Abstract
Background
Reduced vitamin A concentration increases the risk of blindness in children infected with the measles virus. Promoting vitamin A supplementation in children with measles contributes to the control of blindness in children, which is a high priority within the World Health Organization (WHO) VISION 2020 The Right to Sight Program.
Objectives
To assess the efficacy of vitamin A in preventing blindness in children with measles without prior clinical features of vitamin A deficiency.
Search methods
We searched CENTRAL 2015, Issue 11, MEDLINE (1950 to December week 3, 2015), Embase (1974 to December 2015) and LILACS (1985 to December 2015).
Selection criteria
Randomised controlled trials (RCTs) assessing the efficacy of vitamin A in preventing blindness in well‐nourished children diagnosed with measles but with no prior clinical features of vitamin A deficiency.
Data collection and analysis
For the original review, two review authors independently assessed studies for eligibility and extracted data on reported outcomes. We contacted trial authors of the included studies for additional information on unpublished data. We included two RCTs which were clinically heterogenous. We presented the continuous outcomes reported as the mean difference (MD) with 95% confidence interval (CI) and dichotomous outcomes as risk ratio (RR) with 95% CI. Due to marked clinical heterogeneity we considered it inappropriate to perform a meta‐analysis.
Main results
For the first publication of this review, two RCTs involving 260 children with measles which compared vitamin A with placebo met the inclusion criteria. Neither study reported blindness or other ocular morbidities as end points. One trial of moderate quality suggested evidence of a significant increase in serum retinol levels in the vitamin A group one week after two doses of vitamin A (MD 9.45 µg/dL, 95% CI 2.19 to 16.71; 17 participants, moderate‐quality evidence), but not six weeks after three doses of vitamin A (MD 2.56 µg/dL, 95% CI ‐5.28 to 10.40; 39 participants, moderate‐quality evidence). There was no significant difference in weight gain six weeks (MD 0.39 kg, ‐0.04 to 0.82; 48 participants, moderate‐quality evidence) and six months (MD 0.52 kg, 95% CI ‐0.08 to 1.12; 36 participants, moderate‐quality evidence) after three doses of vitamin A.
The second trial found no significant difference in serum retinol levels two weeks after a single dose of vitamin A (MD 2.67 µg/dL, 95% CI ‐0.29 to 5.63; 155 participants, moderate‐quality evidence). Percentage of undernutrition between the two groups did not differ significantly at one week (RR 0.93, 95% CI 0.56 to 1.54, 145 participants) and two weeks (RR 0.82, 95% CI 0.52 to 1.29, 147 participants) after a single dose of vitamin A. No adverse event was reported in either study. We did not find any new RCTS for this second update.
Authors' conclusions
We did not find any trials assessing whether or not vitamin A supplementation in children with measles prevents blindness, as neither study reported blindness or other ocular morbidities as end points.
Keywords: Adolescent; Child; Child, Preschool; Humans; Infant; Blindness; Blindness/etiology; Blindness/prevention & control; Measles; Measles/complications; Randomized Controlled Trials as Topic; Vitamin A; Vitamin A/administration & dosage; Vitamin A/blood; Vitamins; Vitamins/administration & dosage; Vitamins/blood
Plain language summary
Vitamin A for preventing blindness in children with measles
Background
Annually 500,000 children become blind worldwide; 75% of them live in low‐income countries. The major causes of blindness in children vary widely from region to region and are related to the standard of living of the community. Scarring of the eyes from measles, vitamin A deficiency, use of harmful traditional eye remedies and eye infection of the newborn, are the major causes of blindness in low‐income countries. Vitamin A is an important nutrient in the body and is required for the normal functioning of the eye. Its deficiency results in poor vision.
Measles infection in children has been associated with vitamin A deficiency and blindness. The control of blindness in children is considered a high priority within the World Health Organization's VISION 2020 The Right to Sight Program. Studies have reported the beneficial effect of vitamin A in reducing disease burden and rate of death in children with measles. This review examined vitamin A use in preventing blindness in children infected with measles without features of vitamin A deficiency.
Study characteristics
We included two randomised controlled trials of moderate quality, including 260 children with measles, comparing children given vitamin A with children not given vitamin A.
Key results
The evidence is current to December 2015. Two doses of vitamin A given on two consecutive days to hospitalised children with measles led to an increase in the blood concentration of vitamin A after one week. However, there is a limitation in that neither of the two included studies reported blindness or other eye problems in children infected with measles. Also, no side effects of the treatment were reported in the included studies. We do not have sufficient evidence to demonstrate the benefit or otherwise of vitamin A in the prevention of blindness in children infected with measles.
Quality of evidence
The quality of the evidence and methodology of both studies was moderate. The sample size of the included studies was relatively small, which could affect the accuracy of the results.
Summary of findings
for the main comparison.
| Vitamin A compared with placebo or no vitamin A for prevention of blindness | ||||||
|
Patient or population: children with measles infection and no clinically demonstrable vitamin A deficiency Settings: resource‐limited countries Intervention: vitamin A Comparison: placebo or no vitamin A | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin A | |||||
| Blindness | See comment | Not estimable | 260 (2 studies) | See comment | None of the studies reported blindness as an end point | |
|
Serum retinol (1 week post‐intervention) (mean X (µg/dL) ± standard error (SE) |
X ± SE in the placebo group was 29.0 ± 2.2 (95% CI 24.8 to 33.3) | X ± SE in the intervention group was 38.5 ±3.0 (95% CI 32.6 to 44.4) | 9.5 higher (2.2 higher to 16.7 higher) | 17 (1 study) | ⊕⊕⊕⊝ moderate | |
| Serum retinol (2 weeks post‐intervention) (mean X (µg/dL) ± standard error SE) | X ± SE in the placebo group was 19.0 ± 0.7 (95% CI 17.6 to 20.3) | X ± SE in the intervention group was 21.6 ± 1.1 (95% CI 19.5 to 23.7) | 2.7 higher (0.3 lower to 5.6 higher) | 155 (1 study) | ⊕⊕⊕⊝ moderate | |
| Serum retinol (6 weeks post‐intervention) (mean X (µg/dL) ± standard error SE) | X ± SE in the placebo group was 28.5 ± 2.4 (95% CI 23.86 to 33.12) | X ± SE in the intervention group was 31.1 ± 3.2 (95% CI 24.7 to 37.4) | 2.6 higher (5.3 lower to 10.4 higher) | 39 (1 study) | ⊕⊕⊕⊝ moderate | |
| Serum retinol (mean change 1 week post‐intervention) (mean X (µg/dL) ± standard error SE) | X ± SE in the placebo group was 17.3 ± 1.9 (95% CI 13.7 to 21.0) | X ± SE in the placebo group was 26.0 ± 3.3 (95% CI 19.6 to 32.4) | 8.6 higher (1.2 higher to 16.0 higher) | 17 (1 study) | ⊕⊕⊕⊝ moderate | |
| Weight gain 6 weeks post‐intervention (mean X (kg) ± standard error SE) | X ± SE in the placebo group was 0.9 ± 0.1 (95% CI 0.6 to 1.2) | X ± SE in the intervention group was 1.3 ± 0.2 (95% CI 1.3 to 1.3) | 0.4 higher (0.04 lower to 0.8 higher) | 48 (1 study) | ⊕⊕⊕⊝ moderate | |
| Weight gain 6 months post‐intervention (mean X (kg) ± standard error SE) | X ± SE in the placebo group was 2.4 ± 0.2 (95% CI 2.0 to 2.8) | X ± SE in the intervention group was 2.9 ± 0.2 (95% CI 2.4 to 3.3) | 0.5 higher (0.1 lower to 1.1 higher) | 36 (1 study) | ⊕⊕⊕⊝ moderate | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; SE: standard error; GRADE: GRADE Working Group grades of evidence (see explanations) | ||||||
Assumed risk and corresponding risk in the table are from a single study in each case, and are not the usual combined mean or median risks across multiple studies.
