Abstract
A community-based cross-sectional study was undertaken by the Cardiology Society of India (Kerala Chapter) to determine the prevalence of coronary artery disease (CAD) and its risk factors. The periodontal health status of the rural and urban participants in the Thiruvananthapuram district of Kerala was evaluated to document any association between periodontal disease (PD) and CAD and to describe any shared risk factors.
The participants were selected using a multistage cluster random sampling method. Socio-demographic data and personal histories were collected using a structured interview schedule and validated tools. Body mass index, blood pressure, electrocardiogram, and biochemical investigations were recorded and analyzed using standard protocols. A modification of the Ramfjord periodontal disease index was used to assess periodontal health.
PD was more frequent among rural (61.4%) than in the urban population (35.5%). The frequencies of CAD associated with PD in the rural and urban populations were 82.6% and 40.5%, respectively. PD was not found to be a significant risk factor for CAD in the univariate regression analysis of urban populations. In the rural population, the odds of PD as a risk factor for CAD were found to be 3.08 (95% CI [1.38–8.38]) and significant (P = .043) in univariate regression analysis and 1.54 (95% CI: 0.44–5.4) and non-significant (P = .503) in the multivariate regression analysis.
In rural areas, male sex and dyslipidemia demonstrated borderline significance as risk factors for CAD. PD was not found to be an independent risk factor after adjusting for age, sex, tobacco use, hypertension, sedentary lifestyle, and dyslipidemia. Male sex and dyslipidemia were identified as shared risk factors between PD and CAD, which could have confounded the significant association between the latter. In urban areas, age, male sex, and dyslipidemia demonstrated an independent association with CAD. This study could not establish an independent association between PD and CAD in either community. Future epidemiological studies should identify and recruit novel environmental factors to understand the interrelationships between PD and CAD and focus on the role of effect modifiers that may have a protective role against PD colluding with CAD.
Keywords: coronary artery disease, modifiable risk factors, periodontal disease, shared risk factors, traditional risk factors
1. Introduction
Coronary artery disease (CAD) is one of the leading causes of death in the Indian population. There is a high prevalence of this disease, which is approximately 11% in urban areas and 7% in rural populations across India.[1,2] There has been a steady rise in CAD cases reported in both urban and rural settings in India among adults aged ≥20 years.[3] The INTERHEART study, including a sizeable number of participants from India, has highlighted the importance of conventional risk factors in the causation of CAD.[4] Kerala, the southernmost state with a population above 33 million, has the highest prevalence of CAD risk factors in India.[5,6] With this background, a study was undertaken by the Cardiology Society of India, Kerala Chapter; Coronary Artery Disease and its Risk Factors Prevalence Study (CSI Kerala CRP Study) to determine the prevalence of CAD and its traditional and modifiable risk factors. A plethora of modifiable and non-modifiable risk factors have been identified and added over the years by the FRAMINGHAM Heart Study Group since its inception.[7] Periodontitis, an inflammatory process involving the connective tissue apparatus of the tooth, has been reported as a modifiable risk factor for CAD. Numerous epidemiological studies investigating the association between periodontal disease (PD) and CAD have reported variable results; some have suggested positive associations,[8–20] while others have not.[21–23] The authors present a cohort to describe the association between PD and CAD, of which 644 cases were diagnosed with “established periodontitis” and 60 cases with “definite CAD.” The periodontal health status of the rural and urban participants in the Thiruvananthapuram district of Kerala was evaluated to document any association between PD and CAD and to describe any shared risk factors.
2. Methods
2.1. Study design and participants
Briefly, this was a cross-sectional community-based study. Subjects aged 20 to 79 years in the urban and rural settings of southern, central, and northern districts of Kerala were selected using a multistage cluster sampling method. Thiruvananthapuram district was randomly selected for the survey in the southern region. The urban and rural study areas in the Thiruvananthapuram district were randomly selected. One subject between 20 and 59 years of age was selected from each household using the KISH method (WHO STEPS Manual),[24] and all subjects between 60 and 79 years of age were included. A detailed description of the design, sample, and methods of the CSI Kerala CRP Study has already been published.[1,2]
2.2. Diagnosis and exclusion criteria
2.2.1. CAD – definition
Coronary artery disease based on any of the following: documented evidence of prior acute coronary syndrome or treatment for CAD; documented history of undergoing coronary angioplasty or coronary artery bypass grafting; ≥50% epicardial coronary stenosis by invasive coronary angiography; electrocardiogram (ECG) showing pathological Q waves (any of Minnesota codes 1-1-1 to 1-1-7 or 1-2-1 to 1-2-5 or 1-2-7), imaging evidence of a region of loss of viable myocardium that is thinned and has a motion abnormality, in the absence of a non-ischemic cause; Rose Angina Questionnaire (RAQ) angina plus ECG changes (any of Minnesota code 4-1-1, 4-1-2, 4-2 or 5-1, 5-2), RAQ angina plus positive treadmill ECG (exercise-induced horizontal or down-sloping ST depression of ≥1 mm at 80 ms from J point), or inducible ischemia on stress imaging.
2.2.2. Oral examination
Periodontal health status was evaluated among rural and urban participants in the Thiruvananthapuram district of Kerala using standard tools. The final tally showed a sample size difference between the total survey participants (N = 1643) and periodontal health study participants (n = 1285). This could be due to the exclusion of 358 participants either due to incomplete data or edentulousness or because they had opted out from undergoing an intraoral examination. Clinical examination of the oral cavity, including hard and soft tissues, was performed using a mouth mirror and periodontal probe under adequate illumination by 2 dental surgeons. A standard millimeter-graded periodontal probe was used to assess the periodontal clinical attachment level, measured from the cementoenamel junction of the tooth to the bottom of the gingival sulcus. A modification of the Ramfjord periodontal disease index was used to assess the periodontal status of teeth. The gingival and periodontal components of the PD index (PDI) were assessed. Six teeth were indexed: the maxillary right first molar, left central incisor, left first premolar, mandibular left first molar, right central incisor, and right first premolar. The end of the periodontal probe was passed with minimal force in the apical direction, maintaining contact with the tooth, and recording the depth of the gingival sulcus. Each indexed tooth was measured on the mesial, distal, buccal, and lingual areas, and the maximum score was recorded.
