Abstract
Background:
Neonatal encephalopathy (NE) is a major cause of long-term neurodevelopmental disability in neonates. We evaluated the ability of serially measured biomarkers of brain injury to predict adverse neurological outcomes in this population.
Methods:
Circulating brain injury biomarkers including BDNF, IL-6, IL-8, IL-10, VEGF, Tau, GFAP, and NRGN were measured at 0, 12, 24, 48, 72, and 96 hours of cooling from 103 infants with NE undergoing TH. The biomarkers’ individual and combinative ability to predict death or severe brain injury and adverse neurodevelopmental outcomes beyond 1 year of age was assessed.
Results:
Early measurements of inflammatory cytokines IL-6, 8, and 10 within 24 HOL (AUC=0.826) and late measurements of Tau from 72–96 HOL (AUC=0.883, OR 4.37) were accurate in predicting severe brain injury seen on MRI. Late measurements of Tau were predictive of adverse neurodevelopmental outcomes (AUC=0.81, OR 2.59).
Conclusions:
Tau was consistently a predictive marker for brain injury in neonates with NE. However, in the first 24 HOL, IL-6, 8, and 10 in combination were most predictive of death or severe brain injury. The results of this study support the use of a serial biomarker panel to assess brain injury over the time course of disease in NE.
Introduction
Neonatal encephalopathy (NE) is a major contributor to adverse neurologic sequelae and long-term neurodevelopmental disability in affected neonates(1–3). It is estimated that 1–3 per 1,000 births result in asphyxia that can lead to NE(4). Although there have been advancements in the identification and treatment of perinatal NE, including the introduction of therapeutic hypothermia, the rate of disability among affected neonates still remains around 45 percent(3,5,6). To reduce the rate of these adverse outcomes, infants who are at an increased risk of brain injury or not responding well to treatment must be identified early for possible adjuvant neuroprotective interventions. Real-time physiological biomarkers are urgently needed to evaluate the extent of brain injury, guide treatment practices, and predict outcomes for neonates with NE.
Currently, there is no blood based biomarker of neonatal brain injury used in clinical practice. However, several candidate circulating plasma biomarkers that can indicate the severity of hypoxic ischemic injury to the brain have been proposed. Both pro- and anti-inflammatory immune modulating cytokines Interleukin (IL) 6, IL-8, and IL-10 are upregulated as a part of the neuroinflammatory cascade trigged by hypoxic conditions in the brain, and have been associated with brain injury in small cohorts of babies with NE(7). Tau protein, Neurogranin (NRGN), and Glial Acidic Fibrillary Protein (GFAP) are brain specific proteins found concentrated within neuron axons, dendrites, and astrocytes, respectively(8–10). Thus, the elevation of these proteins in the blood can be a sign of neuronal or astrocytic damage or apoptosis(8,9,11,12). Growth Factors Vascular Endothelial Growth Factor (VEGF) and Brain Derived Neurotrophic Factor (BDNF) are also released as a response to neuronal injury(7,11). Thus, these markers can reflect distinct aspects of pathogenesis when assessed quantitatively in the blood of affected newborns. Recent studies have examined the use of circulating plasma biomarkers as a real-time indicator of brain injury in neonates with NE undergoing therapeutic hypothermia (TH) and have investigated the efficacy of these markers to predict adverse CNS outcomes in infants with NE(7,8,10,13,14). However, the temporality of serial measurements and combinative value of these biomarkers during TH and post-rewarming has not been well described. This study aims to investigate a panel of serially measured candidate biomarkers and to determine whether they alone, or in combination, are predictive of neurological outcomes.
Methods
Study Population
This prospective cohort study was conducted at Children’s National Medical Center in a level IV NICU in the United States of America. Infants admitted for initiation of TH between 2010 and 2016 were screened for enrollment. Criteria for enrollment eligibility included term and near-term infants (>35 weeks gestation) with a diagnosis of moderate or severe NE as determined by modified Sarnat staging(4,15), who were treated with TH according to the National Institutes of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) protocol(4). This study was approved by the Children’s National Institutional Review Board and written informed consent was obtained from the parents of participants. Baseline (at time of cooling initiation) biomarker data and clinical characteristics of a subset of this cohort was previously published as part of a multi-center study(16).
Specimen collection and biomarker determinations
Plasma samples were collected from salvaged routine clinical laboratory specimens of infants that met study criteria. Per hospital protocol, routine clinical blood samples are processed and centrifuged at 4000 RPM for 5 minutes. Excess plasma not utilized for biochemical analyses are then stored at −80°C for a minimum of 48 hours in accordance with the College of American Pathologists Laboratory Standards before disposal. Prior to their disposal from the laboratory, the study team identified and retrieved samples from the enrolled patients at each appropriate time point. With the initiation of therapeutic hypothermia set as timepoint 0, specimens were collected at 0, 12, 24, 72, and 96 hours of TH when available. Upon collection, samples were thawed once for transfer to storage cryovials and then stored at −80°C prior to analysis. Inflammatory cytokines and neurotrophic factors, BDNF, IL-6, IL-8, IL-10, and VEGF, were measured using a custom multiplex enzyme-linked immunosorbent assay (ELISA) on the Meso Scale Discovery (MSD) platform (Meso Scale Discovery Inc, Rockville, MD). Calibrators for the analytes were produced using a commercially provided diluent (MSD R50AG-2) and a detection antibody cocktail was likewise prepared in another commercially available diluent (MSD R51BA-5). Plasma samples were diluted 5-fold. The lower limits of detection (LLOD) for the BDNF, IL-6, IL-8, IL-10, and VEGF assays were 48.47 pg/mL, 0.47 pg/mL, 0.56 pg/mL, 1.26 pg/mL, and 1.59 pg/mL, respectively, with interassay coefficients of variation (CV) of 9.7%, 4.7%, 1.8%, 1.7%, and 2.7%, respectively. Any samples that were above the ULOQ were reassayed with a greater dilution to obtain the correct concentration. Any sample CV% > 20 were repeated.
