Vaccines against SARS-CoV-2 represent a pivotal and effective countermeasure to contain the COVID-19 pandemic. Four vaccines are approved by the European Medicines Agency: two messenger RNA-based vaccines encoding the spike protein of SARS-CoV-2 (BNT162b2, Pfizer–BioNTech; mRNA-1273, Moderna) and two adenoviral vector-basedvaccines encoding the spike protein (ChAdOx1 nCoV-19, AstraZeneca; Ad.26.COV2.S, Janssen).1
As of Sept 23, 2021, more than 83 million vaccine doses were administered in Italy, with approximately a fifth of recipients receiving ChAdOx1 nCoV-19 vaccine.2 Here, we report three cases of cutaneous vasculitis developing in previously healthy individuals shortly after vaccination with ChAdOx1 nCoV-19.
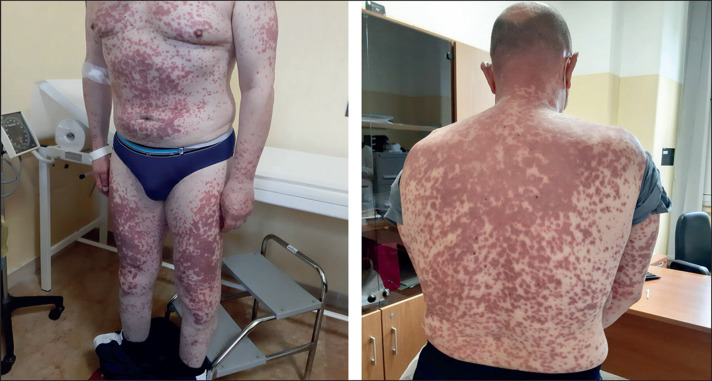
The clinical features of the patients are summarised in the appendix (p 1). Briefly, patient 1 was a 57-year-old man with a history of hypertension but no previous personal or family history of autoimmunity. Purpura developed 14 days following the first vaccine dose, initially affecting the lower limbs and rapidly spreading to the abdomen, torso, and head (figure ). He received treatment with 1 mg/kg prednisone, which led to progressive resolution of skin lesions over 3 weeks. Patient 2 was a 58-year-old man, whose previous medical history was also unremarkable with no history of autoimmunity. Purpura developed 7 days following the second dose of vaccine, spreading from the lower limbs to the abdomen and trunk (appendix p 2). He received 0·5 mg/kg prednisone, to no clinical benefit, and then 1 mg/kg prednisone, with progressive resolution of skin lesions over 10 days. Patient 3 was a 53-year-old woman with no underlying health conditions or history of autoimmunity. Purpura developed 6 days following the first dose, affecting the lower and upper limbs. She received treatment with 1 mg/kg prednisone, which led to a progressive resolution of skin lesions over 2 weeks.
Figure.
Purpura in patient 1
All cases were investigated for laboratory abnormalities or organ involvements that are typically associated with small-vessel vasculitis. However, laboratory tests showed only non-specific increases in erythrocyte sedimentation rate and C-reactive protein (CRP); anti-neutrophil cytoplasmic antibodies, cytoplasmic anti-neutrophil cytoplasmic antibodies, perinuclear anti-neutrophil cytoplasmic antibodies, rheumatoid factor, cryoglobulins, antinuclear antibodies, anti-DNA, C3, C4, IgA, and serology for hepatitis B virus and hepatitis C virus were negative or normal. Chest imaging (ie, x-ray or CT), urinalysis, and a search for stool blood were also negative. A 5 mm skin punch biopsy was performed in patient 3, which showed only a mild lymphocytic perivascular infiltrate (appendix p 3). A histological diagnosis of leukocytoclastic vasculitis could not be formally confirmed in the absence of neutrophils, yet disruption of the vessel wall, or fibrinoid necrosis, the clinical findings in these three patients were clearly indicative of this condition.
Although we cannot exclude the possibility that the onset of vasculitis following vaccination was coincidental, striking similarities between these three patients argue for pathogenic causality. Specifically, vasculitis developed in healthy individuals with no personal or family history of autoimmunity; clinical manifestations were similar and characterised by widespread cutaneous vasculitis with no visceral involvement; and there was a temporal association between vaccination and the development of clinical manifestations, with no other intercurrent inciting events. All patients underwent serologic testing for SARS-CoV-2 infection before vaccination and tested negative, indicating no previous primary infection: hence, vasculitis might have been triggered by maladaptive individual immune responses to a component of the vaccine.
