Abstract
Background. Noroviruses are the most important viral causes of gastroenteritis-related morbidity and mortality. A randomized, double-blind, placebo-controlled study evaluated an adjuvanted bivalent intramuscular norovirus virus-like particle (VLP) vaccine.
Methods. Forty-eight adults aged 18–49 years received either 2 doses containing genotype GI.1 VLP and a consensus GII.4 VLP or 2 doses of placebo. Doses (5 µg, 15 µg, 50 µg, or 150 µg of each VLP) were administered 4 weeks apart in the first stage. Subsequently, 54 adults, aged 18–49 (n = 16), 50–64 (n = 19), and 65–85 (n = 19) years, received 2 doses of vaccine containing 50 µg of each VLP. Total and class-specific antibody responses, as well as histoblood group antigen (HBGA) blocking antibody responses, were measured before and after each dose.
Results. Local reactions were mainly injection site pain/tenderness, with no reported fever or vaccine-related serious adverse events. One dose of vaccine containing 50 µg of each VLP increased GI.1 geometric mean titers (GMTs) by 118-fold, 83-fold, and 24-fold and increased GII.4 GMTs by 49-fold, 25-fold, and 9-fold in subjects aged 18–49, 50–64, and 65–83 years, respectively. Serum antibody responses peaked at day 7 after the first dose, with no evidence of boosting following a second dose. Most subjects achieved HBGA-blocking antibody titers of ≥200.
Conclusions. The vaccine was well tolerated and immunogenic. Rapid immune response to a single dose may be particularly useful in military personnel and travelers and in the control of outbreaks.
Clinical Trials Registration. NCT01168401.
Keywords: gastroenteritis, norovirus, vaccine, virus-like particle
Noroviruses are recognized as the most important cause of outbreaks and sporadic cases of viral gastroenteritis throughout the world. The proportional incidence of norovirus-associated disease will continue to increase with the successful introduction of rotavirus vaccines. Outbreaks of norovirus infection are extremely difficult to control because of the combination of high levels of virus, prolonged shedding, environmental stability, resistance to disinfection, low infectious dose, and contribution of virus-induced vomiting to the spread of the infectious agent. It is estimated that, in the United States, noroviruses are responsible for 5600–70 000 hospitalizations and 560–800 deaths annually [1–3]. In developing countries, it is estimated that up to 200 000 deaths due to norovirus occur annually among children <5 years of age [4].
Development of effective vaccines to prevent norovirus disease and transmission is an important step toward control of this global public health problem. Expression of the norovirus capsid protein VP1 leads to formation of virus-like particles (VLPs) that have emerged as promising vaccine candidates. Both orally and nasally administered VLPs have been well tolerated and immunogenic in phase 1 trials [5, 6]. Importantly, a nasally administered investigational GI.1 norovirus VLP vaccine was recently shown to confer 47% protection against norovirus gastroenteritis in healthy adults in a human experimental challenge model [7].
To explore development of an injectable norovirus vaccine, we performed a phase 1 dose-escalation and age-escalation study with a bivalent, MPL (3-O-desacyl-4′-monophosphoryl lipid A; GlaxoSmithKline)–adjuvanted formulation of a norovirus VLP vaccine. The vaccine included 2 genotypes, GI.1 and GII.4, representing the 2 major norovirus genogroups infecting humans, and was administered intramuscularly to cohorts of healthy adults spanning a wide age range of 18–85 years.
MATERIALS AND METHODS
Participants
The study was conducted as a randomized, multisite, dose-escalation, and age-escalation study of the safety and immunogenicity of the investigational bivalent norovirus vaccine at the University of Rochester, the Saint Louis University Medical Center, and Walter Reed Army Hospital. The study was approved by the respective institutional review board at each institution, including the Naval Medical Research Center, in compliance with all applicable federal regulations governing the protection of human subjects, and was registered at ClinialTrials.gov (NCT01168401). All volunteers provided written informed consent prior to enrollment.
Eligible subjects were healthy adults aged ≥18 years with normal baseline clinical laboratory results at screening. Subjects were excluded if they had unstable medical conditions; serological evidence of hepatitis B virus infection, hepatitis C virus infection, or HIV infection; any condition likely to affect immune response (immunodeficiency or taking immunosuppressant medication); or a history of autoimmune or neuroinflammatory disease. Both men and women agreed to be abstinent or use contraceptive measures as necessary for up to 60 days after the last vaccination. Secretor status was determined as previously described [7] for all subjects after enrollment.
Vaccines
The vaccine candidate is a bivalent VLP formulation designed to elicit a broad antibody response to both genotype GI.1 and GII.4 noroviruses. The GI.1 component was based on the prototypic Norwalk virus, while the GII.4 component was based on a consensus sequence between 3 GII.4 viruses, 2006a (Yerseke), 2006b (Den Haag), and 2002 (Houston). Previous studies in animals had shown that this VLP elicited a broad antibody response against multiple GII.4 genotypes [8]. Different formulations were prepared such that a single dose contained 5 µg, 15 µg, 50 µg, or 150 µg of each of the 2 VLP components. All formulations were adjuvanted with a constant amount of 50 µg of MPL adjuvant and 0.5 mg of aluminum hydroxide (Brenntag Biosector, Denmark). All subjects in the dose-escalation and age-escalation stages were to receive 2 doses of a single vaccine formulation or placebo by intramuscular injection, 28 days apart.
Study Design
The study was performed in 2 stages. The first stage, a dose-escalation stage, was conducted in subjects 18–49 years of age (cohort A). Approximately 10 subjects in each dose group, beginning with the group receiving 5 µg per component, were randomly assigned at a ratio of 5 to 1 to receive vaccine or placebo and observed through day 7 following the second dose (ie, day 35). After review of safety data by an independent central safety monitor, the group receiving the next highest dose of each VLP component was enrolled and evaluated in similar fashion; the process was repeated for the 50-µg and 150-µg doses. Following assessment of serological assays in the dose-escalation stage, the vaccine formulation containing 50 µg of each VLP component (hereafter, the 50/50-µg formulation) was selected for further development, in stage 2. Additional groups of 50–64-year–old subjects (cohort B), 65–85-year-old subjects (cohort C), and 18–49-year-old subjects (cohort D) were enrolled and randomly assigned at a ratio of 1 to 1 to receive bivalent vaccine or saline placebo for further safety and immunogenicity evaluation.
All subjects were monitored clinically for reactions to injections after each vaccination. Participants recorded solicited injection site reactions (redness, swelling, pain, and tenderness) and systemic reactions (oral temperature, headache, fatigue, muscle aches, chills, joint aches, and gastrointestinal symptoms of nausea, vomiting, diarrhea, and abdominal cramps/pain) on memory aid forms for days 0–7 after vaccination. Other adverse events (AEs) were reported throughout the study period. All reactions and events were graded for severity (mild, moderate, or severe) by the participants, and the investigator assessed the relationship of the reactions and events to vaccination.
Serological Analysis
Blood samples were collected before the first vaccination (day 0) and on days 7, 21, 28, 35, 56, 180, and 393 after the first dose of vaccine, for evaluation of immunogenicity. Serum antibodies to the GI.1 and GII.4 vaccine antigens were determined using 5 serological assays. Total immunoglobulin (Pan-Ig) and class-specific (immunoglobulin G [IgG] and immunoglobulin A [IgA]) norovirus-specific antibodies were determined by enzyme linked immunoassay (ELISA) and duplex, time-resolved, dissociation-enhanced lanthanide fluorescence immunoassay, respectively [9, 10]. Histoblood group antigen (HBGA) blocking antibody titers were determined using a previously described assay [11]; the glycans in the assay were H type 1 and H type 3 for GI.1 and GII.4 VLP binding, respectively. GI.1 VLP– and GII.4 VLP–specific hemagglutination inhibiting antibody titers were also evaluated (data not shown). Blood group and secretor status were also determined for all subjects by previously described methods [7].
Statistical Methods
The sample size was selected to obtain adequate indications of safety, reactogenicity, and immune response data within the constraints of a phase 1 trial. Reactogenicity and safety data were assessed on the basis of incidence and severity and are described with respect to dose and age group. No formal statistical analyses were performed. Antibody titers were reported as seroresponse rates ([SRRs]; or percentages with ≥4-fold rises), geometric mean titers (GMTs), and geometric mean fold rises (GMFRs) over baseline. The log transformation of the antibody titers and fold rises of antibody titers were compared between groups, using fixed-effects analysis of variance. Data are expressed as point estimates and 95% confidence estimates. All data analyses and eventual statistical computations were conducted with SAS, version 9.3.
RESULTS
Participant Demographic Characteristics
A total of 102 subjects were enrolled into the study: 48 subjects 18–49 years of age were included in the dose-escalation stage (cohort A), and 54 subjects in different age groups were included in cohorts B, C, and D for subsequent evaluation of the 50/50-µg formulation. The demographic characteristics of each active vaccine group and the combined placebo recipients across all cohorts, as well as their blood group distribution and secretor status, are shown in Table 1. The demographic makeup and blood group and secretor status of each study cohort were similar. Because 18–49-year-old subjects in cohorts A and D were recruited in different locations, they had slightly different racial and blood group distributions: in cohort A, subjects enrolled at Rochester and Saint Louis were primarily female (68%–77%) and white (68%–73%), while cohort D subjects, enrolled at Walter Reed Army Hospital by the Naval Medical Research Center, were primarily male (63%) and black (63%).
Table 1.
