Abstract
This viewpoint devises recommendations for future studies utilizing 13C isotopically non-stationary metabolic flux analysis to characterize plant metabolism. Most importantly, it highlights the necessity for model validation.
Keywords: Carbon flux estimation, carbon metabolism, 13C labelling, 13CO2, complex models, 13C tracer experiments, labelling lag, metabolic flux analysis, model validation, photosynthesis
13C isotopically non-stationary metabolic flux analysis (13C-INST-MFA) is an emerging technique for estimations of metabolic fluxes and pool sizes. Within the plant sciences, two studies utilizing this technique to characterize carbon metabolism have been published so far. Here, I examine these studies carefully. Readers unfamiliar with 13C-INST-MFA will obtain a critical understanding of the method and its findings. Readers working with 13C-INST-MFA are recommended to enter a phase of model validation to devise clear-cut protocols enabling robust estimations of specific fluxes.
13C isotopically non-stationary metabolic flux analysis (13C-INST-MFA) is an emerging technique for estimations of metabolic fluxes and pool sizes. Within the plant sciences, two studies utilizing this technique to characterize carbon metabolism have been published so far. Here, I examine these studies carefully. Readers unfamiliar with 13C-INST-MFA will obtain a critical understanding of the method and its findings. Readers working with 13C-INST-MFA are recommended to enter a phase of model validation to devise clear-cut protocols enabling robust estimations of specific fluxes.
Realistic reaction networks
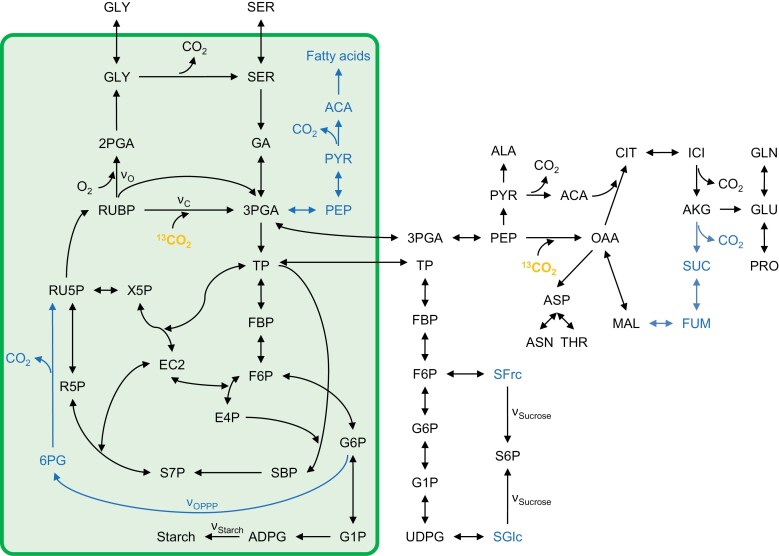
In 13C-INST-MFA, a list of coded reactions specifies by which routes carbon can move from labelled or unlabelled sources through metabolic networks into sinks (Fig. 1). The reaction network of both studies allows direct export of 3-phosphoglycerate (3PGA) from chloroplasts to the cytosol. In the light, however, 3PGA export is believed to be restricted due to the chloroplast to cytosol pH gradient (Flügge et al., 1983, and references therein). Additionally, cytosolic reactions catalysed by glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate kinase are missing [conversion of glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate to 3PGA; triose phosphate (TP) to 3PGA]. Thus, 13C flux into glycolysis and the tricarboxylic acid cycle may follow unrealistic routes and has no cytosolic connection with sucrose biosynthesis. Furthermore, fractional refixation of respired CO2 is not considered (Loreto et al., 1999), and numerous reversible reactions were programmed as irreversible, or vice versa. This includes reactions of the Calvin–Benson cycle catalysed by phosphoglycerate kinase and glyceraldehyde-3-phosphate dehydrogenase (conversion of 3PGA to 1,3-bisphosphoglycerate to glyceraldehyde 3-phosphate; 3PGA to TP), fructose bisphosphatase (conversion of fructose 1,6-bisphosphate to fructose 6-phosphate; FBP to F6P), and fructose-bisphosphate aldolase [conversion of dihydroxyacetone phosphate and erythrose 4-phosphate (E4P) to sedoheptulose 1,7-bisphosphate; TP and E4P to SBP]. Lastly, mesophyll chloroplasts reportedly lack enolase (van der Straeten et al., 1991; Prabhakar et al., 2009; Fukayama et al., 2015). Thus, stromal conversion of 3PGA to phosphoenolpyruvate (PEP) is likely to be infeasible, and fatty acid biosynthesis probably relies on PEP import from the cytosol. Future studies are encouraged to implement more realistic reaction networks representing carbon metabolism with all its intrinsic restrictions and freedom. Incorporation of cytosolic glyceraldehyde-3-phosphate dehydrogenases and phosphoglycerate kinase may enhance the utility of the model since these reactions proposedly constitute a central hub in leaf energy metabolism (Wieloch, 2021).
