Abstract
Galidesivir (BCX4430) is an adenosine nucleoside analog that is broadly active in cell culture against several RNA viruses of various families. This activity has also been shown in animal models of viral disease associated with Ebola, Marburg, yellow fever, Zika, and Rift Valley fever viruses. In many cases, the compound is more efficacious in animal models than cell culture activity would predict. Based on favorable data from in vivo animal studies, galidesivir has recently undergone evaluation in several phase I clinical trials, including against severe acute respiratory syndrome coronavirus 2, and as a medical countermeasure for the treatment of Marburg virus disease.
Keywords: Antiviral, RNA viruses, Yellow fever, Marburg virus, Nucleoside analog
1. Introduction
The need for broadly active antivirals that are effective against acute viral diseases has been made evident by several historical epidemics, most recently the global COVID-19 pandemic declared by the World Health Organization in March 2020. In animal studies, galidesivir (BCX4430) has demonstrated activity against a variety of serious pathogens, including Ebola (EBOV), Marburg (MARV), yellow fever (YFV), Zika (ZIKV), and Rift Valley fever (RVFV) viruses. Galidesivir has also demonstrated broad-spectrum activity in vitro against more than 20 RNA viruses in nine different families, including coronaviruses, filoviruses, togaviruses, phenuiviruses, arenaviruses, paramyxoviruses, pneumoviruses, orthomyxoviruses, picornaviruses, and flaviviruses. The activity of galidesivir in animal models is often more potent than predicted by cell culture activity (Julander et al., 2014; Warren et al., 2014). Clinical development efforts are ongoing. The purpose of this article is to provide an update on current progress in the development of galidesivir by presenting an overview of its known mechanism of action (MoA) and pharmacokinetic (PK) profile; exploring its antiviral activity and efficacy in in vitro and in vivo studies; and providing a brief overview of results from clinical studies.
2. Mechanism of action
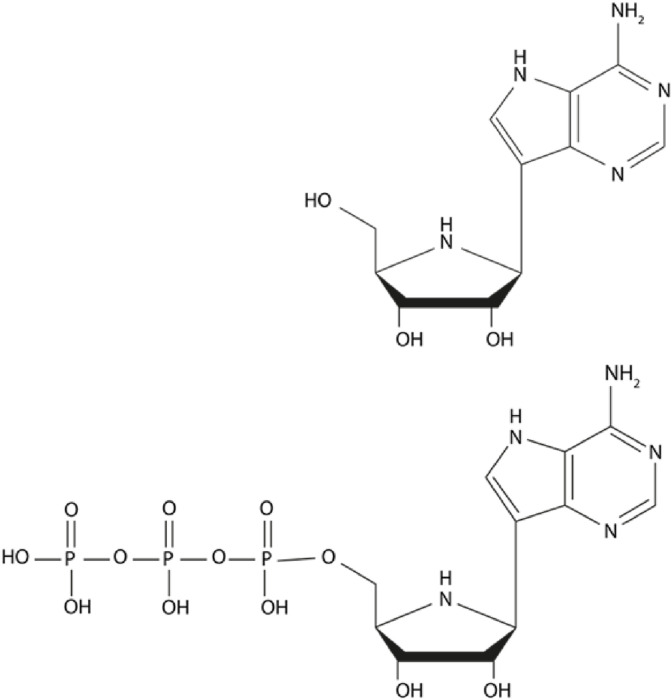
Galidesivir is a nucleoside analog that targets the RNA-dependent RNA polymerase (RdRp) of RNA viruses. The parent compound must be phosphorylated by cellular kinases to a triphosphate (BCX4430-TP or BCX6870) before it can serve as a nucleotide analog of adenosine triphosphate (Fig. 1 ). Some cells, such as the Vero cell line, do not efficiently convert the parent compound to the active triphosphate form and will thus will demonstrate lower activity as compared with cells that produce the triphosphate more efficiently (Julander et al., 2017a, Julander et al., 2017b).
Fig. 1.
Structure of galidesivir, an adenosine nucleoside analog (upper panel), and its active triphosphate form (lower panel).
Once phosphorylation has occurred, BCX4430-TP is incorporated into the viral RNA, causing premature chain termination. BCX4430-TP has demonstrated preference for viral RNA polymerase over host polymerase (Warren et al., 2014). The MoA of galidesivir was further demonstrated in a study utilizing a galidesivir-resistant tick-borne encephalitis virus (TBEV) strain. Resistance to the compound was conferred by a single amino acid substitution in the active site of the viral RdRp, indicative of viral response to targeted drug pressure. The resulting mutant TBEV strain was approximately 7-fold less sensitive to galidesivir compared with the wild-type virus. Notably, the mutation conferring drug resistance led to a considerable loss of viral fitness in vivo (Eyer et al., 2019).