Scoring criteria[25]
Score 0 – Absence of signs of inflammation.
Score 1 – Mild to moderate inflammatory gingival changes not extending around the tooth.
Score 2 – Mild to moderate inflammatory gingival changes extending all around the tooth.
Score 3 – Severe gingivitis
Score 4 – Gingival crevice in any of the 4 measured areas, extending apically to the cemento-enamel junction, but not more than 3 mm.
Score 5 – Gingival crevice in any of the 4 measured areas extending apically to the cemento-enamel junction between 3 and 6 mm.
Score 6 – Gingival crevice in any of the 4 measured areas extending apically more than 6 mm from the cemento-enamel junction.
Although gingivitis is an early form of periodontitis, only established periodontitis cases that manifest a periodontal attachment loss of ≥3 mm were diagnosed with PD. Participants were categorized based on their PDI scores. PDI scores of 4, 5, and 6 were diagnostic of periodontal attachment loss, and hence PD. CAD risk assessment was performed in the PD group.
2.2.3. Assessment of conventional risk factors
Data on conventional risk factors such as age, sex, socioeconomic status, educational status, sedentary lifestyle, and tobacco use were collected using a structured interview schedule and validated tools.[1,2] Body mass index (BMI) and blood pressure were recorded for all participants during the survey. BMI was calculated as weight in kg/(height in meters squared); weight was measured using a portable electronic weighing scale in kilograms to the nearest 0.5 kg and height was measured using a wall-mounted stadiometer to the nearest centimeter.[26] Blood pressure was recorded with an electronic blood pressure apparatus in the sitting position on the left arm resting on the table at the heart level. Diabetes and dyslipidemia were diagnosed using biochemical investigations. The methods and definitions of conventional cardiovascular risk factors were described in detail in a previously published article.[1]
2.2.4. Diagnosis of CAD
Resting 12 lead ECG was performed on all subjects by trained technicians with 3-channel digital ECG recorders with provision for display. Five consecutive complexes were recorded for each lead. Minnesota coding[27] and application of CAD criteria were performed by an experienced cardiologist in the respective region, and subjects diagnosed with CAD were re-evaluated by another cardiologist in a blinded manner. The other methods used in diagnosis are described in the definition of CAD. Eighty cases of probable CAD diagnosed based on the RAQ[28,29] were excluded from the study to avoid systematic errors.
2.3. Statistical analysis
The data were entered into Microsoft Office Excel 2007 and analyzed using the analytical software IBM SPSS Statistics for Windows, Version 11.0.∗ Categorical data are presented as numbers and percentages. Differences between the groups were analyzed using univariate and multivariate logistic regressions. All variables, which, according to univariate logistic regression analysis were significant at the level of P ≤ .10 were included in multivariate analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. A P value of ≤.05, was considered significant in multivariate logistic regression analysis. Differences between groups for categorical variables were analyzed using stratified analysis and the chi-square test. Analyses were performed in both rural and urban settings. ∗IBM Corp. IBM SPSS Statistics for Windows, version 11.0, (Internet). (IBM Corporation Armonk, NY, USA) 2012. Available from: https://www.ibm.com/analytics/us/en/technology/spss/.
2.4. Ethical clearance
The present study was conducted in compliance with the principles of the Declaration of Helsinki. The study was approved by the Ethics Committee of the Cardiology Society of India (Kerala Chapter). Informed written consent was obtained from all the participants.
3. Results
3.1. Clinical characteristics of study groups
The study included 1285 subjects, 725 hailed from a rural area, 560 from an urban area, 227 men and 498 women in rural areas, and 292 men and 268 women in urban areas in the age group of 20 to 79 years. Data on diabetes and dyslipidemia were missing in 17 (8 from rural and 9 from urban) and 20 (7 from rural and 13 from urban) subjects, respectively, and they were excluded from further analysis. Consequently, the missing data for diabetes and dyslipidemia were addressed using the available data on each variable. Risk assessment in this study was performed using univariate and multivariate logistic regression analyses.
3.2. Prevalence of the various risk factors of CAD
Table 1 depicts the prevalence of CAD and the risk factors in rural and urban settings. The risk factors analyzed were age, sex, socioeconomic status (below poverty line [BPL] or above poverty line), educational status (< or >10 years of education), sedentary lifestyle, BMI, tobacco use, hypertension, diabetes mellitus, dyslipidemia, and PD. The frequencies of CAD associated with PD were 82.6% (19 out of 23) in the rural population and 40.5% (15 out of 37) in the urban population.
Table 1.
