Abstract
Intravitreal vascular endothelial growth factor (VEGF) receptor blockade is used for a variety of retinal pathologies. These include age-related macular degeneration (AMD), diabetic macular edema (DME) and central retinal vein obstruction. Reports of absorption of intravitreal agents into systemic circulation have increased in number and confirmation of depletion of VEGF has been confirmed. Increasingly there are studies and case reports showing worsening hypertension, proteinuria, renal dysfunction and glomerular disease. The pathognomonic findings of systemic VEGF blockade, thrombotic microangiopathies (TMAs), are also being increasingly reported. One lesion that occurs in conjunction with TMAs that has been described is collapsing focal segmental glomerulosclerosis (cFSGS). cFSGS has been postulated to occur due to TMA-induced chronic glomerular hypoxia. In this updated review we discuss the mechanistic, pharmacological, epidemiological and clinical evidence of intravitreal VEGF toxicity. We review cases of biopsy-proven toxicity presented by our group and other investigators. We also present the third reported case of cFSGS in the setting of intravitreal VEGF blockade with a chronic TMA component that was crucially found on biopsy. This patient is a 74-year-old nondiabetic male receiving aflibercept for AMD. Of the two prior cases of cFSGS in the setting of VEGF blockade, one had AMD and the other had DME. This case solidifies the finding of cFSGS and its association with chronic TMA as a lesion that may be frequently encountered in patients receiving intravitreal VEGF inhibitors.
Keywords: aflibercept, age-related macular degeneration, collapsing focal and segmental glomerulosclerosis, diabetic macular edema, diabetic retinopathy, intravitreal VEGF nephrotoxicity, nephrotic syndrome, thrombotic microangiopathy, vascular endothelial growth factor (VEGF) blockade
INTRODUCTION
Vascular endothelial growth factor (VEGF) inhibition has been used for nearly 30 years in oncologic indications [1]. The sequelae of their use are well known and well documented in that setting. Systemic hypertension (HTN), increased risk of venous thromboembolism, myocardial infarctions (MIs), cardiovascular events, proteinuria exacerbation (or de novo proteinuria) and glomerular diseases have been reported [1]. Intravitreal injections of VEGF inhibitors (VEGFis) were thought to not result in significant systemic absorption, with levels estimated as <200-fold the levels achieved with systemic injections [2]. Then pharmacokinetic studies showed that intravitreal injections result in systemic levels of VEGFis ≥50% inhibitory concentration (IC50) of these drugs [3, 4]. Further work demonstrated significant circulating VEGF depletion with the use of the more potent VEGFis [bevacizumab (Bev) and aflibercept (Aflib)] [5]. We aim to provide a systematic review of the data regarding this new class of potentially nephrotoxic drugs, updating significant developments and publications since our last reviews in Clinical Kidney Journal and other journals on this subject.
VEGF SIGNALING AND ONCOLOGIC USES OF VEGF INHIBITION
VEGF signaling is increasingly recognized as a key mediator in cellular proliferation and has been targeted directly via inhibition of cellular receptors, ligands and downstream mediators [6]. VEGF receptors are targeted by ramucirumab, ligand binding is accomplished by Bev, Aflib, ranibizumab (Ran) and pegaptanib [2]. Finally, downstream tyrosine kinase pathways are inhibited by a myriad of different inhibitors used for a variety of oncologic indications [5].
The most common systemic agents in use for the blockade of VEGF are Bev and Aflib. They are typically used in non-small cell lung cancer, renal cell carcinoma, breast cancer, colorectal cancer, glioblastoma and other solid organ malignancies [1]. There is an extensive body of literature, previously reviewed, that shows that VEGF inhibition systemically is known to carry a high risk of worsening HTN due to nitric oxide inhibition [2]. Interestingly, renal injury patterns in systemic VEGF manifestations are varied, but typically fall into thrombotic microangiopathy (TMA), collapsing focal segmental glomerulosclerosis (cFSGS) and nephrotic syndrome due to minimal change disease or other glomerulopathies [5].
RENAL VEGF SIGNALING AND PATHOPHYSIOLOGY IN ITS BLOCKADE
VEGF signaling is necessary for healthy podocyte function and endothelial function. The signaling mechanism of VEGF is thought to be paracrine in the podocyte and mediated via VEGF ligand binding to VEGF receptor 2 in endothelial cells and podocytes [6]. VEGF signaling controls renin–angiotensin–aldosterone receptor signaling, podocyte survival through Akt and actin cytoskeletal organization in the podocyte through CD2-associated protein (CD2AP) [6]. In the endothelial cells, nitric oxide signaling, endothelial cell survival and thrombosis via diacylglycerol kinase epsilon expression are subject to VEGF signaling [7–9]. See Figure 1 for a schematic of VEGF signaling in podocytes and endothelial cells.
