Abstract
Aggressive use of antiretroviral therapy has led to excellent viral suppression within the systemic circulation. However, despite these advances, HIV reservoirs still persist. The persistence of HIV within the brain can lead to the development of HIV-associated neurocognitive disorders (HAND). Although the causes of the development of neurocognitive disorders is likely multifactorial, the inability of antiretroviral therapy to achieve adequate concentrations within the brain is likely a major contributing factor. Information about antiretroviral drug exposure within the brain is limited. Clinically, drug concentrations within the cerebrospinal fluid (CSF) are used as markers for central nervous system (CNS) drug exposure. However, significant differences exist; CSF concentration is often a poor predictor of drug exposure within the brain. This article reviews the current information regarding antiretroviral exposure within the brain in humans as well as preclinical animals and discusses the impact of co-morbidities on antiretroviral efficacy within the brain. A more thorough understanding of antiretroviral penetration into the brain is an essential component to the development of better therapeutic strategies for neuroAIDS.
Keywords: NeuroAIDS, antiretroviral, brain, concentration, human immunodeficiency virus, HIV-associated neurocognitive dysfunction
Introduction
Within days of peripheral infection, HIV and SIV can enter the central nervous system (CNS) to establish the brain as a viral reservoir and results in immune activation and neuroinflammation [1,2]. Furthermore, even though aggressive use of combination antiretroviral (ARV) therapy (cART) typically results in effective viral suppression within the periphery, HIV infection persists within the CNS [3,4]. Viral persistence in the CNS is associated with adverse outcomes in about one half of infected individuals [5,6] and HIV-associated neurocognitive disorders (HAND) remain a significant health problem for individuals living with HIV [5,7]. NeuroAIDS is associated with blood-brain barrier (BBB) dysfunction [8,9], increased monocyte transmigration [10–16], altered cytokine expression [17], CNS inflammation, gliosis, neuronal injury [18,19] and increased neurobehavioral deficits in a region-dependent manner [20]. The neuropathology of neuroAIDS is complex and includes neurotoxicity from viral replication occurring prior to initiation of ARV therapy, inflammation/immune activation, and comorbidities (e.g. age, drug abuse, co-infections, potential toxicity of ARV drugs). There also is persistent low level viral replication in the CNS even with otherwise successful antiretroviral therapy [4,21]. Insufficient inhibition of viral replication can result in HIV egress from the CNS into peripheral organs, thereby re-seeding the virus in the periphery [22]. Poor antiretroviral penetration into the brain likely contributes to the local viral replication. This review focuses on antiretroviral penetration into the brain, summarizing what is currently known about antiretroviral concentrations achieved in brain tissue as well as the impact of comorbidities or other factors that may influence therapeutic drug concentrations in the brain. Although outside the focus of this review, there is a growing body of literature examining potential toxic effects of antiretrovirals in the brain [23], further supporting the need for careful evaluation of CNS drug penetration. Although the review primarily focuses on HIV, some parallels to other viruses, like SARS-CoV-2, may potentially be drawn. Although much is still unknown, there is evidence that at least one of the SARS-CoV-2 proteins crosses the blood-brain barrier (BBB) to enter the brain via adsorptive transcytosis and infection results in brain inflammation and immune activation [24,25].
Drug delivery to the brain
For drugs to enter the CNS, they must either traverse the blood-brain barrier (BBB) to enter the brain or traverse the blood-CSF barrier (BCSFB) to enter the CSF. The relatively poor penetration of drugs into the brain is due to the BBB preventing uptake of most therapeutic drugs [26,27]. The primary cellular components of the BBB are brain microvascular endothelial cells. These cells are connected via tight junctions and associated junctional proteins. The junction proteins are responsible for impeding paracellular diffusion of ions and polar solutes and for preventing macromolecular flux into the brain via the paracellular route [28]. In addition to brain microvascular endothelial cells, the neurovascular unit of the BBB is comprised of pericytes and astrocytes, which strongly influence the formation and maintenance of the BBB [29,30]. Drug penetration into the CSF, however, is a function of drug flux across the epithelial cells of the choroid plexus, which is the primary interface of the BCSFB and is the site of CSF production. The BCSFB is leakier than the BBB and is more permeable to macromolecules [28,31].
In addition to paracellular pathways in which drugs or other molecules traverse the BBB by passing between the cells of the BBB, substances also can traverse the BBB by passing through the cells (transcellularly). There are several different ways in which substances can traverse the BBB transcellularly. Small lipophilic molecules can move relatively freely through lipid membranes of the endothelial cells. Other molecules, such as HIV-1 proteins Tat and gp120, can traverse the BBB via adsorptive endocytosis [32,33]. Additionally, solute carriers, transporters of the SLC family, may be localized on luminal or abluminal membranes of brain endothelial cells. These proteins regulate entry of many substances including ions, nutrients, glucose, amino acids, nucleosides as well as xenobiotics [32,34].
There are also energy dependent, active efflux mechanisms that can either facilitate or impair molecular transcellular flux [30,32,34,35]. Efflux transporters, such as P-glycoprotein (P-gp; MDR1) or Breast Cancer Resistance Protein (BCRP; ABCG2) at the BBB are responsible for expulsion of substances from the brain back into the blood, thereby limiting overall flux of the compound into the brain [36,37]. They can limit overall brain penetration of substrate drugs and alterations in these proteins can have significant effects on tissue penetration of substrate drugs. For example, amprenavir, an HIV protease inhibitor and substrate for P-glycoprotein, brain penetration is significantly increased by chemical inhibition or genetic manipulation (knockout) of the efflux protein P-glycoprotein [38]. Other factors influencing a drug’s ability to cross the BBB include molecular weight, lipophilicity (log P), plasma protein binding, and ionization state [39,40].
Measuring ARV penetration into the brain and CSF
Cerebrospinal fluid (CSF) concentrations are the most commonly used clinical marker of drug exposure in the CNS. However, CSF is not brain tissue and significant differences in drug concentrations between CSF and the brain can exist. As discussed above, the BBB mediates drug distribution between the blood and brain, whereas the blood-CSF barrier (BCSFB) governs drug flux into the CSF. Furthermore, there are differences in the localization and expression of transport proteins between the BBB and the BCSFB [41]. Perhaps because of the differential expression of transport proteins between the BBB and BCSFB, for many drugs with high efflux activities (by transporters such as P-glycoprotein or BCRP), CSF tends to over-predict brain exposure [42–45]. For example, over-prediction of brain concentrations by CSF was demonstrated in a study with amprenavir; brain concentrations (3.33 ± 0.6 nCi/g) were ~7-times lower than in CSF (23.3 ± 11.2 nCi/g) in mice [38]. Furthermore, pharmacologic manipulation of P-glycoprotein, as would occur with drug-drug interactions, resulted in differential changes in fold concentration of drug between CSF and brain. CSF concentrations increased 3.3-fold following P-glycoprotein inhibition, but brain concentrations increased by 13-fold [38], illustrating the high likelihood of misinterpretation of brain concentration when trying to use CSF as a surrogate marker.
CNS Penetration Effectiveness (CPE) scores have been developed for HIV antiretroviral medications with the intent of providing a guide for prescribers to determine which regimens have the greatest likelihood of achieving therapeutic concentrations in the CNS [46]. CPE scores were developed based on the physiochemical, pharmacokinetic and pharmacodynamic properties of each antiretroviral drug in CSF from clinical studies. However, CPE does not convey any information about concentrations of the drugs within brain and CSF may not be representative of drug exposure at the sites within the CNS where pathogens reside, where drug action is most relevant. Perhaps even more importantly, drug penetration into CSF does not correlate with neurocognitive outcomes. While CPE scores have been positively correlated with viral suppression in the CSF [46], evidence is conflicting about the extent to which high CPE scores reduce incidence of HAND or neurologic toxicities. While some studies have found regimens with high CPE scores can improve neurocognitive performance [47,48], others have reported no relationship or even a deleterious relationship between highly penetrating regimens and neurocognitive outcomes [49–51]. Furthermore, an analysis of over 61,000 individuals found that individuals on antiretroviral regimens with high (>9) CPE scores were at higher risk of HIV dementia compared to individuals on a regimen with a low (<8) score (Hazard Ratio 1.74; 95% CI, 1.15, 2.65) [52]. Another study, a prospective randomized trial of 59 subjects, stopped early by the Data Safety Monitoring Board for futility, found no improvement in neurocognitive performance when subjects were assigned CNS-targeted therapy [53]. The lack of correlation between neurocognitive performance and CPE score, which is largely based on CSF drug exposures, may be due to differential drug penetration between CSF and anatomical compartments across the CNS. This highlights the need to better understand the penetration of antiretrovirals into the brain tissue itself.
Direct measurement of drug concentrations in human brain tissue is limited to tissues collected postmortem. Interspecies differences in metabolism and distribution can complicate extrapolation from animal models so validation in human tissues is essential [54]. Postmortem tissues, available from tissue banks and other donor programs, are a valuable resource to address this limitation and validate preclinical assessments. Comparisons of drug tissue penetration across species and/or across different doses can be made by expressing the data as tissue to plasma ratios (Fig. 1). While postmortem studies can provide critical insight into drug penetration into and distribution within the brain as well as the CSF, these studies are also inherently limited because of their retrospective nature and because of the inability to control for factors such as postmortem redistribution and variability in dosing history prior to death. Furthermore, although brain tissue from preclinical models can be perfused to flush the blood from within the vasculature, this is not possible with postmortem human brain tissue. Cerebral blood volume has been estimated to be 1 – 10 mLs per 100 g brain tissue [55–61], and, therefore, the lack of ability to flush the blood from the brain vasculature may result in an overestimate of parenchymal drug exposure compared to animal models in which clearing the vasculature by perfusion is much more feasible. Thus far, very few studies examining antiretroviral drug concentrations in postmortem human brain tissue have been conducted, so data is limited.
Fig 1.
