Abstract
Objective
During the COVID-19 pandemic, social distancing and self-isolation called for innovative, readily implementable, and effective short-term health solutions. The objective of this study was to assess the feasibility of self-assessment of vital signs and symptoms with electronic transmission of results, by self-isolating individuals with positive SARS-CoV-2 polymerase chain reaction (PCR) test. The secondary objective was to describe the association between the presence of abnormal vital signs and severe symptoms as well as their evolution over time.
Method
Participants with positive SARS-CoV-2 PCR test were asked to perform twice daily standardized vital signs measurements and self-assessment of symptoms for 14 consecutive days. All data were transmitted electronically through a mobile application and a web-based platform. Participants were provided with decision support tools based on the severity of their condition and a weekly nurse practitioner telephone follow-up. Abnormal values for vital signs and severe symptoms were determined. Per participant and per days, proportions of abnormal vital signs and severe symptoms were calculated.
Results
Data from 46 participants (mean age 54.1 ± 6.9 years, 54% male) were available for analysis. On average, participants performed the standardized self-assessment for 12.3 ± 3.4 days (89% performed at least 7 measurement days and 61% completed all 14 days). The highest proportions abnormal values for vital signs were for oximetry (20.1%) and respiratory rate (12.1%). The highest proportions of severe symptoms were for fatigue (16.9%) and myalgia. (10.2%). The combined proportion of abnormal vital signs and severe symptoms was maximal on day 1 with 20.3% of total measurements, with a linear decrease to 3.5% on day 14.
Conclusion
Remote initiation of home measurements of vital signs and symptoms, self-management of these measures, accompanied by a decision support tool and supported by preplanned nurse follow-up are feasible. This could allow to opening up new insight for the care of sick individuals.
Keywords: Self-assessment, Vital signs, Symptom severity, E-health, Mobile application, Web-based platform, COVID-19
1. Introduction
1.1. Background
The pressure exerted on the health care system from COVID-19 pandemic is unprecedented. Little is known about the evolution of symptoms in self-isolating individuals and the trajectory, which can lead to hospitalization. The contexts of social distancing and self-isolation have called for innovative and easily implementable short-term healthcare solutions. In a context where the population is asked to avoid overcrowding hospitals, the use of telemedicine has become a leading solution [1], [2], [3]. A well-established health telemonitoring tool is the self-measurement of blood pressure (BP) at home. In Canada, more than a third of adults aged 40 and over have high BP [4] and about half of hypertensive people have a BP monitor at home [5]. However, for self-isolating individuals with COVID-19, BP measurement alone would be insufficient to fully monitor the clinical condition and the evolution of signs and symptoms related to this condition.
Indeed, to ensure close clinical surveillance of individuals with COVID-19, all clinically important vital signs would need to be monitored. In addition to daily home BP and heart rate (HR) monitoring, oximetry, body temperature and respiratory rate (RR) should be monitored. Moreover, the relationship between the intensity of COVID-19 symptoms and abnormal vital signs is poorly described. In the Canadian province of Québec, individuals who perceive that their state of health is deteriorating were invited not to present themselves to emergency departments but to call a telephone helpline for advice. However, it is likely that without vital signs, many would nonetheless be oriented to the emergency department, adding to the over crowdedness of the system. Indeed, 57% of individuals who consult the emergency department for symptoms of COVID-19 have a heart rate below 100 beats/min, 63% have a respiratory rate below 24 breaths/min and 80% have an oxygen saturation above 90% [6]. In addition, many cases of severely hypoxic individuals presenting with very mild symptoms were also reported [7], [8]. It seems therefore urgent to rapidly develop alternatives to measure and document the evolution of signs and symptoms, in an attempt to enhance triage and to act before clinical deterioration.
Comprehensive self-assessment of vital signs and symptoms twice daily, of accompanied with algorithms using severity indicators could inform patients on when they should consult the emergency department. Clinical surveillance data can be transmitted by various telemedicine tools that allow monitoring by healthcare professionals. This could help triage but also reduce the risk of delayed hospitalization which is associated with a worse evolution.
