Abstract
Circadian disruption is pervasive and can occur at multiple organizational levels, contributing to poor health outcomes at individual and population levels. Evidence points to a bidirectional relationship, in that circadian disruption increases disease severity and many diseases can disrupt circadian rhythms. Importantly, circadian disruption can increase the risk for the expression and development of neurologic, psychiatric, cardiometabolic, and immune disorders. Thus, harnessing the rich findings from preclinical and translational research in circadian biology to enhance health via circadian-based approaches represents a unique opportunity for personalized/precision medicine and overall societal well-being. In this Review, we discuss the implications of circadian disruption for human health using a bench-to-bedside approach. Evidence from preclinical and translational science is applied to a clinical and population-based approach. Given the broad implications of circadian regulation for human health, this Review focuses its discussion on selected examples in neurologic, psychiatric, metabolic, cardiovascular, allergic, and immunologic disorders that highlight the interrelatedness between circadian disruption and human disease and the potential of circadian-based interventions, such as bright light therapy and exogenous melatonin, as well as chronotherapy to improve and/or modify disease outcomes.
Circadian (circa, “about,” and diem, “day”) rhythms are endogenous oscillations with an approximately 24-hour cycle. Circadian rhythms in physiology and behavior are organized by a central “master” clock, the suprachiasmatic nucleus (SCN) located in the anterior hypothalamus, that coordinates alignment between external synchronizing agents with circadian clocks in other brain regions, as well as in peripheral tissues. Circadian rhythms are modulated by endogenous (genetic, physiological) as well as environmental (light) and behavioral (activity, feeding) factors. In this Review, we use “circadian disruption” as a nonspecific umbrella term to describe a disturbance, dysregulation, or problem that negatively affects circadian function, but as Vetter has pointed out, there is a need for clearer terminology and quantification of circadian disruption (1). It is becoming increasingly clear that circadian disruption in humans can result in broad and significant consequences for mental and physical health (2, 3). Furthermore, changes in circadian function are often accompanied by sleep-wake disturbances, which also contribute to poor health outcomes. The interrelationship between circadian rhythms and human disease can create a vicious cycle between disease expression and circadian disruption, as exemplified in immunologic (4, 5), cardiometabolic (6), neurodegenerative, and psychiatric disorders (7).
General mechanisms of circadian disruption
In humans, measures of circadian disruption include the phase (timing), relationship between internal-internal or internal-external rhythms (alignment), the period and amplitude of circadian rhythms. Disturbance of circadian phase alignment and amplitude are the most common measures that have been associated with adverse health consequences. As outlined in Figure 1, circadian disruption can occur at multiple levels, from intrinsic changes at the molecular, cellular, tissue, or system level to misalignment among different organizational levels and/or with behavioral and environmental cycles. The “molecular circadian clock” refers to genes that maintain autoregulatory feedback loops in which oscillating outputs regulate their own expression (circadian locomotor output cycles kaput [CLOCK], brain and muscle ARNT-like [BMAL], period [PER], rev-erb/nuclear receptor subfamily 1, group D [NR1D], and cryptochrome [CRY]; ref. 4).
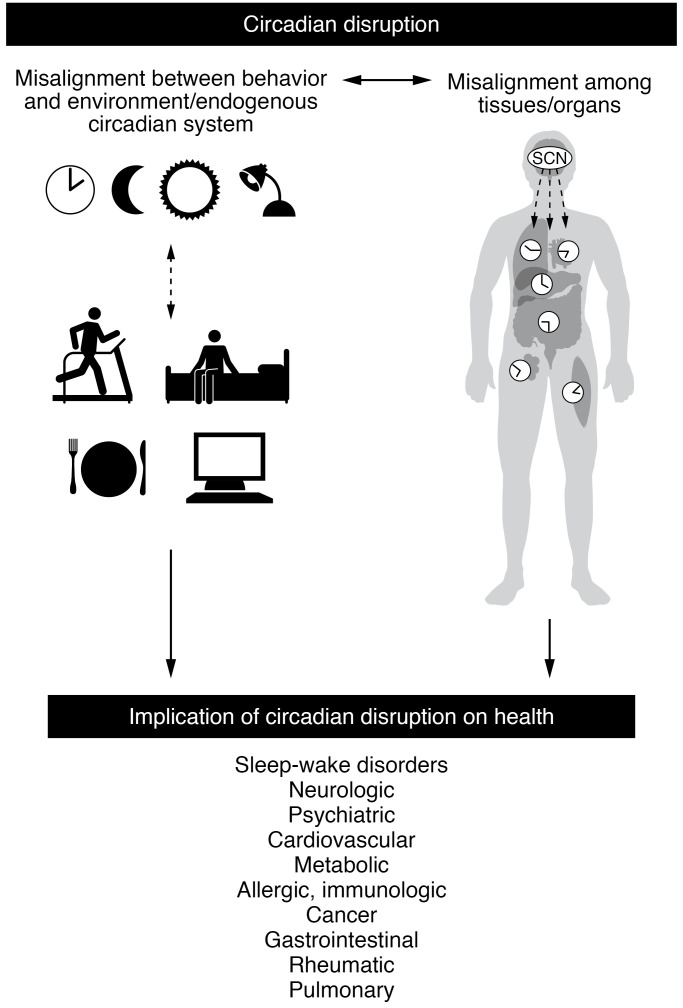
Figure 1. Schematic of the interrelationships between circadian disruption and human disease.
As depicted on the top left, circadian disruption can result from misalignment of external factors (such as the natural light/dark cycle, social and work requirements, and behaviors such as sleep and meal timing) with the master circadian clock located in the suprachiasmatic nucleus (SCN), as well as with endogenous circadian clocks in other tissues. Common examples of chronic external-internal misalignment include social jet lag, shift work, and misalignment due to inappropriately timed light exposure (evening or night). The top right image depicts the SCN and the peripheral clocks throughout the body. The SCN integrates light and nonphotic stimuli to synchronize the timing of other brain and body clocks with the environment and behaviors. Disturbances at these various organizational levels result in circadian disruption. Disturbance in the phase and amplitude of circadian rhythms can occur at the molecular, cellular, and organismal levels. On the bottom are examples of the impact of circadian disruption and the bidirectionality between circadian disruption and the classical circadian sleep-wake disorders and other chronic medical disorders.
Biomarkers of circadian rhythm and circadian disruption
Although the SCN rhythm cannot be directly measured in humans, the timing of melatonin onset and amplitude measured in plasma or saliva, or the melatonin metabolite 6-sulfatoxymelatonin in urine, are gold standard biomarkers of circadian rhythms. The collection of 24-hour urine samples is particularly useful to assess circadian amplitude of melatonin in special populations, such as pediatric patients in whom blood or salivary samples might be difficult to obtain (8), and has been shown to be a useful treatment-responsive biomarker of circadian disruption in neurologic diseases, such as Alzheimer’s disease (AD) (9) and pediatric epilepsy (10). Although less direct, other commonly used measures include rest/activity cycles derived from actigraphy, and other hormonal, metabolic, and cardiovascular rhythms (7).
Public health implications of circadian disruption
The public health implications of circadian disruption are enormous owing to the number of people affected and the mounting evidence of circadian disruption’s association with serious adverse health outcomes. Populations in the US at risk for circadian disruption include the approximately 16% of adults who usually work a nondaytime schedule (11), the approximately 70% who work indoors (12), the estimated 99% affected by light pollution (13), and the growing sector of adults aged 65 and older (14). This topic is also important for policy decisions that affect the general population, such as the public and scientific debate regarding daylight saving time and its potential health and safety risks (15). “Chronotype” refers to one’s preferred timing of sleep and activity, and evening chronotypes (“night owls”) are more likely to experience circadian disruption because of a mismatch between their internal clock and their social or professional responsibilities, and have increased risk of all-cause mortality compared with morning types (16). Shift work increases risk for poor mental and physical health and is considered “probably carcinogenic” (7), and therefore has implications for both individual health and occupational health policies.
Circadian knowledge from basic science is robust, but less is available in the areas of clinical and population health (17). There remains a notable gap in translating this science to directly impact health, both for individuals and for population-based decisions. Part of this gap stems from the lack of multicenter randomized clinical trials and longitudinal population studies that directly focus on circadian disorders, as well as the limited number of well-established patient-reported outcomes and scalable human biomarkers of circadian disruption. Additionally, research targeting clinical circadian rhythm requires an age-based approach, as there are prominent differences in circadian phase and amplitude, as well as stability of rhythms across the lifespan.
In this Review, we use an evidence-based “bench-to-bedside” approach, applying research in circadian disruption to the risk for, and therapeutics for, some of the most prevalent and pressing human diseases. We apply this model to highlight selected examples of research findings from preclinical and clinical work that are ripe for translation and implementation in the clinic, and development of guidelines for public health. Lastly, we discuss strategies for integrating biological timing in medicine to optimize and personalize the care of individuals with circadian rhythm sleep-wake disorders, as well as disruption of the circadian system within the context of chronic disease.
The following discussion is organized by key disease areas of circadian disruption in human health: neurologic, psychiatric, metabolic, cardiovascular, and allergic/immunologic. We highlight the epidemiologic and background evidence supporting the connection between circadian disruption and human health and then apply the bench-to-bedside model to discuss examples of targetable mechanisms to enhance circadian health in these disorders.
Neurologic disorders
Circadian disruption is common in neurologic disorders, affecting the development, expression, and severity of disease. There is mounting evidence linking circadian disruption with cerebrovascular disease, epilepsy, migraine, multiple sclerosis, neurodegenerative disorders, and neurodevelopmental disorders (7). Beyond its association with disease severity, circadian disruption increases the risk for neurologic disorders across the lifespan (18).
Neurodevelopmental disorders
Circadian disruption and poor sleep quality are common in neurodevelopmental disorders, including autism spectrum disorders (ASDs) and rare genetic neurodevelopmental disorders such as Angelman, Williams, Prader-Willi, fragile X, and Smith-Magenis syndromes. ASDs are relatively common and have been associated with several biomarkers of circadian disruption, including misaligned cortisol rhythm and lower-amplitude melatonin rhythm (19). A leading hypothesis is that ASD induces dysfunction of melatonin synthesis (19). In fact, single-nucleotide polymorphisms (SNPs), such as in melatonin receptors MTNR1A and MTNR1B, may cause ASD (20), and other clock gene SNPs appear to modulate the association between severity of ASD and severity of the sleep disruption/circadian disruption (20). Smith-Magenis syndrome (SMS) is caused by a deletion in 17p11.2, which encompasses the gene encoding retinoic acid induced 1 (RAI1) (21). Mice deficient in RAI1 have a shortened period, which appears to be due to RAI1’s activation of core clock gene transcription (22). In humans, RAI1 is implicated in the regulation of melatonin secretion (21). Children with SMS exhibit an inversion of the endogenous melatonin rhythm and sleep/wake cycle (23).
