Abstract
Objective:
To examine the associations of colorectal cancer (CRC) screening with stages of weight control among Korean Americans (KAs) using the transtheoretical model and provide implications for their weight control practice.
Methods:
A quantitative, cross-sectional survey was employed to collect data on current weight-control behaviors and intentions, CRC screening history, previous cancer diagnosis, body mass index, number of chronic conditions, perceived health status, health insurance, and sociodemographics. Purposive sampling was implemented to recruit KA participants in the Atlanta metropolitan area in the U.S. from May 2015 to February 2016. A total of 433 KAs aged 50 to 75 years completed a self-report survey questionnaire. Descriptive and bivariate analyses and multiple logistic regressions were performed using Stata Version 14/MP software.
Results:
Applying the stages of the transtheoretical model 53% were positioned in the first two stages (precontemplation and contemplation) of weight control with 47% being in the last two stages (action and maintenance). Participants who had been screened for CRC were more likely to be in the last two stages of weight control compared to those who had not been screened (OR = 2.49; p = .003).
Conclusions:
The findings suggest that preventive healthcare such as CRC screening may provide the opportunity for health education interventions to help encourage weight-control efforts and behaviors in the KA community. Future research is warranted to investigate the underlying mechanism behind the link between CRC screening and weight control to guide the development of interventions for eliminating health disparities.
Keywords: Colorectal Cancer Screening, Korean Americans, Transtheoretical Model, Weight Control
Introduction
In the U.S. more than two-thirds of adults are obese or overweight [1]. The obesity epidemic is a serious public health threat because it can lead to various adverse health outcomes [2]. Research has confirmed that intentional weight loss and maintenance can reduce the risks of obesity-related chronic diseases, such as hypertension, type 2 diabetes, cardiovascular diseases, and certain types of cancer (e.g., colorectal cancer) [3–5]. Research has also indicated that intentional weight control (IWC) is closely connected to improvements in individuals‟ quality of life and well-being [6]. Furthermore, previous studies showed that IWC could be motivated by several factors, including health concerns, medical triggers, social pressure, and improving appearance [7–9]. Yet, limited research has investigated the underlying mechanism behind IWC especially among historically underrepresented racial and ethnic groups in the U.S., while obesity-related chronic diseases remain a high burden on them [10, 11].
A growing body of literature has revealed evidence of a link between cancer screening and IWC. A National Health Interview Survey showed that colorectal cancer (CRC) screening uptake correlates with health behavior patterns, including smoking, alcohol consumption, fruit and vegetable consumption, and physical activity among individuals ages 50 and older [12]. The aggregated data from the National Health and Nutrition Examination Survey (1999–2008) demonstrated that CRC screening in combination with lifestyle modification (i.e., reduction in obesity, physical inactivity, intake of red meat, processed meat, alcohol, and cigarette smoking) is a more effective approach in preventing CRC and chronic diseases in comparison to receiving CRC screening alone [13]. Moreover, prior studies indicate that cancer screening may provide “teachable moments” to positively influence health behavior change (e.g., IWC) for racial and ethnic minorities in the U.S [7, 14].
Korean Americans (KAs), one of the fastest-growing Asian American subgroups in the U.S., have reported high rates of obesity-related chronic diseases (e.g., type 2 diabetes, hypertension, cardiovascular disease, CRC) and poor management of those chronic conditions [15–18]. A recent study showed that KAs were less likely to receive lifestyle advice from primary care providers compared to Native Korean patients living in South Korea (30.6% vs. 57.6%) [19]. Additionally, while CRC is the second most commonly diagnosed cancer and the leading cause of death among KAs, their CRC screening rates (25–50%) remain lower than those of non-Latino whites (57.7%), falling far short of the national CRC screening goal (70.5%) specified by Healthy People 2020 [20].
A clearer understanding of factors associated with IWC behavior for KAs would help to develop health promotion interventions for reducing their obesity and its related chronic diseases as well as improving their prevention efforts. Specifically, the examination of the association between CRC screening and IWC may inform the development of effective strategies aimed at assisting KAs to accept their physician‟s recommendations of CRC screening and weight control [21]. The recommended guidelines for CRC screening include having a fecal occult blood test (FOBT) annually, sigmoidoscopy every five years, or colonoscopy every ten years for individuals aged 50 to 75 [22]. In regards to IWC, the U.S. Preventive Services Task Force recommends intensive, multicomponent behavioral interventions for all obese adults at their annual medical check-up [23]. To our knowledge, this study is the first to assess the relationship between CRC screening and IWC among KAs. The findings of this study can help better understand the motivational factors for weight control behavior among KAs at risk of obesity-related chronic diseases. The findings also may provide other Asian American subgroups useful implications for encouraging preventive health behaviors including cancer screening and weight control as an effort to reduce obesity-related chronic diseases.