The main objective of this study is to assess the feasibility of a comprehensive self-assessment of vital signs coupled with a self-assessment of symptoms and an electronic transmission of results by self-isolating individuals with COVID-19, all initiated remotely. The secondary objective is to describe the link between abnormal vital signs and symptoms as well as their evolution following initial diagnosis.
2. Method
2.1. Study design and recruitment of participants
The HYTECC study (among HYperTEnsives under Confinement, self-assessment of vital signs and symptoms of COVID-19) is a prospective cohort study which took place in Laval (province of Quebec, Canada), one of the most COVID-19 affected cities in the country in cases per capita. Individuals positive SARS-CoV-2 polymerase chain reaction (PCR) test were referred by the local public health authority to the principal investigator on the day of diagnosis for possible inclusion in the HYTECC study. To be eligible, participants required the following inclusion criteria: aged between 40 and 69 years old, known diagnosis of hypertension, able to understand instructions in the French language, able to receive a package by an express mail service, have access to a mobile phone, computer and internet access, and have no known diagnosis of heart failure or chronic obstructive pulmonary disease. During the initial phone contact and following electronic consent, participants were directed to a secure online platform to obtain all the information needed to participate in the study.
The study obtained ethical certification from the Centre de Santé et Services Sociaux de Laval and the Centre Intégré de Santé et Services Sociaux de l’Estrie (Université de Sherbrooke).
2.2. Self-assessment of vital signs and symptoms
Participants were required to measure vital signs, including BP, HR, RR, oxygen saturation and body temperature, twice daily for 14 consecutive days and to report COVID-19 associated symptoms. For vital signs measurements, participants received by express mail within 48 h of enrollment, a kit containing an electronic BP monitor (LifeSource UA-651BLE™), an oximeter (Contect™) and an electronic oral thermometer. Participants were instructed to download the free Sphygmo™ software app on their mobile phone or tablet. This app allows recording of vital sign data by participants and the electronic transmission to a secure web database. Written guidance and telephone support were provided to participants on the correct method to perform the various vital signs measurements and data collection.
Abnormal values were established for each of the vital signs: systolic BP (SBP) < 100 mm Hg, HR > 99 beats per minute, RR > 19 breaths per minutes, oxygen saturation < 95% [9] and oral body temperature > 37.9 °C [10]. Symptom assessment was performed using an online electronic questionnaire. Participants were asked to rate the intensity of their COVID-19 symptoms according to four levels: 0-not present, 1-weakly present, 2-moderately present or 3-strongly present. The following symptoms, which were rated as moderately present (2) or strongly present (3) were considered severe symptoms: coughing, difficulty in breathing, fatigue and muscle pain.
The participants were informed, both in writing and verbally, that there would be no daily monitoring of transmitted data and that they were to assess the severity of their condition and whether or not they should consult a healthcare professional. To assist them in their decision, an algorithm was provided, guided by the presence or absence of abnormal vital signs or severe symptoms. In addition, each participant received two phone calls from a nurse practitioner around day 3 and day 10 of the study. Participants were then asked again about their symptoms and the nurse could respond to the concerns of the patients or provide advice as needed. All patients were also encouraged to call local COVID-19 help lines for any questions about their health. Finally, participants were informed that they could reach by phone or email, at any time, a member of the research team if they had any technical issues with the devices (BP monitor or oximeter), the software app or the web-based platform.
2.3. Other data
Upon electronic consent, participants were required to complete an electronic questionnaire on health history, lifestyle habits and anthropometric data.
2.4. Outcome measures
Outcome measures were the proportion of participants who completed 7 and 14 days of self-assessment, abnormal vital signs (BP, HR, RR, body temperature and oxygen saturation) and patient-reported symptoms per days, and correlation between the abnormal vital signs and participant reported severe symptoms.
