Abstract
Leptin has emerged over the past 2 decades as a key hormone secreted by adipose tissue that conveys information on energy stores. Leptin is considered an important regulator of both neuroendocrine function and energy homeostasis. Numerous studies (mainly precLinicaL and much less in humans) have investigated the mechanisms of leptin’s actions both in the healthy state as well as in a wide range of metabolic diseases. In this review, the authors present leptin physiology and review the main findings from animal studies, observational and interventional studies, and clinical trials in humans that have investigated the role of leptin in metabolism and cardiometabolic diseases (energy deficiency, obesity, diabetes, cardiovascular diseases, nonalcoholic fatty liver disease). The authors discuss the similarities and discrepancies between animal and human biology and present clinical applications of leptin, directions for future research, and current approaches for the development of the next-generation leptin analogs.
Keywords: appetite, CVD, diabetes, dyslipidemia, hypertension, NAFLD
Leptin, a molecule which is secreted mainly by adipose tissue and which circulates at levels proportional to percentage of body fat mass or acute changes in caloric intake, is considered an important regulator of metabolism and energy homeostasis. Research studies have investigated the impacts of leptin on several disease states of energy deficiency and energy excess, including obesity and its comorbidities, but the role of leptin still remains to be fully defined in humans, given that leptin biology in humans does not fully reflect findings in preclinical models (Central Illustration).
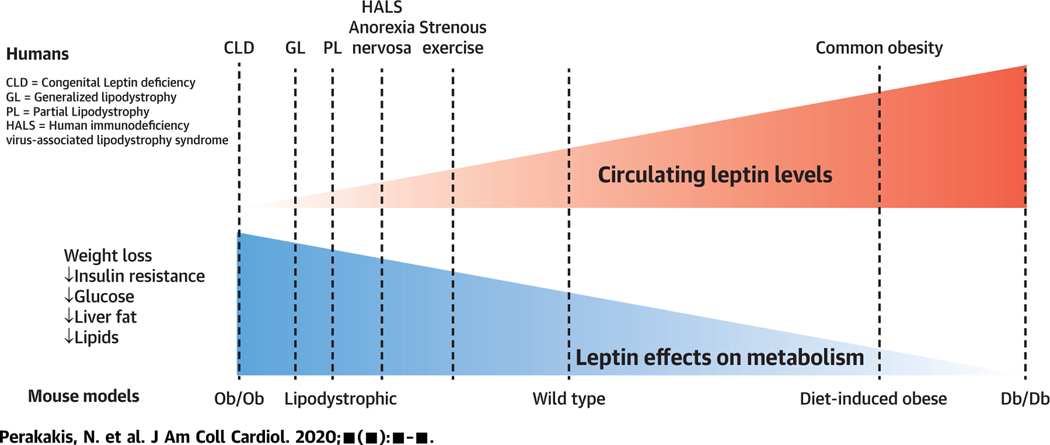
CENTRAL ILLUSTRATION.
Effects of Leptin on Metabolism
Leptin demonstrates metabolic effects that are inversely associated with its circulating levels. In conditions ofabsolute Leptin deficiency, Leptin treatment is highly effective, whereas its efficacy is progressively diminished by increasing leptin levels, probably due to tolerance or resistance to its functions. CLD = congenital leptin deficiency; GL = generalized lipodystrophy; HALS = human immunodeficiency virus-associated lipodystrophy syndrome; PL = partial lipodystrophy.
In this review, we first discuss the essentials of leptin physiology and review the literature related to preclinical studies, observational studies, and clinical trials on leptin therapeutics. We particularly focus on the effects of leptin on neuroendocrine function, energy homeostasis, and metabolic and cardiometabolic diseases in humans. Finally, we present directions of future research in terms of both physiology studies and clinical trials in humans as well as current efforts for the development of next-generation, safer, and more efficient, leptin analogs.
LEPTIN PHYSIOLOGY
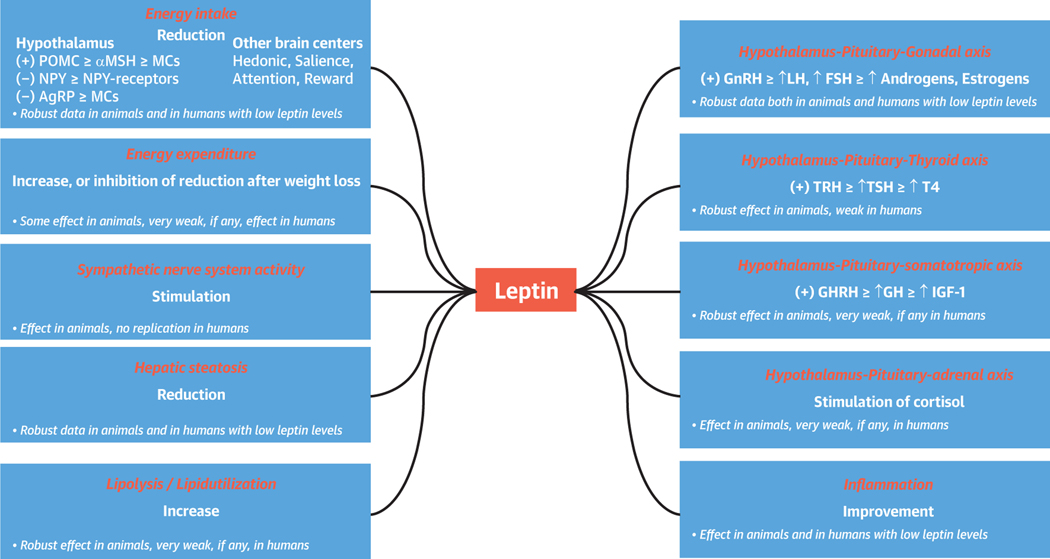
Leptin is mainly secreted by adipose tissue and circulates at a level proportional to the overall amount of energy stored as fat in the body (1). Leptin levels are secondarily regulated by several other factors and are particularly sensitive to acute changes in energy intake, decreasing to ~10% to 20% after only 3 days of fasting, long before levels of adipose tissue have been so reduced (1,2). Leptin acts primarily in the brain and only secondarily in the periphery (3) (Figure 1).
FIGURE 1. Main Effects of Leptin on Metabolism and Endocrine Function in Mouse Models and Humans.
In animals, Leptin has demonstrated robust effects on energy intake and expenditure as well as in sympathetic nervous system activity, Lipid metabolism, inflammation, liver function, and hypothalamic-pituitary function. In humans, leptin treatment is effective mainly in conditions of complete or mild leptin deficiency, in which it decreases energy intake, induces lipid catabolism, improves immune response, and restores the hypothalamus-pituitary-gonadal axis. AgRP = agouti-related peptide; αMSH = α-melanocyte stimulating hormone; FSH = follicle-stimulating hormone; GH = growth hormone; GHRH = growth hormone-releasing hormone; GnRH = gonadotropin-releasing hormone; IGF = insulin-like growth factor; LH = luteinizing hormone; MCs = melanocortins; NPY = neuropeptide Y; POMC = pro-opiomelanocortin; T4 = thyroxine; TRH; TRH = thyrotropin-releasing hormone; TSH = thyroid- stimulating hormone.
Leptin is transported through the blood-brain barrier (BBB) (4–6) and also acts directly in some areas of the hypothalamus which are not protected by the BBB through binding and activating the long form of the leptin receptor (ObRb) (7). On the basis of preclinical experiments, leptin mainly acts in the arcuate nucleus of the hypothalamus to activate pro-opiomelanocortin (POMC)-containing neurons, which produce anorexigenic molecules like αMSH (α-melanocyte stimulating hormone), and to deactivate the orexigenic neuropeptide Y (NPY)- and agouti-related peptide (AgRP)-containing neurons (8–10). Thus, when energy stores are low, such as with low body fat levels or when fasting, leptin levels decrease, leading to lower activity of POMC neurons and increased activity of NPY and AgRP neurons and thus increased appetite and food intake. In the opposite case, when energy stores are abundant, such as in cases of high body fat levels, it would have been expected that the elevated leptin levels would result in decreased energy intake by acting at the level of the hypothalamus. However, in common obesity, leptin effects on energy intake are limited (see Observational Clinical Studies in Humans). In humans, leptin is also known to have direct or indirect impacts on other areas of the brain outside of the hypothalamus, which have bearings on appetite and other behaviors, such as areas related to saliency, decision making, and reward processing (11–13). Leptin has been found to alter functional connectivity between these brain areas, suggesting that it may also alter the way that the brain communicates (13,14). How these may impact normal physiology and the 2 extremes of energy homeostasis (i.e., anorexia nervosa or the female triad and obesity—a state of leptin resistance or tolerance) and how these may relate to the other systems impacted by obesity and leptin need to be further defined by future studies.
Leptin has also an important impact on hypothalamic pituitary axes in both animals and humans. More specifically, leptin has well-documented effects on the hypothalamic-pituitary-gonadal axis in states of energy deficiency (1); administration of leptin in physiological replacement doses for 3 days in leptin deficiency states (e.g., induced hypoleptinemic state due to fasting) in healthy people causes release of GnRH (gonadotropin-releasing hormone), which can stimulate luteinizing hormone and follicle-stimulating hormone to produce androgens or estrogens (1). Leptin administration for several months in mildly hypoleptinemic women with hypothalamic amenorrhea due to strenuous exercise restores menstruation and ovulation (15,16). In these women, leptin administration results in higher luteinizing hormone-to-follicle-stimulating hormone ratio and higher peak concentrations of estradiol and inhibin A (17), all important components of a normal reproductive cycle. Similarly, when leptin is administered in patients with congenital leptin deficiency (CLD), existing disruptions to the gonadal system are corrected (18–21). These effects are aligned with a role of leptin in regulating the neuroendocrine response to energy deprivation (22), during which the gonadal system may be downregulated to conserve energy by diminishing procreation in states of low energy stores that would make sustaining a pregnancy challenging for both the fetus and the mother.