GRADE Working Group grades of evidence High quality (⊕⊕⊕⊕): Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality (⊕⊕⊕⊝): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality (⊕⊕⊝⊝): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality (⊕⊝⊝⊝): We are very uncertain about the estimate.
Background
Description of the condition
The World Health Organization (WHO) defines blindness as a corrected visual acuity in the better eye of less than 3/60 (Gilbert 2001). The measles virus causes blindness by reducing the serum concentration of vitamin A, which is needed for maintenance of epithelial surfaces such as corneas. Vitamin A deficiency subsequently causes dryness and scarring of the cornea. Serum vitamin A concentrations in well‐nourished children with measles have been reported to be lower than those in malnourished children without measles (Chan 1990).
The major causes of blindness in children vary widely from region to region and are related to the level of socio‐economic development of the community. In high‐income countries, lesions of the optic nerve and higher visual pathways predominate as the cause of blindness, while corneal scarring from measles, vitamin A deficiency, use of harmful traditional eye remedies and ophthalmia neonatorium (newborn conjunctivitis) are the major causes in low‐income countries (Gilbert 2001).
The prevalence of blindness also has a direct correlation with the level of socio‐economic development and the under five mortality rate (Gilbert 2003). The prevalence ranges from about 3 per 10,000 in high‐income communities to 15 per 10,000 in low‐income communities. Annually 500,000 children become blind worldwide, 75% of them living in low‐income countries (Gilbert 2003; Nemer 2001). Blind children have a high death rate and the prevalence, therefore, markedly underestimates the burden of disease (Gilbert 2003). Vitamin A deficiency has been strongly implicated as a major cause of blindness in children, especially in low‐income countries.
Description of the intervention
Vitamin A is a fat‐soluble substance stored in the liver and is released as needed into the blood stream (Al‐Kubaisy 2002). It is required for the maintenance of epithelial surfaces, immune competence, normal functioning of the retina, growth and development and reproduction (Potter 1997). As vitamin A levels decrease, total body reserves of vitamin A are depleted first, followed by a diminished concentration of serum retinol. This leads to abnormalities in tissue function. Xerophthalmia (drying of the conjunctiva from changes resulting from vitamin A deficiency) results in ocular manifestations: night blindness, corneal ulceration, scarring and consequent blindness (Al‐Kubaisy 2002; Potter 1997). The WHO cut‐off value indicative of sub‐clinical vitamin A deficiency is a serum retinol level of < 20 µG/dL (0.7 µmol/L) (Al‐Kubaisy 2002).
Vitamin A deficiency is a major cause of paediatric ocular morbidity and the leading cause of childhood blindness. Annually, over five million children develop xerophthalmia and 250,000 children become blind. Vitamin A deficiency is caused by dietary inadequacy, unmet physiological needs and cultural factors.
Measles is a precipitating factor in blindness from vitamin A deficiency, particularly in Africa (Sommer 1990). Measles causes corneal blindness through several mechanisms, including vitamin A deficiency (Gilbert 2003). When mild or severe forms of vitamin A deficiency are present, it is associated with increased morbidity and mortality from respiratory and diarrhoeal complications of measles. These complications not only increase the requirement for vitamin A but decrease its intake by reduced appetite (Nemer 2001).
Vitamin A deficiency is widespread and particularly prevalent in Africa and South East Asia, where about three million children under the age of five show signs of xerophthalmia. In 1998 the WHO estimated that vitamin A deficiency was a problem in 118 countries. Annually, an estimated 250,000 to 500,000 children with the severest deficiencies become blind and even larger numbers die of preventable infectious diseases such as diarrhoea and measles (Nemer 2001).
How the intervention might work
Supplying vitamin A to children suffering measles may reverse the mechanism of blindness. Some evidence suggests that vitamin A supplements may be a cheap and effective way of preventing death and complications in children with measles (Chan 1990).
Why it is important to do this review
The control of blindness in children is considered a high priority within the WHO's VISION 2020 The Right to Sight Program (Gilbert 2001). The benefit of vitamin A in reducing mortality in children with measles has been widely reported (Yang 2011). We aim to determine the benefit or otherwise of vitamin A in preventing blindness in children with measles infection.
Objectives
To assess the efficacy of vitamin A in preventing blindness in children with measles without prior clinical features of vitamin A deficiency.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) that assess the efficacy of vitamin A in preventing blindness in children diagnosed with measles but with no prior clinical features of vitamin A deficiency and who are not malnourished. We excluded studies with participants that had clinically demonstrable vitamin A deficiency.
Types of participants
Children 18 years or younger diagnosed with measles, with no prior clinical features of vitamin A deficiency. We excluded studies that included children with ocular abnormalities unrelated to vitamin A deficiency.
Types of interventions
Vitamin A versus placebo or no vitamin A.
Types of outcome measures
Primary outcomes
Blindness as defined by the WHO: corrected visual acuity in the better eye of less than 3/60 (Gilbert 2001).
Secondary outcomes
Other clinical manifestations of vitamin A deficiencies.
Night blindness
Conjunctival xerosis
Bitot's spot
Corneal xerosis
Xerophthalmia
Corneal ulceration
Corneal scars
Serum retinol level
Nutritional status
-
Adverse events
Vitamin A toxicity
Other adverse events
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2015, Issue 11, part of the Cochrane Library, www.thecochranelibrary.com (accessed 15 December 2015), which includes the Cochrane Acute Respiratory Infections Group Specialised Register, MEDLINE (1950 to December week 3, 2015), Embase (1974 to December 2015) and LILACS (1985 to December 2015). We used the following search strategy to search MEDLINE and CENTRAL. We did not use a filter to identify randomised trials in MEDLINE as there were too few studies. We adapted the search terms accordingly for Embase (Appendix 1) and LILACS (Appendix 2).
