Abstract
Background:
Although an efficacious dementia-risk score system, CAIDE was derived using midlife risk factors in a population with low educational attainment that does not reflect today’s US population, and requires laboratory biomarkers, which are not always available.
Objective:
Develop and validate a modified CAIDE (mCAIDE) system and test its ability to predict presence, severity, and etiology of cognitive impairment in older adults.
Methods:
Population consisted of 449 participants in dementia research (N=230; community sample; 67.9±10.0y old, 29.6% male, 13.7±4.1y education) or receiving dementia clinical services (N=219; clinical sample; 74.3±9.8y old, 50.2% male, 15.5±2.6y education). The mCAIDE, which includes self-reported and performance-based rather than blood-derived measures, was developed in the community sample and tested in the independent clinical sample. Validity against Framingham, Hachinski, and CAIDE risk scores was assessed.
Results:
Higher mCAIDE quartiles were associated with lower performance on global and domain-specific cognitive tests. Each one-point increase in mCAIDE increased the odds of MCI by up to 65%, those of AD by 69%, and those for non-AD dementia by >85%, with highest scores in cases with vascular etiologies. Being in the highest mCAIDE risk group improved ability to discriminate dementia from MCI and controls and MCI from controls, with a cut-off of ≥7 points offering the highest sensitivity, specificity, and positive and negative predictive values
Conclusion:
mCAIDE is a robust indicator of cognitive impairment in community-dwelling seniors, which can discriminate well between dementia severity including MCI vs controls. The mCAIDE may be a valuable tool for case ascertainment in research studies, helping flag primary care patients for cognitive testing, and identify those in need of lifestyle interventions for symptomatic control.
Keywords: dementia risk scores, CAIDE, cognitive impairment, case discrimination, dementia screening
Introduction
Alzheimer’s disease (AD), vascular contributions to cognitive impairment and dementia (VCID), and other dementias will exceed 115 million cases by 2050 [1]. To meet this challenge, clinicians and researchers seek to identify potentially modifiable risk factors (e.g., cardiovascular, metabolic) that could play significant roles in the development of AD and VCID. After age, cardiovascular risk factors including hypertension, hypercholesterolemia, and obesity represent the most important risk factors for AD and VCID, possibly working through vascular and inflammatory mechanisms and interaction with apolipoprotein E (ApoE) and amyloid deposition [2–12]. Although each risk factor increases dementia risk, they may also act in an additive or synergistic fashion.
To capture the role of risk factors, risk score paradigms have been developed including the Framingham Heart Study [13–15] and the Cardiovascular Risk Factors, Aging, and Dementia (CAIDE) [16]. The CAIDE was found to predict dementia risk 20y later in a dose-response manner and is associated with a greater cognitive decline [16, 17]. The CAIDE dementia risk score system can also take presence of ApoE ε4 allele into account, however inclusion of ApoE only increases predictability in the high-risk group and genetic testing is often not available in clinical or community-based samples. The CAIDE performs similarly to the Framingham vascular risk scores [17]. However, CAIDE was developed based on midlife (39–64yrs) risk factors and may not be representative of characteristics of today’s US population particularly regarding educational attainment. In the original CAIDE, 67% of the Finish cohort had less 10yrs of education while in 2019, 94% of the US population completed high school [18]. Since years of education contributed significantly to the computation of CAIDE, it is likely that the predictive value of the CAIDE in the US and other countries may be less than ideal. Furthermore, the CAIDE requires lab measurements that may not always be readily available at the initial evaluations in clinical practice or in community-based research conducted in the field. To address this unmet need for more easily measurable tools that better reflect the current US demographics, we developed a modified CAIDE (mCAIDE) score based on readily assessable and self-reported measurements that can be implemented with ease in dementia screening programs, epidemiological studies, or in clinical practice (i.e., Medicare Annual Wellness visits) and tested its effectiveness in two independent samples of older adults.
Materials and Methods
Study population
Two independent study populations were used in this cross-sectional analysis. The first population, used to develop the mCAIDE risk score (training dataset), consisted of community-dwelling older adults (community sample) residing in New York City who participated in a community-based dementia screening program. A detailed description of the cohort and study procedures has been published previously [19]. Briefly, subjects aged ≥40yrs were recruited and underwent brief cognitive testing, physical functional assessments, physical exams, and provided self-reported information on sociodemographic characteristics, personal and family medical history, and mood. Exclusion criteria included being <40 years old, speaking a language other than English or Spanish, or the presence of a medical (i.e., cancer), neurologic (e.g., multiple sclerosis, seizures) or psychiatric (i.e., schizophrenia) condition that would interfere with the clinical or cognitive assessment. When available, knowledgeable informants contributed information on participants’ cognitive function, behavior, mood, functionality, and general health. For the current study, participants with data on two cognitive tests (the Montreal Cognitive Assessment (MoCA) and animal naming) were included in the analysis (n=230). All subjects provided written informed consent and the study was approved by the NYU School of Medicine Institutional Review Board.
The second population (testing dataset) consisted of older adults attending a South Florida academic dementia center as patients or participants in brain health and dementia studies. For the current study, 219 had complete medical records allowing calculation of mCAIDE scores including 41 cognitively normal (controls); 52 mild cognitive impairment due to AD (MCI-AD); 14 MCI due to Dementia with Lewy Bodies (MCI-DLB); 37 AD; 56 DLB; and 19 VCID (clinical sample). These conditions share many common features including age at presentation, clinical-cognitive features, and comorbid pathologies. Individuals with missing variables that did not permit calculation of mCAIDE (N=163) and individuals with young-onset (i.e., frontotemporal degeneration (FTD)) or unusual presentations (e.g., normal pressure hydrocephalus, traumatic brain injury) (N=18) were excluded from further consideration. Most participants lived at home, either alone (13%), or with a spouse (76%) with very few being institutionalized (3%). Participation of an informant was required in both clinic patients and research participants and informants provided the individuals’ cognitive, physical, behavioral, and mental health utilized during the diagnostic process. A waiver of consent was obtained from clinic patients and research participants provided written informed consent. The study was approved by the University of Miami Institutional Review Board.
Outcome variables
Two cognitive measures were used in the training dataset: the MoCA [20] to measure global cognitive function (range: 0–30; lower total scores indicate impaired overall cognition) and the 1-minute animal naming test to measure verbal fluency. These tests were selected due to their ability to detect earlier cognitive impairment expected in this community dwelling population. Individuals were assigned as being cognitively impaired or cognitively unimpaired by a cognitive neurologist specializing in dementia evaluation and treatment (JEG) using published education-adjusted normative values. As the research goal of this part of the study was to screen for risk of cognitive impairment, formal diagnoses were not available for this sample.
The testing dataset included a battery of 11 performance-based cognitive tests modeled on the National Alzheimer’s Coordinating Center’s (NACC) Uniform Data Set [21]: MoCA; Noise Pareidolia Test, a measure of visual perception [22]; Numbers forward and backward tests as measures of attention and working memory [23]; Hopkins Verbal Learning Tests of immediate and delayed recall as measures of episodic memory [24]; Trail Making A and B, as measures of visual attention, processing speed, and executive function [25]; animal naming, a measure of verbal fluency; Multilingual Naming Test (MINT), a measure of semantic memory [26]; and the Number Symbol Coding Task, a measure of executive function [27]. Z-scores for individual cognitive test were derived based on the mean and SD of the entire sample and averaged to create an aggregate standardized composite Z-score which was used as an overall measure of cognitive function [17]. Diagnoses (Control; MCI; AD; DLB and VCID) were determined based on performance on neuropsychologic testing, report from caregivers, a neurological examination using standard criteria for MCI [28], AD [29], DLB [30], and VCID [31].
Predictor variable
The predictor of interest was based on CAIDE as proposed by Kivipelto and colleagues [16] but modified to reflect the age and educational distribution in our sample (training dataset) and to substitute more invasive lab tests or long survey-based measures with brief self-reported or performance-based measures that were more feasible in the context of our community-based dementia screening study or could be readily used in office practice. Measurements of blood total cholesterol were replaced by self-reported high cholesterol, which was based on the medical history questionnaire. The CAIDE physical activity level was replaced with a brief performance-based measure of physical function – the mini Physical Performance Testing (mPPT) [32] using a cutoff of ≥12 to capture fitness/physical activity. The modified CAIDE (mCAIDE) risk score includes sex (male vs. female), age (<65yrs vs 65–73yrs vs >73yrs), education (<12yrs vs 12–16yrs vs >16yrs), systolic blood pressure (≤140 vs >140 mmHg), body mass index (BMI) (>30 vs. ≤30 kg/m2), self-reported diagnosis of high cholesterol (yes vs no) and mPPT score (≥12 vs <12).