Fig. 1.
Reaction networks of published 13C-INST-MFA studies. Black: network as programmed by Ma et al. (2014; study 1) including reactions of the Calvin–Benson cycle, photorespiration, starch and sucrose biosynthesis, glycolysis, the tricarboxylic acid cycle, and amino acid biosynthesis. Blue: add-ons to the network of study 1 by Xu et al. (2021; study 2) including reactions of the oxidative pentose phosphate pathway, fatty acid biosynthesis, and the tricarboxylic acid cycle. Orange: 13C-enriched compound (label) entering the reaction network. Reactions inside the green box were programmed as chloroplast localized, while reactions outside the box were programmed as either cytosolic or without a compartment identifier. Abbreviations: 2PGA, 2-phosphoglycerate; 3PGA, 3-phosphoglycerate; 6PG, 6-phosphogluconate; ACA, acetyl-CoA; ADPG, ADP-glucose; AKG, α-ketoglutarate; ALA, alanine; ASN, asparagine; ASP, aspartate; CIT, citrate; E4P, erythrose 4-phosphate; EC2, enzyme-bound two-carbon fragment; F6P, fructose 6-phosphate; FBP, fructose 1,6-bisphosphate; FUM, fumarate; G1P, glucose 1-phosphate; G6P, glucose 6-phosphate; GA, glycerate; GLN, glutamine; GLU, glutamate; GLY, glycine; ICI, isocitrate; MAL, malate; OAA, oxaloacetate; PEP, phosphoenolpyruvate; PRO, proline; PYR, pyruvate; R5P, ribose 5-phosphate; RU5P, ribulose 5-phosphate; RUBP, ribulose 1,5-bisphosphate; S6P, sucrose 6-phosphate; S7P, sedoheptulose 7-phosphate; SBP, sedoheptulose 1,7-bisphosphate; SER, serine; SFrc, fructosyl moiety of S6P; SGlc, glucosyl moiety of S6P; SUC, succinate; THR, threonine; TP; triose phosphate (glyceraldehyde 3-phosphate and dihydroxyacetone phosphate); UDPG, UDP-glucose; X5P, xylulose 5-phosphate; νC, Rubisco carboxylation flux; νO, Rubisco oxygenation flux; νOPPP, OPPP flux; νSucrose, flux into sucrose; νStarch, flux into starch.
Constrained fluxes
INCA allows users to constrain fluxes and pool sizes, for example based on independent physiological measurements or theoretical considerations. Users may specify constants or intervals, or choose not to impose any constraints. In all models (both studies), net CO2 assimilation was fixed at pre-determined values scaling fluxes up to reasonable values (supporting interpretation of results) while maintaining flux ratios. Additionally, study 1 fixed the absolute flux into starch (Fig. 1, νStarch) and flux ratios between sucrose and amino acid biosynthesis according to physiological measurements. Similarly, study 2 fixed absolute fluxes into starch, sucrose (νSucrose), and amino acid biosynthesis, and the ratio of Rubisco oxygenation to carboxylation. This practice is potentially problematic since it may affect modelled flux ratios. Additionally, the necessity for constraints poses an important question. If large fluxes need to be fixed (νStarch, νSucrose, and νO/νC), can one rely on 13C-INST-MFA to return credible results for unconstrained fluxes including smaller fluxes of interest (νOPPP)? Thus, future studies are encouraged to present models without constraints alongside constrained models to show that data-driven flux estimation is feasible. Ideally, 13C data should drive the estimations with a minimum of imposed constraints.