Leptin has also an impact on the hypothalamic-pituitary-growth hormone axis in animals, which entails growth hormone (GH)-releasing hormone, leading to growth hormone production and subsequently leading to increased secretion of insulin-like growth factor (IGF) and regulation of the levels of IGF binding proteins (23). In humans, leptin may alter secretion of IGF-1 in men but not in women and has some limited effects on the IGF binding proteins in both sexes. However, leptin does not have any direct effects on GH pulsatility in humans, and when all data in men and women are considered together, its effects in regulating the GH-IGF axis in humans are minor and not as clinically important (1,15,24–26). Similarly, leptin effects both on the hypothalamic-pituitary-thyroid axis (1,16,24,27) and the hypothalamic-pituitary-adrenal axis (18,19,28) are very limited in humans, in contrast to the reported findings in rodent models (18,19,29). Thus, leptin exerts most of its effects on the hypothalamic-pituitary-gonadal axis in humans, and mainly in those with energy deprivation. Importantly, leptin has no role in regulating neuroendocrine axes in humans with energy sufficiency or excess (i.e., obesity). These findings indicate teleologically the development of redundant systems in humans for the regulation of those important for survival axes.
Leptin has also been proposed to be linked with sympathetic nervous system (SNS) activity, mainly on the basis of data from preclinical studies (30–32). These effects of leptin on SNS have not yet been replicated in humans (33,34), showcasing another difference between leptin biology in animals and humans.
Leptin has been linked with immune function, and hypoleptinemia in uncontrolled human studies has been associated with impaired immune response. Normalization of leptin levels in leptin-deficient states up-regulates genes related to cell survival and hormonal response and down-regulates genes related to apoptosis in immune cells as well as activates leptin-signaling pathways in peripheral blood mononuclear cells and restores CD4(+) T cell counts (35). In contrast no immunological effects or changes in inflammatory markers (interleukin-6, interleukin-10, C-reactive protein, sTNFR [soluble tumor necrosis factor receptor]-I, sTNFR-II, MCP1 [monocyte chemoattractant protein 1], sICAM-1 [soluble intercellular adhesion molecule 1]) were observed after leptin administration in the hyperleptinemic (i.e., obese state) (36).
ANIMAL STUDIES
Most animal studies with leptin have been performed in 2 mouse models: The ob/ob mice and the diet-induced obese (DIO) mice. Leptin was first discovered in 1994 by identifying the gene that was mutated in ob/ob mice and was responsible for their phenotype (37). Ob/ob mice have no leptin and are severely obese, are hyperphagic, have lower body temperature and energy expenditure, have reduced physical activity compared with lean mice (37,38). They are also hyperglycemic, hyperinsulinemic, hyperlipidemic, and infertile, and they have an impaired immune system as well as hepatic steatosis (39,40). Leptin administration in ob/ob mice demonstrated spectacular results by completely reversing most of the observed abnormalities, even in adult mice. Specifically, in leptin-deficient ob/ob mice, but not in DIO mice, leptin administration normalized body weight by reducing fat mass without affecting lean mass (41,42). It also increased exercise and reduced blood glucose and insulin levels before any significant changes in body weight (41,42) and improved hepatic steatosis (40) as well as improved myocardial metabolism and mitochondrial function (43). On the one hand, the main mechanisms involved in the weight and metabolic regulatory effects of leptin in the ob/ob mice involved: 1) a reduction in energy intake by massive reduction of appetite (41,42) through activation of POMC and inhibition of NPY and AGRP neurons in the hypothalamus (44,45) and through regulation of other neuronal circuits (s. above physiology of leptin); 2) an increase in energy expenditure (40,45–48) by stimulating SNS activity in cardiovascular system or by increasing thermogenesis through stimulation of sympathetic signaling in brown adipose tissue and of browning in white adipose tissue (49–51); 3) an increase in lipolysis as well as a shift from carbohydrate to lipid utilization as energy fuel (40,47,52); and 4) an increase in glucose turnover and glucose uptake in brown adipose tissue, brain and heart (53). On the other hand, several studies performed mainly in ob/ob mice have shown that leptin may promote atherogenesis, platelet aggregation, inflammation, oxidative stress, and endothelial dysfunction, leading to plaque vulnerability and increased risk of thrombosis (54,55). Finally, it may increase blood pressure by stimulating SNS activity (56).
In contrast to ob/ob mice, the effects of leptin on weight regulation were not fully replicated in DIO mice, which is an animal model that reflects better the biology of human common obesity (57,58) (Table 1). Specifically, in DIO rat and mouse models, the response to leptin was poor and demanded high supraphysiological leptin doses to achieve minimal weight loss effects compared with what was observed in ob/ob mice (31,59,60).
TABLE 1.
Impact of Leptin Treatment in Conditions of Leptin Deficiency and Leptin Excess in Mouse Models and Humans With Metabolic Diseases
| Leptin Levels | Weight | IR | Glucose | TGs | NAFLD | SNS Activity BP* | Energy Intake | Energy Expenditure | Lipid Metabolism† | |
|---|---|---|---|---|---|---|---|---|---|---|
| Animal studies | ||||||||||
| Ob/ob mice | Extremely low | ↓↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓ | ↑↑ | ↓↓↓ | ↑↑ | ↑↑ |
| DIO mice | High | ↔↓ | ↔↓ | ↔↓ | ↔↓ | +/↓ | ↑ | ↔↓ | ↔ | NA |
| Human studies | ||||||||||
| CLD | Extremely low | ↓↓↓ | ↓ | ↓ | ↓ | ↓ | ↔‡ | ↓↓↓ | ↔§ | ↑↑ |
| GL | Extremely low | ↓ | ↓↓↓ | ↓ | ↓↓↓ | ↓↓↓ | ↔↓ | ↓ | ↔ | ↑ |
| PL | Very low | ↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↔↓ | ↓ | ↔ | ↑ |
| HALS | Low | ↔ | ↓ | ↓ | ↓↓ | ↔↓ | NA | ↔ | ↔ | NA |
| Lean with Low % of body fat due to exercise | Low | ↓ | NA | ↔ | NA | NA | ↔ | ↓ | ↔ | ↑ |
| Common obesity | High | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
The arrows indicate the impact of leptin treatment.
SNS activity has been assessed in human clinical studies indirectly, by measuring arterial blood pressure and heart rate.
Lipid metabolism may refer either to increased lipolysis or reduced de novo lipogenesis, or generally to alterations in circulating lipids.
One study reported transient increase of blood pressure and heart rate with short-term leptin treatment but no effect in the longer term and after weight loss (141).
Leptin therapy does not induce energy expenditure but prevents the decrease in metabolic rate that results by weight loss.
BP = blood pressure; CLD = congenital leptin deficiency; DIO = diet-induced obese; GL = generalized lipodystrophy; HALS = human immunodeficiency virus-associated lipodystrophy syndrome; IR = insulin resistance; NA = not available; PL = partial lipodystrophy; SNS = sympathetic nervous system; TG = triglyceride.
Findings from DIO mice together with the results from human studies with obese patients (see the following sections) suggest the presence of leptin tolerance, which is reminiscent of insulin resistance observed in obesity and cardiometabolic disorders. However, a main difference between them is that in insulin resistance a dose response exists, as higher doses of insulin can reduce glucose levels (an important outcome) in patients with diabetes. In contrast, very high leptin doses do not affect body weight (see the following sections). Leptin tolerance remains to be fully defined, but based on animal studies, which are very difficult to perform in humans due to technical limitations, is apparently multifactorial and it: 1) may involve reduced leptin transport across the BBB; 2) may involve impaired receptor trafficking (intracellular transport of the receptor from cytosol to cell membrane) (5); 3) may involve suppression of leptin receptor signaling either due to mutations of important proteins participating in the signaling pathways of leptin (SOCS3 [suppressor of cytokine signaling 3], PTP1B [protein-tyrosine phosphatase 1B], Shp2 [Src homology 2 domain-containing phosphatase 2]) or due to increased hypothalamic inflammation and endoplasmic reticulum stress related to lipotoxicity by chronic overnutrition (61–63); or 4) may also merely reflect the fact that leptin’s role in humans is simply permissive and leptin functions only in hypoleptinemic and normoleptinemic states. The latter seems to be also the overwhelmingly likely explanation, given that the findings from animal studies for the other factors are not consistent. Specifically, it has been suggested, but not always consistently replicated, that in mouse models of obesity with hyperleptinemia, the response to certain functions of leptin (e.g., of sympathetic nerve activity and blood pressure) may still be preserved, especially when leptin is administrated centrally in order to overcome part of the observed leptin tolerance (31,64,65). However, these effects have no impact on body weight or appetite. Additionally, recent imaging studies showed normal distribution of leptin across the brain in obese mice (66,67), arguing against major abnormalities in leptin transport.
Although the obese mouse models of leptin excess such as DIO mice may be more related to the pathophysiology of human obesity, animal models in general, albeit useful to raise hypotheses, have important limitations in their translational capacity to human biology of leptin. First, certain mutations responsible for the mouse phenotypes are extremely rare in obese humans. In addition, appetite regulation in humans is a much more complex process controlled not only by basic homeostatic mechanisms but also by other brain centers (emotion centers, cognitive control, reward system) compared with mice (68). Finally, thermoregulation and thermogenesis through brown adipose tissue significantly differs between humans and mice (69). These differences may explain the rather limited direct translation of the findings in mouse studies related to leptin treatment to humans.
OBSERVATIONAL CLINICAL STUDIES IN HUMANS
Leptin levels are directly proportional to percentage of fat mass (70,71). Consequently, leptin is also higher in women than in men due to their higher percentage of body fat and their different hormonal profile (estrogens in women vs. testosterone in men), and is higher in patients with insulin resistance and type 2 diabetes mellitus (72). Additionally, leptin levels correlate positively with lipids and lipoprotein levels, but most probably not independently of body mass index (BMI) (for which most studies adjust for) or percentage fat mass (for which most studies have not adjusted for, even if they adjust for BMI, thus leading to uncontrolled confounding) (73).