FIGURE 1:
Molecular physiology of VEGF signaling in podocytes and endothelial cells and renal pathophysiology that ensues with VEGF blockade. (A) Molecular physiology and (B) pathophysiology with VEGF blockade. VEGF-A signaling to renal podocytes may be paracrine or mediated through VEGF-2 receptors. Akt, protein kinase B (PKB); C-MIP, C-Maf-inducing protein; DAG, diacyl glycerol; DGKE, diglyceride kinase epsilon; F-Act, F-actin; Fyn, proto-oncogene tyrosine-protein kinase fyn; GN, glomerulonephritis; Nck, NCK tyrosine kinase; NFkB, nuclear factor kappa light chain enhancer of activated B cells; NP1, neuronal pentraxin 1; N-WASP, Neural Wiskott–Aldrich syndrome protein; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; RAS, rat sarcoma protein; Red P, phosphoryl group; RelA, v-rel avian reticuloendotheliosis viral oncogene homolog A; SOS, son of sevenless; sVEG2R, soluble VEGF receptor 2; VEGF-A, VEGF receptor A; VEGFR2, VEGF receptor 2; Tie2, tyrosine-protein kinase receptor TIE-2. Twin nucleic acid strands = messenger RNA. Adapted from Hanna et al. (open access license) [6].
VEGF SIGNALING FOR OPHTHALMOLOGIC USES
The uses of VEGFis via the intravitreal route are classically diabetic retinopathy (DR) and associated neovascularization, central retinal vein obstruction and age-related macular degeneration (AMD) [2]. These agents have been valuable tools for stopping the angiogenesis underlying the neovascularization that results in destruction of the retina’s ability to sense and record light [10]. The first approved agent for AMD/DR was Bev (Avastin), which is an immunoglobulin G anti-VEGF2 antibody with standard structure [10]. Its manufacturer is Genentech and it is generally used off-label and has no US Food and Drug Administration (FDA) approval [10].
This was followed by the introduction of Aflib (Eylea), which is a dimerized soluble VEGF2 trap with four high-affinity binding sites for VEGF [2, 6, 10]. Its manufacturer is Regeneron and it was approved by the FDA in 2011 for DR/diabetic macular edema (DME) [2]. The latest agent is Ran (Lucentis), which is chemically distinct as a light chain anti-VEGF antibody. It was also introduced by Genentech and was approved by the FDA in 2012 for DR/DME [2].
PHARMACOKINETICS OF DRUG ABSORPTION AFTER INTRAVITREAL INJECTION
Ophthalmologic pharmacokinetics were not originally obtained when Bev was first introduced for DR/DME and AMD [2]. This began in 2004–10. Thus the first opportunity to explore the pharmacokinetics of these agents on a large scale was during the approval process of Aflib/Ran [2]. There was early concern for patients to develop side effects that were seen with systemic VEGF blockade use and registries were spontaneously created initially [2].
The US FDA in its approvals for Aflib and Ran stated that although some absorption was detected, it was 200-fold lower than the level expected to cause significant blockade of VEGF [11, 12]. The stated IC50 obtained was quite high, and whether the value referenced 50% inhibition of intravascular VEGF versus total somatic VEGF is unclear [11, 12].
Avery et al. [3, 4] in 2014 and again in 2017 showed reproducible, consistent evidence that intravitreal injections of VEGFis resulted in significant systemic exposure. The IC50 used to compare these data was the IC50 for 50% inhibition of intravascular VEGF [3, 4]. Supporting animal data showed intravascular levels of VEGF decreased and that high-potency VEGFis like Aflib were found bound to simian glomeruli 7 days later [13]. The levels of VEGF inhibition were not uniform across agents, with Aflib presenting the most severe VEGF blockade for the longest time [3, 4, 13], Bev presenting moderate VEGF blockade for a prolonged time [3, 4, 13] and Ran presenting the least intravascular VEGF blockade for the shortest period of time postinjection [3, 4, 13]. The systemic concentrations for all three agents were at or greater than the IC50 at the peak systemic concentration after intravitreal injection (see Table 1 for comparison of the intravitreal dosing, range of systemic drug levels and IC50 used in the pharmacokinetic evaluation) [3, 4, 13]. Multiple studies also confirm depleted VEGF levels intravascularly after intravitreal injection [14–18].
Table 1.
Pharmacokinetics of intravitreal VEGFi’s
| Agent | Oncologic dosing (mg/kg every 2 weeks) | Intravitreal dosing (every 2 weeks) | Serum (drug) post-intravitreal injection (nmol) | IC50 ( nmol) | Half life | Days drug >IC50 |
|---|---|---|---|---|---|---|
| Bev | 5–15 | 1.25–2.5 mg | 0.37–1.58 | 0.67 | 18.7 | 15–20 |
| Aflib | 2–7 | 2–4 mg | 0.04–0.76 | 0.06 | 18.7 | 22–33 |
| Ran | Not applicable | 0.3–0.5 mg | 0.0015–0.08 | 0.06 | 5.8 | 1–2 |
POPULATION STUDIES REGARDING VEGF BLOCKADE EFFECTS
While systemic VEGF inhibition for solid malignancies has been known to cause worsening HTN, proteinuria and thromboembolism, intravitreal VEGF inhibition has had mixed results on detectable effects on a population level. Hanhart et al. [19–21] demonstrated an increased all-cause mortality, post-cardiovascular event mortality and post-cerebrovascular event mortality. In addition, Avery et al. [22] reported an increased risk of stroke. Dalvin et al. [23] and Starr et al. [24] did not reproduce these effects. Further research is needed to investigate whether these effects are more prominent in certain subgroups of patients with comorbidities that may predispose to renal dysfunction, HTN, proteinuria, cardiovascular events, cerebrovascular events or other deleterious sequelae.