Similarly, a custom duplex ELISA was developed to measure GFAP and NRGN (MSD, Rockville, MD) as previously described(17,18). Plasma samples were diluted 2-fold. The LLOD for the GFAP and NRGN assays were 0.014 and 0.016 ng/mL, with interassay CV of 2.6% and 2.8%, respectively.
Tau was measured using a commercial ELISA (MSD, Rockville, MD, Human Total Tau Kit, Cat # N451LAA-1). Plasma samples were diluted 4-fold and assayed according to manufacturer instructions. The LLOD for Tau was 82.03 pg/mL, with an interassay CV of 5.1%.
Magnetic resonance imaging
Per institutional protocol, post-cooling MRIs were performed on each neonate at target age 4–7 days of life. All scans were performed on a 3 Tesla scanner (Discovery MR750, GE Healthcare, Milwaukee, WI) and included the following sequences: 3D T1-weighted Spoiled Gradient Recalled, double acquisition axial FSE T2 proton density, axial T2 propeller (in cases of patient motion), axial T2-Star Weighted Susceptibility Imaging, coronal T1 FLAIR propeller, and axial 30-direction DTI. MRIs were reviewed and scored by a single experienced neuroradiologist who was blinded to clinical and biomarker data. Brain injury severity was scored according to Barkovich and severe brain injury was defined as a basal ganglia score ≥ 3 or watershed score ≥4(19).
Neurodevelopmental Assessment
Neurodevelopmental outcomes were assessed using the Bayley-III Scales of Infant and Toddler Development according to our institutional protocol for follow-up after TH. The Bayley-III measures cognitive, language, and gross and fine motor domains, providing composite scores where 100 (±15 SD) is the normative mean(20). Infants are routinely assessed at 9, 15, 18 and 30 months to follow developmental progress. Testing was performed and scored by a certified developmental psychologist who was blinded to biomarker data. Significant neurodevelopmental delay was classified according to the latest follow-up visit available beyond 1 year and defined as Bayley-III Cognitive Composite <85 or Bayley III Motor Composite <85 (21–24). Infants were considered not to have significant developmental delay if both scores were >=85
Statistical Analysis
Standard descriptive statistics included means (standard deviations) and medians (ranges) as appropriate for continuous variables, as well as counts (percentages) for categorical data. The bivariate association between biomarker levels and outcome groups were assessed with Mann-Whitney U tests. Adverse outcomes were defined as 1) death or severe brain injury by MRI and 2) death or significant neurodevelopmental delay. Logistic regression analyses were used to assess the ability of individual biomarkers to predict adverse outcome at each timepoint. Additionally, in order to assess the value of serial measures within each patient, summary functions including minimum, maximum, early average (of all values within 24 hours), late average (of values beyond 72 hours) and overall average were evaluated for predictive value. Finally, cumulative models were developed to assess the additive predictive value of multiple biomarkers, selected based on their demonstrated individual predictive ability. Multivariable models were also performed, adjusting for covariates including Sarnat grade (moderate versus severe), Apgar score at 5 minutes, presenting pH, electrographic seizure and socioeconomic status (private versus public insurance; for developmental outcome analyses), using step-wise regression. Model fitting was evaluated by accuracy of prediction, C-statistic (area under the curve – AUC; where values close to 1 represent perfect model prediction) and Akaike information criterion (AIC; where lower values represent higher quality of the model). Data were log-transformed for statistical analyses and p-values <0.05 were considered statistically significant. Statistical analysis was performed using SAS 9.4 (SAS Institute,Cary, NC).
Results
A cohort of 103 singleton neonates with NE were enrolled in this study. The study population characteristics included infants with a mean birth weight of 3.25 ± 0.69 kg, a mean gestational age of 38.7 ± 1.6 weeks, and were 50% male. These infants had a median 5-min Apgar of 4 with an interquartile range (IQR) of 2 – 5, and a median presenting pH of 6.97 (IQR 6.83, 7.12). Nineteen infants (18%) had severe encephalopathy. The median number of hours from birth to the initiation of cooling was 4.5 hours (IQR 3.79, 5.43). Fifteen infants died and nineteen infants had severe injury by MRI. Characteristics of the study population are described in Table 1. Sample sizes available for each assay are available in Supplemental Table 1.
Table 1.
