The vaccine had an acceptable tolerability profile and induced rapid, robust humoral immune responses after 1 intramuscular dose of vaccine candidate. Seroresponses were evident 8 days after vaccination as panimmunoglobulin, IgA, and histo-blood group antigen–blocking antibodies against both vaccine GI.1 and GII.4c genotypes. Salivary IgA levels were approximately 1000-fold lower than serum concentrations, and moderately or strongly correlated with the serum IgA titers at all time-points.
Keywords: norovirus, vaccine, IgA, salivary, immunogenicity
A bivalent norovirus virus-like particle vaccine administered intramuscularly induced salivary immunoglobulin A (IgA) responses to the homologous vaccine antigen that correlated with observed serum IgA responses.
Noroviruses (NoVs) are responsible for “winter vomiting disease” and are a major cause of acute gastroenteritis (AGE) around the world, including foodborne AGE in all age groups [1], and have supplanted rotavirus as the leading cause of pediatric AGE in those countries with routine rotavirus vaccination [2]. There are 7 known norovirus genogroups. Human disease is caused primarily by strains belonging to genogroups GI or GII, with the GII.4 genotype currently being responsible for the majority of NoV illnesses [3–5].
Although norovirus AGE can cause mild and self-limited illness, it is also associated with a higher risk of severe or fatal consequences in vulnerable age groups such as the very young, particularly in developing countries, and in the elderly, especially those with underlying medical conditions such as chronic renal or cardiac disease [6]. NoVs are highly infectious and transmitted person-to-person by the fecal–oral route or by aerosolized vomitus, through contaminated food or water, and via environmental exposure. It is a common cause of gastroenteritis associated with foodborne outbreaks and with travel. Vaccination represents a suitable approach to avoid infection, and several vaccine candidates are in various stages of development [4]. The candidate the furthest along in clinical development contains 2 distinct virus-like particles (VLPs) representing the 2 major genogroups that infect people: a Norwalk virus GI.1 VLP and a consensus GII.4 (GII.4c) VLP derived from 3 GII.4 variants [7–12]. In this phase 2 study of a candidate formulation, we performed an exploratory analysis of mucosal immunity measured as salivary IgA responses.
METHODS
Study Design
This open-label phase 2 study was performed in 1 center (Benchmark Research, Austin, Texas) from 26 February–13 October 2015. The study was approved by the institutional review board of the study center, and was performed according to the prevailing Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was registered on ClinicalTrials.gov (NCT02475278).
The primary study objective was to collect serum samples to evaluate serologic assays and to establish proficiency panels for serologic assays used for the assessment of immune responses to an intramuscular norovirus GI.1/GII.4c bivalent VLP vaccine candidate. As an exploratory objective, we analyzed mucosal immune responses to the vaccination, measured as salivary IgA.
Study Participants and Procedures
Eligible participants were men or women 18–49 years of age who were in good health at the time of enrollment based on medical history and physical examination, had a body mass index <35 kg/m2, were able to comply with trial procedures, and were available for the entire duration of the trial. The main exclusion criteria included any history of gastroenteritis within 14 days of enrollment, any known current or chronic medical condition (particularly those likely to affect immune function), any history of allergic reaction to vaccination, and any other recent vaccinations or participation in another clinical trial within 30 days of study start. Breastfeeding women were excluded. Sexually active female participants were required to have a negative pregnancy test and had to agree to use an acceptable form of contraception until 6 months after vaccination. All volunteers provided written informed consent before enrollment.
After screening and enrollment, baseline blood and saliva samples were collected on day 1 before administration of a single dose of the candidate vaccine by intramuscular injection in the deltoid muscle. Participants returned to the clinic to provide the large-volume (~50 mL) blood samples for the test panel, as well as saliva samples, on days 8, 15, and 29; safety assessments were also performed at these visits. A final safety evaluation was performed on day 183, approximately 6 months after vaccination.
Each 0.5-mL dose of candidate vaccine (lot number 3-FIN-1897) contained 15 µg GI.1 genotype VLP, 50 µg GII.4c VLP, and 0.5 mg aluminum hydroxide (Brenntag Biosector A/S, Denmark).