Effects of constrained fluxes on fluxes of interest
In principle, constraining fluxes or pool sizes can affect estimates of fluxes or pool sizes of interest due to interconnectivities within the reaction network (Fig. 1). In study 2, νO/νC was constrained to be between 0.2 and 0.25. Modelling returned a νO/νC ratio of 0.2, and an RL and νOPPP of 5.2 μmol CO2 g–1 FW h–1 and 4.6 μmol CO2 g–1 FW h–1, respectively. When left unconstrained, modelling returned a physiologically unrealistic νO/νC ratio of 0.09, and an RL and νOPPP of 12.1 μmol CO2 g–1 FW h–1 and 10.5 μmol CO2 g–1 FW h–1, respectively. This indicates negative correlations between νO/νC and RL and νO/νC and νOPPP (the lower photorespiration, the higher day respiration). Hence, fixing νO/νC at values >0.2 may cause RL→0 and νOPPP→0. Note that under normal growth conditions, νO/νC ratios of 0.34 are common (Sharkey, 1988; Cegelski and Schaefer, 2006; Pärnik et al., 2007). Thus, future studies are encouraged to include sensitivity analyses investigating dependence between constrained fluxes and fluxes of interest.
Validation of results by independent methods
INCA-based 13C-INST-MFA returns a comprehensive dataset containing estimates of (i) forward and reverse fluxes of all reactions and (ii) pool sizes of all metabolites specified in the reaction network (Fig. 1). Some of these items are accessible to other analytical techniques which, in principle, enables independent validation of 13C-INST-MFA results. Study 1 made no attempt to confirm modelled νO/νC estimates by independent methods. However, estimated ratios were within the physiologically reasonable range. In contrast, study 2 tested the model estimate for RL (5.2 μmol CO2 g–1 FW h–1) by the Laisk method which returned an RL estimate of 9.3 μmol CO2 g–1 FW h–1 (Brooks and Farquhar, 1985). However, corresponding 95% confidence intervals showed no overlap (3.5–8.05 μmol CO2 g–1 FW h–1 versus 8.1–10.7 μmol CO2 g–1 FW h–1). Thus, these estimates are statistically different at the 0.05 significance level. Additionally, the model estimate for νOPPP in chloroplasts was compared with an estimate of flux through the cytosolic OPPP (Sharkey et al., 2020). However, there is no reason to believe that these pathways carry the same flux. Thus, validation of estimates from 13C-INST-MFA by independent methods has not yet been achieved. However, independently determined fluxes currently used as constraints (νStarch and νSucrose) can be utilized to test the method by leaving them unconstrained and comparing modelled and measured values. Additionally, νO/νC ratios may help to test the method since several alternative methods can provide independent estimates (Busch, 2013).