Clinical characteristics by outcome group
| Variable | Total (n=103) | Survived with Normal/Mild MRI (n=69) | Died or Severe Injury by MRI (n=34) |
|---|---|---|---|
|
| |||
| Gestational Age (weeks) 1 | 38.7 ± 1.6 | 38.7 ± 1.6 | 38.7 ± 1.5 |
|
| |||
| Birthweight (kg) 1 | 3.25 ± 0.69 | 3.31 ± 0.75 | 3.11 ± 0.55 |
|
| |||
| Sex (male) 2 | 52, 50% | 39, 56% | 13, 38% |
|
| |||
| Delivery Method 2 | |||
| Cesarean | 65, 63% | 41, 59% | 24, 71% |
| Spontaneous Vaginal | 38, 37% | 28, 41% | 10, 29% |
|
| |||
| APGAR Score 3 | |||
| 1 min | 1 (1, 2) | 2 (1, 2) | 1 (1, 2) |
| 5 min | 4 (2, 5) | 4 (3, 6) | 3 (2, 4) |
|
| |||
| Initial pH 3 | 6.97 (6.83, 7.12) | 7.02 (6.90, 7.13) | 6.91 (6.80, 7.01) |
|
| |||
| Encephalopathy grade 2 | |||
| Moderate | 82, 82% | 66, 96% | 18, 53% |
| Severe | 19, 18% | 3, 4% | 16, 47% |
|
| |||
| EEG Confirmed Seizures 2 | 29, 28% | 12, 17% | 17, 50% |
|
| |||
| Age at MRI (days) 3 | 5 (4, 7.5) | 5 (4, 8) | 4 (4, 5.5) |
mean (SD);
n, %;
median (IQR)
Prediction of death or severe injury by MRI
Biomarker data stratified by outcome group are shown in figure 1. All cytokines peaked in the first 24 hours of cooling in the majority of patients, 83%, 69% and 87% for IL-6, IL-8, and IL-10 respectively. Conversely, the majority (56%) of patients had peak Tau measured during or after rewarming. Cytokine levels differed by outcome group in the first 0–12 hours, while Tau differed at 12–96 hours (Figure 1). Bivariate model results are summarized in Table 2. For averaged early IL-6 measures, the best fit model included the quadratic term of IL-6 denoting a non-linear relationship (i.e. extreme low and high values are associated with adverse outcomes). Overall, biomarker measurements of Tau were most predictive of adverse outcome across timepoints and summary functions. Observed relationships were not solely driven by levels in patients who died, as levels also generally differed across survivors with and without brain injury (Supplemental Table 2).
Fig 1.
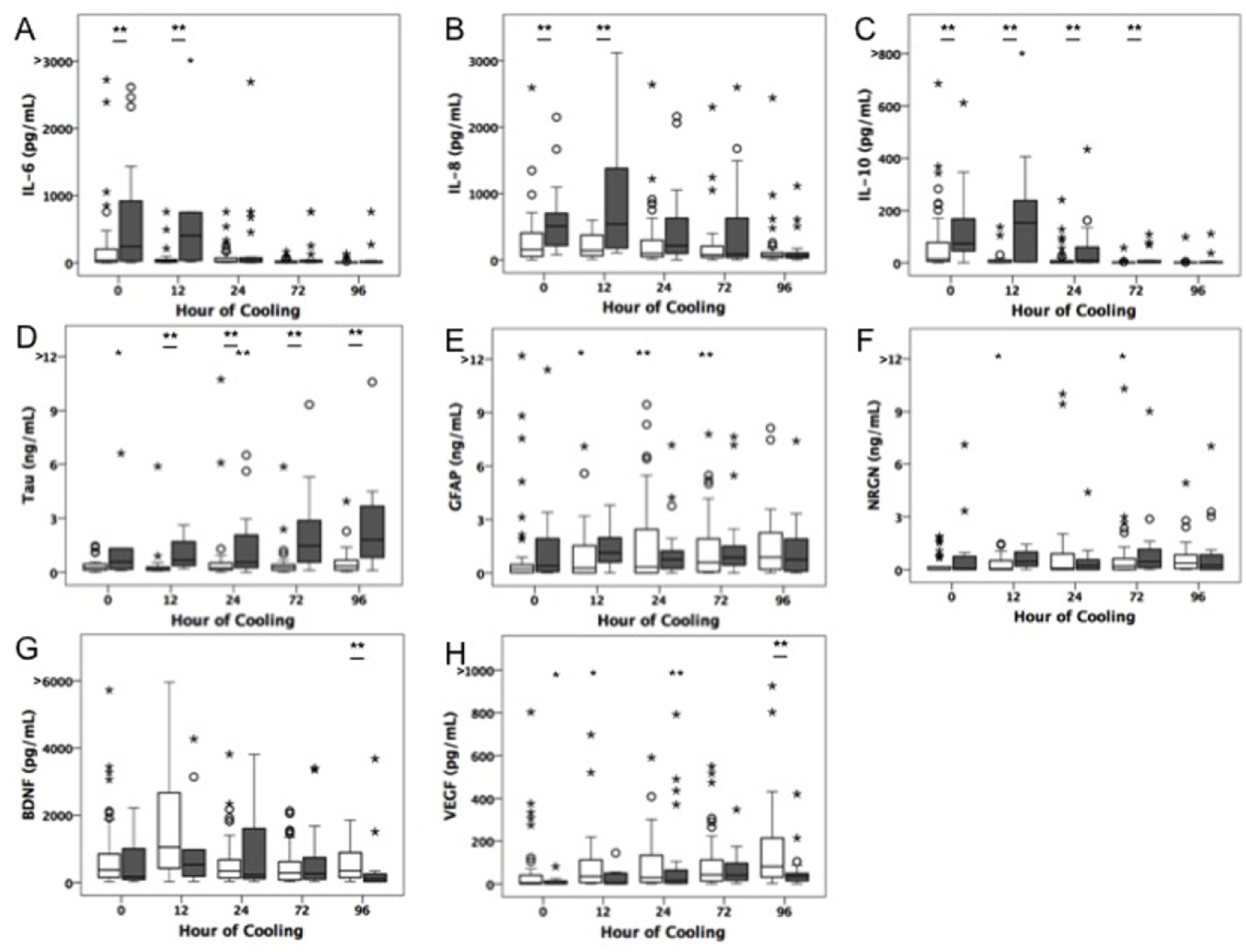
Box plots depicting medians and interquartile ranges of raw biomarker data over time. Data from infants who died or had severe brain injury by MRI (gray boxes) are compared to those who survived with normal or mild injury by MRI. Outliers and extremes represented by open circles and asterisks respectively. § denotes significance (P<0.05) by logistic regression analyses.