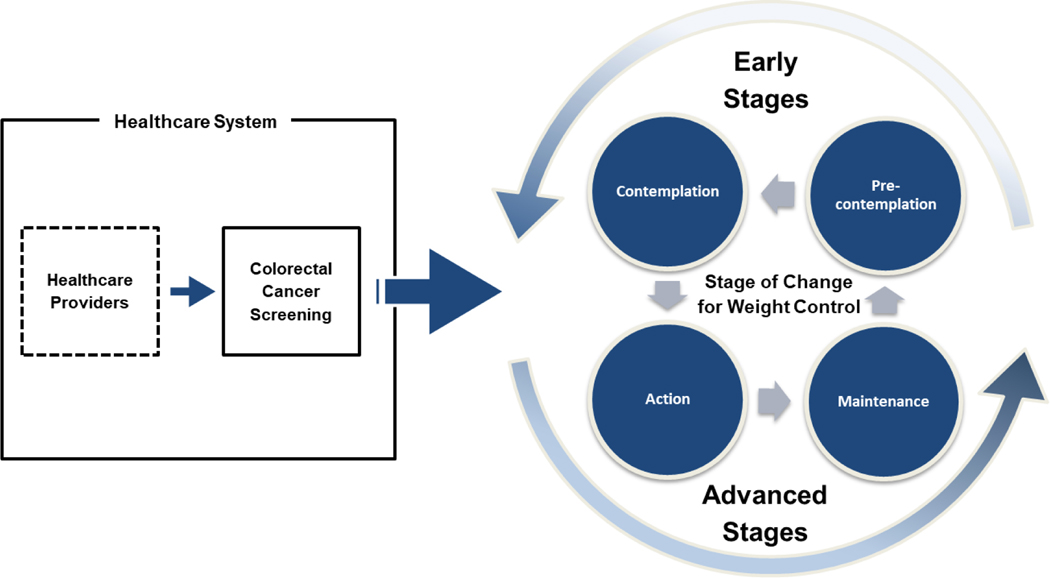
In order to investigate the association between CRC screening and IWC among KAs, the present study employed the transtheoretical model (TTM) as a conceptual framework. The TTM has been widely used in health promotion contexts to assess the progress of health behavior change, including weight control, smoking cessation, exercising, and screening uptake for diverse populations [24, 25]. In this study, the TTM guided building analytic models, selecting study variables, and interpreting IWC as a process of change through a series of four stages (see Figure 1) [26]. The first stage of change in IWC, precontemplation, refers to individuals who do not have any intention to control their weight in the next six months. The second stage of change, contemplation, refers to individuals who are considering or have intention to control their weight in the next six months but are not currently taking any active steps to do so. The third stage of change, action, refers to individuals who are actively trying to control their weight and have done so for less than six months. The fourth and last stage of change, maintenance, refers to individuals who have successfully maintained their weight control for six months or longer.
Figure 1.
Conceptual framework of transtheoretical model.
Note. Early stage: pre-contemplation/contemplation, advanced stage: action/maintenance
The purpose of this study was to assess the relationships between CRC screening uptake and stages of IWC among KAs aged 50–75 years. The research questions were as follows:
How do characteristics of the participants differ by stages of IWC?
Is CRC screening associated with stages of IWC among the participants?
Methods
Design
A cross-sectional survey design was used to collect quantitative data regarding stages of IWC, CRC screening history, health-related information, and sociodemographics from study participants.
Sample
Purposive sampling was implemented to recruit KA participants in the Atlanta metropolitan area from May 2015 to February 2016. Inclusion criteria were individuals who self-identify as Korean American, age 50 to 75, and reside in the state of Georgia. For recruitment, a list of KA community organizations (i.e., senior centers, churches/temples, and associations) was developed, and each organization was contacted by the research team via phone or email to obtain its permission for data collection. At organizations which agreed to participate, self-report survey questionnaires were administered among individuals who were eligible and completed an informed consent form. Out of 526 KAs who met the criteria, a total of 433 participants (82.3%) completed the survey. The questionnaire took about 45 minutes to complete. All measures of questions (originally written in English) were translated into Korean using back-translation. The Korean-translated questionnaire was pilot-tested with six KA men and women. A university institutional review board approved this study.