The prevalence of coronary artery disease and risk factors in the rural and urban areas.
| RURAL CAD | URBAN CAD | |||||
| Yes (n = 23) | No (n = 702) | Total (n = 725) | Yes (n = 37) | No (n = 523) | Total (n = 560) | |
| Risk factor | No (%) | No (%) | No (%) | No (%) | No (%) | No (%) |
| Age ≥ 45 | 19 (82.6) | 410 (58.4) | 429 (59.2) | 35 (94.6) | 373 (71.3) | 408 (72.9) |
| Sex-male | 14 (60.9) | 213 (30.3) | 227 (31.3) | 28 (75.7) | 264 (50.5) | 292 (52.1) |
| Sex-female | 9 (39.1) | 489 (69.7) | 498 (68.7) | 9 (24.3) | 259 (49.5) | 268 (47.9) |
| Socio-economic status (BPL) | 7 (30.4) | 278 (39.6) | 285 (39.3) | 2 (5.4) | 18 (3.4) | 20 (3.6) |
| Education < 10 yrs | 15 (65.2) | 427 (60.8) | 442 (61) | 6 (16.2) | 80 (15.3) | 86 (15.4) |
| Sedentary lifestyle | 16 (69.6) | 282 (40.2) | 298 (41.1) | 12 (32.4) | 190 (36.3) | 202 (36.1) |
| BMI ≥ 25 kg/m2 | 9 (39.1) | 300 (42.7) | 309 (42.6) | 19 (51.4) | 275 (52.6) | 294 (52.5) |
| Tobacco use | 9 (39.1) | 162 (23.1) | 171 (23.6) | 14 (37.8) | 105 (20.1) | 119 (21.3) |
| Hypertension | 14 (60.9) | 220 (31.3) | 234 (32.3) | 23 (62.2) | 235 (44.9) | 258 (46.1) |
| Periodontal disease | 19 (82.6) | 426 (60.7) | 445 (61.4) | 15 (40.5) | 84 (35.2) | 199 (35.5) |
| Diabetes∗ | 9 (39.1) | 172 (24.8) | 181 (25.2) | 20 (55.6) | 172 (33.4) | 192 (34.8) |
| Dyslipidemia∗ | 9 (39.1) | 148 (21.3) | 157 (21.9) | 23 (65.7) | 159 (31.1) | 182 (33.3) |
All results were presented as numbers or percentages.
The missing data for diabetes and dyslipidemia were addressed using the available data
The missing data for diabetes and dyslipidemia were addressed using the available data on each variable. For diabetes, rural – YES (n = 23), NO (n = 694), and TOTAL (n = 717). Urban – YES (n = 36), NO (n = 515), and TOTAL (n = 551). For dyslipidemia, rural – YES (n = 23), NO (n = 695), and TOTAL (n = 718). Urban – YES (n = 35), NO (n = 512), and TOTAL (n = 547).
BMI = body mass index, BPL = below poverty line, CAD = coronary artery disease,
3.3. Univariate regression for the relationship between the various risk factors and CAD
Table 2 outlines the univariate logistic regression models of risk factors in rural and urban populations. All variables that were significant at the level of P ≤ .10, were included in the multivariate logistic regression analysis. Age, sex, tobacco use, hypertension, dyslipidemia, and sedentary lifestyle were statistically significant in both populations; PD was significant in the rural group, while diabetes was significant in the urban group. Odds ratios and confidence intervals for various risk factors were recorded. The odds of PD as a risk factor for CAD in the rural population were found to be 3.08 (95% CI [1.38–8.38]) and significant (P = .043). PD was not found to be a significant risk factor for CAD in urban populations.
Table 2.
Univariate logistic regression in the rural and urban areas.
| RURAL CAD | URBAN CAD | |||||
| Risk factor | Odds ratio | Confidence interval | P value | Odds ratio | Confidence interval | P value |
| Age | 3.38 | 1.14–10.05 | .028 | 7.04 | 1.67–29.63 | .008 |
| Sex | 3.57 | 1.52–8.38 | .003 | 3.05 | 1.41–6.59 | .005 |
| Tobacco use | 2.14 | 0.91–5.04 | .081 | 2.42 | 1.21–4.87 | .013 |
| Hypertension | 3.41 | 1.45–8.00 | .005 | 2.01 | 1.01–4.00 | .046 |
| Diabetes | 1.95 | 0.83–4.59 | .125 | 2.49 | 1.26–4.93 | .009 |
| Dyslipidemia | 2.38 | 1.01–5.60 | .048 | 4.26 | 2.07–8.76 | <.001 |
| Periodontal disease | 3.08 | 1.04–9.14 | .043 | 1.26 | 0.64–2.48 | .511 |
| Sedentary lifestyle | 3.40 | 1.38–8.38 | .008 | 0.84 | 0.41–1.71 | .063 |
| Education | 1.21 | 0.50–2.89 | .671 | 1.07 | 0.43–2.65 | .881 |
| BMI | 0.86 | 0.37–2.02 | .731 | 0.95 | 0.49–1.85 | .885 |
| Socio-economic status (BPL) | 0.67 | 0.27–1.64 | .379 | 1.60 | 0.36–7.19 | .538 |
Factors that are significant at the level of P ≤ .10 are in bold.
BMI= body mass index, BPL = below poverty line, CAD = coronary artery disease.
3.4. Multivariate regression for the relationship between the various risk factors and CAD
Table 3 presents the multivariate regression analysis of risk factors. All variables that were significant at the level of P ≤ .05, were selected. In the rural population, none of the factors showed significance at the level of P ≤ .05; however, there was a borderline significance for male sex and dyslipidemia (0.065 and 0.069). The odds ratio for PD as a risk factor for CAD was 1.54 (95% CI: 0.44–5.4) and non-significant (P = .503). In the urban population, age, male sex, and dyslipidemia were significant at the level of P ≤ .05.
Table 3.
Multivariate logistic regression in the rural and urban areas.
| CAD | |||
| Risk facto r | Odds ratio | Confidence interval | P value |
| RURAL | |||
| Sex-male | 3.07 | 0.93–10.10 | .065 |
| Dyslipidemia | 2.37 | 0.94–5.99 | .069 |
| URBAN | |||
| Age | 8.13 | 1.03–64.16 | .047 |
| Sex-male | 4.42 | 1.69–11.56 | .002 |
| Dyslipidemia | 4.38 | 2.02–9.46 | <.001 |
In the rural population, borderline significance was noted for male sex and dyslipidemia.