2.5. Statistical analysis
Descriptive statistics were used to present baseline characteristics for participants. Comparison between groups were performed using the independent Student’s t-test for continuous parametric data, and the Fisher’s exact test for categorical data. The linear correlation test (Pearson’s R), at the patient level, was calculated between the proportions of abnormal values of vital signs and severe symptoms. Data analysis was performed using Rstudio Team (2020)1 .
3. Results
3.1. Participant characteristics
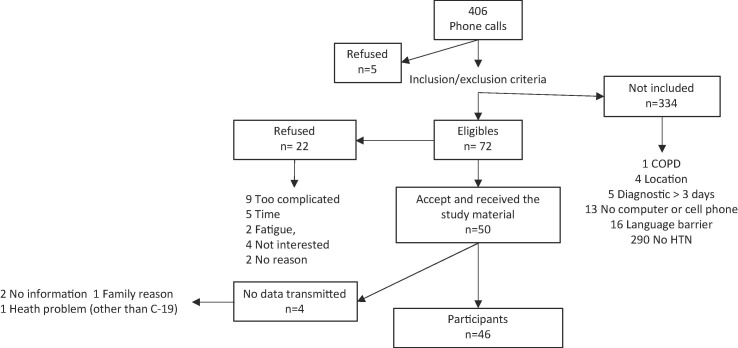
Recruitment took place from December 2020 to March 2021. Phone calls were made to 406 potential participants, from which 72 met the inclusion / exclusion criteria with 50 agreeing to participate in the study. The main reason for non-eligibility was the absence of hypertension (Fig. 1 recruitment flow chart). Out of the 50 who agreed to participate, 46 transmitted a complete set of data and were included for analysis. The mean age was 54.1 ± 6.9 years and 54% were men. The average BMI was 31.1 m2 / kg. They had been diagnosed with hypertension for an average of 9.5 ± 5.8 years, and 30.4% were diagnosed with diabetes. Symptoms associated with COVID-19 started on average 4.5 ± 3.4 days before entering the study (Table 2 , characteristics of participants). Among the participants, 8.7% (n = 4) reported, during the study, that the course and severity of their health condition associated with COVID-19 required medical attention.
Fig. 1.
Recruitment flowchart for the HYTECC study: individuals positive for the SARS-CoV-2 polymerase chain reaction (PCR) test were referred by the local public health authority to the principal investigator on the day of diagnosis for possible inclusion in the study.
Table 2.
Measurements reaching abnormal values for vital signs.
| Vital signs | Abnormal values | Total measures | Number of abnormal values n(%) |
|---|---|---|---|
| Systolic BP | < 100 mm Hg | 1109 | 35 (3.2) |
| Heart beat/minute | > 99 BPM | 1109 | 74 (6.7) |
| Body temperature | > 37.9 °C | 1006 | 32 (3.2) |
| Respiratory rate | > 19 / minute | 804 | 97 (12.1) |
| Oximetry | < 95 % | 987 | 198 (20.1) |
3.2. Days of measurements completed
Participants completed an average of 12.3 ± 3.4 days of vital signs measurements and 10.8 ± 4.7 days of symptom self-reporting. In total, 89% completed at least 7 days of vital signs measurements and 61% completed 14 days. For symptom self-reporting, the proportions were 78% and 46% for 7 and 14 days respectively. Among the participants, 11% (n = 5) could not complete the 14 days of the protocol for valid reasons (family member death, intensive care hospitalization, pneumonia, equipment breakdown and early return to work for an essential worker with atypical working hours).
