Kidney transplant recipients (KTR) are at increased risk of severe coronavirus disease 2019 (COVID-19) infection [1]. Although these patients are obviously at high-priority for vaccination, there are some concerns concerning the efficacy of vaccines. Recent reports confirmed reduced seroresponse of KTR to COVID-19 vaccine [2]. Less than 50% of KTR exhibit antibody positivity after two injections of vaccine [2]. Nevertheless, factors associated with poor response are not well described.
We studied 153 KTR COVID-19 naïve (no clinical history, negative serology) KTR. All the patients received two doses of the BNT162b2 mRNA COVID-19 vaccine. Humoral response [SARS-Cov-2 immunoassay, Abbott® designed to detect IgG antibodies to the receptor-binding domain (RBD) of the S1 subunit of the spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] and factors associated with this response were studied. As a relevant threshold cannot be determined, we used that provided by the manufacturer, and one more empirically defined by a value above 660 UA/mL (upper quartile of antibody titers). We also analysed a subgroup of patients without any detectable response after vaccine.
Among 153 patients, 81 (53%) had an antibody titer ≥50 UA/mL and 38 (25%) >660 UA/mL (high responders), mean ± SD 2.5 ± 1.2 months after the second dose.
Among 72 patients (47%) with an antibody titer <50 UA/mL, 32 (21%) had no detectable antibodies.
Age and gender did not differ between responders and non-responders (Table 1). Transplant duration was significantly longer in responders [median (interquartile range) 158 (60–194) versus 28 (15–84) months, P < 0.001].
Table 1.
Clinical and biological characteristics of responders and non-responders patients (thresholds 50 UA/mL, 2.5 ± 1.2 months after the second dose)
| Non-responders | Responders | P | |
|---|---|---|---|
| (<50 UA/mL) | (≥50 UA/mL) | ||
| n (%) | 72 (47) | 81 (53) | |
| Age (years) | 64 (57–71) | 63 (54–72) | 0.963 |
| Gender (% male) | 62% | 59% | 0.895 |
| Transplant vintage (months) | 28 (15–84) | 158 (60–194) | 0.145 |
| eGFR (mL/min/1.73 m2) | 48 (32–58) | 62 (38–81) | 0.007 |
| CNI use (%) | 90 | 72 | 0.106 |
| MPA use (%) | 76 | 32 | <0.001 |
| mTORi use (%) | 6 | 36 | <0.001 |
| Belatacept use (%) | 6 | 0 | 0.047 |
| CD3 T-cell counta | 657 (442–990) | 971 (793–1380) | <0.001 |
| CD4 T-cell counta | 420 (312–575) | 554 (438–767) | <0.001 |
| CD8 T-cell counta | 215 (143–427) | 330 (238–498) | <0.001 |
| CD19 cell counta | 78 (37–152) | 86 (52–134) | 0.338 |
For continuous variables, median and interquartiles 25–75. aPer mm3.
Estimated glomerular filtration rate (eGFR) was lower in non-responders [48 (32–58) versus 62 (38–81) mL/min/1.73 m2, P < 0.001]. Antibody titers were strongly related to eGFR (r = 0.314, P < 0.001). Seventy-four percent of patients with satisfactory renal function (eGFR >60 mL/min/1.73 m2) achieved significant humoral response, whereas only 37% of those with either intermediate (eGFR 30–60 mL/min/1.73 m2) or low (eGFR <30 mL/min/1.73 m2) renal function did.
Non-responders were more frequently under mycophenolic acid (MPA) (76% versus 32%, P < 0.001), whereas responders were more likely to receive mTOR inhibitors (mTORi) (36% versus 6%, P < 0.001). There was a trend towards fewer use of belatacept in responders (0% versus 6%, P = 0.047). Calcineurin inhibitors (CNIs) use was similar in responders and non-responders (Table 1).
CD3, CD4 and CD8 counts were lower in non-responders (Table 1).