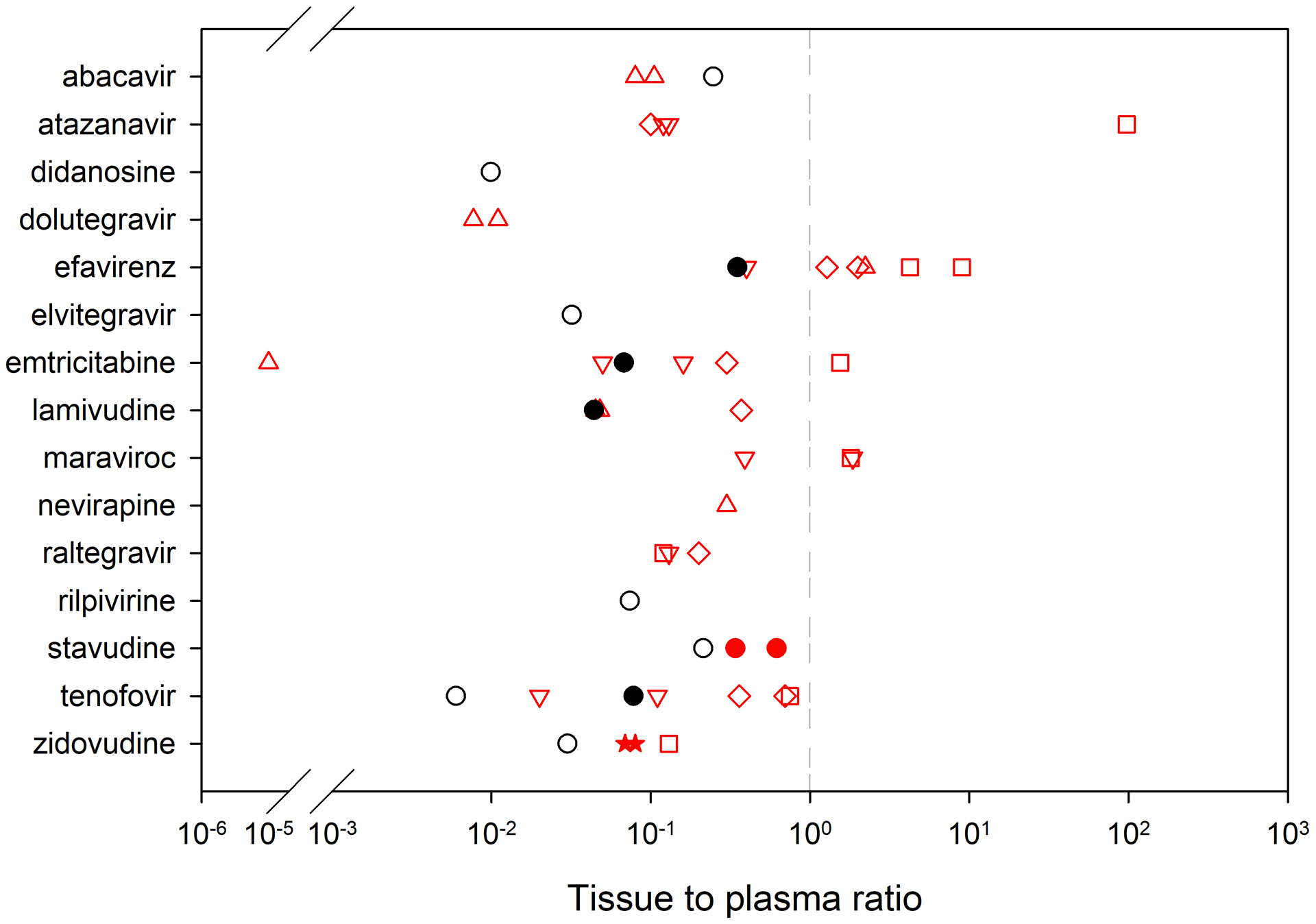
Brain tissue to plasma ratio across species. The dashed line represents a tissue to plasma ratio = 1 (where tissue concentrations = plasma concentrations). Up triangle = mice; Down triangle = humanized mice; Circle = rats; Stars = rabbits; Square = non-human primates; Diamonds = humans; Red = multiple dose; Black = single dose; Filled-in = Area Under the Curve (AUC); Open = concentration from a single time point. Data are from references [65, 66, 117–122, 67, 75–79, 81, 116].
Preclinical models allow for the design of more controlled studies, larger sample sizes, and more robust pharmacokinetic studies, although, depending on the assay methods used, this will require the use of multiple animals. One method that has been commonly used is liquid chromatography-mass spectrometry (LC-MS) based measurements of drug concentrations within tissue homogenate. Using this method, antiretroviral concentrations have been assessed in nonhuman primates (NHP), rats, and mice, including humanized mouse models (Table 1). Furthermore, additional information about the spatial distribution of drugs can be obtained using mass spectrometry imaging [62–65]. This method allows for simultaneous measurement of drug concentrations across multiple brain regions [65–67]. However, both of these analytical methods are an endpoint analyses, so assessment of drug distribution across different time points requires the use of multiple animals and can be time- and resource-intensive.
Table 1.
Antiretroviral penetration into the brain
| Concentrations | Dose | Species | Regional differences | Ref |
|---|---|---|---|---|
| Entry Inhibitors | ||||
| Maraviroc | ||||
| Plasma: 31.8 (18.0 – 80.6) ng/mL# CSF: 0.50 (0.50 – 4.96) ng/mL# Brain tissue: 57.5 (37.6 – 108) ng/g# Tissue to plasma ratio: 1.81 (0.76 – 2.14)# |
150 mg/d oral | NHP, n = 9; 6M/3F; 5 SHIV−, 4 SHIV+, | [66] | |
| Plasma: 5.67 (0.94 – 23.4) ng/mL# Brain tissue: 12.3 (4.44 – 19.7) ng/g# Tissue to plasma ratio: 1.86 (0.64 – 4.84)# |
62 mg/kg oral gavage | BLT mouse, n = 13; 6 HIV−, 7 HIV+ | [66] | |
| Plasma: 1.26 (0.50 – 5.73) ng/mL# Brain tissue: 0.22 (0.20 – 1.16) ng/g# Tissue to plasma ratio: 0.39 (0.25 – 0.44)# |
62 mg/kg oral gavage | Hu-HSC-RAG mouse, n = 12; 6 HIV−, 6 HIV+ | [66] | |
| Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI) | ||||
| Efavirenz | ||||
| Plasma: 3342 ng/mL (310, 4860)% Brain tissue: 1227–4854 ng/g† Tissue to plasma ratio 1.28 (1.08 – 1.79)^ |
600 mg daily | Human, postmortem, plasma, n = 11, brain tissue n = 4 | 24% variability across 13 brain compartments | [79] |
| Brain tissue: 35.9 (29.3–40.8) ng/mL# | 600 mg daily | Human, postmortem, n = 2 | No differences observed between WM, GM, CGM | [80] |
| Brain tissue: ~ 3000 ng/g& Tissue to plasma ratio: ~ 2& |
Human, postmortem, n = 4& | [81] | ||
| Plasma: 187 (71.6 – 339) ng/mL# CSF: 0.94 (0.50 – 1.89) ng/mL# Brain tissue: 775 (318 – 1453) ng/g# Tissue to plasma ratio: 4.26 (4.07 – 4.54)# |
200 mg/d oral gavage to steady state | NHP, n = 9; 5M/4F; 4 SHIV−, 5 SHIV+ | [66] | |
| Plasma: 2.5 (0.5 – 10.7) ng/mL# Brain tissue: 0.58 (0.27 – 19.8) ng/g# Tissue to plasma ratio 1.14 (0.25 – 1.90)# |
10 mg/kg/d oral gavage to steady state | Hu-HSC-RAG mouse, n = 12, 6 HIV− 6 HIV+ | [66] | |
| Plasma: ~ 7000 ng/mL& Brain tissue: ~ 2000 ng/mL& Tissue to plasma ratio: 2.23 |
50 mg/kg/day once daily as oral gavage x 3 d, harvest 4 h post-dose | Mice, n = 6, male | [123] | |
| Plasma: Cmax 3246.07 ± 480.54 ng/mL@ Brain tissue: Cmax 428.54 ± 33.34 ng/g@ Plasma AUC 0–24h: 4255.38 ng h/mL Brain tissue: AUC 0–24h: 1509.14 ng h/g |
50 mg/kg single dose, IP | Rat, n = 3, female | Widespread throughout; highly distributed in cerebral cortex, CC, basal forebrain, globus pallidus, HPF | [65] |
| Nevirapine | ||||
| Brain tissue 25.0 (25.0 – 73.2) ng/g#, ‡ | Human postmortem, n = 4 | No differences observed between WM, GM, CGM | [80] | |
| Plasma: Cmax 6320 ± 176 ng/mL* Brain tissue: Cmax 1923 ± 68.4 ng/mL* |
50 mg/kg single dose, IP | Rat, n = 3, female | Neocortex, thalamus, corticofugal pathways, hippocampus, CC and associated WM | [121] |
| Rilpivirine | ||||
| Plasma: 1767 ± 241 ng/mL* Brain tissue: 132 ± 10 ng/mg* Tissue to plasma ratio: 0.074 at Cmax |
50 mg/kg single dose, IP | Rat, n = 3, female | most in HPF, CC | [119] |
| Nucleoside Reverse Transcriptase Inhibitors (NRTI) | ||||
| Abacavir | ||||
| Brain tissue 25.0 (25.0–174.5)#, ‡ | Human postmortem, n = 3 | No differences observed between WM, GM, CGM | [80] | |
| Plasma (Cmax): 3369 ± 237 ng/mL* Brain tissue (Cmax): 831 ± 86.3 ng/mL * Tissue to plasma ratio: 0.247 |
50 mg/kg single dose, IP | Rat, n = 3, female | [67] | |
| Plasma: 2790.6 ± 607.0 ng/mL@ Striatum: 134.4 ± 26.1, Hippocampus: 129 ± 26.4 ng/g@ Tissue to plasma ratio: 0.105 (striatum)&, 0.08& (hippocampus) |
2.5 mg/day (123.5 mg/kg/day) continuous 5 d subcutaneous delivery via osmotic pump | Mouse, n = 9, females | Similar distribution in striatum and hippocampus | [100] |
| Didanosine | ||||
| Plasma (Cmax): 4389 ± 291 ng/mL* Brain tissue (Cmax): 43.37 ± 10.5 ng/mL * Tissue to plasma ratio: 0.0099 |
50 mg/kg single dose, IP | Rat, n = 3, female | [67] | |
| Emtricitabine | ||||
| Brain tissue: 111.4 (25.0–361.7) ng/mL#, ‡ | Human postmortem, n = 4 | No differences observed between WM, GM, CGM | [80] | |
| Brain tissue: ~30 ng/g& Tissue to plasma ratio: ~0.3& |
Human postmortem, n = 3& | [81] | ||
| Plasma: 13.5 (8.46 – 20.1) ng/mL# CSF: 3.97 (2.50 – 6.48)# Brain tissue: 26.3 (15.9 – 31.9)# Tissue to plasma ratio: 1.55 (1.20 – 2.43)# |
16 mg/kg subcutaneously daily | NHP, n = 18; 12M/6F; 10 SHIV−, 8 SHIV+ | [66] | |
| Plasma: ~500 ng/mL& Brain tissue: ~0.02 ng/mL& Tissue to plasma ratio: 0.00001 |
30 mg/kg/d, once daily as oral gavage for 3d, sacrificed 4 h post-dose | Mice, n = 6, male | [123] | |
| Plasma: Cmax 6470.33 ± 500.57 ng/mL@ Brain tissue: Cmax 591.57 ± 46.28 ng/g@ |
50 mg/kg single dose, IP | Rat, n = 3, female | thalamus, hypothalamus and cerebral cortex | [65] |
| Plasma: 46.6 (27.8 – 79.2) ng/mL# Brain tissue: 8.33 (4.29 – 14.2) ng/g# Tissue to plasma ratio: 0.16 (0.10 – 0.18)# |
240 mg/kg by oral gavage | BLT mouse, n = 13; 6 HIV−, 7 HIV+ | [66] | |
| Plasma 24.0 (19.8 – 68.4) ng/mL# Brain tissue: 1.78 (0.20 – 2.46) ng/g# Tissue to plasma ratio: 0.05 (0.01 – 0.12)# |
240 mg/kg by oral gavage | hu-HSC-RAG mouse, n = 12; 6 HIV−, 6 HIV+ | [66] | |
| Lamivudine | ||||
| Brain tissue 63.4 (25.0–271.8) ng/mL#, ‡ | Human postmortem, n = 4 | No differences observed between WM, GM, CGM | [80] | |
| Plasma: 1315 ng/mL (657, 4522)% CSF: 566 ng/mL (360, 1638)% Brain tissue: 328–784 ng/g† Tissue to plasma ratio: 0.37 (0.23–0.64)^ |
300 mg daily | Human postmortem, plasma and CSF n = 14; for tissue, n = 4 | 27% variability across 13 brain compartments | [79] |
| Plasma: 829.7 ± 320.9 ng/mL@ Striatum: 25.9 ± 3.5, Hippocampus: 27.3 ± 3.4 ng/g@ Tissue to plasma ratio: ~ 0.048 (striatum)&, ~0.045 (hippocampus)& |
1.2 mg/day (61.7 mg/kg/day) continuous 5 d subcutaneous delivery via osmotic pump | Mouse, n = 9, females | Similar distribution in striatum and hippocampus | [100] |
| Plasma Cmax: 25,846 ± 1961 ng/mL* Brain tissue Cmax: 272 ± 45.9 ng/mL* Tissue to plasma ratio: 0.011 (from Cmax), 0.044 (from AUC 0–24h), calculated from mean values |