CAD = coronary artery disease.
3.5. Stratified analysis of covariates
Table 4 shows the stratified analysis of the risk factors. It was found that the area of living and socioeconomic status were determinants of the association between PD and CAD.
Table 4.
Stratified analysis demonstrating the covariates associated with periodontal disease and coronary artery disease.
| Association between PD and CAD | |||||||||
| N | PD risk factor | CAD | PD risk factor | CAD | |||||
| Risk factor | Yes | Yes | % | No | Yes | % | P value∗ | ||
| Area of living | Rural | 725 | 445 | 19 | 4.3 | 280 | 4 | 1.4 | .034 |
| Urban | 560 | 199 | 15 | 7.5 | 361 | 22 | 6.1 | .510 | |
| Socio-economic | No | 540 | 193 | 13 | 6.7 | 347 | 22 | 6.3 | .858 |
| class-BPL (Urban) | Yes | 20 | 6 | 2 | 33.3 | 14 | 0 | 0.0 | .023 |
All results were presented as numbers or percentages.
BPL = below poverty line, CAD = coronary artery disease, PD = periodontal disease.
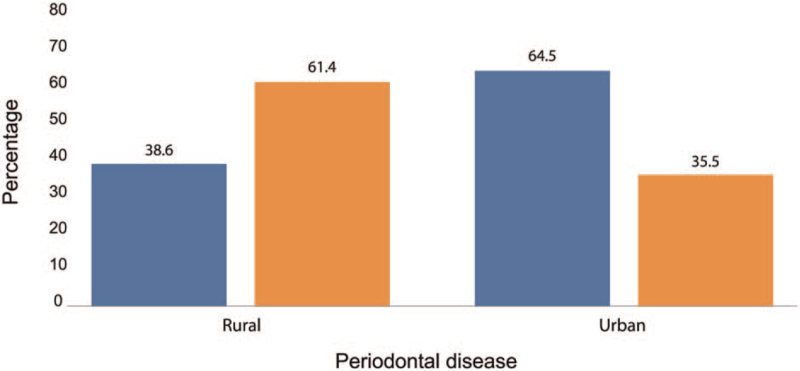
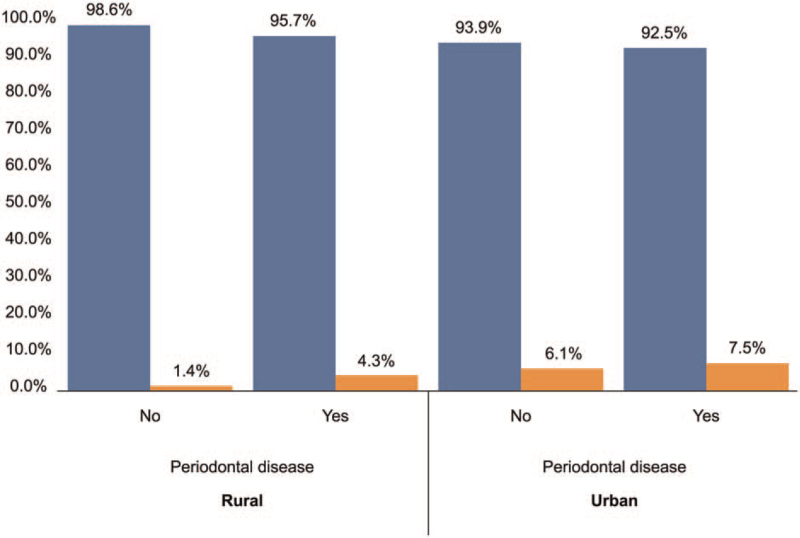
4. Discussion
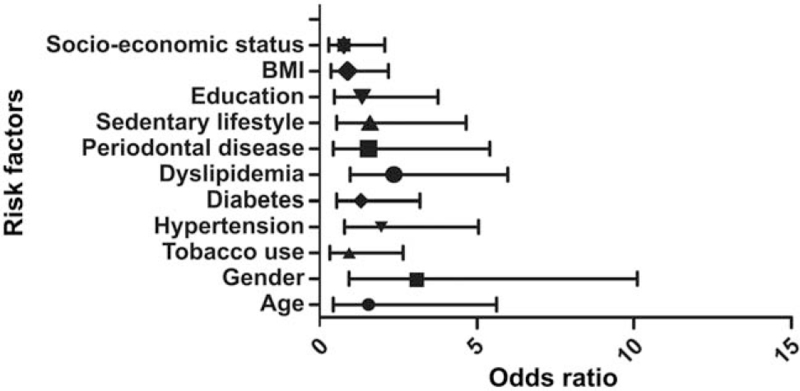
The prevalence of CAD among rural and urban participants (n = 1285) in the Thiruvananthapuram district of Kerala was 3.2% and 6.6%, respectively. PD was more frequent among rural (61.4%) than in the urban population (35.5%) (Fig. 1). The frequency of CAD was found to be greater in participants with PD than in those without PD in both rural and urban areas. The differences in CAD frequency between participants with and without PD were greater in rural areas than in urban areas (Fig. 2). The frequencies of CAD associated with PD in the rural and urban populations were 82.6% and 40.5%, respectively (Table 1). PD was not found to be a significant risk factor for CAD in the univariate regression analysis of urban populations (Table 2). The odds of PD as a risk factor of CAD in the rural population were found to be 3.08 (95% CI [1.38–8.38]) and significant (P = .043) in univariate regression analysis and 1.54 (95% CI: 0.44–5.4) and non-significant (P = .503) in the multivariate regression analysis. The odds ratios and confidence intervals for the various risk factors in the multivariate regression analysis are shown in Figure 3. Male sex and dyslipidemia were identified as shared risk factors between PD and CAD, which could have confounded the significant relationship between the latter (Table 3).
