Abstract
Alopecia occurs frequently in captive populations of nonhuman primates. Because multiple factors can play a role in alopecia, a better understanding of its etiology will help identify potential welfare concerns. The purpose of this study was to investigate risk factors for alopecia in a breeding colony of baboons with a focus on pregnancy and age. Alopecia was scored on a scale of 0 (no alopecia) to 5 (severe alopecia) in 253 female baboons during routine physicals. The subjects ranged in age from 4 to 23 y (Mean = 9.6) and were categorized as pregnant (n = 83), nursing (n = 60) or control (n = 110). Resulting alopecia scores were combined into 2 categories (mild = 0 or 1; moderate = 2 or 3); no animals scored a 4 or 5. Significantly more pregnant females had moderate alopecia than did control females. There was no effect of age on alopecia. An unexpected outcome was that among nursing females, more of those with female infants had moderate alopecia than did those with male infants. The impact of the infant’s sex on alopecia may be due to sex differences in maternal contact or maternal investment. This information adds to our understanding of alopecia risk factors in captive nonhuman primates.
Alopecia, or hair loss, is frequently reported to occur in captive populations of nonhuman primates (NHP). The degree and pattern of hair loss can vary from small, localized patches to large bare areas covering most of the body. Although hair-plucking is often the default diagnosis,37 alopecia is a multifaceted condition that is affected by a variety of both intrinsic and environmental variables including species, sex, age, social rank, pregnancy, housing, season, behavior, stress, hormonal changes, nutritional deficits, infection, and parasites.5,6,9,10,12-14,21,25,28,30-33,37-41,43,47-49,53,56,60,62 Because coat quality and alopecia can be impacted by multiple factors, identification of potential causes can be challenging.
Hair undergoes cyclic regrowth that is typically viewed as recurring phases: the anagen, catagen, and telogen phases, followed by hair shedding.15,20,44,49 The anagen phase is the active growth phase involving cell renewal. The duration of this phase can determine hair length.15,20,44,50,51 In humans, hair grows at a rate of approximately 0.65 to 2.2 cm/mo.35 Catagen is the degradation phase during which the hair follicle undergoes a form of programmed cell death, is shortened in length and reduced in volume.15,20,50 The telogen phase is the resting phase during which the hair shaft matures into a club hair under which a new germ layer develops.15,50 This is followed by hair shedding.15,20,49 In humans, most of an individual’s hair (approximately 60% to 90%) is in the anagen phase, approximately 5% to 35% is in the telogen phase, and the remaining percentages are in the catagen and hair shedding phases.15,20,44,50,51
Telogen effluvium is a disorder characterized by significant hair shedding resulting from a disruption of the hair cycle and excessive loss of telogen hairs.36 Different functional types of telogen effluvium range from chronic to acute and can be influenced by factors such as a major illness, high fever, physiologic stress, drug use, or malnutrition.20,36,52 In women, telogen effluvium is also associated with pregnancy.17,20,45 In the later stage of pregnancy, the percentage of hairs in the telogen phase can increase to 30% to 50%,44,51 followed by accelerated diffuse hair shedding.44 Hair loss resulting from postpartum telogen effluvium can begin approximately 1 to 5 mo after delivery and can last approximately 2 to 6 mo.15,17,45,54 Although the duration and location of postpartum alopecia can vary,54 the hair cycle eventually returns to normal.44
As with humans, NHP can also experience hair loss during and after pregnancy. In rhesus monkeys (Macaca mulatta), hair loss was observed throughout the gestation and perinatal periods, becoming most pronounced during the final months of pregnancy and into the month after parturition.6,13,14 Overall, pregnant females were significantly more likely to have alopecia than were nonpregnant females.6,40,41 Regrowth occurs shortly after parturition,13 with one study reporting only a moderate difference in coat condition between postpartum females and control females that were not pregnant or nursing.40 However, not all females experience hair loss during pregnancy or the perinatal period. Such differences in hair loss may be due in part to differences in maternal investment (i.e., the provision of resources to offspring at some cost to the parent) during pregnancy.14 To our knowledge, genetic effects on alopecia have not been investigated.