MEDLINE (OVID)
1 exp Measles/ 2 exp Measles virus/ 3 measles.tw. 4 rubeola.tw. 5 morbilli*.tw. 6 or/1‐5 7 exp Vitamin A/ 8 vitamin a.tw,nm. 9 retinol.tw,nm. 10 exp Dietary Supplements/ 11 or/7‐10 12 exp Blindness/ 13 Xerophthalmia/ 14 Night Blindness/ 15 (bitot* adj1 spot*).tw. 16 xerosis*.tw. 17 keratomalacia.tw. 18 blind*.tw. 19 xerophthalmia*.tw. 20 exp Vision Disorders/ 21 (vision* or visual* or eye* or sight*).tw. 22 or/12‐21 23 6 and 11 and 22
Searching other resources
There were no publication or language restrictions. We also searched the following ongoing database registers: www.controlled‐trials.com/, www.clinicaltrials.gov, www.trialscentral.org/ and www.gsk‐clinicalstudyregister.com/ (15 December 2015). We also contacted experts in the field for information on ongoing and unpublished trials. We did not find any ongoing trials in the database registers. Efforts at contacting experts also proved unsuccessful as some of the email contacts were no longer active. We did not receive any response from those whose emails were still active.
Data collection and analysis
Selection of studies
For the original review, two review authors (SB, OO) reviewed the results from the initial literature search, excluded non‐relevant studies, retrieved the full text of these articles and designed a study eligibility form (Bello 2011) Two review authors (SB, MM) reviewed the full texts of the publications using the eligibility form. For this update, two review authors (SB, OO) screened the search results for relevant studies. We did not identify any new trials for inclusion or exclusion.
Data extraction and management
Two review authors (SB, OO) designed and piloted a data extraction form. The following were included in the data extraction form.
Verification of the eligibility of study, including the inclusion and exclusion criteria.
Study characteristics, including the quality criteria.
Information on the participants: number in each group, number lost to follow‐up, duration of follow‐up.
The interventions given, including dose and preparation/form of vitamin A used.
Outcome measures of interest to the review.
Publication status.
Date and location of the study.
One review author (MM) supervised data extraction. Two review authors (SB, OO) independently extracted the data.
Assessment of risk of bias in included studies
Two review authors (SB, OO) used a quality assessment form to rank the studies as low, moderate and high risk of bias, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the quality of the studies using the following criteria.
Generation of allocation sequence; secure or not.
Allocation concealment; whether adequate, inadequate or unclear.
Blinding of care giver; yes, no or unclear.
Blinding of outcome assessors; yes, no or unclear.
Differential loss to follow‐up/attrition/exclusion; whether all randomised participants were included in the analysis.
Measures of treatment effect
Both studies used per protocol analysis. They reported mean and standard error (SE) of the mean for serum retinol levels and weight gain. We converted the SE of the mean to standard deviation (SD) by multiplying SE by √n for separate arms. We report the per protocol analysis found in both studies and the mean difference (MD) with 95% confidence interval (CI). Due to the clinical heterogeneity of the included studies, we did not pool any of the estimates.
Unit of analysis issues
Not applicable.
Dealing with missing data
We reported the per protocol analysis found in both studies and the MD with 95% CI. Where we could not obtain missing data, we conducted the analysis using only the data available, as presented by the trial authors. In this circumstance, we assumed that the data are missing at random.
Assessment of heterogeneity
We planned to estimate the I2 statistic, with values of 30% to 59%, 60% to 89% and 90% to 100% representing moderate, substantial and considerable levels of heterogeneity, respectively. However, investigation of heterogeneity was not feasible because we did not pool any of the estimates due to clinical heterogeneity of the included studies,
Assessment of reporting biases
We could not explore the presence of publication bias by looking for funnel plot asymmetry because the number of included studies was too few.
Data synthesis
The data of included studies could not be aggregated into a meta‐analysis because the studies had different interventions. We used the SE of the mean to obtain standard deviation (SD) where SD was not reported by study authors according to Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We extracted the MD and SE of the mean for some outcomes and calculated 95% CIs of the MDs using generic inverse variance. We also used the Mantel‐Haenszel method to analyse the risk ratio (RR) for dichotomous outcomes such as undernutrition post‐intervention.
GRADE and 'Summary of findings' table
We created a Table 1 using the following outcomes: blindness, serum retinol (one week post‐intervention), serum retinol (two weeks post‐intervention), serum retinol (six weeks post‐intervention), serum retinol (mean change one week post‐intervention), weight gain six weeks and six months post‐intervention. We used the five GRADE (Atkins 2004) considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEproGDT software (GRADEproGDT 2015). We justified all decisions to down‐ or up‐grade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary
Subgroup analysis and investigation of heterogeneity
Data were available for two studies that were not combined in a meta‐analysis. As a result, investigation of heterogeneity as well as subgroup analysis were not feasible.
Sensitivity analysis
We did not perform a sensitivity analysis because we included only two small trials.
Results
Description of studies
Results of the search
The original searches identified 147 records. The first updated searches retrieved a further 25 records from the electronic databases while this second updated search retrieved 27 records. The following results were obtained from the updated database searches: MEDLINE (Ovid) from 1 January 2013 to December week 3 2015 (nine search results), Embase.com from 1 January 2011 to November 2013 (six search results), CENTRAL 2015, Issue 11 limited to year published 2013 to 2015 (zero search results), LILACS limited to year published 2013 to 2015 (12 search results).
Included studies
For the first publication of this review (Bello 2011), we retrieved seven full articles out of which two studies (in four publications) were found eligible and included in the review (Coutsoudis 1991; Rosale 1996). No new trials were found for the 2014 update (Bello 2014) For this 2015 update, we obtained an additional reference for one of the studies (Rosale 1996). Both of these studies were randomised, double‐blind, placebo‐controlled trials of vitamin A. One trial was conducted in Durban, South Africa in 1989 (Coutsoudis 1991), while the other trial was carried out in Ndola, Zambia in 1991 (Rosale 1996). Total sample size for both studies was 260. Coutsoudis 1991 enrolled a total of 60 children and Rosale 1996 enrolled 200 children; involving 29 and 90 children in the vitamin A arm, respectively.
In the Coutsoudis 1991 study, participants were aged four to 24 months and in the Rosale 1996 study, participants were aged five months to 17 years. In both studies the children had measles, however, Coutsoudis 1991 enrolled children whose illness was severe enough to warrant hospital admission in contrast to Rosale 1996 who enrolled children with mild illness and excluded cases that required hospital admission. Both studies excluded children with clinical signs of vitamin A deficiency and severe undernutrition. In addition to clinical judgement, Rosale 1996 confirmed measles cases by a four‐fold increase in measles antibody titre two weeks after enrolment.
The intervention given in both studies was vitamin A. Coutsoudis 1991 administered standard WHO recommended dosage (54.5 mg for children < 12 months, 109 mg for children > 12 months) at admission and on days two, eight and week six, while Rosale 1996 administered a single dose of 200,000 IU (international units) (210 µmol). Co‐interventions consisted essentially of standard treatment administered to both groups in both studies. In addition, the formulation used by one study (Rosale 1996) contained vitamin E (40 µG/mL).
None of the studies reported ocular morbidities. Rosale 1996 conducted eye examination and conjunctival impression cytology at baseline and during follow‐up. However, we only had access to assessment of conjunctivitis from the eye examination. Both studies reported other measles‐related complications seen, and serum retinol levels post‐intervention. Also, both studies assessed nutritional status post‐intervention.
Excluded studies
We excluded two studies for the reasons documented in the Characteristics of excluded studies table. One was an advocacy document and not a trial (CID 1993).