Demographic Characteristics, Secretor Status, and Blood Typing of Subjects Who Received 2 Doses of Either Equal Amounts of Norovirus GI.1 Virus-Like Particle (VLP) and a Consensus Norovirus GII.4 VLP or Placebo
| Variable | Cohort A Dose Group |
50/50-µg Dose Cohort |
||||||
|---|---|---|---|---|---|---|---|---|
| 5 µg | 15 µg | 50 µg | 150 µg | D | B | C | Placebo | |
| Target age range, y | 18–49 | 18–49 | 18–49 | 18–49 | 18–49 | 50–64 | 65–85 | 18–85 |
| Subjects, no. | 10 | 10 | 10 | 9 | 8 | 9 | 10 | 36 |
| Demographic characteristic | ||||||||
| Age, y, mean (range) | 33.2 (23–46) | 31.9 (19–46) | 31.9 (22–42) | 32.8 (23–49) | 32 (20–48) | 55.2 (51–59) | 68.5 (65–83) | 48.1 (19–73) |
| Male sex | 3 (30) | 4(40) | 3 (30) | 2 (22) | 5 (63) | 4 (44) | 2 (20) | 11 (31) |
| Ethnicity | ||||||||
| American Indian | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3) |
| Asian | 0 | 0 | 2 (20) | 2 (22) | 0 | 0 | 0 | 0 |
| Black | 2 (20) | 2 (20) | 2 (20) | 1 (11) | 5 (63) | 0 | 0 | 7 (19) |
| White | 8 (80) | 7 (70) | 6 (60) | 6 (67) | 3 (38) | 9 (100) | 10 (100) | 27 (75) |
| Multiracial | 0 | 1 (10) | 0 | 0 | 0 | 0 | 0 | 1 (3) |
| Norovirus secretor | 7 (70) | 8 (80) | 6 (60) | 9 (100) | 7 (88) | 6 (67) | 8 (80) | 28 (78) |
| Blood group | ||||||||
| A | 3 (30) | 3 (30) | 5 (50) | 3 (33) | 1 (13) | 2 (22) | 3 (30) | 12 (33) |
| B | 0 | 1 (10) | 0 | 1 (11) | 2 (25) | 2 (22) | 0 | 5 (14) |
| O | 6 (60) | 4 (40) | 5 (50) | 5 (56) | 5 (63) | 5 (56) | 7 (70) | 16 (44) |
| AB | 1 (10) | 2 (20) | 0 | 0 | 0 | 0 | 0 | 3 (8) |
Data are no. (%) of subjects, unless otherwise indicated.
Safety and Reactogenicity
There were no serious AEs related to vaccination during the 1-year safety surveillance for the whole study. In the dose-escalation stage (cohort A), 2 cases of severe diarrhea (≥6 stools in 24 hours) were reported, one on day 4 after receipt of a first dose of placebo (saline) and one on day 1 after a second dose of vaccine containing 5 µg per VLP. Local and systemic reactogenicity were more frequent in vaccine recipients, compared with placebo recipients, with no dose relationship (Figure 1). There was no pattern of increasing solicited local or systemic AEs as VLP dose increased, nor was there an increase following receipt of dose 2, compared with after receipt of dose 1. The incidence and severity of most local AEs peaked on the day of vaccination or the day afterward and resolved by day 7. Most local AEs were mild, and none were severe. Local reactions primarily consisted of pain and/or tenderness at the injection site. The majority of systemic reactions were reported as mild, the most frequent being mild headache. No fever was reported in the 7 days after either vaccination.