3. Distribution, metabolism, and tolerability of galidesivir
Intracellular galidesivir is rapidly and efficiently converted to the triphosphate form, especially in the liver (Taylor et al., 2016). In a study investigating the PK profile of galidesivir, it was shown that the active triphosphate BCX4430-TP remained in the liver of rats with a half-life of approximately 6 h (Warren et al., 2014); further PK studies in hamsters and NHPs are ongoing. The presence of BCX4430-TP in the liver may have important implications for the treatment of hepatotropic infections such as yellow fever.
Galidesivir administered intraperitoneally (IP) in hamsters confirmed the data generated in rats treated intravenously (IV), with replication of the initial rapid uptake by cells; conversion to the active triphosphate form; intracellular catabolism of BCX4430-TP back to the parent compound; and slower excretion of the parent compound into the plasma. An increase in plasma levels of the parent compound was observed 8 h after IP treatment, likely representing its excretion from the liver (Westover et al., 2018). Similarly, in cynomolgus macaques, an increase in plasma concentrations of galidesivir was observed between 12 and 24 h after intramuscular (IM) injection (Warren et al., 2014). The mechanism of slow release into the central circulation after IP or IM injection has not yet been elucidated.
Tissue distribution studies of [14C] galidesivir in rats demonstrated similar compound distribution to various tissues after either IV or IM administration. The concentration of galidesivir in the brain was lower than that in the blood (ratio of ≤0.10), suggesting that there is very limited central nervous system (CNS) penetration in normal healthy animals. However, preclinical studies in rhesus macaques to assess the safety and antiviral efficacy of galidesivir against ZIKV infection showed that galidesivir was safe when administered twice-daily at 100 mg/kg initiated 1.5 h after virus challenge and prevented or rapidly reduced viral burden in the blood between 1 and 10 days after virus challenge and in the CNS when monitored weekly between 0 and 28 days after infection (Lim et al., 2020).
In hamsters, toxicity with galidesivir was registered at a dose of 300 mg/kg/day administered intraperitoneally over 7 days. Toxicity did not result in fatal outcomes, but manifest as significant alterations in the mean weight change compared with animals receiving lower doses (Julander et al., 2014). Interestingly, the weight loss observed in this study at a dose of 300 mg/kg/day occurred from the final day of treatment and continued for a further 4 days after treatment had ended. Subsequently, these animals experienced a steady weight gain until the end of the observation period; however, their average weights at this time point remained lower than that of groups treated with lower doses. Some weight loss was also observed in animals treated with 250 mg/kg/day, but the change in average weight was not significantly different compared with lower doses. A dose of 200 mg/kg/day had no observable effect on average weight or outward appearance of the hamsters, and as such was deemed the maximum tolerated dose (Julander et al., 2014). A dose of 300 mg/kg/day was tolerated in mice when administered intramuscularly for 8 days (Julander et al., 2017a, Julander et al., 2017b), which is equivalent by surface area conversion to a dose of 180 mg/kg/day in hamsters.
Reproductive toxicity studies have been conducted with galidesivir in pregnant rats and pregnant rabbits to evaluate effects on embryo-fetal development. Daily treatment administered intravenously from gestation day 6 (GD6) to GD17 in rats and from GD7 to GD19 in rabbits demonstrated no evidence of galidesivir-related embryo lethality, fetotoxicity, or teratogenicity at dosages of ≤75 mg/kg/day in rats and ≤25 mg/kg/day in rabbits. Importantly, the observed maternal and fetal drug exposure levels were similar in each species, indicating that galidesivir is effectively transferred across the placenta (Lim et al., 2020).
4. Antiviral activity of galidesivir
Galidesivir has been tested against a wide range of viruses in cell culture, and consistent with this treatment being an inhibitor of viral RdRp, its activity is restricted to RNA viruses. In vitro, galidesivir appears to be more potent against certain viral families than others (Warren et al., 2014), with half maximal effective concentration (EC50) values typically in the low to mid micromolar range. Of the viruses that have been tested in vitro, little to weak activity of galidesivir was reported for members of the Togaviridae and Arenaviridae families, whereas more potent activity was observed against viruses in the Filoviridae, Flaviviridae, Phenuiviridae, Paramyxoviridae, Orthomyxoviridae, Pneumoviridae, and Picornaviridae families (Table 1 ). From studies in mice, hamsters, and non-human primates (NHPs), the effective and maximum tolerated doses of galidesivir have been demonstrated across animal species. The strategy of administering a loading dose followed by a lower maintenance dose has proven effective for galidesivir therapy in various animal models (Lim et al., 2020; Westover et al., 2018). Future clinical studies will inform the relevant dose and dosing schedule of galidesivir by indication.
Table 1.