Table 2.
Bivariate analysis of biomarkers by time point predicting death or severe brain injury on MRI
| Time Point | Biomarker | OR | Cutoff value* | Cutoff value (in original scale) | AUC | P value | Accuracy (%) | Sensitivity (%) | Specificity (%) | Sample Size (n) | Event (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | IL6 | 1.84 | 6.631 | 758.24 | 0.683 | 0.021 | 75.76 | 42 | 89 | 66 | 19 |
| IL8 | 2.23 | 6.236 | 510.81 | 0.741 | 0.006 | 77.27 | 53 | 87 | 66 | 19 | |
| IL10 | 1.55 | 3.704 | 40.61 | 0.714 | 0.012 | 72.72 | 79 | 70 | 66 | 19 | |
| T12 | IL6 | 3.77 | 5.401 | 221.63 | 0.792 | 0.007 | 83.33 | 60 | 92 | 36 | 10 |
| IL8 | 3.93 | 6.447 | 630.81 | 0.812 | 0.009 | 86.11 | 50 | 100 | 36 | 10 | |
| IL10 | 2.14 | 4.969 | 143.88 | 0.827 | 0.002 | 88.89 | 60 | 100 | 36 | 10 | |
| Tau | 2.87 | 6.291 | 539.69 | 0.818 | 0.024 | 80.65 | 67 | 82 | 31 | 9 | |
| T24 | IL10 | 1.38 | 4.085 | 59.44 | 0.665 | 0.019 | 72.29 | 27 | 93 | 83 | 26 |
| Tau | 1.74 | 6.862 | 955.28 | 0.742 | 0.010 | 77.42 | 48 | 93 | 62 | 21 | |
| T72 | IL10 | 1.99 | 1.464 | 4.32 | 0.718 | 0.002 | 80.00 | 45 | 93 | 80 | 22 |
| Tau | 3.67 | 6.314 | 552.25 | 0.864 | 0.001 | 87.04 | 86 | 88 | 54 | 14 | |
| T96 | Tau | 3.36 | 6.603 | 737.30 | 0.847 | 0.003 | 81.40 | 86 | 79 | 43 | 14 |
| BDNF | 0.51 | 4.168 | 64.59 | 0.737 | 0.005 | 76.19 | 40 | 93 | 63 | 20 | |
| VEGF | 0.61 | 3.269 | 26.29 | 0.698 | 0.023 | 73.02 | 45 | 86 | 63 | 20 | |
| Early Average | IL8 | 1.72 | 6.332 | 562.28 | 0.721 | 0.016 | 76.84 | 42 | 94 | 95 | 31 |
| IL10 | 1.46 | 3.656 | 38.71 | 0.667 | 0.007 | 76.84 | 52 | 89 | 95 | 31 | |
| Tau | 1.74 | 6.229 | 507.25 | 0.687 | <0.001 | 75.33 | 58 | 84 | 77 | 26 | |
| Late Average | IL10 | 1.64 | 1.464 | 4.32 | 0.682 | 0.008 | 74.44 | 36 | 92 | 90 | 28 |
| Tau | 3.84 | 6.513 | 673.84 | 0.880 | <0.001 | 84.29 | 89 | 83 | 70 | 22 | |
| BDNF | 0.78 | 3.689 | 40.00 | 0.603 | 0.153 | 70.00 | 25 | 90 | 90 | 28 | |
| VEGF | 0.85 | 1.845 | 6.33 | 0.563 | 0.309 | 67.78 | 18 | 90 | 90 | 28 | |
| Minimum | IL6 | 1.44 | 3.795 | 44.48 | 0.642 | 0.027 | 73.81 | 19 | 98 | 84 | 26 |
| IL10 | 1.90 | 0.555 | 1.74 | 0.676 | 0.005 | 78.57 | 50 | 91 | 84 | 26 | |
| Tau | 2.71 | 5.725 | 306.43 | 0.811 | 0.004 | 79.66 | 74 | 83 | 59 | 19 | |
| Maximum | Tau | 4.68 | 7.514 | 1833.53 | 0.886 | 0.000 | 86.44 | 74 | 93 | 59 | 19 |
Cutoff value selected based on highest accuracy (correct classification)
Several multivariable models were derived considering early data alone (within 24 hours) as well as cumulative data over time to assess the combinative value of biomarkers. When early measurements of IL-8, and Tau were combined, the model demonstrated improved predictive ability (AUC=0.737, AIC=91.92, accuracy 77.3%) when compared to early Tau alone (AUC=0.677, AIC=92.43, accuracy 72%). The combination of early IL6, IL8 and IL10 best predicted death or severe brain injury by MRI (AUC=0.826, AIC=81.48, n=75 Table 3) with an accuracy of 78.7%. The results remained consistent after adjusting for clinical covariates in the model (severe encephalopathy grade OR 25.26, 95% CI 2.22–287.96, p=0.009 and electrographic seizure OR 1.83, 95% CI 1.83–47.38, p=0.007 were selected for inclusion in the final adjusted model). In the patients who had both early and late measures available, late measures of Tau alone was a better single predictor of outcome compared to early combined cytokines (OR 4.37, 95%CI 1.99–9.59, p<0.001, AUC=0.883, AIC =48.91, Accuracy 82.5%, n=57). The best cumulative model (AUC=0.915, AIC 46.05, Accuracy 86%, n=57, Table 3) including early and late biomarker data included early IL8 (OR 3.05, 95%CI 1.01–9.26, p=0.048) and late Tau measures (OR 3.81, 95%CI 1.57–9.22, p=0.016). After evaluating clinical covariates via step-wise regression, no clinical variables were selected in for inclusion in the final model, suggesting that Tau measures were the strongest independent predictor of outcome.