Several studies have investigated associations of leptin levels with arterial hypertension as well as with cardiovascular diseases (CVDs) such as coronary heart disease (CHD), stroke, and carotid artery disease, but they usually demonstrate the same methodological limitations mentioned previously. Most studies suggest a positive association of leptin levels with blood pressure and hypertension in adults (74,75), but in most if not all studies residual uncontrolled confounding by percentage of fat mass remains an important factor. For CHD, the results are so far inconclusive. Several studies have reported higher levels of leptin in patients with CHD compared with control subjects (76,77), as well as increases of leptin after myocardial infarction (77) and percutaneous coronary intervention (78). However, other studies could not confirm this association or have even demonstrated an inverse relationship (79,80). Along these lines, the most recent meta-analysis reported an inverse association between leptin and CHD, which did not remain significant after adjusting for additional cardiovascular risk factors, such as BMI, lipids, systolic blood pressure, and smoking status (79). In prospective cohorts, leptin levels were associated with major adverse cardiac events in the short term (2 years) (81) but not in the long term (7 to 12 years), but in general concerns about uncontrolled confounding remain (82,83).
Similarly, the results of studies investigating the relation of leptin levels with incident stroke, as well as with carotid intima-media thickness are inconsistent, with some of them reporting positive associations (84–86) and others no associations (79,87). Age, sex, type of stroke, severity of CHD, definition of major adverse cardiovascular events, and duration of follow-up differ between studies, and most importantly, lack of control for the confounding role of percentage of fat mass may explain their contradictory findings (88).
Taking into consideration the contradictory findings, it is difficult to assess whether high leptin levels should be considered an independent risk factor of CVD on the basis of observational studies, which have largely not adjusted for the most important confounder and strong correlator to leptin (i.e., the percentage of fat mass); thus, interventional studies in humans are clearly needed herein. Similarly, it seems premature to consider leptin levels a potential biomarker with predictive value for cardiovascular outcomes beyond its role as a biomarker of percentage fat mass, and through this association, with other CVD risk factors associated with fat mass. Interventional studies involving leptin administration are needed to extend and fully clarify these observations.
Given also the rapidly increasing prevalence of nonalcoholic fatty liver disease (NAFLD), which is associated not only with higher liver-related mortality, but also with CVD-related mortality, several studies have investigated whether leptin can be a useful biomarker for the noninvasive diagnosis and staging of the disease (89–93). As mentioned previously, experimentally leptin replacement reverses hepatic steatosis in ob/ob mice, which have complete leptin deficiency, but leptin excess may promote liver inflammation and fibrosis when leptin is raised to supraphysiological levels (94). In humans, leptin levels are higher in patients with NAFLD than in control subjects and progressively increase with increased severity of the disease (95). Similar to CVD, their predictive value for the noninvasive diagnosis and staging of NAFLD has not been shown to be superior compared with BMI, or presence of type 2 diabetes mellitus, and is lower compared with the other major adipokine (i.e., adiponectin), which is a marker of intra-abdominal obesity, insulin resistance, and inflammatory status of the organism (90).
Altogether, leptin is a reliable marker of percentage of fat mass, and its blood levels increase as cardiometabolic risk increases and NAFLD advances. However, leptin should not be considered a reliable independent marker of or independent prognostic factor for cardiometabolic risk, and interventional studies are needed to prove causality.
CLINICAL TRIALS ON LEPTIN TREATMENT IN LEPTIN DEFICIENCY AND DISEASES WITH CARDIOMETABOLIC ABNORMALITIES IN CONDITIONS OF LEPTIN DEFICIENCY
The pharmacokinetic studies on leptin administration in humans (96–98) and in human physiology studies (1,24) w e performed were soon followed by physiology studies (1,24) and then proof-of-concept studies in strenuously exercising women athletes with hypothalamic amenorrhea (15) and neuroendocrine or metabolic issues (16,99), and were followed by the first clinical trials (initially all open label) with leptin administration to subjects with CLD due to mutations in the leptin gene (18,19) and later in severely hypoleptinemic patients due to congenital or acquired lipodystrophy (100–102). Both CLD and congenital lipodystrophy are extremely rare. CLD is characterized by rapid weight gain in childhood mainly due to hyperphagia, which results to morbid obesity, dyslipidemia, mild insulin resistance, glucose intolerance, and steatosis. Lipodystrophy refers to a group of inherited or acquired disorders characterized by the complete (generalized lipodystrophy [GL]) or partial (partial lipodystrophy [PL]) absence of adipose tissue and ectopic fat accumulation, mainly in the liver. People with GL are not morbidly obese, but they have dyslipidemia, severe insulin resistance, hyperglycemia, often heart muscle hypertrophy, and hepatic steatosis that can rapidly progress to nonalcoholic steatohepatitis and liver fibrosis (92). People with PL have similar but more moderate metabolic complications, which depend also on the type of PL (103). Finally, similar metabolic complications are observed in people with human immunodeficiency virus who develop lipodystrophy due to highly active antiretroviral therapy (human immunodeficiency virus-associated lipodystrophy syndrome [HALS]) (101,102,104).
Treatment of these patients with leptin had major and sustainable results. Specifically, in children with CLD, profound weight loss with fat mass loss due to reduced appetite (but not of energy expenditure) was observed (18,19). Additionally, dyslipidemia, insulin resistance, and hyperglycemia were corrected and immune function was improved (18,19). Leptin treatment in people with GL resulted in mild reductions of weight and profound decrease of hyperglycemia and hyperlipidemia (105). Hepatic volume, hepatic fat, alanine aminotransferase, and aspartate aminotransferase levels and ballooning were also reduced, but lobular and portal inflammation as well as fibrosis remained largely unchanged, as reviewed in Polyzos et al. (92). In people with PL, leptin administration led to significant but more modest changes compared with GL (106). Similar to CLD, energy intake was reduced whereas energy expenditure was not increased both in GL and PL (107–109). As all these studies were open label, the possibility that other factors (e.g., confinement to a Clinical Research Center, controlling diet and exercise or other parameters) may also have played a role remains. Interestingly, the improvement in insulin sensitivity and in hepatic and circulating triglycerides were at least partially sustained in these subjects when food intake was controlled, which suggests that the reduced energy intake is not the only mechanism responsible for the metabolic improvement (108). Here, the lip-ocatabolic effects of leptin may contribute to the weight loss. Specifically, leptin replacement in CLD induces lipolysis and oxidation indicated by an increase in free fatty acids, acylcarnitines, and ketone bodies (110). In GL or PL, leptin has a more modest impact, indicated by changes in acylcarnitines and byproducts of branched-chain amino acids and protein degradation (111). In contrast to results from mouse studies and observational human studies, leptin treatment neither in CLD nor in GL or PL increased blood pressure levels. On the contrary, in a mixed population of GL and PL, in which one-third of the subjects had hypertension, leptin treatment was associated with a small but significant reduction of systolic blood pressure after 12 months (112). Additionally, and again in contrast to mouse studies, leptin treatment in lipodystrophic patients did not affect insulin secretion and did not suppress beta cell function (113). Leptin also exerted beneficial immunomodulatory effects by normalizing T lymphocyte number and relative percentages (114), as observed in subjects with acquired leptin deficiency but no cardiometabolic complications (35). Finally, randomized clinical trials (RCTs) involving leptin treatment in subjects with HALS with less significant hypoleptinemia had similar but more modest results in all metabolic parameters. Specifically, insulin resistance, visceral fat, and triglycerides were reduced; high-density lipoprotein was increased; and inflammatory markers (number of CD4+ cells, tumor necrosis factor-α, interleukin-6, C-reactive protein, human immunodeficiency virus viral load) were not affected (102,115).
Altogether, leptin treatment was extremely effective at correcting metabolic abnormalities in children with CLD and people with GL. Additionally, it was effective (but more modestly) at correcting metabolic abnormalities in PL and HALS, whereas leptin deficiency was less pronounced (Table 1) and circulating leptin levels were not as low.
CLINICAL TRIALS IN COMMON OBESITY
From the first observational studies, it became obvious that circulating leptin levels reflect fat mass and are thus increased in people with obesity, which in turn indicates a state of resistance or tolerance to leptin function. Because insulin resistance is often also observed in obese subjects, researchers were hoping that leptin administration to achieve higher circulating levels in obese population, as it happens with insulin’s effects on glucose, will still be able to exert at least part of its beneficial cardiometabolic functions, especially if administered in very high doses. In this context, several studies have investigated the effect of leptin treatment in subjects with common obesity (Table 2). The specific study designs aimed to evaluate escalating doses of leptin or leptin analogs, through several routes of administration and through shorter or longer durations of treatment and in different metabolic conditions, such as treatment with or without dietary caloric restriction and before or after an initial weight loss (Table 2). The first multicenter RCT with subjects treated with escalating doses of leptin that also followed a mild hypocaloric diet (~500-kcal deficit) for 24 weeks failed in terms of its primary outcome but concluded, through a post hoc secondary analysis, that leptin may be effective for some obese people treated with higher leptin doses (116). Subsequent in vitro, ex vivo, and in vivo studies in humans have shown that all important leptin signaling pathways are saturable at 30 to 50 ng/ml free leptin levels in peripheral tissues (adipose tissue, muscle, peripheral blood mononuclear cells) and without any difference between obese versus lean subjects (36,117). In line with these findings, administration of leptin in both low (118) and very high doses, in order for its free plasma concentrations to reach clearly supraphysiological levels, did not show any significant effect on weight loss in obese individuals (36,119). Another line of research focused on evaluating leptin efficacy in obese subjects after an initial attempt to reduce their leptin tolerance through diet or other interventions (120–122). Two RCTs that included exclusively subjects that have lost weight during a 3- to 4-week lead-in diet period could not show any beneficial effects of leptin treatment compared with placebo on weight loss (120,121). Similarly, leptin administration had no effect on body weight in morbidly obese patients that have lost significant body weight after Roux-en-Y gastric bypass (122).