Summary of the efficacy of galidesivir in cell culture assays against viruses of various families.
| Family | Species | Cell | EC50 (μM) | SI50a | Reference |
|---|---|---|---|---|---|
| Arenaviridae | LASV | HeLa | 43.0 | >2.3 | Warren et al. (2014) |
| JUNV | HeLa | 42.2 | >2.4 | Warren et al. (2014) | |
| Phenuiviridae | RVFV | HeLa Vero 76 |
41.6 20.4 |
>2.4 5.2 |
(Warren et al., 2014; Westover et al., 2018) |
| Peribunyaviridae | LACV | Vero 76 | 13.4 | >7.5 | Warren et al. (2014) |
| Hantaviridae | MPRLV | Vero E6 | 40.1 | >6.2 | Warren et al. (2014) |
| Coronaviridae | MERS-CoV | Vero E6 | 68.4 | >1.5 | Warren et al. (2014) |
| SARS-CoV | Vero 76 | 57.7 | >5.1 | Warren et al. (2014) | |
| Filoviridae | MARV | HeLa | 4.4–6.7 | 38–55 | Warren et al. (2014) |
| EBOV | HeLa | 11.8 | >8.5 | Warren et al. (2014) | |
| SUDV | HeLa | 3.4 | >29.4 | Warren et al. (2014) | |
| Flaviviridae | YFV | Vero, Vero 76 |
24.5 14.1 |
38.6 >7.1 |
(Julander et al., 2014; Warren et al., 2014) |
| JEV | Vero 76 | 43.6 | >2.3 | Warren et al. (2014) | |
| DENV | Vero 76 | 32.8 | >9.0 | Warren et al. (2014) | |
| ZIKV | Vero76, Huh-7, RD | 3.8–11.7b,c | Julander et al. (2017) | ||
| WNV | PSd | 2.3 | >42.9 | Eyer et al. (2017) | |
| TBEV | PSd | 1.5 | >67.6 | Eyer et al. (2017) | |
| LIV | PSd | 12.3 | >8.1 | Eyer et al. (2017) | |
| KFDV | PSd | 11.4 | >8.8 | Eyer et al. (2017) | |
| Orthomyxoviridae | IAV | MDCK | 10.7 | >27.7 | Warren et al. (2014) |
| Paramyxoviridae | NiV | HeLa | 41.9 | >2.4 | Warren et al. (2014) |
| MeV | Vero 76 | 6.19 | >47.8 | Warren et al. (2014) | |
| Picornaviridae | HRV2 | HeLa-Ohio | 3.4 | >87.1 | Warren et al. (2014) |
| Pneumoviridae | RSV | MA104 | 11.0 | >8.1 | Warren et al. (2014) |
| Togaviridae | VEEV | HeLa | >100 | 1 | Warren et al. (2014) |
| EEEV | Vero 76 | 43.2 | >2.3 | Warren et al. (2014) | |
| WEEV | Vero 76 | 21.3 | >4.7 | Warren et al. (2014) | |
| CHIKV | HeLa | >100 | 1 | Warren et al. (2014) |
SI (CC50/EC50) of galidesivir in cell culture.
Range of EC50 depending on viral strain and cell line used.
SI50 not given in article; SI90 ranged from 5.5 to 20.5 depending on viral strain and cell line used.
Porcine kidney stable (PS) cells.
It is important to note that the efficiency at which galidesivir is converted to the active triphosphate form varies depending on the cell line used to evaluate antiviral activity, and this process appears to be more efficient in animal tissues in vivo than in cell culture (Warren et al., 2014); therefore, in vitro results may not directly correlate with in vivo potency.
4.1. Arenaviridae
Several members of the Arenaviridae are zoonotic viruses with potential for causing severe, acute hemorraghic illness in humans. Lassavirus (LASV) and Junín (JUNV) virus are etiological agents of hemorrgahic fever in West Africa and South America, respectively (Gomez et al., 2011; WHO, 2017).
4.1.1. LASV and JUNV
Galidesivir has been screened in Vero cells against two arenaviruses; LASV and JUNV (Table 1). Moderate antiviral activity was observed against both with EC50's of 43.0 μM and 42.2 μM, respectively, and CC50's > 100 μM (Warren et al., 2014).
4.2. Phenuiviridae
4.2.1. RVFV
RVFV is a mosquito-borne virus commonly associated with a self-limiting febrile illness, although some patients develop severe hemorrhagic fever with high likelihood of fatal outcome. The primary route of transmission to humans is through contact with infected livestock (Wright et al., 2019).
Galidesivir has been tested against RVFV in cell culture, yielding EC50 values in the range of 20.4 μM–41.6 μM, and CC50 > 100 μM (Westover et al., 2018; Warren et al., 2014). In vitro evaluation of galidesivir against RVFV has been performed in Vero cells, which as mentioned previously do not phosphorylate galidesivir to the active triphosphate form very efficiently. The relatively high micromolar range observed for galidesivir against RVFV in cell culture may indicate only modest antiviral effect, however studies of galidesivir in animal models of Rift Valley fever demonstrate higher in vivo potency than predicted by in vitro results.