Hair loss has also been associated with aging. In humans, scalp hair density and mean anagen growth rate gradually decrease with age.58 Female pattern hair loss, characterized by diffuse hair thinning over the scalp, increases with advancing age,57 while the hair density, diameter, and tensile strength decrease.29,42 In NHP, hair coat condition also tends to worsen with age;6,12,38,49,56 some studies have reported that monkeys with alopecia were often significantly older than those without alopecia.25,31 Older animals also had an increase in the percentage of hairs in the telogen phase, which is a pattern of hair loss that is characteristic of chronic telogen effluvium.25 One study comparing geriatric (mean age = 25.4 y) and adult (mean age = 9.8 y) rhesus monkeys reported that the older monkeys had higher frequencies of skin abnormalities (for example, scaling, wrinkling, laxity of the skin) as well as alopecia.26 However, in other studies, hair coat quality did not change linearly with age,32,62 and still others reported either no effect of age on alopecia37,59 or higher levels of alopecia in the younger animals.41
Hair loss is readily apparent, and its persistence may result in management or clinical responses that are not consistent with animal wellbeing.4 Therefore, a better understanding of the etiology of alopecia in captive NHP will help to identify welfare concerns. Because alopecia commonly occurs in pregnant women and NHPs,6,13,14,17,20,40,44,45,51 the purpose of this study is to further assess the incidence of alopecia in NHP during pregnancy. Due to its association with alopecia, age is also included as a variable. Much of the research on alopecia in NHP has focused on macaque monkeys.5,6,10,12-14,21,30-33,37-40,47,48,53,56,60,62 Even though baboons are commonly used as models in a wide range of research areas,11 less is known about alopecia in these animals.41 Therefore, this study assesses animals from the genus Papio to attain a broader perspective of the impact of pregnancy and age on alopecia. We predict that both age and pregnancy will have an impact on hair loss.
Materials and Methods
Subjects.
This study evaluated adult female olive baboons (Papio hamadryas anubis) and olive/yellow baboons (P. h. cynocephalus) crosses (n = 253). The subjects ranged in age from 4 to 23 y (mean = 9.6 y). Of the 253 subjects, 83 were pregnant, 60 had nursing infants, and 110 were neither pregnant nor nursing (controls). The gestation period in baboons lasts approximately 6 mo.2,19,61
Housing.
The subjects were socially housed in 35 groups consisting of one adult male, 5 to 13 adult females, and their associated offspring. They were housed in outdoor enclosures measuring approximately 6 × 9 m, containing heated indoor access measuring approximately 6 × 3 m, as well as perches, hanging barrels, and various enrichment items. The subjects were fed Purina 5 LEO biscuits (Purina Mills, Gray Summit, MO) 2 times per day. Their diet was supplemented with fruit, vegetables, grains, and cereals. The facility is accredited by AAALAC International, and the animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals.27 The research was approved by the Texas Biomedical Research Institute’s Institutional Animal Care and Use Committee and complied with the laws and regulations of the United States Animal Welfare Act.
Procedures.
Assessments were conducted opportunistically when the animals were sedated for routine physical examinations that were performed between March 2018 and February 2019. The pregnant females were sampled at an average of 80 d prior to delivery (range: 7 to 170 d). Nursing females were sampled at an average of 120 d postpartum (range: 12 to 306 d). Alopecia was scored on a scale of 0 to 5 using the “rule of nines” method, with 0 being no alopecia and 5 being severe alopecia.3 Each animal was scored once. A single observer scored all of the animals. The date of parturition and sex of the fetus (pregnant females) or infant (nursing females) were obtained from the animal records.
Data analysis.
The majority of the baboons had an alopecia score of 1 or 2, with few having scores of 0 or 3, and none having a score of 4 or 5 (Figure 1). Therefore, the subjects were consolidated into 2 alopecia categories: mild (score of 0 or 1) and moderate (score of 2 or 3) for further analyses. Statistics were conducted with SYSTAT 13 (SYSTAT Software, Chicago, IL). Statistical significance was based on a P-value of < 0.05.
Figure 1.