Safety and Reactogenicity
All vaccinees were monitored for 30 minutes after vaccination for any immediate reactions. Each participant then completed a 7-day diary card that solicited local (pain, swelling, induration, erythema at the injection site) and systemic (headache, fatigue, myalgia, arthralgia, vomiting diarrhea) adverse events (AEs) and their severity. Maximum diameters of any swelling, induration, and erythema were measured and any reaction >10 cm was considered severe. Pain and solicited systemic AEs were considered severe if, without treatment, they prevented normal daily activity. Oral temperature was recorded daily, with temperatures ≥38.0°C (100.4°F) considered as fever. Unsolicited AEs were recorded up to 28 days after vaccination and serious AEs (SAEs) were recorded throughout the study duration.
Immunogenicity
Humoral immune responses were assessed as total serum immunoglobulin (pan-Ig) and immunoglobulin A (IgA) antibodies against the GI.1 and GII.4c antigens measured by enzyme-linked immunosorbent assay and as histo-blood group antigen (HBGA)–blocking antibodies as previously described [11]. Salivary IgA antibody levels were measured using a previously described assay using GI.1 and GII.4c VLPs as virus-specific antigens [13]. Individual norovirus-specific salivary IgA levels were normalized by the total salivary IgA level—that is, the ratio of the respective antigen-specific IgA level (GI.1 or GII.4) with the total IgA levels, prior to being used in any analysis.
Statistical Analysis
All safety, reactogenicity, and immunogenicity results are descriptive. No formal sample size calculations were performed; the sample size was selected to provide adequate serum for assay validation and establishment of proficiency panels with an expected drop-out rate of 10%. All serum antibody responses are expressed as geometric mean titer (GMT) or geometric mean HBGA–blocking antibody titer, with 95% confidence intervals (95% CIs) calculated based upon the Clopper-Pearson methodology for each time point. Geometric mean fold rises over baseline (day 1) and response rates (percentages of each group displaying a ≥4-fold increase in titer from baseline) were calculated at each time point.
For salivary IgA conventional descriptive statistics (including arithmetic mean and standard deviation) instead of GMTs were derived for the normalized mucosal IgA antibody levels. Log-transformed data of normalized salivary IgA titers were plotted against serum IgA titers for all visits. The Pearson correlation coefficient for the 2 assays (with the corresponding 95% CI) and the associated P value were calculated using SAS for Windows version 9.2.
RESULTS
Fifty-nine persons were screened and 50 were enrolled (mean age, 30.8 years; 28 were men; 36 were white and 14 black/African American) and received 1 dose of the vaccine candidate. One subject was lost to follow-up and 1 was withdrawn following incarceration, leaving 48 who completed the study according to protocol and were included in the safety analyses. Two additional subjects were excluded from the immunogenicity analyses on study days where their blood or saliva samples were missing. No subject reported an episode of AGE during the 4-week study period.
Humoral Immunogenicity
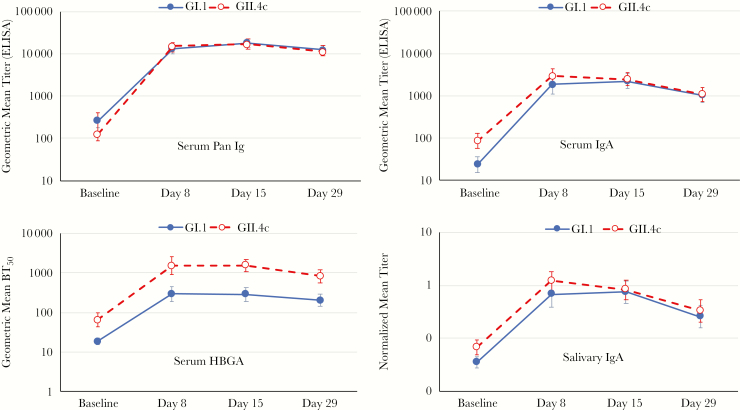
Vaccination elicited rapid increases in antibody levels against both vaccine VLP antigens, with large increases in serum pan-Ig, IgA, and HBGA–blocking antibodies by day 8 that plateaued at day 15 and slightly waned by day 29 (Figure 1). Serum IgA antibody titers against both GI.1 and GII.4c were lower than pan-Ig but the profiles of pan-Ig and IgA responses to the 2 different genotypes were similar, with no meaningful differences between the 2. Peak pan-Ig response rates of 97.9% were achieved against both GI.1 and GII.4c at day 15 (Table 1). Serum HBGA–blocking antibody titers to GII.4c were consistently higher than to GI.1, but the pattern of rapid increase and slight waning was similar for both VLP antigens. By day 8 there was a 15.8-fold increase in HBGA–blocking antibody levels against GI.1 and 23.4-fold increase against GII.4c, which waned to levels 10.7- and 12.3-fold higher than baseline by day 29, respectively.