Data analysis
Using the training dataset, we compared individuals with and without cognitive impairment on variables included in the mCAIDE risk score and race and ethnicity (analysis 1). Depending on the nature of the variables the two groups were compared on, this was done by either Student’s T test (continuous) or chi square analysis (categorical). In this dataset, cognitive impairment was defined as being impaired on both the MoCA (<26 vs. ≥26) and Animal Naming (<13 vs. ≥13). We then modeled the likelihood of cognitive impairment based on the set of factors included in the original CAIDE (but modified to fit the demographic characteristics of our sample and to include non-invasive measures) with logistic regression analysis (analysis 2). Estimates were standardized so that the smallest estimate had a value of 1. We then applied this multiplication factor to all the other estimates and rounded them to the closest integer to obtain scores for each factor. The total mCAIDE risk score was computed by summing up these individual scores (range 0–14) and was used in analyses in its numeric form as well as an ordinal level variable (e.g., tertiles, quartiles). To further describe the relationship between mCAIDE and cognitive performance (i.e., MoCA and Animal Naming), generalized linear regression models (analysis 3) were run to compare higher quartiles of mCAIDE versus bottom quartile (quartiles 2–4 vs. quartile 1) on mean MoCA and Animal Naming scores. To avoid over adjustment by age, sex, and education, whose effect is accounted for in the mCAIDE risk score, no further adjustment was performed. Odds/risk for cognitive impairment associated with mCAIDE score was estimated with logistic regression (analysis 4) and the risk of cognitive impairment was compared between different levels of mCAIDE risk profile using the following formula: p = (e (β0 + β1*mCAIDE) / (1 + e (β0 + β1*mCAIDE)), where p is the probability of cognitive impairment, β0 is the intercept slope, and β1 is the slope for mCAIDE [16] (analysis 5).
We then replicated analyses 3–5 in the testing dataset. For analysis 3, we assessed the relationship between mCAIDE quartiles and each of the 11 performance-based cognitive tests available in this dataset and the average standardized composite Z-score across these individual tests. Multiple-comparison correction was applied using the Bonferroni approach (α/n≤0.004). For analysis 4, cognitive impairment was defined as being in the bottom quartile of the aggregate Z-score and similar models were run as in the training dataset. Using generalized linear regression analysis, we next validated the mCAIDE by assessing it against other vascular risk systems: the modified Hachinski risk score [33] and the Framingham vascular risk score [15] as well as against the original CAIDE score, all available in the testing dataset. The modified Hachinski risk score is calculated based on the weighted presence of 8 features: abrupt onset, stepwise deterioration, somatic complaints, emotional lability, history or presence of hypertension, history of stroke, focal neurologic symptoms, and focal neurologic signs [33] (range: 0–12) with higher scores indicating a higher risk of vascular dementia. The Framingham vascular risk score estimates 10-year risk for myocardial infarction or death based the following features: age, sex, smoking status, total cholesterol, HDL cholesterol, systolic blood pressure, and antihypertensive medications [15]. The original CAIDE risk score was calculated based on the factors proposed by Kivipelto and colleagues [16], with the exception of APOE Ɛ4 status and follow-up time deemed not feasible/ applicable given the cross-sectional, community-based nature of our study (training sample). These measures are further described in Table 1. We also tested the ability of mCAIDE to differentiate between levels of severity and causes of cognitive impairment with logistic regression and ROC analysis while comparing it against the modified Hachinski, the Framingham, and the original CAIDE risk scores. For analysis 5, we estimated the overall risk of cognitive impairment and case discrimination by mCAIDE risk profile with logistic regression. P values were derived by comparing tertiles of mCAIDE risk score. All analyses were performed using the SAS system version 9.4 (SAS Institute Inc., Cary, NC, USA).
Table 1.
Description of study-derived risk scores including components, source, and samples used to derive the scores.
| mCAIDE | CAIDE | Modified Hachinski | Framingham vascular risk | |
|---|---|---|---|---|
| Factors | ||||
| Age | ≥73 vs. 65–72 vs. <65y | >53 vs. 47–53 vs. <47y | - | Value |
| Education | <12 vs. 12–16 vs. >16y | 0–6 vs. 7–9 vs. ≥10y | - | - |
| Sex | Men vs. Women | Men vs. Women | - | Men vs. Women |
| Systolic blood pressure | ≥140 vs. <140 mm Hg | >140 vs ≤140 mm Hg | - | Value |
| Body mass index | >30 vs. ≤30 kg/m2 | >30 vs ≤30 kg/m2 | - | - |
| Total cholesterol | Yes vs. No | >6.5 vs ≤6.5 mmol/L | - | Value |
| Physical activity/fitness | Mini PPT < 12 vs. ≥12 | Inactive vs. active | - | - |
| Smoking status | - | - | - | Smoker vs. non-smoker |
| HDL cholesterol | - | - | - | Value |
| Antihypertensives use | - | - | - | Yes vs. no |
| Abrupt onset | - | - | Present vs. Absent | - |
| Stepwise deterioration | - | - | Present vs. Absent | - |
| Somatic complaints | - | - | Present vs. Absent | - |
| Emotional lability | - | - | Present vs. Absent | - |
| History or presence of hypertension | - | - | Present vs. Absent | - |
| History of stroke | - | - | Present vs. Absent | - |
| Focal neurologic symptoms | - | - | Present vs. Absent | - |
| Focal neurologic signs | - | - | Present vs. Absent | - |
| Source | ||||
| Self-report | X | X | - | X |
| Informant report | - | - | X | X |
| Neurological evaluation | - | - | X | - |
| Physical examination | X | X | - | - |
| Performance | X | - | - | - |
| Blood | - | X | - | X |
| Sample used to derive | Community | Clinical | Clinical | Clinical |
Results
Sample Characteristics
A total of 449 community-dwelling older adults were included in this cross-sectional analysis. A description of the total sample is presented in Table 2. The clinic sample was slightly older, more educated, more likely to be male, White, and to report hypercholesterolemia and scored higher on mCAIDE than the community sample. Cognitively impaired participants were older, performed poorer on physical performance testing, and scored higher on mCAIDE than the cognitively unimpaired participants in both cohorts. In addition, cognitively impaired participants had lower education (15.0±2.6 vs. 16.1±2.4, p=0.013), and higher systolic blood pressure (146.5±22.7 vs. 137.4±18.3, p=0.003 in the clinical sample. These differences are not unexpected based on the different nature of the two samples (community vs. clinical).
Table 2.
Sociodemographic, physical, functional, and overall health by cognitive impairment status by sample
| Total sample | Cognitively impaired (Mean±SD) | Cognitively not impaired (Mean±SD) | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Pop 1 (N=230) | Pop 2 (N=219) | Pop 1 (N=54) | Pop 2 (N=54) | Pop 1 (N=176) | Pop 2 (N=165) | Pop 1 | Pop 2 | |
| Age, years | 67.92±10.01 | 74.34±9.84 | 71.26±8.71 | 79.52±7.56 | 66.90±10.18 | 72.65±9.92 | 0.005 | <0.001 |
| Years of education | 13.73±4.10 | 15.53±2.58 | 12.96±4.31 | 14.95±2.62 | 13.96±4.02 | 16.12±2.41 | 0.117 | 0.013 |
| Men, n (%) | 68 (29.6%) | 110 (50.2%) | 16 (29.6%) | 28 (51.9%) | 52 (29.6%) | 82 (49.7%) | 0.991 | 0.783 |
| SBP, mmHg | 137.94±21.24 | 139.64±19.83 | 136.40±20.03 | 146.50±22.71 | 138.40±21.63 | 137.40±18.32 | 0.531 | 0.003 |
| BMI, kg/m 2 | 27.81±5.13 | 27.64±12.62 | 28.29±5.22 | 29.03±24.00 | 27.67±5.11 | 27.18±4.82 | 0.436 | 0.356 |
| Medical history: high cholesterol | 98 (42.6%) | 116 (53.0%) | 25 (37.9%) | 29 (53.7%) | 84 (43.5%) | 87 (52.7%) | 0.423 | 0.901 |
| Mini PPT total | 12.22±2.53 | 9.84±3.46 | 11.00±2.60 | 8.22±3.36 | 12.59±2.40 | 10.37±3.33 | <0.001 | <0.001 |
| Race, n (%) | 0.096 | 0.421 | ||||||
| Race, White, n (%) | 74 (32.5%) | 73 (89.0%) | 15 (23.4%) | 12 (100%) | 73 (37.4%) | 61 (87.1%) | ||
| Race, Black, n (%) | 49 (21.5%) | 5 (6.1%) | 17 (26.6%) | - | 36 (18.5%) | 5 (7.1%) | ||
| Race, Hispanic, n (%) | 105 (46.1%) | 4 (4.9%) | 32 (50.0%) | - | 86 (44.1%) | 4 (5.7%) | ||
| mCAIDE | 5.41±2.89 | 7.92±2.79 | 6.43±2.93 | 9.20±2.36 | 5.10±2.83 | 7.56±2.80 | 0.003 | <0.001 |
| MCI, n (%) | - | 66 (30.1%) | - | 1 (2.1%) | - | 65 (38.0%) | - | <0.001 |
| Dementia, n (%) | - | 112 (51.1%) | - | 47 (97.9%) | - | 65 (38.0) | - | |
Notes: Pop 1 = community sample. Cognitive impairment defined as being impaired on both MoCA and Animal Naming; Pop 2 = clinical sample. Cognitive impairment was defined as being in the bottom quartile of the global Z-score.