Figure 1.
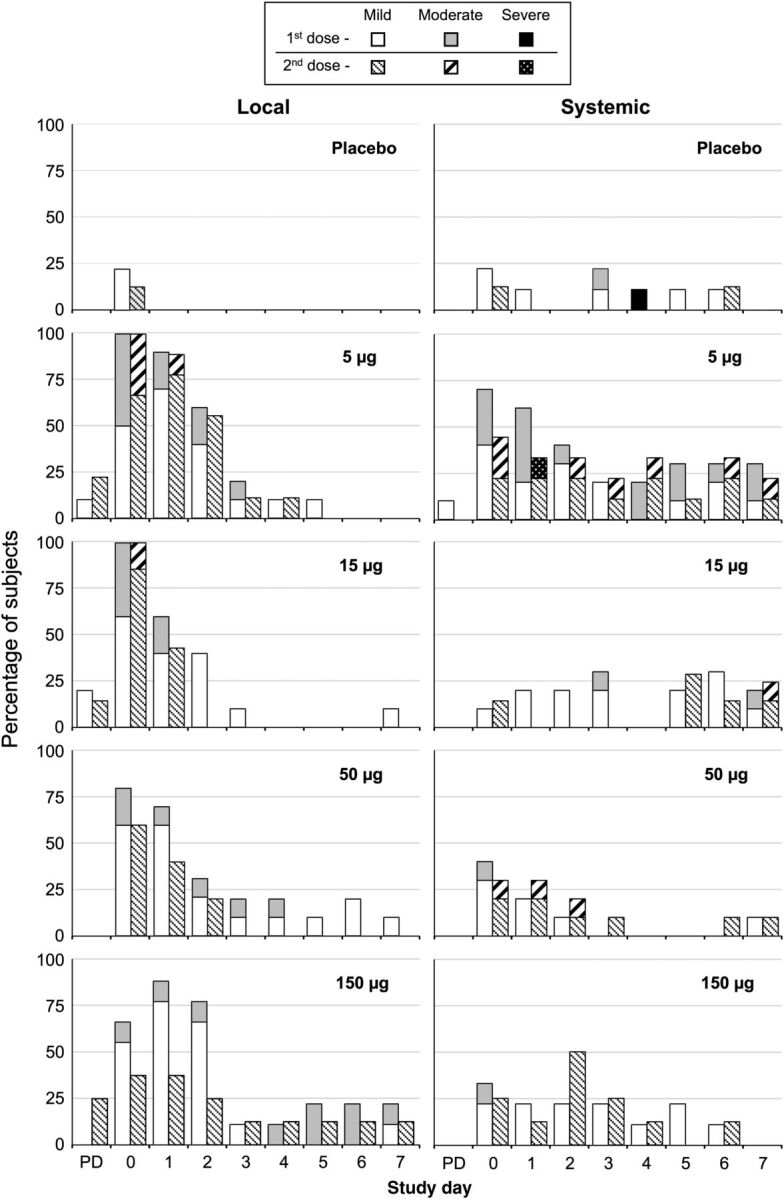
Dose-related reactogenicity of the bivalent norovirus virus-like particle vaccine formulated with aluminum hydroxide and MPL (3-O-desacyl-4′-monophosphoryl lipid A; GlaxoSmithKline) in 18–49 year olds (cohort A). Frequencies of local and systemic reactogenicity events are shown by day after doses 1 and 2, with maximum severity during the observation period assessed as mild, moderate, or severe. The most common local complaints were pain and tenderness at the injection site, and the most common systemic complaint was headache. Post dosing assessment, performed 30 minutes after dosing. Abbreviation: PD, post dosing assessment.
A similar profile of low reactogenicity and good tolerance was observed with the 50/50-µg vaccine in the older age groups (cohorts B and C). The most frequent local reactions were mild pain and/or tenderness at the injection site, and the most common systemic event was mild headache (Supplementary Materials).
Stage 1: Analysis of Immunogenicity by Dose Escalation
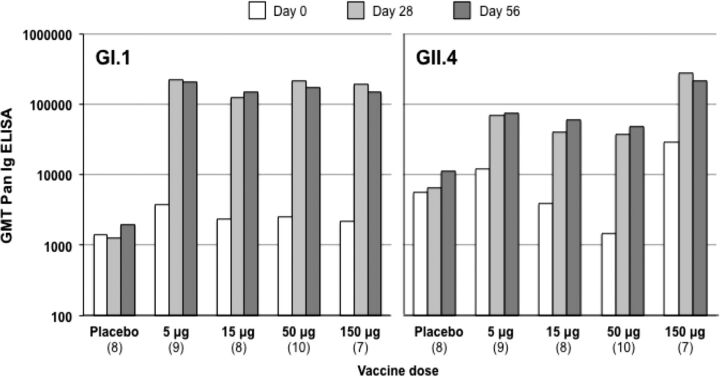
As illustrated in Figure 2, all subjects in the dose-escalation stage (cohort A), who received 2 doses of 5–150 µg of each VLP, demonstrated immune responses to both GI.1 and GII.4 VLPs after receipt of the first dose. All dose groups elicited similar responses of similar magnitude to the GI.1 VLP expressed as GMTs, GMFRs, or SRRs, compared with baseline (Table 2). Variations in baseline GMTs were observed between dose groups. For GI.1, all subjects (100%) displayed a seroresponse (≥4-fold increase in titer from baseline) after the first dose, independent of dose. GMTs were not further increased after the second dose; there was no indication of boosting. Responses to GI.1 were of higher magnitude than those to the GII.4 component.
Figure 2.
Geometric mean titers (GMTs) of enzyme-linked immunosorbent assay of total immunoglobulin (Pan-Ig) antibodies to norovirus GI.1 and GII.4 virus-like particles (VLPs) in cohort A, with dose escalation, assessed before (day 0) and 4 weeks after the first dose (day 28) and 4 weeks after the second dose (day 56). Data in parentheses show the number of subjects per group. Abbreviation: ELISA, enzyme-linked immunosorbent assay.
Table 2.
