Supplemental Digital Content is available in the text.
Keywords: Clinical Dementia Rating, cognitive function, Digit Symbol Substitution Test, mild cognitive impairment, mental status and dementia tests, Montreal Cognitive Assessment, pharmacology, vortioxetine
Abstract
This study investigated the effects of vortioxetine on cognitive function in adults with mild cognitive impairment (MCI). This single-arm, open-label, phase II study enrolled 111 adults with MCI without depressive symptoms to receive 5–10 mg/day vortioxetine for 6 months. Main outcomes assessed: cognitive function [Montreal Cognitive Assessment (MoCA); Digit Symbol Substitution Test (DSST)], disease severity [Clinical Dementia Rating (CDR)], clinician-assessed improvement and safety. Mean MoCA score increased from 24.2 points (baseline) to 29.7 points (month 6), placing most subjects within the cognitively normal range (≥26 points). Compared with baseline, MoCA and DSST scores were significantly improved at months 1, 3 and 6 (P < 0.001 for all). Global CDR scores significantly improved from baseline to month 6 (mean change −0.37 points; P < 0.001), representing an improvement from very mild impairment (0.50 points) to cognitively normal status (0.13 points), mainly in CDR memory scores. At month 6, 89.6% of subjects had improved disease severity. Adverse events and adverse drug reactions were reported in 9.9% (n = 11) and 2.7% (n = 3) of subjects, respectively. Vortioxetine treatment was associated with significant improvement in cognitive function and a favorable safety profile in community-dwelling older adults with MCI. Given the lack of evidence for efficacious pharmacologic interventions for MCI, our results are encouraging and warrant further investigation.
Introduction
Mild cognitive impairment (MCI) is defined as a decline in cognitive function beyond the effect of normal aging, but which does not severely compromise activities of daily living (Petersen et al., 1999). From population-based epidemiologic studies, the estimated prevalence of MCI ranges from 3 to 42% in older adults (Ward et al., 2012). In a meta-analysis, the MCI incidence rate for elderly aged 75 to >85 years was estimated at 22.5–60.1 per 1000 person-years, with the incidence of MCI increasing as age progresses (Gillis et al., 2019).
MCI is a clinically meaningful stage that often precedes dementia or Alzheimer’s disease (Petersen et al., 2009). Studies indicate a significantly elevated risk of dementia in individuals with MCI (Roberts et al., 2014); although some individuals with MCI appear to remain stable or improve over time, >50% progress to dementia within 5 years (Gauthier et al., 2006). Individuals with MCI may have a higher annual conversation rate to Alzheimer’s disease (3–10% among those identified in community settings and 10–15% in speciality clinics) (Michaud et al., 2017). In addition, progressive cognitive impairment also results in deterioration of functional independence and quality of life. It is estimated that a third of individuals with MCI face challenges in managing everyday tasks that depend heavily on memory and complex reasoning (Aretouli and Brandt, 2010). There are currently no pharmacologic or other treatments approved specifically for MCI, and no strong evidence to support interventions studied to date (Petersen et al., 2018; Kasper et al., 2020). Approaches currently explored include potential disease-modifying drugs and cognitive interventions (Karakaya et al., 2013), but randomized controlled trials (RCTs) have not identified efficacious pharmacologic interventions for MCI. For example, findings from RCTs and meta-analyses of RCTs of cholinesterase inhibitors, including donepezil, rivastigmine and galantamine, have been inconclusive, providing little or no strong evidence that these significantly improve cognition or function in individuals with MCI (Salloway et al., 2004; Feldman et al., 2007; Winblad et al., 2008; Doody et al., 2009; Russ and Morling, 2012; Fitzpatrick-Lewis et al., 2015; Matsunaga et al., 2019).
Cognitive function is a broad construct, encompassing a wide range of domains, including memory, attention, perception, problem-solving, psychomotor ability and social intactness (McDougall, 1990). Compared with healthy individuals, individuals with MCI demonstrate diminished function across multiple aspects of cognitive performance, including memory, planning and organization, language, visuospatial skills and divided attention (Farias et al., 2006; Aretouli and Brandt, 2010). Due to the multidomain nature of cognitive performance, a range of assessment tools may be used to measure the degree of impairment across various aspects of cognition (McDougall, 1990). The Montreal Cognitive Assessment (MoCA) and Digit Symbol Substitution Test (DSST) are objective neuropsychologic tests that assess a range of cognitive domains supporting independent functioning, including executive function, memory, attention and visuospatial perception and cognition (Wechsler, 1944; Nasreddine et al., 2005; Dickinson et al., 2007; Jaeger, 2018). Compared with the Mini-Mental State Exam (MMSE), a general cognitive instrument, the MoCA has higher sensitivity (90 versus 18%) and comparable specificity (87 versus 100%), making it suitable for detecting MCI (Nasreddine et al., 2005). In a systematic review, 80% of included studies demonstrated that MoCA was superior to MMSE in detecting MCI in elderly individuals (Pinto et al., 2019). The Clinical Dementia Rating (CDR) scale is used for clinical staging of dementia and cognitive impairment (Morris, 1993). The CDR assesses impairment in six distinct domains of cognitive and functional performance and synthesizes these domain scores into a measure of global disease severity.