Derivation of mCAIDE
Table 3 presents the logistic regression model used to derive mCAIDE and the associated weights for each component. Applying the modeling approach described by the CAIDE authors [16], we obtained weights that were similar to those assigned in the CAIDE study for sex, systolic blood pressure, BMI, and hypercholesterolemia but were lower for age and education and higher for physical activity, likely reflecting demographic and measurement differences from the CAIDE samples and US population, as well as differences between cross-sectional versus longitudinal analytic approaches. Weights were obtained by multiplying each β coefficient by the constant 3.75, which is the amount needed to transform the smallest β coefficient into the integer number 1 (0.267*3.75=1), similarly to how the CAIDE weights were created. For example, for systolic blood pressure of ≥140 mmHg, the weight of 2 was obtained by multiplying the corresponding β coefficient by 3.75 and then rounding to the closest integer. The range for total risk scores is similar (0–14 for mCAIDE vs. 0–15 for CAIDE). This point system derived from the community sample was then applied to the clinical sample to assign a mCAIDE risk score to each individual.
Table 3.
Logistic regression modeling of mCAIDE effects on likelihood of cognitive impairment (N=230).
| Beta±SE | P value | OR (95%CI) | Score | ||
|---|---|---|---|---|---|
| Intercept | −1.362±0.345 | <0.001 | |||
| Sex | |||||
| Female | 0 | 0 | |||
| Male | 0.376±0.385 | 0.329 | 1.457 (0.685–3.098) | 1 | |
| Age | |||||
| <65 years | 0 | 0 | |||
| 65–72 years | 0.294±0.240 | 0.219 | 3.110 (1.230–7.862) | 1 | |
| ≥73 years | 0.546±0.261 | 0.037 | 4.001 (1.484–10.789) | 2 | |
| Education | |||||
| >16 years | 0 | 0 | |||
| 12–16 years | −0.267±0.224 | 0.234 | 0.939 (0.397–2.223) | 1 | |
| <12 years | 0.471±0.270 | 0.081 | 1.964 (0.723–5.338) | 2 | |
| Systolic blood pressure | |||||
| <140 mmHg | 0 | 0 | |||
| ≥140 mmHg | −0.425±0.358 | 0.236 | 0.654 (0.324–1.320) | 2 | |
| Body mass index | |||||
| ≤30 kg/m2 | 0 | 0 | |||
| >30 kg/m2 | 0.450±0.368 | 0.222 | 1.567 (0.762–3.225) | 2 | |
| Self-reported high cholesterol | |||||
| No | 0 | 0 | |||
| Yes | −0.586±0.360 | 0.103 | 0.556 (0.275–1.127) | 2 | |
| Mini PPT | |||||
| ≥12 (fit) | 0 | 0 | |||
| <12 (unfit) | 0.920±0.368 | 0.013 | 2.509 (1.219–5.163) | 3 | |
| Range | 0–14 |
Notes: Estimates were standardized so that the smallest estimate (0.267) had a value of 1 (multiplication factor of 3.7). Each estimate was then multiplied by this factor and rounded to the closest integer and that became the score for each category (e.g., for education of <12 years, estimate=0.471 × 3.7=1.743, was rounded up to a score of 2).
As measures of validation, we assessed correlations of mCAIDE with Framingham and Hachinski risk scores in the clinical sample. Both risk scores are based on a combination of cardiovascular risk factors. The mCAIDE was correlated with both Framingham (r=0.61, p<0.001) and Hachinski risk scores (r=0.34, p<0.001). The mCAIDE discriminated between cognitive impairment and no impairment in the community sample. The mCAIDE discriminated cases (MCI or dementia) and controls similarly to Framingham and better than Hachinski in the clinical sample (Figure 1). In addition, mCAIDE was correlated in the clinical sample with CAIDE (r=0.54, p<0.001) while its ability to predict cases was slightly better (ROCmCAIDE=0.78, 95%CI: 0.71–0.85 vs. ROCCAIDE=0.71, 95%CI: 0.61–0.80).
Figure 1.
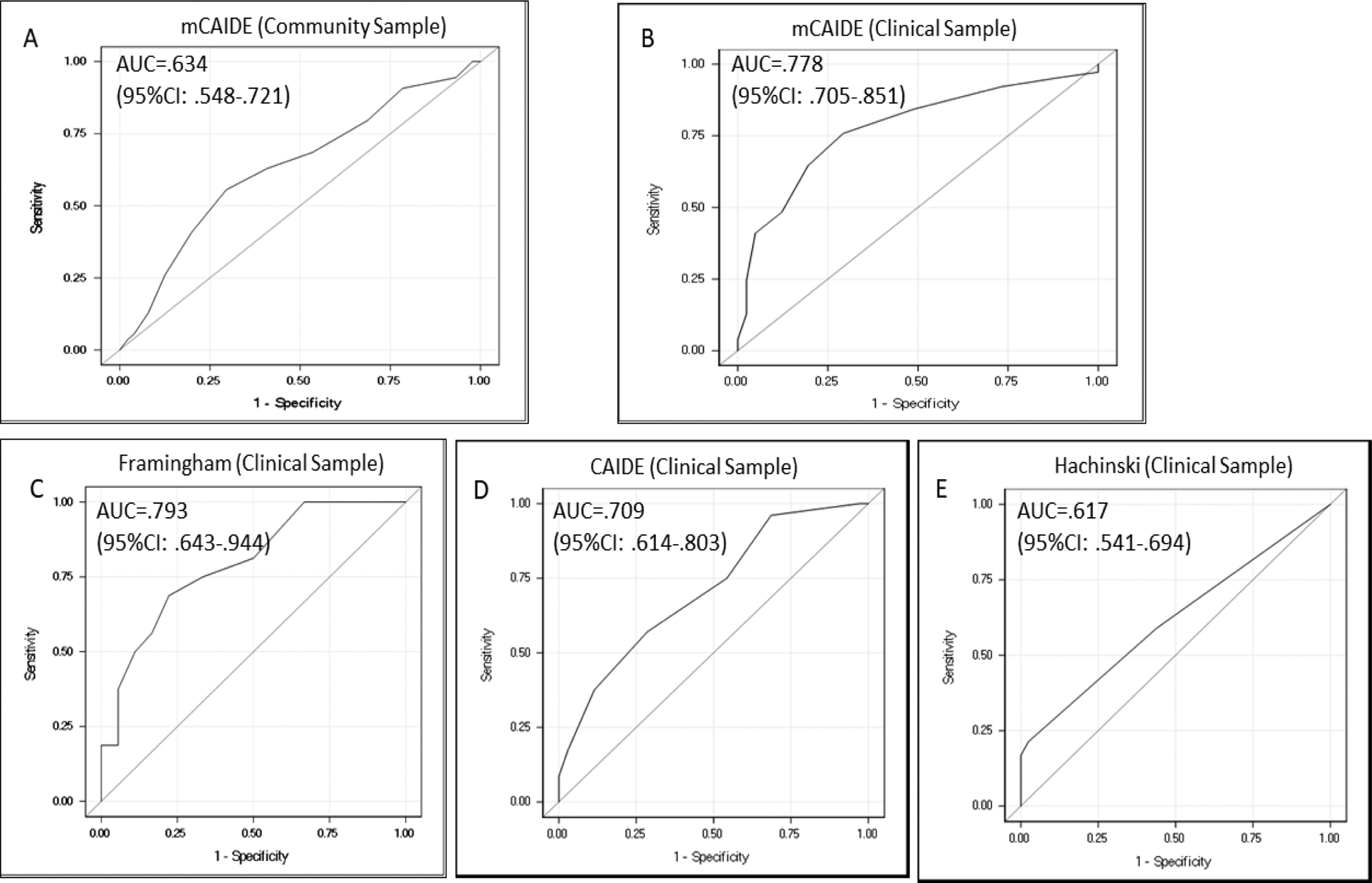
Case discrimination (cognitively impaired vs controls) by mCAIDE, CAIDE, Framingham, and Hachinski risk score.