Figure 1.
Area-wise prevalence of PD. Percentage of PD cases in rural population = 61.4 and percentage of PD cases in urban population = 35.5. PD = periodontal disease.
Figure 2.
Graph demonstrating a comparison of coronary artery disease risk owing to periodontal disease in the rural and urban population. Percentage of CAD cases with PD in rural population = 1.4, percentage of CAD cases without PD in rural population = 4.3, percentage of CAD cases with PD in urban population = 7.5, and percentage of CAD cases without PD urban population = 6.1 The frequency of CAD was found to be greater in participants with PD than in those without PD in both rural and urban areas. The differences in CAD frequency between participants with and without PD were greater in rural areas than in urban areas. CAD = coronary artery disease, PD = periodontal disease.
Figure 3.
Forest plot demonstrating the multivariate regression analysis in the rural area using Enter method. The odds ratio and CI of various risk factors are outlined. The odds ratio for PD as a risk factor of CAD in multivariate regression analysis was 1.54 (95% CI: 0.44–5.4) and found to be non-significant (P = .503). CAD = coronary artery disease, CI confidence interval, PD = periodontal disease.
Periodontitis is a destructive inflammatory disease of the supporting tissues of the teeth and is caused by a group of specific microorganisms resulting in progressive destruction of the periodontal ligament and alveolar bone with periodontal pocket formation, gingival recession, or both.[30–32] The proposed causal or modulating role of periodontitis in CAD includes (a) locally produced inflammatory mediators in periodontitis, such as C-reactive protein, interleukins (IL-1β, IL-6, IL-8), and TNF-α, which can cause systemic effects.[33,34] (b) Periodontal pathogens that are translocated into systemic circulation induce systemic inflammation and contribute to atheroma formation and thrombogenesis.[35–37] (c) Systemic antibodies against periodontal pathogens can cross-react with antigens in cardiac tissues.[38,39] (d) Shared genetic factors suggest a mechanistic link between PD and CAD.[40,41] (e) Periodontal viruses such as Epstein–Barr virus and cytomegalovirus have been suggested as risk factors for CAD.[42,43] The pathophysiology and microbiology related to both these disease entities have been discussed in detail by Lockhart et al.[44]
Numerous studies have reported an association between PD and CAD, whereas other studies did not. DeStefano et al concluded that there was a 25% increased risk of CAD in patients with PD.[8] A study by Beck et al suggested that persons with radiographic evidence of PD were 0.5 to 2.8 times more likely to develop CAD.[9] Likewise, Genco et al concluded that the alveolar bone level was predictive of CAD in persons younger than 60 years of age.[10] Some studies have reported an independent or partial association,[11–20] whereas others reported a modest or no association.[21–23] Thus, the magnitude of risk varies and appears to be modest, and definitive evidence on the association between PD and CAD is not forthcoming.
The mechanisms underlying these epidemiological associations have been explained in genetic studies. Various novel genetic loci that explain the genetic architecture of CAD have been identified.[40] Shared associations between PD and CAD for various genetic risk variants located at CAD genetic risk loci (ANRIL, VAMP3, VAMP8, PLG, etc) have been reported. Recently, evidence has been provided for a shared association between PD and CAD for the genetic risk variant SNP rs1561198 located at the genetic loci VAMP8, SNP rs 10864294 located at VAMP3, and SNP rs 4252120 located at PLG. VAMP3 and VAMP8 are expressed in platelets and play an important role in thrombosis and wound healing. VAMP3 and VAMP8 proteins can be corrupted by specific pathogens, such as chlamydia, to establish within a host cell. The observed genetic association between CAD and PD is still speculative and is explained by the effects of these proteins on the functions of thrombosis and wound healing, as well as its role in bacterial invasion into the host cells, which has some relevance in the pathogenesis of CAD and PD.[41] Hence, epidemiological and genetic studies should complement each other to provide robust scientific evidence.
In a recent study by Bell et al, Mendelian randomization (MR) analysis using data from genome-wide association studies was used to evaluate the causal relationship between PD and CAD. Significant genetic risk variants identified to be associated with aggressive periodontitis and chronic periodontitis in previous genome-wide association studies were selected. The 5 selected single nucleotide polymorphisms (SNPs) were SNP rs 1537415 (genetic locus 9q34.3), SNP rs 4284742 (genetic locus 19q13.41), SNP rs 2738058 (genetic locus 8p23.1), SNP rs 16870060 (genetic locus 8q22.3), and SNP rs729876 (genetic locus 16p13.2). This study used a 2-sample MR approach, using summary data, where SNP exposure (PD) and SNP outcome (CAD) were obtained from different sources and analyzed using inverse-variance weighted meta-analysis. Estimates obtained from inverse-variance weighted MR did not reveal an association between PD and CAD. This study also examined the shared genetic contributions of periodontitis and all cardiovascular traits but found no significant evidence of genetic overlap. The authors concluded that the significant association presented in observational studies may represent confounding.[45]
In the present study, the odds of PD as a risk factor for CAD in the rural population were found to be 3.08 (95% CI [1.38–8.38]) and significant (P = .043) in univariate regression analysis. PD was not found to be an independent significant risk factor for CAD in multiple regression analysis after adjusting for age, sex, tobacco use, hypertension, sedentary lifestyle, and dyslipidemia. The significant association of PD found in univariate regression analysis might have resulted from its association with other confounding factors that were significantly related to CAD, such as male sex and dyslipidemia, regardless of their borderline significance. (Table 3). There is always a possibility that a significant relationship according to multivariate analysis can also be confounded by factors not included in this study. In the present study, male sex and dyslipidemia were identified as shared risk factors between PD and CAD.