Measures
Stages of IWC.
The outcome variable for this study was four stages of IWC among the participants: precontemplation, contemplation, action, and maintenance. The stage was determined by current weight-control behavior and intentions for the future [26]. To determine the stage, the participants were asked to answer the following four questions, each with “yes” or “no” responses: (1) “In the past month, have you been actively trying to lose weight?”, (2) “In the past month, have you been actively trying to keep from gaining weight?”, (3) “Are you seriously considering trying to lose weight to reach your goal in the next six months?”, and (4) “Have you maintained your desired weight for more than six months?”
For example, precontemplation was assigned to participants who had answered “no” to questions 1, 2, and 3, signifying that they had no intention of controlling their weight within the past month or in the next six months. Contemplation was assigned to participants who had answered “no” to questions 1 and 2 and “yes” to question 3, signifying that they had not been actively trying to control their weight, but were seriously considering doing so in the next six months. Action was assigned to participants who had answered “yes” to either question 1 or 2 and “no” to question 4, signifying that they had been actively trying to control their weight for less than six months. Lastly, maintenance was assigned to participants who had answered “yes” to either question 1 or 2 and “yes” to question 4, signifying that they had successfully maintained their weight loss for at least six months.
CRC screening.
The primary independent variable for this study was uptake of CRC screening for lifetime. Participants were asked to report their CRC screening with one item, “Have you ever had any of the following three CRC screening tests: a fecal occult blood test (FOBT), sigmoidoscopy, or colonoscopy?” The stool DNA test, a relatively new screening method for CRC and not widely used at the time of data collection, was not listed in the options. The CRC screening uptake was assigned as a dummy variable with a value of 1 if the participant had received any of the three CRC screening tests or a value of 0 if otherwise.
Health-related information.
Participants were asked to report health-related information, including prior cancer diagnosis, body mass index (BMI), number of chronic conditions, self-rated health status, and access to healthcare services (i.e., having health insurance, having a primary care doctor). Previous cancer diagnosis history was measured with one item, “Have you ever had cancer of any kind?” To assess BMI using a formula (weight [kg]/{height [m]}2 or 703 × weight [lbs]/{height [in]}2), participants were asked to report their height (“meter” or “inch”) and body weight (“kg” or “lb”). The number of chronic conditions was computed with one item, “Do you have the following chronic conditions? (please check all that apply to you) along with options of “high blood pressure,” “diabetes,” “heart disease,” “stroke,” “arthritis,” “asthma,” “stomach-related disease,” and “other (please specify).” The self-rated health status was measured with one item, “How do you rate your current health?” Participants were asked to choose an answer from a 5-Likert scale (very bad, bad, fair, good, or very good). Finally, to measure access to healthcare services, participants were asked to report whether they had health insurance using an item, “Do you have health insurance?” and whether they had a primary care doctor using an item, “Do you have a primary care doctor?”
Sociodemographic information.
Participants were asked to report sociodemographic information including age (years), sex (male or female), educational achievement (<college vs. ≥college), marital status (not married/partnered vs. married/partnered), and annual household income (<$20,000, $20,000 - $39,999, $40,000 - $59,999, $60,000 - $79,999, $80,000 - $99,999, ≥$100,000). Participants were also asked to report length (in months) of residence in the U.S. and to rate English proficiency (“poor,” “bad,” “fair,” “good,” or “excellent”).
Statistical Analysis
Descriptive analyses were conducted to examine characteristics (i.e., sociodemographic, health-related information, CRC screening, and stages of IWC) of the participants. Bivariate analyses were performed to assess the characteristics of the participants by their IWC stages. Finally, the multiple logistic regression models were used to identify correlates significantly associated with being in a higher IWC stage compared to being in a lower one. Model fitting was implemented by using Pearson chi-squared goodness-of-fit test and robust standard errors to adjust for possible interclass correlation. Odds ratios (ORs) with 95% confidence intervals were conducted. All statistical analyses were completed using Stata Version 14/MP software [27].