Vortioxetine, a selective serotonin reuptake inhibitor and serotonin modulator with multiple pharmacologic actions, is currently approved for treating major depressive disorder (MDD). Besides inhibiting the serotonin transporter and modulating various serotonin receptors, vortioxetine has indirect effects on other systems, including the dopaminergic, noradrenergic and histaminergic systems (Bennabi et al., 2019). It has been proposed that vortioxetine’s multimodal activity underlies its effects on cognitive function (McIntyre et al., 2014; Baune et al., 2018; Bennabi et al., 2019), through putative mechanisms such as increasing glutamate neurotransmission and neuroplasticity in regions such as the prefrontal cortex (Sanchez et al., 2015; Baune et al., 2018).
Research in MDD patients indicates that vortioxetine has positive effects on cognitive performance. In a network meta-analysis of 12 RCTs, vortioxetine was the only antidepressant that significantly improved cognitive outcomes (DSST performance) compared with placebo (Baune et al., 2018). Three placebo-controlled RCTs showed that vortioxetine was associated with significantly improved DSST performance and that this effect was largely independent of vortioxetine’s effect on depressive symptoms (Katona et al., 2012; McIntyre et al., 2014; Mahableshwarkar et al., 2015). Vortioxetine’s positive effect on cognitive function has also been observed in subjects with no history of MDD. In an RCT, healthy controls receiving vortioxetine demonstrated significant improvements in the trail-making test (executive function, visuospatial processing) and in the subject-rated Perceived Deficits Questionnaire (Smith et al., 2018). In light of vortioxetine’s effects on cognitive function, this study was designed to investigate the effect of vortioxetine in individuals diagnosed with MCI.
Methods
Study design
This study was a single-arm, open-label, phase II study of vortioxetine treatment in adults diagnosed with MCI, without depressive symptoms. The study was conducted from October 2019 to August 2020 at one site in Singapore. Subjects were recruited via a community-based cognitive screening program for older adults. The study protocol was approved by Parkway Independent Ethics Committee before study initiation. The study was conducted in accordance with the principles of the Declaration of Helsinki and locally applicable regulatory requirements. The study was approved for conduct in Singapore by the Health Sciences Authority (CTA1900075). All subjects provided written informed consent before any study-related activities were undertaken.
Subjects
Adults were eligible for the study if they were diagnosed with MCI (global CDR score ≥0.5), had a baseline MoCA score between 20 and 25 (≤10 years of education) or 20 and 26 (>10 years of education), a Patient Health Questionnaire 9 (PHQ-9) score of ≤4 and had provided written informed consent. Exclusion criteria included: diagnosis of any dementia; history of seizures, alcoholism, drug abuse and Parkinson’s disease; known intolerance to vortioxetine; concomitant use of medications such as antidementia medications, anticonvulsants, antiParkinson medications, hypnotics, anxiolytics, antipsychotics, centrally-acting antihypertensives and cognition enhancers; and pregnant/lactating women.
Treatment and assessment schedule
Subjects were initiated on vortioxetine (5 mg/day) at baseline. This daily dose could be up-titrated at months 1 and 3 in 5 mg increments at the discretion of the study investigators if the subject’s MoCA score did not improve from the previous visit. Assessments were performed at baseline and months 1, 3 and 6. The compliance rate was assessed based on subject interviews and counting of leftover pills.
Outcome measures
The effects of vortioxetine were assessed using MoCA, DSST, the Clinician Interview-Based Impression of Change plus Caregiver Input (CIBIC+) CDR, and the Functional Activities Questionnaire (FAQ).
The MoCA is a cognitive assessment tool comprising various tasks that assess a range of cognitive functions, including memory, executive function, attention, concentration, orientation, language and visuospatial cognition (Nasreddine et al., 2005). Its score range spans 0–30 points (lower scores denote more severe cognitive impairment). Studies demonstrate that using country-specific score cutoffs increases MoCA’s sensitivity in detecting MCI (Lee et al., 2008; Wen et al., 2008; Ng et al., 2013). This study used a country-specific score window of 20–25 (≤10 years of education) or 20–26 (>10 years of education) that was previously identified as supporting the diagnosis of MCI (Ng et al., 2013).
The DSST is a cognitive assessment tool that assesses a variety of functions, including executive function, processing speed, attention, spatial perception and visual scanning (Wechsler, 1944; Dickinson et al., 2007; Jaeger, 2018). The DSST score equals the number of correctly matched number-symbol pairs, and spans from 0 points to a maximum of 90 points, with higher scores denoting better cognitive function. The DSST is a sensitive assessment tool for monitoring changes in cognitive performance over time, and has been used in a wide range of clinical populations, including subjects with MCI, MDD and other cognitive disorders (Wechsler, 1944; Dickinson et al., 2007; Jaeger, 2018). In one study, the mean DSST score for a population of community-dwelling older individuals was 51.8 points; in contrast, individuals within this population with self-reported subjective cognitive decline had a mean DSST score of 37.9, almost 14 points lower (Brody et al., 2019). In association with an individual’s modified MMSE score, a mean DSST score of 41.7 (SD 12.1) suggests normal cognitive performance, whereas a mean DSST score of 28.3 (SD 11.0) suggests impaired cognitive function associated with greater risk of MCI (Rosano et al., 2016).