CLINICAL STUDIES REGARDING INTRAVITREAL VEGF BLOCKADE EFFECTS
Clinical studies have been more suggestive in demonstrating systemic effects after intravitreal VEGF injections [2]. There were two negative studies by Lee et al. [25] and Risimic et al. [26] for worsening HTN risk, but two studies by Bagheri et al. [27] and Rasier et al. [28] showed worsening HTN risk. Anjali et al. [29] demonstrated a link between the need for more intravitreal VEGFis and higher blood pressure.
Most studies have demonstrated no difference in kidney function after intravitreal VEGF injection [30] and Glassman et al. [31] demonstrated no change in proteinuria category after intravitreal VEGF injection. Finally, O’Neill et al. [32] found no link between the number of VEGFis given intravitreally and proteinuria worsening, although recently Bagheri et al. [27] noted that 45% of patients experienced worsening urine microalbumin:creatinine ratios after intravitreal injections, although not statistically significant. In another observational study, published as an abstract in Nephrology Dialysis and Transplantation, Maisarah et al. [33] showed a 4% risk of acute kidney injury and increased urine protein:creatinine ratio (UPCR) in patients after intravitreal VEGFis. Although a limited study, it presents an opposite result from Glassman et al. [31], O’Neill et al. [32] and Kumasaka et al. [34]. A more robust study by Chung et al. [35] shows worsening proteinuria after intravitreal injections, predominantly in patients with high-grade pre-existing proteinuria.
The general conclusion from the differing results is that the systemic effects of intravitreal VEGF inhibition are more subtle than the systemic side effects of systemic VEGF inhibition [2]. Chung et al. [35] established the hypothesis that patients with worse renal disease, proteinuria, HTN and possibly other unknown parameters may be differentially severely affected by VEGF blockade. See Table 2 for studies demonstrating population and systemic effects of intravitreal VEGF blockade.
Table 2.
Review of literature on systemic effects of intravitreal anti-VEGF injection
| Systemic effect, pathology study type, study name, reference |
|---|
|
| Absorption in AMD, dec. systemic VEGF (Bev, Aflb) > Ran, prospective observational study, Avery et al. [3] |
| Absorption in AMD/DME/CRVO, dec. systemic VEGF, prospective observational study, Avery et al. [4] (Bev, Aflib)>Ran |
| Dec. systemic VEGF (Bev, Aflib), prospective non-randomized clinical study, Hirano et al. [14] |
| Dec. systemic VEGF (Bev, Aflib) > Ran, prospective randomized clinical study, Jampol et al. [15] |
| Absorption of drug in AMD, dec. systemic VEGF, retrospective study of RCT data, Rogers et al. [16] |
| Bev > Ran, dec. in systemic VEGF, prospective observational study, Yoon et al. [17] |
| Dec. systemic VEGF (Bev, Aflib), prospective randomized observational study, Zehetner et al. [18] |
|
| Absorption of drug, binding at glomerulus, animal (simian) study, Tschulakow et al. [13] |
|
| Limited short-term increase in blood pressure at 1 h, prospective observational study, Lee et al. [25] |
| No significant change in blood pressure, observational study, Risimic et al. [26] |
| Long- and short-term increase in systolic blood pressure, observational study, Rasier et al. [28] |
| Higher blood pressure linked to need for more VEGFi, retrospective study, Anjali et al. [29] |
|
| Increased proteinuria 45% of patients (not statistically significant), prospective observational study, Bagheri et al. [27] |
| Significant increase in diastolic blood pressure |
| Significant increase in hemoglobin and platelets |
| No change in eGFR 7–30 days after injection (Bev, Aflib and Ran), retrospective observational study, Kameda et al. [30] |
| No long-term change in HTN or category of albuminuria, planned retrospective analysis of trial, Glassman et al. [31] |
| No association with number of VEGFi injections and proteinuria, retrospective observational study, O’Neill et al. [32] |
| 4% of patients with AKI and elevated UPCR after VEGFi, retrospective observational study, Maisarah et al. [33] |
| Significant increase in UPCR in patients with pre-existing proteinuria, prospective observational study, Chung et al. [35] |
|
| Increased all-cause mortality in AMD patients, retrospective observational studya, Hanhart et al. [19] |
| Increased risk of mortality after MI in AMD patients, retrospective observational studyb, Hanhart et al. [20] |
| Increased risk of mortality after CVA in AMD patients, retrospective observational studyb, Hanhart et al. [21] |
| Increased risk of CVA in DME patients, meta-analysis, Avery and Gordon [22] |
| No finding of CVA, MI and AC mortality in AMD patients, retrospective observational studyb, Dalvin et al. [23] |
| No finding of increased CVA in DME patients, retrospective bservational studyb, Starr et al. [24] |
Number of injections.
Age- and gender-matched controls served as a comparator group.
Age- and gender-matched controls with a cardiovascular or cerebrovascular event served as a comparator group. CRVO, central retinal vein obstruction; CVA, cerebrovascular accident; dec., decreased; eGFR, estimated glomerular filtration rate; RCT, randomized controlled trial. Green lettering = positive result linking VEGFi and renal outcome; orange lettering = equivocal result; red lettering = negative result.
CASE REPORTS DEMONSTRATING RENAL PATHOLOGY AFTER INTRAVITREAL VEGF INJECTIONS
Currently there are 32 cases documenting worsening HTN, proteinuria exacerbation and glomerular diseases after intravitreal VEGF blockade [2, 5, 6, 10, 36–53]. There is an additional two cases of cFSGS [5, 46], which is a lesion related to hypoxia from chronic renal TMA [54]. Two of the newer cases are noted in this updated review: a case linking cerebral hemorrhage with intravitreal VEGF injection and HTN [44] with evidence of depleted serum VEGF, as well as this current case with both cFSGS and TMA simultaneously present in the same kidney biopsy. This case also features a finding of systemic VEGF depletion, strongly suggesting a role for intravitreal VEGF blockade in the pathology.
We present the case of this patient who is a nondiabetic man receiving intravitreal Aflib for AMD. The finding of both cFSGS and TMA on biopsy in this setting is instructional, novel and reinforces the link between both pathologies and intravitreal VEGF blockade. See Table 3 for a compilation of the aforementioned clinical cases.
Table 3. Clinical reports of intravitreal VEGFi toxicity.
| References | Patients, n | Agent used | Clinical effect(s), renal pathology |
|---|---|---|---|
| Shye et al. [5] | 3 |
Case 1 Bev→Ran Case 2 Bev Case 3 Bev→Ran |
All: increased proteinuria, CKD progression, HD Case 1: worsening proteinuria, CKD progression, HD Case 2: DN + FSGS with collapsing features + AIN (biopsy+) Case 3: DN + AIN + low systemic VEGF level (biopsy+) |
| Hanna et al. [6] | 4 | Bev and Ran |
Case 1: de novo MCD (biopsy+) Cases 2–4: increased proteinuria, CKD progression, HTN worsening |
| Hanna et al. [10] | 1 | Bev→Ran |
Worsening HTN and proteinuria, lessened with Ran use versus Bev |
| Cheungpasitporn et al. [36] | 2 | Bev |
Case 1: MGN Case 2: TMA (biopsy+) |
| Scott et al. [37] | 3 | Bev | Decreased eGFR |
| Georgalas et al. [38] | 2 | Ran and Bev | Decreased eGFR; HD started |
| Hanna et al. [39] | 1 | Bev | Case 1: scleroderma renal crisis and TMA induced after intravitreal VEGFi and oral corticosteroids. |
| Jamrozy-Witkowska et al. [41] | 1 | NR | Decreased eGFR |
| Kenworthy et al. [42] | 1 | Bev | Increased proteinuria |
| Khneizer et al. [43] | 1 | Bev | MGN (biopsy+) |
| Miwako et al. [44] | 1 | Aflib | Case 1: hypertensive hemorrhage with undetectable VEGF plasma levels after intravitreal injection (preprint) |
| Morales et al. [45] | 1 | Ran | DN (biopsy+) |
| Nobakht et al. [46] | 1 | Bev→Ran→Aflib | cFSGS (biopsy+) + low systemic VEGF level |
| Pelle et al. [47] | 1 | Ran | TMA (biopsy+) |
| Perez-Valdivia et al. [48] | 1 | Bev | Relapsed MCD (biopsy+) |
| Hanna et al. [49] | 3 |
Bev (Cases 1,2) Aflib (Case 3) |
Cases 1 and 2: DN and chronic TMA (biopsy+) Case 3: FSGS with chronic TMA features (biopsy +) |
| Sato et al. [50] | 1 | Bev | Relapsed MCD (biopsy+) |
| Touzani et al. [51] | 1 | Bev | Endotheliosis/possible TMA (biopsy+) |
| Tran et al. [52] | 1 | Bev | AIN (biopsy+) |
| Yen and Zhang [53] | 1 | Bev | TMA (biopsy+) |
| Phadke-Hanna et al. (this study) | 1 | Ran→Aflib |
cFSGS + chronic TMA (biopsy+) Low serum VEGF level Worsening renal disease and HTN with switch from low potency agent (Ran) to high potency agent (Aflib) |
AIN, acute interstitial nephritis; biopsy+, biopsy obtained; CKD, chronic kidney disease; DM, diabetes mellitus; DN, diabetic nephropathy; HD, hemodialysis started; MCD, minimal change disease; MGN, membranous GN; . Biopsy only if biopsy+ stated. Adapted from Shye et al. [15] with permission and updated.
CASE REPORT
A 74-year-old Caucasian male was evaluated for rapidly worsening creatinine and uncontrolled HTN over a period of 6–8 months. The patient had a history of HTN for 35 years, was nondiabetic with hemoglobin A1c at 5.1% 2 months prior to presentation and he had never smoked. His blood pressure (8 months prior) was 140–160/70–80 mmHg on nifedipine XL 30 mg/day, losartan 100 mg/day, diltiazem 300 mg/day, hydralazine 100 mg thrice daily and furosemide 40 mg/day. At presentation, his blood pressure was noted to be 220/110 mmHg. The patient’s creatinine progressively worsened from 1.4–1.7 mg/dL at baseline to 2.4 mg/dL within 4 months and 5.2 mg/dL at 6 months. Urine protein was 1+ (at baseline) on dipstick, but at presentation showed albuminuria and a spot UPCR of 5.2 without hematuria.