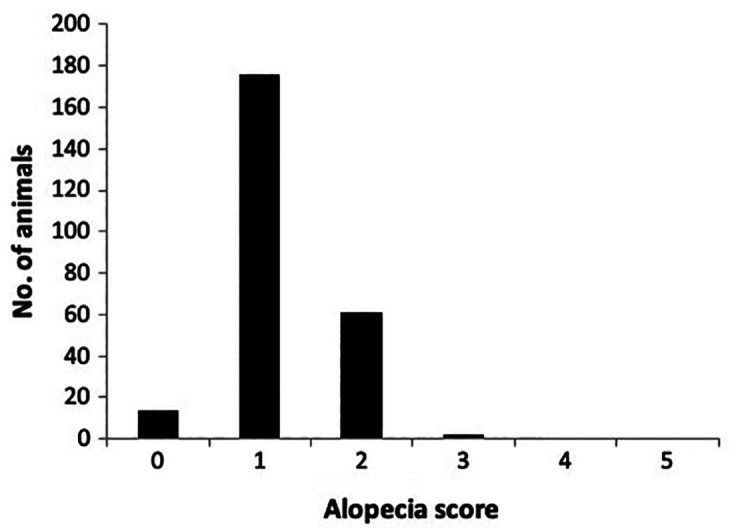
Overall alopecia scores
A χ2 test was used to examine study group (pregnant, nursing, control) differences in alopecia levels (mild, moderate). Because age was not normally distributed in the study groups, a nonparametric Kruskal–Wallis H test was used to examine study group differences in age, and a Mann–Whitney U test was used to compare alopecia level (mild, moderate) with age.
Because research has shown that hair loss in rhesus monkeys is most pronounced during the final months of pregnancy, with hair growing back postpartum,6,13,14 logistic regressions were conducted separately on the pregnant and nursing study groups to further assess the effect of pregnancy on mild or moderate alopecia levels. Variables specific to the 2 study groups included days prior to birth (pregnant females) and days postpartum (nursing females). Additional group-specific variables included sex of the fetus (pregnant females) and sex of the infant (nursing females). For the logistic regressions, the group-specific variables and their interactions were initially entered into each model. A backward elimination procedure was used to determine the “best-fit” model. Variables were assessed for their contribution to the model by evaluating the change in total variance accounted for (R squared) when that term was removed. Terms with the highest P values were removed first.
Results
Across Groups.
Alopecia scores were significantly different across study groups (χ2(2,253) = 10.960, P < 0.005). A significantly larger proportion of pregnant females had moderate alopecia as compared to control females (χ2(1,193) = 10.993, P < 0.005). Moderate alopecia was not significantly different between nursing and control females (χ2(1,170) = 3.120, P < 0.10; Figure 2). There was no significant difference in age across study groups (H(2) = 1.994, P > 0.30) and there was no effect of age on alopecia level (U = 5,879, P > 0.80).
Figure 2.
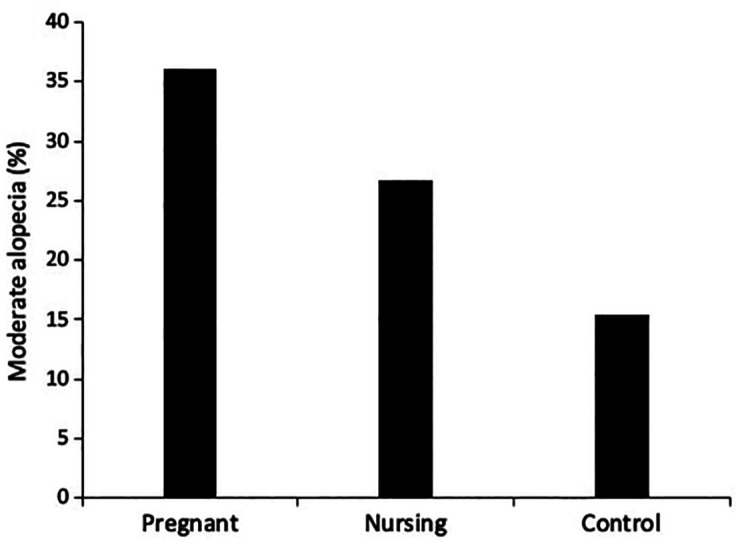
A comparison of alopecia across groups
Within Groups.