The CIBIC+ is an interview-based, physician-rated assessment of disease improvement relative to the initial assessment at baseline (Schneider et al., 1997). The assessment takes cognitive (specifically attention, concentration, orientation, memory, language, motor activity, judgment and problem solving), behavioral, functional and general performance into consideration. Informed by subject and informant interviews, CIBIC+ is based on a seven-point ordinal scale, from ‘marked worsening’ to ‘very much improved’.
The CDR is a physician-rated assessment used to characterize disease severity in six domains of cognitive and functional performance: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. A global CDR score is produced from the individual scores of the six domains using an algorithm (Morris, 1993). Both the global and individual domain scores use a five-point scale (0, 0.5, 1, 2, 3) (0: ‘normal functioning’; 0.5: ‘very mild impairment/dementia’; 3: ‘severe impairment/dementia’). A global score of 0.5 is commonly used as a cutoff value supporting the diagnosis of MCI (Hughes et al., 1982; Petersen et al., 1999).
The FAQ is a 10-item, informant-reported questionnaire used to assess the functional ability for activities of daily living (Pfeffer et al., 1982; Tabert et al., 2002; Teng et al., 2010). The cumulative score range is 0–30, with higher scores denoting higher levels of functional dependency.
Safety assessments included adverse events and adverse drug reactions (ADRs), defined as adverse events that were assessed by the investigators to be possibly related to the study treatment.
Statistical analyses
The primary outcome measure was the change in mean MoCA score from baseline to month 6. The changes in mean MoCA score from baseline to each visit were also evaluated. Assuming a two-unit improvement in mean MoCA score from baseline to month 6 (Wong et al., 2017; Wu et al., 2019) and a seven-unit SD of this improvement, 99 subjects would be required for detecting this difference with 80% power at 5% significance level. The final sample size of 124 subjects was planned based on an expected attrition rate of 20%.
Efficacy analyses were performed for both the intention-to-treat (ITT) and per-protocol analysis sets. The ITT analysis set consisted of subjects who received at least one dose of vortioxetine and had preintervention and at least one postintervention assessment of efficacy. The per-protocol analysis set was a subset of the ITT analysis set and consisted of subjects who completed all scheduled visits without major protocol deviations or violations. Safety analyses were performed for the safety analysis set, which included all subjects who had at least one safety follow-up. Statistical significance of changes from baseline to month 6 and other time-points in primary (MoCA) and secondary outcomes (DSST, CIBIC+, CDR, FAQ) was evaluated using paired t-tests. Subject demographics, study treatment and relevant efficacy and safety outcomes were summarized using descriptive statistics. SAS Version 9.2 (SAS Institute Inc; Cary, North Carolina, USA) was used for all statistical analyses.
Results
Patient disposition and baseline characteristics
A total of 111 subjects were enrolled in the study, of which 40.5% of subjects eventually discontinued. The number of subjects discontinued and remaining in the study at each time-point are presented in Supplementary Table 1, Supplemental digital content 1, http://links.lww.com/ICP/A88. Only a minority of discontinuations (15.6%) were due to adverse events. The majority (71.1%) of discontinuations were due to other reasons. The higher-than-expected number of discontinuations was attributed to disruptions and restrictions mandated due to COVID-19. The ITT population consisted of 83 subjects, and the per-protocol population consisted of 55 subjects.
The study cohort was mostly elderly (median age 70 years), female (65.8%) and had a median of 10 years of education (Table 1). All subjects were Asian. Most were not working or retired (74.7%) and were married (86.5%).
Table 1.
Subject characteristics
| Summary of demographic and baseline characteristics | N = 111 |
|---|---|
| Age (years) | |
| Mean (SD) | 69 (9.6) |
| Median (min, max) | 70 (42, 92) |
| Sex | |
| Male | 38 (34.2) |
| Female | 73 (65.8) |
| Years of education (years) | |
| Mean (SD) | 9.5 (5.5) |
| Median (min, max) | 10 (0, 20) |
| Work status | |
| Employed | 22 (19.8) |
| Self-employed | 6 (5.4) |
| Retired | 42 (37.8) |
| Not employed | 41 (36.9) |
| Marital status | |
| Single | 4 (3.6) |
| Married | 96 (86.5) |
| Divorced | 3 (2.7) |
| Widow/widower | 8 (7.2) |
| PHQ-9 score | |
| Median (min, max) | 0 (0, 4) |
| MoCA score | |
| Mean (SD) | 24.1 (1.7) |
| Median (min, max) | 24 (20, 26) |
| DSST score (n = 110) | |
| Mean (SD) | 33.0 (13.0) |
| Median (min, max) | 31.5 (9, 73) |
| CDR global score | |
| Mean (SD) | 0.5 (0) |
| Median (min, max) | 0.5 (0.5, 0.5) |
| FAQ score (n = 10) | |
| Mean (SD) | 6.6 (7.1) |
| Median (min, max) | 6 (0, 24) |
n (%) unless otherwise stated. Percentages are based on the total number of enrolled subjects (N = 111).
CDR, Clinical Dementia Rating; DSST, Digit Symbol Substitution Test; FAQ, Functional Activities Questionnaire; MoCA, Montreal Cognitive Assessment; PHQ-9, Patient Health Questionnaire 9.