In the urban population, PD was not found to be a significant risk factor for CAD. Age, male sex, and dyslipidemia were found to be significant according to the multivariate logistic regression model. A total of 72.9% of the urban population was >45 years, compared to 59.2% of the rural population (Table 1). Nonetheless, the prevalence of PD in urban participants aged >45 years was found to be 43%. This may be one of the reasons why PD is non-significant in urban populations. The role of effect modifiers has been speculated to be another reason for the non-significant results.
Further, the authors conducted a stratified analysis, to explore the covariates that were determinants of the association between PD and CAD (Table 4). The area of living was found to be a significant covariate. The rural participants with PD had a higher risk of CAD (P = .034) than their urban counterparts (P = .510). Stratified analysis also demonstrated an increased risk of CAD among the low socioeconomic class in the presence of PD (P = .023).
It is speculated that the higher prevalence of PD and associated CAD risk may be attributable to socioeconomic backwardness within the rural community. Most of the rural participants were fishermen from coastal areas. Socioeconomic status based on BPL/above poverty line categorization revealed a huge gap between rural and urban areas. Of the rural population, 39.3% belonged to the BPL category, compared to 3.6% in the urban area. A striking difference was also noted in the level of education between the 2 groups (Table 1).
The lower prevalence of PD in the urban area would imply a lower associated CAD risk, which could be due to better educational and socioeconomic standards, which in turn contribute to increased oral hygiene awareness, better health care, and dietary and lifestyle practices. These factors might play a role as effect modifiers to overcome the risk associated with PD. The authors presume that meticulous oral hygiene and periodontal therapy would reduce the bacterial load and infectivity of periodontal pathogens and the risk of bacteremia and CAD, even in the presence of periodontal attachment loss.
DeStefano et al suggested that PD may be a surrogate for lifestyle affecting personal hygiene and health care, which could explain the relationship between PD and CAD.[8] The present study also endorsed the finding that lifestyle and socioeconomic factors are putative determinants of their association. Modifiable risk factors such as PD and socioeconomic status, in comparison with traditional robust risk factors, could be prevented or improved by interventions at the personal or organizational level through the implementation of broad social and health policies.
The authors adopted stringent sample selection methods and diagnostic criteria to define CAD and PD. Eighty cases of probable CAD diagnosed based on RAQ were excluded from the present study for a conclusive diagnosis, to avoid systematic errors. A modification of the Ramfjord periodontal disease index was used for the periodontal charting of teeth. The use of this index obviated the necessity for radiographic evaluation of teeth and suited the requirements of a “field study,” in terms of logistics and convenience. Only “established periodontitis” cases that manifest a periodontal attachment loss ≥3 mm were diagnosed with PD. PDI scores of 4, 5, and 6, which exhibited periodontal attachment loss ≥3 mm, were diagnostic of PD.
Future epidemiological studies should identify and recruit novel environmental factors to understand the interrelationships between PD and CAD and focus on the role of effect modifiers that may have a protective role against PD colluding with CAD. A uniform, standard protocol to define both PD and CAD is inevitable, and exposure variables such as gingivitis, self-reported tooth loss, self-reported oral health status, edentulousness of unspecified etiology, serum IgG levels against periodontal pathogens, etc, have been reported hitherto in cohorts and meta-analyses, to define PD, a reason for demonstrating diversified results.
4.1. Limitation of the study
Unlike the traditional risk factors considered, PD and socioeconomic standards carry a modifiable risk, as it is possible to improve oral hygiene and modify lifestyle, eventually underrating or overrating the real risk. The differences arising from ill-defined sample selection and data collection protocol for both the exposure and outcome variables, viz. PD and CAD, could be a reason for the varied results, that is, independent, partial, or no association between PD and CAD, in different studies.
The present observational study could not establish an independent association of PD with CAD in both rural and urban communities and contrasts the independent positive association reported in various studies. Although PD was significant in the rural population in univariate regression analysis, the positive association could have been confounded by apparent or obscure sources and therefore underpins a role for shared risk factors for both diseases. Recent genetic studies have also pointed towards this direction.
5. Conclusion
In rural areas, male sex and dyslipidemia demonstrated borderline significance as risk factors for CAD. PD was not found to be an independent, significant risk factor after adjusting for age, sex, tobacco use, hypertension, sedentary lifestyle, and dyslipidemia. Male sex and dyslipidemia were identified as shared risk factors between PD and CAD, which could have confounded the significant association between the latter. In urban areas, age, male sex, and dyslipidemia demonstrated an independent association with CAD. The present study could not establish an independent association between PD and CAD in either rural or urban areas. Future epidemiological studies should identify and recruit novel environmental factors to understand the interrelationships between PD and CAD and focus on the role of effect modifiers that may have a protective role against PD colluding with CAD. (Dataset final for recoding.xlsx, Supplemental Digital Content, Dataset for journal.xlsx, Supplemental Digital Content, Logistic regression areawise.doc, Supplemental Digital Content, Statistical analyses for journal.doc, Supplemental Digital Content).
Acknowledgments
We thank the office bearers and staff members of CSI Kerala, Dr N.O. Varghese, former Principal, Government Dental College, Thiruvananthapuram, Kerala, India, and staff members and residents for their support in conducting this study.
Author contributions
Conceptualization: Chacko Pearl Dain, Sanjay Ganapathi, Zachariah Geevar, Sivadasanpillai Harikrishnan.