Dose-Related Response to Norovirus GI.1 and GII.4 Virus-Like Particles in Healthy Adults Aged 18–49 Years in Cohort A, by Results of Enzyme-Linked Immunosorbent Assay of the Total Immunoglobulin Response
| Group | Subjects, No. | G1.1 |
GII.4 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 28 |
Day 56 |
Day 0 | Day 28 |
Day 56 |
||||||||||
| GMT | GMT | GMFRa | Subjects, %b | GMT | GMFRa | Subjects, %b | GMT | GMT | GMFRa | Subjects, %b | GMT | GMFRa | Subjects, %b | ||
| Placebo | 8 | 1396 | 1280 | 1.2 | 0 | 1974 | 1.4 | 25 | 5583 | 6451 | 1.4 | 13 | 11 167 | 2.0 | 13 |
| Vaccine dose | |||||||||||||||
| 5 µg | 9 | 3763 | 222 952 | 59.3 | 100 | 206 425 | 54.9 | 100 | 11 945 | 70 225 | 5.9 | 56 | 75 848 | 6.3 | 56 |
| 15 µg | 8 | 2348 | 126 338 | 53.8 | 100 | 148 394 | 70.7 | 100 | 3948 | 40 960 | 10.4 | 88 | 60 866 | 10.8 | 86 |
| 50 µg | 10 | 2560 | 216 188 | 84.4 | 100 | 176 957 | 64.0 | 100 | 1470 | 38 217 | 26.0 | 80 | 47 781 | 21.8 | 89 |
| 150 µg | 7 | 2153 | 194 840 | 90.5 | 100 | 150 242 | 69.8 | 100 | 28 963 | 275 545 | 9.5 | 75 | 212 474 | 7.3 | 75 |
Times correspond to before (day 0) and 4 weeks after the first dose (day 28) and 4 weeks after the second dose (day 56).
Abbreviation: GMT, geometric mean titer.
a Geometric mean fold rise (GMFR) is defined as the geometric mean of the ratio between the titer on the given day and the titer at baseline.
b Defined as the percentage with a ≥4-fold increase in titer from baseline.
For GII.4, there was more variation in baseline GMTs between groups, and GMFRs were more variable than for GI.1 (Table 2). Not all subjects displayed a seroresponse to GII.4, with only 56% responding in the 5/5-µg group and 75%–88% responding in the higher-dose groups, and these proportions and final GMTs were not increased by a second dose. The point estimate of the GMT for GII.4 was highest for the 150/150-µg group. However, taking into account baseline titers and measuring GMFR (to normalize variation), the response to 1 dose of the GII.4 VLP was highest for the 50/50-µg dose; the GMFR was 9.5-fold for the 150-µg VLP dose, compared with 26-fold for the 50-µg dose. Administration of a second dose did not significantly enhance responses.
On the basis of these responses in 18–49 year olds and the reactogenicity profile, further study cohorts subsequently received the 50/50-µg dose of each VLP component in the bivalent vaccine.
Stage 2
Analysis of Immunogenicity by Age Group
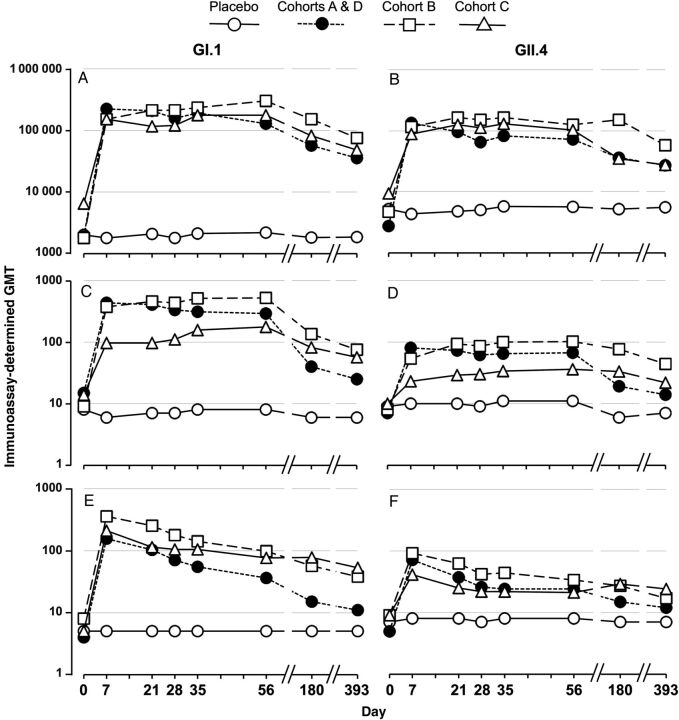
Subjects aged 50–64 years (cohort B), 65–85 years (cohort C), and the additional 18–49 year olds (cohort D) were subsequently recruited and randomly assigned to receive 2 doses of the 50/50-µg bivalent vaccine formulation or placebo, with each dose separated by 28 days. As in the dose-escalation cohort, the first dose induced large serum antibody responses in total Pan-Ig, IgG, and IgA to both components in all 3 age groups with each of these assays (Figure 3). These responses peaked at the first time point measured (day 7) after the first dose. There was no significant increase in response to the second dose in any of the age groups. Response patterns to both VLP components of the vaccine were similar, but point estimates of titers to the GII.4 component were lower than those to the GI.1 component in each assay. Pan-Ig and IgA responses to both VLP components were similar in the 3 age groups, but IgG responses showed some age-dependent variation, with IgG GMTs lower in subjects aged 65–83 years. The sample sizes evaluated did not have the power to identify significant differences between age groups nor between responses to the 2 different VLPs at the levels observed. It is notable, however, that baseline GII.4 titers were higher than GI.1 titers, which may reflect more-recent prior exposure to the GII.4 genotype.