Risk of bias in included studies
The quality of both studies was moderate (Figure 1; Figure 2).
1.
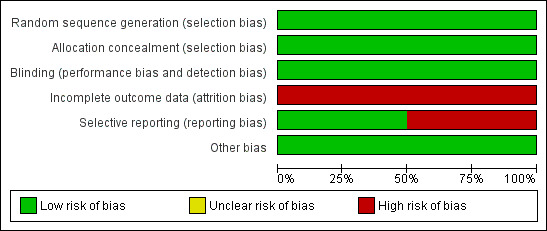
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
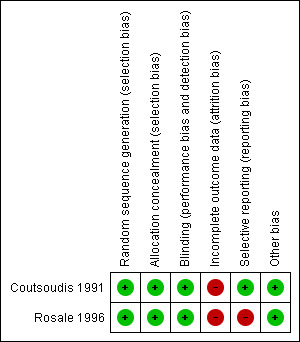
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Both included studies generated allocation sequence by using a random numbers table. Allocation concealment was adequate in both trials. Dispenser bottles were number‐coded in both studies.
Blinding
Both studies were double‐blind.
Incomplete outcome data
Neither study included all randomised participants in the final analysis. Rosale 1996 included 77.5% of enrolled participants in the final analysis, while Coutsoudis 1991 included 28% (one week post‐intervention), 65% (for serum retinol) and 80% (for weight gain) at six weeks and 60% at six months post‐intervention. All participants enrolled were well accounted for by both studies. There was evidence for a differential loss to follow‐up between groups in both studies.
Selective reporting
Coutsoudis 1991 reported all outcomes stated in the objectives of the study based on the study report, while Rosale 1996 indicated that eye examination and conjunctival impression cytology were done at follow‐up visits, but we only had access to information on the assessment of conjunctivitis.
Other potential sources of bias
None known
Effects of interventions
See: Table 1
Both studies were clinically heterogenous in several ways (see Characteristics of included studies table). Only the time of study and duration of study and the geographical location were similar. The age groups enrolled, the formulation of vitamin A used, doses of vitamin A given and the time point of outcome assessment were widely different between the two studies. The Coutsoudis 1991 study was hospital‐based. Neither included study reported ocular morbidities. We could therefore not assess the following outcomes: blindness, night blindness, conjunctival xerosis, Bitot's spot, corneal xerosis, xerophthalmia, corneal ulceration and corneal scars. No adverse event was reported in either study. Only serum retinol levels post‐intervention were reported in both studies. A measure of nutritional status (weight gain) was reported in both studies (Coutsoudis 1991; Rosale 1996).
Primary outcome
Blindness as defined by the WHO
Neither included trial reported on this outcome as an end point in children infected with measles.
Secondary outcomes
Neither included trial reported on other ocular morbidities as end points in children infected with measles.
Other clinical manifestations of vitamin A deficiencies:
1. Night blindness
Neither included trial reported on this outcome.
2. Conjunctival xerosis
Neither included trial reported on this outcome. One study reported that no cases of conjunctivitis were observed in both groups during the follow‐up assessments (Rosale 1996).
3. Bitot's spot
Neither included trial reported on this outcome.
4. Corneal xerosis
Neither included trial reported on this outcome.
5. Xerophthalmia
Neither included trial reported on this outcome.
6. Corneal ulceration
Neither included trial reported on this outcome.
7. Corneal scars
Neither included trial reported on this outcome.
8. Serum retinol level
Rosale measured and reported a summary estimate for serum retinol level at two weeks post‐intervention (Rosale 1996). There was no significant difference in the mean serum retinol level of both groups (mean difference (MD) 2.67 µg/dL, 95% CI ‐0.29 to 5.63; 155 participants, moderate‐quality evidence) (Analysis 1.1). Coutsoudis reported a significantly higher serum retinol level (measured on day eight) in the vitamin A group (MD 9.45 µg/dL, 95% CI 2.19 to 16.71; 17 participants, moderate‐quality evidence) (Analysis 1.2) (Coutsoudis 1991). The mean change in serum retinol level on day eight compared to baseline was also significantly higher in the vitamin A group (MD 8.62 µg/dL, 95% CI 1.22 to 16.02; 17 participants, moderate‐quality evidence) (Analysis 1.4). However, there was no strong evidence to show that there was a difference in the serum retinol level between the vitamin A and the placebo groups on day 42 post‐intervention (MD 2.56 µg/dL, ‐5.28 to 10.40; 39 participants, moderate‐quality evidence) (Analysis 1.3).
1.1. Analysis.
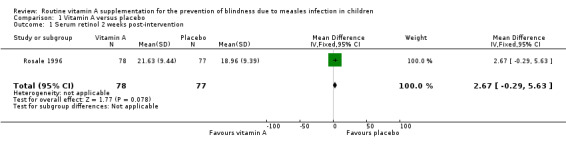
Comparison 1 Vitamin A versus placebo, Outcome 1 Serum retinol 2 weeks post‐intervention.
1.2. Analysis.
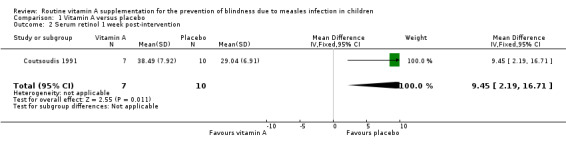
Comparison 1 Vitamin A versus placebo, Outcome 2 Serum retinol 1 week post‐intervention.
1.4. Analysis.
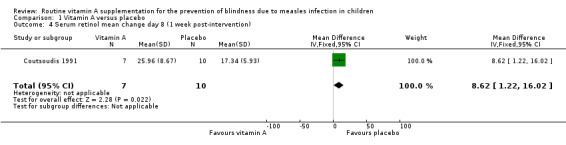
Comparison 1 Vitamin A versus placebo, Outcome 4 Serum retinol mean change day 8 (1 week post‐intervention).
1.3. Analysis.
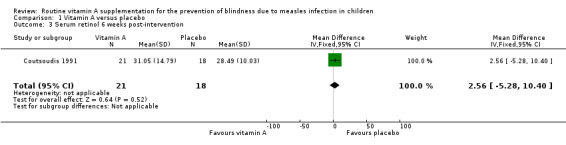
Comparison 1 Vitamin A versus placebo, Outcome 3 Serum retinol 6 weeks post‐intervention.
9. Nutritional status
One study (Coutsoudis 1991) measured and reported weight gain post‐intervention. There was no significant difference in weight gain between both groups at six weeks (MD 0.39 kg, 95% CI ‐0.04 to 0.82, moderate‐quality evidence) (Analysis 1.5) and six months (MD 0.52 kg, 95% CI ‐0.08 to 1.12, moderate‐quality evidence) (Analysis 1.6).
1.5. Analysis.
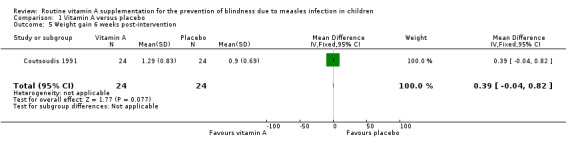
Comparison 1 Vitamin A versus placebo, Outcome 5 Weight gain 6 weeks post‐intervention.