Results
Sample Characteristics by Stages of Weight Control
Descriptive statistics for the study participants are presented in Table 1. The mean age of the participants was 58.8 years old, and more than 60% were female. About 59% reported achieving a college education or higher, and 80% were married or partnered. Approximately 16% had an annual household income of less than $20,000 and 7.1% had an annual household income greater than $100,000. Theaverage period of U.S. residence was 267.1 months (22.3 years), and only 11.8% self-rated their English proficiency as good or excellent. Study participants reported having an average BMI of 23.5 and at least one chronic condition. More than 10% of study participants reported having a history of cancer, approximately 73% had health insurance, and 63% had a primary care doctor. The majority (91.5%) of study participants rated their health status as fair or better. Furthermore, about a third (34%) of study participants have been screened for CRC. Results showed that less than half (48.1%) of study participants were in the precontemplation stage, 4.7% were in the contemplation stage, 30.0% were in the action stage, and 17.1% were in the maintenance stage. For analysis the first two stages of IWC (precontemplation and contemplation) were combined into one stage (52.8%; hereafter, early stage) and the last two stages of IWC (action and maintenance) were combined (47.1%; hereafter, advanced stage).
Table 1.
Characteristics of the study sample (N = 433).
| Variable | % (N) |
|---|---|
| Age | 58.8 (SD = ±7.2) |
| Sex | |
| Male | 38.3 (166) |
| Female | 61.7 (267) |
| Education | |
| <College | 41.4 (175) |
| ≥College | 58.6 (248) |
| Marital status | |
| Not married/partnered | 16.1 (69) |
| Married/partnered | 84.0 (361) |
| Annual household income | |
| <$20,000 | 15.9 (63) |
| $20,000–$39,999 | 24.9 (99) |
| $40,000–$59,999 | 24.4 (97) |
| $60,000–$79,999 | 17.6 (70) |
| $80,000–$99,999 | 10.1 (40) |
| ≥$100,000 | 7.1 (28) |
| Length of residence in the U.S. (Months) | 267.1 (SD = ±125.6) |
| Self-rated English proficiency | |
| Poor | 11.3 (49) |
| Bad | 36.5 (158) |
| Fair | 40.4 (175) |
| Good | 8.6 (37) |
| Excellent | 3.2 (14) |
| Prior cancer diagnosis | |
| No | 89.1 (376) |
| Yes | 10.9 (46) |
| Body mass index | 23.5 (SD = ±3.58) |
| Number of chronic conditions | 1.0 (SD = ±1.1) |
| Self-rated health status | |
| Poor | 12 (5) |
| Bad | 7.2 (31) |
| Fair | 57.9 (248) |
| Good | 28.0 (120) |
| Excellent | 5.6 (24) |
| Health insurance | |
| No | 26.8 (116) |
| Yes | 73.2 (317) |
| Having a primary care doctor | |
| No | 37.4 (162) |
| Yes | 62.6 (271) |
| Colorectal cancer screening | |
| No | 66.0 (249) |
| Yes | 34.0 (128) |
| Stages of weight control | |
| Early stage of intentional weight control | 52.8 (225) |
| Precontemplati on | 48.1 (205) |
| Contemplation | 4.7 (20) |
| Advanced stage of intentional weight control | 47.2 (201) |
| Action | 30.1 (128) |
| Maintenance | 17.1 (73) |
Note. The percent and total number of each variable may not equal 100% due to missing values.
The bivariate statistics present differences in the prevalence of sociodemographic and health-related variables for participants being in the advanced stage versus the early stage of IWC (Table 2). The advanced stage was more common among those who were female (p = .001), had any CRC screening test (p< .001), had a cancer history (p = .01), had a greater BMI (p < .001), had more chronic conditions (p = .002), and had a primary care doctor (p = .004).
Table 2.
Characteristics of the study sample by stages of intentional weight control (N = 433).
| % (N) or Mean (±SD) Stages of Intentional Weight Control | |||
|---|---|---|---|
|
|
|||
| Variable | Early Stage (Precontemplation/Contemplation) (n = 225) | Advanced Stage (Action/Maintenance) (n = 201) | p-value |
| Age | 58.84 (±7.0) | 58.74 (±7.4) | 0.888 |
| Sex | 0.001 | ||
| Male | 45.78 (103) | 30.35 (61) | |
| Female | 54.22 (122) | 69.65 (140) | |
| Education | 0.293 | ||
| <College | 43.78 (95) | 38.69 (77) | |
| ≥College | 56.22 (122) | 61.31 (122) | |
| Marital status | 0.998 | ||
| Not married/partnered | 16.07 (36) | 16.08 (32) | |
| Married/partnered | 83.93 (188) | 83.92 (167) | |
| Annual household income | 0.892 | ||
| <$20,000 | 14.56 (30) | 16.67 (31) | |
| $20,000–$39,999 | 24.27 (50) | 25.27 (47) | |
| $40,000–$59,999 | 27.18 (56) | 22.04 (41) | |
| $60,000–$79,999 | 17.96 (37) | 17.74 (33) | |
| $80,000–$99,999 | 9.71 (20) | 10.75 (20) | |
| ≥$100,000 | 6.31 (13) | 7.53 (14) | |
| Length of residence in the U.S. (Months)’ | 266.6 (±118) | 265.8 (±134) | 0.945 |
| Self-rated English proficiency | 0.483 | ||
| Poor | 10.22 (23) | 11.94 (24) | |
| Bad | 40.44 (91) | 32.34 (65) | |
| Fair | 32.34 (85) | 32.34 (89) | |
| Good | 8.44 (19) | 8.96 (18) | |
| Excellent | 3.11 (7) | 2.49 (5) | |
| Prior cancer diagnosis | 0.010 | ||
| No | 92.79 (206) | 85.00 (170) | |
| Yes | 7.21 (16) | 15.00 (30) | |
| Body mass index | 22.4 (±2.59) | 24.7 (±4.06) | <0.001 |
| Number of chronic conditions | 0.87 (±1.09) | 1.21 (±1.13) | 0.002 |
| Self-rated health status | 0.062 | ||
| Poor | 0.9 (2) | 15 (3) | |
| Bad | 4.07 (9) | 11 (22) | |
| Fair | 61.54 (136) | 54 (108) | |
| Good | 27.15 (60) | 29 (58) | |
| Excellent | 6.33 (14) | 4.5 (9) | |
| Health insurance | 0.057 | ||
| No | 31.11 (70) | 22.89 (46) | |
| Yes | 68.89 (155) | 77.11 (155) | |
| Having a primary care doctor | 0.004 | ||
| No | 44.44 (100) | 30.85 (62) | |
| Yes | 55.56 (125) | 69.15 (139) | |
| Colorectal cancer screening | <0.001 | ||
| No | 74.63 (150) | 56.25 (99) | |
| Yes | 25.37 (51) | 43.75 (77) | |
Note. The total sample size of each variable may not be the same as the total sample size of the study due to missing values. P-values for Pearson Chi-squared test for categorical variables and t-test with unequal variances for continuous variables.
Association between CRC Screening and Stages of Weight Control
As shown Table 3, the multiple logistic regression models show that the advanced stage of IWC was observed more often among individuals who had been screened for CRC than those who had not (OR = 2.49; p = .003). Moreover, study participants with a college education or higher (OR = 2.06; p = .030), a greater BMI (OR = 1.54; p< .001), and an increased number of chronic conditions (OR = 1.39; p = .036) were more likely to be in the advanced stage of IWC. The advanced stage of IWC was less likely to be observed in study participants who were men (OR = .19; p< .001)with increased age (OR = .93; p = .013).
Table 3.
Logistic regression analyses of factors associated with stages of intentional weight control (N = 433).
| Advanced Stage (Action/Maintenance) vs. Early Stage (Precontemplation/Contemplation) | ||||
|---|---|---|---|---|
|
|
||||
| Variable | Odds Ratios | [95% CI] | p-value | |
| Colorectal cancer screening (ref. No) | 2.49 | 1.354 | 4.576 | 0.003 |
| Age | 0.93 | 0.883 | 0.985 | 0.013 |
| Male (ref. Female) | 0.19 | 0.101 | 0.370 | 0.000 |
| Annual household income (ref. <$20,000) | ||||
| $20,000–$39,999 | 0.63 | 0.255 | 1.573 | 0.325 |
| $40,000–$59,999 | 0.36 | 0.124 | 1.036 | 0.058 |
| $60,000–$79,999 | 0.48 | 0.150 | 1.509 | 0.208 |
| $80,000–$99,999 | 0.45 | 0.126 | 1.628 | 0.225 |
| ≥$100,000 | 0.63 | 0.161 | 2.487 | 0.513 |
| Education (ref. <College) | 2.06 | 1.071 | 3.945 | 0.030 |
| Married/Partnered (ref. Not married/partnered) | 1.73 | 0.749 | 4.014 | 0.199 |
| Length of residence in the U.S. (Months)’ | 1.00 | 0.997 | 1.002 | 0.495 |
| Self-rated English proficiency (ref. Poor) | ||||
| Bad | 0.63 | 0.221 | 1.816 | 0.396 |
| Fair | 0.71 | 0.246 | 2.074 | 0.536 |
| Good | 0.47 | 0.113 | 1.985 | 0.306 |
| Excellent | 0.35 | 0.041 | 3.006 | 0.339 |
| Prior cancer diagnosis (ref. No) | 1.42 | 0.610 | 3.297 | 0.417 |
| Body mass index | 1.54 | 1.356 | 1.756 | 0.000 |
| Number of chronic conditions | 1.39 | 1.022 | 1.892 | 0.036 |
| Self-rated health status (ref. Poor) | ||||
| Bad | 0.30 | 0.023 | 3.887 | 0.357 |
| Fair | 0.49 | 0.050 | 4.695 | 0.533 |
| Good | 0.85 | 0.084 | 8.475 | 0.887 |
| Excellent | 0.40 | 0.032 | 4.922 | 0.472 |
| Health insurance (ref. No) | 1.29 | 0.664 | 2.518 | 0.450 |
| Having a primary doctor (ref. No) | 1.26 | 0.648 | 2.432 | 0.500 |
Discussion
This study sought to examine the associations between CRC screening and the stages of IWC among KAs aged 50–75 years old using the TTM. Study findings add to our understanding of weight control intent among KAs who participate in health behaviors such as CRC screening.