Metabolically inactive pools or injection of carbon from unlabelled sources
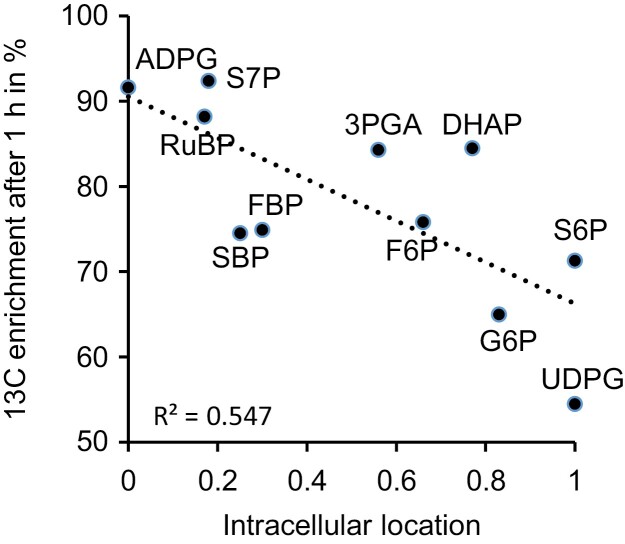
In 13C-INST-MFA, 12C is progressively flushed out of the metabolic network and replaced by 13C from the labelling compound, such as 13CO2 (Fig. 1). Both studies reported fast initial labelling of metabolite pools. After several minutes, however, labelling slowed and, even after 1 h, a significant fraction of the pools remained unlabelled. This was attributed to metabolically inactive pools (i.e. metabolite pools disconnected from the flux of incoming 13C) and modelled accordingly by including a dilution term for each metabolite (accounting for apparently constant offsets between measured and modelled 13C enrichments). Alternatively, labelling lags may be explained in combination by breakdown of weakly labelled cytosolic sucrose into glucose and fructose, phosphorylation by hexokinase and fructokinase, and reinjection of glucose-6-phosphate-derived carbon into chloroplasts via a cytosolic OPPP not shown in Fig. 1 (Sharkey et al., 2020). Figure 2 shows reported 13C enrichments of metabolites of the Calvin–Benson cycle, and starch and sucrose biosynthesis 1 h into 13CO2 labelling of Arabidopsis thaliana rosettes (Szecowka et al., 2013). Additionally, these authors reported subcellular distributions of metabolites given on the x-axis from fully plastidial (x=0) to fully cytosolic (x=1). Interestingly, plastidial metabolites are more strongly 13C labelled than cytosolic metabolites. Metabolite distribution explains 55% of the labelling variability (P<0.01, n=11). Since it is not apparent why sizes of metabolically inactive pools would correlate with plastid–cytosol metabolite distribution, this corroborates the idea of injection of weakly labelled carbon into cytosolic metabolism. Future 13C-INST-MFA studies are encouraged to further explore this by expanding their reaction networks by sucrose breakdown pathways and a cytosolic OPPP. Additionally, sucrose, glucose, and fructose are large carbon pools with significant vacuolar contributions (Szecowka et al., 2013). Thus, cytosol–vacuole transmembrane transport of these metabolites may need to be considered.
Fig. 2.
13C enrichment of metabolite pools in Arabidopsis thaliana rosettes 1 h into 13CO2 labelling as a function of the intracellular metabolite distribution from fully plastidial (x=0) to fully cytosolic (x=1). Abbreviations: 3PGA, 3-phosphoglycerate; ADPG, ADP-glucose; DHAP, dihydroxyacetone phosphate; F6P, fructose 6-phosphate; FBP, fructose 1,6-bisphosphate; G6P, glucose 6-phosphate; RuBP, ribulose 1,5-bisphosphate; S6P, sucrose 6-phosphate; S7P, sedoheptulose 7-phosphate; SBP, sedoheptulose 1,7-bisphosphate; UDPG, UDP-glucose. Re-analysed data from Szecowka et al. (2013). Sucrose and glucose 1-phosphate were excluded from the analysis since the former has a large vacuolar fraction and the latter reportedly exhibits an anomalous labelling behaviour (Szecowka et al., 2013; Xu et al., 2021).
Future focus
To date, evidence that 13C-INST-MFA returns reliable flux and pool size estimates is not available. Therefore, the field is recommended to enter a phase of validation of the complex models used in 13C-INST-MFA to devise clear-cut protocols enabling robust estimations of specific fluxes.
Glossary
Abbreviations
- INCA
isotopomer network compartmental analysis
- INST-MFA
isotopically non-stationary metabolic flux analysis
- OPPP
oxidative pentose phosphate pathway
- PEP
phosphoenolpyruvate
- 3PGA
3-phosphoglycerate
- RL
day respiration
- TP
triose phosphate
- ν O/ν C
Rubisco oxygenation to carboxylation ratio
- ν OPPP
OPPP flux
- ν Sucrose
flux into sucrose
- ν Starch
flux into starch
Data availability
The data supporting the findings of this study have been published by Ma et al. (2014), Szecowka et al. (2013), and Xu et al. (2021).