3.3. Abnormal vital signs and severe symptoms
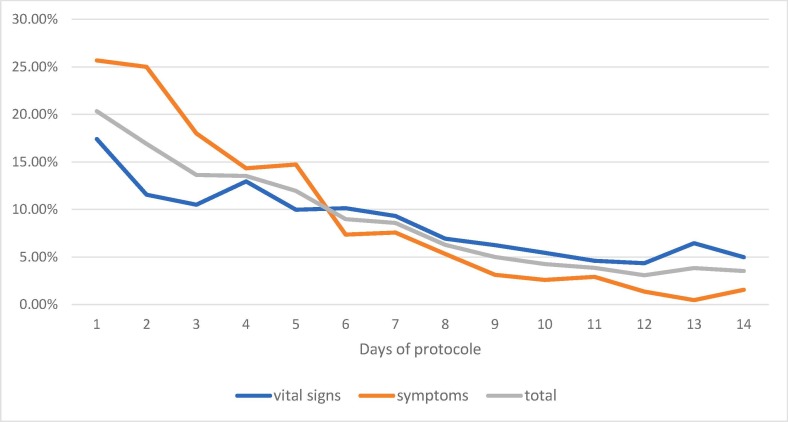
A total of 8527 measurements were included that allowed the calculation of proportions of abnormal vital signs and severe symptoms. Of these, 766 (8.9%) were either abnormal vital signs, 436 (5.1%) or severe symptoms, 330 (3.8%). In terms of vital signs, oximetry (20.1%) and respiratory rate (12.1%) are the results that most often reached or surpassed the abnormal value. (Table 2). The symptoms that most often reached the severe values are fatigue (16.9%) and muscle pain (10.2% (Table 3 .) The overall proportions of abnormal vital signs and severe symptoms are higher during the first 7 days (range 8.6% − 20.3%, median 13.53%) than during days 8 to 14 (range 3.5% − 6.3%, median 3.86%) (Fig. 2 ).
Table 3.
Measurements reaching severe values for symptoms.
| Symptoms | Severe values | Total measures | Number of severe values n(%) |
|---|---|---|---|
| Cough | 2 or 3 on a self-rated symptom intensity scale of 0 to 3* | 878 | 58 (6.9) |
| Breathing symptoms | 878 | 34 (4.0) | |
| Fatigue | 878 | 142 (16.9) | |
| Muscle pain | 878 | 86 (10.2) |
* Not present = 0, weakly present = 1, moderately present = 2, strongly present = 3.
Fig. 2.
Per day, proportions of abnormal vital signs and severe symptoms for total participants. The overall proportions of abnormal vital signs and severe symptoms are higher during the first 7 days (range 8.6% − 20.3%, median 13.53%) than during days 8 to 14 (range 3.5% − 6.3%, median 3.86%).
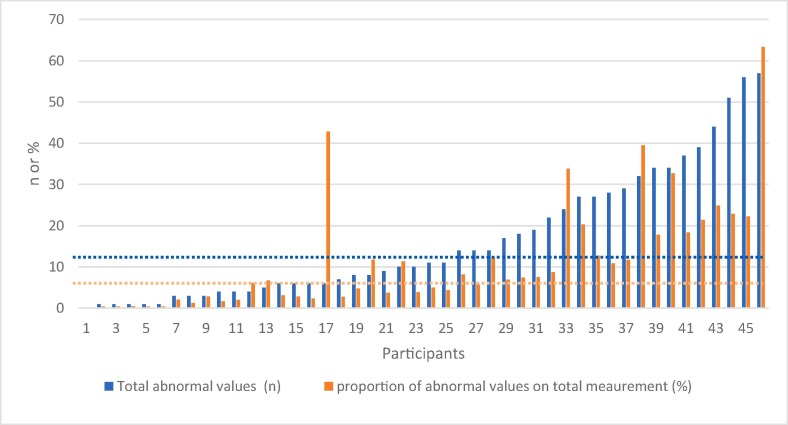
Of the participants, 45 out of 46 obtained at least 1 abnormal vital signs value or severe symptoms, ranging from 1 to 57, median 11 (Fig. 3 ).
Fig. 3.