The main finding of this study is that the study participants with CRC screening history were more than two times likely than those without one to be in the advanced stage of IWC, holding all other variables constant. This may be because participants in the advanced stage of IWC were more internally motivated to manage their overall health, including being screened for CRC. Also, CRC screening may play a crucial role in providing opportunities for KAs to better understand the importance of primary prevention efforts (e.g., IWC) in lowering their risks for obesity-related chronic diseases. Healthcare providers could assess KA patients about their weight control intentions as this may indicate the patients‟ ability to follow through on other preventative health screenings. The finding also suggests that health professionals need to develop approaches to increasing CRC screening and IWC among KAs through primary- and secondary-combined collaborative prevention efforts.
Secondly, this study found that the study participants in the advanced stage of IWC tended to be women who had been screened for CRC, had increased risks for poorer health (i.e., increased BMI, more chronic conditions, and previous cancer diagnosis), and had greater access to healthcare services (i.e., reported having a primary care doctor). Prior research has indicated that due to sociocultural factors and family pressures Asian American women tend to be highly weight conscious [28, 29]. Cultural messages that emphasize a strict thin body ideal combined with midlife aging-related physical changes (i.e., increased BMI) may result in body dissatisfaction among middle-aged women [30]. These race- and age-specific perspectives may enhance KA women‟s interest in learning about prevention efforts that would lower their risks for obesity-related chronic diseases.
Furthermore, because the participants in the advanced stage of IWC had greater health-related concerns and needs compared to those in the early stage of IWC, they may have more opportunities for discussing IWC with their healthcare providers. Research has demonstrated that there is a lack of understanding among older Asian Americans regarding prevention care, which results in their tendency to see a doctor only after symptoms have become apparent [31]. This suggests that healthcare providers should consider the characteristics of KAs found in this study when discussing IWC, especially with KA men having no CRC screening history. Moreover, health professionals at primary care settings should address the importance of preventive health behaviors and adherence to healthcare services in reducing the risks of obesity-related chronic diseases with all of their patients.
Finally, the findings offer implications for practice and research directed at promoting IWC among middle-aged and older KAs. Community health educators should develop educational programs that target KAs who lack an understanding of preventive healthcare and do not comply with the recommended guidelines for CRC screening and IWC. Interventions for IWC promotion among middle-aged and older KAs could be tailored according to the degree of their readiness for change. Future research should explore the barriers that prohibit KAs in the community from engaging in and maintaining IWC and adhering to recommended CRC screening. Future research could also assess the associations between IWC and other cancer screenings (e.g., mammography, Pap tests) and preventive health behaviors (e.g., physical activities) to develop interventions that may decrease the burden of obesity and other chronic diseases among the KA population.
Limitations
Findings of this study should be carefully interpreted due to several limitations. A cross-sectional study design does not allow for causal associations between CRC screening and stages of IWC. Future studies with randomized controlled trials can better explain the causal relationships.