At baseline, all subjects had MoCA scores supporting the diagnosis of MCI (20.0–26.0 points), and PHQ-9 scores suggesting that no subjects had depression (0–4.0 points). The median DSST score was 31.5 points, suggesting significantly impaired cognitive function, and in the range of scores associated with subjective cognitive decline and greater risk of MCI (Rosano et al., 2016; Brody et al., 2019). All subjects had a baseline global CDR score of 0.50, supporting the diagnosis of MCI (Hughes et al., 1982; Petersen et al., 2009). Of the six CDR domains, only the memory domain scores indicated very mild impairment (median: 0.50 points). The scores in each of the other domains (median: 0 points) indicated normal functioning at baseline. The baseline FAQ score (median: 6.0 points) was indicative of some level of functional impairment but was only based on 10 informants.
Study treatment
The average daily dose of vortioxetine received was 5 mg. Except for one subject who was up-titrated to 10 mg daily at month 3, all subjects received 5 mg vortioxetine per day throughout the study. The median time on treatment was more than 5 months and the median treatment compliance rate was approximately 95%.
Effect of vortioxetine on measures of cognitive function
Montreal Cognitive Assessment
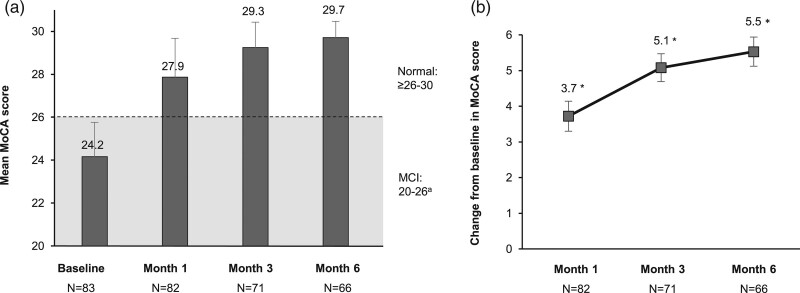
The mean MoCA score increased from 24.2 points at baseline to 29.7 points at month 6. At the end of the study, all subjects had MoCA scores ≥26 points, indicating that the majority of subjects had returned to a level of normal cognitive function (Fig. 1a). Compared with baseline, the mean MoCA score was significantly improved at months 1, 3 and 6 (Fig. 1b; P < 0.001 for all time-points).
Fig. 1.
Montreal Cognitive Assessment (MoCA) scores (intention-to-treat analysis set). (a) Mean MoCA scores at baseline, month 1, month 3 and month 6. Error bars represent SD. (b) Mean change from baseline in MoCA scores. Error bars represent 95% confidence interval (CI) of mean. aMoCA score range for mild cognitive impairment: 20–25 (≤ 10 years of education); 26 (>10 years of education); normal: ≥25/26–30; dementia: <20. *P < 0.001 (paired t-test).
Digit Symbol Substitution Test
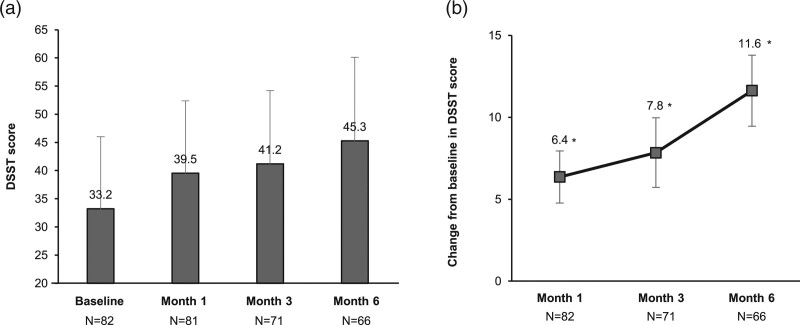
Compared with baseline, the mean DSST score was significantly increased at months 1, 3 and 6 (Fig. 2; P < 0.001 for all time-points). At month 6, the mean DSST score had increased by 11.6 (95% CI, 9.5–13.8) points, indicating significant improvements in cognitive function.
Fig. 2.
Digit Symbol Substitution Test (DSST) scores (intention-to-treat analysis set). (a) Mean DSST scores at baseline, month 1, month 3 and month 6. Error bars represent SD. (b) Mean change from baseline in DSST scores. Error bars represent 95% confidence interval (CI) of mean. *P < 0.001 (paired t-test).
Clinician Interview-Based Impression of Change plus Caregiver Input CDR, and the, Clinical Dementia Rating and Functional Activities Questionnaire
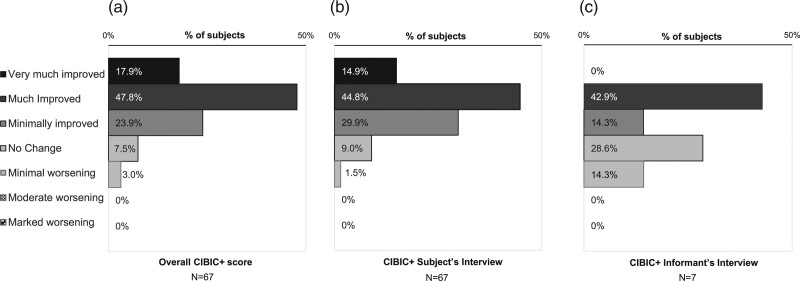
At the end of the study, the CIBIC+ assessment was improved in the majority of subjects, indicating global improvements in disease state (Fig. 3). Nearly all (89.6%) subjects demonstrated improvement in the overall CIBIC+ assessment, with almost two-thirds (65.7%) of all subjects being much or very much improved. Less than 10% experienced no change, and only 3% were assessed to have minimal worsening (Fig. 3a). A similar trend was observed in the assessments from the CIBIC+ subject’s interview (Fig. 3b). The low number of responses for the informant’s interview (N = 7) precluded meaningful interpretation of these data.