Sex of the infant was associated with greater alopecia in nursing females. Significantly more mothers that were nursing female infants had moderate alopecia (40%) in comparison to those that were nursing male infants (13%; b = –1.466, P < 0.05). No other predictors were significant.
Discussion
This study supports previous findings that alopecia is a common condition in healthy, pregnant, nonhuman primates.5,13,14,40,41 Although the percentage of pregnant baboons with moderate alopecia (36%) was lower than that reported in pregnant rhesus macaques (63%),40 significantly more pregnant baboons had moderate alopecia in comparison to nonpregnant animals. This result supports previous findings of higher levels of hair loss in pregnant or postpartum human and nonhuman primates.6,13-15,17,20,40,41,44,45,51,54 The baboons in the present study may be experiencing a form of pregnancy-related telogen effluvium. However, the hair gradually grows back postpartum, as observed in the nursing females whose percentage with moderate alopecia was not significantly different from that of nonpregnant controls.
In contrast, age did not contribute to alopecia levels. This finding contradicts previous studies reporting that alopecia typically increases with age in both humans29,42,57,58 and NHPs.6,12,38,49,56 The broad age range of animals sampled in the current study (4 to 23 y) should have been sufficient for detecting an age effect. These data suggest that baboons maintain their hair coat as they age. A previous study on baboon alopecia reported that younger animals were more likely to experience hair loss than were older animals.41 However, the age range in that study included both juveniles as young as 2 to 3 y of age and males. These 2 differences may influence the relationship between age and alopecia in baboons.
A surprising finding in this study is that the infant’s sex influences alopecia. A greater proportion of mothers nursing female infants had moderate alopecia as compared with those nursing male infants. However, the sex of the fetus did not affect alopecia in pregnant females, suggesting that the effect of infant sex on alopecia occurs postpartum. Postpartum alopecia could be due to the friction and rubbing of the hair when infants are in physical contact with their mothers, either causing additional alopecia or restricting postpartum regrowth. In this case, differences in alopecia based on the infant’s sex may be due to sex differences in maternal contact. Often male infants have less contact with their mothers than do female infants, as reported in captive Japanese macaques (Macaca fuscata)16 and captive baboons.7 Similarly, in wild baboons, mothers of male infants were more tolerant of infant independence than were mothers of female infants.46 Male infants were also more active and had more infant-initiated changes in mother-infant contact.46 However, this male-biased separation was not reported in all studies. In captive hamadryas baboons (Papio hamadryas hamadryas), mothers broke contact with their female infants more often than with their male infants22 and sex differences in separation from the mother was not observed in captive rhesus macaques14 or wild yellow baboons.1
Alternatively, differences in maternal alopecia based on the infant’s sex could be due to differential maternal investment. For example, in alopecic rhesus monkey mothers, hair cortisol concentrations (indicators of stress) in pregnancy were positively correlated with infant birth weight, infant growth rate, and milk yield volume; this association was not present in mothers without alopecia.14 Maternal investment can be measured by variables such as infant birth weight, infant growth weight, milk production, milk content, and interbirth interval.8,14,18,23,24,34,55 Although some studies reported no infant sex differences in birth or growth weight in captive macaque monkeys,34,55 other studies reported that male rhesus infants weighed significantly more than females at birth, with relative weight increasing during the first year,8 suggesting that in this case maternal investment was directed toward male offspring. In contrast, other studies reported that rhesus monkey mothers rearing daughters had higher calcium concentrations in milk than did mothers rearing sons24 and in low-ranking mothers, daughters made more nipple contacts, suggesting that female infants were obtaining more milk.18 However, one study reported that although rhesus monkey mothers of females produced more milk, mothers of sons produced milk of higher energy density; therefore, available milk energy was the same for sons and daughters.23 Another indicator of maternal investment is interbirth interval, or the timing between subsequent births. Although studies of macaque monkeys reported no overall differences in interbirth interval,18,55 low-ranking mothers raising female infants typically did not give birth the following year; this did not occur in high-ranking mothers or mothers with sons.18,34 These results suggest that raising female infants can be more costly, at least in low-ranking mothers. Due to colony management procedures, maternal rank, natural interbirth interval, and infant birth weight and growth rates are not known for the animals in the present study. However, these important variables should be assessed in future studies.