1.6. Analysis.
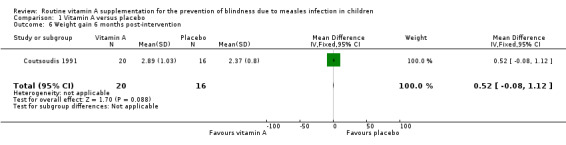
Comparison 1 Vitamin A versus placebo, Outcome 6 Weight gain 6 months post‐intervention.
It is possible that a larger effect of serum retinol and weight gain could have been observed if the sample size of both studies was larger. This is depicted in the wide CIs (smaller precision) of the reported estimates.
One study (Rosale 1996) reported the weight for age (W/A) undernutrition in proportions at baseline and at week one and two. The proportions of vitamin A and placebo groups with undernutrition at baseline were 35.6% and 35.5%. and at week one (30.6% and 37.3%) (risk ratio (RR) = 0.93, 95% CI 0.56 to 1.54), and week two (28.6% and 30.7%) (RR = 0.82, 95% CI 0.52 to 1.29), respectively.
10. Adverse events
Neither included trial reported on this outcome.
Discussion
Summary of main results
The serum retinol level increased significantly one week after two doses of vitamin given on two consecutive days at the WHO recommended dosage. A single dose of 200,000 IU did not increase the serum retinol significantly two weeks after administration. However, administration of three doses of vitamin within one week did not result in a significant increase in serum retinol level six weeks post‐intervention. Likewise, there was no significant difference in weight gain between the vitamin A group and the placebo group six weeks and six months post‐administration of three doses of vitamin A. Also, there was no significant difference in the percentage of undernutrition at one week and two weeks post‐administration of a single dose of 200,000 IU of vitamin A.
Blindness, other ocular morbidities and adverse events were not reported in the included studies.
Overall completeness and applicability of evidence
None of the studies included assessed the primary outcome of this review. There is therefore insufficient evidence to address this question.
Quality of the evidence
The quality of the evidence and methodology of both studies was moderate. There was a possible reporting bias in Rosale 1996 because ocular examinations were carried out but not reported. There was also incomplete outcome data bias in both studies.
Potential biases in the review process
The sample size in the included studies was small and this could affect the precision of the estimates given. We reported the per protocol analysis as given in the studies. This could have produced an over‐estimate of effects of intervention. One study indicated that eye examination including conjunctival impression cytology was performed, but we had access to only assessment of conjunctivitis (Rosale 1996). We were unable to obtain information from the trial authors about the outcome of conjunctival impression cytology done.
Agreements and disagreements with other studies or reviews
We found insufficient data in these trials to attempt any comparison with other studies.
Authors' conclusions
Implications for practice.
None of the included studies assessed blindness (primary outcome of this review) and other ocular morbidities as end points. There is insufficient evidence to demonstrate the benefit or otherwise of vitamin A in the prevention of blindness in children infected with measles. There is a need for more high‐quality randomised controlled trials that evaluate the efficacy of vitamin A in the prevention of blindness in children infected with measles.
Implications for research.
New placebo‐controlled vitamin A studies in children with measles will pose a significant ethical challenge since the beneficial effect of vitamin A on measles mortality and morbidity has been demonstrated in a Cochrane Review (Yang 2011). In light of dose‐related differences in serum level of vitamin A, there could be some benefit in conducting more randomised controlled trials to assess the efficacy of different dosage schedules (single, double or triple doses of vitamin A) for the prevention of blindness and other ocular morbidities in measles infection. Serum retinol levels and other study outcomes should also be measured at similar time points during follow‐up to ensure comparability of the study results. Studies should also address dosage for level of severity and age groups. Larger studies would enable analysis of these subgroups.
Feedback
Routine vitamin A supplementation for the prevention of blindness due to measles infection in children, 22 April 2014
Summary
I am the author of two of the papers reviewed in this Cochrane meta‐analysis: Routine vitamin A supplementation for the prevention of blindness due to measles infection in children. DOI: 10.1002/14651858.CD007719.pub3
The results from the study by Rosales et al. were published in two manuscripts:
Rosales 1: Efficacy of a single oral dose of 200,000 IU of oil‐soluble vitamin A in measles‐associated morbidity. Rosales FJ, Kjolhede C, Goodman S. Am J Epidemiol. 1996 Mar 1;143(5):413‐22 Rosales 2: A single 210‐mumol oral dose of retinol does not enhance the immune response in children with measles. Rosales FJ, Kjolhede C. J Nutr. 1994 Sep;124(9):1604‐14.
I would like to use this opportunity to correct some errors on the information reported in the above meta‐analysis and its evaluation of the results and information reported in the manuscripts from the study by Rosales et al.
1. Sample size. In the meta‐analysis it has been misallocated the sample size of 200 measles patients to the study by Coutsoudis et al. (Am J Clin Nutr. 1991 Nov;54(5):890‐5). On page 8 of the Cochrane meta‐analysis is stated that “Coutsoudis 1991 enrolled 200 children and Rosales 1996 enrolled 60 children; the number enrolled in the vitamin A arm was 90 and 29 respectively.” However in Rosales1&2, it is clearly indicated that the total population enrolled was 200 with 110 measles patients enrolled in the placebo group and 90 in the Vitamin A supplemented group.
2. Dosing of vitamin A. On page 8 of the Cochrane met analysis is indicated that Coutsoudis 1991 provided vitamin A supplements on days two, eight and week six, but the information provided in the manuscript (Am J Clin Nutr. 1991 Nov;54(5):890‐5) states that vitamin A was administered at admission and at 2 and 8 days, and that on discharged at the 6th week appointment.
3. Measles induced ocular morbidities. The Cochrane meta‐analysis suggests that none of the studies reported on ocular morbidities. However, Rosales1 reports the findings on measles conjunctivitis. In Rosales et al study measles conjunctivitis was measured from baseline and throughout the experimental period by eye exams. The results are presented on table 1 (Rosales1), and it shows that no conjunctivitis was observed in either group during the weekly follow‐ups after baseline.
4. Anthropometric measurements and assessments. The Cochrane meta‐analysis suggests that only Coutsoudis et al. reported on weight changes. It indicated that “One study (Coutsoudis 1991) measured nutritional status post‐intervention.” But Rosales2 also provides information on the nutritional status of the studied population; table 1 shows the anthropometric characteristics of the patients enrolled; undernutrition was defined based on weight‐for‐age indicator (W/A), and table 1 shows that undernutrition remained unchanged throughout the study period and did not differ significantly between the two groups.
5. Selective reporting. The meta‐analysis also indicated that Rosales et al study was affected by selective reporting bias. The meta‐analysis suggests that only Coutsoudis et al, but not Rosales et al reported all the data collected: “One study (Coutsoudis 1991) reported all outcomes stated in the objectives of the study while the other (Rosale 1996) indicated that eye examination was done at follow‐up visits but ocular outcomes were not reported.” This is not correct: Rosales1 clearly reported on measles conjunctivitis, which was measles induced. In Rosales et al study measles conjunctivitis was measured from baseline and throughout the experimental period by eye exams. The results are presented on table 1 (Rosales1), and it shows that no conjunctivitis was observed in either group during the weekly follow‐ups after baseline. The same argument can be made for the reporting of anthropometric measures, table 1 in Rosales2.