TABLE 2.
Clinical Trials Investigating the Effect of Leptin Administration in Common Obesity
| First Author (Year) (Ref. #) | Study Design | Subjects | N | Type of Leptin | Duration | Dose | Leptin Weight Loss | Other Effects |
|---|---|---|---|---|---|---|---|---|
| Heymsfield et al. (1999) (116) | RCT, double blind placebo controlled | Overweight/obese BMI 27.6–36 kg/m2 | 12 6 8 13 8 |
r-met hu leptin | 24 weeks | Placebo 0.01 mg/kg/day 0.03 mg/kg/day 0.10 mg/kg/day 0.30 mg/kg/day +hypocaloric diet (−500 kcal) |
−1.3 kg/m2 (4.9 kg) −0.7 kg/m2 (5.4 kg) −1.4 kg/m2 (4.1 kg) −2.4 kg/m2 (5.5 kg) −7.1 kg/m2 (8.5 kg) |
• No significant differences in weight loss between treatment groups reported • Linear regression models: reduction of weight and fat mass but not fat free mass with increasing dose |
| Moon et al. (2011) (36) | RCT, double blind placebo controlled | Obese with T2D BMI 33.2 ± 3.8 kg/m2 | 71 | r-met hu leptin | 16 weeks | Placebo 10 mg twice daily |
−0.5 ± 0.2 kg/m2
−0.7 ± 0.1 kg/m2 => No significant effect of leptin on weight loss |
• HbA1c slightly reduced (p = 0.03) Placebo: 8.01 ± 0.93%; leptin: 7.96 ± 1.12% • No changes in inflammatory markers • All metreleptin signaling pathways saturable at 50 ng/ml of free leptin |
| Mittendorfer et al. (2011) (119) | RCT, placebo controlled | Obese with newly diagnosed T2D | 18 | r-met hu leptin | 14 days | Placebo 15 mg twice daily 40 mg twice daily |
=> No changes in BMI in all groups | • No effect on total body mass, fat mass, fat free mass, % of body fat • No effects on insulin sensitivity on hyperinsulinemic-euglycemic clamp |
| Lejeune et al. (2003) (123) | RCT, double blind placebo controlled | Overweight/obese BMI 25–32 kg/m2 | 24 | PEG-OB | 6 weeks of treatment with VLCD followed by 8 weeks of follow-up | VLCD + placebo 80 mg/week for 6 weeks | 0–6 weeks of treatment: −3.6 kg/m2 −4.3 kg/m2 => Reduction (p = 0.049) of body weight with leptin Follow-up: +0.6 kg/m2 +1.1 kg/m2 => Increase of body weight in subjects previously treated with leptin during weight maintenance |
• 0–6 weeks of treatment: no effect of leptin on fat mass, % of body fat, fat-free mass, REE, RQ, hunger • Follow up: no effect of leptin on total body mass, fat mass, fat-free mass, % of fat mass, hunger |
| Hukshorn et al. (2000) (118) | RCT, double blind placebo controllec | Overweight/obese BMI ≥27 kg/m2 | 30 | PEG-OB | 12 weeks | Placebo 20 mg/week +hypocaloric diet (−500 kcal) | −6.4 kg −4.3 kg => No changes in weight |
• No effect on body fat %, 24-h EE, SMR, RQ, appetite (ad libitum evening meal) • No effect on glucose, insulin, glucose disappearance rate, FFAs, glycerol, TGs, TC, LDL-C, HDL-C |
| Hukshorn et al. (2002) (120) | RCT, double blind placebo controlled | Overweight/obese BMI ≥27 kg/m2 + loss of ≥1.75 kg after a 4-week lead-in diet period | 28 | PEG-OB | 4 weeks of diet followed by 8 weeks of treatment | Placebo 60 mg/week | −3.8 kg −4.8 kg => No changes in body weight |
• No effects on glucose, insulin, HOMA-IR, FFAs, glycerol, TGs, TC • No effects on inflammatory markers (sTNF, CRP) |
| Zelissen et al. (2005) (121) | RCT, double blind placebo controlled | Overweight/obese BMI 27.5–37.5 kg/m2 + loss of 0–5 kg and BMI >25 kg/m2 after a 3 week lead-in diet period | 284 | r-met hu leptin | 3 weeks of diet followed by 12 weeks of treatment | Placebo 10 mg every morning 10 mg every night 10 mg twice daily |
−2.6 kg −2.8 kg −2.7 kg −3.4 kg => No changes in body weight |
• No change in SBP, DBP, heart rate, TGs, TCs, LDL-C, HDL-C, fasting glucose, fasting insulin |
| Rosenbaum et al. (2005) (125) | Single-arm open label stud | 3 nonobese, 7 obese | 10 | r-met hu leptin | Diet until 10% weight loss achieved followed by 5 weeks treatment | Dose calculated to maintain blood leptin concentrations at levels prior to weight loss | −2.1 kg compared with treatment start | • 1.0 kg fat mass loss and 1.1 kg free fat mass loss compared with treatment start • Returned of nonresting EE, SNS tone to pre-weight loss levels |
| Rosenbaum et al. (2008) (133) Kissileff etal (2012) (124) |
Single-blind placebo-controlled crossover study design | Obese subjects | 10 | r-met hu Leptin | Diet until 10% weight loss achieved followed by 5 weeks treatment | Placebo Leptin dose calculated to maintain blood leptin concentrations at levels prior to weight loss | No significant changes in weight between leptin and placebo (−1.8-kg difference). | • EE and VAS for satiation were lower in the placebo group than in the leptin group • Leptin reverses changes in neural activity in response to visual food stimuli due to weight loss |
| Korner et al. (2013) (122) | RCT double-blind placebo-controlled crossover study design | Post-RYGB, weight-stable, relative hypoleptinemic subjects | 27 | r-met hu Leptin | 16 weeks | Placebo 0.05 mg/kg twice daily |
No differences in body weight | • No effect of leptin on fat mass, REE, thyroid hormones, cortisol levels |
| Chrysafi et al. (2020) (34) | Crossover study design |
5 obese men, 5 lean men, 5 lean women | 15 | r-met hu Leptin |
(a) 24 h (fed state) (b) 72 h (fasting) Leptin administrated at 8 AM/day [1 dose in (a), 4 doses in (b)] |
0.01 mg/kg 0.1 mg/kg 0.3 mg/kg (total 9 visits for each subject) |
No differences in body weight with escalating leptin doses | • No effect of leptin on energy expenditure, SNS activity, adrenal hormones, lipid utilization. |
CRP = C-reactive protein; DBP = diastolic blood pressure; EE = energy expenditure; FFA = free fatty acid; HbA1c, glycosylated hemoglobin; HDL-C = high-density lipoprotein cholesterol; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; LDL-C = low-density lipoprotein cholesterol; PEG-OB = •••; RCT = randomized clinical trial; REE = resting energy expenditure; RQ = respiratory quotient; RYGB = Roux-en-Y gastric bypass; SBP = systolic blood pressure; SMR = sleeping metabolic rate; sTNF, soluble tumor necrosis factor; T2D, type 2 diabetes mellitus; TC = total cholesterol; TG = triglyceride; VAS = •••; VLCD = very low-caloric diet (provided 2.1 MJ/day).
In line with the lack of any effect of leptin on body weight, no changes in body composition (body fat mass, fat free mass) (118,119,122,123), energy expenditure (118,122,123), appetite (118,123), respiratory quotient as marker of macronutrient utilization (118,123), glucose homeostasis (insulin, glucose, insulin sensitivity) (118–121), lipid profile (118,120,121), heart rate (121), blood pressure (121), cortisol (122), and inflammatory markers (36,120) were observed with leptin treatment compared with placebo in obese hyperleptinemic individuals. Only 1 single-arm open label study and a single-blind placebo-controlled trial, of sequential design, performed by the same group, showed an increase in nonresting energy expenditure and SNS tone in obese subjects that were treated with leptin after a 10% weight loss achieved with diet (124–126). Additionally, it showed improvements in visual analog scales for appetite and a leptin-mediated reversal of the changes in neural activity in response to visual food stimuli due to weight loss (124,126). These effects led to a 2.1-kg weight loss in the open-label study (125) and to nonsignificant weight changes compared with placebo after 5 weeks of treatment in the single-blind placebo-controlled crossover study, but the sequential design of these studies has raised the notion that observed changes may have simply reflected the evolution of normal physiology of energy deprivation over time (124).
Altogether, clinical studies so far have shown no major weight-regulatory or metabolic benefit with leptin treatment in obese hyperleptinemic individuals and need to be expanded in the future for a better understanding of leptin biology and its therapeutic potential in humans.
NOVEL FINDINGS FROM CLINICAL TRIALS IN LEAN AND OBESE POPULATIONS
The lack of an effect of leptin treatment on weight in patients with obesity has led to the question of whether leptin’s role in human metabolism is limited in regulating energy homeostasis in the rare cases of CLD and lipodystrophies (e.g., in the extremes of leptin deficiency). In this context, we have recently reported the effects on metabolic outcomes of: 1) leptin administration for 3 days in lean and obese subjects during hypoleptinemia induced by complete fasting; 2) a single-dose leptin administration in lean and obese subjects in the fed state; and 3) leptin administration for up to 9 months in mildly hypoleptinemic women with a low percentage of body fat due to strenuous exercise (34). The results of our studies showed that short-term leptin administration reduces food intake during refeeding after complete fasting in a lean, and thus leptin sensitive, group of subjects. Otherwise, leptin administration in the short term had no impact on body weight, energy expenditure, and SNS activity both during fasting and in fed state, in obese and lean subjects and in 3 different leptin doses (physiological, supra-physiological, and pharmacological). Long-term leptin treatment in women with mild hypoleptinemia due to low body fat (athletes), who had stable and within-normal-range BMI at least 6 months prior to treatment initiation, led to significant body weight loss, which was exclusively loss of fat mass. These weight-regulatory effects of leptin were most probably achieved by reducing energy intake and transiently increasing lipid catabolism. In contrast, leptin treatment did not affect energy expenditure, SNS activity, adrenal function, heart rate, and blood pressure. The weight and fat mass loss observed with long-term leptin treatment were completely reversible after discontinuation of leptin treatment and return of leptin levels to the normal range. These results support a role for leptin to regulate not only neuroendocrine (mainly reproductive) function, but also energy homeostasis in low-leptin states. Additionally, these results support the hypothesis of a gradual loss of function for leptin when moving from conditions of leptin deficiency to conditions of leptin sufficiency and leptin excess (Central Illustration).