50 mg/kg single dose, IP | Rat, n = 3, female | CC, globus pallidus, striatum, neocortex | [120] |
| Stavudine | ||||
| Drug concentrations below limit of quantification | Human postmortem, n = 2 | [80] | ||
| Plasma (Cmax): 6064 ± 202 ng/mL* Brain tissue (Cmax): 1300 ±121 ng/mL * Tissue to plasma ratio: 0.214 |
50 mg/kg single dose, IP | Rat, n = 3, female | [67] | |
| Brain (dialysate): 290 ± 52 ng/mL * Plasma: 850 ± 90 ng/mL* Brain to plasma ratio: 0.34 ± 0.04 |
1.75 mg/kg/hr, continuous infusion | Rat, n = 7, male | [78] | |
| Caudate putamen (dialysate): 1.1 ± 0.13 ng/mL* Cortex (dialysate): 1.4 ± 0.82 ng/mL* Brain to plasma ratio: 0.62 ± 0.17 (putamen), 0.62 ± 0.11 |
5 mg/kg, i.v. bolus | Rat, n = 4 (putamen), n = 7 (cortex), male | No differences between putamen and cortex | [75] |
| Tenofovir | ||||
| Plasma: 1024 ng/mL (247, 2683)% CSF: 138 ng/mL (77–675) ng/mL% Brain tissue: 328–784 ng/g† Tissue to plasma ratio: 0.36 (0.14–1.24)^ |
300 mg daily | Human postmortem, plasma and CSF n = 11; for tissue, n = 4 | 49% variability across 13 brain compartments | [79] |
| Brain tissue 147.9 (80.6–291.8) ng/g# | Human postmortem, n = 7 | No differences observed between WM, GM, CGM | [80] | |
| Brain tissue: ~ 80 ng/g& Tissue to plasma ratio: ~ 0.9& |
Human postmortem, sample size not reported | [81] | ||
| Plasma: 60.3 (47.8 – 84.4) ng/mL$ CSF: 2.04 (1.40–2.82) ng/mL$ Brain tissue: 51.3 (34.9 – 57.5) ng/g$ Tissue to plasma ratio: 0.75 (0.59 – 0.92)$ |
30 mg/kg daily subcutaneously to steady state | NHP, n = 18; 12M/6F; 10 SHIV−, 8 SHIV+ | [66] | |
| Plasma: Cmax 5651.72 ± 672.87 ng/mL@ Brain tissue: Cmax 51.06 ± 29.23 ng/g@ Tissue to plasma ratio: 0.009 |
50 mg/kg single dose, IP | Rat, n = 3, female | Striatum, corticospinal tracts, globus pallidus and cerebral cortex | [65] |
| Plasma: 125 (89.5 – 241) ng/mL$ Brain tissue: 14.3 (11.9 – 47.9) ng/g$ Tissue to plasma ratio: 0.11 (0.07 – 0.14)$ |
208 mg/kg/day oral gavage to steady state |
BLT mouse, n = 13; 6 HIV−, 7 HIV+ | [66] | |
| Plasma: 150 (77.1 – 368) ng/mL$ Brain tissue: 4.49 (0.62 – 18.8)$ Tissue to plasma ratio: 0.02 (0.01 – 0.11)$ |
208 mg/kg/day oral gavage to steady state |
Hu-HSC-RAG mouse, n = 12; 6 HIV−, 6 HIV+ | [66] | |
| Plasma Cmax: 9784.2 ± 4722.7 ng/mL* Brain tissue Cmax: 54.5 ± 7.1 ng/g* Tissue to plasma ratio: 0.006 |
50 mg/kg single IP injection, given as TAF | Rat, n = 3, female | poor BBB penetration; but widely distributed | [116] |
| Zidovudine | ||||
| Plasma Cmax: 55,976 ± 5128 ng/mL* Brain tissue Cmax: 692 ± 74.11 ng/mL* Tissue to plasma ratio 0.012 (from Cmax), 0.032 (from AUC 0–24h), calculated from mean values |
50 mg/kg single dose, IP | Rat, n = 3, female | CC, globus pallidus, striatum, neocortex | [120] |
| Blood: 112 ± 63.8 μM* Brain (dialysate): 13.8 ± 10.4 μM* Brain (dialysate) to blood ratio: 0.13 ± 0.06* |
5 mg/kg, i.v. loading dose, then 15 mg/kg/h continuous infusion | NHP, n = 5, male | [117] | |
| Thalamus (dialysate) to plasma ratio (AUC) 0.052 ± 0.027 0.067 ± 0.030 0.064 ± 0.013 0.092 ± 0.039 |
IV bolus 5 mg/kg 10 mg/kg 20 mg/kg 30 mg/kg |
Rabbit, n = 3 per dosing regimen, male | [76] | |
| Thalamus (dialysate) to plasma ratio (AUC), 0.08 ± 0.019 | 10 mg/kg, i.v. bolus | Rabbit, n = 6, male | [77] | |
| Integrase Inhibitors | ||||
| Dolutegravir | ||||
| Plasma: 433.2 ± 80.9 ng/mL@ Striatum: 4.6 ± 1.1, Hippocampus: 4.8 ± 1.1 ng/g@ Tissue to plasma ratio: ~0.011 (striatum)&, ~0.011 (hippocampus)& |
0.2 mg/day (10.3 mg/kg/day) continuous 5 d subcutaneous delivery via osmotic pump | Mouse, n = 9, females | Similar distribution in striatum and hippocampus | [100] |
| Plasma: ~50,000 ng/mL& Brain: ~400 ng/mL& Tissue to plasma ratio: 0.0077 |
10 mg/kg/day, once daily as oral gavage for 3 d, sacrificed 4 h post-dose | Mouse, n = 6, male | [123] | |
| Elvitegravir | ||||
| Plasma Cmax: 30760.9 ± 3351.2 ng/mL* Brain tissue Cmax: 976.5 ± 105.2 ng/g* Tissue to plasma ratio: 0.032 |
50 mg/kg single dose, IP | Rat, n = 3, female | [116] | |
| Raltegravir | ||||
| Plasma: 157 (78.6 – 297) ng/mL# CSF: 0.50 (0.50 – 1.05)# Brain tissue: 21.8 (14.2 – 67.1) ng/g# Tissue to plasma ratio: 0.12 (0.05 – 0.21)# |
200 mg/day oral | NHP, n = 9, 6M/3F; 5 SHIV−, 4 SHIV+ | [66] | |
| Brain tissue: ~80 ng/g& Tissue to plasma ratio: ~0.2& |
Human, postmortem, sample size not reported | [81] | ||
| Plasma: 21.9 (10.5 – 32.2) ng/mL# Brain tissue: 2.29 (1.53 – 3.17) ng/g# Tissue to plasma ratio: 0.13 (0.07 – 0.17)# |
56 mg/kg oral gavage | BLT mouse, n = 13; 6 HIV−, 7 HIV+ | [66] | |
| Brain tissue: 0.22 (0.19 – 0.26) ng/g# Tissue to plasma ratio: 0.13 (0.05 – 0.41)# |
56 mg/kg oral gavage | Hu-HSC-RAG mouse, n = 12; 6 HIV−, 6 HIV+ | [66] | |
| Protease Inhibitors | ||||
| Atazanavir | ||||
| Plasma: 2.40 (0.50 – 106) ng/mL# CSF: 0.50 (0.50 – 4.96) ng/mL# Brain tissue: 84.1 (47.2 – 269) ng/g# Tissue to plasma ratio: 97.4 (0.41–166)# |
270 mg/kg oral | NHP, n = 9; 6M/3F; 5 SHIV−, 4 SHIV+ | [66] | |
| Brain tissue: ~400 ng/g& Tissue to plasma ratio: 0.1& |
Human, postmortem, n = 1& | [81] | ||
| Plasma: 9.80 (8.64–14.4) ng/mL# Brain tissue: 2.10 (0.71–10.1) ng/g# Tissue to plasma ratio: 0.12 (0.04–0.28)# |
140 mg/kg oral gavage | BLT mouse, n = 13, 6 HIV−, 7 HIV+ | [66] | |
| Plasma: 9.91 (2.50 – 18.7) ng/mL# Brain tissue: 0.98 (0.49 – 1.54) ng/g# Tissue to plasma ratio: 0.13 (0.06 – 0.76)# |
140 mg/kg oral gavage | Hu-HSC-RAG mouse, n = 12, 6 HIV−, 6 HIV+ | [66] | |
| Nelfinavir | ||||
| Brain tissue: 54.7 (25.0–168.2) ng/g#, ‡ | Human postmortem, n = 4 | No differences observed between WM, GM, CGM | [80] | |
| Lopinavir | ||||
| Brain tissue, White Matter: 250.5 (25.0, 956.23)#, ‡ | Human postmortem, n = 4 | High conc in WM, not detected in GM or CGM | [80] | |
| Saquinavir | ||||
| Brain tissue: 208.3 (116.5–360.5) ng/g# | Human postmortem, n = 2 | No differences observed between WM, GM, CGM” | [80] | |
Mean ± SD,
Median (IQR),
Mean ± SEM,
Mean (range),
Median (25th, 75th percentile),
Geometric Mean Ratio (95% Confidence Interval),
Range of median values across multiple brain regions
values approximated from publication figure
Lower Limit of Quantification (LLOQ) for this assay was 25 ng/g
Abbreviations: AUC, Area Under the Curve; BLT, bone liver thymus mouse; CC, corpus callosum; CGM, cortical gray matter; CSF, cerebrospinal fluid; GM, gray matter; hippocampal formation (HPF), Hu-HSC-RAG, human stem cell hematopoietic/Rag2-; IP, intraperitoneal: IQR, interquartile range; NHP, nonhuman primate; SHIV, Simian-Human Immunodeficiency Virus; TAF, tenofovir alafenamide; WM, white matter
Positron Emission Tomography (PET) can also be used as a non-invasive way to estimate brain exposure and distribution of compounds [68]. Most PET studies administer small doses (< 5% occupancy of the protein target) of the radioligand to avoid pharmacologic activity [69]. The PET imaging data is used to characterize the drug’s concentrations and distribution within tissue compartments through pharmacokinetic modeling [68]. The major limitation of PET studies is that parent radioactive drug versus metabolite cannot be distinguished and therefore additional characterization of metabolism of the drug is necessary for appropriate interpretation of the data [68].