Infection of hamsters with RVFV results in a rapidly lethal disease, with most animals requiring humane euthanasia within 3 days of challenge. Galidesivir administered intraperitoneally with a loading dose of 400 mg/kg and a subsequent daily dose of 100 mg/kg significantly delayed mortality by approximately 1 week and resulted in protection of 50%–70% from mortality (Westover et al., 2018). In most treated animals, treatment reduced RVFV loads in serum, liver, spleen, and brain to baseline levels. In this study, galidesivir typically outperformed the positive control compound, ribavirin. Similar efficacy was observed in a mouse model of RVFV infection (Warren et al., 2014).
As discussed previously, a maximum tolerated dose of 200 mg/kg/day, dosed twice daily (BID), over 7 days had been demonstrated for galidesivir in healthy hamsters (Julander et al., 2014). The tolerance of a 400 mg/kg loading dose in the context of the RVFV model may be explained by the use of IM rather than IP delivery, or because of the single use of this high loading dose was followed by a lower maintenance dose that was below the maximum tolerated dose.
4.3. Peribunyaviridiae
4.3.1. La Crosse virus
La Crosse virus (LACV) is endemic to several parts of the USA, and is the causative agent of La Crosse encephalitis, a disease which can manifest with severe neuroinvasive symptoms (Harding et al., 2018). Galidesivir exhibited antiviral activity against LACV in Vero cells, with a favorable SI of >7.5 (Table 1) (Warren et al., 2014). The in vivo activity of galidesivir against LACV has not been explored.
4.4. Hantaviridae
4.4.1. Maporal virus
Maporal virus (MPRLV) is a New World hantavirus that shares close phylogeny with Andes virus, a primary agent of a rare zoonotic disease named Hantavirus cardiopulmonary syndrome, with case-fatality rates of up to 40% (Avsic-Zupanc et al., 2019; Buys et al., 2011). In vitro, galidesivir exhibited moderate antiviral activity against Maporal virus cultured in Vero cells (Table 1) (Warren et al., 2014).
4.5. Coronaviridae
In 2002, an outbreak of severe acute respiratory syndrome (SARS), first identified in China, was found to be caused by a novel coronavirus subsequently named SARS-CoV. Ten years later, Middle East respiratory syndrome coronavirus (MERS-CoV) was identified as the causative agent of outbreaks of viral respiratory illness, largely contained to the Arabian Peninsula (Cui et al., 2019). SARS-CoV-2 is the third highly pathogenic coronavirus to have emerged since the start of the new millennium. Approved antiviral compounds effective against these viruses have been lacking; in October 2020 remdesivir became the first FDA-approved therapeutic for COVID-19 (FDA and research, 2020).
4.5.1. SARS-CoV and MERS-CoV
Galidesivir exhibited moderate to low antiviral activity against SARS-CoV and MERS-CoV cultured in Vero cells, with SI's of >5.1 and > 1.5, respectively (Table 1) (Warren et al., 2014).
4.5.2. SARS-CoV-2
Recently, there has been a focus on evaluating the antiviral activity of galidesivir against SARS-CoV-2, the etiologic agent responsible for the recent COVID-19 pandemic. Various computational modeling studies demonstrated potential for galidesivir to bind effectively to the SARS-CoV-2 virus RdRp (Aftab et al., 2020; Elfiky, 2020); additional studies are ongoing to more broadly characterize the antiviral activity of galidesivir against SARS-CoV-2.
4.6. Filoviridae
MARV and EBOV are members of the Filoviridae and are pathogens with the potential to cause outbreaks of life-threatening hemorrhagic fever in humans. Both viruses are associated with case-fatality rates ranging from 20% to 90%, highlighting the need for development of effective medical countermeasures (CDC, 2014; WHO, 2021a, WHO, 2021b).
4.6.1. MARV
Potent inhibition against MARV was observed in an antiviral screen of galidesivir, with EC50's ranging from 4.4 μM to 6.7 μM, and EC90 ‘s ranging from 10.5 μM to 16.1 μM across three different MARV strains. For all strains, the corresponding CC50 was >200 μM (Warren et al., 2014).
The seminal work evaluating the efficacy of galidesivir in vivo was performed against filoviruses (Warren et al., 2014). In the guinea pig model of MARV disease, galidesivir conferred significant protection when twice daily treatment with 15 mg/kg was initiated within 48 h of viral challenge by IP injection, or within 72 h of exposure to aerosolized viral particles. These results justified studies in a macaque model of MARV disease, a NHP model that accurately reproduces pathology as it presents in fatal human cases. Galidesivir exhibited potent activity when twice daily treatment with 50 mg/kg was initiated 24 or 48 h after virus challenge, reducing viremia and other disease parameters, and resulting in a 100% survival rate among treated animals. These results confirm the activity observed in cell culture and provided some data to justify further studies in other animal models, which could possibly be used to fulfil the FDAs “two-animal rule”, and potentially in EBOV- or MARV-infected patients during future clinical evaluation.