Figure 3.
Serum antibody responses to the vaccine containing 50 μg of norovirus GI.1 virus-like particle (VLP) and 50 μg of consensus norovirus GII.4 VLP in 18–49 year olds (cohorts A and D), 50–64 year olds (cohort B), and 65–85 year olds (cohort C). Placebo recipients are combined across all cohorts. Subjects received 2 doses of vaccine separated by 28 days (day 0 and day 28). Geometric mean titers (GMTs) of antibody are shown to GI.1 (A, C, E) and GII.4 consensus (B, D, F) VLPs as assessed by total immunoglobulin (A and B), immunoglobulin G (C and D), and immunoglobulin A (E and F) immunoassays.
Analysis of Immunogenicity by Measuring the HBGA-Blocking Antibody Response
The ability of serum antibody to block attachment of VLPs to HBGAs, which has been proposed as a surrogate measure of neutralizing activity [12], was also assessed (Table 3). The vaccine induced a vigorous HBGA-blocking antibody response after the first dose in all 3 age cohorts, with little increase after the second dose. The majority of subjects in all 3 age groups developed antibody titers of ≥200 against both GI.1 and GII.4 viruses after the first dose.
Table 3.
Histoblood Group Antigen–Blocking Geometric Mean Titers (GMTs) and Proportions of Subjects Achieving Titers of ≥200 Against Norovirus GI.1 and GII.4 Virus-Like Particles
| Antigen, Time | Cohorts A and D |
Cohort B |
Cohort C |
|||
|---|---|---|---|---|---|---|
| GMT (95% CI) | Subjects With GMT ≥200, % | GMT (95% CI) | Subjects With GMT ≥200, % | GMT (95% CI) | Subjects With GMT ≥200, % | |
| GI.1 | ||||||
| Day 0 | 16 (12–22) | 0 | 17 (8–34) | 0 | 17 (11–27) | 0 |
| Day 28 | 260 (166–405) | 61 | 251 (170–370) | 75 | 216 (126–369) | 56 |
| Day 56 | 191 (139–262) | 35 | 276 (171–444) | 63 | 278 (228–338) | 89 |
| GII.4 | ||||||
| Day 0 | 25 (15–41) | 0 | 49 (16–150) | 13 | 57 (16–200) | 33 |
| Day 28 | 249 (161–386) | 56 | 545 (272–1092) | 100 | 293 (171–500) | 67 |
| Day 56 | 172 (123–239) | 35 | 472 (228–979) | 75 | 318 (203–499) | 78 |
Subjects in cohorts A and D were aged 18–49 years, those in cohort B were aged 50–64 years, and those in cohort C were aged 65–83 years.
Abbreviation: CI, confidence interval.
Analysis of Immunogenicity by Blood Group and Secretor Status
Blood group (type B or AB vs A or O) and secretor status (FUT2 gene negative vs positive) have been identified as factors that reduce susceptibility to infection by some genotypes of norovirus, including GI.1 and GII.4 [13, 14]. We show overall Pan-Ig antibody titers of all cohorts in response to the VLP vaccine, by blood group and secretor status, in Table 4. Individuals who were secretor negative and who may be intrinsically less susceptible to infection with some norovirus genotypes, had lower titers of Pan-Ig at baseline, particularly to the GII.4 consensus VLP. The numbers of individuals who were blood group B or AB were too small (n = 4) to draw conclusions, although they also appeared to have lower titers of baseline antibody. Both groups responded similarly to the first dose of vaccine, and there were no statistically significant differences in either the GMFR in antibody titer or the titers achieved.
Table 4.