Brain microdialysis is an in vivo technique which allows for the quantification of analytes within the extracellular fluid (ECF) of the brain [70]. With microdialysis, a probe is surgically implanted into the brain region of interest and unbound concentrations can be measured dynamically. A key advantage to microdialysis is that repeated sampling can be performed in the same animal, which saves animals and also allows for assessment of both intra- and inter-animal variability. One challenge with microdialysis is that highly lipophilic drugs can adsorb to the microdialysis tubing and probes and, if not recognized and addressed, can lead to incorrect interpretation of quantitative information [70–73]. Microdialysis is commonly used to measure drug concentrations in animal models, although under rare circumstances it can be used clinically to measure brain concentrations of drugs during certain surgeries, like brain resections for refractory epilepsy, tumor resections, or for patients in a neurocritical care unit [74]. In regards to analysis of antiretroviral therapy, the brain penetration of some of the early HIV drugs like zidovudine and stavudine were studied using microdialysis in the 1990s and early 2000s [75–78]. However, microdialysis has not been widely used for assessment of antiretroviral brain concentrations in more recent years, perhaps because of the development of imaging technology, as discussed above.
Variable antiretroviral concentrations in brain of human and preclinical species.
A number of studies have directly measured antiretroviral concentrations in brain from both human and preclinical species. These data are summarized in Table 1.
As discussed above, quantification of antiretroviral exposure in tissue collected postmortem is one approach to directly assess drug exposure in the brain in humans. In 2019, Nicol et al. measured postmortem antiretroviral concentrations in brain tissue, CSF and plasma. The investigators were able to measure antiretroviral brain concentrations from four individuals. In examining brain penetration, expressed as tissue to plasma ratios, tenofovir penetration was 0.36 (0.14–1.24; Geometric Mean Ratio (GMR) (95% Confidence Interval)), lamivudine was 0.27 (0.01 – 1.04), and efavirenz was 1.28 (1.08–1.79). [79]. Additionally, the investigators measured drug concentrations across 13 distinct brain regions (frontal lobe, corpus callosum (CC), parietal lobe, occipital lobe, globus pallidus, hippocampus, cerebellum, temporal lobe, midbrain, pons, medulla oblongata, meninges, and the choroid plexus) to examine if there were regional differences in drug concentrations. However, because of the small sample size, no definitive conclusions could be made regarding regional differences in drug concentrations.
Another study has described antiretroviral brain concentrations across three brain regions - white matter, globus pallidus, and cortical gray matter - from a total of 11 postmortem donors of the California NeuroAIDS Tissue Network [80]. No significant differences in drug concentrations were reported between the brain regions. The largest observed difference was that lopinavir was more concentrated in the white matter than the other regions, although this did not reach statistical significance. The investigators had access to recent neurocognitive exams and plasma viral loads. They reported that higher antiretroviral concentrations in brain tissue (when pooling all drugs together) were associated with lower viral RNA in plasma. However, higher concentrations were also associated with poorer performance on neurocognitive exams, perhaps suggesting potential toxicity of the antiretroviral drugs. However, because of the small sample size, the investigators pooled all data together, which precludes the ability to explore which specific drugs might be driving poorer neurocognition. Furthermore, information regarding recent antiretroviral dosing (e.g., time of last dose or recent adherence patterns) was unknown. Lastly, although their cohort included a total of 11 individuals, the number of people on any individual antiretroviral was four or fewer, with the exception of tenofovir (n = 7) [80].
Devanathan et al. (2020) also have reported antiretroviral concentrations obtained from human postmortem tissues samples; and compared them to previously published concentration data in non-human primates and humanized mouse models [81]. Tissues were obtained from the National NeuroAIDS Tissue Consortium, National Neurological AIDS Bank and the National Research Disease Interchange. Frontal cortex, cerebellum, basal ganglia, and parietal cortex tissues were assessed but only median values across all brain regions were reported so localization of drug could not be determined. Sample sizes were not reported, but from the published figure, it appears that the number of samples per drug ranges from one to four. Brain tissue to plasma ratios were ≤ 0.3 for atazanavir, raltegravir, and emtricitabine, tenofovir was ~ 0.7 and for efavirenz was just over 1.0. In this study, plasma and brain concentrations were strongly correlated for emtricitabine, tenofovir, and efavirenz with correlation coefficients ≥ 0.8 (unable to assess for raltegravir and atazanavir since they only had one participant on each drug).
Taken together, in the three studies using human tissues described above, antiretroviral concentrations have been measured in fewer than 20 individuals (with a maximum of 15 for any single drug). Furthermore, although examination of the regional distribution of antiretroviral drugs was intended in each study, definitive conclusions were limited by small sample sizes. To gain a clearer understanding of antiretroviral brain penetration and any associations with viral load or neurocognition, future studies should include larger samples sizes with investigator access to a more detailed medical and prescription dosing history, if possible. Notably, there is also a lack of data in human brain for integrase inhibitors, which have become standard first-line treatment of HIV.
Influence of age, drugs of abuse, pre-existing conditions on antiretroviral penetration into the brain.
Chronic neuroinflammation.
Chronic inflammation is associated with the neuropathology of HIV [82,83]. Inflammation and associated inflammatory factors mediate CNS damage and are driven by residual viral replication, persistently elevated levels of viral proteins despite systemic viral suppression, immune dysfunction and positive feedback loops [82]. With a focus on how neuroinflammation may impact therapeutic drug efficacy within the brain, neuroinflammation is known to alter the expression of tight junctions and compromise BBB integrity [84–86]. Additionally, inflammatory cytokines alter the expression and function of drug metabolizing enzymes and drug transport proteins, leading to alterations in plasma drug concentrations and target site concentrations, which may impact the efficacy of these drugs [87–89].
CNS Opportunistic Infections.
Although there is limited information on the effect of CNS co-infections on antiretroviral exposure in the brain, co-infections that are common among individuals with HIV, such as cryptococcal meningitis or tuberculosis meningitis, have been shown to affect drug distribution. Using PET to investigate drug penetration in the setting of tuberculosis meningitis, Tucker et al. (2019) observed that penetration of 11C-rifampin into brain lesions was limited and heterogeneously distributed [90]. Furthermore, the penetration of 11C-rifampin significantly decreased two weeks after initiation of treatment. Although not tested experimentally, the investigators postulated that the decrease in rifampin brain penetration after two weeks of therapy could be because of initial repair of the leaky BBB and/or induction of P-glycoprotein, with resultant increased efflux of rifampin. How a tuberculosis meningitis co-infection in the setting of HIV might impact antiretroviral and tuberculosis medications is not currently known. Tuberculosis has an estimated prevalence of 23.5% among people living with HIV and is, therefore, a common opportunistic infection within this patient population [91]. Although the prevalence of tuberculosis-meningitis specifically in this group is unclear, the impact of tuberculosis on the pharmacology of antiretrovirals within the brain is an important area to study.
Cryptococcal meningitis is the most common CNS co-infection in individuals living with HIV. One study has investigated antiretroviral concentrations within the CNS in the setting of cryptococcal meningitis co-infection [79]. Using plasma and CSF collected postmortem, Nicol et al. reported increased penetration (threefold to fivefold) of tenofovir and lamivudine in the CSF of 14 individuals with cryptococcal meningitis [79]. However, as described above, drug exposure within the CSF may not be a good surrogate for drug exposure throughout actual brain tissue. These same investigators also had access to brain tissue from four individuals, three of whom also had cryptococcal meningitis. However, because of the small sample sizes, the investigators did not make statistical comparisons. Further studies need to be conducted to investigate the impact of CNS co-infections on therapeutic drug penetration into the brain.
Opioid Use.