In conclusion, our study found that pregnancy was a risk factor for alopecia in captive baboons. However, in our population, age did not contribute to alopecia, which may be unique to baboons. Moderate alopecia was shown to be more common in mothers with female infants than in those with male infants. This difference could be due, in part, to greater contact with female infants or to increased maternal investment directed toward daughters. The current study focused on pregnancy as a risk factor for alopecia, but another useful study would be to examine whether alopecia in baboons correlates with measures of welfare, such as indicators of stress. Future research in this area could be useful for evaluation of animal wellbeing.
Acknowledgments
The author thanks Jarom DeCrescenzo, Amberlee Dugosh, Carrie McCabe, and Drs John Dutton, Elizabeth Clemmons, Patrice Frost, Melissa De la Garza, and Shannan Hall-Ursone for their assistance with alopecia scoring. Thanks also to Dr Kris Coleman for her thoughtful comments on an earlier draft. This study was supported by National Institutes of Health grant number 2P51OD011133 to Texas Biomedical Research Institute.
References
- 1.Altmann J.1980.Baboon mothers and infants. Chicago (IL):University of Chicago Press [Google Scholar]
- 2.Altmann J, Alberts SC.2003.Variability in reproductive success viewed from a life history perspective in baboons.Am J Hum Biol 15:401–409. 10.1002/ajhb.10157. [DOI] [PubMed] [Google Scholar]
- 3.Baker KC, Bloomsmith MA, Coleman K, Crockett CM, Worlein J, Lutz CK, McCowan B, Pierre P, Weed J. 2017.The behavioral management consortium- A partnership for promoting consensus and best practices, p9–23. In:Schapiro SJ, editor.Handbook of primate behavioral management. New York (NY):CRC Press. [Google Scholar]
- 4.Baker KC, Crockett CM, Bloomsmith MA, Coleman K, Bellanca RU.2006.Behavioral and clinical management of alopecia in nonhuman primates.Am J Primatol 68S1:89. [Google Scholar]
- 5.Beisner BA, Isbell LA.2008.Ground substrate affects activity budgets and hair loss in outdoor captive groups of rhesus macaques (Macaca mulatta).Am J Primatol 70:1160–1168. 10.1002/ajp.20615. [DOI] [PubMed] [Google Scholar]
- 6.Beisner BA, Isbell LA.2009.Factors influencing hair loss among female captive rhesus macaques (Macaca mulatta).Appl Anim Behav Sci 119:91–100. 10.1016/j.applanim.2009.03.016. [DOI] [Google Scholar]
- 7.Bentley-Condit VK.2003.Sex differences in captive olive baboon behavior during the first fourteen days of life.Int J Primatol 24:1093–1112. 10.1023/A:1026232413614. [DOI] [Google Scholar]
- 8.Bercovitch FB, Widdig A, Nurnberg P.2000.Maternal investment in rhesus macaques (Macaca mulatta): reproductive costs and consequences of raising sons.Behav Ecol Sociobiol 48:1–11. 10.1007/s002650000204. [DOI] [Google Scholar]
- 9.Brand CM, Marchant LF.2018.Prevalence and characteristics of hair plucking in captive bonobos (Pan paniscus) in North American zoos.Am J Primatol 80:e22751. 10.1002/ajp.22751. [DOI] [PubMed] [Google Scholar]
- 10.Conti G, Hansman C, Heckman JJ, Novak MFX, Ruggiero A, Suomi SJ.2012.Primate evidence on the late health effects of early-life adversity.Proc Natl Acad Sci USA 109:8866–8871. 10.1073/pnas.1205340109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox LA, Comuzzie AG, Havill LM, Karere GM, Spradling KD, Mahaney MC, Nathanielsz PW, Nicolella DP, Shade RE, Voruganti S, VandeBerg JL.2013.Baboons as a model to study genetics and epigenetics of human disease.ILAR J 54:106–121. 10.1093/ilar/ilt038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crockett CM, Bentson KL, Bellanca RU.2007.Alopecia and overgrooming in laboratory monkeys vary by species but not sex, suggesting a different etiology than self biting.Am J Primatol 69S1:87–88. [Google Scholar]
- 13.Davis EB, Suomi SJ.2006.Hair loss and replacement cycles in socially housed, pregnant, rhesus macaques.Am J Primatol 68S1:58. [Google Scholar]
- 14.Dettmer AM, Rosenberg K, Menard MT, El-Mallah SN, Woodward RA, Suomi SJ, Meyer JS.2017.Differential relationships between chronic hormone profiles in pregnancy and maternal investment in rhesus monkey mothers with hair loss in the neonatal period.Am J Primatol 79:1–8. 10.1002/ajp.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eastham JH.2001.Postpartum alopecia.Ann Pharmacother 35:255–258. 10.1345/aph.10153. [DOI] [PubMed] [Google Scholar]
- 16.Eaton GG, Johnson DF, Glick BB, Worlein JM.1985.Development in Japanese macaques (Macaca fuscata): Sexually dimorphic behavior during the first year of life.Primates 26:238–247. 10.1007/BF02382400. [DOI] [Google Scholar]
- 17.Gjerdingen DK, Froberg DG, Chaloner KM, McGovern PM.1993.Changes in women’s physical health during the first postpartum year.Arch Fam Med 2:277–283. 10.1001/archfami.2.3.277. [DOI] [PubMed] [Google Scholar]
- 18.Gomendio M.1990.The influence of maternal rank and infant sex on maternal investment trends in rhesus macaques: birth sex ratios, inter-birth intervals and suckling patterns.Behav Ecol Sociobiol 27:365–375. 10.1007/BF00164008. [DOI] [Google Scholar]
- 19.Hall KRL, DeVore I. 1965.Baboon social behavior, p53–110. In:DeVore I, editor.Primate behavior field studies of monkeys and apes. New York (NY):Holt, Rinehart and Winston. [Google Scholar]
- 20.Headington JT.1993.Telogen effluvium. New concepts and review.Arch Dermatol 129:356–363. 10.1001/archderm.1993.01680240096017. [DOI] [PubMed] [Google Scholar]
- 21.Heagerty A, Wales RA, Prongay K, Gottlieb DH, Coleman K.2017.Social hair pulling in captive rhesus macaques (Macaca mulatta).Am J Primatol 79:1–26. 10.1002/ajp.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernández-Lloreda MV, Colmenares F.2005.Mother-infant relationships in baboons (Papio hamadryas): predictors of differences and discontinuities in developmental pathways.J Comp Psychol 119:311–324. 10.1037/0735-7036.119.3.311. [DOI] [PubMed] [Google Scholar]
- 23.Hinde K.2009.Richer milk for sons but more milk for daughters: sex-biased investment during lactation varies with maternal life history in rhesus macaques.Am J Hum Biol 21:512–519. 10.1002/ajhb.20917. [DOI] [PubMed] [Google Scholar]
- 24.Hinde K, Foster AB, Landis LM, Rendina D, Oftedal OT, Power ML.2013.Daughter dearest: sex-biased calcium in mother’s milk among rhesus macaques.Am J Phys Anthropol 151:144–150. 10.1002/ajpa.22229. [DOI] [PubMed] [Google Scholar]
- 25.Horenstein VD-P, Williams LE, Brady AR, Abee CR, Horenstein MG. 2005.Age related diffuse chronic telogen effluvium-type alopecia in female squirrel monkeys (Saimiri boliviensis boliviensis).Comp Med 55:169–174. [PubMed] [Google Scholar]
- 26.Huneke RB, Foltz CJ, VandeWoude S, Mandrell TD, Garman RH.1996.Characterization of dermatologic changes in geriatric rhesus macaques.J Med Primatol 25:404–413. 10.1111/j.1600-0684.1996.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 27.Institute for Laboratory Animal Research.2011.Guide for the care and use of laboratory animals,8th ed.Washington (DC):National Academies Press. [Google Scholar]
- 28.Isbell LA.1995.Seasonal and social correlates of changes in hair, skin, and scrotal condition in vervet monkeys (Cercopithecus aethiops) of Amboseli National Park, Kenya.Am J Primatol 36:61–70. 10.1002/ajp.1350360105. [DOI] [PubMed] [Google Scholar]