Panel a – case discrimination by mCAIDE (community sample). Panel b – case discrimination by mCAIDE (clinical sample). Panel c– case discrimination by Framingham score (clinical sample). Panel d – case discrimination by CAIDE risk score (clinical sample). Panel e – case discrimination by Hachinski risk score (clinical sample). In the community sample, case discrimination was based on presence of cognitive impairment (versus no cognitive impairment) while in the clinical sample on presence of MCI or dementia (versus controls) as determined by diagnosis.
Relationship of mCAIDE to Cognitive Performance
Mean differences in performance on global and domain-specific cognitive tests across mCAIDE quartiles as well as coefficient estimates from generalized linear models are presented in Table 4. Being in higher mCAIDE quartiles was associated with lower global cognitive performance (i.e., MoCA) and verbal fluency in the community sample, although for MoCA only the top quartile group was different from the bottom quartile group while for Animal Naming a clearer stepwise relationship was observed (βQ2vsQ1=−2.7±1.1, p=0.017; βQ3vsQ1=−3.2±1.2, p=0.008; and βQ4vsQ1=−4.9±1.1, p<0.001). In the clinical sample, higher mCAIDE risk scores were associated with lower global cognition (i.e., MoCA), and poorer performance on tests of episodic and working memory, language, attention, processing speed, executive function, visual perception, and visual-spatial abilities after correction for multiple testing. When performance on all tests was aggregated, we found overall cognitive function to decrease with increasing mCAIDE score (βQ3vsQ1=−3.7±0.8, p<0.001 and βQ4vsQ1=−4.9±0.9, p<0.001). Overall cognitive performance was similar between mCAIDE risk scores of 6–8 and ≤5 (βQ2vsQ1=−0.7±0.8, p=0.396).
Table 4.
Cognitive performance by quartiles of the modified CAID dementia risk score
| Bottom quartile (Q1) | Q2 | Q3 | Top quartile (Q4) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean±SD | β±SE | P | Mean±SD | β±SE | P | Mean±SD | β±SE | P | Mean±SD | β±SE | P | |
| Community sample | ||||||||||||
| MoCA | 23.4±0.6 | ref | - | 23.5±0.7 | 0.2±0.9 | 0.855 | 22.6±0.7 | −0.7±0.8 | 0.455 | 20.1±0.7 | −3.3±0.9 | <0.001 |
| Animals | 19.9±0.8 | ref | - | 17.2±0.8 | −2.7±1.1 | 0.017 | 16.7±0.9 | −3.2±1.2 | 0.008 | 15.0±0.8 | −4.9±1.1 | <0.001 |
| Clinical sample | ||||||||||||
| MoCA | 24.0±0.8 | ref | - | 21.4±0.9 | −2.5±1.2 | 0.038 | 17.6±0.7 | −6.4±1.1 | <0.001 | 14.8±0.9 | −9.1±1.2 | <0.001 |
| Noise | 19.0±0.5 | ref | - | 18.1±0.5 | −0.9±0.7 | 0.207 | 17.1±0.4 | −1.9±0.6 | 0.002 | 16.6±0.5 | −2.4±0.7 | <0.001 |
| Num-F | 7.1±0.2 | ref | - | 7.0±0.2 | −0.1±0.3 | 0.715 | 6.5±0.2 | −0.6±0.3 | 0.025 | 5.8±0.2 | −1.3±0.3 | <0.001 |
| Num-B | 5.0±0.2 | ref | - | 4.9±0.2 | −0.0±0.3 | 0.884 | 4.3±0.2 | −0.7±0.3 | 0.010 | 4.0±0.2 | −1.0±0.3 | 0.002 |
| HVLT-R | 19.5±0.9 | ref | - | 17.1±0.9 | −2.4±1.3 | 0.075 | 12.8±0.7 | −6.7±1.2 | <0.001 | 11.9±1.0 | −7.6±1.3 | <0.001 |
| HVLT-D | 6.9±0.5 | ref | - | 5.2±0.5 | −1.7±0.7 | 0.018 | 2.9±0.4 | −4.0±0.6 | <0.001 | 2.6±0.5 | −4.3±0.7 | <0.001 |
| TMT A | 35.0±5.5 | ref | - | 52.2±5.6 | 17.1±7.9 | 0.030 | 63.7±4.4 | 28.7±7.0 | <0.001 | 76.7±5.8 | 41.6±8.0 | <0.001 |
| TMT B | 69.4±6.2 | ref | - | 102.4±6.7 | 33.1±9.1 | <0.001 | 138.7±5.4 | 69.3±8.2 | <0.001 | 145.5±7.8 | 76.1±9.9 | <0.001 |
| Animals | 18.3±0.8 | ref | - | 16.3±0.8 | −2.0±1.1 | 0.079 | 12.4±0.6 | −5.9±1.0 | <0.001 | 10.6±0.9 | −7.8±1.2 | <0.001 |
| MINT | 14.8±0.3 | ref | - | 14.3±0.3 | −0.5±0.5 | 0.303 | 13.1±0.3 | −1.8±0.4 | <0.001 | 12.9±0.4 | −1.9±0.5 | <0.001 |
| Num-Sym | 41.7±1.8 | ref | - | 32.7±1.9 | −8.9±2.7 | 0.001 | 27.2±1.6 | −14.4±2.4 | <0.001 | 22.5±2.1 | −19.2±2.8 | <0.001 |
| z-score | 4.4±0.6 | ref | - | 3.7±0.6 | −0.7±0.8 | 0.396 | 0.7±0.5 | −3.7±0.8 | <0.001 | −0.4±0.7 | −4.9±0.9 | <0.001 |
Legend: MoCA=MoCA global; Noise=Noise Pareidolia; Num-F=Numbers Forward; Num-B=Numbers Backward; HVLT-R=Hopkins Verbal Learning Test - Recall; HVLT-D=HVLT Delayed; TMA=Trail Making A; TMB=Trail Making B; Animals=Animal Naming; MINT=Multilingual Naming Test; Num-Sym=Number Symbol Coding Task.
Notes: Mean scores are presented in both samples. Unadjusted values. Bolded p values are significant at p<0.005 in the community sample and significant after adjustment for multiplicity of testing with the Bonferroni correction (p<0.004) in the clinical sample.
Each point increase in mCAIDE was associated with a 17% increase in the odds for cognitive impairment (OR=1.17; 95%CI: 1.05–1.30) in the community sample and with a 24% increase (OR=1.24; 95%CI: 1.10–1.41) in the clinical sample (Figure 2). The effect in the clinic sample was higher than in the community sample, possibly due to the more extensive cognitive testing and the ability to assign diagnoses.
Figure 2.