Fig. 3.
Clinician Interview-Based Impression of Change plus Caregiver Input (CIBIC+) assessment at end of study (intention-to-treat analysis set).
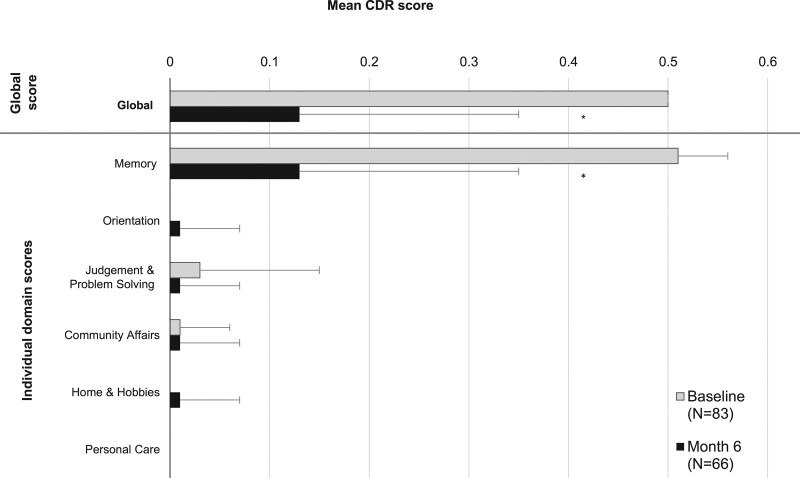
Compared with baseline, the mean global CDR score was significantly improved at month 6 (Fig. 4; −0.37 points; P < 0.001). Mean global CDR score improved from 0.50 (very mild impairment) at baseline to 0.13 (cognitively normal range) at the end of the study. Significant improvement was observed only in the memory domain, from very mild impairment at baseline (mean: 0.51 points) to the cognitively normal range (mean: 0.13 points) at month 6. Mean scores in the other domains indicated no appreciable impairment at baseline and throughout the study.
Fig. 4.
Mean Clinical Dementia Rating (CDR) scores at baseline and at month 6 (intention-to-treat analysis set). Error bars represent SD. N at baseline = 83; N at month 6 = 66. *P < 0.001 (paired t-test).
There were no significant changes from baseline in mean FAQ score. Due to the low number of available responses at baseline (N = 9) and end of study (N = 4), we were unable to meaningfully interpret potential changes in functional ability.
For all study assessments, findings in the ITT analysis set and per-protocol analysis set (data not shown) were similar.
Safety
A total of 11 subjects (9.9%) reported adverse events. Three subjects (2.7%) reported four ADRs, none of which were serious or severe (Supplementary Table 2, Supplemental digital content 1, http://links.lww.com/ICP/A88). The most common ADR reported was nausea (two subjects; 1.8%). The others were headache (one subject; 0.9%) and gastritis (one subject; 0.9%). There were no changes to study product administration due to the occurrence of ADRs.
Discussion
This study assessed the effects of vortioxetine on cognitive function in community-dwelling older adults diagnosed with MCI, without depressive symptoms. In this study, vortioxetine treatment was associated with large and clinically meaningful improvements in cognitive function and a favorable safety profile. The findings of this study provide encouraging initial evidence for vortioxetine’s effect in individuals with MCI, a condition which currently has no approved pharmacologic treatments (Petersen et al., 2018; Kasper et al., 2020).
All enrolled subjects had baseline MoCA and global CDR scores consistent with the diagnosis of MCI. By the end of the study, both MoCA and global CDR scores were significantly improved, and most subjects had MoCA and global CDR scores in the cognitively normal range. These findings on vortioxetine-associated cognitive effects were further reinforced by the significant improvement (>10 points) seen in DSST performance, another widely used cognitive outcome measure.
Compared with baseline, the mean MoCA score was significantly improved at all time points. In our study, mean MoCA score was significantly increased from baseline (24.2 points) to month 3 (29.7 points). This improvement in cognitive performance was comparable to that observed in a study of 10 mg vortioxetine treatment in MDD patients, where mean MoCA score significantly improved from baseline (26.7 points) to month 3 (29.1 points) (Fernandez-Miranda et al., 2017).
Similarly, significant improvements in DSST performance from baseline were observed at all time points. The observed increase in mean DSST score at month 3 (7.8 points) was within the range of improvements at week 8 in three RCTs of 5–20 mg vortioxetine treatment in MDD patients (2.8–9.0 points) (Katona et al., 2012; McIntyre et al., 2014; Mahableshwarkar et al., 2015). As our study excluded subjects with depression, the findings appear consistent with the direct effects of vortioxetine on cognition, rather than indirect effects through the improvement of depressive symptoms. Within the three RCTs that showed significantly greater improvement in DSST scores with vortioxetine compared with placebo, subgroup and path analyses indicated that these improvements were largely independent of vortioxetine’s effect on depressive symptoms (Katona et al., 2012; McIntyre et al., 2014; Mahableshwarkar et al., 2015).