Per participant: combined total of abnormal vital signs and severe symptoms and combined proportion of abnormal vital signs and severe symptoms on total measurements. The blue dotted line represents the median value (10.5) for the total abnormal values and severe symptoms. The orange dotted line represents the median value (6.8) for the proportion of abnormal values and severe symptoms. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Association between abnormal vital signs and severe symptoms
For each of the vital signs and symptoms, per participant and per days, the proportions of measurements carried out that reached or surpassed the abnormal or severity values were calculated. The statistically significant correlations between the proportions of abnormal vital signs and the proportions of severe symptoms are presented in Table 4 . The highest correlations are between body temperature and muscle pain (0.43), heart rate and cough (0.61), oximetry and fatigue (0.44) and oximetry and muscle pain (0.55).
Table 4.
Correlation between proportions of abnormal vital signs and proportion of severe symptoms.
| Vital signs | Symptoms | Correlations ® | p values |
|---|---|---|---|
| Body temperature | Breathing symptoms | 0.36 | 0.015 |
| Fatigue | 0.38 | 0.009 | |
| Muscle pain | 0.43 | 0.003 | |
| Heart rate | Muscle pain | 0.36 | 0.014 |
| Fatigue | 0.36 | 0.014 | |
| Cough | 0.61 | < 0.001 | |
| Oximetry | Cough | 0.30 | 0.044 |
| Breathing symptoms | 0.33 | 0.029 | |
| Fatigue | 0.44 | 0.003 | |
| Muscle pain | 0.55 | < 0.001 | |
| Respiratory rate | Breathing symptoms | 0.32 | 0.054 |
3.5. The technical challenges for participants
Among the 46 participants, 11(24%) sent an email and two participants (4%) made a phone call for technical assistance. The reasons for contact were: precisions regarding how to link the software app to the web-based platform (6), the self-assessment questionnaire (3), the delay in receiving the material by express mail (1), how to record vital sign data in the software app (1), how to link the BP device to the app (1), and how to link the software app to the web-based platform (1).
4. Discussion
To the best of our knowledge, this is the first study demonstrating the feasibility of remote implementation and use of a telehealth system that includes comprehensive self-assessment of vital signs and symptoms, with electronic transmission and concomitant self-management of patient data supported by preplanned nurse follow-up. In the United Kingdom, home oximetry in individuals 65 years of age and over is part of the guidelines for the follow-up of people with symptomatic COVID-19. Tools to help decision-making based on oxygen saturation and the presence of symptoms are provided. The modalities and frequencies of the follow-up by the primary care provider are then personalized [11]. This fits into the Bosch model of Care [12] in which the patient’s self-management of various health conditions is emphasized. This would promote autonomy by allowing a better management of a patient’s condition, while being likely to contribute to improving the quality of life as well as promoting better health care efficiency.
The secondary objective of the study was to describe the link between abnormal vital signs and severe symptoms associated with COVID-19. Like other studies [13], [14], [15], oxygen saturation and RR appear to be the most altered vital signs in COVID-19. Systolic BP and HR appear to be poor predictors of deterioration of the clinical condition in COVID-19. This is consistent with a recent study in which the Shock Index (SBP + HR) demonstrated no predictive value of deterioration in COVID-19, unlike in patients with sepsis [16]. In terms of symptoms, fatigue and myalgia presented the highest proportions of severe intensity and these are the two symptoms most strongly correlated with abnormal oxygen saturation. For its part, breathlessness symptom presented a low proportion of severe intensity. This is consistent with the fact that individuals can present to the emergency department with low oxygen saturation levels, without severe respiratory symptoms, a phenomenon described as “happy hypoxia” [7], [8].
In terms of the progression over time of abnormal vital signs and severe symptoms, the peak was on day 1 of inclusion in the study. Considering that participants started their symptoms on average 4.5 days before entering the study (Table 1), this corresponds to the typical peak of intensity of symptoms from days 1 to 5. This is concordant with a cohort study held in Geneva, among 669 participants, where participants diagnosed with COVID-19 received every 48 h phone calls from day 1 of symptom onset [17].
Table 1.