Using multiple logistic regression, shorter transplant duration (P = 0.003), lower eGFR (P < 0.001), MPA use (P < 0.001) and lower CD4 count (P = 0.016) were all associated with lack of response to vaccine (Table 2). This model strongly predicts vaccine response [area under the curve 0.89 (0.82–0.93), P < 0.001] (Figure 1).
Table 2.
Factors associated with response to BNT162b2 mRNA COVID-19 vaccine (logistic regression) (thresholds 50 UA/mL, 2.5 ± 1.2 months after the second dose)
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Parameters | OR (CI 95%) | P | OR (CI 95%) | P |
| Age (/year) | 1.00 (0.97–1.03) | 0.991 | – | – |
| Gender (male) | 1.40 (0.71–2.77) | 0.329 | – | – |
| Transplant duration (/year) | 1.24 (1.12–1.32) | <0.001 | 1.12 (1.04–1.20) | 0.003 |
| eGFR (/mL/min/1.73 m2) | 1.03 (1.01–1.05) | <0.001 | 1.04 (1.02–1.07) | <0.001 |
| CNI | 0.27 (0.11–0.68) | 0.005 | 1.24 (0.24–6.38) | 0.798 |
| mTORi | 9.06 (2.99–24.48) | <0.001 | 1.71 (0.32–9.17) | 0.530 |
| MPA | 0.13 (0.06–0.27) | <0.001 | 0.14 (0.05–0.42) | <0.001 |
| CD3 cell counta | 1.10 (1.05–1.15) | <0.001 | – | – |
| CD4 cell counta | 1.13 (1.05–1.20) | <0.001 | 1.65 (1.12–2.19) | 0.016 |
| CD8 cell counta | 1.13 (1.05–1.22) | 0.003 | – | – |
| NK cell counta | 1.09 (0.89–1.30) | 0.362 | – | – |
| B-cell counta | 1.01 (0.89–1.14) | 0.841 | – | – |
CD3, CD4 and CD8 cell counts were closely related. Trivariate stepwise analysis including the three T lymphocytes subsets only retains CD4 cell count as associated with vaccine response. As a consequence, only CD4 cell count was maintained in the model. a/50 mm3. OR, odds ratio; CI, confidence interval; NK, natural killer.
FIGURE 1:
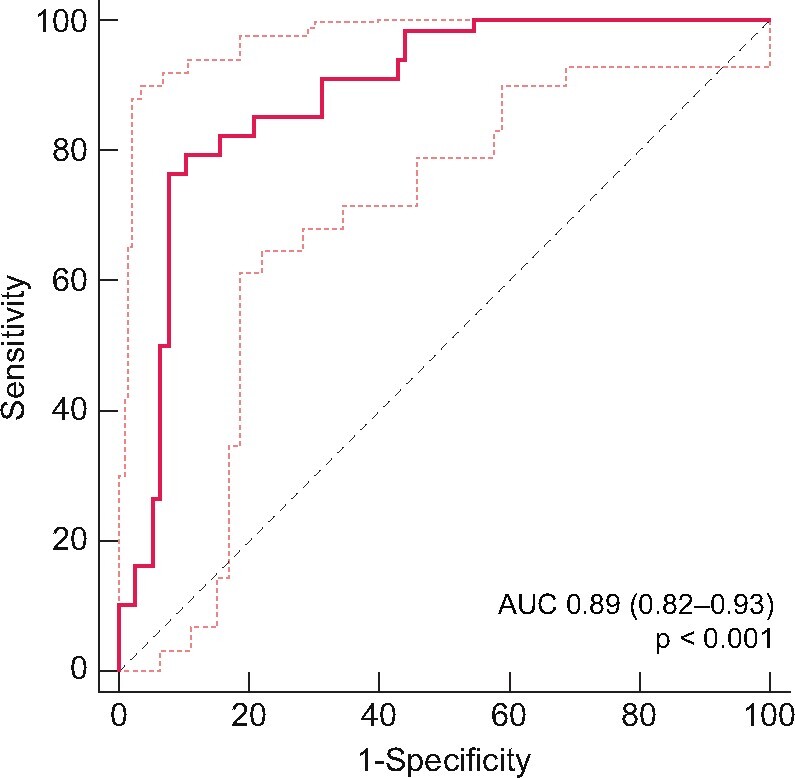
Receiver operating characteristic curve illustrating the ability of a multiple logistic regression model predicting response to COVID-19 vaccine (thresholds 50 UA/mL, 2.5 ± 1.2 months after the second dose). AUC, area under the curve.