The opioid epidemic in the United States is reaching devastating levels. In 2017, the World Drug Report documented that worldwide use of opioids had reached approximately 53.4 million people worldwide. This represented an increase of over 50% than in the prior year [92]. Injection drug use and addiction to prescription opioids are associated with increases in risky behaviors which can lead to increased infection rates. Additionally, substance abuse is associated with decreased adherence to antiretroviral medications, which results in increased viral loads and poorer health outcomes. One of the major comorbidities of HIV is HAND, which is exacerbated by opioid misuse and abuse [93–95]. The neuropathology of HIV in the presence of opioids centers is complex and consists of altered BBB integrity, which leads to increased immune cell migration into the brain, direct action on microglia and astrocytes, increases in reactive oxygen species and reactive nitrogen species, proinflammatory cytokine release as well as increases in the release of HIV-1 proteins, such as Tat and gp120, which also promote inflammation. This exaggerated neuropathology with opioids and HIV has been extensively reviewed elsewhere (reviewed in [96,97]). Furthermore, coadministration of antiretroviral drugs and substances of abuse can result in drug-drug interactions that can impact the pharmacokinetics of the antiretroviral drugs as well as the interacting substance. Many of these drug-drug interactions are mediated through alterations in drug metabolism. The interactions can result in decreased efficacy or increased potential for toxicity of the drugs [98,99].
The impact of opioid use on antiretroviral concentrations specifically within the brain, however, has not been well studied. To our knowledge, there is only one study, to date, examining the impact of opioids on antiretroviral concentrations within the brain. Leibrand et al. demonstrated that 5 days of continuous morphine administration resulted in brain region specific changes in select antiretroviral concentrations in a HIV Tat transgenic mouse model. Dolutegravir concentrations were significantly lower in striatum and hippocampus in morphine exposed mice. Abacavir concentrations also were significantly lower but only within the striatum and lamivudine concentrations were not significantly altered by morphine exposure in either brain region [100]. There also was a morphine-associated increase in P-glycoprotein expression and function in these animals. The antiretroviral drugs dolutegravir and abacavir, which were decreased in the brain are also substrates for P-glycoprotein; lamivudine concentrations were not influenced by morphine and it is not a P-glycoprotein substrate. Future studies should focus on the impact of opioids on other antiretroviral drugs and also should consider the impact of other substances of abuse on antiretroviral concentrations within the brain.
Age.
With improved drugs and management, the life expectancy of people living with HIV is markedly improved. In 2018, approximately 51% of individuals with a diagnosis of HIV were 50 years old and older [101]. By 2035, the proportion of infected individuals living with HIV is expected to reach 70% [102]. Aging impacts drug therapy in multiple ways; it is strongly associated with comorbidity, polypharmacy, and increased adverse effects to medications. Age-related changes in physiology such as altered body composition, metabolism and renal function can lead to altered pharmacokinetics [103] as well as altered pharmacodynamic responses.
Increased age is also associated with multiple changes within the blood-brain barrier, which lead to altered permeability. Aging has been associated with decreases in tight junction protein expression [104–106] and increases in permeability to paracellular compounds [104,105,107], which may occur in a region-specific manner [105]. Age also has been associated with changes in functional transport, with a shift from receptor-mediated transcytosis to caveolar transcytosis of the BBB, which impacts the flux of plasma proteins into the brain and can allow the entry of neurotoxic endogenous proteins such as albumin and fibrinogen [108]. Additionally, aging is also associated with changes in drug transport proteins, including the drug efflux protein P-glycoprotein. Several studies from humans and animal models have demonstrated an association between age and P-glycoprotein expression and/or function. In general, this an inverse relationship between P-glycoprotein and age [109–114], although in some studies there appears to be a biphasic expression pattern over time [109–113]. Additionally, one study examined P-glycoprotein function in male and female volunteers using PET imaging. This study found that P-glycoprotein function decreased in males, but not females with age [115].
Despite studies demonstrating alterations in BBB and drug transport expression with aging, there is a lack of data examining the impact of aging on antiretroviral penetration into the brain. With increased paracellular permeability and decreased expression and/or function of P-glycoprotein, it could be hypothesized that this would result in an increase in penetration of select antiretroviral drugs into the brain. However, conclusions should not be drawn until tested experimentally. Furthermore, the impact of aging on brain expression and function of other transport proteins commonly involved in antiretroviral pharmacology, such as BCRP should be examined in future studies as well.
Conclusions
Significant advances have been made over the last four decades in the treatment and prevention of HIV. Yet, NeuroAIDS remains a significant problem for a significant proportion of individuals living with HIV. A critical weapon against NeuroAIDS is the use of antiretroviral drugs that optimally target brain regions most affected by HIV. This effort is limited, however, by a lack of understanding of the extent of penetration and/or regional specificity of antiretrovirals within the brain. Future studies using postmortem tissues and non-invasive imaging technology will provide critical insight to extrapolate and validate findings from preclinical animal models. Additionally, more work is needed to quantify antiretroviral penetration into specific cell-types that are predominantly infected in the brain, including macrophages and microglia. Understanding regional and cellular localization of antiretroviral drugs may help to distinguish between direct toxic effects of antiretrovirals versus indirect effects from persistent viral replication due to suboptimal drug concentrations. Because most antiretroviral drugs inhibit replication inside the cells, methods to specifically quantify intracellular concentrations (rather than brain homogenate) could improve prediction of HIV efficacy within the brain. Addressing these questions will fill a critical gap in the efforts to reduce morbidity due to NeuroAIDS.
Acknowledgements:
This work was supported by funds from the National Institutes of Health (NIH): R21 DA045630 (MPM), P30 DA033934 Pilot Award (MPM), R21 NS108344 (MRN)
Abbreviations:
- AUC
Area Under the Curve
- BBB
blood-brain barrier
- BCRP
breast cancer resistance protein
- BCSFB
blood- cerebrospinal fluid barrier
- cART
combination antiretroviral therapy
- CC
corpus collosum
- CNS
central nervous system
- CPE
CNS Penetration Effectiveness
- CSF
cerebrospinal fluid
- ECF
extracellular fluid
- HAND
HIV associated neurocognitive disorders
- HIV
human immunodeficiency virus
- HPF
hippocampal formation
- IP
intraperitoneal
- IQR
interquartile range
- LC-MS
liquid chromatography-mass spectrometry
- NHP
nonhuman primate
- PET
positron emission tomography
- SHIV
simian-human immunodeficiency virus
References
- [1].Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, Suwanwela NC, Jagodzinski L, Michael N, Spudich S, Van Griensven F, De Souza M, Kim J, Ananworanich J, Central nervous system viral invasion and inflammation during acute HIV infection, J. Infect. Dis 206 (2012) 275–282. 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Witwer KW, Gama L, Li M, Bartizal CM, Queen SE, Varrone JJ, Brice AK, Graham DR, Tarwater PM, Mankowski JL, Christine Zink M, Clements JE, Coordinated regulation of SIV replication and immune responses in the CNS, PLoS One. 4 (2009). 10.1371/journal.pone.0008129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kruize Z, Kootstra NA, The Role of Macrophages in HIV-1 Persistence and Pathogenesis., Front. Microbiol 10 (2019) 2828. 10.3389/fmicb.2019.02828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Spudich S, HIV and Neurocognitive Dysfunction, Curr. HIV/AIDS Rep 10 (2013) 235–243. http://link.springer.com/10.1007/s11904-013-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ellis R, Langford D, Masliah E, HIV and antiretroviral therapy in the brain: neuronal injury and repair., Nat. Rev. Neurosci 8 (2007) 33–44. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=17180161&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- [6].Vivithanaporn P, Gill MJ, Power C, Impact of current antiretroviral therapies on neuroAIDS, Expert Rev. Anti. Infect. Ther 9 (2011) 371–374. http://informahealthcare.com/doi/abs/10.1586/eri.10.179. [DOI] [PubMed] [Google Scholar]
- [7].Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L, HIV-associated cognitive impairment before and after the advent of combination therapy., J. Neurovirol 8 (2002) 136–142. 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- [8].Petito CK, Cash KS, Blood-brain barrier abnormalities in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain., Ann. Neurol 32 (1992) 658–66. 10.1002/ana.410320509. [DOI] [PubMed] [Google Scholar]
- [9].Power C, a Kong P, Crawford TO, Wesselingh S, Glass JD, McArthur JC, Trapp BD, Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood-brain barrier., Ann. Neurol 34 (1993) 339–350. 10.1002/ana.410340307. [DOI] [PubMed] [Google Scholar]
- [10].Saukkonen JJ, Furfaro S, Mahoney KM, Strieter RM, Burdick M, a Wright E, Kornfeld H, Berman JS, In vitro transendothelial migration of blood T lymphocytes from HIV-infected individuals, AIDS. 11 (1997) 1595–1601. 10.1097/00002030-199713000-00008. [DOI] [PubMed] [Google Scholar]