One strategy to suppress the morning level of melatonin in SMS includes administration of a β1-adrenergic antagonist (24). Melatonin supplementation is also a core strategy to realign the circadian system and improve sleep and cognitive outcome measures (25). Melatonin supplementation for realignment (i.e., phase shifting) typically occurs at lower doses of ≤1 mg of immediate-release formulations given several hours before bedtime (26), whereas doses greater than 1 mg of extended-release versions just before bedtime are used for the soporific effect (27). In ASD, Malow et al. found in children with autism that treatment with melatonin significantly improved sleep quality using oral pediatric prolonged-release melatonin at starting doses of 2 mg titrated up to 5 mg after 3 weeks and up to 5–10 mg/d if needed after that (28).
Neurodegenerative disorders
Increasing evidence indicates a bidirectional relationship between circadian disruption and neurodegeneration, such as in traumatic brain injury, AD, Parkinson’s disease (PD), and Huntington’s disease. Circadian misalignment and decreased amplitude of several rhythms have been shown to predict the development of neurogenerative diseases, such as AD and PD (29–31). In this section we focus on the most common age-related neurodegenerative diseases, AD and PD.
PD.
In mouse models of PD, SCN neurons had decreased activity, and this may contribute to lower amplitude and/or misalignment of circadian rhythms (32). Dopamine has an important role in the regulation of centrally generated circadian rhythmicity via direct effects on retinal circadian rhythms (33), as well as via its effect on melanopsin-containing photoreceptors in the retina (34). In nonhuman primate models, depletion of dopamine disrupted rhythmicity, and this appeared to be further exacerbated by misalignment of environmental and behavioral rhythms (35). Circadian disruption in patients with PD can occur at any stage of the disease, and can include changes in the phase and amplitude of rest/activity and melatonin rhythms (36). The circadian disruption may even occur prior to the onset of clinical motor symptoms, suggesting a possible role in disease pathogenesis (37, 38).
Given circadian rhythmicity in the pharmacokinetics of levodopa (39), a dopamine precursor indicated for the management of PD symptoms, timing of dosing could potentially help optimize effectiveness and decrease side effects. Mathematical models are being developed to integrate biomarkers of circadian disruption, such as clock gene expression and timing of light exposure, to predict the impact on neurotransmitters, such as dopamine, and help in timing treatment and choosing drug targets (40). Tested circadian-based therapeutics include bright light therapy, which improves mood (41), sleep quality, and daytime alertness (42). Interestingly, bright light therapy twice daily for 14 days also increased objective levels of physical activity (42) as well as enhanced sleep and improved disease control (43). In PD, melatonin has been primarily used for its soporific effects (44).
Dementia and AD.
AD is associated with circadian disruption at multiple organizational levels, including loss of SCN neurons (31) and impaired function of light input pathways (degeneration of the intrinsically photosensitive retinal ganglion cells; ref. 45). Environmentally there is decreased exposure to light and structured activity. These and other factors can contribute to the low amplitude and misalignment of circadian rhythms at multiple levels of organization (7, 31, 46–53), which in turn have been shown to correlate with the degree of neurologic impairment (54, 55). In mice, misalignment between feeding rhythms and the SCN results in memory impairment (56), which improves with timed feeding schedules (57). Furthermore, there is evidence for misalignment of neuronal activity in different brain regions, such as between the SCN and hippocampus (58, 59). The evidence that circadian disruption increases the risk for cognitive decline and AD (30) raises the possibility that improving circadian function may decelerate age-related cognitive impairment.
Light therapy, specifically morning blue-enriched light, might be effective for improving sleep quality and cognitive function (60), and evidence indicates that light therapy reduces neuroinflammation and oxidative stress to improve sleep and cognition (61). Indeed, these circadian-based therapies, including sleep behavior modifications (62), are increasingly becoming part of standard of care for AD, and might combat cognitive decline (30). Increasing daytime light exposure might improve rhythmicity and help consolidate sleep (63). In a systemic review and meta-analysis, melatonin supplementation appeared to significantly improve scores on the Mini–Mental State Examination in nine studies of mild AD (64). However, the evidence is weak, and a recent Cochrane review found that melatonin, even at higher doses, did not improve sleep measures compared with placebo (65).
Psychiatric disorders
Circadian disruption is common in psychiatric disease, including schizophrenia and mood disorders, such as depression, bipolar disorder, and seasonal affective disorder (21, 66, 67). In this section, we focus on mood disorders as common examples of the role of circadian disruption in the development of psychiatric disorders and the potential of circadian-based psychiatric therapeutics. Other published reviews in this area (21, 66, 67) delve deeper into psychiatric disorders that are outside the scope of this Review, including substance use disorders (21).
Mood disorders
The link between circadian disruption and mood disorders is well established (68–70). Seasonal affective disorder is characterized by the onset or worsening of depression during the fall and winter months, when the daily duration of daylight decreases (66), and patients are symptomatic as a result of seasonally induced circadian misalignment. Accurate assessment of circadian phase and therapy to shift phase might improve depressive symptoms (68). Another example is delayed sleep-wake phase disorder (DSWPD), in which misalignment between the onset of melatonin and bedtime resulted in increased odds of depressive symptoms (OR = 4.31, 95% CI = 1.75–10.64) and DSWPD exacerbation (71). “Circadian depression” is a recently coined term used to describe this potential clinical phenotype that requires circadian-targeted treatment for specific mood disorders, as evidenced by specific rhythmicity/seasonality to symptoms, clinical course, objective circadian parameters, and treatment responses (72).
Preclinical evidence further demonstrates the bidirectional relationship between mood disorders and the circadian system in mice, in which manipulation of serotonin receptors themselves induced circadian disruption (73). External circadian factors, such as light at the wrong time in a mouse model, also induce signs of depression (74), mediated by intrinsically photosensitive retinal ganglion cells and their projections to hypothalamic, preoptic, and limbic regions, such as the amygdala (75). In humans, functional MRI studies found that light suppresses amygdala activity, but improves connectivity within the prefrontal cortex (76), which suggests a potential mechanistic explanation of the effect of light on mood. Other circadian mechanistic contributors to mood disorders in humans include altered clock gene expression (77) and genetic polymorphisms in clock genes (78) (e.g., PER3 rs57875989 is associated with anxiety; ref. 79). As a separate point, synchronizing timing of the brain clock with the external environment can decrease depression (80). A recent study of thousands of patients in several large cohorts identified that earlier sleep midpoint was associated with a 23% lower risk of depression (80).
Circadian-based approach to psychiatric therapy
The findings described above support the potential of circadian-based treatment approaches for psychiatric disease. In fact, psychiatric drugs, such as selective serotonin reuptake inhibitors, have differential effects in various light conditions in humans (81). Chronotype also influences therapeutic efficacy. For example, patients with bipolar disease who respond to lithium tend to be “morning larks” (82), and lithium might strengthen circadian amplitude in those that are responders (83, 84). Interestingly, lithium-induced circadian disruption in some nonresponders might be overcome by circadian entrainment with synchronizing agents or zeitgebers (83). The use of bright light therapy is relevant across many mood disorders, such as major depression (85), bipolar disorder (86–88), and seasonal affective disorder (89, 90). It is important to note that the efficacy of bright light therapy in clinical trials for mood disorders is variable, and is dependent on the type of light used, the timing of usage, and patient adherence (91). It is worth a brief mention of burgeoning literature on attention deficit hyperactivity disorder (ADHD) that suggests that phase advancing via bright light can correct circadian disruption without inducing changes in sleep and improves symptoms of ADHD (92), which might also be accomplished by phase advancing with melatonin (93).
Metabolic disorders
The intimate relationship between the circadian system and metabolism is crucial for optimizing energy extraction and utilization from food during the active period and processing stored energy during the rest phase to maintain stable glucose levels during the overnight fast. The impact of circadian disruption on metabolic disorders has been an area of intense research. Several high-impact reviews (94–97) summarize the close coupling of circadian regulation and metabolism. In this Review, we focus on the negative-feedback loop between circadian disruption, diabetes, and obesity.
Metabolism and diabetes
It is well established that circadian dysregulation affects metabolism at the genetic, cellular, and system levels, resulting in impaired glucose tolerance and insulin resistance and increased risk of metabolic syndrome and diabetes (98). Core clock gene disruption, such as in ClockΔ19 mutant mice, demonstrates the profound impact of the Clock gene mutation on metabolism, with the development of obesity and metabolic syndrome (99). Loss of Bmal1 increases insulin sensitivity (95), as well as tissue-specific mechanisms of misalignment (100). In fact, social jet lag (the shifting of bedtimes between work and free days) has also been associated with the metabolic syndrome and with increased glycosylated hemoglobin (101). Misalignment in those with later chronotype might explain why they are more likely to be overweight and have type 2 diabetes (102, 103). Even among patients with type 2 diabetes, those who are evening types have poorer glycemic control (104–106). These studies suggest that discrepancies between internal timing and social timing can lead to metabolic impairment.
The timing of food consumption also directly impacts blood glucose levels. For example, eating the same meal at breakfast, lunch, or dinner will result in the lowest glucose spike at breakfast, doubled at dinner (94). In fact, a continuous glucose infusion demonstrated that blood glucose levels are highest overnight when the body is not expecting to receive glucose. Experimental human studies that induced circadian disruption recapitulated the increased glucose levels, despite controlled food intake and increased insulin levels (6, 107). Circadian misalignment also reduces glucose tolerance and impairs insulin sensitivity (108–110), providing a potential mechanism to explain the increased risk of diabetes in shift workers (107). Another population at risk for circadian misalignment is pregnant women, and a higher risk of gestational diabetes was observed in those with a later sleep midpoint (111).
The timing, intensity, and wavelength of light exposure can also modulate metabolic function, with evening blue-enriched light having the strongest negative impact by increasing blood glucose levels (112). Indeed, several studies have demonstrated that dim light conditions during the daytime and/or excessive evening light disrupts metabolic clocks, such as in patients with diabetes in whom blood sugar levels are more difficult to control (113, 114). Enhanced daytime light exposure and promotion of dim light in the evening to improve circadian alignment is one strategy that might improve metabolism and cardiometabolic disease control (114). In addition, recent evidence from preclinical and clinical studies indicates that time-restricted feeding and timed exposure to light may be important strategies to align the timing of energy intake with clock-regulated metabolic rhythms. Such circadian-based approaches have the potential to improve metabolic health and maybe even weight regulation (115–117).