PERSPECTIVES
Based on the results from clinical studies, a human leptin analog (metreleptin [Myalept], which has a methionine added to the leptin molecule to improve molecular folding and production efficiency) was first approved in Japan in 2013, followed by the United States in 2014 and Europe in 2018. The indication for metreleptin in the United States is the presence of complication such as diabetes and hypertriglyceridemia associated with congenital or acquired GL. In Europe, the indication is extended also to patients with PL (familial or acquired) in adults and children 12 years of age and older, for whom standard treatments have failed to achieve adequate metabolic control. The lack of response to leptin treatment in common obesity has led to intensified efforts: 1) to further decipher the biological mechanisms involved in leptin’s actions; 2) to fully clarify whether leptin resistance or leptin tolerance (permissive action of leptin only in energy deficiency states) exists in humans; and 3) to design novel and more effective drugs, given that metreleptin use has been limited by the Food and Drug Administration to complete leptin deficiency states only (Figure 2).
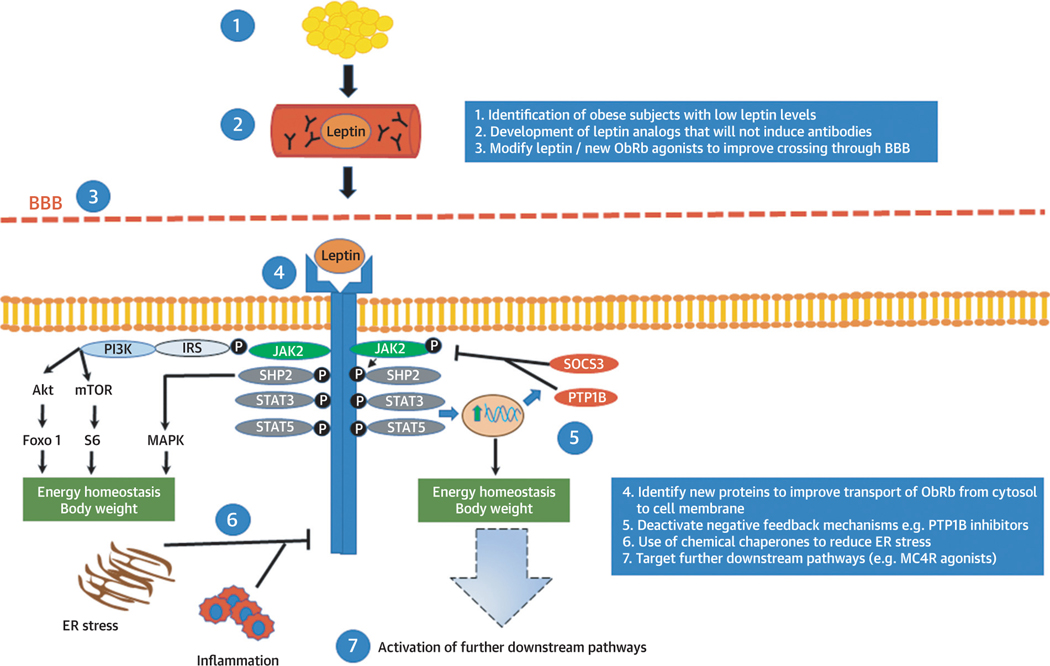
FIGURE 2.
Current Approaches Aiming to Improve the Efficiency of Leptin Treatments in Common Obesity
Leptin is secreted mainly by adipose tissue and circulates in the bloodstream crossing the blood-brain barrier (BBB) and acting in the brain. Leptin’s binding on its receptor activates signaling pathways that are related to energy homeostasis and body weight regulation in humans. In common obesity, leptin treatment does not induce changes in body weight, suggesting potentially the presence of leptin tolerance or resistance. Current efforts aim to increase the efficiency of leptin treatment by following several approaches, such as by selecting obese patients with low leptin levels and thus higher chances for response or by improving transport of leptin- based treatments in the brain or by targeting the downstream signaling pathways related to leptin. Akt = ■■■; ER = endoplasmic reticulum; Foxo1, Forkhead box protein O1; IRS = insulin receptor substrate; JAK2 = Janus kinase 2; MAPK = mitogen-activated protein kinase; MC4R, melanocortin 4 receptor; mTOR = mammalian target of rapamycin; ObRb = long form of the leptin receptor; PI3K = phosphoinositide 3-kinase; PTP1B = protein-tyrosine phosphatase 1B; S6 = substrate 6; SHP2 = Src homology 2 domain-containing phosphatase 2; SOCS3 = suppressor of cytokine signaling 3; STAT = signal transducer and activator of transcription.
In this context, recent approaches aim to identify individuals among the population with common obesity who have low leptin levels and thus theoretically less leptin tolerance and more room for leptin to act when its levels are raised from low (below physiological level) to normal. Along these lines, a recent study identified a long noncoding RNA (IncOb) that controls leptin gene expression. DIO-mice lacking IncOb are obese and lose weight after leptin treatment. Importantly, polymorphisms in the IncOb in humans are associated with obesity and low leptin levels (127). Additionally, in a post hoc exploratory pooled analysis of old studies in obese subjects treated with leptin, published only in abstract form, women with <16 ng/ml and men with <5 ng/ml leptin levels may be losing more weight when treated with leptin compared with placebo (approximately −4% vs. −2% weight change from baseline after 24 weeks of treatment) (128). However, there are a number of caveats in this approach. First, at this point in time, it is difficult to define a universal threshold for leptin levels as an indicator of leptin responsiveness not only for technical reasons (leptin assays are not standardized across studies and data on specific leptin thresholds from 1 study cannot be directly extrapolated to others), but also for important scientific reasons. As indicated in our recent study (34), leptin treatment reduces body weight in lean individuals, but baseline leptin levels before treatment initiation cannot predict the magnitude of weight loss with leptin administration. Finally, even if we manage to identify a target subset of the population with high chances of response to leptin, and not withstanding leptin’s effect to decrease exclusively fat mass, leptin treatment will still have to prove to be equal or superior to available weight loss medications that can currently lead to up to 5% to 10% weight loss (129,130).
A second approach that has been discussed, on the basis of the proposed existence of leptin resistance or tolerance, involves combination of leptin treatment with another agent that may act as leptin sensitizer. Studies both in rodents and humans have shown, for example, that amylin can act additively, but not synergistically, with leptin to activate signaling pathways in peripheral tissues (131) and in concert with leptin in the brain to regulate feeding (132). In a 24-week randomized, double-blind clinical study, combination of pramlintide (amylin analog) with leptin led to 12.7% weight loss, significantly more compared with either treatment alone (133,134). However, a phase II clinical trial evaluating pramlintide with metreleptin was terminated 2011 due to the development of leptin antibodies.
This raises another important point that has to be addressed in potential future studies with leptin or leptin analogs. Development of non-neutralizing antibodies against drugs is observed frequently and has in most cases no clinical relevance. However, antibodies may lead to neutralization of treatment or, more importantly, to adverse effects due to neutralization of the endogenous secreted protein. In an analysis of 579 patients with obesity and 134 patients with lipodystrophy treated with metreleptin, the vast majority of patients developed antibodies, which similar to other protein therapeutics, were almost exclusively non-neutralizing. Only 3 patients with obesity may have developed in vitro neutralizing antibodies coincident with weight gain and only 4 patients with GL coincident with worsened metabolic profile (135). Although a clear cause-and-effect relationship has not been demonstrated, this was sufficient to terminate clinical studies with leptin in common obesity and prevent the approval of metreleptin for treatment of PL and delay it for GL. Thus, future research is now focusing on the development of leptin analogs, including monoclonal antibodies activating the leptin receptor, that do not induce the production of endogenous antibodies, which after they are tested in clinical experimental settings they may proceed to larger clinical trials.
A third experimental approach focuses on developing treatments that will address 1 or more pathophysiological mechanisms that may be involved in potential leptin resistance. In this context, the following solutions are being evaluated: 1) chemical modification of leptin and intranasal delivery methods or novel agonists of leptin ObRb with shorter sequences to facilitate the crossing across the BBB (136,137); 2) identification of novel molecules, such as endospanin-1, that control ObRb trafficking (transport of the receptor from cytosol to cell membrane in order to be functional) (138); 3) inhibition of endogenous negative feedback mechanisms of leptin signaling, which are mainly controlled by SOCS3 and PTP1B, although the first PTP1B inhibitors showed poor selectivity and in vivo efficacy (139); and 4) decrease of endoplasmatic reticulum stress with the use of chemical chaperones, which stabilize protein folding and reduce defected protein aggregation (140). All of the previous have shown promising results in DIO mouse models, but the investigation and translation of their effects in humans has been limited so far.
In conclusion, leptin clearly controls energy homeostasis and neuroendocrine, mainly reproductive, function in states of energy deprivation in humans. Leptin treatment has major and sustainable metabolic effects in people with low leptin levels (obese with CLD, GL, PL, HALS), who are, however, a small fraction of the cardiometabolically unhealthy population. Leptin treatment is ineffective in common obesity, which is characterized by leptin excess. Whether leptin resistance is an important factor that contributes to the development and progression of obesity versus whether leptin only has a permissive role acting in energy deficiency states, and thus obesity is simply a leptin-tolerant state remains to be fully elucidated.