Nine months prior to presentation the patient was diagnosed with bilateral macular degeneration and was started on intravitreal Aflib for 6 months. He was then switched to intravitreal Ran 8 weeks prior to presentation. Extensive serological workup including antinuclear antibodies, anti-double-stranded DNA, anti-myeloperoxidase antibodies, anti-prtoteinase 3 antibodies, anti-glomerular basement membrane antibodies, serum electrophoresis and immunofixation, kappa:lambda light chain ratio, anti-phospholipase A2 receptor antibodies, human immunodeficiency virus and hepatitis B and C were all negative. Renal ultrasound with duplex showed the right kidney was 11.2 cm with peak systemic velocity at 183 cm/s in the right main renal artery and the left kidney was 11.5 cm with peak systemic velocity at 186 cm/s in the left main renal artery, suggesting no evidence of clinically significant renal artery stenosis. Serum aldosterone was 4 ng/dL and plasma renin was undetectable. The plasma VEGF level was 33 pg/mL.
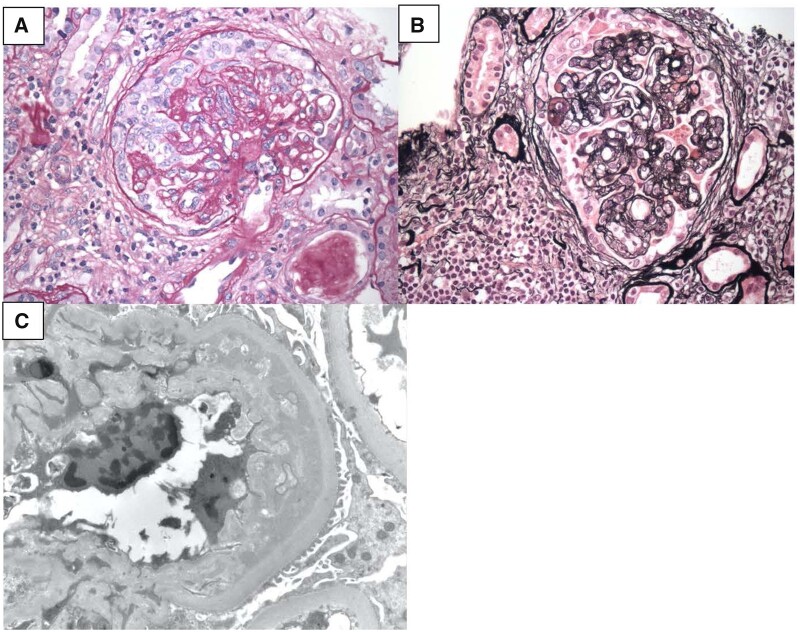
Given the rapidly worsening creatinine, the patient underwent kidney biopsy that revealed glomeruli with early collapsing features, minimal podocyte proliferation and double contouring of the glomerular basement membrane. Interstitial scarring was 20–25% of the biopsy sample. Electron microscopy confirmed extensive foot process effacement. A diagnosis of FSGS was made with features of chronic TMA. We attributed the temporal relationship of uncontrolled blood pressure and worsening creatinine from collapsing FSGS to the use of intravitreal VEGFi. Figure 2 reviews the renal biopsy slides showing cFSGS and TMA seen in our patient’s history.
FIGURE 2:
Biopsy data showing cFSGS and TMA after intravitreal VEGF injections. (A) Periodic acid–Schiff stain, 40×, showing features of collapsing FSGS. (B) Silver stain, 40×, showing double contouring of glomerular basement membrane seen in chronic TMA lesions. (C) Electron microscopy showing splitting/double contouring of glomerular basement membrane seen in chronic TMA lesions.
Ran was discontinued after discussion with the patient’s ophthalmologist. Prednisone was started at 60 mg/day with a tapering schedule over 8–10 weeks. At the time of the last clinic visit (3 weeks after stopping the medication), the patient’s blood pressure was 130–140/70–80 mmHg and creatinine had improved to 3.2 mg/dL. If the patient does not respond, supportive care will be provided and one consideration will be the use of eculizumab as therapy for the secondary TMA, as systemic VEGF depletion is expected to result in a complement-mediated TMA.
DISCUSSION
The presented case is valuable as it confirms several prior observations about the results of intravitreal VEGF blockade [55]. The risk of high-potency VEGFis when given intravitreally is observed here [55]. The association of cFSGS and TMA is confirmed and the risk of intravitreal VEGF blockade to induce TMA through VEGF depletion is also confirmed [54]. TMA (and cFSGS by association with TMA) are further suggested as possible pathognomonic lesions of VEGF blockade [46]. This case supports the hypothesis that these lesions are the result of nephropathy due to VEGF depletion that occurs in patients receiving systemic and intravitreal VEGFis [2].