The significant improvements in DSST performance seen in our study appear clinically meaningful in a community setting. Taking as a reference point a large, nationally-representative US health survey of community-dwelling older adults, baseline DSST performance in our study (mean: 33.0 points) was similar to that of the US survey participants with subjective cognitive decline (mean: 37.9 points) (Brody et al., 2019). After vortioxetine treatment, DSST performance in our study (mean: 45.5 points; median age: 70 years) improved to a level comparable to that in the overall population in the US survey (mean: 47.9 points; age range: 70–79 years) (Brody et al., 2019). Encouragingly, the improvement in our study appears larger than that observed in earlier trials involving other classes of pharmacologic treatments for MCI. For example, the >10-point improvement in mean DSST score is approximately four times larger than in placebo-controlled RCTs of galantamine (nonstatistically-significant mean improvement of 2.3–2.4 points at month 12), suggesting that vortioxetine could have a potentially large effect on cognitive function (Winblad et al., 2008). We also considered the possible contribution of practice effects, which have been described for a number of neuropsychologic tests (Calamia et al., 2012). In a study of MCI subjects with baseline DSST scores similar to that of our study (31.1 versus 31.5 points), practice effects were associated with a 2.4-point improvement in DSST scores (2–3 week retest interval) (Jutten et al., 2018). As improvements in DSST performance in our study appear larger than those previously reported for practice effects (Jutten et al., 2018), in our view it is less likely that improvements observed in our study were from practice effects alone.
The cognitive improvements observed in objective neuropsychologic tests were further supported by physician-based assessments of improvements in disease state and severity. The CIBIC+ overall assessment demonstrated that nearly all subjects experienced global improvements in disease state. Additionally, significant improvement in mean global CDR score indicated that disease severity improved with vortioxetine treatment, from very mild impairment consistent with MCI to cognitively normal status.
Taken together, the MoCA, DSST, CIBIC+ and global CDR assessments suggest vortioxetine treatment was associated with improvements across a range of cognitive domains. This comprises memory, as well as attention, orientation, executive function and concentration. These findings are consistent with the multidomain effects described in a post hoc analysis of an RCT of vortioxetine treatment in MDD patients (Harrison et al., 2016). In this analysis, improved DSST performance was attributed to vortioxetine’s positive effect on memory, executive function, attention and processing speed (Harrison et al., 2016). Vortioxetine thus appears to have a positive effect on multiple cognitive domains relevant to the demands of daily living in the community setting.
CDR domain-specific scores demonstrated that memory was improved, from very mild impairment consistent with MCI toward a cognitively normal state. This observed effect on memory is consistent with RCTs, where a significant improvement in memory-related assessments such as the Rey Auditory Verbal Learning Test were observed in vortioxetine-treated MDD patients (Katona et al., 2012; McIntyre et al., 2014). As all other domain scores indicated no appreciable impairment at baseline, it was not possible to draw conclusions about the effect of vortioxetine on these domains.
Treatment with vortioxetine for up to 6 months was associated with a favorable safety profile. The observed proportion of subjects with adverse events in our study (9.9%) was lower than that estimated in a pooled safety analysis of 11 RCTs in MDD patients (64.9% of patients with treatment-emergent adverse events; average vortioxetine dose 5 mg/day, duration 6–8 weeks) (Baldwin et al., 2016). Nausea was the most common ADR in our study, consistent with vortioxetine’s expected side effect profile from clinical studies (Baldwin et al., 2016).
This study had a number of limitations. First, the study was an open-label, single-arm study without a comparator group. Although these results indicate that vortioxetine treatment had positive effects on cognitive function, it is possible that nontreatment-specific factors related to study participation, such as increased interaction with clinicians and frequent assessment or practice effects, could have also contributed to improvement (Sanderson et al., 2013). It is also possible that, in some cases, the MCI was temporary and could have resolved without treatment. Some longitudinal studies have documented reversion to normal cognition in a proportion of subjects diagnosed with MCI, although subjects who revert to normal cognition may remain at higher risk of progression to dementia than individuals who have never been diagnosed with MCI (Canevelli et al., 2016; Aerts et al., 2017; Petersen et al., 2018). Nevertheless, the significant improvements in cognition observed are promising and appear to be consistent with the direct effect of vortioxetine on cognition, as demonstrated in RCTs conducted with MDD patients (Katona et al., 2012; McIntyre et al., 2014; Mahableshwarkar et al., 2015). Controlled studies (placebo or other investigational treatments) would be needed to quantify the effects of vortioxetine treatment on cognition and function in subjects across the spectrum of MCI.
Although the overall sample size was reduced due to a higher-than-expected withdrawal rate in this study, improvements in key outcomes were statistically significant at all time points in both the ITT and per-protocol analyses. Furthermore, mean MoCA and DSST scores were already significantly improved by month 1, when three-quarters (74.8%) of subjects were still in the study. The majority of withdrawals were attributed to COVID-19-related disruptions and restrictions, rather than adverse events.
Subjects in the study received relatively lower doses of vortioxetine (5 mg/day for all except one subject, who was up-titrated to 10 mg/day at month 3) The recommended starting dose for the elderly is 5 mg, compared with the recommended starting dose of 10 mg for adults (H. Lundbeck A/S, 2021). Encouragingly, improvements in cognitive function were readily perceived even with this low dose, and adverse effects were minimal.