Future research should focus on: 1) any effects of leptin on the cardiovascular system and CVDs, either through or independently from its weight- or lipid-regulatory effects; 2) leptin’s effects in central nervous system areas controlling feeding behavior in humans beyond the hypothalamus as well as leptin’s effects in peripheral tissues; and 3) novel drug development that will not be inducing leptin antibodies and which will be either targeting the leptin receptor or pathways downstream of the leptin receptor. Finally, another area of considerable clinical interest derives from findings from in proof-of-concept studies that leptin exerts significant bone metabolism effects (16,99) in leptin-deficient humans. Leptin treatment, in the context of randomized clinical trials, improves bone mineral density and content in strenuously exercising women with hypothalamic amenorrhea that are at high risk for developing osteoporosis and stress fractures (16,99).
In conclusion, given the differences between human studies and animal studies, which similar to observational studies in humans have been extremely useful to raise hypotheses but cannot prove causality and elucidate human leptin biology, the field needs new leptin analogs to be used in the context of first human physiology studies and later proof-of-concept randomized clinical trials that can prove causality, elucidate molecular pathways, and demonstrate efficacy in humans (i.e., the species of interest). The availability of novel leptin analogs that may apparently not be inducing anti-leptin antibodies provides an opportunity in that direction.
HIGHLIGHTS.
Leptin is an important regulator of neuroendocrine function and energy homeostasis, and blood levels of leptin reflect energy stores, fat mass, or energy deprivation.
Treatment with leptin can be effective in patients with certain cardiometabolic diseases associated with leptin deficiency but not in common obesity.
Additional physiological studies and clinical trials are needed to explore potential clinical applications of next-generation leptin analogs.
ABBREVIATIONS AND ACRONYMS
- AgRP
agouti-related peptide
- BBB
blood-brain barrier
- BMI
body mass index
- CHD
coronary heart disease
- CLD
congenital leptin deficiency
- CVD
cardiovascular disease
- DIO
diet-induced obese
- GH
growth hormone
- GL
generalized lipodystrophy
- HALS
human immunodeficiency virus- associated lipodystrophy syndrome
- IGF
insulin-like growth factor
- NAFLD
nonalcoholic fatty liver disease
- NPY
neuropeptide Y
- ObRb
long form of the leptin receptor
- PL
partial lipodystrophy
- POMC
pro-opiomelanocortin
- RCT
randomized clinical trial
- SNS
sympathetic nervous system
Footnotes
AUTHOR DISCLOSURES
Dr. Perakakis is funded by Deutsche Forschungsgemeinschaft (German Research Foundation) grant 389891681 (PE 2431/3–1:1). Dr. Farr is currently an employee of Bristol Myers Squibb, but this work was performed in the capacity of her previous employment at Beth Israel Deaconess Medical Center. Dr. Mantzoros is supported by National Institutes of Health grant K24DK081913; is a shareholder and consultant for Coherus Inc.; has served on the advisory board for Novo Nordisk; has received institutional funding from Novo Nordisk; has served as Visiting Professor and consultant for Regeneron, Aegherion, and Ansh Inc.; has received education fees from CardioMetabolic Health Conference (The Metabolic Institute ofAmerica); has received personal fees from Alexion; and has received nonfinancial support from Amarin, Janssen, and Boehringer Ingelheim, outside the submitted work.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the AuthorCenter.
REFERENCES
- 1.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in heaLthy men. J CLin Invest 2003;111:1409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum Leptin in normaL human subjects. J Clin Endocrinol Metab 1996;81:3419–23. [DOI] [PubMed] [Google Scholar]
- 3.Farr OM, Tsoukas MA, Mantzoros CS. Leptin and the brain: influences on brain development, cognitive functioning and psychiatric disorders. Metabolism 2015;64:114–30. [DOI] [PubMed] [Google Scholar]
- 4.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturabLe system independent of insulin. Peptides 1996;17: 305–11. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D Jr. Cerebrospinal fluid leptin levels: relationship to pLasma LeveLs and to adiposity in humans. Nat Med 1996;2:589–93. [DOI] [PubMed] [Google Scholar]
- 6.Caro JF, Kolaczynski JW, Nyce MR, et al. Decreased cerebrospinaL-fluid/serum Leptin ratio in obesity: a possibLe mechanism for Leptin resistance. Lancet 1996;348:159–61. [DOI] [PubMed] [Google Scholar]
- 7.Faouzi M, Leshan R, Bjornholm M, Hennessey T, Jones J, Munzberg H. DifferentiaL accessibiLity of circuLating Leptin to individuaL hypothaLamic sites. EndocrinoLogy 2007;148:5414–23. [DOI] [PubMed] [Google Scholar]
- 8.Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-reLated protein (AGRP) brain circuitry in normaL, anorectic, and monosodium gLutamate-treated mice. Proc NatL Acad Sci U S A 1998;95: 15043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huszar D, Lynch CA, Fairchild-Huntress V, et al. Targeted disruption of the meLanocortin-4 receptor resuLts in obesity in mice. CeLL 1997;88:131–41. [DOI] [PubMed] [Google Scholar]
- 10.Cone RD. Anatomy and regulation of the centraL meLanocortin system. Nat Neurosci 2005; 8:571–8. [DOI] [PubMed] [Google Scholar]
- 11.Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science 2007; 317:1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank S, Heni M, Moss A, et al. Leptin therapy in a congenital leptin-deficient patient leads to acute and Long-term changes in homeostatic, reward, and food-reLated brain areas. J CLin EndocrinoL Metab 2011;96:E1283–7. [DOI] [PubMed] [Google Scholar]
- 13.Farr OM, Fiorenza C, Papageorgiou P, et al. Leptin therapy aLters appetite and neuraL responses to food stimuLi in brain areas of Leptin-sensitive subjects without aLtering brain structure. J CLin EndocrinoL Metab 2014;99:E2529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinkle W, Cordell M, Leibel R, Rosenbaum M, Hirsch J. Effects of reduced weight maintenance and leptin repletion on functional connectivity of the hypothalamus in obese humans. PLoS One 2013;8:e59114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 2004;351:987–97. [DOI] [PubMed] [Google Scholar]
- 16.Chou SH, Chamberland JP, Liu X, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A 2011;108: 6585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouzoni E, Perakakis N, Mantzoros CS. Circulating profile of activin-follistatin-inhibin axis in women with hypothalamic amenorrhea in response to leptin treatment. Metabolism 2020: 154392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 1999; 341:879–84. [DOI] [PubMed] [Google Scholar]
- 19.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hypores-ponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 2002;110:1093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Licinio J, Caglayan S, Ozata M, et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci U S A 2004;101:4531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quennell JH, Mulligan AC, Tups A, et al. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 2009; 150:2805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet 2005; 366:74–85. [DOI] [PubMed] [Google Scholar]
- 23.Carro E, Senaris R, Considine RV, Casanueva FF, Dieguez C. Regulation of in vivo growth hormone secretion by leptin. Endocrinology 1997;138:2203–6. [DOI] [PubMed] [Google Scholar]
- 24.Chan JL, Matarese G, Shetty GK, et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad SciUSA 2006;103:8481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan JL, Williams CJ, Raciti P, et al. Leptin does not mediate short-term fasting-induced changes in growth hormone pulsatility but increases IGF-I in leptin deficiency states. J Clin Endocrinol Metab 2008;93:2819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilitsi E, Peradze N, Perakakis N, Mantzoros CS. Circulating levels of the components of the GH/IGF-1/IGFBPs axis totaL and intact IGF-binding proteins (IGFBP) 3 and IGFBP 4 and total IGFBP 5, as well as PAPPA, PAPPA2 and Stanniocalcin-2 levels are not altered in response to energy deprivation and/or metreLeptin administration in humans. MetaboLism 2019;97:32–9. [DOI] [PubMed] [Google Scholar]
- 27.Dardeno TA, Chou SH, Moon HS, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physioLogy and therapeutics. Front Neuroendocrinol 2010;31:377–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CLement K, Vaisse C, LahLou N, et al. A mutation in the human Leptin receptor gene causes obesity and pituitary dysfunction. Nature 1998;392:398–401. [DOI] [PubMed] [Google Scholar]
- 29.van der Kroon PH, BoLdewijn H, LangeveLd-Soeter N. CongenitaL hypothyroidism in Latent obese (ob/ob) mice. Int J Obes 1982;6:83–90. [PubMed] [Google Scholar]
- 30.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regionaL sympathetic nerve activation by leptin. J Clin Invest 1997;100:270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 2005;54:2012–8. [DOI] [PubMed] [Google Scholar]
- 32.Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 2000; 100:197–207. [DOI] [PubMed] [Google Scholar]
- 33.Chan JL, Mietus JE, Raciti PM, Goldberger AL, Mantzoros CS. Short-term fasting-induced autonomic activation and changes in catecholamine levels are not mediated by changes in leptin levels in healthy humans. Clin Endocrinol 2007;66: 49–57. [DOI] [PubMed] [Google Scholar]
- 34.Chrysafi P, Perakakis N, Farr OM, et al. Leptin alters energy intake and fat mass but not energy expenditure in lean subjects. Nat Commun 2020; 11:5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matarese G, La Rocca C, Moon HS, et al. Selective capacity of metreleptin administration to reconstitute CD4+ T-cell number in females with acquired hypoleptinemia. Proc Natl Acad SciUSA 2013;110:E818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moon HS, Matarese G, Brennan AM, et al. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes 2011;60:1647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–32. [DOI] [PubMed] [Google Scholar]