Data curation: Chacko Pearl Dain, Sanjay Ganapathi, Zachariah Geevar, Sivadasanpillai Harikrishnan.
Formal analysis: Chacko Pearl Dain, Sanjay Ganapathi, Sivadasanpillai Harikrishnan, Jayanthi Viswanathan Ammu, Manas Chacko.
Funding acquisition: Zachariah Geevar.
Investigation: Chacko Pearl Dain, Sanjay Ganapathi, Zachariah Geevar, Sivadasanpillai Harikrishnan.
Methodology: Chacko Pearl Dain, Sanjay Ganapathi, Zachariah Geevar, Sivadasanpillai Harikrishnan.
Project administration: Sanjay Ganapathi, Zachariah Geevar, Sivadasanpillai Harikrishnan.
Resources: Sanjay Ganapathi, Zachariah Geevar, Sivadasanpillai Harikrishnan.
Software: Jayanthi Viswanathan Ammu, Manas Chacko.
Supervision: Sanjay Ganapathi, Zachariah Geevar, Sivadasanpillai Harikrishnan.
Validation: Chacko Pearl Dain, Jayanthi Viswanathan Ammu, Manas Chacko.
Visualization: Chacko Pearl Dain, Sivadasanpillai Harikrishnan, Jayanthi Viswanathan Ammu, Manas Chacko.
Writing – original draft: Chacko Pearl Dain.
Writing – review & editing: Chacko Pearl Dain, Sanjay Ganapathi, Zachariah Geevar, Sivadasanpillai Harikrishnan.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, BPL = below poverty line, CAD = coronary artery disease, CI = confidence interval, ECG = electrocardiogram, MR = Mendelian randomization, PD = periodontal disease, PDI = periodontal disease index, RAQ = Rose angina questionnaire, SNP = single nucleotide polymorphism.
How to cite this article: Dain CP, Ganapathi S, Geevar Z, Harikrishnan S, Ammu JV, Chacko M. The traditional and modifiable risk factors of coronary artery disease – a community-based cross-sectional study among 2 populations. Medicine. 2021;100:39(e27350).
The study was approved by the Ethics Committee of the Cardiology Society of India (Kerala Chapter). Informed written consent was obtained from all the participants.
The study was funded by the Cardiology Society of India (Kerala Chapter). The funding source had no role in the design, data collection, analysis, interpretation, or writing of the article.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
References
- [1].Zachariah G, Harikrishnan S, Krishnan MN, et al. Prevalence of coronary artery disease and coronary risk factors in Kerala, South India: a population survey-design and methods. Indian Heart J 2013;65:243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Krishnan MN, Zachariah G, Venugopal K, et al. Prevalence of coronary artery disease and its risk factors in Kerala, South India: a community-based cross-sectional study. BMC Cardiovasc Disord 2016;16:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gupta R, Joshi P, Mohan V, Reddy KS, Yusuf S. Epidemiology and causation of coronary heart disease and stroke in India. Heart 2008;94:16–26. [DOI] [PubMed] [Google Scholar]
- [4].Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52. [DOI] [PubMed] [Google Scholar]
- [5].Thankappan KR, Shah B, Mathur P, et al. Risk factor profile for chronic non-communicable diseases: results of a community-based study in Kerala, India. Indian J Med Res 2010;131:53–63. [PubMed] [Google Scholar]
- [6].Sivasankaran S, Thankappan KR. Prevention of non-communicable diseases requires a life course approach: a case study from Kerala. Indian J Med Res 2013;137:874–7. [PMC free article] [PubMed] [Google Scholar]
- [7].Kannel WB, Dawbter TR, Kagan A, Revotskie N, Stokes J, 3rd. Factors of risk in the development of coronary heart disease – six year follow-up experience. The Framingham Study. Ann Intern Med 1961;55:33–50. [DOI] [PubMed] [Google Scholar]
- [8].DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. BMJ 1993;306:688–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol 1996;67:1123–37. [DOI] [PubMed] [Google Scholar]
- [10].Genco RJ, Chadda S, Grossi S, et al. Periodontal disease is a predictor of cardiovascular disease in a Native American population. J Dent Res 1997;76:3158. [Google Scholar]
- [11].Mattila KJ, Valtonen V, Nieminen M, Huttunen JK. Dental infections and the risk of new coronary events: prospective study of patients with documented coronary artery disease. Clin Infect Dis 1995;20:588–90. [DOI] [PubMed] [Google Scholar]
- [12].Blaziot A, Vergnes JN, Nuwwareh S, Amar J, Sixou M. Periodontal diseases and cardiovascular events: meta-analysis of observational studies. Int Dent J 2009;59:197–209. [PubMed] [Google Scholar]
- [13].Arbes SJ, Jr, Slade GD, Beck JD. Association between extent of periodontal attachment loss and self-reported history of heart attack: an analysis of NHANES III data. J Dent Res 1999;78:1777–82. [DOI] [PubMed] [Google Scholar]
- [14].Morrison HI, Ellison LF. Periodontal disease and risk of fatal coronary heart and cerebrovascular diseases. J Cardiovasc Risk 1996;6:07–11. [DOI] [PubMed] [Google Scholar]
- [15].Janket SJ, Baird AE, Chuang SK, Jones JA. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;95:559–69. [DOI] [PubMed] [Google Scholar]
- [16].Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease and stroke: a systematic review. Ann Periodontol 2003;8:38–53. [DOI] [PubMed] [Google Scholar]
- [17].Khader YS, Albashaireh ZS, Alonari MA. Periodontal diseases and the risk of coronary heart and cerebrovascular diseases: a meta-analysis. J Periodontol 2004;75:1046–53. [DOI] [PubMed] [Google Scholar]