Participants characteristics.
| Participants’ characteristics n = 44* | % or mean (±SD) |
|---|---|
| Age, years | 54.1 (6.9) |
| Sex, male | 54 % |
| BMI, m2/kg | 31.1 (5.3) |
| Duration of hypertension, years | 9.5 (5.8) |
| Diabetes | 31.8 % |
| At least one symptom of COVID-19 | 86.3% |
| Days since onset of the COVID-19 symptoms | 4.5 (3.2) |
| Currently smoker | 9.1 % |
| 7 alcohol consumption per week | 18.2% |
| 150 min of physical activity per week | 68.8% |
| Number of drugs per day | 3.8 (2.3) |
| Two or more blood pressure medications | 32.6 % |
*2 participants did not complete the health questionnaire.
The high success rates of completed measurements observed at 7 and 14 days, performed by patients with symptomatic COVID-19 without the usual primary care follow-up, are particularly encouraging for the implementation of such protocol to other health conditions. The easily accessible technical support could be a contributing factor for the completion by the participants of such protocol. Finally, the total material cost per participant, including its delivery is relatively inexpensive and provides reusable material.
4.1. Limitation
One of the limitations of the study is the number of participants. A generalization of the results to other settings and populations is limited, especially for data on the association between abnormal for vital signs and severe symptoms. In addition, the high proportion of participants with diabetes (31.8% vs. 8.1% for this age group in the population) may have influenced the impact of COVID-19 on participants [18]. Diabetes is thought to be one of the predictors of severity for COVID-19 [19], [20].
5. Conclusion
While it is important to increase, facilitate and optimize the use of simple and accessible technological tools in health, this should also be done with the involvement of patients with self-management of their condition. The extraordinary context of the COVID-19 pandemic has prompted the implementation of innovative measures. This study, carried out in the context of a pandemic, made it possible to demonstrate the feasibility of a completely remote initiation of a telehealth protocol for sick and symptomatic individuals, including a complete measurement of vital signs twice a day for a period of 14 days.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The study was partially funded by Université de Sherbrooke, the Réseau de Recherche en Interventions en Sciences Infirmières du Québec (RRISIQ) and the Centre de Santé et Service Sociaux de Laval. We are also grateful to the Centre de Santé et Service Sociaux de Laval for their valuable help for the recruitment of participants. Finally, we are gratefull for the great work of Sara Maria Viens-Vega and Sandra Lucier, nurse practionners who made the follow-up calls for our participants.
Footnotes
RStudio: Integrated Development for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/.
References
- 1.Rockwell K.L., Gilroy A.S. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am. J. Managed Care. 2020;26(4):147–148. doi: 10.37765/ajmc.2020.42784. [DOI] [PubMed] [Google Scholar]
- 2.Treskes R.W., van Winden L.A.M., van Keulen N., van der Velde E.T., Beeres S.L.M.A., Atsma D.E., Schalij M.J. Effect of smartphone-enabled health monitoring devices vs regular follow-up on blood pressure control among patients after myocardial infarction: a randomized clinical trial. JAMA Network Open. 2020;3(4):e202165. doi: 10.1001/jamanetworkopen.2020.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenhalgh T., Koh G.C.H., Car J. Covid-19: a remote assessment in primary care. BMJ. 2020;368 doi: 10.1136/bmj.m1182. [DOI] [PubMed] [Google Scholar]
- 4.Padwal R.S., Bienek A., McAlister F.A., Campbell N.R.C. Epidemiology of hypertension in Canada: an update. Can. J. Cardiol. 2016;32(5):687–694. doi: 10.1016/j.cjca.2015.07.734. [DOI] [PubMed] [Google Scholar]
- 5.Bancej C.M., Campbell N., McKay D.W., Nichol M., Walker R.L., Kaczorowski J. Home blood pressure monitoring among Canadian adults with hypertension: results from the 2009 Survey on Living with Chronic Diseases in Canada. Can. J. Cardiol. 2010;26(5):e152–e157. doi: 10.1016/s0828-282x(10)70382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson S., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tobin M.J., Laghi F., Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am. J. Respir. Crit. Care Med. 2020;202(3):356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhont S., Derom E., Van Braeckel E., Depuydt P., Lambrecht B.N. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir. Res. 2020;21(1) doi: 10.1186/s12931-020-01462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NHS. Pulse oximetry to detect early deterioration of patients with COVID-19 in primary and community care settings. 2020 [cited 2020 august 20]; Guidance]. Available from: https://www.england.nhs.uk/coronavirus/publication/pulse-oximetry-to-detect-early-deterioration-of-patients-with-covid-19-in-primary-and-community-care-settings/.