6. Potential biases in the review process. The authors of the meta‐analysis determined that due to the sample size of the included studies was small, this could have affected the precision of the estimates given. However, Rosales et al is the largest randomized placebo‐controlled clinical study reported so far among non‐hospitalized patients on the effects of vitamin A treatment of measles infection. Moreover, the clinical outcomes were rigorously defined and measured. It is quite possible that hospitalized cases as in the study by Coutsoudis et al were relative more severe patients (e.g., requiring hospitalization) than those seen in the study Rosales et al study, and that their severity made them more likely to benefit (increased in plasma retinol) from vitamin A treatment as reported by Coutsoudis. However, if the favorable effect of vitamin A during measles is mediated by replenishing the measles‐induced hyporetinolemia (i.e., plasma retinol <20 µg/ dl), the patients in Rosales et al study should have benefited from receiving vitamin A. Eighty percent of patients had serum retinol levels less than 20 µg/dl, and, among them, half had levels below 10 µg/dl (Rosales1). Thus, an explanation for the modest effect of vitamin A observed in Rosales et al study (no difference in plasma retinol between the control and vitamin A supplemented groups) could not be advocated to these patients being less hyporetinolemic than those in Coutsoudis 1991. But rather, it could be to the differences in dosage of vitamin A as explained by Rosales1. In Rosales et al study, measles patients received a single dose of 200,000 IU (210µmol) of vitamin A in oil, as recommended by WHO for non‐xerophthalmic measles patients, whereas Coutsoudis 1991 administered dosagt (54.5 mg for children (104 µmol) < 12 months, 109 mg (208 µmol) for children > 12 months) on admission and on days two and eight. The total amount of vitamin A received within a week by measles patients in Coutsoudis et al study was three (3)‐times more than that received by measles patients in Rosales et al. These studies should not be compared because of the magnitude of the differences in dosing of vitamin A. Finally, it needs to be realized that the best preventive therapy for reducing measles‐related morbidity is measles vaccine immunization.
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Francisco J. Rosales Affiliation: Abbott Laboratories Role: Medical Director
Reply
I am the author of two of the papers you reviewed:
Rosales1: Efficacy of a single oral dose of 200,000 IU of oil‐soluble vitamin A in measles‐associated morbidity. Rosales FJ, Kjolhede C, Goodman S. Am J Epidemiol. 1996 Mar 1;143(5):413‐22
Rosales2: A single 210‐mumol oral dose of retinol does not enhance the immune response in children with measles. Rosales FJ, Kjolhede C. J Nutr. 1994 Sep;124(9):1604‐14.
1. I was disturbed and confused by your evaluation of the reported information in the above publications. Especially when you misallocated the sample size of the study reported above, 200 meales patients, to the other study by Coutsoudis et al. (Am J Clin Nutr. 1991 Nov;54(5):890‐5). In you manuscript it reads, “Coutsoudis 1991 enrolled 200 children and Rosales 1996 enrolled 60 children; the number enrolled in the vitamin A arm was 90 and 29 respectively.” However in Rosales1&2, it is clearly indicated that the total population was 200 with 110 measles patients enrolled in the placebo group and 90 in the Vitamin A supplemented group.
Reply: We agree there was an error of reference interchange in the first paragraph under the section 'included studies'. However, samples sizes were correctly reported for outcomes. We would correct the reference error.
2. Dosing of vitamin A. On page 8 of the Cochrane met analysis is indicated that Coutsoudis 1991 provided vitamin A supplements on days two, eight and week six, but the information provided in the manuscript (Am J Clin Nutr. 1991 Nov;54(5):890‐5) states that vitamin A was administered at admission and at 2 and 8 days, and that on discharged at the 6th week appointment
Reply: Thank you. We missed out 'at admission'
3. Measles induced ocular morbidities. The Cochrane meta‐analysis suggests that none of the studies reported on ocular morbidities. However, Rosales1 reports the findings on measles conjunctivitis. In Rosales et al study measles conjunctivitis was measured from baseline and throughout the experimental period by eye exams. The results are presented on table 1 (Rosales1), and it shows that no conjunctivitis was observed in either group during the weekly follow‐ups after baseline.
5. Selective reporting. The meta‐analysis also indicated that Rosales et al study was affected by selective reporting bias. The meta‐analysis suggests that only Coutsoudis et al, but not Rosales et al reported all the data collected: “One study (Coutsoudis 1991) reported all outcomes stated in the objectives of the study while the other (Rosale 1996) indicated that eye examination was done at follow‐up visits but ocular outcomes were not reported.” This is not correct: Rosales1 clearly reported on measles conjunctivitis, which was measles induced. In Rosales et al study measles conjunctivitis was measured from baseline and throughout the experimental period by eye exams. The results are presented on table 1 (Rosales1), and it shows that no conjunctivitis was observed in either group during the weekly follow‐ups after baseline. The same argument can be made for the reporting of anthropometric measures, table 1 in Rosales2.
Reply: We do not agree with the author. The authors clearly stated that 'At the end of one month, each child received a large dose of vitamin A and an eye examination which included a conjunctival impression cytology sample'. Our impression was that the presence or absence of conjunctival morbidities (e.g. xerophthalmia) might be demonstrable with examination of conjunctival impression cytology sample. The result of the conjunctival impression cytology was apparently missing. However, we are happy to report it as so if the author asserts that only conjunctivitis was assessed in the eye examination.
4. In addition, your reports indicated that “One study (Coutsoudis 1991) measured nutritional status post‐intervention.” But Rosales2 also provide information on the nutritional status of the studied population; table 1 provides the anthropometric characteristics of the patients enrolled; undernutrition was defined based on weight‐for‐age indicator (W/A); table 1 showed that undernutrition remained unchanged throughout the study period and did not differ significantly between the two groups.
Reply: We agree with the author. Part of the challenges was that some outcomes were mentioned in the methods section of the primary report but were not reported in the result section. For example, only the baseline undernutrition was reported in the primary report, with no mention of follow‐up results. We would add the information accordingly.
6. Finally, your report calls for “Potential biases in the review process” due to sample size in the included studies was small and this could affect the precision of the estimates given. However, Rosales1&2 is the largest study reported so far among non‐hospitalized patients on the effect of vitamin A treatment of measles infection. Moreover, the clinical outcomes were rigorously defined and measured.
Reply: We do not agree with the author. Our conclusion on sample size was based on the confidence intervals of the reported outcomes.
7. However, you need to realize that both Rosales 1996 and Coutsoudis 1991 were designed to measure the effect of vitamin A measles‐related morbidity like pneumonia and diarrhea and not the effect of vitamin A supplementation on measles‐related ocular morbidities. Thus, the main issue in your review is that you and your associates did not have access to the right information.
Reply: We agree that both studies did not address the primary objective of our review. This challenge was clearly stated under the section 'implication for practice'.