Effect of Secretor Status and Blood Group on Total Immunoglobulin Response Before and After Receipt of Norovirus Vaccine Containing GI.1 and GII.4 Virus-Like Particles
| Variable, Antigen | Day 0 | Day 28 |
Day 56 |
Day 0 | Day 28 |
Day 56 |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| GMT | GMT | GMFRa | GMT | GMFRa | GMT | GMT | GMFRa | GMT | GMFRa | |
| Secretor status | Negative (n = 10) | Positive (n = 25) | ||||||||
| GI.1 | 1576 | 163 840 | 104 | 206 425 | 128 | 3108 | 155 002 | 49.9 | 159 360 | 51.3 |
| GII.4 | 485 | 50 428 | 104 | 44 239 | 69.1 | 10 240 | 114 257 | 11.2 | 117 470 | 11.5 |
| Blood group | B/AB (n = 4) | A/O (n = 31) | ||||||||
| GI.1 | 1076 | 163 840 | 152.2 | 137 772 | 128 | 2863 | 156 675 | 54.7 | 175 599 | 59.7 |
| GII.4 | 1076 | 97 420 | 90.5 | 81 920 | 76.1 | 5120 | 89 584 | 17.5 | 91 952 | 15.3 |
Times correspond to before (day 0) and 4 weeks after the first dose (day 28) and 4 weeks after the second dose (day 56).
Abbreviation: GMT, geometric mean titer.
a Geometric mean fold rise (GMFR) is defined as the geometric mean of the ratio between the titer on the given day and the titer at baseline.
DISCUSSION
In this placebo-controlled dose- and age-escalation trial, the adjuvanted, bivalent norovirus VLP vaccine was well tolerated at all of the doses and ages evaluated. Although the group sizes in the dose-escalation stage were too small to make definitive comparisons, the rates of local and systemic reactogenicity events did not vary substantially by dose among these 18–49 year olds. Interestingly, the highest immune responses for Pan-Ig, IgA, IgG, and HBGA were observed for the GI.1 VLP antigen in the 150/150-µg dose, whereas the highest immune responses to the GII.4 VLP antigen were observed with the 50/50-µg dose. On the basis of the immune responses to and reactogenicity profiles of the different doses, the 50/50-µg vaccine was selected for further evaluation in the different age groups. The results in the age-escalation stage with the 50/50-µg vaccine confirmed the generally acceptable reactogenicity profile after 1 or 2 doses in all ages. The intranasal formulations previously studied included the adjuvant MPL [7]. To maximize the protection afforded by the intramuscular vaccine, the MPL component was retained in these new experimental formulations. The reactogenicity profile observed in this study is not different from what has been reported with other MPL/alum-adjuvanted vaccines, including a hepatitis B vaccine (HBV) for nonresponders to the standard HBV formulations [15], in which inclusion of the adjuvant significantly increased the incidence of solicited reactions (from 68% to 94.6%; P < .001), mainly because of an increase in injection site reactions (from 48.4% to 90.4%). Similarly, adolescent girls given a widely used VLP human papillomavirus vaccine containing MPL reported mild or moderate local pain after 81%–86% of doses, and headache, fatigue, and myalgia after 29%–33%, 29%–32%, and 25%–33% of doses, respectively [16].
The bivalent vaccine was effective at inducing serum antibody responses at all dose levels and in all of the adult age groups evaluated. In contrast to earlier studies evaluating oral or nasal administration of norovirus VLPs [5, 6], the serum antibody response to the intramuscular vaccine were higher and extremely rapid, peaking at day 7 after the first dose of the 50/50-µg formulation, with no evidence of boosting when a second dose was administered 28 days later. These results are suggestive of previous priming by natural exposure to related noroviruses. Of note, vigorous immune responses to the bivalent vaccine were detected in subjects who would be considered relatively resistant to norovirus infection on the basis of blood group and secretor status [13], suggesting that natural priming may occur by infection with noroviruses from different genotypes with effective boosting to vaccination. Rapid immune response to a single dose of vaccine may be particularly useful in military personnel and travelers and in the control of outbreaks.
Antibody levels resulting in protection against infection or disease due to noroviruses have not been definitively established, and assessment of neutralizing activity is not possible because of the lack of an in vitro culture model for norovirus. However, since noroviruses most likely initiate infection by attachment to human HBGAs, assessment of the ability to block this interaction may be a surrogate for neutralizing activity. In the challenge model, a serum HBGA-blocking titer of ≥200 was associated previously with significant protection against norovirus-induced disease [7, 11]. The achievement of this titer in the majority of subjects in each age group in this study suggests that intramuscular delivery of norovirus vaccine may protect from norovirus gastroenteritis. In addition, the postvaccination antibody titers were similar to those observed after norovirus infection in the placebo group of a previous GI.1 challenge trial [7]. The combination of tolerability profile, immune responses to an initial dose, and achievement of antibody levels higher than those previously observed with the nasal vaccine previously shown to be effective in prevention of GI.1 norovirus illness [7] are highly encouraging. Further studies of the intramuscular vaccine to demonstrate protective efficacy and evaluate immunogenicity with different ratios of the GI.1 and GII.4 VLPs are currently underway.