It is important to note that these lesions of TMA and cFSGS have now been documented in patients with DR [5] and AMD [46]. This strongly suggests that the link between these cases is the intravitreally injected VEGF blockade. For several years, observed systemic VEGF suppression was not linked to known clinical outcomes [2, 30–32].
As this review suggests, these cases, and increasingly large-scale studies, show clinical outcomes and population-based outcomes demonstrating pathophysiological effects after intravitreal VEGF injections [2, 5, 6, 49]. There are other studies that do not demonstrate these outcomes [30–32], and this suggests that there exists a subgroup or subgroups of patients at risk for renal injury from intravitreal VEGF blockade. These/ subgroups could be patients who are exposed to higher doses, experience greater drug absorption or have more severe nephropathy or HTN as comorbidities [10]. It is also possible that mutations that modify the risk of TMA (like alternative complement pathway mutations) may have a role to play in disease causation.
Recommendations were published previously describing monitoring of patients receiving intravitreal VEGF blockade. An increase in blood pressure of ≥20 mmHg, an increase in serum creatinine of ≥25% and an increase in proteinuria ≥25% are all suggested to trigger further investigation. As more patients undergo renal biopsies, the true scale of risk from intravitreal VEGF inhibition will become clearer.
Further studies are required to confirm the rate of glomerular disease occurence in these patients, as well as the absolute risk of HTN in patients receiving VEGF inhibition [33]. Studies in diabetics are likely to yield a higher event rate, since they have worse baseline nephropathy [2], although it is likely that some patients receiving these agents for AMD are at risk as well [46]. The use of lower-potency agents like Ran theoretically offers a strategy to limit the risks of worsening renal disease and HTN while preserving vision [10]. Ran, however, also needs to be thoroughly studied to make sure that the risk factor profile it offers is superior to higher-potency VEGF blocking agents [2, 49]. Another important avenue is to determine if detectable serum VEGF depletion (especially VEGF-C) translates into a subgroup of patients receiving intravitreal VEGF blockers who may be predisposed to poorer clinical outcomes [44].
ACKNOWLEDGEMENTS
Consent was obtained from the patient and documented on the condition that no identifiable data be published. This research work does not contain human subject research material, as it is an individual anonymized case series. The work herein conforms with the Declaration of Istanbul.
CONFLICT OF INTEREST STATEMENT
I.K. is supported in part by funds from the National Insitutes of Health (R01-DK077162), the Allan Smidt Charitable Fund, the Factor Family Foundation and the Ralph Block Family Foundation. K.K.Z. is supported by the National Institute on Aging of the National Institutes of Health (R21-AG047036) and the National Institute of Diabetes, Digestive and Kidney Disease (R01-DK078106, R01-DK096920, U01-DK102163 and K24-DK091419), as well as philanthropist grants from Mr. Harold Simmons and Mr. Louis Chang. The authors declare no conflicts of interest.
REFERENCES
- 1.Hanna RM, Lopez E, Wilson J. et al. Minimal change disease onset observed after bevacizumab administration. Clin Kidney J 2016; 9: 239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanna RM, Barsoum M, Arman F. et al. Nephrotoxicity induced by intravitreal vascular endothelial growth factor inhibitors: emerging evidence. Kidney Int 2019; 96: 572–580 [DOI] [PubMed] [Google Scholar]
- 3.Avery RL, Castellarin AA, Steinle NC. et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 2014; 98: 1636–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avery RL, Castellarin AA, Steinle NC. et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina 2017; 37: 1847–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shye M, Hanna RM, Patel SS. et al. Worsening proteinuria and renal function after intravitreal vascular endothelial growth factor blockade for diabetic proliferative retinopathy. Clin Kidney J 2020; 13: 969–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna RM, Lopez E, Hasnain H. et al. Three patients with injection of intravitreal vascular endothelial growth facto inhibitors and subsequent exacerbation of chronic proteinuria and hypertension. Clin Kidney J 2018; 12: 92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izzedine H.Anti-VEGF cancer therapy in nephrology practice. Int J Nephrol 2014; 2014: 143426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izzedine H, Brocheriou I, Deray G, Rixe O.. Thrombotic microangiopathy and anti-VEGF agents. Nephrol Dial Transplant 2007; 22: 1481–1482 [DOI] [PubMed] [Google Scholar]
- 9.Izzedine H, Sene D, Hadoux J. et al. Thrombotic microangiopathy related to anti-VEGF agents: intensive versus conservative treatment? Ann Oncol 2011; 22: 487–490 [DOI] [PubMed] [Google Scholar]
- 10.Hanna RM, Abdelnour L, Hasnain H. et al. Intravitreal bevacizumab-induced exacerbation of proteinuria in diabetic nephropathy, and amelioration by switching to ranibizumab. SAGE Open Med Case Rep 2020; 8: 2050313X20907033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eylea. FDA package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125387lbl.pdf