The ChAdOx1 nCoV-19 vaccine contains recombinant adenoviral vectors encoding the spike protein of SARS-CoV-2, stabilisers, and immune adjuvants. It is possible that molecular mimicry might develop between the peptides that are expressed in the viral spike protein and in the host endothelial cells, particularly following non-specific adjuvant effects. Vasculitis can develop during COVID-19 because of direct endothelial damage,3, 4, 5 and coagulation disorders can develop following vaccination with ChAdOx1 nCoV-19 because of platelet-activating antibodies against platelet factor 4 (PF4).6 Thereby, we speculate that maladaptive immune activation induced by vaccination affects the endothelial layer or the coagulation cascade, ultimately inducing vasculitis in predisposed individuals.
SARS-CoV-2 infection has resulted in more than 4 million deaths worldwide, often due to excessive or aberrant host immune responses.7 The benefits of vaccination outweigh the risks,8 yet vaccination of millions of individuals is unavoidably complicated by sparse immune-mediated adverse events, since proinflammatory stimulation can expose individual predisposition to the development of maladaptive immune responses.9, 10, 11
GC led the study and wrote the report. GC, GDL, SC, RP, FC, and LD took clinical care of patients, obtained data, and contributed to drafting the manuscript. NR conducted histology evaluations. Written informed consent for publication was obtained from the patients. We declare no competing interests.
Supplementary Material
References
- 1.Olliaro P, Torreele E, Vaillant M. COVID-19 vaccine efficacy and effectiveness-the elephant (not) in the room. Lancet Microbe. 2021;2:e279–e280. doi: 10.1016/S2666-5247(21)00069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Presidenza del Consiglio dei Ministri Report vaccini anti COVID-19. Sept 16, 2021. https://www.governo.it/it/cscovid19/report-vaccini
- 3.McGonagle D, Bridgewood C, Ramanan AV, Meaney JFM, Watad A. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol. 2021;3:e224–e233. doi: 10.1016/S2665-9913(20)30420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta P, Haskard DO, Laffan MA, Chambers RC, Hunt BJ. Thromboses and COVID-19: reducing inflammation in addition to thromboprophylaxis. Lancet Rheumatol. 2021;3:e171–e172. doi: 10.1016/S2665-9913(21)00003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalli G, Colafrancesco S, Emmi G, et al. Interleukin 1alpha: a comprehensive review on the role of IL-1alpha in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun Rev. 2021;20 doi: 10.1016/j.autrev.2021.102763. [DOI] [PubMed] [Google Scholar]
- 6.Eichinger S, Warkentin TE, Greinacher A. Thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Reply. N Engl J Med. 2021;385:e11. doi: 10.1056/NEJMc2107227. [DOI] [PubMed] [Google Scholar]
- 7.Cavalli G, Larcher A, Tomelleri A, et al. Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study. Lancet Rheumatol. 2021;3:e253–e261. doi: 10.1016/S2665-9913(21)00012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubbert-Roth A, Vuilleumier N, Ludewig B, Schmiedeberg K, Haller C, von Kempis J. Anti-SARS-CoV-2 mRNA vaccine in patients with rheumatoid arthritis. Lancet Rheumatol. 2021;3:e470–e472. doi: 10.1016/S2665-9913(21)00186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boekel L, Hooijberg F, van Kempen ZLE, et al. Perspective of patients with autoimmune diseases on COVID-19 vaccination. Lancet Rheumatol. 2021;3:e241–e243. doi: 10.1016/S2665-9913(21)00037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boekel L, Kummer LY, van Dam KPJ, et al. Adverse events after first COVID-19 vaccination in patients with autoimmune diseases. Lancet Rheumatol. 2021;3:e542–e545. doi: 10.1016/S2665-9913(21)00181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu TY, D'Silva KM, Patel NJ, Fu X, Wallace ZS, Sparks JA. Incident systemic rheumatic disease following COVID-19. Lancet Rheumatol. 2021;3:e402–e404. doi: 10.1016/S2665-9913(21)00106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.