8. Bottom line; please provide me with the professional courtesy of correcting the misrepresentations on the studies by Rosales et al. Thank you
Reply: We are happy to do this as appropriate.
Contributors
Segun Bello
What's new
| Date | Event | Description |
|---|---|---|
| 15 December 2015 | New search has been performed | Our conclusions remain unchanged. |
| 15 December 2015 | New citation required but conclusions have not changed | Searches updated. We did not identify any new trials for inclusion in this update. |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 4, 2011
| Date | Event | Description |
|---|---|---|
| 27 November 2013 | New search has been performed | Searches updated and no new trials were identified for inclusion in this update. |
| 27 November 2013 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
Acknowledgements
The review authors wish to thank the following people for commenting on the draft protocol: Chanpen Choprapawon, Rita Sitorus, Francisco Espinosa, Nelcy Rodriguez and Anthony Harnden and the draft of the original review: Emmanuel Effa, Chanpen Choprapawon, Rita Sitorus, Elaine Beller and Matthew Thompson. We also acknowledge the efforts of the Review for Africa Program (RAP Nigeria) and the Nigerian Branch of the South African Cochrane Centre in securing a dedicated time for the authors to complete the original review.
Appendices
Appendix 1. Embase (Elsevier) search strategy
19. #6 AND #11 AND #18 18. #12 OR #13 OR #14 OR #15 OR #16 OR #17 17. blind*:ab,ti OR xerosis*:ab,ti OR keratomalacia:ab,ti OR xerophthalmia*:ab,ti OR vision*:ab,ti OR visual*:ab,ti OR eye*:ab,ti OR sight*:ab,ti 16. 'xerosis'/de 15. (bitot* NEAR/1 spot*):ab,ti 14. 'night blindness'/de 13. 'xerophthalmia'/de 12. 'blindness'/exp OR 'visual impairment'/de OR 'visual disorder'/de 11. #7 OR #8 OR #9 OR #10 10. 'nutrient'/de OR 'vitamin'/de OR 'carotenoid'/exp 9. retinol:ab,ti 8. 'vitamin a':ab,ti 7. 'retinol'/exp 6. #1 OR #2 OR #3 OR #4 OR #5 5. morbilli*:ab,ti 4. rubeola:ab,ti 3. measles:ab,ti 2. 'measles virus'/de 1. 'measles'/exp
Appendix 2. LILACS (BIREME) search strategy
(mh:measles OR measles OR sarampión OR sarampo OR rubeola OR mh:c02.782.580.600.500.500* OR mh:"Measles virus" OR mh:b04.820.455.600.650.500.500* OR mh:b04.909.777.455.600.650.500.500* OR morbilli* OR mh:blindness OR ceguera OR cegueira OR mh:c10.597.751.941.162* OR mh:c11.966.075* OR mh:c23.888.592.763.941.162* OR blind* OR mh:xerophthalmia OR xeroftalmia OR xerophthalm* OR mh:"Night Blindness" OR bitot* OR xerosis OR xeroses OR keratomalacia) AND (mh:"Vitamin A" OR "vitamin A" OR "vitamina A" OR retinol OR mh:d02.455.326.271.665.202.495.818* OR mh:d02.455.426.392.368.367.379.249.700.860* OR mh:d02.455.849.131.495.818* OR mh:d23.767.261.700.860* OR mh:"Dietary Supplements" OR mh:j02.500.456*) AND db:("LILACS")
Data and analyses
Comparison 1. Vitamin A versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum retinol 2 weeks post‐intervention | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | 2.67 [‐0.29, 5.63] |
| 2 Serum retinol 1 week post‐intervention | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 9.45 [2.19, 16.71] |
| 3 Serum retinol 6 weeks post‐intervention | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 2.56 [‐5.28, 10.40] |
| 4 Serum retinol mean change day 8 (1 week post‐intervention) | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 8.62 [1.22, 16.02] |
| 5 Weight gain 6 weeks post‐intervention | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.04, 0.82] |
| 6 Weight gain 6 months post‐intervention | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.52 [‐0.08, 1.12] |
| 7 Undernutrition 1 week post‐intervention | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.56, 1.54] |
| 8 Undernutrition 2 weeks post‐intervention | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.52, 1.29] |
1.7. Analysis.
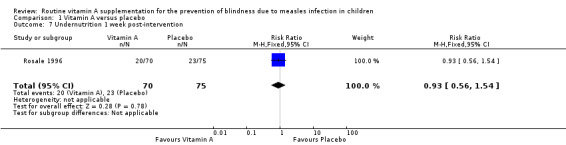
Comparison 1 Vitamin A versus placebo, Outcome 7 Undernutrition 1 week post‐intervention.
1.8. Analysis.
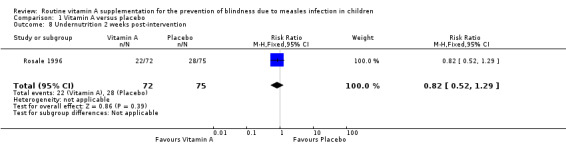
Comparison 1 Vitamin A versus placebo, Outcome 8 Undernutrition 2 weeks post‐intervention.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Coutsoudis 1991.
| Methods | Randomised, double‐blind, placebo‐controlled trial Allocation sequence was generated using a table of random numbers Unit of randomisation was individual participants Treatment and placebo dropper (dispenser bottle) were number‐coded Study duration was 7 months |
|
| Participants | Inclusion criteria: measles severe enough to warrant hospital admission, measles cases with pneumonia and diarrhoea, age between 4 and 24 months Exclusion criteria: mild cases of measles (without pneumonia and diarrhoea), children > 24 months, rash > 5 days, vitamin A administration before admission, children with laryngotracheobronchitis |
|
| Interventions | Vitamin A versus placebo syrup Investigators used the WHO‐recommended dose for vitamin A (54.5 mg for children < 12 months, 109 mg for children ≥ 12 months) Vitamin A given at admission, on days 2, 8 and 42 Follow‐up was 6 months |
|
| Outcomes | Extent of pneumonia, duration of fever, diarrhoea and pneumonia, incidence of herpes stomatitis and laryngotracheobronchitis. Serum zinc, serum vitamin E, serum retinol, serum retinol‐binding protein (RBP), serum albumin and pre‐albumin, weight gain Outcomes were measured on days 8, 42 and 6 months post‐intervention |
|
| Notes | Study was carried out in 1989 Normal‐phase, high‐pressure liquid chromatography (HPLC) using fluorescent detection was used to estimate serum retinol level |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate: allocation sequence was generated by table of random numbers |
| Allocation concealment (selection bias) | Low risk | Adequate: treatment and placebo dropper (dispenser bottle) was number‐coded |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Adequate: double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data for different outcomes at different times. Differential loss to follow‐up between groups |
| Selective reporting (reporting bias) | Low risk | All pre‐stated outcomes were reported |
| Other bias | Low risk | None known |
Rosale 1996.