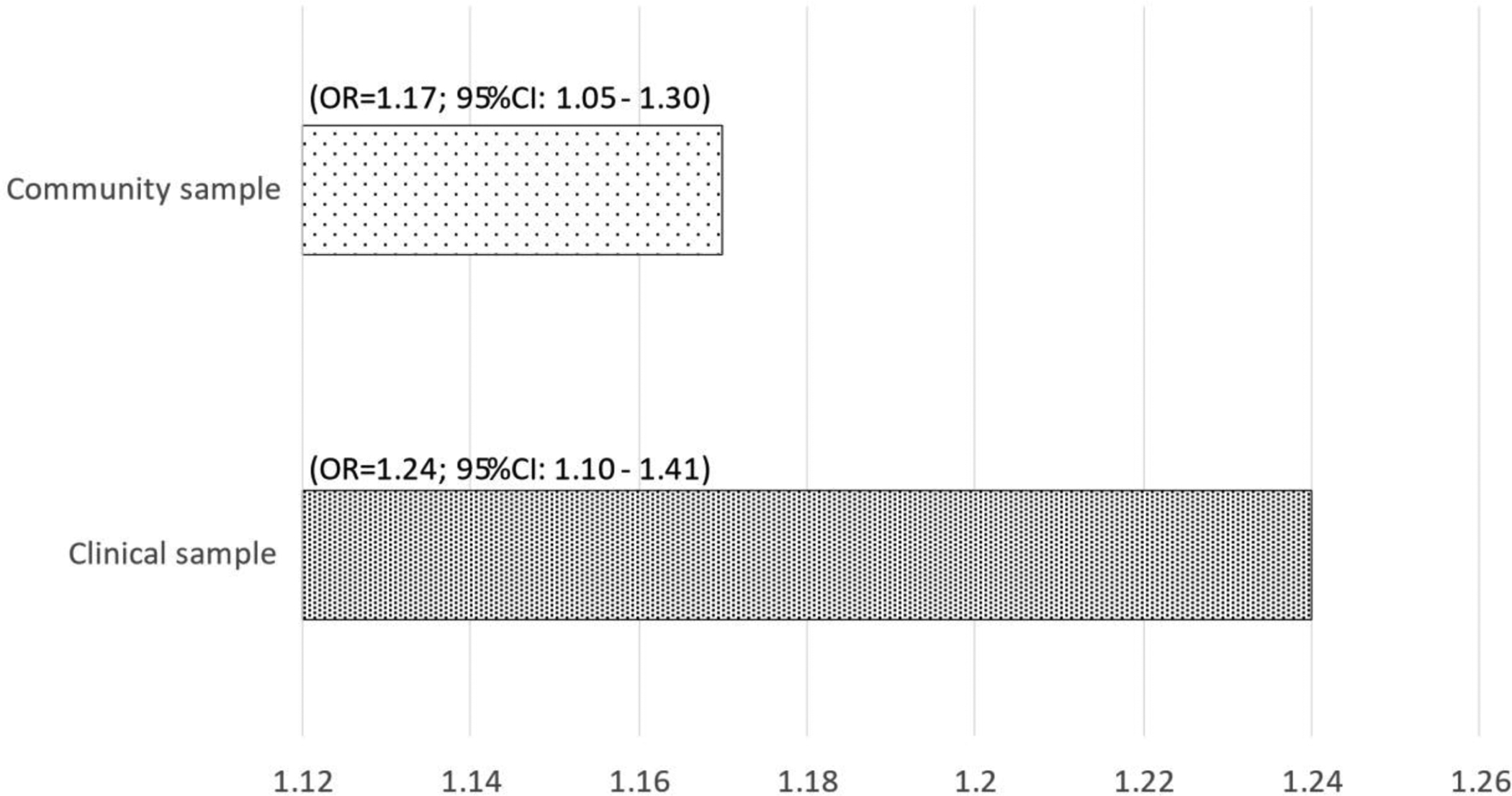
Odd ratios of cognitive impairment associated with one-unit increase in mCAIDE.
Notes: Cognitive impairment defined as being impaired on both MoCA and Animal Naming; Pop. 2=clinical sample. Cognitive impairment was defined as being in the bottom quartile of the global Z-score.
Relationship of mCAIDE to Diagnoses
Findings related to the ability of mCAIDE to discriminate between severity stage and dementia etiologies are presented in Table 5. Average mCAIDE score increased with severity of cognitive impairment. Odds for MCI-AD were increased by 22%, those for MCI-DLB by 65%; those of AD by 69%, while those for non-AD dementia (DLB or VCID) by more than 85% for each point increase in mCAIDE. We found mCAIDE to discriminate well between controls and dementia cases regardless of cause (AUCs above 0.80). The mCAIDE also showed good discrimination between MCI-DLB and controls (AUC=0.80, 95%CI: 0.68–0.92), as well as between VCID and both MCI etiologies (AUCMCI-AD=0.76; 95%CI: 0.64–0.88 and AUCMCI-DLB=0.74; 95%CI: 0.57–0.91). A cutoff score of ≥7 on the mCAIDE (67.1% of the sample) offered the best combination of sensitivity (0.76), specificity (0.71), and positive predictive value (PPV; 0.92). This cut-off score had a specificity of 0.71 for discriminating MCI-DLB, AD, LBD, and VCID from controls with sensitivities of 0.79, 0.87, 0.86, and 0.90, respectively. The best combinations of PPV and NPV were found for AD vs controls (0.73 and 0.85, respectively) and for LBD vs controls (0.80 and 0.78, respectively).
Table 5.
Ability of mCAIDE to discriminate between severity stages and etiologies of dementia (clinical sample).
| mCAIDE risk score | Cognitively normal (N=41) | MCI-AD (N=52) | MCI-DLB (N=14) | AD (N=37) | DLB (N=56) | VCID (N=19) |
|---|---|---|---|---|---|---|
| Average score | 5.8±0.4 (ref) |
7.2±0.3 (p=0.010) |
8.1±0.7 (p=0.004) |
8.7±0.4 (p<0.001) |
8.9±0.3 (p<0.001) |
10.1±0.6 (p<0.001) |
| OR | ref | 1.221 (1.031–1.445) |
1.645 (1.178–2.297) |
1.688 (1.324–2.151) |
1.851 (1.442–2.377) |
1.925 (1.402–2.643) |
| AUC | ref | 0.632 (0.520–0.744) |
0.800 (0.679–0.920) |
0.819 (0.718–0.920) |
0.845 (0.764–0.926) |
0.885 (0.785–0.985) |
| OR | - | ref | 1.113 (0.905–1.368) |
1.207 (1.030–1.414) |
1.269 (1.091–1.476) |
1.380 (1.128–1.689) |
| AUC | - | ref | 0.613 (0.476–0.751) |
0.657 (0.541–0.773) |
0.685 (0.581–0.789) |
0.759 (0.636–0.883) |
| OR | - | - | ref | 1.120 (0.859–1.460) |
1.189 (0.911–1.551) |
1.423 (1.027–1.973) |
| AUC | - | - | ref | 0.614 (0.444–0.784) |
0.631 (0.473–0.789) |
0.737 (0.565–0.909) |
| OR | - | - | - | ref | 1.048 (0.877–1.252) |
1.279 (0.991–1.652) |
| AUC | - | - | - | ref | 0.518 (0.399–0.638) |
0.636 (0.490–0.782) |
| OR | - | - | - | - | ref | 1.245 (0.979–1.584) |
| AUC | - | - | - | - | ref | 0.639 (0.480–0.798) |
Notes: OR of cognitive impairment are shown. For OR and AUC, 95%CI is shown in parentheses. Unadjusted values.
Finally, presented in Table 6 are examples of risk profiles based on mCAIDE scores of 0, 7, and 14 representing the lowest, the middle, and the highest mCAIDE scores, respectively. A low risk profile would be best described as a younger, educated, and fit female with BMI, blood pressure, and cholesterol within normal limits. In contrast, the highest risk might be seen in older, less educated, and less physically active males, with elevated blood pressure, BMI, and cholesterol levels. Several scenarios conferring an intermediate risk profile are presented separately for females and males. In both samples, a dose-response relationship was found for risk of cognitive impairment and mCAIDE risk profile. In addition, we found that mCAIDE’s ability to discriminate MCI cases from both non-AD dementia cases and controls may be best captured at the highest mCAIDE risk profile (p HighvsLow mCAIDE=0.003 for VCID vs. MCI-AD; p=0.037 for VCID vs MCI-DLB; p=0.035 for MCI-DLB vs controls. In addition, we found that mCAIDE’s ability to discriminate MCI cases from controls was improved at the highest mCAIDE risk level (p=0.018 for MCI-AD and p=0.035 for MCI-DLB).
Table 6.
Risk of cognitive impairment across mCAIDE risk profiles
| Low risk profile (score=0) | Intermediate risk profile (score=7) | High risk profile (score= 14) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Female | Female | Male | Male | |||||
| Age, years | <65 | 65–72 | 65–72 | ≥73 | |||||
| Education, years | ≥16 | 12–16 | 12–16 | <12 | |||||
| Systolic blood pressure, mmHg | <140 | Scenarios | Scenarios | ≥140 | |||||
| <140 | ≥140 | <140 | <140 | ≥140 | ≥140 | ||||
| Body-mass index, kg/m2 | <30 | <30 | <30 | ≥30 | ≥30 | ≥30 | <30 | ≥30 | |
| High cholesterol, self-reported | No | Yes | No | No | Yes | No | Yes | Yes | |
| Physical activity, mini PPT | Active | Inactive | Inactive | Inactive | Active | Active | Active | Inactive | |
| Risk of … | |||||||||
| Cognitive impairment | Community sample | 12.4% | 27.2% | 53.1% | |||||
| Clinical sample | 3.9% | 16.7% | 49.4% | ||||||
| DLB vs MCI-AD (clinical sample) | 13.6% (ref) | 52.4% (p=0.051) | 81.5% (p=0.043) | ||||||
| VCID vs. MCI-AD (clinical sample) | 2.2% (ref) | 17.5% (p=0.864) | 66.9% (p=0.003) | ||||||
| VCID vs. MCI-DLB (clinical sample) | 5.2% (ref) | 39.4% (p=0.252) | 88.5% (p=0.037) | ||||||
| MCI-AD vs controls (clinical sample) | 25.9% (ref) | 58.4% (p=0.274) | 85.0% (p=0.018) | ||||||
| MCI-DLB vs controls (clinical sample) | 1.1% (ref) | 26.6% (p=0.551) | 92.2% (p=0.035) | ||||||
Notes: Cognitive impairment defined as impairment in both MoCA and Animal Naming in the community sample and as being in the bottom quartile of the global Z-score in the clinical sample. Unadjusted values.