- 12.Lucentis. FDA package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/125156lbl.pdf
- 13.Tschulakow A, Christner S, Julien S. et al. Effects of a single intravitreal injection of aflibercept and ranibizumab on glomeruli of monkeys. PLoS One 2014; 9: e113701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirano T, Toriyama Y, Iesato Y. et al. Changes in plasma vascular endothelial growth factor level after intravitreal injection of bevacizumab, aflibercept, or ranibizumab for diabetic macular edema. Retina 2018; 38: 1801–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jampol LM, Glassman AR, Liu D. et al. Plasma vascular endothelial growth factor concentrations after intravitreous anti-vascular endothelial growth factor therapy for diabetic macular edema. Ophthalmology 2018; 125: 1054–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers CS, Reeves BC, Downes S. et al. Serum vascular endothelial growth factor levels in the IVAN trial; relationships with drug, dosing, and systemic serious adverse events. Ophthalmol Retina 2018; 2: 118–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon MH, Young JK, So YL. et al. Effects of intravitreal injection of bevacizumab or eanibizumab on systemic circulation. J Korean Ophthalmol Soc 2016; 57: 429 [Google Scholar]
- 18.Zehetner C, Kralinger MT, Modi YS. et al. Systemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age-related macular degeneration: a randomised, prospective trial. Acta Ophthalmol 2015; 93: e154–e159 [DOI] [PubMed] [Google Scholar]
- 19.Hanhart J, Comaneshter DS, Freier Dror Y. et al. Mortality in patients treated with intravitreal bevacizumab for age-related macular degeneration. BMC Ophthalmol 2017; 17: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanhart J, Comaneshter DS, Freier-Dror Y. et al. Mortality associated with bevacizumab intravitreal injections in age-related macular degeneration patients after acute myocardial infarct: a retrospective population-based survival analysis. Graefes Arch Clin Exp Ophthalmol 2018; 256: 651–663 [DOI] [PubMed] [Google Scholar]
- 21.Hanhart J, Comaneshter DS, Vinker S.. Mortality after a cerebrovascular event in age-related macular degeneration patients treated with bevacizumab ocular injections. Acta Ophthalmol 2018; 96: e732–e739 [DOI] [PubMed] [Google Scholar]
- 22.Avery RL, Gordon GM.. Systemic safety of prolonged monthly anti-vascular endothelial growth factor therapy for diabetic macular edema: a systematic review and meta-analysis. JAMA Ophthalmol 2016; 134: 21–29 [DOI] [PubMed] [Google Scholar]
- 23.Dalvin LA, Starr MR, AbouChehade JE. et al. Association of intravitreal anti-vascular endothelial growth factor therapy with risk of stroke, myocardial infarction, and death in patients with exudative age-related macular degeneration. JAMA Ophthalmol 2019; 137: 483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starr MR, Dalvin LA, AbouChehade JE. et al. Classification of strokes in patients receiving intravitreal anti-vascular endothelial growth factor. Ophthalmic Surg Lasers Imaging Retina 2019; 50: e140–e157 [DOI] [PubMed] [Google Scholar]
- 25.Lee K, Yang H, Lim H. et al. A prospective study of blood pressure and intraocular pressure changes in hypertensive and nonhypertensive patients after intravitreal bevacizumab injection. Retina 2009; 29: 1409–1417 [DOI] [PubMed] [Google Scholar]
- 26.Risimic D, Milenkovic S, Nikolic D. et al. Influence of intravitreal injection of bevacizumab on systemic blood pressure changes in patients with exudative form of age-related macular degeneration. Hellenic J Cardiol 2013; 54: 435–440 [PubMed] [Google Scholar]
- 27.Bagheri SD, Afarid M, Sagheb MM.. Proteinuria and renal dysfunction after intravitreal injection of bevacizumab in patients with diabetic nephropathy: a prospective observational study. Galen Med J 2018; 7: e1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasier R, Artunay O, Yuzbasioglu E. et al. The effect of intravitreal bevacizumab (avastin) administration on systemic hypertension. Eye (Lond) 2009; 23: 1714–1718 [DOI] [PubMed] [Google Scholar]
- 29.Anjali RS, Van Horn AN, Lilia V. et al. Blood pressure is associated with receiving intravitreal anti-vascular endothelial growth factor treatment in patients with diabetes. Opththalmol Retina 2019; 3: 410–416 [DOI] [PubMed] [Google Scholar]
- 30.Kameda Y, Babazono T, Uchigata Y. et al. Renal function after intravitreal administration of vascular endothelial growth factor inhibitors in patients with diabetes and chronic kidney disease. J Diabetes Investig 2018; 9: 937–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glassman AR, Liu D, Jampol LM. et al. Changes in blood pressure and urine albumin-creatinine ratio in a randomized clinical trial comparing aflibercept, bevacizumab, and ranibizumab for diabetic macular edema. Invest Ophthalmol Vis Sci 2018; 59: 1199–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neill RA, Gallagher P, Douglas T. et al. Evaluation of long-term intravitreal anti-vascular endothelial growth factor injections on renal function in patients with and without diabetic kidney disease. BMC Nephrol 2019; 20: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maisarah J, Kamalden TAFT, Ismail NAS. et al. Adverse renal outcome following administration of intravitreal anti-vascular endothelial growth factor inhibitors in a single tertiary centre in Malaysia. Nephrol Dial Transplant 2020; 35(Suppl 3): gfaa142.P0590 [Google Scholar]