4.6.2. EBOV
As observed with Marburgvirus, galidesivir exhibited potent in vitro antiviral activity against ebolavirus species (Table 1).
The activity of galidesivir against EBOV was confirmed in a small animal model; high survival rates were observed in EBOV-infected mice receiving 30 mg/kg of galidesivir by IM injection or by peroral administration following a lethal viral challenge (Warren et al., 2014). Equally high survival rates were observed with delayed treatment in a lethal NHP model; all treated animals survived when receiving a loading dose of galidesivir of 100 mg/kg two days after viral challenge, with subsequent continued treatment for 11 days at a dose of 25 mg/kg BID (Warren, unpublished data).
4.7. Flaviviridae
The Flaviviridae encompass several vector-borne viruses with potential for causing mild to severe illness in humans. With geographical expansion of their vectors driven by factors such as climate change, urbanization, and global travel, there is a threat of emergence of flavivirus-related disease in novel regions, as witnessed in Brazil and other regions of the Americas with the introduction and subsequent outbreak of ZIKV in 2013–2014 (Pierson and Diamond, 2020).
4.7.1. YFV
In Vero cells galidesivir demonstrated strong inhibition of yellow fever virus with a SI > 7 (Table 1). The efficacy of galidesivir against yellow fever was subsequently confirmed in a small animal model (Julander et al., 2014). Treatment of YFV-infected hamsters with a galidesivir dose of 200 mg/kg/day, dosed BID, conferred high survival rates and significant improvements in disease related parameters compared to placebo-treated control animals, when treatment was initiated as late as 3 days post infection. A dose of 4 mg/kg/day approached a 50% effective concentration with treatment initiated at the time of virus challenge. Considering a maximum tolerated dose of 200 mg/kg/day, this provided a SI of 50, consistent with the notion that activity in cell culture assays may underestimate in vivo potency.
The efficacy of different dosing regimens were also tested in the hamster model of YFV. (Julander et al., 2014). A comparison of once daily (QD) versus BID treatment administered via the IP route demonstrated a slightly better response when an equivalent dose was given more frequently (Julander et al., 2014). This study also demonstrated that a shortened treatment regimen of 4 days was equivalent to a 7-day BID treatment, when treatment was initiated 4 h prior to viral challenge. Further studies demonstrated that BID galidesivir provided significant survival benefits when treatment initiation was delayed up to 4 days after infection (Julander et al., 2014).
4.7.2. ZIKV
In vitro, galidesivir was a strong inhibitor of ZIKV cultured in Vero, Huh-7 and RD cells (Julander and Siddharthan, 2017). Greater activity was consistent in Huh-7 cells as compared with RD or Vero 76 cells, suggesting a more efficient conversion in the former cell line (Julander et al., 2017a, Julander et al., 2017b).
Efficacy was confirmed in a lethal mouse model of ZIKV infection, demonstrating improved survival and delayed mean day to death, weight change, and viremia in treated animals (Julander et al., 2017a, Julander et al., 2017b). A dose of 300 mg/kg/day of galidesivir at day 1 after infection protected 6 of 7 mice from mortality and significantly extended the mean day to death when treatment was initiated 3, 5, or 7 days after infection. Furthermore, this activity of galidesivir is impressive compared with other antiviral compounds that have been tested in immunocompromised mouse models, such as sofosbuvir and ribavirin (Julander and Siddharthan, 2017). In a lethal mouse model of ZIKV infection, sofosbuvir resulted in approximately 50% survival when treatment was initiated one day after infection, whereas ribavirin, though effective in cell culture, did not protect mice against lethal ZIKV challenge (Bullard-Feibelman et al., 2017; Julander et al., 2017a, Julander et al., 2017b).
Four preclinical studies were conducted in NHPs to assess the safety, antiviral efficacy, and dosing strategies of galidesivir against peripheral and reproductive tract ZIKV infection (Lim et al., 2020). Collectively, 70 rhesus macaques were infected by various routes using Puerto Rican or Thai isolates of ZIKV. Galidesivir was evaluated after administration as early as 90 min and as late as 72 h after virus challenge, and as late as 5 days after intravaginal challenge. A dose of 100 mg/kg was administered beginning various times before and after challenge with some test groups receiving a maintenance dose of 25 mg/kg administered twice daily for 9 days. Galidesivir dosing in rhesus macaques was safe and offered significant post-exposure protection against infection. The potent anti-ZIKV efficacy of galidesivir observed in the blood and CNS warrant continued evaluation (Lim et al., 2020).
4.7.3. Dengue virus, West Nile virus and Japanese encephalitis virus
The in vitro antiviral activity of galidesivir has been tested against several other flaviviruses including Dengue (DNV), Japanese encephalitis virus (JEV) and West Nile virus (WNV). Inhibition of DNV and JEV was observed in Vero cells with SI's of >9.0 and > 2.3, respectively (Warren et al., 2014). Compound related antiviral activity was also demonstrated against WNV cultured in PS cells, with an EC50 of 2.3 μM and CC50 > 100 μM (Eyer et al., 2017).