- 29.Kim SN, Lee SY, Choi MH, Joo KM, Kim SH, Koh JS, Park WS.2013.Characteristic features of ageing in Korean women’s hair and scalp.Br J Dermatol 168:1215–1223. 10.1111/bjd.12185. [DOI] [PubMed] [Google Scholar]
- 30.Kimura T.2008.Systemic alopecia resulting from hyperadrenocorticism in a Japanese monkey.Lab Primate Newsletter 47:5–9. [Google Scholar]
- 31.Kramer J, Fahey M, Santos R, Carville A, Wachtman L, Mansfield K.2010.Alopecia in rhesus macaques correlates with immunophenotypic alterations in dermal inflammatory infiltrates consistent with hypersensitivity etiology.J Med Primatol 39:112–122. 10.1111/j.1600-0684.2010.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroeker R, Bellanca RU, Lee GH, Thom JP, Worlein JM.2013.Alopecia in three macaque species housed in a laboratory environment.Am J Primatol 76:325–334. 10.1002/ajp.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroeker R, Lee GH, Bellanca RU, Thom JP, Worlein JM.2017.Prior facility affects alopecia in adulthood for rhesus macaques.Am J Primatol 79:1–9. 10.1002/ajp.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurita H, Matsui T, Shimomura T, Fujita T, Oka T.2012.Maternal investment in sons and daughters in provisioned, free-ranging Japanese macaques (Macaca fuscata).Anthropol Sci 120:33–38. 10.1537/ase.110120. [DOI] [Google Scholar]
- 35.LeBeau MA, Montgomery MA, Brewer JD.2011.The role of variations in growth rate and sample collection on interpreting results of segmental analyses of hair.Forensic Sci Int 210:110–116. 10.1016/j.forsciint.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Liyanage D, Sinclair R.2016.Telogen effluvium.Cosmetics 3:1–8. 10.3390/cosmetics3020013. [DOI] [Google Scholar]
- 37.Luchins KR, Baker KC, Gilbert MH, Blanchard JL, Liu DX, Myers L, Bohm RP.2011.Application of the diagnostic evaluation for alopecia in traditional veterinary species to laboratory rhesus macaques (Macaca mulatta).J Am Assoc Lab Anim Sci 50:926–938. [PMC free article] [PubMed] [Google Scholar]
- 38.Lutz CK, Coleman K, Worlein JM, Kroeker R, Menard MT, Rosenberg K, Meyer JS, Novak MA.2016.Factors influencing alopecia and hair cortisol in rhesus macaques (Macaca mulatta).J Med Primatol 45:180–188. 10.1111/jmp.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutz CK, Coleman K, Worlein J, Novak MA.2013.Hair loss and hair-pulling in rhesus macaques (Macaca mulatta).J Am Assoc Lab Anim Sci 52:454–457. [PMC free article] [PubMed] [Google Scholar]
- 40.Lutz CK, Menard MT, Rosenberg K, Meyer JS, Novak MA.2019.Alopecia in rhesus macaques (Macaca mulatta): association with pregnancy and chronic stress.J Med Primatol 48:251–256. 10.1111/jmp.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lutz CK, Sharp RM.2015.Alopecia in outdoor group- and corral- housed baboons (Papio hamadryas spp.).J Am Assoc Lab Anim Sci 54:384–388. [PMC free article] [PubMed] [Google Scholar]
- 42.Mai W, Sun Y, Liu X, Lin D, Lu D.2019.Characteristic findings by phototrichogram in southern Chinese women with female pattern hair loss.Skin Res Technol 25:447–455. 10.1111/srt.12672. [DOI] [PubMed] [Google Scholar]
- 43.Miller RE, Albert SG, Boever WJ.1983.Hypothyroidism in a chimpanzee.J Am Vet Med Assoc 183:1326–1328. [PubMed] [Google Scholar]
- 44.Millikan L.2006.Hirsutism, postpartum telogen effluvium, and male pattern alopecia.J Cosmet Dermatol 5:81–86. 10.1111/j.1473-2165.2006.00229.x. [DOI] [PubMed] [Google Scholar]