Table 3.
Multivariable models for prediction of death or severe brain injury by MRI
| Variable Name | Regression Coefficient | Standard Error | P Value | Odds Ratio | Lower OR | Upper OR | |
|---|---|---|---|---|---|---|---|
| Best Early Model | Log IL6 Early Average | −2.65 | 0.740 | <0.001 | |||
| Log IL6 Early Average | 0.28 | 0.085 | 0.001 | ||||
| Log IL8 Early Average | 0.84 | 0.240 | 0.042 | 1.63 | 1.02 | 2.62 | |
| Log IL10 Early Average | 0.49 | 0.350 | 0.016 | 2.33 | 1.17 | 4.61 | |
| Best Cumulative Model | Log IL8 Early Average | 0.91 | 0.460 | 0.048 | 3.05 | 1.01 | 9.26 |
| Log Tau Late Average | 1.43 | 0.452 | 0.016 | 3.81 | 1.57 | 9.22 |
Prediction of death or significant neurodevelopmental delay
A subset (n=48, 54.5% of survivors) of subjects had available neurodevelopmental outcome after 1 year of age. Of these infants with neurodevelopmental outcomes, 43 had moderate HIE and 5 had severe HIE. Eleven infants met the study definition of significant developmental delay with either poor cognitive outcomes (n=6) and/or poor motor outcomes (n=9). The cohort lost to follow-up was similar with regards to baseline and clinical characteristics including gestational age, sex, presenting pH/ base deficit, Apgar score at 5 minutes, Sarnat grade of encephalopathy and presence of electrographic seizures (p>0.05). More infants lost to follow-up had public insurance compared to private insurance, but this difference was not statistically significant (57% of lost to follow-up vs 42% retained cohort; p=0.058). In the cohort with follow-up data, late biomarker measurements (72 and 96 hours of TH) of IL10 and Tau were consistently predictive of neurodevelopmental outcome (Table 4). Measures of GFAP and NRGN at 96 hours of life were inversely related to adverse outcome (Figure 2). Late Tau alone was the strongest predictor of neurodevelopmental outcome with an accuracy of 81% (aOR 2.59, 95% CI 1.36–4.95, β 0.953, SE 0.330, AUC=0.81, AIC=45.17, n=42) and the addition of clinical covariates, other biomarkers or early Tau measures did not improve prediction.
Table 4.
Bivariate analysis of biomarkers by time point for prediction of death or significant neurodevelopmental delay
| Time Point | Biomarker | OR | Cutoff value* | AUC | P value | Accuracy (%) | Sensitivity (%) | Specificity (%) | Sample Size (n) | Event (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| T72 | IL10 | 1.89 | 0.850 | 0.67 | 0.021 | 76.60 | 63 | 86 | 47 | 19 |
| Tau | 3.07 | 7.284 | 0.82 | 0.009 | 86.67 | 70 | 95 | 30 | 10 | |
| T96 | IL10 | 1.76 | 0.560 | 0.72 | 0.039 | 79.07 | 67 | 86 | 43 | 15 |
| Tau | 2.13 | 6.710 | 0.77 | 0.034 | 77.42 | 89 | 73 | 31 | 9 | |
| GFAP | 0.64 | −2.767 | 0.72 | 0.032 | 70.00 | 31 | 96 | 40 | 16 | |
| NRGN | 0.65 | −4.140 | 0.71 | 0.039 | 72.50 | 38 | 96 | 40 | 16 | |
| Late Average | IL10 | 1.74 | 0.660 | 0.68 | 0.026 | 75.47 | 67 | 81 | 53 | 21 |
| Tau | 2.51 | 7.007 | 0.80 | 0.004 | 79.55 | 80 | 79 | 44 | 15 | |
| Minimum | Tau | 1.94 | 7.284 | 0.70 | 0.044 | 72.97 | 31 | 96 | 37 | 13 |
| Maximum | Tau | 1.79 | 7.514 | 0.71 | 0.042 | 72.97 | 69 | 75 | 37 | 13 |
Cutoff value selected based on highest accuracy (correct classification)
Fig 2.