- [18].Dhadse P, Gattani D, Mishra R. The link between periodontal disease and cardiovascular disease: how far we have come in last two decades? J Indian Soc Periodontol 2010;14:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wu T, Trevisan M, Genco RJ, Dorn JP, Falkner KL, Sempos CT. Periodontal disease and risk of cerebrovascular disease: the first national health and nutrition examination survey and its follow-up study. Arch Intern Med 2000;160:2749–55. [DOI] [PubMed] [Google Scholar]
- [20].Briggs JE, McKeown PP, Crawford VL, et al. Angiographically confirmed coronary heart disease and periodontal disease in middle-aged males. J Periodontol 2006;77:95–102. [DOI] [PubMed] [Google Scholar]
- [21].Howell TH, Ridker PM, Ajani UA, Hennekens CH, Christen WG. Periodontal disease and risk of subsequent cardiovascular disease in US male physicians. J Am Coll Cardiol 2001;37:445–50. [DOI] [PubMed] [Google Scholar]
- [22].Joshipura KJ, Rimm EB, Douglass CW, Trichopoulos D, Ascherio A, Willett WC. Poor oral health and coronary heart disease. J Dent Res 1996;75:1631–6. [DOI] [PubMed] [Google Scholar]
- [23].Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Periodontal disease and coronary heart disease risk. JAMA 2000;284:1406–10. [DOI] [PubMed] [Google Scholar]
- [24]. World Health Organization, WHO STEPS Manual, WHO STEPS Surveillance, Part 2: Planning and Set up; Section 2: Preparing the sample; 2008: 2-12-24-25. [Google Scholar]
- [25].Peter S. Peter S. Indices in dental epidemiology. Essentials of Public Health Dentistry 6th ed.New Delhi: Arya Medi Publishing House Pvt. Ltd; 2017. 495–500. [Google Scholar]
- [26].World Health Organization. WHO STEPS Part 3, Training and practical Guides. Section 3: Guide to physical measurements (Step 2); 2008: 3-3-1–3-3-14. [Google Scholar]
- [27].Springer, Ronald JP, Richards SC, Zhu-Ming Z. The Minnesota Code Manual of Electrocardiographic Findings. Second edition2010. [Google Scholar]
- [28].Rose GA. The diagnosis of ischemic heart pain and intermittent claudication in field surveys. Bull World Health Organ 1962;27:645–58. [PMC free article] [PubMed] [Google Scholar]
- [29].Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ 1968;56:01–188. [PubMed] [Google Scholar]
- [30].Aiuto FD. Periodontitis and atherogenesis: causal association or simple coincidence. J Clin Periodontol 2004;31:402–11. [DOI] [PubMed] [Google Scholar]
- [31].Sanz M, D’Aiuto F, Deanfield J, Fernandez-Aviles F. European workshop in periodontal health and cardiovascular disease-scientific evidence on the association between periodontal and cardiovascular diseases: a review of the literature. Eur Heart J Suppl 2010;12:B3–12. [Google Scholar]
- [32].Friedewald VE, Kornman KS, Beck JD, et al. The American Journal of Cardiology and Journal of Periodontology Editors’ Consensus: periodontitis and atherosclerotic cardiovascular disease. Am J Cardiol 2009;104:59–68. [DOI] [PubMed] [Google Scholar]
- [33].Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Clin Periodontol 2013;40:S51–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gulati M, Anand V, Jain N, et al. Essentials of periodontal medicine in preventive medicine. Int J Prev Med 2013;4:988–94. [PMC free article] [PubMed] [Google Scholar]
- [35].Herrera D, Molina A, Buhlin K, Klinge B. Periodontal diseases and association with atherosclerotic disease. Periodontology 2000 2020;83:66–89. [DOI] [PubMed] [Google Scholar]
- [36].Reyes L, Herrera D, Kozarov E, Roldan S, Progulske-Fox A. Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology. J Clin Periodontol 2013;40:S30–50. [DOI] [PubMed] [Google Scholar]
- [37].Chandy S, Joseph K, Sankaranarayanan A, et al. Evaluation of C-reactive protein and fibrinogen in patients with chronic and aggressive periodontitis: a clinico-biochemical study. J Clin Diagn Res 2017;11:zc41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].de Boer SP, Cheng JM, Range H, et al. Antibodies to periodontal pathogens are associated with coronary plaque remodeling but not with vulnerability or burden. Atherosclerosis 2014;237:84–91. [DOI] [PubMed] [Google Scholar]
- [39].Teles R, Wang CY. Mechanisms involved in the association between periodontal diseases and cardiovascular disease. Oral Dis 2011;17:450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res 2018;122:433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Munz M, Richter GM, Loos BG, et al. Meta-analysis of genome-wide association studies of aggressive and chronic periodontitis identifies two novel risk loci. Eur J Hum Genet 2019;27:102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ilango P, Mahendra J, Mahendra L, et al. Evidence linking the role of periodontal viruses in coronary artery disease with and without periodontitis. J Periodontol 2021;92:113–22. [DOI] [PubMed] [Google Scholar]
- [43].Zhu W, Liu S. The role of human cytomegalovirus in atherosclerosis: a systematic review. Acta Biochim Biophys Sin 2020;52:339–53. [DOI] [PubMed] [Google Scholar]
- [44].Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation 2012;125:2520–44. [DOI] [PubMed] [Google Scholar]
- [45].Bell S, Gibson JT, Harshfield EL, Markus HS. Is periodontitis a risk factor for ischaemic stroke, coronary artery disease and subclinical atherosclerosis? A Mendelian randomization study. Atherosclerosis 2020;313:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.