- 38.Lindstrom P. The physiology of obese-hyperglycemic mice [ob/ob mice]. Scienti-ficWorld Journal 2007;7:666–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen P, Zhao C, Cai X, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 2001;108:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh A, Wirtz M, Parker N, et al. Leptin- mediated changes in hepatic mitochondrial metabolism, structure, and protein levels. Proc Natl Acad SciUSA 2009;106:13100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995;269: 543–6. [DOI] [PubMed] [Google Scholar]
- 42.Weigle DS, Bukowski TR, Foster DC, et al. Recombinant ob protein reduces feeding and body weight in the ob/ob mouse. J Clin Invest 1995;96: 2065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sloan C, Tuinei J, Nemetz K, et al. Central leptin signaling is required to normalize myocardial fatty acid oxidation rates in caloric-restricted ob/ob mice. Diabetes 2011;60:1424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science 1996; 274:1704–7. [DOI] [PubMed] [Google Scholar]
- 45.Vaisse C, Halaas JL, Horvath CM, Darnell JE Jr., Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 1996;14:95–7. [DOI] [PubMed] [Google Scholar]
- 46.Carpino G, Morini S, Ginanni Corradini S, et al. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig Liver Dis 2005;37:349–56. [DOI] [PubMed] [Google Scholar]
- 47.Hwa JJ, Fawzi AB, Graziano MP, et al. Leptin increases energy expenditure and selectively promotes fat metabolism in ob/ob mice. Am J Physiol 1997;272:R1204–9. [DOI] [PubMed] [Google Scholar]
- 48.Mistry AM, Swick AG, Romsos DR. Leptin rapidly lowers food intake and elevates metabolic rates in lean and ob/ob mice. J Nutr 1997;127: 2065–72. [DOI] [PubMed] [Google Scholar]
- 49.Commins SP, Watson PM, Padgett MA, Dudley A, Argyropoulos G, Gettys TW. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology 1999;140: 292–300. [DOI] [PubMed] [Google Scholar]
- 50.Dodd GT, Decherf S, Loh K, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 2015;160:88–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ukropec J, Anunciado RV, Ravussin Y, Kozak LP. Leptin is required for uncoupling protein-1-independent thermogenesis during cold stress. Endocrinology 2006;147:2468–80. [DOI] [PubMed] [Google Scholar]
- 52.Zeng W, Pirzgalska RM, Pereira MM, et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 2015;163:84–94. [DOI] [PubMed] [Google Scholar]
- 53.Burcelin R, Kamohara S, Li J, Tannenbaum GS, Charron MJ, Friedman JM. Acute intravenous leptin infusion increases glucose turnover but not skeletal muscle glucose uptake in ob/ob mice. Diabetes 1999;48:1264–9. [DOI] [PubMed] [Google Scholar]
- 54.Beltowski J. Leptin and atherosclerosis. Atherosclerosis 2006;189:47–60. [DOI] [PubMed] [Google Scholar]
- 55.Konstantinides S, Schafer K, Koschnick S, Loskutoff DJ. Leptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. J Clin Invest 2001;108:1533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simonds SE, Pryor JT, Cowley MA. Does leptin cause an increase in blood pressure in animals and humans? Curr Opin Nephrol Hypertens 2017;26: 20–5. [DOI] [PubMed] [Google Scholar]
- 57.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad SciUSA 1997;94:8878–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masuzaki H, Ogawa Y, Hosoda K, Kawada T, Fushiki T, Nakao K. Augmented expression of the obese gene in the adipose tissue from rats fed high-fat diet. Biochem Biophys Res Commun 1995; 216:355–8. [DOI] [PubMed] [Google Scholar]
- 59.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 1995;269:546–9. [DOI] [PubMed] [Google Scholar]
- 60.Cusin I, Rohner-Jeanrenaud F, Stricker-Krongrad A, Jeanrenaud B. The weight-reducing effect of an intracerebroventricular bolus injection of leptin in genetically obese fa/fa rats. Reduced sensitivity compared with lean animals. Diabetes 1996;45:1446–50. [DOI] [PubMed] [Google Scholar]
- 61.Bence KK, Delibegovic M, Xue B, et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 2006;12:917–24. [DOI] [PubMed] [Google Scholar]
- 62.Bjorbak C, Lavery HJ, Bates SH, et al. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem 2000;275:40649–57. [DOI] [PubMed] [Google Scholar]
- 63.Lu D, Willard D, Patel IR, et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 1994;371:799–802. [DOI] [PubMed] [Google Scholar]
- 64.Aizawa-Abe M, Ogawa Y, Masuzaki H, et al. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest 2000;105: 1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simonds SE, Pryor JT, Ravussin E, et al. Leptin mediates the increase in blood pressure associated with obesity. Cell 2014;159:1404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harrison L, Schriever SC, Feuchtinger A, et al. Fluorescent blood-brain barrier tracing shows intact leptin transport in obese mice. Int J Obes (Lond) 2019;43:1305–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kleinert M, Kotzbeck P, Altendorfer-Kroath T, Birngruber T, Tschop MH, Clemmensen C. Time-resolved hypothalamic open flow micro-perfusion reveals normal leptin transport across the blood-brain barrier in leptin resistant mice. Mol Metab 2018;13:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farr OM, Li CR, Mantzoros CS. Central nervous system regulation of eating: Insights from human brain imaging. Metabolism 2016;65:699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herz CT, Kiefer FW. Adipose tissue browning in mice and humans. J Endocrinol 2019;241: R97–109. [PubMed] [Google Scholar]
- 70.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996;334:292–5. [DOI] [PubMed] [Google Scholar]
- 71.Marshall JA, Grunwald GK, Donahoo WT, Scarbro S, Shetterly SM. Percent body fat and lean mass explain the gender difference in leptin: analysis and interpretation of leptin in Hispanic and non-Hispanic white adults. Obes Res 2000;8: 543–52. [DOI] [PubMed] [Google Scholar]
- 72.Bidulescu A, Dinh PC Jr., Sarwary S, et al. Associations of leptin and adiponectin with incident type 2 diabetes and interactions among African Americans: the Jackson heart study. BMC Endocr Disord 2020;20:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ostlund RE Jr., Yang JW, Klein S, Gingerich R. Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab 1996;81:3909–13. [DOI] [PubMed] [Google Scholar]
- 74.Shankar A, Xiao J. Positive relationship between plasma leptin level and hypertension. Hypertension 2010;56:623–8. [DOI] [PubMed] [Google Scholar]
- 75.Ma D, Feitosa MF, Wilk JB, et al. Leptin is associated with blood pressure and hypertension in women from the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension 2009;53:473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shanker J, Rao VS, Ravindran V, Dhanalakshmi B, Hebbagodi S, Kakkar VV. Relationship of adiponectin and leptin to coronary artery disease, classical cardiovascular risk factors and atherothrombotic biomarkers in the IARS cohort. Thromb Haemost 2012;108:769–80. [DOI] [PubMed] [Google Scholar]
- 77.Taneli F, Yegane S, Ulman C, et al. Increased serum leptin concentrations in patients with chronic stable angina pectoris and ST-elevated myocardial infarction. Angiology 2006;57:267–72. [DOI] [PubMed] [Google Scholar]
- 78.Azar RR, Sarkis A, Salameh E, et al. Percutaneous coronary intervention increases leptin and decreases adiponectin levels. Clin Endocrinol (Oxf) 2006;65:712–6. [DOI] [PubMed] [Google Scholar]
- 79.Yang H, Guo W, Li J, et al. Leptin concentration and risk of coronary heart disease and stroke: A systematic review and meta-analysis. PLoS One 2017;12:e0166360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoefle G, Saely CH, Risch L, et al. Leptin, leptin soluble receptor and coronary atherosclerosis. Eur J Clin Invest 2007;37:629–36. [DOI] [PubMed] [Google Scholar]
- 81.Puurunen VP, Kiviniemi A, Lepojarvi S, et al. Leptin predicts short-term major adverse cardiac events in patients with coronary artery disease. Ann Med 2017;49:448–54. [DOI] [PubMed] [Google Scholar]
- 82.Martin SS, Blaha MJ, Muse ED, et al. Leptin and incident cardiovascular disease: the Multiethnic Study of Atherosclerosis (MESA). Atherosclerosis 2015;239:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seven E, Husemoen LL, Sehested TS, et al. Adipocytokines, C-reactive protein, and cardiovascular disease: a population-based prospective study. PLoS One 2015;10:e0128987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sierra-Johnson J, Romero-Corral A, Lopez-Jimenez F, et al. Relation of increased leptin concentrations to history of myocardial infarction and stroke in the United States population. Am J Cardiol 2007;100:234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Adiposity, adipokines, and risk of incident stroke in older men. Stroke 2013;44: 3–8. [DOI] [PubMed] [Google Scholar]
- 86.Bevan S, Meidtner K, Lorenz M, Sitzer M, Grant PJ, Markus HS. Adiponectin level as a consequence of genetic variation, but not leptin Level or leptin: adiponectin ratio, is a risk factor for carotid intima-media thickness. Stroke 2011;42: 1510–4. [DOI] [PubMed] [Google Scholar]
- 87.Saber H, Himali JJ, Shoamanesh A, et al. Serum leptin levels and the risk of stroke: the Framingham study. Stroke 2015;46:2881–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Katsiki N, Mikhailidis DP, Banach M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol Sin 2018;39:1176–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perakakis N, Stefanakis K, Mantzoros CS. The role of omics in the pathophysiology, diagnosis and treatment of non-alcoholic fatty liver disease. Metabolism 2020;111S:154320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perakakis N, Polyzos SA, Yazdani A, et al. Non-invasive diagnosis of non-alcoholic steatohepatitis and fibrosis with the use of omics and supervised learning: a proof of concept study. Metabolism 2019;101:154005. [DOI] [PubMed] [Google Scholar]
- 91.Polyzos SA, Perakakis N, Boutari C, et al. Targeted analysis of three hormonal systems identifies molecules associated with the presence and severity of NAFLD. J Clin Endocrinol Metab 2020; 105:e399–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Polyzos SA, Perakakis N, Mantzoros CS. Fatty liver in lipodystrophy: a review with a focus on therapeutic perspectives of adiponectin and/or leptin replacement. Metabolism 2019;96:66–82. [DOI] [PubMed] [Google Scholar]
- 93.Boutari C, Perakakis N, Mantzoros CS. Association of adipokines with development and progression of nonalcoholic fatty liver disease. Endocrinol Metab (Seoul) 2018;33:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Polyzos SA, Kountouras J, Mantzoros CS. Leptin in nonalcoholic fatty liver disease: a narrative review. Metabolism 2015;64:60–78. [DOI] [PubMed] [Google Scholar]
- 95.Polyzos SA, Aronis KN, Kountouras J, Raptis DD, Vasiloglou MF, Mantzoros CS. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia 2016;59:30–43. [DOI] [PubMed] [Google Scholar]
- 96.Wong SL, DePaoli AM, Lee JH, Mantzoros CS. Leptin hormonal kinetics in the fed state: effects of adiposity, age, and gender on endogenous leptin production and clearance rates. J Clin Endocrinol Metab 2004;89:2672–7. [DOI] [PubMed] [Google Scholar]
- 97.Chan JL, Wong SL, Orlova C, Raciti P, Mantzoros CS. Pharmacokinetics of recombinant methionyl human leptin after subcutaneous administration: variation of concentration-dependent parameters according to assay. J Clin Endocrinol Metab 2007;92:2307–11. [DOI] [PubMed] [Google Scholar]
- 98.Chan JL, Wong SL, Mantzoros CS. Pharmacokinetics of subcutaneous recombinant methionyl human leptin administration in healthy subjects in the fed and fasting states: regulation by gender and adiposity. Clin Pharmacokinet 2008;47: 753–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sienkiewicz E, Magkos F, Aronis KN, et al. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism 2011;60: 1211–21. [DOI] [PubMed] [Google Scholar]
- 100.Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med 2002;346:570–8. [DOI] [PubMed] [Google Scholar]
- 101.Addy CL, Gavrila A, Tsiodras S, Brodovicz K, Karchmer AW, Mantzoros CS. Hypoadiponectinemia is associated with insulin resistance, hypertriglyceridemia, and fat redistribution in human immunodeficiency virus-infected patients treated with highly active antiretroviral therapy. J Clin Endocrinol Metab 2003;88:627–36. [DOI] [PubMed] [Google Scholar]
- 102.Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab 2006;91:2605–11. [DOI] [PubMed] [Google Scholar]
- 103.Tsoukas MA, Farr OM, Mantzoros CS. Leptin in congenital and HIV-associated lipodystrophy. Metabolism 2015;64:47–59. [DOI] [PubMed] [Google Scholar]
- 104.Magkos F, Brennan A, Sweeney L, et al. Leptin replacement improves postprandial glycemia and insulin sensitivity in human immunodeficiency virus-infected lipoatrophic men treated with pioglitazone: a pilot study. Metabolism 2011; 60:1045–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brown RJ, Oral EA, Cochran E, et al. Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine 2018;60:479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oral EA, Gorden P, Cochran E, et al. Long-term effectiveness and safety of metreleptin in the treatment of patients with partial lipodystrophy. Endocrine 2019;64:500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McDuffie JR, Riggs PA, Calis KA, et al. Effects of exogenous leptin on satiety and satiation in patients with lipodystrophy and leptin insufficiency. J Clin Endocrinol Metab 2004;89: 4258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brown RJ, Valencia A, Startzell M, et al. Metreleptin-mediated improvements in insulin sensitivity are independent of food intake in humans with lipodystrophy. J Clin Invest 2018; 128:3504–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Javor ED, Cochran EK, Musso C, Young JR, Depaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes 2005;54:1994–2002. [DOI] [PubMed] [Google Scholar]
- 110.Lawler K, Huang-Doran I, Sonoyama T, et al. Leptin-mediated changes in the human metabolome. J Clin Endocrinol Metab 2020;105: 2541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grewal S, Gubbi S, Fosam A, et al. Metabolomic analysis of the effects of leptin replacement therapy in patients with lipodystrophy. J Endocr Soc 2020;4:bvz022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown RJ, Meehan CA, Gorden P. Leptin does not mediate hypertension associated with human obesity. Cell 2015;162:465–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Muniyappa R, Brown RJ, Mari A, et al. Effects of leptin replacement therapy on pancreatic beta-cell function in patients with lipodystrophy. Diabetes Care 2014;37:1101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oral EA, Javor ED, Ding L, et al. Leptin replacement therapy modulates circulating lymphocyte subsets and cytokine responsiveness in severe lipodystrophy. J Clin Endocrinol Metab 2006;91:621–8. [DOI] [PubMed] [Google Scholar]
- 115.Mulligan K, Khatami H, Schwarz JM, et al. The effects of recombinant human leptin on visceral fat, dyslipidemia, and insulin resistance in patients with human immunodeficiency virus-associated lipoatrophy and hypoleptinemia. J Clin Endocrinol Metab 2009;94:1137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 1999;282:1568–75. [DOI] [PubMed] [Google Scholar]
- 117.Moon HS, Huh JY, Dincer F, Schneider BE, Hasselgren PO, Mantzoros CS. Identification and saturable nature of signaling pathways induced by metreleptin in humans: comparative evaluation of in vivo, ex vivo, and in vitro administration. Diabetes 2015;64:828–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hukshorn CJ, Saris WH, Westerterp-Plantenga MS, Farid AR, Smith FJ, Campfield LA. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab 2000;85: 4003–9. [DOI] [PubMed] [Google Scholar]
- 119.Mittendorfer B, Horowitz JF, DePaoli AM, McCamish MA, Patterson BW, Klein S. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes 2011;60:1474–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hukshorn CJ, van Dielen FM, Buurman WA, Westerterp-Plantenga MS, Campfield LA, Saris WH. The effect of pegylated recombinant human leptin (PEG-OB) on weight loss and inflammatory status in obese subjects. Int J Obes Relat Metab Disord 2002;26:504–9. [DOI] [PubMed] [Google Scholar]
- 121.Zelissen PM, Stenlof K, Lean ME, et al. Effect of three treatment schedules of recombinant methionyl human leptin on body weight in obese adults: a randomized, placebo-controlled trial. Diabetes Obes Metab 2005;7:755–61. [DOI] [PubMed] [Google Scholar]
- 122.Korner J, Conroy R, Febres G, et al. Randomized double-blind placebo-controlled study of leptin administration after gastric bypass. Obesity (Silver Spring) 2013;21:951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lejeune MP, Hukshorn CJ, Saris WH, West-erterp-Plantenga MS. Effect of dietary restraint during and following pegylated recombinant leptin (PEG-OB) treatment of overweight men. Int J Obes Relat Metab Disord 2003;27:1494–9. [DOI] [PubMed] [Google Scholar]
- 124.Kissileff HR, Thornton JC, Torres MI, et al. Leptin reverses declines in satiation in weight-reduced obese humans. Am J Clin Nutr 2012;95: 309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 2005;115:3579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 2008;118: 2583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dallner OS, Marinis JM, Lu YH, et al. Dysregulation of a long noncoding RNA reduces leptin leading to a leptin-responsive form of obesity. Nat Med 2019;25:507–16. [DOI] [PubMed] [Google Scholar]
- 128.DePaoli A, Long A, Fine GM, Stewart M, O’Rahilly S. Efficacy of metreleptin for weight loss in overweight and obese adults with low leptin levels. Diabetes 2018;67 Suppl 1:296-LB. [Google Scholar]
- 129.Upadhyay J, Polyzos SA, Perakakis N, et al. Pharmacotherapy of type 2 diabetes: an update. Metabolism 2018;78:13–42. [DOI] [PubMed] [Google Scholar]
- 130.Pilitsi E, Farr OM, Polyzos SA, et al. Pharmacotherapy of obesity: available medications and drugs under investigation. Metabolism 2019;92: 170–92. [DOI] [PubMed] [Google Scholar]
- 131.Moon HS, Chamberland JP, Diakopoulos KN, et al. Leptin and amylin act in an additive manner to activate overlapping signaling pathways in peripheral tissues: in vitro and ex vivo studies in humans. Diabetes Care 2011;34:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Z, Kelly L, Heiman M, Greengard P, Friedman JM. Hypothalamic amylin acts in concert with leptin to regulate food intake. Cell Metab 2015;22:1059–67. [DOI] [PubMed] [Google Scholar]
- 133.Roth JD, Roland BL, Cole RL, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci U S A 2008; 105:7257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ravussin E, Smith SR, Mitchell JA, et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 2009;17:1736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chan JL, Koda J, Heilig JS, et al. Immunogenicity associated with metreleptin treatment in patients with obesity or lipodystrophy. Clin Endocrinol (Oxf) 2016;85:137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yuan D, Yi X, Zhao Y, et al. Intranasal delivery of N-terminal modified leptin-pluronic conjugate for treatment of obesity. J Control Release 2017; 263:172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang A, Anderson BM, Novakovic ZM, Grasso P. [D-Leu-4]-OB3 and MA-[D-Leu-4]-OB3, small molecule synthetic peptide leptin mimetics, improve glycemic control in diet-induced obese (DIO) mice. Peptides 2018;101:51–9. [DOI] [PubMed] [Google Scholar]
- 138.Vauthier V, Roujeau C, Chen P, et al. Endo- spanin1 affects oppositely body weight regulation and glucose homeostasis by differentially regulating central leptin signaling. Mol Metab 2017;6:159–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Digenio A, Pham NC, Watts LM, et al. Antisense inhibition of protein tyrosine phosphatase 1B With IONIS-PTP-1BRx improves insulin sensitivity and reduces weight in overweight patients with type 2 diabetes. Diabetes Care 2018;41: 807–14. [DOI] [PubMed] [Google Scholar]
- 140.Kars M, Yang L, Gregor MF, et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 2010;59: 1899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.von Schnurbein J, Manzoor J, Brandt S, et al. Leptin is not essential for obesity-associated hypertension. Obes Facts 2019;12:460–75. [DOI] [PMC free article] [PubMed] [Google Scholar]