References
- Brooks A, Farquhar GD. 1985. Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 165, 397–406. [DOI] [PubMed] [Google Scholar]
- Busch FA. 2013. Current methods for estimating the rate of photorespiration in leaves. Plant Biology 15, 648–655. [DOI] [PubMed] [Google Scholar]
- Cegelski L, Schaefer J. 2006. NMR determination of photorespiration in intact leaves using in vivo13CO2 labeling. Journal of Magnetic Resonance 178, 1–10. [DOI] [PubMed] [Google Scholar]
- Cheah YE, Young JD. 2018. Isotopically nonstationary metabolic flux analysis (INST-MFA): putting theory into practice. Current Opinion in Biotechnology 54, 80–87. [DOI] [PubMed] [Google Scholar]
- Flügge UI, Gerber J, Heldt HW. 1983. Regulation of the reconstituted chloroplast phosphate translocator by an H+ gradient. Biochimica et Biophysica Acta 725, 229–237. [Google Scholar]
- Fukayama H, Masumoto C, Taniguchi Y, Baba-Kasai A, Katoh Y, Ohkawa H, Miyao M. 2015. Characterization and expression analyses of two plastidic enolase genes in rice. Bioscience, Biotechnology, and Biochemistry 79, 402–409. [DOI] [PubMed] [Google Scholar]
- Loreto F, Delfine S, Di Marco G. 1999. Estimation of photorespiratory carbon dioxide recycling during photosynthesis. Functional Plant Biology 26, 733–736. [Google Scholar]
- Ma F, Jazmin LJ, Young JD, Allen DK. 2014. Isotopically nonstationary 13C flux analysis of changes in Arabidopsis thaliana leaf metabolism due to high light acclimation. Proceedings of the National Academy of Sciences, USA 111, 16967–16972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Jazmin LJ, Young JD, Allen DK. 2017. Isotopically nonstationary metabolic flux analysis (INST-MFA) of photosynthesis and photorespiration in plants. In: Fernie AR, Bauwe H, Weber APM, eds. Photorespiration: methods and protocols. New York: Springer, 167–194. [DOI] [PubMed] [Google Scholar]
- Pärnik T, Ivanova H, Keerberg O. 2007. Photorespiratory and respiratory decarboxylations in leaves of C3 plants under different CO2 concentrations and irradiances. Plant, Cell & Environment 30, 1535–1544. [DOI] [PubMed] [Google Scholar]
- Prabhakar V, Löttgert T, Gigolashvili T, Bell K, Flügge UI, Häusler RE. 2009. Molecular and functional characterization of the plastid-localized phosphoenolpyruvate enolase (ENO1) from Arabidopsis thaliana. FEBS Letters 583, 983–991. [DOI] [PubMed] [Google Scholar]
- Sharkey TD. 1988. Estimating the rate of photorespiration in leaves. Physiologia Plantarum 73, 147–152. [Google Scholar]
- Sharkey TD, Preiser AL, Weraduwage SM, Gog L. 2020. Source of 12C in Calvin–Benson cycle intermediates and isoprene emitted from plant leaves fed with 13CO2. The Biochemical Journal 477, 3237–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szecowka M, Heise R, Tohge T, et al. . 2013. Metabolic fluxes in an illuminated Arabidopsis rosette. The Plant Cell 25, 694–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Straeten D, Rodrigues-Pousada RA, Goodman HM, Van Montagu M. 1991. Plant enolase: gene structure, expression, and evolution. The Plant Cell 3, 719–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieloch T. 2021. A cytosolic oxidation–reduction cycle in plant leaves. Journal of Experimental Botany 72, 4186–4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Fu X, Sharkey TD, Shachar-Hill Y, Walker BJ. 2021. The metabolic origins of non-photorespiratory CO2 release during photosynthesis: a metabolic flux analysis. Plant Physiology 186, 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JD. 2014. INCA: a computational platform for isotopically non-stationary metabolic flux analysis. Bioinformatics 30, 1333–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study have been published by Ma et al. (2014), Szecowka et al. (2013), and Xu et al. (2021).