- [11].Eugenin EA, Clements JE, Christine Zink M, Berman JW, Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism, J. Neurosci 31 (2011) 9456–9465. 10.1523/JNEUROSCI.1460-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Eugenin EA, CCL2/Monocyte Chemoattractant Protein-1 Mediates Enhanced Transmigration of Human Immunodeficiency Virus (HIV)-Infected Leukocytes across the Blood-Brain Barrier: A Potential Mechanism of HIV-CNS Invasion and NeuroAIDS, J. Neurosci 26 (2006) 1098–1106. http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Persidsky Y, Zheng J, Miller D, Gendelman HE, Mononuclear phagocytes mediate blood-brain barrier compromise and neuronal injury during HIV-1-associated dementia., J. Leukoc. Biol 68 (2000) 413–422. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=10985259&retmode=ref&cmd=prlinks. [PubMed] [Google Scholar]
- [14].Buckner CM, Calderon TM, Williams DW, Belbin TJ, Berman JW, Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: Implications for NeuroAIDS, Cell. Immunol 267 (2011) 109–123. 10.1016/j.cellimm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Coley JS, Calderon TM, Gaskill PJ, Eugenin EA, Berman JW, Dopamine Increases CD14+CD16+ Monocyte Migration and Adhesion in the Context of Substance Abuse and HIV Neuropathogenesis, PLoS One. 10 (2015) e0117450. 10.1371/journal.pone.0117450.s003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Williams DW, Eugenin EA, Calderon TM, Berman JW, Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis., J. Leukoc. Biol 91 (2012) 401–15. 10.1189/jlb.0811394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chaudhuri A, Duan F, Morsey B, Persidsky Y, Kanmogne GD, HIV-1 activates proinflammatory and interferon-inducible genes in human brain microvascular endothelial cells: putative mechanisms of blood-brain barrier dysfunction., J. Cereb. Blood Flow Metab 28 (2008) 697–711. 10.1038/sj.jcbfm.9600567. [DOI] [PubMed] [Google Scholar]
- [18].Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, Persidsky Y, Volsky DJ, Knapp PE, HIV-1 neuropathogenesis: Glial mechanisms revealed through substance abuse, J. Neurochem 100 (2007) 567–586. 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dutta R, Roy S, Chronic morphine and HIV-1 Tat promote differential central nervous system trafficking of CD3+ and Ly6C+ immune cells in a murine Streptococcus pneumoniae infection model, J. Neuroinflammation 12 (2015) 120. 10.1186/s12974-015-0341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nath A, Neurologic Complications of Human Immunodeficiency Virus Infection, Contin. Lifelong Learn. Neurol 21 (2015) 1557–1576. 10.1212/CON.0000000000000244. [DOI] [PubMed] [Google Scholar]
- [21].Peluso MJ, Ferretti F, Peterson J, Lee E, Fuchs D, Boschini A, Gisslén M, Angoff N, Price RW, Cinque P, Spudich S, Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load, Aids. 26 (2012) 1765–1774. 10.1097/QAD.0b013e328355e6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lutgen V, Narasipura SD, Barbian HJ, Richards M, Wallace J, Razmpour R, Buzhdygan T, Ramirez SH, Prevedel L, Eugenin EA, Al-Harthi L, HIV infects astrocytes in vivo and egresses from the brain to the periphery, PLOS Pathog. 16 (2020) e1008381. 10.1371/journal.ppat.1008381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lanman T, Letendre S, Ma Q, Bang A, Ellis R, CNS Neurotoxicity of Antiretrovirals., J. Neuroimmune Pharmacol (2019). 10.1007/s11481-019-09886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rhea EM, Logsdon AF, Hansen KM, Williams LM, Reed MJ, Baumann KK, Holden SJ, Raber J, Banks WA, Erickson MA, The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice, Nat. Neurosci (2020). 10.1038/s41593-020-00771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bodnar B, Patel K, Ho W, Luo JJ, Hu W, Cellular mechanisms underlying neurological/neuropsychiatric manifestations of COVID-19, J. Med. Virol (2020). 10.1002/jmv.26720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pardridge WM, Drug transport across the blood–brain barrier, J. Cereb. Blood Flow Metab 32 (2012) 1959–1972. 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pardridge WM, The blood-brain barrier: Bottleneck in brain drug development, NeuroRX. 2 (2005) 3–14. 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ, Structure and function of the blood-brain barrier., Neurobiol. Dis 37 (2010) 13–25. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=19664713&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- [29].Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C, Pericytes regulate the blood-brain barrier, Nature. 468 (2010) 557–561. 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- [30].Abbott NJ, Rönnbäck L, Hansson E, Astrocyte-endothelial interactions at the blood-brain barrier., Nat. Rev. Neurosci 7 (2006) 41–53. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=16371949&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- [31].Strazielle N, Ghersi-Egea JF, Physiology of Blood-Brain Interfaces in Relation to Brain Disposition of Small Compounds and Macromolecules., Mol. Pharm (2013). http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=23298398&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- [32].Erickson MA, Banks WA, Neuroimmune Axes of the Blood-Brain Barriers and Blood-Brain Interfaces: Bases for Physiological Regulation, Disease States, and Pharmacological Interventions., Pharmacol. Rev 70 (2018) 278–314. 10.1124/pr.117.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mann DA, Frankel AD, Endocytosis and targeting of exogenous HIV-1 Tat protein., EMBO J. 10 (1991) 1733–1739. 10.1002/j.1460-2075.1991.tb07697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Abbott NJ, Blood–brain barrier structure and function and the challenges for CNS drug delivery, J. Inherit. Metab. Dis (2013). http://link.springer.com/10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- [35].V Zlokovic B, The blood-brain barrier in health and chronic neurodegenerative disorders., Neuron. 57 (2008) 178–201. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=18215617&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- [36].Miller DS, Bauer B, Hartz AMS, Modulation of P-glycoprotein at the blood-brain barrier: Opportunities to improve central nervous system pharmacotherapy, Pharmacol. Rev 60 (2008) 196–209. 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Giri N, Shaik N, Pan G, Terasaki T, Mukai C, Kitagaki S, Miyakoshi N, Elmquist WF, Investigation of the Role of Breast Cancer Resistance Protein (Bcrp/Abcg2) on Pharmacokinetics and Central Nervous System Penetration of Abacavir and Zidovudine in the Mouse, Drug Metabol. Dispos 36 (2008) 1476–1484. 10.1124/dmd.108.020974.roviral. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Polli JW, Jarrett JL, Studenberg SD, Humphreys JE, Dennis SW, Brouwer KR, Woolley JL, Role of P-glycoprotein on the CNS disposition of amprenavir (141W94), an HIV protease inhibitor., Pharm. Res 16 (1999) 1206–12. http://www.ncbi.nlm.nih.gov/pubmed/10468021. [DOI] [PubMed] [Google Scholar]
- [39].Eisfeld C, Reichelt D, Evers S, Husstedt I, CSF penetration by antiretroviral drugs, CNS Drugs. 27 (2013) 31–55. 10.1007/s40263-012-0018-x. [DOI] [PubMed] [Google Scholar]
- [40].Banks WA, Characteristics of compounds that cross the blood-brain barrier, BMC Neurol. 9 (2009) 5–9. 10.1186/1471-2377-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Morris ME, Rodriguez-Cruz V, Felmlee MA, SLC and ABC Transporters: Expression, Localization, and Species Differences at the Blood-Brain and the Blood-Cerebrospinal Fluid Barriers, AAPS J. 19 (2017) 1317–1331. 10.1208/s12248-017-0110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu X, Smith BJ, Chen C, Callegari E, Becker SL, Chen X, Cianfrogna J, Doran AC, Doran SD, Gibbs JP, Hosea N, Liu J, Nelson FR, Szewc MA, Van Deusen J, Evaluation of cerebrospinal fluid concentration and plasma free concentration as a surrogate measurement for brain free concentration, Drug Metab. Dispos 34 (2006) 1443–1447. 10.1124/dmd.105.008201. [DOI] [PubMed] [Google Scholar]
- [43].Kodaira H, Kusuhara H, Fuse E, Ushiki J, Sugiyama Y, Quantitative investigation of the brain-to-cerebrospinal fluid unbound drug concentration ratio under steady-state conditions in rats using a pharmacokinetic model and scaling factors for active efflux transporters, Drug Metab. Dispos 42 (2014) 983–989. 10.1124/dmd.113.056606. [DOI] [PubMed] [Google Scholar]
- [44].Friden M, Winiwarter S, Jerndal G, Bengtsson O, Hong W, Bredberg U, Hammarlund-Udenaes M, Antonsson M, Structure-brain exposure relationships in rat and human using a novel data set of unbound drug concentrations in brain interstitial and cerebrospinal fluids, J. Med. Chem 52 (2009) 6233–6243. 10.1021/jm901036q. [DOI] [PubMed] [Google Scholar]
- [45].Kodaira H, Kusuhara H, Fujita T, Ushiki J, Fuse E, Sugiyama Y, Quantitative Evaluation of the Impact of Active Efflux by P-Glycoprotein and Breast Cancer Resistance Protein at the BBB on the Predictability of the Unbound Concentrations of Drugs in the Brain Using Cerebrospinal Fluid Concentration as a Surrogate, J. Pharmacol. Exp. Ther 339 (2011) 935–944. 10.1124/jpet.111.180398. [DOI] [PubMed] [Google Scholar]