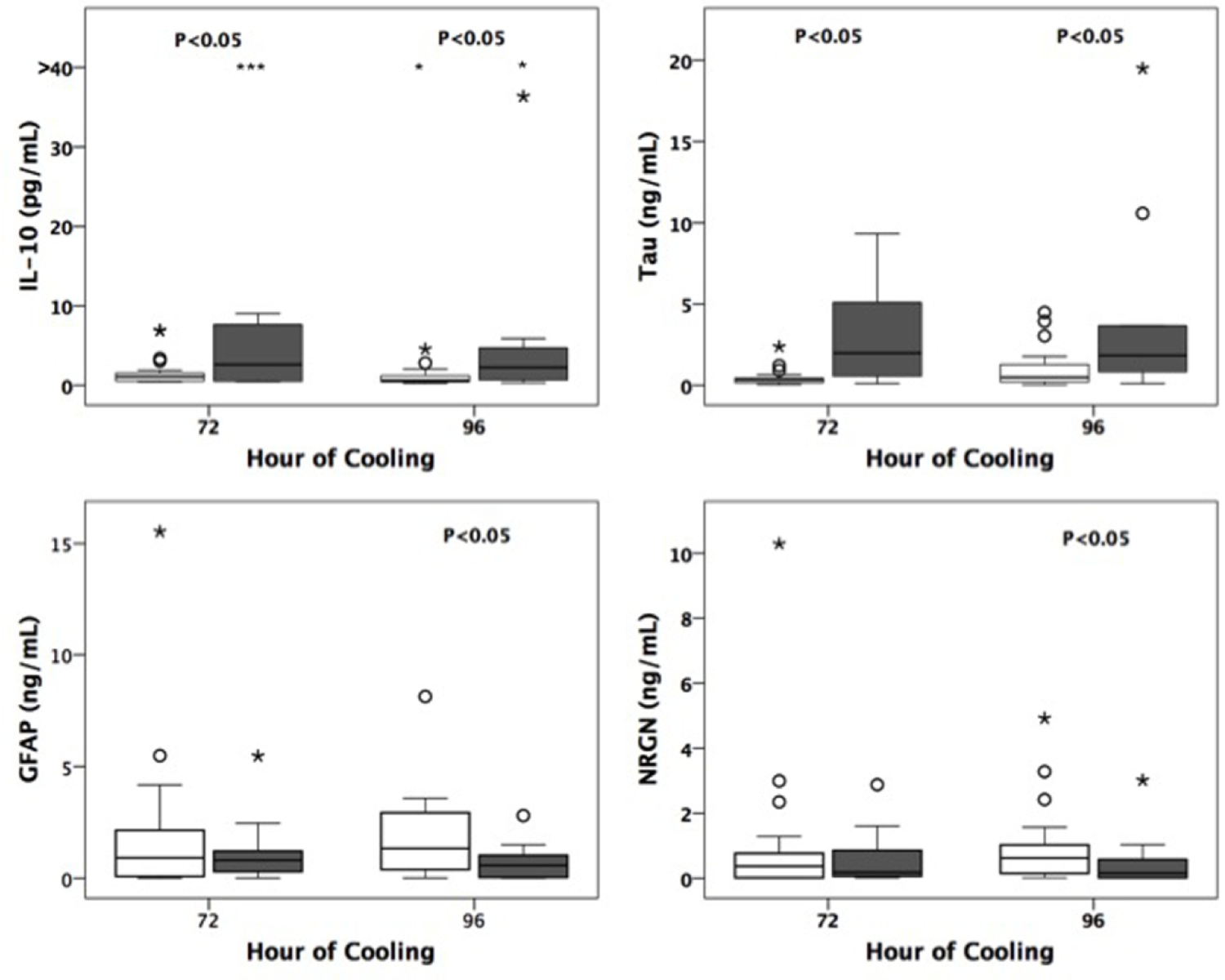
Box plots depicting medians and interquartile ranges of raw biomarker data over time. Data from infants who died or had significant developmental delay (gray boxes) are compared to those who survived with normal outcomes at 15–30 months. Outliers and extremes represented by open circles and asterisks respectively. § denotes significance (P<0.05) by logistic regression analyses.
Discussion
The results from this study illustrate the importance of serial measurements of multiple biomarkers to predict outcomes in infants with NE. As a stand-alone biomarker, Tau protein was consistently a predictive marker for brain injury on MRI, although later measurements of Tau at 72 and 96 hours of life had the best predictive ability compared to earlier measurements within the first 24 hours. Evidence for Tau protein as a putative biomarker of brain injury in NE was also observed in its ability to predict adverse neurodevelopmental outcomes when measured after 72 hours. However, when considering biomarkers measured within the first 24 hours of life, early measurements of cytokines IL-6, 8, and 10 in combination were most predictive of death or severe brain injury by MRI. These data support the use of a serial biomarker panel to assess brain injury over the time course of disease in NE. Cytokines have high predictive value when measured within the first day of presentation, which is helpful in making timely treatment decisions. The later predictive value of Tau is helpful in confirming the accuracy of prediction and to give appropriate counseling and rehabilitative interventions. That these relationships remained independently predictive of outcomes even in the context of common clinical indicators of severity (encephalopathy grade, Apgar score, initial pH, EEG seizures) further supports the potential for biomarkers to aid in the clinical care of neonates with NE.
Previous studies have examined the correlation of blood-based biomarker measurements and neurological outcome in newborns with NE. These studies identified important candidate biomarkers of brain injury including inflammatory cytokines, Tau, GFAP, VEGF, BDNF, and S-100B as indicators of clinical severity and adverse sequelae(6–9,11–14). Although the relationship between biomarkers and neurological outcome has been suggested, the temporality of these markers and their combinative value for predicting outcomes has not been well established. Most studies have measured biomarker values as single measures or within limited time-frames, such as just within the first 24 hours of life(8,9,11–14). While other studies measured values for up to one week after life, few have attempted to compare alternate summary functions or evaluate combinative values across biomarkers (7–9). Additionally, only recent studies have focused on cohorts of infants with NE that have been treated with TH, which is the current standard of care(6,7,9,13,14).