Supplementary Material
Notes
Acknowledgments. We thank each of the clinical sites, for recruiting and providing care for the study participants, specifically the staff at Saint Louis University Center for Vaccine Development (Linda Eggemeyer, Kathleen Geldmacher, Irene Graham, and Edwin Anderson), the staff of the University of Rochester Medical Center (Carolyn Nolan, Doreen Francis, and Diane O'Brien), the staff at the Naval Medical Research Center and the Walter Reed Army Medical Center (Mark Riddle, Chad Porter, Paul Keiser, Christopher Soltis, David Tribble, Devon Bryant, Zhu Lei, Robin Nielsen, and Wendy Munera); the clinical and laboratory personnel who supported the study (Baylor College of Medicine; Frederick H. Neill and Sasirekha Ramani); the members of the Safety Monitoring Committee (Nancy Browning and Robert B Belshe); staff at EMMES, for their help with data management and analysis (Jill Barrett, Tom Greene); Julie Cordova and Robin Mertens, for their operational expertise; Keith Veitch, for editorial assistance; and Robert Bargatze and Charles Richardson of Takeda Vaccines (Montana), for their support of research and development.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, or the US government.
Financial support. This work was supported by Takeda Vaccines.
Potential conflicts of interest. J. J. T., R. L. A., and S. E. F. have received research grants from Takeda Vaccines (Montana), for performing this study. S. E. F., J. J. T., and R. L. A. have received financial support to attend scientific meetings to present the data. R. G. and P. M. M., are all full-time employees of Takeda Vaccines (Montana). A. B. and R. C. are all full-time employees of Takeda Pharmaceuticals International (Zurich). All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lopman BA, Hall AJ, Curns AT, Parashar UD. Increasing rates of gastroenteritis hospital discharges in US adults and the contribution of norovirus, 1996–2007. Clin Infect Dis. 2011;52:466–74. doi: 10.1093/cid/ciq163. [DOI] [PubMed] [Google Scholar]
- 2.Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and Norovirus among gastroenteritis-associated deaths in the United States 199–2007. Clin Infect Dis. 2012;55:213–23. doi: 10.1093/cid/cis386. [DOI] [PubMed] [Google Scholar]
- 3.Hall AJ, Lopman BA, Payne DC, et al. Norovirus disease in the United States. Emerg Infect Dis. 2013;19:1198–205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. Systematic literature review of role of Noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14:1224–31. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tacket CO, Sztein MB, Losonsky G, Wasserman S, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol. 2003;108:241–7. doi: 10.1016/s1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 6.El-Kamary SS, Pasetti MF, Mendelman PM, et al. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis. 2010;202:1649–58. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atmar RL, Bernstein DI, Harro CD, et al. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med. 2011;365:2178–87. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parra GI, Bok K, Taylor R, et al. Immunogenicty and specificity of norovirus consensus GII.4 virus-like particles in monovalent and bivalent vaccine formulations. Vaccine. 2012;30:3580–6. doi: 10.1016/j.vaccine.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham DY, Jiang X, Tanaka T, Opekun AR, Madore HP, Estes MK. Norwalk virus infection of volunteers: new insights based on improved assays. J Infect Dis. 1994;170:34–43. doi: 10.1093/infdis/170.1.34. [DOI] [PubMed] [Google Scholar]
- 10.Kavanagh O, Estes MK, Reeck A, et al. Serologic responses to experimental norwalk virus infection measured using a quantitative duplex time-resolved fluorescence immunoassay. Clin Vac Immunol. 2011;18:1187–90. doi: 10.1128/CVI.00039-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reeck A, Kavanagh O, Estes MK, et al. Serological correlate of protection against Norovirus-induced gastroenteritis. J Infect Dis. 2010;202:1212–8. doi: 10.1086/656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LoBue AD, Lindesmith L, Yount B, et al. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine. 2006;24:5220–34. doi: 10.1016/j.vaccine.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 13.Hutson AM, Airaud F, LePendu J, Estes MK, Atmar RL. Norwalk virus infection associates with secretor status genotyped from sera. J Med Virol. 2005;77:116–20. doi: 10.1002/jmv.20423. [DOI] [PubMed] [Google Scholar]
- 14.Frenck R, Bernstein DI, Xia M, et al. Predicting susceptibility to Norovirus GII.4 by use of a challenge model involving humans. J Infect Dis. 2012;206:1386–93. doi: 10.1093/infdis/jis514. [DOI] [PubMed] [Google Scholar]
- 15.Levie K, Gjorup I, Skinhoj P, Stoffel M. A 2-dose regimen of a crcombinant hepatitis B vaccine with the immune stimulant ASO4 compared with the standard 3-dose regimen of Engerix-B in healthy young adults. Scand J Infect Dis. 2002;34:610–4. doi: 10.1080/00365540110080881. [DOI] [PubMed] [Google Scholar]
- 16.Romanosski B, Schwarz TF, Ferguson LM, et al. Immunogenicity and safety of the HPV-16/AS0–4-adjuvanted vaccine admi9nistered as a 2-dose schedule compared t the licensed 3-dose schedule: results from a randomized study Human. Vaccines. 2011;7:1374–86. doi: 10.4161/hv.7.12.18322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.