- 34.Kumasaka R, Nakamura N, Shirato K. et al. Side effects of therapy: case 1. Nephrotic syndrome associated with gefitinib therapy. J Clin Oncol 2004; 22: 2504–2505 [DOI] [PubMed] [Google Scholar]
- 35.Chung YR, Kim YH, Byeon HE. et al. Effect of a single intravitreal injection of vevacizumab on proteinuria in patients with diabetes. Translat Vis Sci Tech 2020; 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheungpasitporn W, Chebib FT, Cornell LD. et al. Intravitreal antivascular endothelial growth factor therapy may induce proteinuria and antibody mediated injury in renal allografts. Transplantation 2015; 99: 2382–2386 [DOI] [PubMed] [Google Scholar]
- 37.Scott IU, Edwards AR, Beck RW. et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology 2007; 114: 1860–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Georgalas I, Papaconstantinou D, Papadopoulos K. et al. Renal injury following intravitreal anti-VEGF administration in diabetic patients with proliferative diabetic retinopathy and chronic kidney disease – a possible side effect? Curr Drug Saf 2014; 9: 156–158 [DOI] [PubMed] [Google Scholar]
- 39.Hanna RM, Abdelnour L, Zuckerman JE. et al. Refractory scleroderma renal crisis precipitated after high-dose oral corticosteroids and concurrent intravitreal injection of bevacizumab. SAGE Open Med Case Rep 2020; 8: 2050313X20952650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanna RM, Selamet U, Hasnain H. et al. Development of focal segmental glomerulosclerosis and thrombotic microangiopathy in a liver transplant patient on sorafenib for hepatocellular carcinoma: a case report. Transplant Proc 2018; 50: 4033–4037 [DOI] [PubMed] [Google Scholar]
- 41.Jamrozy-Witkowska A, Kowalska K, Jankowska-Lech I. et al. [Complications of intravitreal injections–own experience]. Klin Oczna 2011; 113: 127–131 [PubMed] [Google Scholar]
- 42.Kenworthy JA, Davis J, Chandra V. et al. Worsening proteinuria following intravitreal anti-VEGF therapy for diabetic macular edema. J Vitreo Retinal Dis 2019; 3: 54–56 [Google Scholar]
- 43.Khneizer G, Al-Taee A, Bastani B.. Self limited membranous nephropathy after intravitreal nephropathy after intravitreal bevacizumab therapy for age related macular degeneration. J Nephropathol 2017; 6: 134–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44., Yoshimoto M, Takeda N, Yoshimoto TMatsumoto S.. Hypertensive cerebral hemorrhage with undetectable plasma VEGF after intravitreal injection of aflibercept for diabetic macular edema. Authorea 2020; doi: 10.22541/au.159986309.90198834 [DOI] [PMC free article] [PubMed]
- 45.Morales E, Moliz C, Gutierrez E.. Renal damage associated to intravitreal administration of ranibizumab. Nefrologia 2017; 37: 653–655 [DOI] [PubMed] [Google Scholar]
- 46.Nobakht NN, Kamgar M, Abdelnour L. et al. Development of collapsing focal and segmental glomerulosclerosis in a patient receiving intravitreal vascular endothelial growth factor blockade. Kidney Int Rep 2019; 4: 1508–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pellé G, Shweke N, Duong Van Huyen JP. et al. Systemic and kidney toxicity of intraocular administration of vascular endothelial growth factor inhibitors. Am J Kidney Dis 2011; 57: 756–759 [DOI] [PubMed] [Google Scholar]
- 48.Perez-Valdivia MA, Lopez-Mendoza M, Toro-Prieto FJ. et al. Relapse of minimal change disease nephrotic syndrome after administering intravitreal bevacizumab. Nefrologia 2014; 34: 421–422 [DOI] [PubMed] [Google Scholar]
- 49.Hanna N-Tt RM, Patel SS, Hou J. et al. Thrombotic microangiopathy and acute kidney injury induced after intravitreal injection of vascular endothelial growth factor inhibitors VEGF blockade-related TMA after intravitreal use. Front Med 2020; 7: 579603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato T, Kawasaki Y, Waragai T. et al. Relapse of minimal change nephrotic syndrome after intravitreal bevacizumab. Pediatr Int 2013; 55: e46–e48 [DOI] [PubMed] [Google Scholar]
- 51.Touzani F, Geers C, Pozdzik A.. Intravitreal injection of anti-VEGF antibody induces glomerular endothelial cells injury. Case Rep Nephrol 2019; 2019: 2919080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tran T.Intravitreal VEGF inhibitor causing allergic interstitial nephritis. Am J Kidney Dis 2017; 69: A99 [Google Scholar]
- 53.Yen W, Zhang P.. Intravitreal injection of avastin (IIA) over time can be associated with thromobtic microangiopathy (TMA) in the native kidney. Presnted at : ASN Kidney Week, 2019 [Google Scholar]
- 54.Buob D, Decambron M, Gnemmi V. et al. Collapsing glomerulopathy is common in the setting of thrombotic microangiopathy of the native kidney. Kidney Int 2016; 90: 1321–1331 [DOI] [PubMed] [Google Scholar]
- 55.Friedlander AUO.Anti-VEGF therapy: higher potency and long-lasting antagonism are not necessarily better. J Clin Invest 2019; 129: 3032–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]