Cytokines have been widely investigated as a surrogate of disease severity and outcome in infants with NE. In hypoxic-ischemic injury, as well as many other types of brain injury, cytokines are an important mediator of the neuroinflammatory cascade(25). They can have pro-or anti-inflammatory properties, contributing to immune cell chemotaxis, cellular adherence, and other immune-modulating properties(25). These cytokines usually peak around the first 12–24 hours after injury, indicating the possible role of different cytokines in the initial inflammatory cascade and repair processes(26). We previously described that higher levels of IL-6, 8, and 10 at 24 hours of TH was associated with adverse neurological outcomes, whereas by 72 hours most cytokine response had dissipated and no longer distinguished outcome groups(6). Other studies have reported comparable findings, indicating that both the measured levels and timing of peak concentrations of cytokines are important considerations in evaluating neurologic severity(7,11). Our study is the first to include and compare serial measurements of inflammatory cytokines IL-6, 8, and 10 at several points both within the first 24 hours and at 72 to 96 hours after injury to evaluate for brain injury and neurodevelopmental outcome. This comparison of early versus late measurements showed that cytokine measurements within the first 24 hours have the best predictive value of subacute brain injury on MRI and supports the use of these biomarkers in initial therapeutic decisions. That the combination of cytokine measures did not predict neurodevelopmental outcomes may be related to the limited sample-size entering these analyses due to loss to follow-up. Given the trends of higher early cytokine levels in the adverse outcome group (supplemental table 3) and reports from others relating cytokine levels to adverse neurodevelopmental outcomes in HIE (7,12,25), the relationship between early cytokine measures and long-term functional outcomes warrants further study.
Perhaps one of the most promising candidate biomarkers of brain injury in NE is Tau, a microtubule-associated protein found in the axons of both neurons and oligodendrocytes(8). The initial injury caused by hypoxic conditions in the brain contribute to the phosphorylation of Tau, leading to neuronal damage and increased levels of Tau detected in the blood(26). Thus, it has been proposed that Tau protein can serve as a marker of extent neuronal injury and has a role in predicting outcomes in NE. A study by Takahashi et. al. measured serum Tau levels in non-cooled neonates with asphyxia on postnatal days 0, 3, and 7 and found that only Tau measurements on days 3 and 7 could predict neurological outcome. Few studies have investigated Tau protein measurements in babies receiving TH. A subset of this cohort was included in a recently reported multicenter study evaluating candidate biomarkers measured in the first 24 hours of life in babies with NE who received TH(16). Plasma Tau was the only biomarker that related to MRI abnormalities and Bayley-III cognitive and motor outcomes. In another previous cohort of cooled babies receiving Epo, Tau was selected amongst a panel of other biomarkers as the most significant in predicting brain injury on MRI and neurodevelopmental outcome(11). This study evaluated Tau at baseline (randomization to Epo versus placebo within 24 hours of life) and day 5, however lacked more frequent measurements of Tau between 48–72 hours to provide more information on its trajectory over time(11). The current study addresses this knowledge gap by investigating the role of Tau more granularly in the evolution of brain injury in NE. Interestingly, this study found that of all the biomarkers included in our analysis, Tau was the best predictor of both MRI injury and neurodevelopmental outcome especially when the measurements were taken at 72 and 96 hours after injury. This supports the previous findings that Tau is a marker of the later biological consequences of hypoxic-ischemic brain injury and that it is a strong predictor for later functional outcomes.
This study included several other candidate biomarkers that have been previously shown to correlate with disease severity and outcome in babies with NE such as NRGN, GFAP(7,9), BDNF(11), and VEGF(7). While our analyses indicated limited evidence for these analytes as brain injury biomarkers, we recognize that additional studies are needed to confirm the associations (or lack of associations) observed in this cohort of babies with NE given that there are limitations to this study. First, the samples collected for the biomarker assays were salvaged from clinical samples which meant that we were unable to obtain a sample at every timepoint for each subject, given some samples did not have adequate volume remaining for analysis. Thus, the use of a convenience sample based on available plasma (with varying sample sizes by analyte and by timepoint) precluded the calculation of an a priori determined sample size. While we included all available specimens in analyses for each individual timepoint, we only included subjects with two or more timepoints in the calculation of summary functions, thus excluding those with incomplete data which can lead to potential selection bias. Second, variable collection and storage processes in the clinical setting (i.e. time between collection and processing, degree of hemolysis, etc) and the introduction of freeze thaw cycles in the storing and analyzing of samples can provide a source of technical variability. Given the goal of this work was to evaluate the individual and combinative value of the time-dependent biomarkers, we analyzed data separately for each timepoint as well as overall summary functions. Given our limited sample size and the exploratory nature of this study, we did not adjust for multiple comparisons and thus these results require confirmatory investigation. Lastly, many of our subjects were lost to follow up which may introduce selection bias and additionally limited our sample size for the neurodevelopmental outcome analyses. (15)
Conclusions
Based on the results of this study, serial measurements of Tau and inflammatory cytokines show differences in their ability to predict brain injury by MRI and neurodevelopmental outcomes based on the time of measurement over the first 96 hours of life. These data support the use of a serial biomarker panel in newborns with moderate to severe NE treated with TH to accurately guide intervention and counseling strategies. Tau protein may have the most utility as a stand-alone biomarker of brain injury and neurodevelopmental impairment in NE. Large scale validation of early cytokine and serial Tau measures are needed prior to integration into the clinical setting for monitoring of disease progression and directing care in newborns with NE.