Obesity
Considering the strong association of obesity with ClockΔ19 mutation in mice, it is not surprising that CLOCK gene variants, such as rs6820823, rs3792603, rs11726609 (118), and rs1801260 (119), are associated with elevated BMI in humans. Polymorphisms of CLOCK, such as rs3749474, might be identified to more effectively personalize weight loss treatment, such as with restriction of dietary fat intake (120). Polymorphisms in genes encoding melatonin receptors are also associated with metabolic dysfunction and obesity (121–125), and the relationship between nighttime food consumption and obesity might be partly mediated by these melatonin receptor polymorphisms (126). Potential mechanisms linking circadian disruption to weight gain and obesity in night shift workers include a decrease in daytime energy expenditure after night shifts (127), compounded by dysregulation of hormonal (leptin/ghrelin) signaling to eat (128).
Shift workers are an at-risk population for metabolic disorders, type 2 diabetes, and obesity (129–131). In a systematic review of shift work and metabolic syndrome, there was an association between metabolic syndrome and shift work (OR = 1.29, 95% CI = 1.06–1.52) (132). Social jet lag is associated with overweight, and the greater the discrepancy in bedtimes, the greater the prevalence (101, 133).
As a method to improve circadian alignment, particularly between central and peripheral metabolic tissues, time-restricted feeding of animals, even animals on a high-fat diet, improved circadian function (134). Time-restricted feeding is also a proposed strategy to prevent metabolic diseases (135, 136). A recent study suggests a correlation between insulin sensitivity and the proportion of calories consumed in the morning (137), which should be considered in relation to melatonin rhythms (138). Taking a step further, a systematic review of time-restricted feeding in humans in seven studies found that it resulted in a mean weight loss of 2.88 kg (95% CI = –3.96 to –1.80) (139), although the effects on weight loss are highly dependent on the specific intervention protocol (135).
Cardiovascular disorders
A landmark 1985 New England Journal of Medicine paper described the timing of myocardial infarctions (MIs; commonly known as heart attacks), with a higher likelihood of events occurring between 6 am and noon (140). Further research identified similar timing of this risk in stroke (141) and ventricular arrhythmias (142). This vulnerable morning time is not explained by behavioral triggers alone, i.e., feeding, sleep, etc. (143–145). Rather, it appears to be due in part to circadian regulation of prothrombotic factors, such as platelets and prothrombotic plasminogen activator inhibitor-1 (PAI-1) (146, 147). Because the circadian system regulates the timing of cardiovascular (CV) function, when circadian disruption is present there is a profound impact on CV health.
Acute CV events and CV risk
Circadian misalignment between environmental or behavioral rhythms and central or peripheral clocks has been shown to increase the risk for cardiovascular disease (CVD). Animal models demonstrate that arrhythmias and CV events manifest in conditions of circadian misalignment (6).
Myocardial infarction and stroke.
In humans, circadian misalignment might induce hypertension and inflammation (as exemplified with biomarkers such as C-reactive protein) (148), which in part may explain the epidemiology data linking shift work with MI and stroke (149). In mouse models of stroke, one functional consequence of longer duration of “shift work” is upregulated inflammatory mediators and ultimately worse stroke severity (150). The modulation and interaction between CVD and circadian disruption is exemplified by the finding that in patients with type 2 diabetes, the CLOCK rs4580704 SNP is associated with increased lifetime risk of stroke (151). Evidence indicates that CV damage and risk for CV events in night shift workers is due to a putative circadian disruption–induced IL-6–mediated inflammatory mechanism (152), as evidenced by increased carotid intimal-medial thickness (153). Other types of circadian disruption, including social jet lag or late chronotype, increase risk factors for CVD, such as increased resting heart rate and hypertension (103, 154, 155), and are associated with an increased prevalence of CV diseases (16).
Hypertension.
Circadian regulation of blood pressure occurs in the nondisrupted state by increased nocturnal parasympathetic (156) tone that provides cardioprotective nocturnal blood pressure dipping (157–159). Misalignment, such as in one small study of social jet lag, can alter parasympathetic tone during sleep (160). A review of studies in shift workers affirms that this circadian disruption induces increases in sympathetic tone and downregulation of cardioprotective parasympathetic tone (161). Indeed, experimental induction of circadian misalignment in humans increases blood pressure (6, 108, 149).
Arrhythmias.
With regard to arrhythmia, 44% of the sinus node transcriptome has diurnal variation (162). In one mouse study, unnaturally restricting feeding to the rest period unmasked long QT syndrome (163). Additionally, housing mice in non-24-hour-light/dark cycles, even under stable entrainment (similar to shift work conditions) induced phase misalignment between the environment and internal circadian timing, and this phase misalignment was the driver of the cardiac abnormalities, including prolonged QT interval (164).
Circadian-based therapeutic strategies for CVD.
Strategies to boost circadian amplitude and stable alignment are potentially beneficial. Considering the vulnerable time window of CV events and a hypothesized circadian link, murine studies identified that expression of the core clock gene Per2 appears to be protective against myocardial ischemic and reperfusion injury (165–167). Central and peripheral PER2 expression can be induced by light activation of melanopsin receptors in retinal ganglion cells (165, 168, 169), and bright light therapy to increase PER2 amplitude might protect against ischemic injury (167, 170) and reduce platelet aggregation (171).
In humans, bright light therapy in the morning can indeed induce PER2 expression in buccal or plasma samples (170). This finding as well as mechanistic animal studies led to a proof-of-concept study in humans using bright light therapy (10,000 lux from 8:30 to 9:00 am every morning for 5 days) to evaluate CV risk pathways. The researchers found they could target the vulnerable morning time window for CV events by decreasing clotting time and downregulating several procoagulant pathways as measured on proteomics of blood samples, including tissue inhibitor of metalloproteinase (TIMP-1), PDGFA/B, and platelet factor 4 (PF4) (171).
Furthermore, manipulation of the timing of therapies, or chronotherapy, for CVD has been gaining attention. Therapeutic attempts to combat hypertension via time-based treatment have received considerable attention; even nighttime exercise has been tested as a possible strategy (172). A recently published study with a large sample of hypertensive patients followed for about 6 years identified that bedtime treatment decreased risk of all measured CV events (stroke, MI, heart failure) (173). Although there has been some controversy over the study design (174), a systematic review of ten published trials also supported evening dosing of blood pressure therapy (175). It should be considered, however, that pharmacokinetics differs between antihypertensives, and translating these findings to clinical practice should consider the half-life and time to peak action. Further prospective studies that evaluate the benefits of evening dosing for specific populations are needed (176). In comorbid conditions such as obstructive sleep apnea, morning dosing might still be preferable (177).
CV risk in children
Although pediatric populations are not well studied, this group offers a unique opportunity for intervention to avoid accumulation of CV damage and to decrease long-term CV risk. With regard to CV circadian rhythms, children are unique in that rhythms vary widely by age (178), and the timing of CV events, such as arrhythmias, in children might be different from that in adults (179). Specific pediatric diseases such as type 1 diabetes and prematurity can further induce internal misalignment and CV risk, creating a feedback loop between disease expression and circadian disruption (180, 181). Obese children have lower-amplitude circadian rhythms, and even when BMI is controlled for, inflammation inversely correlates with the strength of the rhythm (182). Cardioprotective physiology, such as nocturnal blood pressure dipping, is also important in children yet appears to be disrupted by several pediatric disease states (183–185). Because adolescents are susceptible to phase delays (8, 186, 187), circadian misalignment is commonly associated with greater metabolic effects in this population (188, 189) and potentially effects on the CV system (190).
Modifiable behavioral factors such as screen time at night, alcohol use, and consistently early school start times (191) might be targeted to modulate this CV risk. Race/ethnicity might also modulate the relationship between circadian misalignment and metabolic effects in adolescents (192), and is an important factor to consider in the study of circadian disruption.
Immunologic disorders
Circadian control of the immune system is an area of important investigation that shows the intimate relationship between the circadian system, the immune system, and sleep (193–202). It follows that circadian disruption has been associated with immune disorders (203). In this Review, we highlight the relationship between circadian disruption, asthma (204), and other allergic diseases (205).
COVID-19
The COVID-19 pandemic offers an example of the interrelationship between human health and circadian disruption in immunology. Specifically, circadian misalignment, such as shift work, might increase the risk of being infected with SARS-CoV-2 (206–208). Although there are no data specific to COVID-19, time of day of infection with pathogens appears to alter susceptibility (209, 210). Upon infection with SARS-CoV-2, severe disease (like other severe illnesses) appears to induce circadian disruption by obliterating normal rest/activity rhythms (211). It is speculated that active SARS-CoV-2 infection might also dampen melatonin rhythm and alter timing of clock gene expression, resulting in misalignment and upregulation of the damaging inflammatory cytokine expression (212). In terms of therapy, the circadian system might have the potential to improve COVID-19 disease outcomes (212–214). For example, morning administration of antiviral therapy (darunavir/ritonavir) might be more effective in reducing inflammation, as measured by biomarker C-reactive protein (215).
Asthma
In Clock- or Bmal1-mutated mice, misalignment between clock genes and organs results in defects of the epithelial barrier of the skin (216), intestine (217), and airways (218). Furthermore, deletion of a downstream target of Bmal1, Rev-Erba, results in clock gene and cellular misalignment, leading to diminished antiviral responses and increased allergen responsiveness, translating to an asthma-like phenotype (219, 220). Misalignment between environmental rhythms and the SCN also appears to increase asthma risk in shift workers (OR = 1.23, 95% CI = 1.03–1.46) (221). The mechanism behind the increased risk for asthma in shift workers might also be one of genetic susceptibility to circadian disruption. For example, there is an association of clock gene variants, such as TIMELESS, with asthma in children (222), and higher odds of asthma are noted in morning-chronotype shift workers who had to work nights (221). This argues for the consideration of circadian chronotype in the design of personalized shift work schedules to improve health. Importantly, hypoxia in asthma or in other conditions is one disease-induced factor that appears to further promote circadian misalignment owing to tissue-specific, time-dependent transcriptional changes (223).
Although there are limited data, time can be harnessed to personalize therapy for asthma, via chronotherapy (Figure 2). For example, the package insert for montelukast, a leukotriene receptor antagonist therapy, suggests that evening administration might improve efficacy (224). Paying attention to time can even adjust the risk of side effects from therapies. Bavishi et al. described an increased rate of cutaneous reaction to allergy shots (subcutaneous allergen immunotherapy) in the afternoon compared with morning (225).
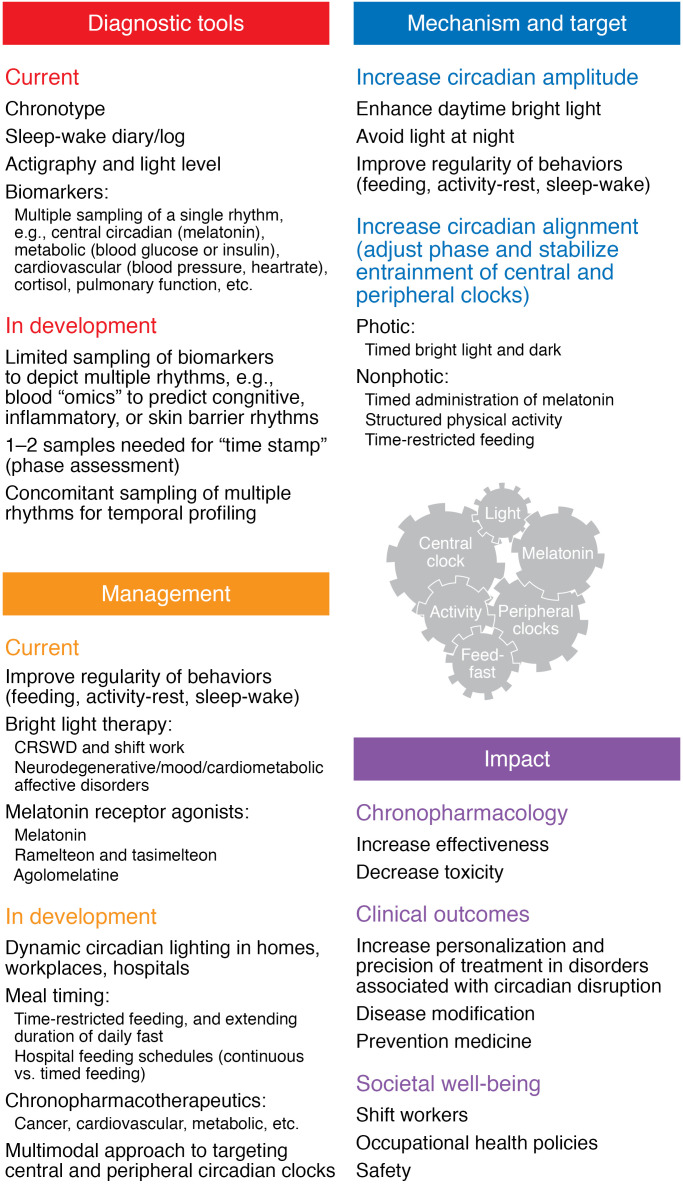
Figure 2. A multimodal approach to address circadian disruption and its effect on health.
Diagnostic approaches, including current tools, rely primarily on self-reported and behavioral measures and require time-intensive multiple sampling of biomarkers to obtain a 24-hour profile. However, tools in development seek to assess circadian phase using a single time sample of multiple rhythms. Circadian-based therapies, such as timed bright light exposure and exogenous melatonin, are commonly used to align the phase of external/behavioral rhythms with endogenous central and peripheral clocks. In addition, time-restricted feeding can enhance alignment among peripheral clocks and improve metabolic health. In development are multimodal approaches to target central and peripheral clocks with dynamic circadian lighting, time-restricted feeding and drugs to align and stabilize circadian entrainment, and chronopharmacotherapy to optimize efficacy and tolerability of medications. CRSWD, circadian rhythm sleep wake disorder.
Other allergic diseases
Here we describe other common allergic diseases, such as anaphylaxis (226), atopic dermatitis (4), and allergic rhinitis (227). In a mouse model of passive cutaneous anaphylaxis (226), multiple mechanisms of circadian disruption lowered the threshold for allergic reaction and altered the rhythmic pattern of reactions; these included mutations of Per2, mast cell–specific Clock mutation, and time-restricted feeding (during the rest interval) in mice (228–230). This supports the hypothesis that circadian disruption due to multiple mechanisms might increase the risk of severe allergic reaction.
Because of the nighttime predominance of allergic disease (4), the degree of nocturnal disruption defines disease severity level for asthma and allergic rhinitis and directly impacts treatment decisions by physicians (231–233). Targeting of allergic disease should include assessment of timing of symptoms, such as asthma flare, classically around 4 am (the timing of lung function nadir and increased lymphocyte counts in the lung) (234, 235). Allergic rhinitis symptoms peak between 4 and 6 am, a time when pollen counts are high; patient is recumbent (sleeping); and when inflammatory rhythms are upregulated — all of which can increase edema of the upper airway (227). Atopic dermatitis (eczema) symptoms also appear to peak in the evening, with overnight scratching peaking approximately 3 hours after bedtime (236).
Preclinical human testing has also garnered evidence supporting] the therapeutic strategy of “drugging the clock,” such as via a selective casein kinase inhibitor. In mast cells, this has similar effects to corticosteroid, i.e., there is suppression of IgE-mediated allergic reactions in mouse mast cells and decreased basophil reactivity in human basophils of allergic rhinitis patients (237). This suggests an exciting role for circadian therapeutics in targeting allergic and immunologic disease. In a mouse model of pulmonary fibrosis, drugging the clock gene by inhibition of Rev-Erba might inhibit myofibroblast activation and potentially prevent fibrosis (238).
Conclusion
The accumulating bench-to-bedside-to-population research highlights the magnitude and interrelatedness of circadian disruption in human disease, and the opportunity for circadian-based approaches to prevent and optimize individual and societal health. In addition to disease-specific effects of circadian disruption, transdiagnostic effects are noted as a result of particular sleep/circadian constructs (e.g., late/evening chronotype, social jet lag). Given this, adverse health outcomes are unlikely to occur in isolation, and therapeutic strategies such as melatonin supplementation or bright light therapy might be used concordantly to treat multiple conditions. However, few clinical trials have been conducted with only weak treatment recommendations for circadian disorders.
The discovery of the genetic underpinnings of circadian rhythms, together with the findings that this clock genetic machinery exists in virtually all cells and tissues and regulates metabolic, immune, and neural processes and pathways, has fundamentally changed our view of the importance of circadian health in medicine. Circadian medicine is a relatively new concept, but is rapidly gaining momentum throughout the world. In parallel with translational research, there is a clear need to identify biomarkers that are clinically relevant, but also scalable at the population level. Rapid development of technology for wearables and environmental sensors, coupled with artificial intelligence, will allow us to assess circadian timing of multiple rhythms at different organizational levels in real time to gain insight into the interaction between environment, behavior, and central and peripheral clocks in health and disease. Finally, clinical trials and a narrowing of the bench-to-bedside gap are needed. For example, although about 1000 genes are involved as drug targets or in drug metabolism/transport (239), only a few of the most commonly prescribed treatments in medicine have time-of-day indications for dosing (240). This is not because the therapies do not work better with timed administration, but rather because clinical trials are lacking to determine optimal timing. We have a good blueprint for how to integrate circadian timing in medicine, and now is the time to move disease-specific research forward.
Acknowledgments
The authors acknowledge funding sources as follows: ABF received funding from the NIH (grant 5K23AR075108); PCZ received funding from the NIH (grants R01-HL140580, P01-AG011412; R01-AG059291, T32-HL007909); KLK received funding from the NIH (grants R01-AG059291, P01-AG011412, and R01-HL141881).
Version 1. 10/01/2021
Electronic publication
Footnotes
Conflict of interest: PCZ owns stock in Teva, receives advisory board honoraria from Eisai and Jazz Pharmaceutical, receives research support from Apnimed, and holds pending patents related to phase-locked loop acoustic stimulation (US20170304587A1), time signature (US20180357360A1), and treatment of sleep-wake disorders comprising modified resistant maltodextrin (US20190151348A1).
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(19):e148286.https://doi.org/10.1172/JCI148286.
References
- 1.Vetter C. Circadian disruption: what do we actually mean? Eur J Neurosci. 2020;51(1):531–550. doi: 10.1111/ejn.14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sletten TL, et al. Health consequences of circadian disruption. Sleep. 2020;43(1):zsz194. doi: 10.1093/sleep/zsz194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chellappa SL, et al. Circadian misalignment increases mood vulnerability in simulated shift work. Sci Rep. 2020;10(1):18614. doi: 10.1038/s41598-020-75245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fishbein AB, et al. Nocturnal eczema: review of sleep and circadian rhythms in children with atopic dermatitis and future research directions. J Allergy Clin Immunol. 2015;136(5):1170–1177. doi: 10.1016/j.jaci.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Wang R, et al. Asthma diagnosis: into the fourth dimension. Thorax. 2021;76(6):624–631. doi: 10.1136/thoraxjnl-2020-216421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheer FA, et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott SM, et al. Circadian disruption and human health: a bidirectional relationship. Eur J Neurosci. 2020;51(1):567–583. doi: 10.1111/ejn.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowley SJ, Eastman CI. Human adolescent phase response curves to bright white light. J Biol Rhythms. 2017;32(4):334–344. doi: 10.1177/0748730417713423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luboshitzky R, et al. Actigraphic sleep-wake patterns and urinary 6-sulfatoxymelatonin excretion in patients with Alzheimer’s disease. Chronobiol Int. 2001;18(3):513–524. doi: 10.1081/CBI-100103973. [DOI] [PubMed] [Google Scholar]
- 10.Uberos J, et al. Normalization of the sleep-wake pattern and melatonin and 6-sulphatoxy-melatonin levels after a therapeutic trial with melatonin in children with severe epilepsy. J Pineal Res. 2011;50(2):192–196. doi: 10.1111/j.1600-079X.2010.00828.x. [DOI] [PubMed] [Google Scholar]
- 11. U.S. Bureau of Labor Statistics. Job Flexibilities and Work Schedules Summary. https://www.bls.gov/news.release/flex2.nr0.htm Updated September 24, 2019. Accessed August 18, 2021.
- 12.Mendell MJ, et al. Improving the health of workers in indoor environments: priority research needs for a national occupational research agenda. Am J Public Health. 2002;92(9):1430–1440. doi: 10.2105/AJPH.92.9.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chepesiuk R. Missing the dark: health effects of light pollution. Environ Health Perspect. 2009;117(1):A20–A27. doi: 10.1289/ehp.117-a20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vincent GK, Velkoff VA. The Next Four Decades: The Older Population in the United States: 2010 to 2050. US Census Bureau; 2010. [Google Scholar]
- 15.Kolla BP, et al. Increased patient safety-related incidents following the transition into daylight savings time. J Gen Intern Med. 2021;36(1):51–54. doi: 10.1007/s11606-020-06090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knutson KL, von Schantz M. Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiol Int. 2018;35(8):1045–1053. doi: 10.1080/07420528.2018.1454458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Aircrew Safety & Health: Circaduab Rhythm Disruption (Jet Lag). https://www.cdc.gov/niosh/topics/aircrew/jet lag.html Updated May 9, 2017. Accessed August 18, 2021.
- 18.Videnovic A, Zee PC. Consequences of circadian disruption on neurologic health. Sleep Med Clin. 2015;10(4):469–480. doi: 10.1016/j.jsmc.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorsung E, et al. Biological timing and neurodevelopmental disorders: a role for circadian dysfunction in autism spectrum disorders. Front Neurosci. 2021;15:642745. doi: 10.3389/fnins.2021.642745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z, et al. Circadian-relevant genes are highly polymorphic in autism spectrum disorder patients. Brain Dev. 2016;38(1):91–99. doi: 10.1016/j.braindev.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Logan RW, McClung CA. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci. 2019;20(1):49–65. doi: 10.1038/s41583-018-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacaria M, et al. Circadian abnormalities in mouse models of Smith-Magenis syndrome: evidence for involvement of RAI1. Am J Med Genet A. 2013;161a(7):1561–1568. doi: 10.1002/ajmg.a.35941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith ACM, et al. Twenty-four-hour motor activity and body temperature patterns suggest altered central circadian timekeeping in Smith-Magenis syndrome, a neurodevelopmental disorder. Am J Med Genet A. 2019;179(2):224–236. doi: 10.1002/ajmg.a.61003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Leersnyder H, et al. β1-Adrenergic antagonists improve sleep and behavioural disturbances in a circadian disorder, Smith-Magenis syndrome. J Med Genet. 2001;38(9):586–590. doi: 10.1136/jmg.38.9.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodford EC, et al. Endogenous melatonin and sleep in individuals with Rare Genetic Neurodevelopmental Disorders (RGND): a systematic review. Sleep Med Rev. 2021;57(rgnd):101433. doi: 10.1016/j.smrv.2021.101433. [DOI] [PubMed] [Google Scholar]
- 26.Mundey K, et al. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28(10):1271–1278. doi: 10.1093/sleep/28.10.1271. [DOI] [PubMed] [Google Scholar]
- 27.Harpsøe NG, et al. Clinical pharmacokinetics of melatonin: a systematic review. Eur J Clin Pharmacol. 2015;71(8):901–909. doi: 10.1007/s00228-015-1873-4. [DOI] [PubMed] [Google Scholar]
- 28.Malow BA, et al. Sleep, growth, and puberty after 2 years of prolonged-release melatonin in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(2):252–261. doi: 10.1016/j.jaac.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leng Y, et al. Association of circadian abnormalities in older adults with an increased risk of developing Parkinson disease. JAMA Neurol. 2020;77(10):1270–1278. doi: 10.1001/jamaneurol.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim AS, et al. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36(7):1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354(6315):1004–1008. doi: 10.1126/science.aah4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kudo T, et al. Circadian dysfunction in a mouse model of Parkinson’s disease. Exp Neurol. 2011;232(1):66–75. doi: 10.1016/j.expneurol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Ruan GX, et al. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008;6(10):e249. doi: 10.1371/journal.pbio.0060249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakamoto K, et al. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur J Neurosci. 2005;22(12):3129–3136. doi: 10.1111/j.1460-9568.2005.04512.x. [DOI] [PubMed] [Google Scholar]
- 35.Fifel K, et al. Alteration of daily and circadian rhythms following dopamine depletion in MPTP treated non-human primates. PLoS One. 2014;9(1):e86240. doi: 10.1371/journal.pone.0086240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Videnovic A, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 2014;71(4):463–469. doi: 10.1001/jamaneurol.2013.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, et al. Recent progress in non-motor features of Parkinson’s disease with a focus on circadian rhythm dysregulation. Neurosci Bull. 2021;37(7):1010–1024. doi: 10.1007/s12264-021-00711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fifel K, Videnovic A. Circadian and sleep dysfunctions in neurodegenerative disorders-an update. Front Neurosci. 2020;14:627330. doi: 10.3389/fnins.2020.627330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyholm D, et al. Circadian rhythmicity in levodopa pharmacokinetics in patients with Parkinson disease. Clin Neuropharmacol. 2010;33(4):181–185. doi: 10.1097/WNF.0b013e3181e70f7a. [DOI] [PubMed] [Google Scholar]
- 40.Kim R, Reed MC. A mathematical model of circadian rhythms and dopamine. Theor Biol Med Model. 2021;18(1):8. doi: 10.1186/s12976-021-00139-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slominski AT, et al. How UV light touches the brain and endocrine system through skin, and why. Endocrinology. 2018;159(5):1992–2007. doi: 10.1210/en.2017-03230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Videnovic A, et al. Timed light therapy for sleep and daytime sleepiness associated with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2017;74(4):411–418. doi: 10.1001/jamaneurol.2016.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endo T, et al. Bright light improves sleep in patients with Parkinson’s disease: possible role of circadian restoration. Sci Rep. 2020;10(1):7982. doi: 10.1038/s41598-020-64645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pérez-Lloret S, Cardinali DP. Melatonin as a chronobiotic and cytoprotective agent in Parkinson’s disease. Front Pharmacol. 2021;12:650597. doi: 10.3389/fphar.2021.650597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.La Morgia C, et al. Retinal ganglion cells and circadian rhythms in Alzheimer’s disease, Parkinson’s disease, and beyond. Front Neurol. 2017;8:162. doi: 10.3389/fneur.2017.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibson EM, et al. Aging in the circadian system: considerations for health, disease prevention and longevity. Exp Gerontol. 2009;44(1-2):51–56. doi: 10.1016/j.exger.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hofman MA, Swaab DF. Alterations in circadian rhythmicity of the vasopressin-producing neurons of the human suprachiasmatic nucleus (SCN) with aging. Brain Res. 1994;651(1-2):134–142. doi: 10.1016/0006-8993(94)90689-0. [DOI] [PubMed] [Google Scholar]
- 48.Yamazaki S, et al. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A. 2002;99(16):10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turner PL, Mainster MA. Circadian photoreception: ageing and the eye’s important role in systemic health. Br J Ophthalmol. 2008;92(11):1439–1444. doi: 10.1136/bjo.2008.141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim AS, et al. Increased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individuals. Sleep. 2012;35(5):633–640. doi: 10.5665/sleep.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang JL, et al. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann Neurol. 2015;78(2):317–322. doi: 10.1002/ana.24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen CY, et al. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2016;113(1):206–211. doi: 10.1073/pnas.1508249112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cermakian N, et al. Circadian clock gene expression in brain regions of Alzheimer’s disease patients and control subjects. J Biol Rhythms. 2011;26(2):160–170. doi: 10.1177/0748730410395732. [DOI] [PubMed] [Google Scholar]
- 54.Park JE, et al. Differential associations of age and Alzheimer’s disease with sleep and rest-activity rhythms across the adult lifespan. Neurobiol Aging. 2021;101:141–149. doi: 10.1016/j.neurobiolaging.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Witting W, et al. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27(6):563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- 56.Loh DH, et al. Misaligned feeding impairs memories. Elife. 2015;4:e09460. doi: 10.7554/eLife.09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song J, et al. Time-restricted feeding alters isoflurane-induced memory deficits. Transl Neurosci. 2020;11(1):341–355. doi: 10.1515/tnsci-2020-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monje FJ, et al. Disrupted ultradian activity rhythms and differential expression of several clock genes in interleukin-6-deficient mice. Front Neurol. 2017;8:99. doi: 10.3389/fneur.2017.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stevanovic K, et al. Disruption of normal circadian clock function in a mouse model of tauopathy. Exp Neurol. 2017;294:58–67. doi: 10.1016/j.expneurol.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 60.Kim SJ, et al. Positive effect of timed blue-enriched white light on sleep and cognition in patients with mild and moderate Alzheimer’s disease. Sci Rep. 2021;11(1):10174. doi: 10.1038/s41598-021-89521-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cardoso FDS, et al. Therapeutic potential of photobiomodulation in Alzheimer’s disease: a systematic review. J Lasers Med Sci. 2020;11(suppl 1):S16–S22. doi: 10.34172/jlms.2020.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cordone S, et al. Sleep-based interventions in Alzheimer’s disease: promising approaches from prevention to treatment along the disease trajectory. Pharmaceuticals (Basel) 2021;14(4):383. doi: 10.3390/ph14040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ancoli-Israel S, et al. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 2003;1(1):22–36. doi: 10.1207/S15402010BSM0101_4. [DOI] [PubMed] [Google Scholar]
- 64.Sumsuzzman DM, et al. Neurocognitive effects of melatonin treatment in healthy adults and individuals with Alzheimer’s disease and insomnia: a systematic review and meta-analysis of randomized controlled trials. Neurosci Biobehav Rev. 2021;127:459–473. doi: 10.1016/j.neubiorev.2021.04.034. [DOI] [PubMed] [Google Scholar]
- 65.McCleery J, et al. Pharmacotherapies for sleep disturbances in dementia. Cochrane Database Syst Rev. 2016;11(11):CD009178. doi: 10.1002/14651858.CD009178.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones SG, Benca RM. Circadian disruption in psychiatric disorders. Sleep Med Clin. 2015;10(4):481–493. doi: 10.1016/j.jsmc.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 67.Walker WH, et al. Circadian rhythm disruption and mental health. Transl Psychiatry. 2020;10(1):28. doi: 10.1038/s41398-020-0694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lewy AJ, et al. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006;103(19):7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Emens JS, et al. Circadian rhythm in negative affect: implications for mood disorders. Psychiatry Res. 2020;293:113337. doi: 10.1016/j.psychres.2020.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robillard R, et al. Sleep-wake cycle and melatonin rhythms in adolescents and young adults with mood disorders: comparison of unipolar and bipolar phenotypes. Eur Psychiatry. 2013;28(7):412–416. doi: 10.1016/j.eurpsy.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 71.Murray JM, et al. Prevalence of circadian misalignment and its association with depressive symptoms in delayed sleep phase disorder. Sleep. 2017;40(1):zsw002. doi: 10.1093/sleep/zsw002. [DOI] [PubMed] [Google Scholar]
- 72.Carpenter JS, et al. Circadian depression: a mood disorder phenotype. Neurosci Biobehav Rev. 2021;126:79–101. doi: 10.1016/j.neubiorev.2021.02.045. [DOI] [PubMed] [Google Scholar]
- 73.Siemann JK, et al. Rhythms, reward, and blues: consequences of circadian photoperiod on affective and reward circuit function. Neuroscience. 2021;457:220–234. doi: 10.1016/j.neuroscience.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.LeGates TA, Kvarta MD. Illuminating a path from light to depression. Nat Neurosci. 2020;23(7):785–787. doi: 10.1038/s41593-020-0659-x. [DOI] [PubMed] [Google Scholar]
- 75.LeGates TA, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594–598. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGlashan EM, et al. Afraid of the dark: light acutely suppresses activity in the human amygdala. PLoS One. 2021;16(6):e0252350. doi: 10.1371/journal.pone.0252350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li JZ, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci U S A. 2013;110(24):9950–9955. doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Partonen T. Clock gene variants in mood and anxiety disorders. J Neural Transm (Vienna) 2012;119(10):1133–1145. doi: 10.1007/s00702-012-0810-2. [DOI] [PubMed] [Google Scholar]
- 79.Liberman AR, et al. Modeling strengthens molecular link between circadian polymorphisms and major mood disorders. J Biol Rhythms. 2018;33(3):318–336. doi: 10.1177/0748730418764540. [DOI] [PubMed] [Google Scholar]
- 80.Daghlas I, et al. Genetically proxied diurnal preference, sleep timing, and risk of major depressive disorder. JAMA Psychiatry. 2021;78(8):903–910. doi: 10.1001/jamapsychiatry.2021.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McGlashan EM, et al. The SSRI citalopram increases the sensitivity of the human circadian system to light in an acute dose. Psychopharmacology (Berl) 2018;235(11):3201–3209. doi: 10.1007/s00213-018-5019-0. [DOI] [PubMed] [Google Scholar]
- 82.McCarthy MJ, et al. Chronotype and cellular circadian rhythms predict the clinical response to lithium maintenance treatment in patients with bipolar disorder. Neuropsychopharmacology. 2019;44(3):620–628. doi: 10.1038/s41386-018-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mishra HK, et al. Circadian rhythms in bipolar disorder patient-derived neurons predict lithium response: preliminary studies. Mol Psychiatry. doi: 10.1038/s41380-021-01048-7. [published online March 5, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCarthy MJ, et al. Genetic and clinical factors predict lithium’s effects on PER2 gene expression rhythms in cells from bipolar disorder patients. Transl Psychiatry. 2013;3(10):e318. doi: 10.1038/tp.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Geoffroy PA, et al. Efficacy of light therapy versus antidepressant drugs, and of the combination versus monotherapy, in major depressive episodes: a systematic review and meta-analysis. Sleep Med Rev. 2019;48:101213. doi: 10.1016/j.smrv.2019.101213. [DOI] [PubMed] [Google Scholar]
- 86.Tseng PT, et al. Light therapy in the treatment of patients with bipolar depression: a meta-analytic study. Eur Neuropsychopharmacol. 2016;26(6):1037–1047. doi: 10.1016/j.euroneuro.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 87.Hirakawa H, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):e01876. doi: 10.1002/brb3.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang S, et al. Bright light therapy in the treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS One. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bedrosian TA, Nelson RJ. Timing of light exposure affects mood and brain circuits. Transl Psychiatry. 2017;7(1):e1017. doi: 10.1038/tp.2016.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maruani J, Geoffroy PA. Bright light as a personalized precision treatment of mood disorders. Front Psychiatry. 2019;10:85. doi: 10.3389/fpsyt.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nussbaumer-Streit B, et al. Light therapy for preventing seasonal affective disorder. Cochrane Database Syst Rev. 2019;3(3):CD011269. doi: 10.1002/14651858.CD011269.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fargason RE, et al. Correcting delayed circadian phase with bright light therapy predicts improvement in ADHD symptoms: A pilot study. J Psychiatr Res. 2017;91:105–110. doi: 10.1016/j.jpsychires.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Andel E, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260–269. doi: 10.1080/07420528.2020.1835943. [DOI] [PubMed] [Google Scholar]
- 94.Panda S. Circadian physiology of metabolism. Science. 2016;354(6315):1008–1015. doi: 10.1126/science.aah4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bass J. Circadian topology of metabolism. Nature. 2012;491(7424):348–356. doi: 10.1038/nature11704. [DOI] [PubMed] [Google Scholar]
- 96.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allada R, Bass J. Circadian mechanisms in medicine. N Eng J Med. 2021;384(6):550–561. doi: 10.1056/NEJMra1802337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Knutson KL, et al. Association between sleep timing, obesity, diabetes: the Hispanic community health study/study of Latinos (HCHS/SOL) Cohort Study. Sleep. 2017;40(4):zsx014. doi: 10.1093/sleep/zsx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maury E. Off the clock: from circadian disruption to metabolic disease. Int J Mol Sci. 2019;20(7):1597. doi: 10.3390/ijms20071597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parsons MJ, et al. Social jet lag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes (Lond) 2015;39(5):842–848. doi: 10.1038/ijo.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu JH, et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J Clin Endocrinol Metab. 2015;100(4):1494–1502. doi: 10.1210/jc.2014-3754. [DOI] [PubMed] [Google Scholar]
- 103.Merikanto I, et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol Int. 2013;30(4):470–477. doi: 10.3109/07420528.2012.741171. [DOI] [PubMed] [Google Scholar]
- 104.Reutrakul S, et al. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. 2013;36(9):2523–2529. doi: 10.2337/dc12-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iwasaki M, et al. Morningness-eveningness questionnaire score correlates with glycated hemoglobin in middle-aged male workers with type 2 diabetes mellitus. J Diabetes Investig. 2013;4(4):376–381. doi: 10.1111/jdi.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Osonoi Y, et al. Morningness-eveningness questionnaire score and metabolic parameters in patients with type 2 diabetes mellitus. Chronobiol Int. 2014;31(9):1017–1023. doi: 10.3109/07420528.2014.943843. [DOI] [PubMed] [Google Scholar]
- 107.Morris CJ, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A. 2015;112(17):E2225–E2234. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leproult R, et al. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63(6):1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qian J, et al. Differential effects of the circadian system and circadian misalignment on insulin sensitivity and insulin secretion in humans. Diabetes Obes Metab. 2018;20(10):2481–2485. doi: 10.1111/dom.13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wefers J, et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc Natl Acad Sci U S A. 2018;115(30):7789–7794. doi: 10.1073/pnas.1722295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Facco FL, et al. Objectively measured short sleep duration and later sleep midpoint in pregnancy are associated with a higher risk of gestational diabetes. Am J Obstet Gynecol. 2017;217(4):447.e1–447.e13. doi: 10.1016/j.ajog.2017.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cheung IN, et al. Morning and evening blue-enriched light exposure alters metabolic function in normal weight adults. PLoS One. 2016;11(5):e0155601. doi: 10.1371/journal.pone.0155601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Plano SA, et al. Circadian and metabolic effects of light: implications in weight homeostasis and health. Front Neurol. 2017;8:558. doi: 10.3389/fneur.2017.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Melanson EL, et al. Daytime bright light exposure, metabolism, and individual differences in wake and sleep energy expenditure during circadian entrainment and misalignment. Neurobiol Sleep Circadian Rhythms. 2018;4:49–56. doi: 10.1016/j.nbscr.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baron KG, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19(7):1374–1381. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 116.Baron KG, et al. Circadian timing and alignment in healthy adults: associations with BMI, body fat, caloric intake and physical activity. Int J Obes (Lond) 2017;41(2):203–209. doi: 10.1038/ijo.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Culnan E, et al. Meal timing relative to DLMO: associations with BMI and body fat. Sleep Health. 2021;7(3):339–344. doi: 10.1016/j.sleh.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Riestra P, et al. Circadian CLOCK gene polymorphisms in relation to sleep patterns and obesity in African Americans: findings from the Jackson heart study. BMC Genet. 2017;18(1):58. doi: 10.1186/s12863-017-0522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meng Y, et al. Sex modifies the association between the CLOCK variant rs1801260 and BMI in school-age children. PLoS One. 2020;15(8):e0236991. doi: 10.1371/journal.pone.0236991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Loria-Kohen V, et al. Polymorphism in the CLOCK gene may influence the effect of fat intake reduction on weight loss. Nutrition. 2016;32(4):453–460. doi: 10.1016/j.nut.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 121.Tchio C, et al. Association between MTNR1B polymorphisms and obesity in African American: findings from the Jackson Heart Study. BMC Med Genomics. 2021;14(1):136. doi: 10.1186/s12920-021-00983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lane JM, et al. Impact of common diabetes risk variant in MTNR1B on sleep, circadian, and melatonin physiology. Diabetes. 2016;65(6):1741–1751. doi: 10.2337/db15-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garaulet M, et al. Common type 2 diabetes risk variant in MTNR1B worsens the deleterious effect of melatonin on glucose tolerance in humans. Metabolism. 2015;64(12):1650–1657. doi: 10.1016/j.metabol.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lyssenko V, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Prokopenko I, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41(1):77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lopez-Minguez J, et al. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: a randomized, cross-over study. Clin Nutr. 2018;37(4):1133–1140. doi: 10.1016/j.clnu.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McHill AW, et al. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci U S A. 2014;111(48):17302–17307. doi: 10.1073/pnas.1412021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qian J, et al. Sex differences in the circadian misalignment effects on energy regulation. Proc Natl Acad Sci U S A. 2019;116(47):23806–23812. doi: 10.1073/pnas.1914003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eriksson AK, et al. Work stress, sense of coherence, and risk of type 2 diabetes in a prospective study of middle-aged Swedish men and women. Diabetes Care. 2013;36(9):2683–2689. doi: 10.2337/dc12-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pan A, et al. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8(12):e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ha M, Park J. Shiftwork and metabolic risk factors of cardiovascular disease. J Occup Health. 2005;47(2):89–95. doi: 10.1539/joh.47.89. [DOI] [PubMed] [Google Scholar]
- 132.Khosravipour M, et al. A systematic review and meta-analysis of the association between shift work and metabolic syndrome: the roles of sleep, gender, and type of shift work. Sleep Med Rev. 2021;57:101427. doi: 10.1016/j.smrv.2021.101427. [DOI] [PubMed] [Google Scholar]
- 133.Roenneberg T, et al. Social jet lag and obesity. Curr Biol. 2012;22(10):939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 134.Chaix A, et al. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20(6):991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sutton EF, et al. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212–1221. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hatori M, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rangaraj VR, et al. Association between timing of energy intake and insulin sensitivity: a cross-sectional study. Nutrients. 2020;12(2):503. doi: 10.3390/nu12020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McHill AW, et al. Later circadian timing of food intake is associated with increased body fat. Am J Clin Nutr. 2017;106(5):1213–1219. doi: 10.3945/ajcn.117.161588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Allaf M, et al. Intermittent fasting for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2021;1(1):Cd013496. doi: 10.1002/14651858.CD013496.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Muller JE, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313(21):1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 141.Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke. 1998;29(5):992–996. doi: 10.1161/01.STR.29.5.992. [DOI] [PubMed] [Google Scholar]
- 142.Twidale N, et al. Morning increase in the time of onset of sustained ventricular tachycardia. Am J Cardiol. 1989;64(18):1204–1206. doi: 10.1016/0002-9149(89)90881-3. [DOI] [PubMed] [Google Scholar]
- 143.Edahiro R, et al. Association of lifestyle-related factors with circadian onset patterns of acute myocardial infarction: a prospective observational study in Japan. BMJ Open. 2014;4(6):e005067. doi: 10.1136/bmjopen-2014-005067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kanth R, et al. Circadian patterns of ST elevation myocardial infarction in the new millennium. Clin Med Res. 2013;11(2):66–72. doi: 10.3121/cmr.2013.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scheer FA, et al. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS One. 2011;6(9):e24549. doi: 10.1371/journal.pone.0024549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scheer FA, Shea SA. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood. 2014;123(4):590–593. doi: 10.1182/blood-2013-07-517060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pritchett D, Reddy AB. Circadian clocks in the hematologic system. J Biol Rhythms. 2015;30(5):374–388. doi: 10.1177/0748730415592729. [DOI] [PubMed] [Google Scholar]
- 148.Morris CJ, et al. Circadian misalignment increases c-reactive protein and blood pressure in chronic shift workers. J Biol Rhythms. 2017;32(2):154–164. doi: 10.1177/0748730417697537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Morris CJ, et al. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113(10):E1402–E1411. doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ramsey AM, et al. Environmental circadian disruption increases stroke severity and dysregulates immune response. J Biol Rhythms. 2020;35(4):368–376. doi: 10.1177/0748730420929450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Corella D, et al. CLOCK gene variation is associated with incidence of type-2 diabetes and cardiovascular diseases in type-2 diabetic subjects: dietary modulation in the PREDIMED randomized trial. Cardiovasc Diabetol. 2016;15:4. doi: 10.1186/s12933-015-0327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Amano H, et al. Interleukin-6 level among shift and night workers in Japan: cross-sectional analysis of the J-HOPE Study. J Atheroscler Thromb. 2018;25(12):1206–1214. doi: 10.5551/jat.42036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rizza S, et al. Carotid intimal medial thickness in rotating night shift is related to IL1β/IL6 axis. Nutr Metab Cardiovasc Dis. 2020;30(10):1826–1832. doi: 10.1016/j.numecd.2020.05.028. [DOI] [PubMed] [Google Scholar]
- 154.Rutters F, et al. Is social jet lag associated with an adverse endocrine, behavioral, and cardiovascular risk profile? J Biol Rhythms. 2014;29(5):377–383. doi: 10.1177/0748730414550199. [DOI] [PubMed] [Google Scholar]
- 155.Lucassen EA, et al. Evening chronotype is associated with changes in eating behavior, more sleep apnea, and increased stress hormones in short sleeping obese individuals. PLoS One. 2013;8(3):e56519. doi: 10.1371/journal.pone.0056519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lecarpentier Y, et al. Molecular mechanisms underlying the circadian rhythm of blood pressure in normotensive subjects. Curr Hypertens Rep. 2020;22(7):50. doi: 10.1007/s11906-020-01063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.de la Sierra A, et al. Nocturnal hypertension or nondipping: which is better associated with the cardiovascular risk profile? Am J Hypertens. 2014;27(5):680–687. doi: 10.1093/ajh/hpt175. [DOI] [PubMed] [Google Scholar]
- 158.Boggia J, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370(9594):1219–1229. doi: 10.1016/S0140-6736(07)61538-4. [DOI] [PubMed] [Google Scholar]
- 159.Lurbe E, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347(11):797–805. doi: 10.1056/NEJMoa013410. [DOI] [PubMed] [Google Scholar]
- 160.Sűdy ÁR, et al. Association of social jet lag with sleep quality and autonomic cardiac control during sleep in young healthy men. Front Neurosci. 2019;13:950. doi: 10.3389/fnins.2019.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fink AM. Measuring the effects of night-shift work on cardiac autonomic modulation: an appraisal of heart rate variability metrics. Int J Occup Med Environ Health. 2020;33(4):409–425. doi: 10.13075/ijomeh.1896.01560. [DOI] [PubMed] [Google Scholar]
- 162.Wang Y, et al. RNAseq shows an all-pervasive day-night rhythm in the transcriptome of the pacemaker of the heart. Sci Rep. 2021;11(1):3565. doi: 10.1038/s41598-021-82202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schroder EA, et al. Light phase-restricted feeding slows basal heart rate to exaggerate the type-3 long QT syndrome phenotype in mice. Am J Physiol Heart Circ Physiol. 2014;307(12):H1777–H1785. doi: 10.1152/ajpheart.00341.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.West AC, et al. Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nat Commun. 2017;8(1):417. doi: 10.1038/s41467-017-00462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Eckle T, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18(5):774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Oyama Y, et al. The circadian PER2 enhancer nobiletin reverses the deleterious effects of midazolam in myocardial ischemia and reperfusion injury. Curr Pharm Des. 2018;24(28):3376–3383. doi: 10.2174/1381612824666180924102530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bartman CM, et al. Intense light-elicited upregulation of miR-21 facilitates glycolysis and cardioprotection through Per2-dependent mechanisms. PLoS One. 2017;12(4):e0176243. doi: 10.1371/journal.pone.0176243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Güler AD, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453(7191):102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bendová Z, Sumová A. Photoperiodic regulation of PER1 and PER2 protein expression in rat peripheral tissues. Physiol Res. 2006;55(6):623–632. doi: 10.33549/physiolres.930849. [DOI] [PubMed] [Google Scholar]
- 170.Oyama Y, et al. Intense light-mediated circadian cardioprotection via transcriptional reprogramming of the endothelium. Cell Rep. 2019;28(6):1471–1484. doi: 10.1016/j.celrep.2019.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Oyama Y, et al. Intense light as anticoagulant therapy in humans. PLoS One. 2020;15(12):e0244792. doi: 10.1371/journal.pone.0244792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Brito LC, et al. Morning versus evening aerobic training effects on blood pressure in treated hypertension. Med Sci Sports Exerc. 2019;51(4):653–662. doi: 10.1249/MSS.0000000000001852. [DOI] [PubMed] [Google Scholar]
- 173.Hermida RC, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J. 2020;41(48):4565–4576. doi: 10.1093/eurheartj/ehz754. [DOI] [PubMed] [Google Scholar]
- 174.Kreutz R, et al. Disregard the reported data from the HYGIA project: blood pressure medication not to be routinely dosed at bedtime. J Hypertens. 2020;38(11):2144–2145. doi: 10.1097/HJH.0000000000002671. [DOI] [PubMed] [Google Scholar]
- 175.Xie Z, et al. Chronotherapy for morning blood pressure surge in hypertensive patients: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2021;21(1):274. doi: 10.1186/s12872-021-02081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bowles NP, et al. Chronotherapy for hypertension. Curr Hypetertens Rep. 2018;20(11):97. doi: 10.1007/s11906-018-0897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Serinel Y, et al. Chronotherapy for hypertension in obstructive sleep apnoea (CHOSA): a randomised, double-blind, placebo-controlled crossover trial. Thorax. 2017;72(6):550–558. doi: 10.1136/thoraxjnl-2016-209504. [DOI] [PubMed] [Google Scholar]
- 178.Bobkowski W, et al. Measures of heart rate variability in 24-h ECGs depend on age but not gender of healthy children. Front Physiol. 2017;8:311. doi: 10.3389/fphys.2017.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Stephenson EA, et al. Circadian and seasonal variation of malignant arrhythmias in a pediatric and congenital heart disease population. J Cardiovasc Electrophysiol. 2002;13(10):1009–1014. doi: 10.1046/j.1540-8167.2002.01009.x. [DOI] [PubMed] [Google Scholar]
- 180.Kardelen F, et al. Heart rate variability and circadian variations in type 1 diabetes mellitus. Pediatr Diabetes. 2006;7(1):45–50. doi: 10.1111/j.1399-543X.2006.00141.x. [DOI] [PubMed] [Google Scholar]
- 181.Wolfenstetter A, et al. Altered cardiovascular rhythmicity in children born small for gestational age. Hypertension. 2012;60(3):865–870. doi: 10.1161/HYPERTENSIONAHA.112.196949. [DOI] [PubMed] [Google Scholar]
- 182.Qian J, et al. Blunted rest-activity rhythms link to higher body mass index and inflammatory markers in children. Sleep. 2021;44(5):zsaa256. doi: 10.1093/sleep/zsaa256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Macumber IR, et al. The association of pediatric obesity with nocturnal non-dipping on 24-hour ambulatory blood pressure monitoring. Am J Hypertens. 2016;29(5):647–652. doi: 10.1093/ajh/hpv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chang JC, et al. Nocturnal blood pressure dipping as a marker of endothelial function and subclinical atherosclerosis in pediatric-onset systemic lupus erythematosus. Arthritis Res Ther. 2020;22(1):129. doi: 10.1186/s13075-020-02224-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dionne JM, et al. Blood pressure abnormalities in children with chronic kidney disease. Blood Press Monit. 2008;13(4):205–209. doi: 10.1097/MBP.0b013e3283052fd0. [DOI] [PubMed] [Google Scholar]
- 186.Crowley SJ, et al. Estimating dim light melatonin onset (DLMO) phase in adolescents using summer or school-year sleep/wake schedules. Sleep. 2006;29(12):1632–1641. doi: 10.1093/sleep/29.12.1632. [DOI] [PubMed] [Google Scholar]
- 187.Crowley SJ, et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS One. 2014;9(11):e112199. doi: 10.1371/journal.pone.0112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Martínez-Lozano N, et al. Evening types have social jet lag and metabolic alterations in school-age children. Sci Rep. 2020;10(1):16747. doi: 10.1038/s41598-020-73297-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mitchell JA, et al. Targeting sleep duration and timing for prevention of adolescent obesity. JAMA Pediatr. 2019;173(11):1018–1020. doi: 10.1001/jamapediatrics.2019.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cespedes Feliciano EM, et al. Chronotype, social jet lag, and cardiometabolic risk factors in early adolescence. JAMA Pediatr. 2019;173(11):1049–1057. doi: 10.1001/jamapediatrics.2019.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Touitou Y, et al. Disruption of adolescents’ circadian clock: the vicious circle of media use, exposure to light at night, sleep loss and risk behaviors. J Physiol Paris. 2016;110(4 pt b):467–479. doi: 10.1016/j.jphysparis.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 192.Mathew GM, et al. Social jet lag, eating behaviours and BMI among adolescents in the USA. Br J Nutr. 2020;124(9):979–987. doi: 10.1017/S0007114520001804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Labrecque N, Cermakian N. Circadian clocks in the immune system. J Biol Rhythms. 2015;30(4):277–290. doi: 10.1177/0748730415577723. [DOI] [PubMed] [Google Scholar]
- 194.Cuesta M, et al. Simulated night shift disrupts circadian rhythms of immune functions in humans. J Immunol. 2016;196(6):2466–2475. doi: 10.4049/jimmunol.1502422. [DOI] [PubMed] [Google Scholar]
- 195.Fortier EE, et al. Circadian variation of the response of T cells to antigen. J Immunol. 2011;187(12):6291–6300. doi: 10.4049/jimmunol.1004030. [DOI] [PubMed] [Google Scholar]
- 196.Nguyen KD, et al. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341(6153):1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Druzd D, et al. Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity. 2017;46(1):120–132. doi: 10.1016/j.immuni.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Scheiermann C, et al. Clocking in to immunity. Nat Rev Immunol. 2018;18(7):423–437. doi: 10.1038/s41577-018-0008-4. [DOI] [PubMed] [Google Scholar]
- 199.Man K, et al. Immunity around the clock. Science. 2016;354(6315):999–1003. doi: 10.1126/science.aah4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yu X, et al. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342(6159):727–730. doi: 10.1126/science.1243884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Spiegel K, et al. Effect of sleep deprivation on response to immunization. JAMA. 2002;288(12):1471–1472. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- 202.Greenberg EN, et al. Circadian control of interferon-sensitive gene expression in murine skin. Proc Natl Acad Sci U S A. 2020;117(11):5761–5771. doi: 10.1073/pnas.1915773117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Castanon-Cervantes O, et al. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185(10):5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Durrington HJ, et al. The circadian clock and asthma. Thorax. 2014;69(1):90–92. doi: 10.1136/thoraxjnl-2013-203482. [DOI] [PubMed] [Google Scholar]
- 205.Nakao A. Circadian regulation of the biology of allergic disease: clock disruption can promote allergy. Front Immunol. 2020;11:1237. doi: 10.3389/fimmu.2020.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Silva FRD, et al. Does the compromised sleep and circadian disruption of night and shiftworkers make them highly vulnerable to 2019 coronavirus disease (COVID-19)? Chronobiol Int. 2020;37(5):607–617. doi: 10.1080/07420528.2020.1756841. [DOI] [PubMed] [Google Scholar]
- 207.Goren A, et al. Clock genes may drive seasonal variation in SARS-CoV-2 infectivity: are we due for a second wave of COVID-19 in the fall? J Biol Regul Homeost Agents. 2020;34(4):1455–1457. doi: 10.23812/20-359-L-35. [DOI] [PubMed] [Google Scholar]
- 208.Fatima Y, et al. Shift work is associated with increased risk of COVID-19: findings from the UK Biobank cohort. J Sleep Res. doi: 10.1111/jsr.13326. [published online March 8, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Edgar RS, et al. Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc Natl Acad Sci U S A. 2016;113(36):10085–10090. doi: 10.1073/pnas.1601895113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhuang X, et al. Circadian control of hepatitis B virus replication. Nat Commun. 2021;12(1):1658. doi: 10.1038/s41467-021-21821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vitale JA, et al. Is disruption of sleep quality a consequence of severe Covid-19 infection? A case-series examination. Chronobiol Int. 2020;37(7):1110–1114. doi: 10.1080/07420528.2020.1775241. [DOI] [PubMed] [Google Scholar]
- 212.Haspel J, et al. A timely call to arms: COVID-19, the circadian clock, and critical care. J Biol Rhythms. 2021;36(1):55–70. doi: 10.1177/0748730421992587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ray S, Reddy AB. COVID-19 management in light of the circadian clock. Nat Rev Mol Cell Biol. 2020;21(9):494–495. doi: 10.1038/s41580-020-0275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sengupta S, et al. Accounting for time: circadian rhythms in the time of COVID-19. J Biol Rhythms. 2021;36(1):4–8. doi: 10.1177/0748730420953335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.De Giorgi A, et al. Morning vs. evening administration of antiviral therapy in COVID-19 patients. A preliminary retrospective study in Ferrara, Italy. Eur Rev Med Pharmacol Sci. 2020;24(15):8219–8225. doi: 10.26355/eurrev_202008_22511. [DOI] [PubMed] [Google Scholar]
- 216.Matsunaga N, et al. 24-Hour rhythm of aquaporin-3 function in the epidermis is regulated by molecular clocks. J Invest Dermatol. 2014;134(6):1636–1644. doi: 10.1038/jid.2014.13. [DOI] [PubMed] [Google Scholar]
- 217.Kyoko OO, et al. Expressions of tight junction proteins Occludin and Claudin-1 are under the circadian control in the mouse large intestine: implications in intestinal permeability and susceptibility to colitis. PLoS One. 2014;9(5):e98016. doi: 10.1371/journal.pone.0098016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gibbs J, et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med. 2014;20(8):919–926. doi: 10.1038/nm.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ehlers A, et al. BMAL1 links the circadian clock to viral airway pathology and asthma phenotypes. Mucosal Immunol. 2018;11(1):97–111. doi: 10.1038/mi.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Durrington HJ, et al. Circadian asthma airway responses are gated by REV-ERBα. Eur Respir J. 2020;56(6):1902407. doi: 10.1183/13993003.02407-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Maidstone RJ, et al. Night shift work is associated with an increased risk of asthma. Thorax. 2021;76(1):53–60. doi: 10.1136/thoraxjnl-2020-215218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Langwinski W, et al. Association of circadian clock TIMELESS variants and expression with asthma risk in children. Clin Respir J. 2020;14(12):1191–1200. doi: 10.1111/crj.13260. [DOI] [PubMed] [Google Scholar]
- 223.Manella G, et al. Hypoxia induces a time- and tissue-specific response that elicits intertissue circadian clock misalignment. Proc Natl Acad Sci U S A. 2020;117(1):779–786. doi: 10.1073/pnas.1914112117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Singulair. Product monograph. Merck Canada Inc.; 2020. Accessed August 18, 2021. https://www.merck.ca/static/pdf/SINGULAIR-PM_E.pdf.
- 225.Bavishi AA, et al. Diurnal variations in subcutaneous allergen immunotherapy reactions. Ann Allergy Asthma Immunol. 2017;118(1):103–107. doi: 10.1016/j.anai.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 226.Nakao A, et al. The circadian clock functions as a potent regulator of allergic reaction. Allergy. 2015;70(5):467–473. doi: 10.1111/all.12596. [DOI] [PubMed] [Google Scholar]
- 227. Padem N, Fishbein A, eds. Allergy and Sleep. Springer; 2019. [Google Scholar]
- 228.Nakamura Y, et al. Circadian regulation of allergic reactions by the mast cell clock in mice. J Allergy Clin Immunol. 2014;133(2):568–575. doi: 10.1016/j.jaci.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 229.Nakamura Y, et al. Circadian clock gene Period2 regulates a time-of-day-dependent variation in cutaneous anaphylactic reaction. J Allergy Clin Immunol. 2011;127(4):1038–1045. doi: 10.1016/j.jaci.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 230.Nakamura Y, et al. Time-restricted feeding in rest phase alters IgE/mast cell-mediated allergic reaction in mice. Allergol Int. 2020;69(2):296–299. doi: 10.1016/j.alit.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 231.Bousquet J, et al. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5 suppl):S147–S334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 232.Cloutier MM, et al. 2020 focused updates to the Asthma Management Guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217–1270. doi: 10.1016/j.jaci.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.National Asthma Education Prevention Program Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma—Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 234.Kraft M, et al. Blood and bronchoalveolar lavage endothelin-1 levels in nocturnal asthma. Am J Respir Crit Care Med. 1994;149(4 pt 1):946–952. doi: 10.1164/ajrccm.149.4.8143060. [DOI] [PubMed] [Google Scholar]
- 235.Barnes P, et al. Nocturnal asthma and changes in circulating epinephrine, histamine, and cortisol. N Engl J Med. 1980;303(5):263–267. doi: 10.1056/NEJM198007313030506. [DOI] [PubMed] [Google Scholar]
- 236.Fishbein AB, et al. Nocturnal movements in children with atopic dermatitis have a timing pattern: a case-control study. J Am Acad Dermatol. 2021;85(2):474–476. doi: 10.1016/j.jaad.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nakamura Y, et al. Inhibition of IgE-mediated allergic reactions by pharmacologically targeting the circadian clock. J Allergy Clin Immunol. 2016;137(4):1226–1235. doi: 10.1016/j.jaci.2015.08.052. [DOI] [PubMed] [Google Scholar]
- 238.Cunningham PS, et al. The circadian clock protein REVERBα inhibits pulmonary fibrosis development. Proc Natl Acad Sci U S A. 2020;117(2):1139–1147. doi: 10.1073/pnas.1912109117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhang R, et al. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111(45):16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ruben MD, et al. Dosing time matters. Science. 2019;365(6453):547–549. doi: 10.1126/science.aax7621. [DOI] [PMC free article] [PubMed] [Google Scholar]