Figure 1.
Geometric mean titers (with 95% confidence interval bars) of panimmunoglobulin and immunoglobulin A antibodies, and geometric mean blocking titers of histo-blood group antigen blocking antibodies against GI.1 and GII.4c virus-like particles at each of the sampling time-points. Abbreviations: BT50, geometric mean blocking titer; ELISA, enzyme-linked immunosorbent assay; HBGA, histo-blood group antigen–blocking; Ig, immunoglobulin; IgA, immunoglobulin A.
Table 1.
Geometric Mean Fold Rises From Baseline and Response Rates for Each Antibody Assay Against GI. 1 and GII.4c in Serum and Saliva at the Indicated Days After Vaccination
| Genotype | Day After Vaccination | Antibody | |||
|---|---|---|---|---|---|
| Serum Pan-Ig (n = 48) | Serum IgA (n = 48) | Serum HBGA–blocking (n = 48) | Saliva IgA (n = 48) | ||
| Geometric mean-fold rise (95% CI) | |||||
| GI.1 | Day 8 | 49.5 (30.6–80.0) |
78.5 (49.1–125.4) |
15.8 (10.2–24.5) |
18.7 (10.9–31.9) |
| Day 15 | 66.7 (41.6–106.7) |
87.8 (57.0–135.4) |
15.1 (10.4–22.1) |
20.1a (12.2–33.1) |
|
| Day 29 | 50.3b (32.1–78.6) |
45.7b (30.7–68.0) |
10.7b (7.7–14.9) |
6.9b (4.1–11.5) |
|
| GII.4c | Day 8 | 117.1 (76.7–178.8) |
33.1 (21.71–50.6) |
23.4 (13.7–39.9) |
17.1 (11.7–25.1) |
| Day 15 | 129.3 (87.1–192.0) |
27.3 (18.4–40.4) |
23.5 (15.5–35.7) |
11.8a (7.8–17.9) |
|
| Day 29 | 85.2b (57.3–126.8) |
12.5b (8.7–18.0) |
12.3b (8.3–18.2) |
4.7b (2.9–7.5) |
|
| Response rate, % (95% CI) | |||||
| GI.1 | Day 8 | 93.8 (82.8–98.7) |
93.8 (82.8–98.7) |
85.4 (72.2–93.9) |
79.2 (65.0–89.5) |
| Day 15 | 97.9 (88.9–99.9) |
97.9 (88.9–99.9) |
87.5 (74.8–95.3) |
87.2a (74.3–95.2) |
|
| Day 29 | 97.8b (88.5–99.9) |
95.7b (85.2–99.5) |
82.6b (68.6–92.2) |
65.2b (49.8–78.6) |
|
| GII.4c | Day 8 | 97.9 (88.9–99.9) |
95.8 (85.7–99.5) |
83.3 (69.8–92.5) |
87.5 (74.8–95.3) |
| Day 15 | 97.9 (88.9–99.9) |
93.8 (82.8–98.7) |
91.7 (80.0–97.7) |
83.0a (69.2–92.4) |
|
| Day 29 | 95.7b (85.2–99.5) |
84.8b (71.1–93.7) |
84.8b (71.1–93.7) |
52.2b (36.9–67.1) |
|
Abbreviations: CI, confidence interval; HBGA, histo-blood group antigen; Ig, immunoglobulin; IgA, immunoglobulin A.
an = 47.
bn = 46.
Normalized salivary IgA levels were much lower than the serum levels, but the response to vaccination paralleled the serum IgA responses for both vaccine antigens. Peak mean fold increase in salivary IgA was 20.1 and 17.1 for GI.1 and GII.4c, respectively, with >87% of subjects displaying a salivary immune response against each VLP.
When the values for normalized IgA for the GI.1 and GII.4c antigens in saliva were plotted against the corresponding serum values for all time-points, there were statistically significant moderate to strong correlations for both: Pearson r = 0.700 (95% CI, .619–.766; P = .001) for GI.1 and Pearson r = 0.795 (95% CI, .736–.842; P = .001) for GII.4c (Supplementary Figure).
Safety and Tolerability
There were no deaths or vaccine-related SAEs reported during the 28-day reporting period, or any withdrawals due to an SAE. The vaccine was well tolerated, with only mild to moderate AEs reported by 34 of the 50 vaccinees (68%), most of which occurred within the first 3 days. The only local reaction was mild/moderate injection site pain, reported by 50% of the participants in days 1–3 and another 6% during days 4–7. The most frequent systemic AEs were fatigue (34%) headache (24%), myalgia (12%), and diarrhea (12%), which also occurred mainly during the first 3 days after vaccination. No fever was reported. Of 19 unsolicited AEs reported by 11 (22%) participants in the 28 days after vaccination, 3 events in 2 participants, cases of diarrhea, headache, and fatigue, were considered to be related to the vaccination.
DISCUSSION
The main purpose of this trial was to collect the large volumes of serum samples necessary to establish proficiency panels of sera to allow postvaccination assessments of immune responses. As such, it was important that we confirmed that rapid and robust humoral immune responses manifested as increases in serum pan-Ig, IgA, and HBGA-blocking antibodies against both vaccine antigens, GI.1 and GII.4c, on day 8 after vaccination. These observations confirm the rapid response to a similar vaccine candidate formulation adjuvanted with monophosphoryl lipid A (MPL) observed in a previous study in adults of the same age [11]. Another study in this age group has confirmed that the response is not affected by the presence of MPL, but does depend upon the relative concentrations of GI.1 and GII.4c VLPs in the formulations, with the present unbalanced formulation of 15 µg GI.1 and 50 µg GII.4c VLP providing the best overall response to both genotypes [12]. The present study also confirmed the good tolerability of the vaccine candidate and did not reveal any new safety concerns beyond those already known for NoV. The most frequent AE was transient mild to moderate pain at the injection site, which occurred and resolved within 7 days in all cases.
Intestinal mucosal immunity may play a primary protective role against norovirus infection, as with other orally ingested pathogens, such as poliovirus and cholera. Higher levels of salivary IgA were associated with a lower risk of illness in persons challenged with Norwalk virus [13]. Similarly, higher levels of serum IgA were associated with protection against infection and illness among placebo recipients in a GII.4 challenge study [14]. A recent report of parenteral immunization of mice with a GII.4 VLP vaccine failed to induce measurable mucosal immune responses [15]. In contrast, the current study demonstrates that mucosal immune responses were induced following vaccination, as demonstrated by rapid increases in salivary IgA against both genotypes, with a profile similar to that of the humoral responses and with moderate to strong correlations between the normalized levels of IgA antibodies in saliva and serum IgA antibody titers.
In summary, we confirmed previous reports that a single dose of NoV vaccine administered by intramuscular injection was well-tolerated, causing only mild to moderate and transient AEs, and no vaccine-related SAEs. The formulation used elicited robust homologous immune responses to the 2 genotype component VLPs by day 8 as evidenced by pan-Ig, IgA, and HBGA–blocking antibody responses. Mucosal immunity as measured by salivary IgA levels was also induced and paralleled in profile the serum IgA responses. Future studies will delineate the influence of the vaccination on intestinal immunity and its role in preventing norovirus infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors are grateful to all the volunteers who participated in the study, to the staff at the study center, and to Frederick Neill and Sasirekha Ramani for assistance with the salivary IgA studies. Keith Veitch (Takeda Vaccines) assisted in preparation of the manuscript and provided general editorial assistance.