4.7.4. Tick-borne flaviviruses
Galidesivir exhibited antiviral activity against tick-born flaviviruses, such as TBEV and Kyasanur forest disease virus (KFDV), in cell culture (Table 1) (Eyer et al., 2017). Another tick-borne encephalitic flavivirus called Louping ill virus (LIV) was also sensitive to the activity of galidesivir; although this virus primarily causes a disease in sheep, it can be transmitted to humans (Eyer et al., 2017). These data underscore the broad activity of galidesivir against a wide range of flaviviruses that are of concern to humans.
4.8. Orthomyxoviridae
4.8.1. Influenza A virus
Influenza A (IAV) and influenza B viruses cause annual seasonal flu epidemics. IAV is additionally associated with sporadic outbreaks of pandemic flu as a consequence of the emergence of novel IAV strains in the human population (Krammer et al., 2018). In cell culture, galidesivir exhibited potent inhibition of IAV (Table 1) (Warren et al., 2014). Further assessment of the activity of galidesivir against influenza viruses has not been reported.
4.9. Paramyxovirdae
The Paramyxovirdae family includes several human pathogens, such as measles virus (MeV), mumps virus and parainfluenza viruses, as well as the zoonotic and highly pathogenic henipaviruses (Plemper, 2020). An effective vaccine against MeV is available, however MeV is still the cause of around 100,000 deaths annually. Because of the highly contagious nature of this pathogen, the required vaccine coverage to achieve herd immunity and prevent sporadic outbreaks is approximately 95%; in many parts of the world vaccine coverage has remained below this target (Plemper, 2020).
4.9.1. MeV and Nipah virus
Galidesivir has been screened against MeV and Nipah virus (NiV) in cell culture (Table 1). Against MeV, potent antiviral activity was observed in Vero cells, with an SI > 47.8. In contrast, inhibition of NiV by galidesivir was low, with an SI > 2.4 (Warren et al., 2014).
4.10. Picornaviridae
4.10.1. Human rhinovirus
Rhinovirus is one of the leading causes of upper respiratory tract infections in humans world-wide and is a primary etiological agent of bronchiolitis in infants (Vandini et al., 2019). In Vero cells, galidesivir was a potent inhibitor of human rhinovirus 2 (HRV2), with an associated SI of >87.1 (Table 1) (Warren et al., 2014). No in vivo studies of galidesivir against HRV2 have been reported.
4.11. Pneumoviridae
4.11.1. Respiratory syncytial virus
Respiratory syncytial virus (RSV) is a common pathogen in humans, infecting 90% of children within their first 2 years of life. RSV is predominantly associated with upper respiratory illness; however, bronchiolitis may develop in some patients (Schweitzer and Justice, 2021). Galidesivir inhibited RSV in Vero cells, with an SI of >8.1 (Table 1) (Warren et al., 2014). No in vivo studies of galidesivir against RSV have been reported.
4.12. Togaviridae
4.12.1. Chikungunya virus
Chikungunya virus (CHIKV) is a mosquito-borne virus that causes a febrile illness with associated debilitating arthralgia, and has in recent years emerged in areas of Asia, Europe, and The Americas. Long-term arthritic sequela is frequently reported following acute CHIKV disease (Vu et al., 2017). In cell culture, galidesivir showed no activity against CHIKV (Table 1), and as such has not been further evaluated for this indication (Warren et al., 2014).
4.12.2. Other togaviruses
Galidesivir was additionally screened against three closely related togaviruses associated with rare cases of encephalitis in humans and horses; Venezuelan encephalitis virus (VEEV), Western encephalitis virus (WEEV) and Eastern encephalitis virus (EEEV) (CDC, 2021; Crosby and Crespo, 2021; Health, 2019). No activity was observed against VEEV in cell culture, while low to moderate activity was observed against EEEV and WEEV (Table 1) (Warren et al., 2014).
5. Clinical development
The safety, tolerability, and PK of galidesivir administered by either IM injection or IV infusion have been assessed in phase I, double-blind, placebo-controlled, dose-ranging studies in healthy subjects (ClinicalTrials.gov, 2016, 2021). Initial results of clinical exposure indicate that galidesivir is safe and generally well tolerated (BioCryst, 2019).