Conclusion
In this open-label, single-arm study, vortioxetine was associated with an overall improvement in cognitive function in community-dwelling older adults with MCI without depression. Our findings also demonstrated that vortioxetine treatment for up to 6 months was associated with a favorable safety profile. Given the lack of evidence for interventions for MCI, the present study’s results are encouraging and warrant further investigation.
Acknowledgements
This study was an investigator-initiated trial with grant support from Lundbeck South East Asia. Medical writing and editorial assistance were provided by Tech Observer Asia Pacific Pte Ltd.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.intclinpsychopharm.com
References
- Aerts L, Heffernan M, Kochan NA, Crawford JD, Draper B, Trollor JN, et al. (2017). Effects of MCI subtype and reversion on progression to dementia in a community sample. Neurology 88:2225–2232. [DOI] [PubMed] [Google Scholar]
- Aretouli E, Brandt J. (2010). Everyday functioning in mild cognitive impairment and its relationship with executive cognition. Int J Geriatr Psychiatry 25:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DS, Chrones L, Florea I, Nielsen R, Nomikos GG, Palo W, Reines E. (2016). The safety and tolerability of vortioxetine: analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol 30:242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune BT, Brignone M, Larsen KG. (2018). A network meta-analysis comparing effects of various antidepressant classes on the digit symbol substitution test (DSST) as a measure of cognitive dysfunction in patients with major depressive disorder. Int J Neuropsychopharmacol 21:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennabi D, Haffen E, Van Waes V. (2019). Vortioxetine for cognitive enhancement in major depression: from animal models to clinical research. Front Psychiatry 10:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DJ, Kramarow EA, Taylor CA, McGuire LC. (2019). Cognitive performance in adults aged 60 and over: National Health and Nutrition Examination Survey, 2011-2014. Natl Health Stat Report 126:1–23. [PubMed] [Google Scholar]
- Calamia M, Markon K, Tranel D. (2012). Scoring higher the second time around: meta-analyses of practice effects in neuropsychological assessment. Clin Neuropsychol 26:543–570. [DOI] [PubMed] [Google Scholar]
- Canevelli M, Grande G, Lacorte E, Quarchioni E, Cesari M, Mariani C, et al. (2016). Spontaneous reversion of mild cognitive impairment to normal cognition: a systematic review of literature and meta-Analysis. J Am Med Dir Assoc 17:943–948. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. (2007). Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry 64:532–542. [DOI] [PubMed] [Google Scholar]
- Doody RS, Ferris SH, Salloway S, Sun Y, Goldman R, Watkins WE, et al. (2009). Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology 72:1555–1561. [DOI] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed BR, Harvey D, Cahn-Weiner D, Decarli C. (2006). MCI is associated with deficits in everyday functioning. Alzheimer Dis Assoc Disord 20:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman HH, Ferris S, Winblad B, Sfikas N, Mancione L, He Y, et al. (2007). Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol 6:501–512. [DOI] [PubMed] [Google Scholar]
- Fernandez-Miranda JJ, Frias-Ortiz DF, Diaz-Fernandez S, Rubio-Rodriguez L. (2017). Cognitive performance in patients with major depressive disorder treated with vortioxetine compared with escitalopram and venlafaxine. Eur Neuropsychopharmacol 27:S794–S795. [Google Scholar]
- Fitzpatrick-Lewis D, Warren R, Ali MU, Sherifali D, Raina P. (2015). Treatment for mild cognitive impairment: a systematic review and meta-analysis. CMAJ Open 3:E419–E427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. ; International Psychogeriatric Association Expert Conference on mild cognitive impairment. (2006). Mild cognitive impairment. Lancet 367:1262–1270. [DOI] [PubMed] [Google Scholar]
- Gillis C, Mirzaei F, Potashman M, Ikram MA, Maserejian N. (2019). The incidence of mild cognitive impairment: a systematic review and data synthesis. Alzheimers Dement (Amst) 11:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- H. Lundbeck A/S (2021). TRINTELLIX (vortioxetine) tablets for oral use: prescribing information. Revised 01/2021. [Online]. https://general.takedapharm.com/TRINTELLIXPI. [Accessed 20 May 2021].