As nonprobability sampling was conducted in a metropolitan area in the U.S., the findings cannot be generalized to all KAs residing in other geographic regions of the country. However, when compared to KAs in other metropolitan areas (i.e., Chicago, Philadelphia and New Jersey) [32, 33], the KAs in the present study reported similar sociodemographic characteristics except for lower English proficiency and higher rates of having health insurance. Moreover, the majority of the participants in this study were recruited from ethnic churches so the findings might not reflect characteristics of KAs who do not belong to community or religious organizations. Research using probability sampling with a larger sample size may lead to a better explanation of the relationship between CRC screening and IWC in this population.
Also, variables used in this study relied on participant self-report. Observational assessments on these variables by health professionals may reduce systematic error (e.g., recall bias) of the measures. In addition, this study investigated the stages of IWC by the CRC screening groups (i.e., „ever screened‟ versus „never screened‟). Division of subgroups (e.g., „ever screened‟ versus „appropriately screened‟) in the CRC screening by compliance with the screening guidelines might reveal better understandings of the associations between stages of IWC and CRC screening among KAs.
Lastly, this study included individuals with normal weight or underweight in the precontemplation pool. Analyses of data excluding this pool might provide different results. Future research may further analyze weight control stages by BMI groups (e.g., underweight only, normal weight only, overweight/obese only, and all combined).
Acknowledgments
Declarations
Funding The first author was supported by the National Institute on Minority Health and Health Disparities (NIMHD) Grant Number U54MD008173, a component of the National Institutes of Health (NIH) and its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIMHD or NIH.
Footnotes
Conflicts of interest/Competing interests
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Centers for Disease Control and Prevention. Obesity and overweight. https://www.cdc.gov/nchs/fastats/obesity-overweight.htm.Accessed February 8, 2020.
- 2.Centers for Disease Control and Prevention. Adult obesity causes & consequences. 2019. https://www.cdc.gov/obesity/adult/causes.html.Accessed February 8, 2020.
- 3.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the management of overweight and obesity in adults. Circulation. 2014;129(25 Suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Institute. Obesity and cancer fact sheet. 2017. https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/obesity-fact-sheet.Accessed February 4, 2020.
- 5.Ma C, Avenell A, Bolland M, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017;359:j4849. doi: 10.1136/bmj.j4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolotkin RL, Andersen JR. A systematic review of reviews: exploring the relationship between obesity, weight loss and health-related quality of life. Clin Obes. 2017;7(5):273–289. doi: 10.1111/cob.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorin AA, Phelan S, Hill JO, Wing RR. Medical triggers are associated with better short- and long-term weight loss outcomes. Prev Med. 2004;39(3):612–616. doi: 10.1016/j.ypmed.2004.02.026 [DOI] [PubMed] [Google Scholar]
- 8.LaRose JG, Leahey TM, Hill JO, Wing RR. Differences in motivations and weight loss behaviors in young adults and older adults in the National Weight Control Registry. Obesity. 2013;21(3):449–453. doi: 10.1002/oby.20053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos I, Sniehotta FF, Marques MM, Carraça EV, Teixeira PJ. Prevalence of personal weight control attempts in adults: a systematic review and meta-analysis. Obesity Reviews. 2017;18(1):32–50. doi: 10.1111/obr.12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon NP, Lin TY, Rau J, Lo JC. Aggregation of Asian-American subgroups masks meaningful differences in health and health risks among Asian ethnicities: an electronic health record based cohort study. BMC Public Health. 2019;19(1):1551. doi: 10.1186/s12889-019-7683-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palaniappan LP, Wong EC, Shin JJ, Fortmann SP, Lauderdale DS. Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int J Obes. 2011;35(3):393–400. doi: 10.1038/ijo.2010.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meissner HI, Yabroff KR, Dodd KW, Leader AE, Ballard-Barbash R, Berrigan D. Are patterns of health behavior associated with cancer screening? Am J Health Promot. 2009;23(3):168–175. doi: 10.4278/ajhp.07082085 [DOI] [PubMed] [Google Scholar]