A randomized, double-blind, placebo-controlled clinical trial of IV galidesivir in the treatment of yellow fever or COVID-19 was initiated in April 2020 in Brazil (ClinicalTrials.gov, 2020). The primary objective of the study was to evaluate safety, while secondary objectives were to evaluate the clinical and antiviral effects of galidesivir administered via IV infusion versus placebo in hospitalized adult subjects. The trial was designed to be conducted in two parts: an initial dose-ranging study in which subjects received IV galidesivir or placebo every 12 h for 7 days, followed by a period during which participants would receive an optimized dosing regimen of galidesivir. Results from the initial dose-ranging part of the trial in COVID-19 subjects showed that galidesivir was safe and generally well tolerated across tested dose levels and that the therapy was associated with a dose-dependent decline in viral levels of SARS-CoV-2 in the respiratory tract. However, the trial was not designed or sized to demonstrate clinical efficacy. Further development of galidesivir for COVID-19 will not be pursued at this time (BioCryst, 2020).
6. Next steps in research
Galidesivir is a broad-spectrum antiviral with demonstrated in vitro and in vivo activity against several RNA viruses of human public health concern. Studies are ongoing to further characterize the in vitro and in vivo antiviral activity of galidesivir against SARS-CoV-2, and work continues on the advanced clinical development of galidesivir as a medical countermeasure for the treatment of MARV disease.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Amanda Mathis, PhD, Y. S. Babu, PhD, Ray Taylor, MBA, and Dennis M. Walling, MD, MMCI, FIDSA are employed by and own stock in BioCryst. James F. Demarest, PhD, is a contract consultant for BioCryst. Justin G. Julander, PhD, and Brian B. Gowen, PhD, have no disclosures.
Acknowledgments
Writing support was provided by Sarah Brun Bar-Yaacov, PhD, and Julia Van Diver, PhD, from Porterhouse Medical Group.
References
- Aftab S.O., Ghouri M.Z., Masood M.U., Haider Z., Khan Z., Ahmad A., Munawar N. Analysis of SARS-CoV-2 RNA-dependent RNA polymerase as a potential therapeutic drug target using a computational approach. J. Transl. Med. 2020;18:275. doi: 10.1186/s12967-020-02439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avsic-Zupanc T., Saksida A., Korva M. Hantavirus infections. Clin. Microbiol. Infect. 2019;21S:e6–e16. doi: 10.1111/1469-0691.12291. [DOI] [PubMed] [Google Scholar]
- BioCryst BioCryst initiates phase 1 clinical trial of galidesivir. 2019. https://ir.biocryst.com/news-releases/news-release-details/biocryst-initiates-phase-1-clinical-trial-galidesivir press release, January 2, 2019.
- BioCryst . 2020. BioCryst Provides Update on Galidesivir Program.https://ir.biocryst.com/news-releases/news-release-details/biocryst-provides-update-galidesivir-program press release, December 22, 2020. [Google Scholar]
- Bullard-Feibelman K.M., Govero J., Zhu Z., Salazar V., Veselinovic M., Diamond M.S., Geiss B.J. The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antivir. Res. 2017;137:134–140. doi: 10.1016/j.antiviral.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys K.K., Jung K.H., Smee D.F., Furuta Y., Gowen B.B. Maporal virus as a surrogate for pathogenic New World hantaviruses and its inhibition by favipiravir. Antivir. Chem. Chemother. 2011;21:193–200. doi: 10.3851/IMP1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . 2014. Marburg Hemorrhagic Fever. [Google Scholar]
- CDC . 2021. Eastern Equine Encephalitis. [Google Scholar]
- Crosby B., Crespo M.E. 2021. Venezuelan Equine Encephalitis, StatPearls, Treasure. Island (FL. [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyer L., Zouharová D., Širmarová J., Fojtíková M., Štefánik M., Haviernik J., Nencka R., de Clercq E., Růžek D. Antiviral activity of the adenosine analogue BCX4430 against West Nile virus and tick-borne flaviviruses. Antivir. Res. 2017;142:63–67. doi: 10.1016/j.antiviral.2017.03.012. [DOI] [PubMed] [Google Scholar]
- Eyer L., Nougairède A., Uhlířová M., Driouich J.S., Zouharová D., Valdés J.J., Haviernik J., Gould E.A., De Clercq E., de Lamballerie X., Ruzek D. An E460D substitution in the NS5 protein of tick-borne encephalitis virus confers resistance to the inhibitor galidesivir (BCX4430) and also attenuates the virus for mice. J. Virol. 2019;93 doi: 10.1128/JVI.00367-19. e00367-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA . Research. 2020. Cross-discipline team leader, division director, and ODE summary review. C.f.d.e.a. [Google Scholar]
- Gomez R.M., Jaquenod de Giusti C., Sanchez Vallduvi M.M., Frik J., Ferrer M.F., Schattner M. Junin virus. A XXI century update. Microb. Infect. 2011;13:303–311. doi: 10.1016/j.micinf.2010.12.006. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov . 2016. A Phase 1 Study to Evaluate the Safety, Tolerability and Pharmacokinetics of BCX4430.https://clinicaltrials.gov/ct2/show/NCT02319772 [Google Scholar]