Discussion
This study was designed to develop a modified CAIDE that could easily be utilized in community-based dementia screening programs and the clinical setting especially when extensive neuropsychological testing is not possible and lab results may not be available at the time of the office visit. Using non-invasive clinical (e.g., systolic blood pressure), self-reported (e.g., history of high cholesterol), and brief performance-based measures (i.e., mPPT) of physical functionality, we found mCAIDE, which was developed in a community-based sample [19, 34] to be a robust indicator of cognitive impairment in community-dwelling otherwise healthy older adults, which discriminates well between dementia severity levels and may be helpful in differentiating MCI due to different etiologies from cognitively normal controls, particularly at the highest levels of mCAIDE. We found mCAIDE’s case discrimination power to be slightly better than that of CAIDE likely due to differences in the contribution and the measurement of individual components and their modification to better fit US populations of older adults. Moreover, our findings also point to an ability to discriminate between MCI and controls and between dementia and MCI that is captured best at the highest levels of mCAIDE risk. A cut-off of ≥7 was found to have the best discrimination value. These results suggest potential for mCAIDE as a tool that HCP could use to determine the need for a cognitive assessment of their patients, particularly of those testing at the higher end of the mCAIDE score, assessment that could be done in house or referred out to dementia clinics
While age and education had the greatest contribution to CAIDE, physical fitness/functionality was the strongest contributor to mCAIDE suggesting that individual contributions can shift due to differences in study population risk factors. This provides support for mCAIDE as an alternative tool to capture the significant contribution of physical functionality/fitness to cognitive impairment and dementia [35, 36].
While CAIDE was found to predict future risk of dementia among middle aged adults with relatively low levels of educational attainment [16], our goal here was to assess its ability to capture concurrent cognitive impairment in a diverse sample of community-dwelling older adults. Additionally, the education cut-offs of CAIDE were not applicable to most US populations. By keeping the same analytical approach to derive the CAIDE risk score while modifying the way some of the risk factors are measured (e.g., labs replaced with self-report), we found evidence that mCAIDE consistently captures overall cognitive impairment as well as impairment in cognitive domains commonly affected in dementia. This increases mCAIDE’s utility in primary care practices, epidemiological surveys, and community field work where labs may not be available. Although the cognitive outcomes being studied differed, mCAIDE was similarly ‘powered’ to detect dementia, with an AUC of at least 0.78, suggesting good discrimination. Our approach to derive a global cognitive score was similar to other studies reporting links between higher dementia risk as measured by the CAIDE and greater decline in executive function, language, and global cognitive scores [17]. The ability of mCAIDE to predict future decline and development of cognitive impairment needs to be evaluated using a longitudinal approach.
Our method of assessing the impact of a late-life risk index of cognitive impairment was similar to the Cardiovascular Health Cognition Study [37]. Although not specifically assessed as a modified CAIDE, selection of risk factors included in the dementia risk score index reflected the sample being studied and included self-reported and performance-based measures of health and functionality. In our study, we included self-reported history of high cholesterol and performance on mPPT, a proxy measure for physical functionality. To keep in line with the dichotomization scheme used in the CAIDE algorithm, we used the published cutoff mPPT score of ≥12 [32]. A total of 30% of our community sample was unfit (mPPT<12), which is comparable with reports from the CDC that >25% of older adults are physically inactive [38]. Test-retest and inter-rater reliability of the mPPT is high with reported Cronbach’s coefficients of 0.96 and 0.99, respectively (p<0.001) [39], and in early-stage AD, mPPT can help identify individuals with insufficient aerobic capacity to perform activities of daily living [40]. Our decision to use mPPT is supported by evidence of significant correlations between physical activity and physical functionality in older adults [41–44]. Self-reported measures of physical activity may be less robust in measuring commonly performed light or moderate activities [45]. Instead, performance-based measures such as the mPPT, which only take 5–10 minutes to complete, can easily be completed in clinical and community-based research settings and provide a valid measure of physical functionality.
There may be some reticence to using self-reported high cholesterol during clinic visits and prevalence studies due to its under-estimation of hypercholesterolemia [46]. However, under-reporting is lower among older adults [47] and may be even lower in dementia patients for whom informants such as family members are valuable sources of health information when making accurate diagnoses and planning treatment strategies [48, 49]. In our test sample, input from collateral sources was required on medical history, likely minimizing under-reporting of high cholesterol.
Age and education ranges for the three categories included in mCAIDE were modified to reflect their respective distribution in our training sample. While these were different from those used in the CAIDE, cutoff points were defined similarly using tertiles. Scores assigned to each risk group category were lower than those in the CAIDE suggesting a lower impact of age and education in predicting concurring cognitive impairment in our study. Reports from longitudinal studies suggesting that age differences in cognition are less robust than those derived from cross-sectional studies [50] would suggest against the former supposition. On the other hand, age is recognized as the strongest risk factor for dementia, therefore supporting a stronger effect on risk of developing dementia than on likelihood of concurrent cognitive impairment. For education, given that comparison groups are widely different between CAIDE and mCAIDE, it is expected that greater differences in dementia risk would exist between those with 0–6yrs vs ≥10yrs of education (i.e., CAIDE) than the differences in cognitive impairment between those with >16yrs vs <12yrs of education (mCAIDE). In addition, education was only marginally significant in our logistic regression model used to derive the mCAIDE, therefore explaining the lower scores assigned for education.
Cardiovascular risk factors are among the strongest predictors of cognitive impairment, particularly of AD and VCID etiology, and may be relevant in DLB which often has comorbid AD and vascular pathology. Observational studies suggest an important role for individual coronary risk factors such as hypertension [51] on cognitive decline in later life. Hypertension may increase the risk of cognitive impairment by 20% [2] and is associated with small vessel disease [3], white matter hyperintensities, and subsequent cognitive decline [4–7]. Hypercholesterolemia may play a role through atherosclerosis and interaction with ApoE and amyloid deposition [8, 9]. There is conflicting evidence on the role obesity may play, however obesity may influence dementia risk through vascular and inflammatory mechanisms [10–12] as well as diminishing physical functionality [52]. In addition, late middle-age cardiovascular risk indexes were found to predict cognitive decline in later life suggesting a cumulative effect of these risk factors on cognition [17]. We found mCAIDE to correlate well with other cardiovascular risk scores (e.g., Framingham and Hachinski) suggesting that its impact on cognitive impairment is due in a large part to cardiovascular risk factors, particularly lifestyle related factors such as physical fitness and functionality. There is consistent evidence for a protective effect of high physical activity against cognitive decline and dementia, particularly for AD [53], which may be due to reduced stress [54] and improved oxygen and nutrients to the brain via increased cerebrovascular integrity [55].
Other dementia risk scores based on vascular risk profiles in late life have been proposed as valuable tools to identify older adults at risk for AD. In a cohort of older adults free of cognitive impairment, the probability of AD increased with higher vascular risk scores either self-reported or lab-derived [56]. However, our interest was in assessing the value of the CAIDE dementia risk score modified to include late-life easily measurable risk factors as an indicator of cognitive impairment in cross-sectional large-scale studies with clinic visits done in the community where more invasive measures of cardiovascular risk may not be feasible. As these analyses are cross-sectional, we have not demonstrated that mCAIDE can predict the risk of future dementia, however, our findings support its use in dementia screening programs as a robust marker of cognitive impairment that deserves further evaluation.
Moreover, our findings suggest that mCAIDE could also assist clinicians during the first diagnostic visit to identify older adults at-risk for cognitive impairment. Despite the requirement for cognitive evaluation as part of the Medicare Annual Wellness Visit, a recent Alzheimer Association Special Report [57] suggests that Annual Wellness Visits are not a common practice and that cognitive evaluations are not often performed or documented. Although 90% of providers believed there were benefits to dementia screening, only 50% assessed cognition as part of their evaluation and only 40% were familiar with cognitive testing tools. Providers however collect many of the mCAIDE items as part of the electronic health record and their physical examinations. Addition of the mPPT to examine physical functionality also dovetails well with the providers evaluation of frailty and falls risk in older adults. Completing the mCAIDE first to identify at-risk individuals could provide a two-staged approach and led to increase cognitive testing which is infrequently documented in electronic health records, particularly in minority and economically disadvantaged populations [58].
These findings need to be interpreted with several potential limitations in mind. Other dementia risk scores that have been proposed were derived based on risk factors identified in the study sample. We took the approach of using the existing CAIDE scoring system, which was developed in a longitudinal study and applying it to a cross-sectional sample. This could explain the lack of significance for some of the risk factors included. Alternatively, the different measurement method may also help explain these results. For example, self-reported hypercholesterolemia may lead to under-reporting of hypercholesterolemia particularly in those older adults for whom reliable informants are not available. Input on patient medical history from a collateral source of information when available can help reduce the likelihood of underreporting. To further reduce this risk when informant input is not available, studies relying on self-reported measures of high cholesterol should also account for statin use, which would help reduce both underreporting and non-committal responses. However, our goal was to assess whether a CAIDE modified to include easily measurable risk factors would be effective at detecting presence, severity, and possibly cause of cognitive impairment in dementia screening research studies and potentially flagging patients in the primary care setting for a cognitive evaluation or be used in the field for data collection. Most cases in our clinical sample were MCI, AD, and DLB. We had fewer VCID cases and therefore the ability of the mCAIDE to discriminate this type of dementia was limited by sample size but ORs/AUCs suggest that mCAIDE should detect VCID. Studies with larger samples of VCID are needed to further confirm this. There were differences between the two datasets analyzed in this study in terms of the racial and ethnic distribution of participants, which suggest the need to assess the mCAIDE in other diverse samples. Last, since this study was community-based, we did not collect genetic (i.e., ApoE) markers or other neurodegenerative disease biomarkers such as measurements of amyloid or tau. This should be an area of future research.
Several strengths of the study should also be acknowledged. A two-step analytic approach was taken in this work increasing the confidence in our results and their applicability to different populations. The mCAIDE was developed in a community-based sample of older adults and then validated in a clinic-based sample. While the community sample was only assessed with two cognitive screens, the clinic-based sample underwent a comprehensive neuropsychological evaluation modeled after the UDS battery and diagnoses were determined based on published guidelines.
Findings from this study support the use of CAIDE modified to include easily measurable risk factors not just as a method to predict risk of dementia but also to determine likelihood of presence of cognitive impairment early in the disease process. With its reliance on sociodemographics, vital signs, self-reported medical history, and a very brief performance-based measure of fitness, the mCAIDE can easily and with little restrictions be applied to other community-based dementia screening programs, epidemiological studies, or clinical patient care. The mCAIDE may help ascertain cases including MCI in population-based research studies, offers a quick method to identify primary care patients in need of a cognitive evaluation, and suggests targets for lifestyle modification interventions to reduce symptoms and slow down dementia progression.
Disclosure statement
MIT, JH, and SC have no conflict of interest to report.
JEG serves on the Board of Directors for the Lewy Body Dementia Association, Lewy Body Resource Center, the South Florida Chapter of the Alzheimer Association and the South Palm Beach County YMCA, as a consultant for Biogen, performs evaluations in his clinical practice (15% effort) and bills for these procedures, is funded by NIH grants (R01 AG040211, R01 NS1010483, R01 AG057681, P30 AG059295, U01 NS100610, U54 AG063546, R01 AG056610, R01 AG054425, R01 AG056531, R01 AG040211, R01 NS1010483, R01 AG057681, P30 AG059295, U01 NS100610, U54 AG063546, R01 AG056610, R01 AG054425, and R01 AG056531) and by the Harry T. Mangurian Foundation, and the Leo and Anne Albert Charitable Trust, and receives research support as Director and Principal Investigator of the Lewy Body Dementia Research Center of Excellence from the Lewy Body Dementia Association.
Study funding:
Supported by grants from the NIH to JEG (R01 AG040211-A1, R01 AG069765-01, and R01 NS1010483) and the Harry T. Mangurian Foundation and the Leo and Anne Albert Charitable Trust.
Data Availability Statement
An anonymized dataset will be shared by request from any qualified investigator. For inquiries regarding the dataset, please contact Magdalena Tolea at mit38@med.miami.edu.
References
- 1.Prince M, Jackson J (2009) World Alzheimer Report 2009. Alzheimer’s Disease International, London. [Google Scholar]
- 2.Haring B, Wu C, Coker LH, Seth A, Snetselaar L, Manson JE, Rossouw JE, Wassertheil-Smoller S (2016), Hypertension, Dietary Sodium, and Cognitive Decline: Results From the Women’s Health Initiative Memory Study. Am J Hypertens 29, 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skoog I (1998) A review on blood pressure and ischaemic white matter lesions. Dement Geriatr Cogn Disord 9 Suppl 1, 13–19. [DOI] [PubMed] [Google Scholar]
- 4.Pantoni L, Poggesi A, Inzitari D (2007) The relation between white-matter lesions and cognition. Curr Opin Neuro 20, 390–397. [DOI] [PubMed] [Google Scholar]
- 5.Jokinen H, Gouw AA, Madureira S, Ylikoski R, van Straaten EC, van der Flier WM, Barkhof F, Scheltens P, Fazekas F, Schmidt R, Verdelho A, Pantoni L, Inzitari D, Erkinjuntti T (2011) Incident lacunes influence cognitive decline: the LADIS study. Neurology 76, 1872–1878. [DOI] [PubMed] [Google Scholar]
- 6.Prins ND, van Dijk EJ, Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM (2004) Cerebral white matter lesions and the risk of dementia. Arch Neurol 61, 1531–1534. [DOI] [PubMed] [Google Scholar]
- 7.Jokinen H, Kalska H, Ylikoski R, Madureira S, Verdelho A, van der Flier WM, Scheltens P, Barkhof F, Visser MC, Fazekas F, Schmidt R, O’Brien J, Waldemar G, Wallin A, Chabriat H, Pantoni L, Inzitari D, Erkinjuntti T (2009) Longitudinal cognitive decline in subcortical ischemic vascular disease--the LADIS Study. Cerebrovasc Dis 27, 384–391. [DOI] [PubMed] [Google Scholar]
- 8.Pappolla MA, Bryant-Thomas TK, Herbert D, Pacheco J, Fabra Garcia M, Manjon M, Girones X, Henry TL, Matsubara E, Zambon D, Wolozin B, Sano M, Cruz-Sanchez FF, Thal LJ, Petanceska SS, Refolo LM (2003) Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology 61, 199–205. [DOI] [PubMed] [Google Scholar]
- 9.Cosentino S, Scarmeas N, Helzner E, Glymour MM, Brandt J, Albert M, Blacker D, Stern Y (2008) APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology 70, 1842–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qizilbash N, Gregson J, Johnson ME, Pearce N, Douglas I, Wing K, Evans SJW, Pocock SJ (2015) BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol 3, 431–436. [DOI] [PubMed] [Google Scholar]
- 11.Chuang YF, An Y, Bilgel M, Wong DF, Troncoso JC, O’Brien RJ, Breitner JC, Ferrucci L, Resnick SM, Thanbisetty M (2016) Midlife adiposity predicts earlier onset of Alzheimer’s dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Mol Psychiatry 21, 910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh-Manoux A, Dugravot A, Shipley M, Brunner EJ, Elbaz A, Sabia S, Kivimaki M (2018) Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimers Dement 14, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97, 1837–1847. [DOI] [PubMed] [Google Scholar]
- 14.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB (1994) Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke 25, 40–43. [DOI] [PubMed] [Google Scholar]