- [46].Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ, Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system, Arch. Neurol 65 (2008) 65–70. 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Carvalhal A, Gill MJ, Letendre SL, Rachlis A, Bekele T, Raboud J, Burchell A, Rourke SB, Central nervous system penetration effectiveness of antiretroviral drugs and neuropsychological impairment in the Ontario HIV Treatment Network Cohort Study, J. Neurovirol 22 (2016) 349–357. 10.1007/s13365-015-0404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ, Clifford DB, Evans S, Collier AC, Taylor M, Ellis R, Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort., AIDS. 25 (2011) 357–365. 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, Ellis RJ, Rodriguez B, Coombs RW, Schifitto G, McArthur JC, Robertson K, AIDS Clinical Trials Group 736 Study Team, Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance., AIDS. 23 (2009) 1359–66. 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Garvey L, Winston A, Walsh J, Post F, Porter K, Gazzard B, Fisher M, Leen C, Pillay D, Hill T, Johnson M, Gilson R, Anderson J, Easterbrook P, Bansi L, Orkin C, Ainsworth J, Palfreeman A, Gompels M, Phillips AN, Sabin CA, Antiretroviral therapy CNS penetration and HIV-1-associated CNS disease, Neurology. 76 (2011) 693–700. 10.1212/WNL.0b013e31820d8b0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Baker LM, Paul RH, Heaps-Woodruff JM, Chang JY, Ortega M, Margolin Z, Usher C, Basco B, Cooley S, Ances BM, The Effect of Central Nervous System Penetration Effectiveness of Highly Active Antiretroviral Therapy on Neuropsychological Performance and Neuroimaging in HIV Infected Individuals, J. Neuroimmune Pharmacol (2015). http://link.springer.com/10.1007/s11481-015-9610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Caniglia EC, Cain LE, Justice A, Tate J, Logan R, Sabin C, Winston A, Van Sighem A, Miro JM, Podzamczer D, Olson A, Arribas JR, Moreno S, Meyer L, Del Romero J, Dabis F, Bucher HC, Wandeler G, Vourli G, Skoutelis A, Lanoy E, Gasnault J, Costagliola D, Hernán M. a., Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions, Neurology. 83 (2014) 134–141. 10.1212/WNL.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ellis RJ, Letendre S, Vaida F, Haubrich R, Heaton RK, Sacktor N, Clifford DB, Best BM, May S, Umlauf A, Cherner M, Sanders C, Ballard C, Simpson DM, Jay C, McCutchan JA, Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder, Clin. Infect. Dis 58 (2014) 1015–1022. 10.1093/cid/cit921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hammer H, Schmidt F, Marx-Stoelting P, Pötz O, Braeuning A, Cross-species analysis of hepatic cytochrome P450 and transport protein expression, Arch. Toxicol (2020). 10.1007/s00204-020-02939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kuhl DE, Reivich M, Alavi A, Nyary I, Staum MM, Local cerebral blood volume determined by three dimensional reconstruction of radionuclide scan data, Circ. Res 36 (1975) 610–619. 10.1161/01.RES.36.5.610. [DOI] [PubMed] [Google Scholar]
- [56].Engvall C, Ryding E, Wirestam R, Holtås S, Ljunggren K, Ohlsson T, Reinstrup P, Human cerebral blood volume (CBV) Measured by dynamic susceptibility contrast MRI and 99mTc-RBC SPECT, J. Neurosurg. Anesthesiol 20 (2008) 41–44. 10.1097/ANA.0b013e31815d4c70. [DOI] [PubMed] [Google Scholar]
- [57].Galloon S, The Volume of the Intracranial Contents, Int. Anesthesiol. Clin 7 (1969) 663–686. 10.1097/00004311-196907030-00012. [DOI] [PubMed] [Google Scholar]
- [58].Ciris PA, Qiu M, Constable RT, Non-invasive quantification of absolute cerebral blood volume during functional activation applicable to the whole human brain, Magn. Reson. Med 71 (2014) 580–590. 10.1002/mrm.24694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tudorica A, Li HF, Hospod F, Delucia-Deranja E, Huang W, Patlak CS, Newman GC, Cerebral blood volume measurements by rapid contrast infusion and T2*-weighted echo planar MRI, Magn. Reson. Med 47 (2002) 1145–1157. 10.1002/mrm.10167. [DOI] [PubMed] [Google Scholar]
- [60].Kolbitsch C, Lorenz IH, Hörmann C, Hinteregger M, Löckinger A, Moser PL, Kremser C, Schocke M, Felber S, Pfeiffer KP, Benzer A, The influence of hyperoxia on regional cerebral blood flow (rCBF), regional cerebral blood volume (rCBV) and cerebral blood flow velocity in the middle cerebral artery (CBFVMCA) in human volunteers, Magn. Reson. Imaging 20 (2002) 535–541. 10.1016/S0730-725X(02)00534-9. [DOI] [PubMed] [Google Scholar]
- [61].Dean BL, Lee C, Kirsch JE, Runge VM, Dempsey RM, Pettigrew LC, Cerebral hemodynamics and cerebral blood volume: MR assessment using gadolinium contrast agents and T1-weighted turbo-FLASH imaging, Am. J. Neuroradiol 13 (1992) 39–48. [PMC free article] [PubMed] [Google Scholar]
- [62].Aikawa H, Hayashi M, Ryu S, Yamashita M, Ohtsuka N, Nishidate M, Fujiwara Y, Hamada A, Visualizing spatial distribution of alectinib in murine brain using quantitative mass spectrometry imaging, Sci. Rep 6 (2016) 1–9. 10.1038/srep23749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sun N, Walch A, Qualitative and quantitative mass spectrometry imaging of drugs and metabolites in tissue at therapeutic levels, Histochem. Cell Biol 140 (2013) 93–104. 10.1007/s00418-013-1127-4. [DOI] [PubMed] [Google Scholar]
- [64].Thompson CG, Bokhart MT, Sykes C, Adamson L, Fedoriw Y, Luciw PA, Muddiman DC, Kashuba ADM, Rosen EP, Mass spectrometry imaging reveals heterogeneous efavirenz distribution within putative HIV reservoirs, Antimicrob. Agents Chemother 59 (2015) 2944–2948. 10.1128/AAC.04952-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ntshangase S, Mdanda S, Singh SD, Naicker T, Kruger HG, Baijnath S, Govender T, Mass Spectrometry Imaging Demonstrates the Regional Brain Distribution Patterns of Three First-Line Antiretroviral Drugs., ACS Omega. 4 (2019) 21169–21177. 10.1021/acsomega.9b02582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Srinivas N, Rosen EP, Gilliland WM Jr, Kovarova M, Remling-Mulder L, De La Cruz G, White N, Adamson L, Schauer AP, Sykes C, Luciw P, Garcia JV, Akkina R, Kashuba ADM, Antiretroviral concentrations and surrogate measures of efficacy in the brain tissue and CSF of preclinical species, Xenobiotica. 49 (2019) 1192–1201. 10.1080/00498254.2018.1539278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mdanda S, Ntshangase S, Singh SD, Naicker T, Kruger HG, Baijnath S, Govender T, Mass spectrometric investigations into the brain delivery of abacavir, stavudine and didanosine in a rodent model, Xenobiotica. 0 (2019) 1–10. 10.1080/00498254.2019.1655605. [DOI] [PubMed] [Google Scholar]
- [68].Varnäs K, Varrone A, Farde L, Modeling of PET data in CNS drug discovery and development, J. Pharmacokinet. Pharmacodyn 40 (2013) 267–279. 10.1007/s10928-013-9320-6. [DOI] [PubMed] [Google Scholar]
- [69].Hooker JM, Carson RE, Human Positron Emission Tomography Neuroimaging, Annu. Rev. Biomed. Eng 21 (2019) 551–581. 10.1146/annurev-bioeng-062117-121056. [DOI] [PubMed] [Google Scholar]
- [70].de Lange EC, de Boer BA, Breimer, Microdialysis for pharmacokinetic analysis of drug transport to the brain., Adv. Drug Deliv. Rev 36 (1999) 211–227. 10.1016/s0169-409x(98)00089-1. [DOI] [PubMed] [Google Scholar]
- [71].de Lange EC, de Boer AG, Breimer DD, Methodological issues in microdialysis sampling for pharmacokinetic studies., Adv. Drug Deliv. Rev 45 (2000) 125–48. 10.1016/s0169-409x(00)00107-1. [DOI] [PubMed] [Google Scholar]
- [72].Hammarlund-Udenaes M, Microdialysis as an Important Technique in Systems Pharmacology—a Historical and Methodological Review, AAPS J. 19 (2017) 1294–1303. 10.1208/s12248-017-0108-2. [DOI] [PubMed] [Google Scholar]
- [73].Sawchuk RJ, Yang Z, Investigation of distribution, transport and uptake of anti-HIV drugs to the central nervous system., Adv. Drug Deliv. Rev 39 (1999) 5–31. 10.1016/s0169-409x(99)00017-4. [DOI] [PubMed] [Google Scholar]
- [74].Shannon RJ, Carpenter KLH, Guilfoyle MR, Helmy A, Hutchinson PJ, Cerebral microdialysis in clinical studies of drugs: Pharmacokinetic applications, J. Pharmacokinet. Pharmacodyn 40 (2013) 343–358. 10.1007/s10928-013-9306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yang Z, Huang Y, Gan G, Sawchuk RJ, Microdialysis evaluation of the brain distribution of stavudine following intranasal and intravenous administration to rats, J. Pharm. Sci 94 (2005) 1577–1588. 10.1002/jps.20334. [DOI] [PubMed] [Google Scholar]
- [76].Wong SL, Wang Y, Sawchuk RJ, Analysis of Zidovudine Distribution to Specific Regions in Rabbit Brain Using Microdialysis, Pharm. Res. An Off. J. Am. Assoc. Pharm. Sci 9 (1992) 332–338. 10.1023/A:1015834701136. [DOI] [PubMed] [Google Scholar]
- [77].Wong SL, Van Belle K, Sawchuk RJ, Distributional transport kinetics of zidovudine between plasma and brain extracellular fluid/cerebrospinal fluid in the rabbit: investigation of the inhibitory effect of probenecid utilizing microdialysis., J. Pharmacol. Exp. Ther 264 (1993) 899–909. [PubMed] [Google Scholar]
- [78].Yang Z, Brundage RC, Barbhaiya RH, Sawchuk RJ, Microdialysis studies of the distribution of stavudine into the central nervous system in the freely-moving rat, Pharm. Res 14 (1997) 865–872. 10.1023/A:1012191515035. [DOI] [PubMed] [Google Scholar]
- [79].Nicol MR, Pastick KA, Taylor J, Namuju OC, Rhein J, Williams DA, Meya DB, Boulware DR, Lukande R, Cerebrospinal Fluid and Brain Tissue Penetration of Tenofovir, Lamivudine, and Efavirenz in Postmortem Tissues with Cryptococcal Meningitis, Clin. Transl. Sci (2019) 445–449. 10.1111/cts.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ferrara M, Bumpus NN, Ma Q, Ellis RJ, Soontornniyomkij V, Fields JA, Bharti A, Achim CL, Moore DJ, Letendre SL, Antiretroviral drug concentrations in brain tissue of adult decedents., AIDS. 34 (2020) 1907–1914. 10.1097/QAD.0000000000002628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Devanathan AS, Pirone JR, Akkina R, Remling-Mulder L, Luciw P, Adamson L, Victor Garcia J, Kovarova M, White NR, Schauer AP, Blake K, Sykes C, Burgunder EM, Srinivas N, Rosen EP, Kashuba ADM, Antiretroviral penetration across three preclinical animal models and humans in eight putative HIV viral reservoirs, Antimicrob. Agents Chemother 64 (2020) 1–7. 10.1128/AAC.01639-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].McArthur JC, Johnson TP, Chronic inflammation mediates brain injury in HIV infection, Curr. Opin. Neurol (2020) 1. 10.1097/WCO.0000000000000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].González-Scarano F, Martín-García J, The neuropathogenesis of AIDS., Nat. Rev. Immunol 5 (2005) 69–81. 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- [84].Luissint A-C, Artus C, Glacial F, Ganeshamoorthy K, Couraud P-O, Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation., Fluids Barriers CNS. 9 (2012) 23. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=23140302&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Dallasta LM, Pisarov LA, Esplen JE, V Werley J, V Moses A, Nelson JA, Achim CL, Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis., Am. J. Pathol 155 (1999) 1915–27. 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Boven LA, Middel J, Verhoef J, De Groot CJA, Nottet HSLM, Monocyte infiltration is highly associated with loss of the tight junction protein zonula occludens in HIV-1-associated dementia, Neuropathol. Appl. Neurobiol 26 (2000) 356–360. 10.1046/j.1365-2990.2000.00255.x. [DOI] [PubMed] [Google Scholar]
- [87].Cressman AM, Petrovic V, Piquette-Miller M, Inflammation-mediated changes in drug transporter expression/activity: Implications for therapeutic drug response, Expert Rev. Clin. Pharmacol 5 (2012) 69–89. 10.1586/ecp.11.66. [DOI] [PubMed] [Google Scholar]
- [88].Roberts DJ, Goralski KB, A critical overview of the influence of inflammation and infection on P-glycoprotein expression and activity in the brain, Expert Opin. Drug Metab. Toxicol 4 (2008) 1245–1264. 10.1517/17425255.4.10.1245. [DOI] [PubMed] [Google Scholar]
- [89].Martinez MN, Greene J, Kenna L, Kissell L, Kuhn M, The impact of infection and inflammation on drug metabolism, active transport, and systemic drug concentrations in veterinary species, Drug Metab. Dispos 48 (2020) 631–644. 10.1124/DMD.120.090704. [DOI] [PubMed] [Google Scholar]
- [90].Tucker EW, Guglieri-Lopez B, Ordonez AA, Ritchie B, Klunk MH, Sharma R, Chang YS, Sanchez-Bautista J, Frey S, Lodge MA, Rowe SP, Holt DP, Gobburu JVS, Peloquin CA, Mathews WB, Dannals RF, Pardo CA, Kannan S, Ivaturi VD, Jain SK, Noninvasive 11 C-rifampin positron emission tomography reveals drug biodistribution in tuberculous meningitis, Sci. Transl. Med 10 (2018) eaau0965. 10.1126/scitranslmed.aau0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Gao J, Zheng P, Fu H, Prevalence of TB/HIV Co-Infection in Countries Except China: A Systematic Review and Meta-Analysis, PLoS One. 8 (2013). 10.1371/journal.pone.0064915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].UNODC, Executive Summary: Conclusions and policy implications, United Nations Off. Drugs Crime. (2019). https://wdr.unodc.org/wdr2019/prelaunch/WDR19_Booklet_1_EXECUTIVE_SUMMARY.pdf. [Google Scholar]
- [93].Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE, Updated research nosology for HIV-associated neurocognitive disorders., Neurology. 69 (2007) 1789–1799. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=17914061&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Anthony IC, Arango J, Stephens B, Simmonds P, Bell JE, The effects of illicit drugs on the HIV infected brain., Front. Biosci 13 (2008) 1294–1307. 10.2741/2762. [DOI] [PubMed] [Google Scholar]
- [95].Hauser KF, Fitting S, Dever SM, Podhaizer EM, Knapp PE, Opiate Drug Use and the Pathophysiology of NeuroAIDS, Curr. HIV Res 10 (2012) 435–452. 10.2174/157016212802138779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Murphy A, Barbaro J, Martínez-Aguado P, Chilunda V, Jaureguiberry-Bravo M, Berman JW, The Effects of Opioids on HIV Neuropathogenesis., Front. Immunol 10 (2019) 2445. 10.3389/fimmu.2019.02445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Fitting S, McRae M, Hauser KF, Opioid and neuroHIV Comorbidity – Current and Future Perspectives, J. Neuroimmune Pharmacol (2020). 10.1007/s11481-020-09941-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].McCance-Katz EF, Sullivan LE, Nallani S, Drug Interactions of Clinical Importance among the Opioids, Methadone and Buprenorphine, and Other Frequently Prescribed Medications: A Review, Am. J. Addict 19 (2010) 4–16. http://doi.wiley.com/10.1111/j.1521-0391.2009.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Gruber VA, McCance-Katz EF, Methadone, buprenorphine, and street drug interactions with antiretroviral medications, Curr. HIV/AIDS Rep 7 (2010) 152–160. 10.1007/s11904-010-0048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Leibrand CR, Paris JJ, Jones AM, Masuda QN, Halquist MS, Kim WK, Knapp PE, Kashuba ADM, Hauser KF, McRae MP, HIV-1 Tat and opioids act independently to limit antiretroviral brain concentrations and reduce blood–brain barrier integrity, J. Neurovirol 25 (2019) 560–577. 10.1007/s13365-019-00757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Centers for Disease Control and Prevention, HIV Surveillance Report 2018 (Updated), (2020) Vol. 31. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (accessed December 23, 2020). [Google Scholar]
- [102].Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Van Sighem A, de Wolf F, Hallett TB, Future challenges for clinical care of an ageing population infected with HIV: a modelling study, Lancet Infect. Dis 15 (2015) 810–818. 10.1016/S1473-3099(15)00056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].McLean AJ, Le Couteur DG, Aging biology and geriatric clinical pharmacology, Pharmacol. Rev 56 (2004) 163–184. 10.1124/pr.56.2.4. [DOI] [PubMed] [Google Scholar]
- [104].Elahy M, Jackaman C, Mamo JCL, Lam V, Dhaliwal SS, Giles C, Nelson D, Takechi R, Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment, Immun. Ageing 12 (2015) 1–9. 10.1186/s12979-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Bake S, Friedman JA, Sohrabji F, Reproductive age-related changes in the blood brain barrier: Expression of IgG and tight junction proteins, Microvasc. Res 78 (2009) 413–424. 10.1016/j.mvr.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mooradian AD, Haas MJ, Chehade JM, Age-related changes in rat cerebral occludin and zonula occludens-1 (ZO-1), Mech. Ageing Dev 124 (2003) 143–146. 10.1016/S0047-6374(02)00041-6. [DOI] [PubMed] [Google Scholar]
- [107].Takechi R, Pallebage-Gamarallage MM, Lam V, Giles C, Mamo JC, Aging-Related Changes in Blood-Brain Barrier Integrity and the Effect of Dietary Fat, Neurodegener. Dis 12 (2013) 125–135. http://www.karger.com?doi=10.1159/000343211. [DOI] [PubMed] [Google Scholar]
- [108].Yang AC, Stevens MY, Chen MB, Lee DP, Stähli D, Gate D, Contrepois K, Chen W, Iram T, Zhang L, Vest RT, Chaney A, Lehallier B, Olsson N, du Bois H, Hsieh R, Cropper HC, Berdnik D, Li L, Wang EY, Traber GM, Bertozzi CR, Luo J, Snyder MP, Elias JE, Quake SR, James ML, Wyss-Coray T, Physiological blood–brain transport is impaired with age by a shift in transcytosis, Nature. 583 (2020) 425–430. 10.1038/s41586-020-2453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Osgood D, Miller MC, Messier AA, Gonzalez L, Silverberg GD, Aging alters mRNA expression of amyloid transporter genes at the blood-brain barrier, Neurobiol. Aging 57 (2017) 178–185. 10.1016/j.neurobiolaging.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Chiu C, Miller MC, Monahan R, Osgood DP, Stopa EG, Silverberg GD, P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer’s disease: Preliminary observations, Neurobiol. Aging 36 (2015) 2475–2482. 10.1016/j.neurobiolaging.2015.05.020. [DOI] [PubMed] [Google Scholar]
- [111].Toornvliet R, van Berckel BNM, Luurtsema G, Lubberink M, Geldof AA, Bosch TM, Oerlemans R, Lammertsma AA, Franssen EJF, Effect of age on functional P-glycoprotein in the blood-brain barrier measured by use of (R)-[11C]verapamil and positron emission tomography, Clin. Pharmacol. Ther 79 (2006) 540–548. 10.1016/j.clpt.2006.02.004. [DOI] [PubMed] [Google Scholar]
- [112].Pan Y, Nicolazzo JA, Impact of aging, Alzheimer’s disease and Parkinson’s disease on the blood-brain barrier transport of therapeutics, Adv. Drug Deliv. Rev 135 (2018) 62–74. 10.1016/j.addr.2018.04.009. [DOI] [PubMed] [Google Scholar]
- [113].Pekcec A, Schneider EL, Baumgärtner W, Stein VM, Tipold A, Potschka H, Age-dependent decline of blood-brain barrier P-glycoprotein expression in the canine brain, Neurobiol. Aging 32 (2011) 1477–1485. 10.1016/j.neurobiolaging.2009.08.014. [DOI] [PubMed] [Google Scholar]
- [114].Bartels AL, de Klerk OL, Kortekaas R, de Vries JJ, Leenders KL, 11C-verapamil to Assess P-gp Function in Human Brain During Aging,Depression and Neurodegenerative Disease, Curr. Top. Med. Chem 10 (2010) 1775–1784. 10.2174/156802610792928059. [DOI] [PubMed] [Google Scholar]
- [115].van Assema DME, Lubberink M, Boellaard R, Schuit RC, Windhorst AD, Scheltens P, Lammertsma AA, van Berckel BNM, P-Glycoprotein Function at the Blood–Brain Barrier: Effects of Age and Gender, Mol. Imaging Biol 14 (2012) 771–776. 10.1007/s11307-012-0556-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ntshangase S, Mdanda S, Naicker T, Kruger HG, Baijnath S, Govender T, Spatial distribution of elvitegravir and tenofovir in rat brain tissue: Application of MALDI-MSI and LC-MS/MS., Rapid Commun. Mass Spectrom (2019) 0–3. 10.1002/rcm.8510. [DOI] [PubMed] [Google Scholar]
- [117].Fox E, Bungay PM, Bacher J, McCully CL, Dedrick RL, Balis FM, Zidovudine concentration in brain extracellular fluid measured by microdialysis: steady-state and transient results in rhesus monkey., J. Pharmacol. Exp. Ther 301 (2002) 1003–11. 10.1124/jpet.301.3.1003. [DOI] [PubMed] [Google Scholar]
- [118].Dyavar SR, Gautam N, Podany AT, Winchester LC, Weinhold JA, Mykris TM, Campbell KM, Alnouti Y, Fletcher CV, Assessing the lymphoid tissue bioavailability of antiretrovirals in human primary lymphoid endothelial cells and in mice., J. Antimicrob. Chemother 74 (2019) 2974–2978. 10.1093/jac/dkz273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Ntshangase S, Mdanda S, Naicker T, Kruger HG, Govender T, Baijnath S, Rilpivirine as a potential candidate for the treatment of HIV-associated neurocognitive disorders (HAND), J. Mol. Histol (2019). 10.1007/s10735-019-09826-y. [DOI] [PubMed] [Google Scholar]
- [120].Mdanda S, Ntshangase S, Singh SD, Naicker T, Kruger HG, Baijnath S, Govender T, Zidovudine and Lamivudine as Potential Agents to Combat HIV-Associated Neurocognitive Disorder, Assay Drug Dev. Technol 17 (2019) 322–329. 10.1089/adt.2019.941. [DOI] [PubMed] [Google Scholar]
- [121].Mdanda S, Ntshangase S, Singh SD, Naicker T, Kruger HG, Baijnath S, Govender T, Investigating time dependent brain distribution of nevirapine via mass spectrometric imaging, J. Mol. Histol 50 (2019) 593–599. 10.1007/s10735-019-09852-w. [DOI] [PubMed] [Google Scholar]
- [122].Leibrand CR, Paris JJ, Jones AM, Masuda QN, Halquist MS, Kim W-K, Knapp PE, Kashuba ADM, Hauser KF, McRae M, HIV-1 Tat and opioids act independently to limit antiretroviral brain concentrations and reduce blood-brain barrier integrity., J. Neurovirol (2019). 10.1007/s13365-019-00757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Dyavar SR, Gautam N, Podany AT, Winchester LC, Weinhold JA, Mykris TM, Campbell KM, Alnouti Y, Fletcher CV, Assessing the lymphoid tissue bioavailability of antiretrovirals in human primary lymphoid endothelial cells and in mice, J. Antimicrob. Chemother 74 (2019) 2974–2978. 10.1093/jac/dkz273. [DOI] [PMC free article] [PubMed] [Google Scholar]