- ClinicalTrials.gov . 2020. A Study to Evaluate the Safety, Pharmacokinetics and Antiviral Effects of Galidesivir in Yellow Fever or COVID-19.https://clinicaltrials.gov/ct2/show/NCT03891420 [Google Scholar]
- ClinicalTrials.gov . 2021. A Study to Evaluate the Single Dose Safety, Tolerability and Pharmacokinetics of IV BCX4430.https://clinicaltrials.gov/ct2/show/NCT03800173 [Google Scholar]
- Harding S., Greig J., Mascarenhas M., Young I., Waddell L.A. La Crosse virus: a scoping review of the global evidence. Epidemiol. Infect. 2018:1–13. doi: 10.1017/S0950268818003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health, M.D.o. Department of Health; 2019. Western Quine Encephalitis Fact Sheet -Minnesota. [Google Scholar]
- Julander J.G., Siddharthan V. Small-animal models of Zika virus. J. Infect. Dis. 2017;216:S919–S927. doi: 10.1093/infdis/jix465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander J.G., Bantia S., Taubenheim B.R., Minning D.M., Kotian P., Morrey J.D., Smee D.F., Sheridan W.P., Babu Y.S. BCX4430, a novel nucleoside analog, effectively treats yellow fever in a hamster model. Antimicrob. Agents Chemother. 2014;58:6607–6614. doi: 10.1128/AAC.03368-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander J.G., Siddharthan V., Evans J., Taylor R., Tolbert K., Apuli C., Stewart J., Collins P., Gebre M., Neilson S., Van Wettere A., Lee Y.M., Sheridan W.P., Morrey J.D., Babu Y.S. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antivir. Res. 2017;137:14–22. doi: 10.1016/j.antiviral.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander J.G., Siddharthan V., Evans J., Taylor R., Tolbert K., Apuli C., Stewart J., Collins P., Gebre M., Neilson S., Van Wettere A., Lee Y.M., Sheridan W.P., Morrey J.D., Babu Y.S. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antivir. Res. 2017;137:14–22. doi: 10.1016/j.antiviral.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F., Smith G.J.D., Fouchier R.A.M., Peiris M., Kedzierska K., Doherty P.C., Palese P., Shaw M.L., Treanor J., Webster R.G., Garcia-Sastre A. Influenza. Nat Rev Dis Primers. 2018;4:3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.Y., Osuna C.E., Best K., Taylor R., Chen E., Yoon G., Kublin J.L., Schalk D., Schultz-Darken N., Capuano S., Safronetz D., Luo M., MacLennan S., Mathis A., Babu Y.S., Sheridan W.P., Perelson A.S., Whitney J.B. A direct-acting antiviral drug abrogates viremia in Zika virus–infected rhesus macaques. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aau9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson T.C., Diamond M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020;5:796–812. doi: 10.1038/s41564-020-0714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemper R.K. Measles resurgence and drug development. Curr. Opin. Virol. 2020;41:8–17. doi: 10.1016/j.coviro.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer J.W., Justice N.A. 2021. Respiratory Syncytial Virus Infection. StatPearls, Treasure Island (FL. [PubMed] [Google Scholar]
- Taylor R., Kotian P., Warren T., Panchal R., Bavari S., Julander J., Dobo S., Rose A., El-Kattan Y., Taubenheim B., Babu Y., Sheridan W.P. BCX4430 – a broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J. Infect. Public Health. 2016;9:220–226. doi: 10.1016/j.jiph.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandini S., Biagi C., Fischer M., Lanari M. 2019. Impact of Rhinovirus Infections in Children. Viruses 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu D.M., Jungkind D., Angelle Desiree L. Chikungunya virus. Clin. Lab. Med. 2017;37:371–382. doi: 10.1016/j.cll.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren T.K., Wells J., Panchal R.G., Stuthman K.S., Garza N.L., Van Tongeren S.A., Dong L., Retterer C.J., Eaton B.P., Pegoraro G., Honnold S., Bantia S., Kotian P., Chen X., Taubenheim B.R., Welch L.S., Minning D.M., Babu Y.S., Sheridan W.P., Bavari S. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508:402–405. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westover J.B., Mathis A., Taylor R., Wandersee L., Bailey K.W., Sefing E.J., Hickerson B.T., Jung K.H., Sheridan W.P., Gowen B.B. Galidesivir limits Rift Valley fever virus infection and disease in Syrian golden hamsters. Antivir. Res. 2018;156:38–45. doi: 10.1016/j.antiviral.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2017. Lassa Fever. [Google Scholar]
- WHO . 2021. Ebola Virus Disease. [Google Scholar]
- WHO . 2021. WHO Disease Outbreak News.https://www.who.int/csr/don/en/ [Google Scholar]
- Wright D., Kortekaas J., Bowden T.A., Warimwe G.M. Rift Valley fever: biology and epidemiology. J. Gen. Virol. 2019;100:1187–1199. doi: 10.1099/jgv.0.001296. [DOI] [PMC free article] [PubMed] [Google Scholar]