- Harrison JE, Lophaven S, Olsen CK. (2016). Which cognitive domains are improved by treatment with vortioxetine? Int J Neuropsychopharmacol 19:pyw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. (1982). A new clinical scale for the staging of dementia. Br J Psychiatry 140:566–572. [DOI] [PubMed] [Google Scholar]
- Jaeger J. (2018). Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol 38:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutten RJ, Harrison J, Lee Meeuw Kjoe PR, Opmeer EM, Schoonenboom NSM, de Jong FJ, et al. (2018). A novel cognitive-functional composite measure to detect changes in early Alzheimer’s disease: test-retest reliability and feasibility. Alzheimers Dement (Amst) 10:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakaya T, Fußer F, Schröder J, Pantel J. (2013). Pharmacological treatment of mild cognitive impairment as a prodromal syndrome of Alzheimer’s disease. Curr Neuropharmacol 11:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S, Bancher C, Eckert A, Förstl H, Frölich L, Hort J, et al. (2020). Management of mild cognitive impairment (MCI): the need for national and international guidelines. World J Biol Psychiatry 21:579–594. [DOI] [PubMed] [Google Scholar]
- Katona C, Hansen T, Olsen CK. (2012). A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol 27:215–223. [DOI] [PubMed] [Google Scholar]
- Lee JY, Lee DW, Cho SJ, Na DL, Jeon HJ, Kim SK, et al. (2008). Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol 21:104–110. [DOI] [PubMed] [Google Scholar]
- Mahableshwarkar AR, Zajecka J, Jacobson W, Chen Y, Keefe RS. (2015). A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology 40:2025–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga S, Fujishiro H, Takechi H. (2019). Efficacy and safety of cholinesterase inhibitors for mild cognitive impairment: a systematic review and meta-analysis. J Alzheimers Dis 71:513–523. [DOI] [PubMed] [Google Scholar]
- McDougall GJ. (1990). A review of screening instruments for assessing cognition and mental status in older adults. Nurse Pract 15:18–28. [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Lophaven S, Olsen CK. (2014). A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol 17:1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud TL, Su D, Siahpush M, Murman DL. (2017). The risk of incident mild cognitive impairment and progression to dementia considering mild cognitive impairment subtypes. Dement Geriatr Cogn Dis Extra 7:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. (1993). The clinical dementia rating (CDR): current version and scoring rules. Neurology 43:2412–2414. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. [DOI] [PubMed] [Google Scholar]
- Ng A, Chew I, Narasimhalu K, Kandiah N. (2013). Effectiveness of Montreal Cognitive Assessment for the diagnosis of mild cognitive impairment and mild Alzheimer’s disease in Singapore. Singapore Med J 54:616–619. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. (2018). Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 90:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, et al. (2009). Mild cognitive impairment: ten years later. Arch Neurol 66:1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. (1999). Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56:303–308. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. (1982). Measurement of functional activities in older adults in the community. J Gerontol 37:323–329. [DOI] [PubMed] [Google Scholar]
- Pinto TCC, Machado L, Bulgacov TM, Rodrigues-Júnior AL, Costa MLG, Ximenes RCC, Sougey EB. (2019). Is the Montreal Cognitive Assessment (MoCA) screening superior to the mini-mental state examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s disease (AD) in the elderly? Int Psychogeriatr 31:491–504. [DOI] [PubMed] [Google Scholar]
- Roberts RO, Knopman DS, Mielke MM, Cha RH, Pankratz VS, Christianson TJ, et al. (2014). Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology 82:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Perera S, Inzitari M, Newman AB, Longstreth WT, Studenski S. (2016). Digit symbol substitution test and future clinical and subclinical disorders of cognition, mobility and mood in older adults. Age Ageing 45:688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ TC, Morling JR. (2012). Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database Syst Rev 2012:CD009132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Ferris S, Kluger A, Goldman R, Griesing T, Kumar D, Richardson S; Donepezil 401 Study Group. (2004). Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology 63:651–657. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Asin KE, Artigas F. (2015). Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther 145:43–57. [DOI] [PubMed] [Google Scholar]
- Sanderson C, Hardy J, Spruyt O, Currow DC. (2013). Placebo and nocebo effects in randomized controlled trials: the implications for research and practice. J Pain Symptom Manage 46:722–730. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, et al. (1997). Validity and reliability of the Alzheimer’s disease cooperative study-clinical global impression of change. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 11 (Suppl 2):S22–S32. [DOI] [PubMed] [Google Scholar]
- Smith J, Browning M, Conen S, Smallman R, Buchbjerg J, Larsen KG, et al. (2018). Vortioxetine reduces BOLD signal during performance of the N-back working memory task: a randomised neuroimaging trial in remitted depressed patients and healthy controls. Mol Psychiatry 23:1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton G, Liu X, et al. (2002). Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology 58:758–764. [DOI] [PubMed] [Google Scholar]
- Teng E, Becker BW, Woo E, Knopman DS, Cummings JL, Lu PH. (2010). Utility of the functional activities questionnaire for distinguishing mild cognitive impairment from very mild Alzheimer disease. Alzheimer Dis Assoc Disord 24:348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A, Arrighi HM, Michels S, Cedarbaum JM. (2012). Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement 8:14–21. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1944). The measurement of adult intelligence. Baltimore: The Williams & Wilkins Company. [Google Scholar]
- Wen HB, Zhang ZX, Niu FS, Li L. (2008). [The application of Montreal Cognitive Assessment in urban Chinese residents of Beijing]. Zhonghua Nei Ke Za Zhi 47:36–39. [PubMed] [Google Scholar]
- Winblad B, Gauthier S, Scinto L, Feldman H, Wilcock GK, Truyen L, et al. ; GAL-INT-11/18 Study Group (2008). Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 70:2024–2035. [DOI] [PubMed] [Google Scholar]
- Wong GKC, Mak JSY, Wong A, Zheng VZY, Poon WS, Abrigo J, Mok VCT. (2017). Minimum clinically important difference of Montreal Cognitive Assessment in aneurysmal subarachnoid hemorrhage patients. J Clin Neurosci 46:41–44. [DOI] [PubMed] [Google Scholar]
- Wu CY, Hung SJ, Lin KC, Chen KH, Chen P, Tsay PK. (2019). Responsiveness, minimal clinically important difference, and validity of the MoCA in stroke rehabilitation. Occup Ther Int 2019:2517658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.