- 10.LaMantia M., Stewart P., Platts-Mills T., Biese K., Forbach C., Zamora E., McCall B., Shofer F., Cairns C., Busby-Whitehead J., Kizer J. Predictive value of initial triage vital signs for critically ill older adults. Western J. Emergency Med. 2013;14(5):453–460. doi: 10.5811/westjem.2013.5.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.T. Greenhalgh et al., Remote management of covid-19 using home pulse oximetry and virtual ward support. BMJ, 2021, p. n677. [DOI] [PubMed]
- 12.B. Klapper, H. Kühne, Patient Self-management by telehealth using the Bosch Model of Care, J. Telemed. Telecare, 16(4) (2010) 193-195. [DOI] [PubMed]
- 13.M.C. Demir, B. Ilhan, Performance of the Pandemic Medical Early Warning Score (PMEWS), Simple Triage Scoring System (STSS) and Confusion, Uremia, Respiratory rate, Blood pressure and age ≥ 65 (CURB-65) score among patients with COVID-19 pneumonia in an emergency department triage setting: a retrospective study. Sao Paulo medical journal = Revista paulista de medicina 139(2) (2021) 70-177. [DOI] [PMC free article] [PubMed]
- 14.T. Mikami, et al., Risk Factors for Mortality in Patients with COVID-19 in New York City, J. General Int. Med. 36(1) (2021) 17–26. [DOI] [PMC free article] [PubMed]
- 15.F.Y. Liu et al., Evaluation of the risk prediction tools for patients with coronavirus disease 2019 in Wuhan, China: a single-centered, retrospective, observational study. Crit. Care Med. 48(11) (2020) e1004–e1011. [DOI] [PMC free article] [PubMed]
- 16.van Rensen I.H.T., Hensgens K.R.C., Lekx A.W., van Osch F.H.M., Knarren L.H.H., van Kampen-van den Boogaart V.E.M., Mehagnoul-Schipper J.D.J., Wyers C.E., van den Bergh J.P., Barten D.G. Early detection of hospitalized patients with COVID-19 at high risk of clinical deterioration: Utility of emergency department shock index. Am. J. Emerg. Med. 2021;49:76–79. doi: 10.1016/j.ajem.2021.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nehme M., Braillard O., Alcoba G., Aebischer Perone S., Courvoisier D., Chappuis F., Guessous I. COVID-19 symptoms: longitudinal evolution and persistence in outpatient settings. Ann. Int. Med. 2021;174(5):723–725. doi: 10.7326/M20-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Statistique Canada. Tableau 13-10-0096-07 Diabète, selon le groupe d'âge. 2021 [cited 2021 27 january]; Available from: https://www150.statcan.gc.ca/t1/tbl1/fr/tv.action?pid=1310009607&pickMembers%5B0%5D=1.6&pickMembers%5B1%5D=3.1&cubeTimeFrame.startYear=2019&cubeTimeFrame.endYear=2020&referencePeriods=20190101%2C20200101.
- 19.Longmore D.K., Miller J.E., Bekkering S., Saner C., Mifsud E., Zhu Y., Saffery R., Nichol A., Colditz G., Short K.R., Burgner D.P. Diabetes and overweight/obesity are independent, nonadditive risk factors for in-hospital severity of COVID-19: an international, multicenter retrospective meta-analysis. Diabetes Care. 2021;44(6):1281–1290. doi: 10.2337/dc20-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez-Solem E., et al. Developing and validating COVID-19 adverse outcome risk prediction models from a bi-national European cohort of 5594 patients. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-81844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]