- 15.D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB (2008) General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 117, 743–753. [DOI] [PubMed] [Google Scholar]
- 16.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J (2006) Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol 5, 735–741. [DOI] [PubMed] [Google Scholar]
- 17.Kaffashian S, Dugravot A, Elbaz A, Shipley MJ, Sabia S, Kivimaki M, Singh-Manoux A (2013) Predicting cognitive decline: a dementia risk score vs. the Framingham vascular risk scores. Neurology 80, 1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussar B, Zhang J, Hein S, Wang K, Roberts A, Cui J, Smith M, Mann FB, Barmer A, Dilig R (2020) The Condition of Education 2020 (NCES 2020–144), U.S. Department of Education, Washington, DC: National Center for Education Statistics. https://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2020144. Accessed on March 14, 2021. [Google Scholar]
- 19.Tolea MI, Galvin JE (2015) Sarcopenia and impairment in cognitive and physical performance. Clin Interv Aging 10, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- 21.Weintraub S, Besser L, Dodge HH, Teylan M, Ferris S, Goldstein FX, Giordani B, Kramer J, Loewenstein D, Marson D, Mungas D, Salmon D, Welsh-Bohmer K, Zhou XH, Shirk SD, Atri A, Kukull WA, Phelps C, Morris JC (2018) Version 3 of the Alzheimer Disease Centers’ Neuropsychological Test Battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord 32, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokoi K, Nishio Y, Uchiyama M, Shimomura T, Iizuka O, Mori E (2014) Hallucinators find meaning in noises: pareidolic illusions in dementia with Lewy bodies. Neuropsychologia 56, 245–254. [DOI] [PubMed] [Google Scholar]
- 23.Wechsler D (2008) Wechsler adult intelligence scale. Fourth edn. Pearson, San Antonio, TX. [Google Scholar]
- 24.Shapiro AM, Benedict RH, Schretlen D, Brandt J (1999) Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol 13, 348–358. [DOI] [PubMed] [Google Scholar]
- 25.Reitan RM (1958) Validity of the trail making test as an indication of organic brain damage. Perceptual and Motor Skills 8, 271–276. [Google Scholar]
- 26.Stasenko A, Jacobs DM, Salmon DP, Gollan TH (2019) The Multilingual Naming Test (MINT) as a Measure of Picture Naming Ability in Alzheimer’s Disease. J Int Neuropsychol Soc 25, 821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galvin JE, Tolea MI, Moore C, Chrisphonte S (2020) The Number Symbol Coding Task: A brief measure of executive function to detect dementia and cognitive impairment. PLoS One 15, e0242233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKhann GM, et al. , The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 2011. 7(3): p. 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee WMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skrobot OA, O’Brien J, Black S, Chen C, DeCarli C, Erkinjuntti T, Ford GA, Kalaria RN, Pantoni L, Pasquier F, Roman GC, Wallin A, Sachdev P, Skoog I, Viccs group, Ben-Shlomo Y, Passmore AP, Love S, Kehoe PG (2017) The Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement 13, 624–633. [DOI] [PubMed] [Google Scholar]
- 32.Wilkins CH, Roe CM, Morris JC (2010) A brief clinical tool to assess physical function: the mini-physical performance test. Arch Gerontol Geriatr 50, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A (1980) Pathological verification of ischemic score in differentiation of dementias. Ann Neurol 7, 486–488. [DOI] [PubMed] [Google Scholar]
- 34.Tolea MI, Chrisphonte S, Galvin JE (2018) Sarcopenic obesity and cognitive performance. Clin Interv Aging 13, 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tolea MI, Morris JC, Galvin JE (2015) Longitudinal associations between physical and cognitive performance among community-dwelling older adults. PLoS One 10, e0122878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolea MI, Galvin JE (2016) The Relationship Between Mobility Dysfunction Staging and Global Cognitive Performance. Alzheimer Dis Assoc Disord 30, 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes DE, Covinsky KE, Whitmer RA, Kuller LH, Lopez OL, Yaffe K (2009) Predicting risk of dementia in older adults: The late-life dementia risk index. Neurology 73, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson KB, Carlson SA, Gunn JP, Galuska DA, O’Connor A, Greenlund KJ, Fulton JE (2016) Physical Inactivity Among Adults Aged 50 Years and Older - United States, 2014. MMWR Morb Mortal Wkly Rep 65, 954–958. [DOI] [PubMed] [Google Scholar]
- 39.Chen DW, Chen JW, Xu W, Liu W, Du WJ, Li HJ (2012) A pilot study for reliability and validity of mini-Physical Performance Test for Chinese Male Elders. Int J Gerontol 6, 16–19. [Google Scholar]
- 40.Vidoni ED, Thomas GP, Honea RA, Loskutova N, Burns JM (2012) Evidence of altered corticomotor system connectivity in early-stage Alzheimer’s disease. J Neurol Phys Ther 36, 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savikangas T, Tirkkonen A, Alen M, Rantanen T, Fielding RA, Rantalainen T, Sipila S (2020) Associations of physical activity in detailed intensity ranges with body composition and physical function. a cross-sectional study among sedentary older adults. Eur Rev Aging Phys Act 17, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller DK, Wolinsky FD, Andresen EM, Malmstrom TK, Miller JP (2008) Adverse outcomes and correlates of change in the Short Physical Performance Battery over 36 months in the African American health project. J Gerontol A Biol Sci Med Sci 63, 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jantunen H, Wasenius N, Salonen MK, Perala MM, Osmond C, Kautiainen H, Simonen M, Pohjolainen P, Kajantie E, Rantanen T, von Bonsdorff MB, Eriksson JG (2017) Objectively measured physical activity and physical performance in old age. Age Ageing 46, 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de la Motte SJ, Welsh MM, Castle V, Burnett D, Gackstetter GD, Littman AJ, Boyko EJ, Hooper TI (2019) Comparing self-reported physical activity and sedentary time to objective fitness measures in a military cohort. J Sci Med Sport 22, 59–64. [DOI] [PubMed] [Google Scholar]
- 45.Jacobs DR Jr, Ainsworth BE, Hartman TJ, Leon AS (1993) A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc 25, 81–91. [DOI] [PubMed] [Google Scholar]
- 46.Natarajan S, Lipsitz SR, Nietert PJ (2002) Self-report of high cholesterol: determinants of validity in U.S. adults. Am J Prev Med 23, 13–21. [DOI] [PubMed] [Google Scholar]
- 47.Peterson KL, Jacobs JP, Allender S, Alston LV, Nichols M (2016) Characterising the extent of misreporting of high blood pressure, high cholesterol, and diabetes using the Australian Health Survey. BMC Public Health 16, 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galvin JE (2018) Using Informant and Performance Screening Methods to Detect Mild Cognitive Impairment and Dementia. Curr Geriatr Rep 7, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omole FS, Sow CM, Fresh E, Babalola D, Strothers H 3rd (2011) Interacting with patients’ family members during the office visit. Am Fam Physician 84, 780–784. [PubMed] [Google Scholar]
- 50.Salthouse TA (2014) Why are there different age relations in cross-sectional and longitudinal comparisons of cognitive functioning? Curr Dir Psychol Sci 23, 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grodstein F (2007) Cardiovascular risk factors and cognitive function. Alzheimers Dement 3, S16–22. [DOI] [PubMed] [Google Scholar]
- 52.Batsis JA, Zbehlik AJ, Barre LK, Bynum JP, Pidgeon D, Bartels SJ (2015) Impact of obesity on disability, function, and physical activity: data from the Osteoarthritis Initiative. Scand J Rheumatol 44, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guure CB, Ibrahim NA, Adam MB, Said SM (2017) Impact of Physical Activity on Cognitive Decline, Dementia, and Its Subtypes: Meta-Analysis of Prospective Studies. Biomed Res Int 2017, 9016924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dorsman KA, Weiner-Light S, Staffaroni AM, Brown JA, Wolf A, Cobigo Y, Walters S, Kramer JH, Casaletto KB (2020) Get Moving! Increases in Physical Activity Are Associated With Increasing Functional Connectivity Trajectories in Typically Aging Adults. Front Aging Neurosci 12, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cortes-Canteli M, Iadecola C (2020) Alzheimer’s Disease and Vascular Aging: JACC Focus Seminar. J Am Coll Cardiol 75, 942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reitz C, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA (2010) A summary risk score for the prediction of Alzheimer disease in elderly persons. Arch Neurol 67, 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alzheimer Association (2020) Alzheimer’s Association Report: 2020 Alzheimer’s Disease facts and figures. Alzheimer’s Dement 16, 391–460. [Google Scholar]
- 58.Maserejian N, Krzywy H, Eaton S, Galvin JE (2021) Cognitive measures lacking in EHR prior to dementia or Alzheimer’s disease diagnosis. Alzheimers Dement (online ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
An anonymized dataset will be shared by request from any qualified investigator. For inquiries regarding the dataset, please contact Magdalena Tolea at mit38@med.miami.edu.