Complete absence of response to vaccine was associated with low CD4 count (P = 0.049) and reduced eGFR (P = 0.006).
Robust vaccine response was associated with better eGFR (P < 0.001), longer transplant duration (P < 0.001) and no MPA use (<0.001).
We report in a single-centre study that only half of KTR have a vaccine response after two doses of BNT162b2 mRNA COVID-19 vaccine. We observed that some factors are strongly associated with vaccine response.
Patients receiving MPA had a lower rate of vaccine response. MPA inhibits both T- and B-cell proliferation. Previous studies reported similar results [3, 4]. Reduced response to other vaccines has been also reported in patients under MPA when compared with those receiving CNI [5–7].
Reduced renal function diminishes the likelihood of achieving seroprotection after complete vaccine. Similar results have been observed for H1N1 vaccination [7]. The mechanisms are not very clear, since significant humoral response is reported in about 90% of dialysis patients [8].
Low CD4 T-cell counts were associated with reduced response to vaccine. Post-transplant low CD4 counts indicate accelerated immune senescence and are associated with increased risk of cancer and infection [9].
We showed that four risk factors explain 90% of response likelihood to BNT162b2 mRNA COVID-19 vaccine in KTR. These factors are not modifiable except for MPA use. Better strategies of vaccination as well as reinforced barrier measures are required in KTR.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Caillard S, Chavarot N, Francois H. et al. ; French SOT COVID Registry. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant 2021; 21: 1295–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grupper A, Rabinowich L, Schwartz D. et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant 2021; doi: 10.1111/ajt.16615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peled Y, Ram E, Lavee J. et al. BNT162b2 vaccination in heart transplant recipients: Clinical experience and antibody response. J Heart Lung Transplant 2021; S1053–S2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rozen-Zvi B, Yahav D, Agur T. et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: A prospective cohort study. Clin Microbiol Infect 2021; doi: 10.1016/j.cmi.2021.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonker EFF, Uijlings MAC, Visser LG. et al. Comparison of the immunogenicity of Dukoral® oral cholera vaccine between renal transplant recipients on either a calcineurin inhibitor or mycophenolate—a controlled trial. Vaccine 2019; 37: 3133–3139 [DOI] [PubMed] [Google Scholar]
- 6.Azevedo LS, Gerhard J, Miraglia JL. et al. Seroconversion of 2009 pandemic influenza A (H1N1) vaccination in kidney transplant patients and the influence of different risk factors. Transplant Infect Dis 2013; 15: 612–618 [DOI] [PubMed] [Google Scholar]
- 7.Mulley WR, Visvanathan K, Hurt AC. et al. Mycophenolate and lower graft function reduce the seroresponse of kidney transplant recipients to pandemic H1N1 vaccination. Kidney Int 2012; 82: 212–219 [DOI] [PubMed] [Google Scholar]
- 8.Longlune N, Nogier MB, Miedougé M. et al. High immunogenicity of a messenger RNA based vaccine against SARS-CoV-2 in chronic dialysis patients. Nephrol Dial Transplant 2021; 31: doi: 10.1093/ndt/gfab193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ducloux D, Courivaud C, Bamoulid J. et al. Prolonged CD4 T cell lymphopenia increases morbidity and mortality after renal transplantation. J Am Soc Nephrol 2010; 21: 868–875 [DOI] [PMC free article] [PubMed] [Google Scholar]


