Original citation: J Clin Invest. 2021;131(13):e150319. https://doi.org/10.1172/JCI150319
Citation for this corrigendum: J Clin Invest. 2021;131(19):e154834. https://doi.org/10.1172/JCI154834
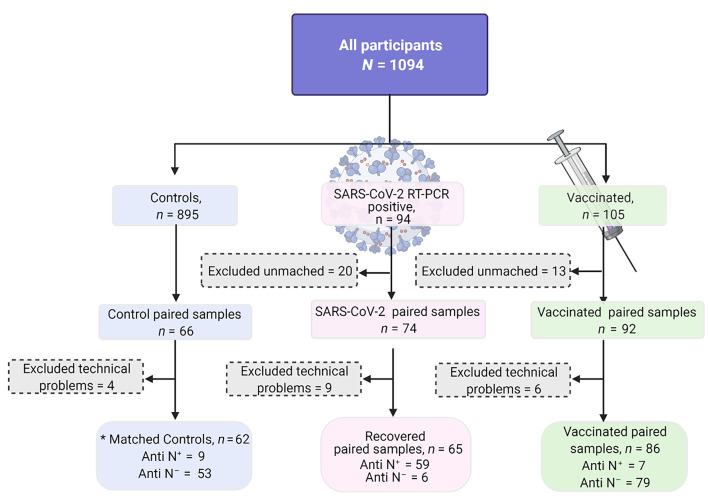
For clarity, the authors are updating Figure 1, the flow chart for this study, to better illustrate the excluded samples and criteria for exclusion. The HTML and PDF versions of the figure and its legend have been updated.
Figure 1. Patient selection flow chart.
Patients were recruited from 8 medical centers in Israel and were all SARS-CoV-2 RT-PCR negative at delivery. The original study groups included all the paired samples from the vaccinated (n = 92) and SARS-CoV-2 (n = 74) groups together with the original matched control group (n = 66). Samples were thereafter excluded for technical testing problems, leading to a total of n = 62, n = 65, and n = 86 analyzed samples for the control, SARS-CoV-2, and vaccinated groups, respectively. Seropositivity for nucleocapsid (N) was set at the level of the top 90% of the PCR-positive recovered group and verified by positivity for S1, S2, and RBD (see Supplemental Figures 1 and 2). The same threshold was used to reveal seropositive cases in the vaccinated and the control groups.
Version 1. 10/01/2021
Electronic publication
Footnotes
See the related article at Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine.