Abstract
BACKGROUND
The angiotensin-converting enzyme (ACE) D allele is more prevalent among African Americans compared with other races and ethnicities and has previously been associated with severe coronavirus disease 2019 (COVID-19) pathogenesis through excessive ACE1 activity. ACE inhibitors/angiotensin receptor blockers (ACE-I/ARB) may counteract this mechanism, but their association with COVID-19 outcomes has not been specifically tested in the African American population.
METHODS
We identified 6218 patients who were admitted into Mount Sinai hospitals with COVID-19 between February 24 and May 31, 2020, in New York City. We evaluated whether the outpatient and in-hospital use of ACE-I/ARB is associated with COVID-19 in-hospital mortality in an African American compared with non–African American population.
RESULTS
Of the 6218 patients with COVID-19, 1138 (18.3%) were ACE-I/ARB users. In a multivariate logistic regression model, ACE-I/ARB use was independently associated with a reduced risk of in-hospital mortality in the entire population (OR, 0.655; 95% CI, 0.505–0.850; P = 0.001), African American population (OR, 0.44; 95% CI, 0.249–0.779; P = 0.005), and non–African American population (OR, 0.748, 95% CI, 0.553–1.012, P = 0.06). In the African American population, in-hospital use of ACE-I/ARB was associated with improved mortality (OR, 0.378; 95% CI, 0.188–0.766; P = 0.006), whereas outpatient use was not (OR, 0.889; 95% CI, 0.375–2.158; P = 0.812). When analyzing each medication class separately, ARB in-hospital use was significantly associated with reduced in-hospital mortality in the African American population (OR, 0.196; 95% CI, 0.074–0.516; P = 0.001), whereas ACE-I use was not associated with impact on mortality in any population.
CONCLUSION
In-hospital use of ARB was associated with a significant reduction in in-hospital mortality among COVID-19–positive African American patients.
FUNDING
None.
Keywords: COVID-19
Keywords: Cardiovascular disease, Drug therapy, Epidemiology
Introduction
The current coronavirus disease 2019 (COVID-19) pandemic is expected to reduce life expectancy in the United States in 2020 by 1.13 years, although life expectancy reductions in African American and Hispanic populations are estimated to be 3 to 4 times higher than among the White population (1). US African American and Hispanic individuals make up approximately 13.4% and 18.5% of the US population, respectively, but they constitute roughly 21% and 22%, respectively, of COVID-19 deaths reported to the National Center for Health Sciences (2). Socioeconomic factors contribute to these disparities, with 19.7% of African American and 16.2% of Hispanic individuals disproportionately represented among essential occupations (3). However, it remains unclear whether population differences in the prevalence of genetic risk variants may also contribute to disparate outcomes.
Several studies have attempted to delineate the genetic basis of susceptibility to COVID-19 clinical syndromes (4–7). Epidemiological analyses have suggested that polymorphisms in the angiotensin-converting enzyme (ACE) gene, which encodes ACE1, are related to the incidence and mortality from COVID-19 at the national level (8–11). The insertion (I) or deletion (D) of a 287 bp sequence in intron 16 of the ACE gene results in 3 possible genotypes: DD homozygotes, II homozygotes, or ID heterozygotes. The D allele has been associated with increased ACE1 expression in a dose-dependent manner, such that compared with II homozygotes, ID heterozygotes and DD homozygotes have 31% and 65% more ACE1 protein expression in blood serum, respectively (12). Data from predominantly European nations suggests that there is a population-level correlation between the prevalence of the D allele and incidence of COVID-19 (10).
SARS-CoV-2 enters cells by binding to ACE2. ACE1 and ACE2 have counterregulatory roles in the renin-angiotensin (RAS) system: ACE1 converts angiotensin I to angiotensin II, the primary effector of the RAS system, and ACE2 inactivates angiotensin II. Since SARS-CoV-2 viral binding and entry via ACE2 leads to decreased ACE2 availability, it has been hypothesized that an imbalance in ACE1 and ACE2 activity (leading to excess angiotensin II) may contribute to the pathogenesis of severe COVID-19 (13). This imbalance may be further exacerbated by increased ACE1 expression in the presence of the ACE D allele. This mechanistic hypothesis predicts that either ACE1 inhibition or angiotensin II receptor blockade would have a beneficial effect on COVID-19 outcomes, especially in populations with high prevalence of the ACE D allele (13).
Early in the COVID-19 pandemic, there were concerns that ACE inhibitors (ACE-I) and angiotensin receptor blockers (ARB), commonly used to manage hypertension, might increase ACE2 expression and thus increase susceptibility to COVID-19. Since then, several groups have assessed the relationship between ACE-I/ARB use and COVID-19 outcomes, with no clear consensus on risk versus benefit (14–16). However, these studies were conducted in predominantly White populations, were focused on estimating risk of COVID-19, and did not account for ethnicity and the ACE D allele prevalence. Differences in the frequency of continuing or discontinuing ACE-I/ARB across hospitalized patients may also have contributed to discrepancies between studies (17). These results supported the joint statement issued by expert cardiology societies that in the absence of experimental or clinical information demonstrating benefit or adverse outcomes, individuals using ACE-I/ARB medications should continue their medications during COVID-19 infection (18).
We recently reported that there was an increased prevalence of the ACE D allele among African Americans compared with other races and ethnicities (13). We therefore hypothesized that there may be a more pronounced benefit from ACE-I/ARB therapy in the African American population than in prior published cohorts. This study assessed whether ACE-I/ARB use among hospitalized African American patients with COVID-19 was associated with clinical benefit and improved outcome.
Results
Participants.
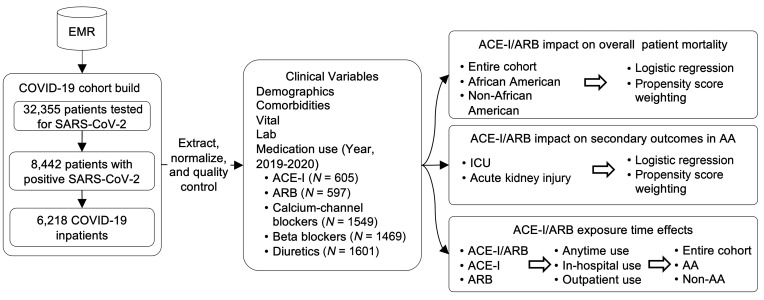
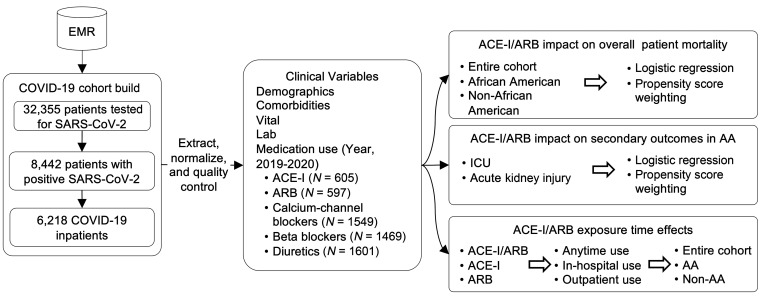
Our cohort included 6218 patients diagnosed with COVID-19 and admitted to 1 of 5 hospitals using the Epic system in the Mount Sinai Health System from February 24 through May 31, 2020. Among these patients, 1139 (18.3%) were ACE-I/ARB users, and 5079 (81.7%) were ACE-I/ARB nonusers since 2019. The data extraction process and study workflow are shown in Figure 1. ACE-I/ARB users were older than nonusers (median age, 68 [IQR, 60–78] vs. 60 [IQR, 43–72]; Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/JCI151418DS1). Compared with nonusers, ACE-I/ARB users were more likely to have cardiovascular comorbidities and risk factors, such as hypertension (60.6% vs. 18.3%; P < 0.001), diabetes mellitus (40.1% vs. 11.3%; P < 0.001), coronary artery disease (24.0% vs. 5.7%; P < 0.001), and heart failure (16.3% vs. 3.0%; P < 0.001). ACE-I/ARB users were also more likely to use other antihypertensive medications, such as calcium channel blockers (53.8% vs. 18.4%; P < 0.001), beta blockers (47.7% vs. 18.2%; P < 0.001), and diuretics (56.4% vs. 18.9%; P < 0.001). As of May 31, 2020, of the 6218 patients in our COVID-19 cohort, 1326 (21.3%) died, 4384 (70.5%) were discharged, and 508 (8.2%) were still hospitalized as of May 31, 2020.
Figure 1. The working flowchart for analyzing ACE-I/ARB effects on COVID-19 in-hospital mortality.
EMR, electronic medical record; AA, African American.
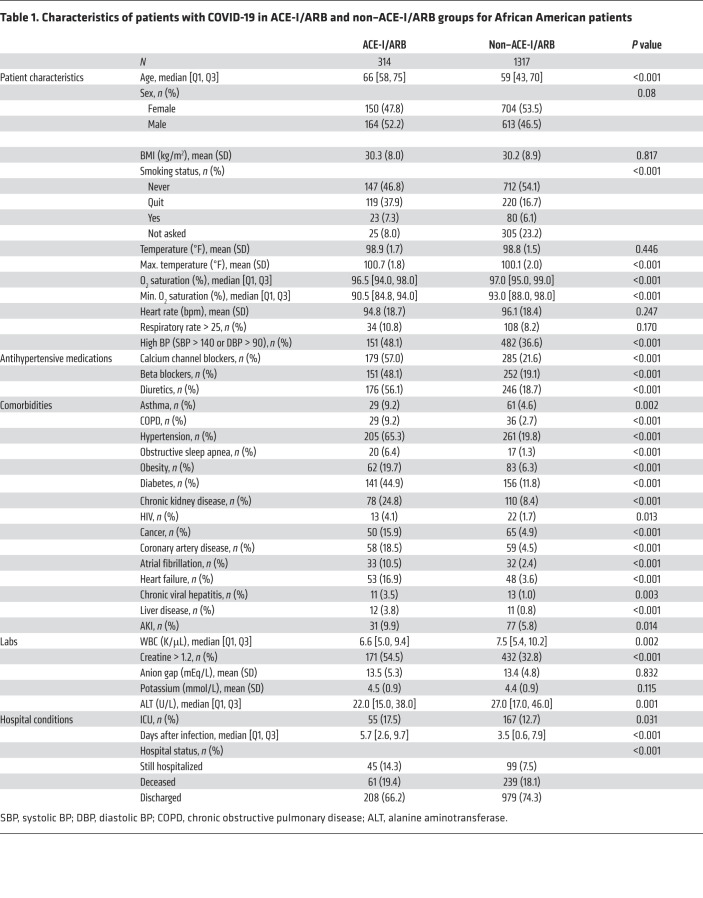
African American patients comprised 26.2% of our COVID-19 cohort (1621 patients). Similar to the entire population, African American ACE-I/ARB users were older than ACE-I/ARB nonusers (median age, 66 [IQR 58–75] vs. 59 [IQR 43–70]) and more likely to have disease comorbidities (Table 1 and Supplemental Table 1). In the African American population, 300 patients (18.4%) died, 1187 patients (72.8%) were discharged, and 114 patients (8.8%) were still hospitalized as of May 31, 2020. A comparison of the characteristics of the African American and non–African American populations is presented in Supplemental Table 2.
Table 1. Characteristics of patients with COVID-19 in ACE-I/ARB and non–ACE-I/ARB groups for African American patients.
ACE-I/ARB associations with in-hospital mortality overall and by race.
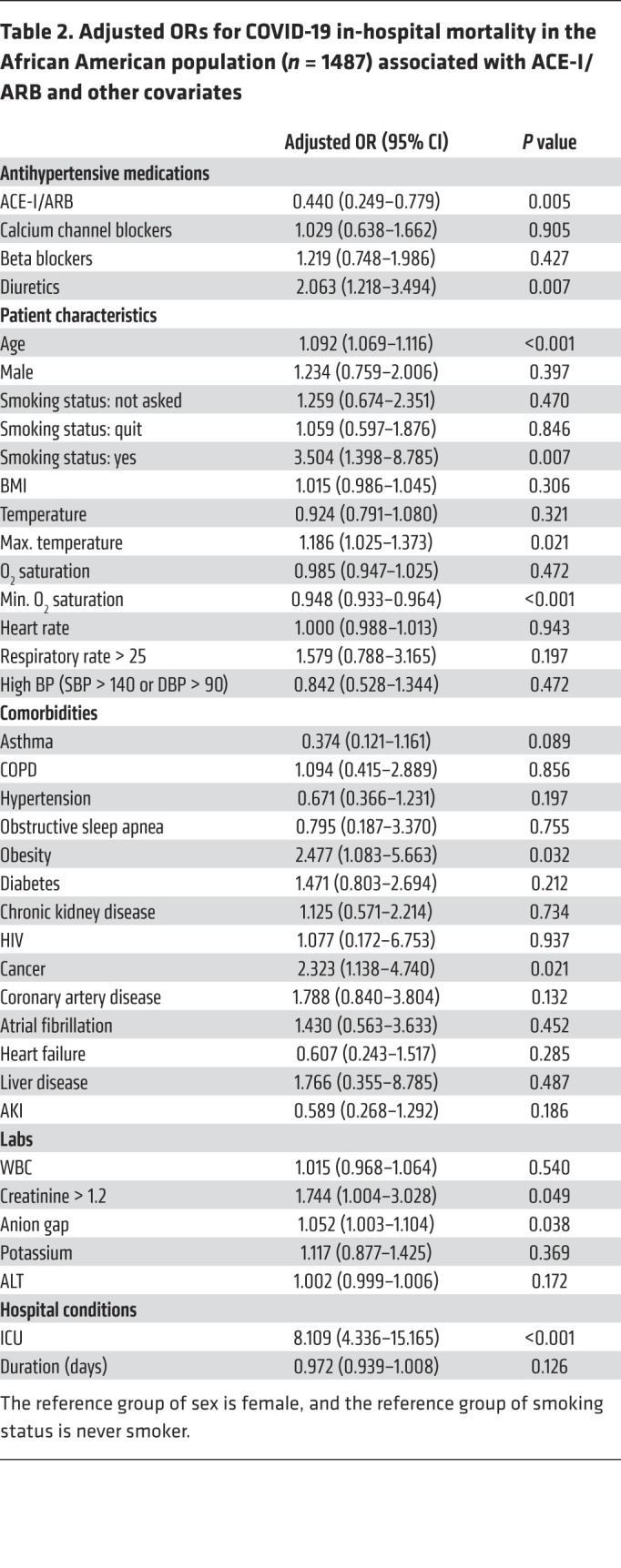
Unadjusted mortality was higher in ACE-I/ARB users compared with nonusers in the entire population (25.2% vs. 20.5%, P < 0.001) and the African American population (19.4% vs. 18.1%, P = 0.296; Table 1 and Supplemental Table 1), especially for hypertension, obesity, and diabetes, which are known risk factors for COVID-19 mortality (19). We therefore adjusted for these confounding factors in this population substantially enriched with chronic conditions and observed that ACE-I/ARB use was independently associated with reduced risk of in-hospital mortality in the entire population (OR, 0.655; 95% CI, 0.505–0.850; P = 0.001) by the multivariable logistic regression model (Supplemental Table 3). Although the interaction term of ACE-I/ARB use and African American population was not significant (OR, 0.736; 95% CI, 0.428–1.266; P = 0.268), the odds of in-hospital death in African American patients who received ACE-I/ARB were smaller compared with non–African American patients who also received ACE-I/ARB (OR, 0.659; Supplemental Table 4). When stratified by subpopulation, there was a significant reduction in in-hospital mortality in the African American population (OR, 0.44; 95% CI, 0.249–0.779; P = 0.005; Table 2), and this did not reach significance in the non–African American population (OR, 0.748; 95% CI, 0.553–1.012; P = 0.06) with the power 1-β = 0.8 in the Wald-based power analysis (Supplemental Table 3). The prescription ratio of ACE-I/ARB was similar between the African American and non–African American populations (Supplemental Table 2). We confirmed this result using inverse propensity-weighted logistic regression after balancing for confounders: ACE-I/ARB use was significantly associated with decreased risk of in-hospital mortality in the entire population (OR, 0.673; 95% CI, 0.511–0.886; P = 0.005) and the African American population (OR, 0.381; 95% CI, 0.204–0.712; P = 0.003) but not the non–African American population (OR, 0.765; 95% CI, 0.556–1.052; P = 0.1).
Table 2. Adjusted ORs for COVID-19 in-hospital mortality in the African American population (n = 1487) associated with ACE-I/ARB and other covariates.
ACE-I/ARB use among African Americans did not affect secondary outcomes.
In the African American population, given that we identified the significant association between ACE-I/ARB use and in-hospital mortality, we further estimated the effects of ACE-I/ARB use on 2 secondary outcomes: intensive care unit (ICU) admission and acute kidney injury (AKI) development. Unadjusted rates of these outcomes were higher among African American ACE-I/ARB users than African American nonusers (death: 19.4% vs. 18.1%, P = 0.296; ICU: 17.5% vs. 12.7%, P = 0.03; AKI: 9.9% vs. 5.8%, P = 0.01; Table 1). Neither ICU admission (OR, 0.709; 95% CI, 0.419–1.198; P = 0.198) nor AKI (OR, 1.306; 95% CI, 0.721–2.364; P = 0.379) were significantly associated with ACE-I/ARB use in the African American population (Supplemental Table 5). We confirmed these findings using inverse propensity-weighted logistic regression as well, where again, ACE-I/ARB use was not significantly associated with ICU admission (OR, 0.733; 95% CI, 0.425–1.264; P = 0.264) or AKI (OR, 1.257; 95% CI, 0.642–2.463; P = 0.505).
In-hospital ARB use reduced risk of COVID-19–caused in-hospital mortality.
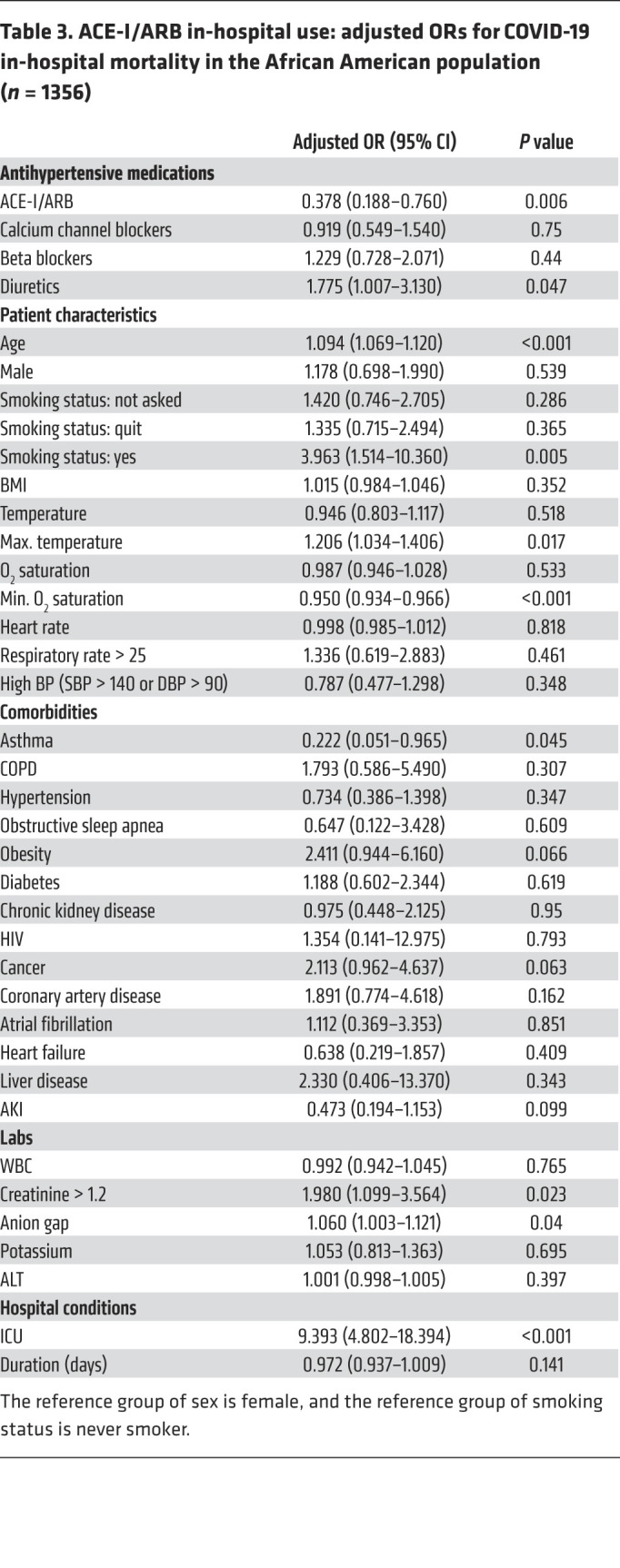
Given the significant impact ACE-I/ARB exposure had on in-hospital mortality of African American patients admitted for COVID-19, we sought to distinguish between in-hospital use of these classes of drugs and outpatient use, and we sought to distinguish between the 2 classes of drugs. ACE-I/ARB in-hospital use was significantly associated with reduced mortality in the entire population (OR, 0.575; 95% CI, 0.419–0.791; P = 0.001), the African American population (OR, 0.378; 95% CI, 0.188–0.766; P = 0.006), and the non–African American population (OR, 0.652; 95% CI, 0.449–0.946; P = 0.024) (Table 3 and Supplemental Table 6). Outpatient ACE-I/ARB use alone was not significantly associated with mortality in the entire population (OR, 1.016; 95% CI, 0.69–1.496; P = 0.936), the African American population (OR, 0.889; 95% CI, 0.375–2.158; P = 0.812), or the non–African American population (OR, 1.101; 95% CI, 0.7–1.73; P = 0.677).
Table 3. ACE-I/ARB in-hospital use: adjusted ORs for COVID-19 in-hospital mortality in the African American population (n = 1356).
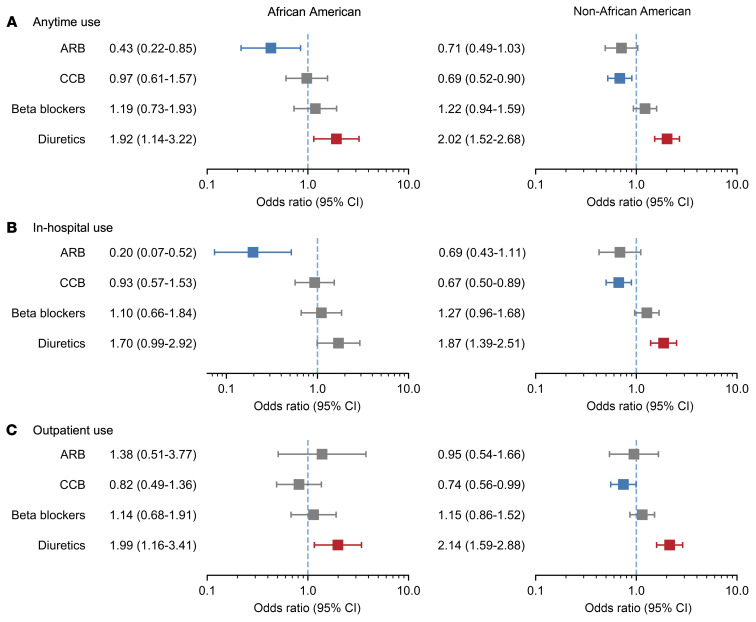
We additionally evaluated ACE-I use and ARB use separately and across different exposure periods (anytime use, in-hospital use, and outpatient use). ARB in-hospital use was significantly associated with mortality in the entire population (OR, 0.560; 95% CI, 0.371–0.846; P = 0.006) and the African American population (OR, 0.196; 95% CI, 0.074–0.516; P = 0.001), but not the non–African American population (OR, 0.687; 95% CI, 0.427–1.106; P = 0.122), which might be due to limited statistical power (1-β = 0.55 in the Wald-based power analysis) (Figure 2); ACE-I use was not significantly associated with mortality in any cohort or any drug exposure period (Supplemental Figure 1). Again, the interaction term of ARB use and African American population was not significant (OR, 0.488; 95% CI, 0.185–1.292; P = 0.149), but the direction of the effect suggests African American individuals who received ARB might have smaller odds (OR, 0.421) of in-hospital death compared with non–African American individuals (Supplemental Table 4). Outpatient use was not significantly associated with in-hospital mortality for either medication class. We confirmed all the results with inverse propensity-weighted logistic regression (Supplemental Table 7).
Figure 2. Different ARB exposure times and other antihypertensive drugs and their associations with COVID-19 in-hospital mortality.
(A) Anytime use, African American (n = 1487), non–African American (n = 4223). (B) In-hospital use, African American (n = 1415), non–African American (n = 4042). (C) Outpatient use, African American (n = 1396), non–African American (n = 3982).
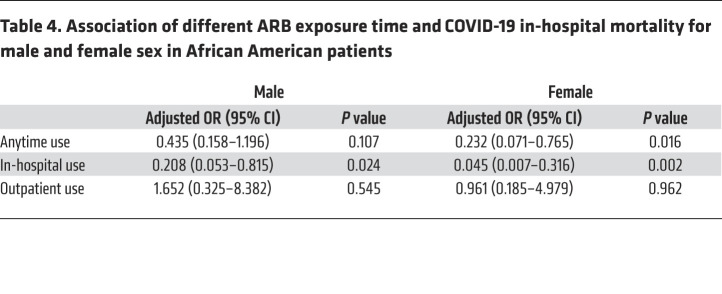
In-hospital ARB use was associated with reduced mortality in male and female African American patients; the magnitude of the protective effect was greater in African American female patients (OR, 0.045; 95% CI, 0.007–0.316; P = 0.002) compared with African American male patients (OR, 0.208; 95% CI, 0.053–0.815; P = 0.024) (Table 4).
Table 4. Association of different ARB exposure time and COVID-19 in-hospital mortality for male and female sex in African American patients.
Discussion
In this retrospective study of a racially and ethnically diverse cohort from New York City comprising 6218 hospitalized patients with COVID-19 seen between February 24 and March 31, 2020, we found that ACE-I/ARB use was significantly associated with reduced in-hospital mortality, particularly among African American patients. ACE-I/ARB users were older and had more comorbidities than nonusers, but after controlling for these adverse risk factors, in-hospital ACE-I/ARB use (as opposed to outpatient-only use) was independently associated with improved outcomes. When analyzing ACE-I and ARB exposures separately, in-hospital ARB use was significantly associated with improved outcomes among African American patients but not non–African American patients. Conversely, ACE-I exposure was not independently associated with outcomes in any cohort.
Early in the COVID-19 pandemic, the finding that SARS-CoV-2 enters cells by binding to the ACE2 protein led to concern that ACE-I/ARB might affect SARS-CoV-2 infectivity or virulence (20). Many clinical studies from the United States, Europe, and China were subsequently reported, examining the association between ACE-I/ARB use and COVID-19 outcomes (21–23). These studies found that ACE-I/ARB usage was associated with neither harm nor benefit in the setting of COVID-19 in the White population; however, 1 meta-analysis grouped studies by geography and found a lower mortality risk associated with ACE-I/ARB use among studies from China (OR = 0.65; 95% CI, 0.46−0.91; P = 0.013) (21), which might be suggested by a higher ACE I/D allele frequency in the Asian population (13, 24–26). There was no significant association among studies from the United States or Europe, suggesting the need to account for race and ethnicity. To our knowledge, this is the first study to report a potential benefit from the use of ACE-I/ARB in an African American population hospitalized with COVID-19 using one of the largest EMR systems in New York City.
Race and ethnicity may be relevant in this context as proxies for genetic ancestry and the probability of carrying the ACE D allele — the deletion allele of the ACE insertion/deletion polymorphism — which is associated with increased ACE1 activity and may contribute to ACE1/ACE2 imbalance in SARS-CoV-2 infection. Epidemiological studies in European, North African, and Asian settings have found significant positive correlations between D allele prevalence and COVID-19 infection and mortality rates at the population level (8–11, 26). While it is not practical at present to determine the ACE genotype of large cohorts of patients with COVID-19, the prevalence of the D allele is known to vary geographically and is highest in Africa and the Middle East (27). Therefore, race may serve as a proxy for the probability of D allele carrier status. Indeed, we recently reported that African Americans have a higher prevalence of the D allele than White Americans (13).
With this premise in mind, we investigated the interaction between African American race and ACE-I/ARB use in relation to inpatient COVID-19 outcomes. Individuals carrying the D allele would be expected to have excessive ACE1 and angiotensin II in the context of COVID-19 infection and may thus derive benefit from either ACE1 inhibition by ACE-I or angiotensin II blockade via ARB (13). Using a multiethnic cohort of 6218 hospitalized patients with COVID-19, we found a substantial association between ACE-I/ARB use and reduced in-hospital mortality overall; this association appeared to be driven by a strong protective association between ACE-I/ARB use and in-hospital mortality in the African American (but not the non–African American) population though the interaction with ACE-I/ARB use, and race was not significant. Although our ability to detect a significant interaction may have been limited by sample size, these findings are consistent with the hypothesis that RAS hyperactivity in an ACE D allele–enriched (e.g., African American) COVID-19–positive cohort may benefit from ACE-I/ARB use (13).
We further analyzed ACE-I or ARB use separately and according to either outpatient or in-hospital exposure. Neither in-hospital nor outpatient ACE-I use was associated with mortality. Conversely, in-hospital ARB use was significantly associated with reduced mortality among African Americans but not non–African Americans. This may suggest that ACE1 inhibition alone is inadequate in the context of ACE2 occupation/downregulation by SARS-CoV-2 virions; excess angiotensin II may still accumulate in the absence of sufficient ACE2 activity. Inhibiting downstream angiotensin II type 1 receptors using ARB may therefore be more effective. Mechanistic and clinical studies to dissect the relationships among COVID-19, ACE1/ACE2 activity, angiotensin II levels, and ARB are warranted. Attention should be paid to underlying ACE genotype in studies of RAS inhibitors in COVID-19 in the future. The ongoing randomized clinical trial of ramipril against a placebo to treat patients with COVID-19 (ClinicalTrials.gov NCT04366050) may provide insightful data on the therapeutic value of ACE inhibitors (28).
Although sex was not significantly associated with mortality rate in our data (Table 3), we detected a sex-specific effect of ARB in protecting against COVID-19 mortality. ARB use was significantly associated with lower mortality in the African American population in female and male patients, and the protective effect showed a stronger signal in female than male patients (OR, 0.045; P = 0.002 vs. OR, 0.208, P = 0.024, respectively) for in-hospital use. The sex-specific effect could be at least partially due to different ACE2 levels in male and female patients. ACE2 is an X-linked gene expressed in many organs (29, 30). It is located on the cell membrane and acts as a brake on angiotensin II by converting it into angiotensin 1–7, resulting in attenuation of its vasoconstrictive and other activities (20). When SARS-CoV-2 binds to ACE2, it inhibits ACE2’s function and makes more angiotensin II available to bind its cognate receptor, angiotensin II type 1 receptor (AT1), consequently leading to inflammation and tissue injury. (20). The expression level, protein level, and activity of ACE2 were found to be higher in male compared with female participants, possibly as a result of sex hormone–dependent regulation (30). As stated above, lower ACE2 activity in female patients leads to more available angiotensin II. Therefore, one possible explanation for the ARB’s sex-specific effect in protection against COVID-19 mortality could be that SARS-CoV-2 infection elevates angiotensin II binding to the angiotensin II receptor more substantially in female patients and is thus more responsive to ARB. As an important caveat, the current knowledge of ACE2 sex differences is mostly derived at normal conditions, and scenarios after SARS-CoV-2 infection and under ARB treatment certainly need further investigation to fully understand the mechanisms by which ARB protects against COVID-19 mortality as well as the sex differences.
Prior clinical studies in the US population have examined associations between ACE-I/ARB use and COVID-19 outcomes without stratification by race (16, 31–33). Most of these studies have found no association between ACE-I/ARB use and outcomes, such as hospitalization, ICU admission, or in-hospital mortality. However, these cohorts had a substantially higher proportion of White (45%–70%) patients than ours (23.3%). One exception was a study by Lam et al., which found lower rates of ICU admission and mortality among patients who continued rather than discontinued ACE-I/ARB treatment upon admission for COVID-19 (31). The Lam et al. cohort was predominantly White (61.2%), but there was a large fraction of patients of “other ethnicity” (26.2%), which could have influenced the overall D allele prevalence in the cohort. Taken together, these results suggest that race and ethnicity — while imperfect as surrogates of D allele prevalence — should be accounted for when assessing ACE-I/ARB associations with COVID-19.
Our study has several limitations. First, it was a retrospective study and cannot establish a causal relationship between ACE-I/ARB and COVID-19 outcomes via the ACE D allele without individual-level germline genetic data and prospective treatment assignment. Second, our study is based on a single-center experience, and these analyses should be repeated in other cohorts. Third, although outpatient medication adherence could not be verified, this EMR data set draws on multiple data sources to bolster accuracy and comprehensiveness. Finally, in the current scope of the study, the genotype information was not sufficient to assess the ACE D/I variant in different ethnic groups; therefore, we propose that future studies will be required to demonstrate the direct linkage of allelic frequency of D allele prevalence to alteration in the RAS and its impact on COVID-19 and angiotensin receptor blocker–mediated improvement in survival in various ethnic groups.
In conclusion, our results agree with current recommendations to continue ACE-I/ARB in hospitalized patients with COVID-19, provided there are no other contraindications. Our results also suggest a potential benefit for ACE-I/ARB among African American patients with COVID-19. We hypothesize that this is due to a higher ACE D allele prevalence in the African American population. Further investigations in COVID-19 cohorts with linked clinical and genomic data are warranted.
Methods
Data source and COVID-19 cohort.
This observational, retrospective cohort study utilized deidentified EMR data (Epic system) from the Mount Sinai Health System. We identified 6218 patients who met the admission criteria for COVID-19 and were admitted to the hospital (including emergency department visits and inpatient admissions) related to COVID-19 at a Mount Sinai facility from February 24 through May 31, 2020. Encounters were considered related to COVID-19 if a COVID-19 test was ordered or a COVID-19 diagnosis was assigned. COVID-19 was diagnosed by real-time reverse-transcriptase PCR–based clinical tests from nasopharyngeal swab specimens.
We extracted patient demographics from the EMR, including age, sex, and self-identified race and ethnicity. Race and ethnicity were summarized into the mutually exclusive categories of African American, Asian, Hispanic, White, unknown, and other. We retrieved disease comorbidities from the database, including asthma, COPD, obstructive sleep apnea, obesity, diabetes mellitus, chronic kidney disease, HIV infection, cancer, coronary artery disease, atrial fibrillation, heart failure, chronic viral hepatitis, and alcoholic nonalcoholic liver disease. Additionally, we extracted laboratory test orders and results from the encounters, including WBC, creatinine, anion gap, potassium, and ALT. We also retrieved the initial vital sign measurements for each encounter, including BMI, temperature, O2 saturation, heart rate, respiratory rate, and BP.
ACE-I/ARB and other antihypertensive medications and outcomes.
We defined ACE-I/ARB exposure as an active prescription from January 1, 2019, up to and including hospitalization for COVID-19 (“anytime use”), with 18.3% in our SARS-CoV-2–positive inpatient population; 9.4% patients were prescribed ACE-I/ARB in our general population in our EMR system from January 1, 2019, to May 31, 2020. We identified 1139 anytime use patients and divided them into 2 subsets: a) documented ACE-I/ARB administration during their hospitalization (“in-hospital use,” n = 605), and b) ACE-I/ARB use prior to admission but not during hospitalization (“outpatient use,” n = 534). We also traced other antihypertensive medications (calcium channel blockers, beta blockers, and diuretics) dispensed from January 1, 2019 until May 31, 2020, that were included in the medication history or usage. We have listed all the antihypertensive medications in Supplemental Table 8.
The primary outcome was COVID-19–caused in-hospital mortality in the entire population and in the African American subpopulation. There were 3 possible outcomes for each hospital encounter: in-hospital mortality, discharge, or continued hospitalization (right censored). The secondary outcomes were ICU admission and AKI, which were evaluated in the African American population as a subanalysis.
We assessed the associations between ACE-I/ARB anytime use, in-hospital use, or outpatient use and COVID-19 in-hospital mortality for the entire population, African American population, or non–African American population compared with patients who were not on ACE-I/ARB. We defined the outpatient use as patients being prescribed ACE-I/ARB during their outpatient visits but without continued in-hospital use, and in-hospital use was defined as patients continuing or being administered ACE-I/ARB. These analyses were also repeated for ACE-I use and ARB use; exposure to either class of medication was determined without regard to exposure to the other class.
Statistics.
We described continuous variables as their medians and IQRs or means and SDs. We described categorical variables as a number and percentage. We measured statistical differences with Kruskal-Wallis or 2-sample t test with 2-side P value for continuous variables and χ2 test for categorical variables.
We evaluated the ACE-I/ARB associations with in-hospital mortality using multivariate logistic regression adjusting for the following confounders: age, hospital stay duration, race, smoking status, BMI, temperature, O2 saturation, heart rate, respiratory rate, hypertension, asthma, COPD, obstructive sleep apnea, obesity, diabetes, chronic kidney disease, HIV, cancer, coronary artery disease, atrial fibrillation, heart failure, chronic viral hepatitis, liver disease, ICU stay, WBC, creatinine, anion gap, potassium, and ALT. We reported the adjusted ORs and corresponding 95% CIs. Statistical significance was defined as a 2-sided P value less than 0.05 unless otherwise stated. We also applied the Wald-based power and sample size formulas for logistic regression to calculate the power given the observed sample size (34). We used a 2-tailed test to estimate the probability of an event under the null hypothesis, which was the in-hospital mortality rate, and we used the binomial distribution for ACE-I/ARB with the prescription rate as the probability. We further analyzed the interaction between ACE-I/ARB and African American race by adding the interaction term in the logistic regression with adjustment by same potential confounders.
We employed inverse propensity-weighted logistic regression as a doubly robust method to balance the potential confounders and further estimate the effect of ACE-I/ARB use on COVID-19 patient outcomes. Specifically, we used the generalized boosted regression to compute propensity scores and balance the covariates based on the average treatment effect on the treated, and then calculated the adjusted ORs and 95% CI using multivariate logistic regression with the balanced covariates. In all the analyses, cases with missing covariates were omitted. We performed all analysis using R (version 4.0.1) and Python (version 3.7).
Study approval.
This study was approved by the Mount Sinai IRB (IRB-17-01245). Because this was not a human subject study, the requirement for informed consent was waived by the IRB.
Author contributions
LL and EES designed the study; SL, ZW, and ES performed data acquisition and quality control; SL performed statistical analysis; TJ, RS, MAK, EG, NRN, RC, EES, and LL contributed clinical interpretation; SL, RS, TJ, and LL drafted the paper; KH, MAK, EG, NRN, RC, and EES revised and approved the paper.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Sema4 IT for computational support hosted on Amazon Web Services.
Version 1. 08/19/2021
In-Press Preview
Version 2. 10/01/2021
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(19):e151418.https://doi.org/10.1172/JCI151418.
Contributor Information
Shilong Li, Email: shilong.li@sema4.com.
Rangaprasad Sarangarajan, Email: rangaprasad.sarangarajan@berghealth.com.
Tomi Jun, Email: tomi.jun@mountsinai.org.
Yu-Han Kao, Email: yu-han.kao@sema4.com.
Zichen Wang, Email: wangzc921@gmail.com.
Ke Hao, Email: ke.hao@sema4.com.
Emilio Schadt, Email: emilio.schadt@sema4.com.
Michael A. Kiebish, Email: michael.kiebish@berghealth.com.
Elder Granger, Email: elder.granger@berghealth.com.
Niven R. Narain, Email: niven.narain@berghealth.com.
Rong Chen, Email: rong.chen@sema4.com.
Eric E. Schadt, Email: eric.schadt@sema4.com.
Li Li, Email: li.li@sema4.com.
References
- 1.Andrasfay T, Goldman N. Reductions in 2020 US life expectancy due to COVID-19 and the disproportionate impact on the Black and Latino populations. Proc Natl Acad Sci U S A. 2021;118(5):e2014746118. doi: 10.1073/pnas.2014746118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Center for Health Statistics. Provisional Death Counts for Coronavirus Disease (COVID-19). https://www.cdc.gov/nchs/nvss/vsrr/covid19/index.htm Updated August 20, 2021. Accessed August 20, 2021.
- 3.Rogers TN, et al. Racial disparities in COVID-19 mortality among essential workers in the United States. World Med Health Policy. doi: 10.1002/wmh3.358. [published online August 5, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeberg H, Pääbo S. The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature. 2020;587(7835):610–612. doi: 10.1038/s41586-020-2818-3. [DOI] [PubMed] [Google Scholar]
- 5.COVID-19 Host Genetics Initiative The COVID-19 Host Genetics Initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. Eur J Hum Genet. 2020;28(6):715–718. doi: 10.1038/s41431-020-0636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pairo-Castineira E, et al. Genetic mechanisms of critical illness in COVID-19. Nature. 2020;591(7848):92–98. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 7.Gemmati D, Tisato V. Genetic hypothesis and pharmacogenetics side of renin-angiotensin-system in COVID-19. Genes (Basel) 2021;11(9):E1044. doi: 10.3390/genes11091044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delanghe JR, et al. ACE polymorphism and COVID-19 outcome. Endocrine. 2020;70(1):13–14. doi: 10.1007/s12020-020-02454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delanghe JR, et al. The host’s angiotensin-converting enzyme polymorphism may explain epidemiological findings in COVID-19 infections. Clin Chim Acta. 2020;505:192–193. doi: 10.1016/j.cca.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellone M, Calvisi SL. ACE polymorphisms and COVID-19-related mortality in Europe. J Mol Med (Berl) 2020;98(11):1505–1509. doi: 10.1007/s00109-020-01981-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pati A, et al. ACE deletion allele is associated with susceptibility to SARS-CoV-2 infection and mortality rate: an epidemiological study in the Asian population. Clin Chim Acta. 2020;510:455–458. doi: 10.1016/j.cca.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigat B, et al. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86(4):1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarangarajan R, et al. Ethnic prevalence of angiotensin-converting enzyme deletion (D) polymorphism and COVID-19 risk: rationale for use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers. J Racial Ethn Health Disparities. 2021;8(4):973–980. doi: 10.1007/s40615-020-00853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopes RD, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325(3):254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mancia G, et al. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds HR, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382(25):2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angeli F, et al. RAAS inhibitors and risk of Covid-19. N Engl J Med. 2020;383(20):1990–1994. doi: 10.1056/NEJMc2030446. [DOI] [PubMed] [Google Scholar]
- 18.Bozkurt B, et al. Joint HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19. J Card Fail. 2020;26(5):370. doi: 10.1016/j.cardfail.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah H, et al. The triumvirate: why hypertension, obesity, and diabetes are risk factors for adverse effects in patients with COVID-19. Acta Diabetol. 2021;58(7):831–843. doi: 10.1007/s00592-020-01636-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaduganathan M, et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, et al. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: a systematic review and meta-analysis. Pharmacol Res. 2020;158:104927. doi: 10.1016/j.phrs.2020.104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flacco ME, et al. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID-19: a meta-analysis cardiac risk factors and prevention. Heart. 2020;106(19):1519–1524. doi: 10.1136/heartjnl-2020-317336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MMY, et al. Renin–angiotensin system blockers, risk of SARS-CoV-2 infection and outcomes from CoViD-19: systematic review and meta-analysis. Eur Hear J Cardiovasc Pharmacother. doi: 10.1093/ehjcvp/pvaa138. [published online December 18, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajeevan H, et al. ALFRED: an allele frequency resource for research and teaching. Nucleic Acids Res. 2012;40(D1):D1010–D1015. doi: 10.1093/nar/gkr924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrap SB, et al. The ACE gene I/D polymorphism is not associated with the blood pressure and cardiovascular benefits of ACE inhibition. Hypertension. 2003;42(3):297–303. doi: 10.1161/01.HYP.0000088322.85804.96. [DOI] [PubMed] [Google Scholar]
- 26.Hatami N, et al. Worldwide ACE (I/D) polymorphism may affect COVID-19 recovery rate: an ecological meta-regression. Endocrine. 2020;68(3):479–484. doi: 10.1007/s12020-020-02381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, et al. Worldwide spatial genetic structure of angiotensin-converting enzyme gene: a new evolutionary ecological evidence for the thrifty genotype hypothesis. Eur J Hum Genet. 2011;19(9):1002–1008. doi: 10.1038/ejhg.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ajmera V, et al. RAMIC: design of a randomized, double-blind, placebo-controlled trial to evaluate the efficacy of ramipril in patients with COVID-19. Contemp Clin Trials. 2021;103:106330. doi: 10.1016/j.cct.2021.106330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salah HM, Mehta JL. Hypothesis: sex-related differences in ACE2 activity may contribute to higher mortality in men versus women with COVID-19. J Cardiovasc Pharmacol Ther. 2021;26(2):114–118. doi: 10.1177/1074248420967792. [DOI] [PubMed] [Google Scholar]
- 30.Viveiros A, et al. Sex differences in COVID-19: candidate pathways, genetics of ACE2, and sex hormones. Am J Physiol Heart Circ Physiol. 2021;320(1):H296–H304. doi: 10.1152/ajpheart.00755.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam KW, et al. Continued in-hospital angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use in hypertensive COVID-19 patients is associated with positive clinical outcome. J Infect Dis. 2020;222(8):1256–1264. doi: 10.1093/infdis/jiaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta N, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):1020–1026. doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khera R, et al. Association of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers with the risk of hospitalization and death in hypertensive patients with Coronavirus Disease-19. J Am Heart Assoc. 2021;10(13):e018086. doi: 10.1161/JAHA.120.018086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demidenko E. Sample size determination for logistic regression revisited. Stat Med. 2007;26(18):3385–3397. doi: 10.1002/sim.2771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.