- 45.Murray JC.1990.Pregnancy and the skin.Dermatol Clin 8:327–334. 10.1016/S0733-8635(18)30504-7. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen N, Gesquiere L, Alberts SC, Altmann J.2012.Sex differences in the motherneonate relationship in wild baboons: social, experiential and hormonal correlates.Anim Behav 83:891–903. 10.1016/j.anbehav.2012.01.003. [DOI] [Google Scholar]
- 47.Novak MA, Hamel AF, Coleman K, Lutz CK, Worlein J, Menard M, Ryan A, Rosenberg K, Meyer JS.2014.Hair loss and hypothalamic-pituitary-adrenocortical axis activity in captive rhesus macaques (Macaca mulatta).J Am Assoc Lab Anim Sci 53:261–266. [PMC free article] [PubMed] [Google Scholar]
- 48.Novak MA, Menard MT, El-Mallah SN, Rosenberg K, Lutz CK, Worlein J, Coleman K, Meyer JS.2017.Assessing significant (>30%) alopecia as a possible biomarker for stress in captive rhesus monkeys (Macaca mulatta).Am J Primatol 79:1–8. 10.1002/ajp.22547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Novak MA, Meyer JS.2009.Alopecia: possible causes and treatments, particularly in captive nonhuman primates.Comp Med 59:18–26. [PMC free article] [PubMed] [Google Scholar]
- 50.Paus R, Cotsarelis G.1999.The biology of hair follicles.N Engl J Med 341:491–497. 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 51.Piérard-Franchimont C, Piérard GE.2013.Alterations in hair follicle dynamics in women.BioMed Res Int 2013:1–5. 10.1155/2013/957432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rebora A.2019.Telogen effluvium: a comprehensive review.Clin Cosmet Investig Dermatol 12:583–590. 10.2147/CCID.S200471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarnowski MB, Jacobsen KR, Lambeth SP, Schapiro SJ.2013.A multi-faceted investigation of hair loss in outdoor group-housed rhesus macaques (Macaca mulatta).Am J Primatol 75S1:61. [Google Scholar]
- 54.Schiff BL, Kern AB.1963.Study of postpartum alopecia.Arch Dermatol 87:609–611. 10.1001/archderm.1963.01590170067011. [DOI] [PubMed] [Google Scholar]
- 55.Small MF, Smith DG.1984.Sex differences in maternal investment by Macaca mulatta.Behav Ecol Sociobiol 14:313–314. 10.1007/BF00299503. [DOI] [Google Scholar]
- 56.Steinmetz HW, Kaumanns W, Dix I, Heistermann M, Fox M, Kaup F-J.2006.Coat condition, housing condition and measurement of faecal cortisol metabolites- a noninvasive study about alopecia in captive rhesus macaques (Macaca mulatta).J Med Primatol 35:3–11. 10.1111/j.1600-0684.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- 57.Su L-H, Chen L-S, Chen H-H.2012.Factors associated with female pattern hair loss and its prevalence in Taiwanese women: a community-based survey.J Am Acad Dermatol 69:e69–e77. 10.1016/j.jaad.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 58.Tajima M, Hamada C, Arai T, Miyazawa M, Shibata R, Ishino A.2007.Characteristic features of Japanese women’s hair with aging and with progressing hair loss.J Dermatol Sci 45:93–103. 10.1016/j.jdermsci.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 59.Vazquez M, Lutz CK.2018.Activity budget and alopecia in captive chimpanzees (Pan troglodytes): impact of sex, age, and group size.Am J Primatol 80S1:17. [Google Scholar]
- 60.Vessey SH, Morrison JA.1970.Molt in free-ranging rhesus monkeys, Macaca mulatta.J Mammal 51:89–93. 10.2307/1378535. [DOI] [Google Scholar]
- 61.Wasser SK, Norton G.1993.Baboons adjust secondary sex ratio in response to predictors of sex-specific offspring survival.Behav Ecol Sociobiol 32:273–281. 10.1007/BF00166517. [DOI] [Google Scholar]
- 62.Zhang P.2011.A non-invasive study of alopecia in Japanese macaques Macaca fuscata.Curr Zool 57:26–35. 10.1093/czoolo/57.1.26. [DOI] [Google Scholar]