| Methods | Randomised controlled, double‐blind trial Allocation sequence generated by table of random numbers Unit of randomisation: individual participants Study duration was 7 months |
|
| Participants | Inclusion criteria: prodromal or effervescent measles, consent by parents, confirmation by a four‐fold increase in measles antibody titre at end of week 2 Exclusion: cases requiring hospitalisation, xerophthalmia, severe undernutrition, refusal to give consent by parents |
|
| Interventions | Vitamin A in oil given as a single dose of 210 μmol (200,000 IU) with vitamin E (42.4 microgram) versus placebo Co‐interventions: eye ointment, paracetamol, aspirin, tetracycline, intramuscular penicillin, oral rehydration fluids, gentian violet, cough mixture Follow‐up was for 4 weeks |
|
| Outcomes | Cough, pneumonia, serum retinol level, nutritional status Outcomes were measured 2 weeks and 42 days post‐intervention |
|
| Notes | Both nutritional status and eye examination were reportedly done at follow‐up visits. In a feedback communication, the author (Frasisco Rosale) indicated that "....undernutrition remained unchanged throughout the study period and did not differ significantly between the two groups" and that "......no cases of conjunctivitis was observed in both groups throughout the follow‐up period" Serum retinol levels were determined by high‐pressure, liquid chromatography |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate: sequence was generated using a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Adequate: codes were used on bottles |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Adequate: double‐masking of the dispenser bottles which were also number‐coded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data for different outcomes at different times. Differential loss to follow‐up between groups |
| Selective reporting (reporting bias) | High risk | Eye examination and conjunctival impression cytology done but we do not have access to information on cytology examination |
| Other bias | Low risk | None known |
IU: international units
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dollimore 1997 | Intervention not targeted at measles participants. No outcome of interest to the review question was measured |
| Hussey 1990 | No outcome of interest to the review question |
Differences between protocol and review
The age of the participants was increased to < 18 years.
Contributions of authors
The final review was written by all review authors. Segun Bello searched the ongoing databases of trials. Segun Bello and Olabisi Oduwole conducted trial selection, data extraction and quality assessment under the guidance of Martin M Meremikwu. Olabisi Oduwole and Regina I Ejemot‐Nwadiaro edited the final draft of this review.
Sources of support
Internal sources
-
Institute of Tropical Diseases Research and Prevention, University of Calabar Teaching Hospital, Calabar, Nigeria.
Training IT support
-
Nigerian Branch, South African Cochrane Centre, Calabar, Nigeria.
Training IT support
External sources
-
Acute Respiratory Infections (ARI) Group editorial base, Australia.
Cochrane materials Information and technical support
Declarations of interest
Segun Bello: none known. Martin M Meremikwu: none known. Regina I Ejemot‐Nwadiaro; none known. Olabisi Oduwole: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Coutsoudis 1991 {published data only}
- Coutsoudis A, Broughton M, Coovadia HM. Vitamin A supplementation reduces measles morbidity in young African children: a randomized placebo‐controlled, double‐blind trial. American Journal of Clinical Nutrition 1991;54:890‐5. [DOI] [PubMed] [Google Scholar]
- Coutsoudis A, Coovadia HM, Broughton M, Salisbury RT, Elison I. Micronutrient utilization during measles treated with vitamin A or placebo. Internet Journal for Vitamin and Nutrition Research 1990;61(1991):199‐204. [PubMed] [Google Scholar]
Rosale 1996 {published data only (unpublished sought but not used)}
- Rosale FJ. Vitamin A supplementation of vitamin A deficient measles patients lowers the risk of measle‐related pneumonia in Zambian children. Journal of Nutrition 2002;132(12):3700‐3. [DOI] [PubMed] [Google Scholar]
- Rosale FJ, Kjolhede C, Goodman S. Efficacy of a single oral dose of 200,000 IU of oil‐soluble vitamin A in measles‐associated morbidity. American Journal of Epidemiology 1996;143(5):413‐22. [DOI] [PubMed] [Google Scholar]
- Rosales FJ, Kjolhede C. A single 210‐μmol oral dose of retinol does not enhance the immune response in children with measles. Journal of Nutrition 1994;124:1604‐14. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dollimore 1997 {published data only}
- Dollimore N, Cutts F, Binka FN, Ross DA, Morris SS, Smith PG. Measles incidence, case fatality, and delayed mortality in children with or without vitamin A supplementation in rural Ghana. American Journal of Epidemiology 1997;146(8):646‐53. [DOI] [PubMed] [Google Scholar]
Hussey 1990 {published data only}
- Hussey GD, Klein M. A randomized, controlled trial of vitamin A in children with severe measles. New England Journal of Medicine 1990;323(3):160‐4. [DOI] [PubMed] [Google Scholar]
Additional references
Al‐Kubaisy 2002
- Al‐Kubaisy W, Al‐Rubaiy MG, Nassief HA. Xerophthalmia among hospitalised Iraqi children. Eastern Mediterranean Health Journal 2002;8:485. [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 1990
- Chan M. Vitamin A and measles in the third world children. BMJ 1990;301:1230‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
CID 1993
- Committee on Infectious Diseases. Vitamin A treatment of measles. Pediatrics 1993;91:1014‐5. [PubMed] [Google Scholar]
Gilbert 2001
- Gilbert C, Allen F. Childhood blindness in the context of vision 2020 ‐ the right to sight. Bulletin of the World Health Organization 2001;79:3. [PMC free article] [PubMed] [Google Scholar]
Gilbert 2003
- Gilbert C. Blindness in children: half of it is avoidable and suitable cost effective interventions are available. BMJ 2003;327(7418):760‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- GRADEproGDT. Guideline Development Tool. www.guidelinedevelopment.org. Evidence Prime, Inc. Hamilton: McMaster University, 2015.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook‐cochrane‐org.
Nemer 2001
- Nemer L, Gelband H, Jha P. The evidence base for interventions to reduce malnutrition in children under‐5 and school‐age children in the low and middle income countries. Commission on Macroeconomics and Health (Working paper series) WHO, Geneva 2001;WGS 11:11‐2. [Google Scholar]
Potter 1997
- Potter AR. Reducing vitamin A deficiency. BMJ 1997;314(7077):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sommer 1990
- Sommer A. Xerophthalmia, keratomalacia and nutritional blindness. International Ophthalmology 1990;14(3):195‐9. [DOI] [PubMed] [Google Scholar]
Yang 2011
- Yang HM, Mao M, Wan C. Vitamin A for treating measles in children. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD001479.pub3] [DOI] [Google Scholar]
References to other published versions of this review
Bello 2009
- Bello S, Meremikwu MM, Ejemot RI. Routine vitamin A supplementation for the prevention of blindness due to measles infection in children. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007719] [DOI] [PubMed] [Google Scholar]
Bello 2011
- Bello S, Meremikwu MM, Ejemot‐Nwadiaro RI, Oduwole O. Routine vitamin A supplementation for the prevention of blindness due to measles infection in children. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD007719.pub2] [DOI] [PubMed] [Google Scholar]
Bello 2014
- Bello S, Meremikwu MM, Ejemot‐Nwadiaro RI, Oduwole O. Routine vitamin A supplementation for the prevention of blindness due to measles infection in children. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD007719.pub3] [DOI] [PubMed] [Google Scholar]