- 13.Joshu CE, Parmigiani G, Colditz GA, Platz EA. Opportunities for the primary prevention of colorectal cancer in the United States. Cancer Prev Res. 2012;5(1):138–145. doi: 10.1158/1940-6207.CAPR-11-0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sriphanlop P, Jandorf L, Thompson H, et al. Preventive health behaviors among low-income African American and Hispanic populations: can colonoscopy screening serve as a teachable moment? J Racial Ethn Health Disparities. 2018;5(1):179–186. doi: 10.1007/s40615-017-0355-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heo H-H, Sentell TL, Li D, et al. Disparities in potentially preventable hospitalizations for chronic conditions among Korean Americans, Hawaii, 2010–2012. Prev Chronic Dis. 2015;12:E152. doi: 10.5888/pcd12.150057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko J, Kim KB, Timmerman GM, et al. Factors predicting sodium intake of Korean Americans with type 2 diabetes. J Immigr Minor Health. 2018;20(3):641–650. doi: 10.1007/s10903-017-0602-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin C-N, Keller C, An K, Sim J. Cardiovascular disease in Korean Americans: A systematic review. J Cardiovasc Nurs. 2018;33(1):82–93. doi: 10.1097/JCN.0000000000000417 [DOI] [PubMed] [Google Scholar]
- 18.Jung MY, Holt CL, Ng D, et al. The Chinese and Korean American immigrant experience: a mixed-methods examination of facilitators and barriers of colorectal cancer screening. Ethn Health. 2018;23(8):847–866. doi: 10.1080/13557858.2017.1296559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim MJ, Lee SJ, Ahn Y-H, Lee H. Lifestyle advice for Korean Americans and native Koreans with hypertension. J Adv Nurs. 2011;67(3):531–539. doi: 10.1111/j.1365-2648.2010.05504.x [DOI] [PubMed] [Google Scholar]
- 20.Office of Disease Prevention and Health Promotion. Cancer. C-16 Increase the proportion of adults who receive a colorectal cancer screening based on the most recent guidelines. 2020. https://www.healthypeople.gov/2020/data-search/Search-the-Data#objid=4054; Accessed February 4, 2020.
- 21.Lee S-Y, Lee EE, Aranda F. Instrument adaptation, modification, and validation for cultural beliefs about colorectal cancer screening among Korean Americans. Cancer Nurs. 2018;41(3):E38–E48. doi: 10.1097/NCC.0000000000000523 [DOI] [PubMed] [Google Scholar]
- 22.American Cancer Society. American cancer society guideline for colorectal cancer screening. 2018https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/acs-recommendations.html.Accessed February 18, 2020.
- 23.U.S. Preventive Services. Final recommendation statement, Obesity in adults: Screening and management. 2012. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/obesity-in-adults-screening-and-management-2012.Accessed February 18, 2020.
- 24.Becker NV, Asch DA, Kullgren JT, et al. Stages of change and patient activation measure scores in the context of incentive-based health interventions. Am J Health Promot. 2015;30(2):133–135. doi: 10.4278/ajhp.141001-QUAN-489 [DOI] [PubMed] [Google Scholar]
- 25.Sbrocco T, Osborn R, Clark RD, et al. Assessing the stages of change among African American women in a weight management program. J Black Psychol. 2011;38(1):81–103. doi: 10.1177/0095798411403618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cancer Prevention Research Center. Weight control: stages of change (Short Form). The University of Rhode Island. https://web.uri.edu/cprc/weight-control-stages-of-change-short-form/.Accessed February 4, 2020. [Google Scholar]
- 27.Stata. Stata 14. https://www.stata.com/stata14/.Accessed February 4, 2020.
- 28.Frederick DA, Kelly MC, Latner JD, Sandhu G, Tsong Y. Body image and face image in Asian American and white women: Examining associations with surveillance, construal of self, perfectionism, and sociocultural pressures. Body image. 2016;16:113–25. [DOI] [PubMed] [Google Scholar]
- 29.Smart R, Tsong Y. Weight, body dissatisfaction, and disordered eating: Asian American women‟s perspectives. Asian American Journal of Psychology. 2014;5(4):344. [Google Scholar]
- 30.Slevec JH, Tiggemann M. Predictors of body dissatisfaction and disordered eating in middle-aged women. Clinical psychology review. 2011;31(4):515–24. [DOI] [PubMed] [Google Scholar]
- 31.Yoo G, Musselman E, Lee I, Yee-Melichar D. Addressing health disparities among older Asian Americans: Data and diversity. Generations. 2015;38:74–81. [Google Scholar]
- 32.Lee SY. Colorectal Cancer Screening among Korean Americans in Chicago: Does It Matter Whether They had the Screening in Korea or the US? Asian Pac J Cancer Prev. 2018;19(5):1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma GX, Lee M, Beeber M, Das R, Feng Z, Wang MQ, et al. Community-Clinical Linkage Intervention to Improve Colorectal Cancer Screening Among Underserved Korean Americans. Cancer Health Disparities. 2019;3:e1–15.. [PMC free article] [PubMed] [Google Scholar]