Disclaimer. The views, opinions, and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy, or decision unless so designated by other documentation. The investigator(s) adhered to the policies regarding the protection of human subjects as prescribed by Code of Federal Regulations (CFR) Title 45, Volume 1, Part 46; Title 32, Chapter 1, Part 219; and Title 21, Chapter 1, Part 50 (Protection of Human Subjects).
Financial support. The study sponsor was Takeda Pharmaceutical International AG, Zurich, Switzerland. This work was supported by the US Army Medical Research and Materiel Command (contract number W81XWH-15-C-0063).
Potential conflicts of interest. J. P. C., F. B., A. B., and P. M. M. are all full-time employees of the study sponsor. R. L. A. has received grant support from Takeda Vaccines, Inc. C. H. reports no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lopman BA, Steele D, Kirkwood CD, Parashar UD. The vast and varied global burden of norovirus: prospects for prevention and control. PLoS Med 2016; 13:e1001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Payne DC, Vinjé J, Szilagyi PG, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med 2013; 368:1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoa Tran TN, Trainor E, Nakagomi T, Cunliffe NA, Nakagomi O. Molecular epidemiology of noroviruses associated with acute sporadic gastroenteritis in children: global distribution of genogroups, genotypes and GII.4 variants. J Clin Virol 2013; 56:185–93. [DOI] [PubMed] [Google Scholar]
- 4.Atmar RL, Ramani S, Estes MK. Human noroviruses: recent advances in a 50-year history. Curr Opin Infect Dis 2018; 31:422–32. [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Sun L, Fang L, et al. Gastroenteritis outbreaks caused by norovirus GII.17, Guangdong province, China, 2014–2015. Emerg Infect Dis 2015; 21:1240–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trivedi TK, Desai R, Hall AJ, Patel M, Parashar UD, Lopman BA. Clinical characteristics of norovirus-associated deaths: a systematic literature review. Am J Infect Control 2013; 41:654–7. [DOI] [PubMed] [Google Scholar]
- 7.Atmar RL, Bernstein DI, Harro CD, et al. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med 2011; 365:2178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parra GI, Bok K, Taylor R, et al. Immunogenicity and specificity of norovirus consensus GII.4 virus-like particles in monovalent and bivalent vaccine formulations. Vaccine 2012; 30:3580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein DI, Atmar RL, Lyon GM, et al. Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J Infect Dis 2015; 211:870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treanor JJ, Atmar RL, Frey SE, et al. A novel intramuscular bivalent norovirus virus-like particle vaccine candidate—reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults. J Infect Dis 2014; 210:1763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atmar RL, Baehner F, Cramer JP, Song E, Borkowski A, Mendelman PM; NOR-201 Study Group . Rapid responses to 2 virus-like particle norovirus vaccine candidate formulations in healthy adults: a randomized controlled trial. J Infect Dis 2016; 214:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leroux-Roels G, Cramer JP, Mendelman PM, et al. Safety and immunogenicity of different formulations of norovirus vaccine candidate in healthy adults: a randomized, controlled, double-blind clinical trial. J Infect Dis 2018; 217:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramani S, Neill FH, Opekun AR, et al. Mucosal and cellular immune responses to Norwalk virus. J Infect Dis 2015; 212:387–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atmar RL, Bernstein DI, Lyon GM, et al. Serological correlates of protection against a GII.4 norovirus. Clin Vaccine Immunol 2015; 22:923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinimäki S, Malm M, Vesikari T, Blazevic V. Parenterally administered norovirus GII.4 virus-like particle vaccine formulated with aluminum hydroxide or monophosphoryl lipid A adjuvants induces systemic but not mucosal immune responses in mice. J Immunol Res 2018; 2018:3487095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.