Supplementary Material
Impact Statement:
While recent studies have evaluated candidate brain injury biomarkers, no biomarker is in current clinical use.
This study supports the use of a serial biomarker panel for ongoing assessment of brain injury neonates with NE.
In combination, IL6, IL8 and IL10 in the first 24 hours of cooling were more predictive of brain injury by MRI than each cytokine alone.
Individually, Tau was overall most consistently predictive of adverse neurological outcomes, particularly when measured at or after rewarming.
Statement of Financial Support:
This study was supported by the Clinical and Translational Science Institute at Children’s National (UL1TR000075, 1KL2RR031987-01) and the National Institutes of Health Intellectual and Developmental Disabilities Research Consortium (U54 HD090257).
Footnotes
Disclosure Statement: The authors have no conflicts of interest to declare.
Category of Study: Clinical Research Article
Consent Statement: Written informed consent for enrollment into this study was obtained from the parents of participants.
References
- 1.Shankaran S, Woldt E, Koepke T, Bedard MP & Nandyal R Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early Hum. Dev 25, 135–148 (1991). [DOI] [PubMed] [Google Scholar]
- 2.Gluckman PD et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet Lond. Engl 365, 663–670 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Kurinczuk JJ, White-Koning M & Badawi N Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev 86, 329–338 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Shankaran S et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med 353, 1574–1584 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Wu YW et al. High-Dose Erythropoietin and Hypothermia for Hypoxic-Ischemic Encephalopathy: A Phase II Trial. Pediatrics 137, (2016). [DOI] [PubMed] [Google Scholar]
- 6.Orrock JE et al. Association of brain injury and neonatal cytokine response during therapeutic hypothermia in newborns with hypoxic-ischemic encephalopathy. Pediatr. Res 79, 742–747 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalak LF et al. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J. Pediatr 164, 468–474.e1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serum tau protein level serves as a predictive factor for neurological prognosis in neonatal asphyxia. - Abstract - Europe PMC at <https://europepmc.org/article/med/24268747> [DOI] [PubMed]
- 9.Ennen CS et al. Glial fibrillary acidic protein as a biomarker for neonatal hypoxic-ischemic encephalopathy treated with whole-body cooling. Am. J. Obstet. Gynecol 205, 251.e1–7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubota Y, Putkey JA & Waxham MN Neurogranin Controls the Spatiotemporal Pattern of Postsynaptic Ca2+/CaM Signaling. Biophys. J 93, 3848–3859 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massaro AN et al. Plasma Biomarkers of Brain Injury in Neonatal Hypoxic-Ischemic Encephalopathy. J. Pediatr 194, 67–75.e1 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Ramaswamy V et al. Systematic review of biomarkers of brain injury in term neonatal encephalopathy. Pediatr. Neurol 40, 215–226 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Massaro AN et al. Biomarkers of brain injury in neonatal encephalopathy treated with hypothermia. J. Pediatr 161, 434–440 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massaro AN et al. Biomarkers S100B and neuron-specific enolase predict outcome in hypothermia-treated encephalopathic newborns*. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc 15, 615–622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarnat HB & Sarnat MS Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol 33, 696–705 (1976). [DOI] [PubMed] [Google Scholar]
- 16.Dietrick B et al. Plasma and CSF Candidate Biomarkers of Neonatal Encephalopathy Severity and Neurodevelopmental Outcomes. J. Pediatr (2020). 10.1016/j.jpeds.2020.06.078 [DOI] [PMC free article] [PubMed]
- 17.Yang J, Korley FK, Dai M & Everett AD Serum neurogranin measurement as a biomarker of acute traumatic brain injury. Clin. Biochem 48, 843–848 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bembea MM et al. Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc 12, 572–579 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barkovich AJ et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am. J. Neuroradiol 19, 143–149 (1998). [PMC free article] [PubMed] [Google Scholar]
- 20.Michalec D in Encycl. Child Behav. Dev (eds. Goldstein S & Naglieri JA) 215–215 (Springer US, 2011). 10.1007/978-0-387-79061-9_295 [DOI] [Google Scholar]
- 21.Anderson PJ et al. Underestimation of developmental delay by the new Bayley-III Scale. Arch. Pediatr. Adolesc. Med 164, 352–356 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Vohr BR et al. Are Outcomes of Extremely Preterm Infants Improving? Impact of Bayley Assessment on Outcomes. J. Pediatr 161, 222–8.e3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalak LF et al. Neurodevelopmental outcomes after hypothermia therapy in the era of Bayley-III. J. Perinatol. Off. J. Calif. Perinat. Assoc 34, 629–633 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore T, Johnson S, Haider S, Hennessy E & Marlow N Relationship between test scores using the second and third editions of the Bayley Scales in extremely preterm children. J. Pediatr 160, 553–558 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Jenkins DD et al. Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab 32, 1888–1896 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu H et al. SBDPs and Tau proteins for diagnosis and hypothermia therapy in neonatal hypoxic ischemic encephalopathy. Exp. Ther. Med 13, 225–229 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


