Abstract
The presence of bridging fibrosis predicts survival of primary biliary cholangitis (PBC). This study aimed to compare serum parameters for the estimation of liver fibrosis and prediction of clinical outcomes in PBC.
Out of 392 patients with PBC, 102 who underwent liver biopsy and in whom fibrosis indices, platelet count, hyaluronic acid, type IV collagen 7 second domain, procollagen type III amino-terminal peptide, tissue inhibitor of metalloproteinases 1, Mac-2 binding protein glycosylation isomer, N-terminal type III collagen propeptide levels; fibrosis index based on 4 factors, aspartate aminotransferase-to-platelet ratio index, and enhanced liver fibrosis (ELF) score were determined, were included. The correlation of histological stages based on both Scheuer and Nakanuma classifications with fibrosis indices was investigated. The Nakanuma system comprises grading for liver fibrosis and bile duct loss. Diagnostic performances of 10 fibrosis indices were evaluated to identify patients with poor prognosis. Moreover, correlations of those with PBC clinical manifestation and survival were also investigated.
Enhances liver fibrosis (ELF) score had the highest correlation coefficient for liver fibrosis evaluated according to either the Scheuer or Nakanuma classification among 10 serum fibrosis indices. It also had the highest diagnostic performance in estimating Scheuer stage III and Nakanuma fibrosis score 2, both of which represent portal-bridging fibrosis. Patients with an ELF score of ≥10.0 had shorter survival and presented more frequently clinical complications than those with an ELF score of <10.0.
ELF score determines the severity of liver fibrosis and predicts the occurrence of complications and survival in patients with PBC.
Keywords: biomarkers, complications, liver fibrosis, primary biliary cholangitis, prognosis
1. Introduction
Primary biliary cholangitis (PBC) is histologically characterized by chronic non-suppurative destructive cholangitis and intrahepatic bile duct destruction, leading to an unfavorable biochemical response and progress to end-stage liver disease that may ultimately require liver transplantation.[1,2] Liver fibrosis is a major independent prognostic factor in patients with chronic liver disease (CLD), including PBC[3] and nonalcoholic steatohepatitis.[4] Repeat liver biopsy helps assess liver fibrosis progression or regression over a period of time and evaluate the effects of drug treatment. Despite being an invasive procedure with possible adverse events and limitations, liver biopsy remains the most reliable method to diagnose CLD.[5] Surrogate disease progression markers are required for multiple reasons, such as providing pathological and prognostic information to patients and serving as end-points in clinical trials.[6] At present, several putative biomarkers have been used for assessing the development of liver fibrosis in PBC.[7] Single biomarkers, such as increased serum levels of hyaluronic acid,[8] laminin,[9,10] type IV collagen 7 s domain (7S collagen),[10] and procollagen type III amino-terminal peptide (PIIINP),[9,11] are notably higher in PBC at an advanced stage than those at an early stage.[12]Wisteria floribunda agglutinin (WFA)-positive Mac-2 bp was reported as a liver fibrosis biomarker for PBC[13,14] and verified in another study.[15] Studies also report that liver stiffness is a good indicator of liver fibrosis[8] and clinical outcomes associated with PBC.[16] Nonetheless, these biomarkers inaccurately reflect the fibrosis progression in PBC owing to the considerable overlapping of data in different histological stages. Liver-related death involves mortality caused by hepatic events, including hepatocellular carcinoma, liver failure, and gastrointestinal bleeding. The enhanced liver fibrosis (ELF) score, which is based on the combination of serum HA, metalloproteinase 1 (TIMP-1), and PIIINP, can be used for the prediction of advanced fibrosis in patients with PBC.[17] On the basis of diagnostic accuracy and cost-effectiveness among novel diagnostic tests and biomarkers, ELF was ranked at highest at a cost-effectiveness threshold value of £20,000 per quality-adjusted life year gained. It is linearly related with Ishak fibrosis stages and predicts the likelihood of clinical progression associated with PBC.[18] The recently developed Nakanuma classification, a new staging and grading system for PBC,[19] is recommended as the Japanese version of clinical practice guidelines for PBC to individually evaluate damage and the loss of small intrahepatic bile ducts, fibrosis, and cholestasis.[20] Therefore, we aimed to compare the performance of 10 liver fibrosis indices in predicting liver fibrosis progression and related clinical outcomes using histological classifications devised by Nakanuma[19] and Scheuer et al[21] for evaluating the severity of intrahepatic bile duct injury and hepatic fibrosis in patients with PBC.
2. Methods
2.1. Patient population
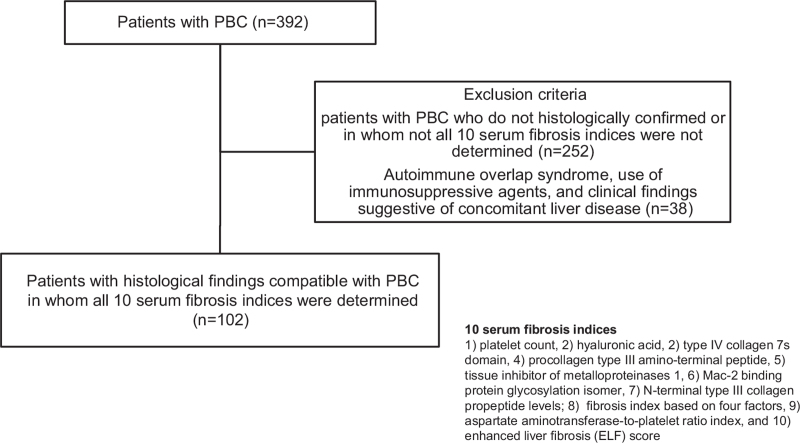
A retrospective observation study that enrolled a single-center cohort comprising 392 patients with PBC was conducted between January 2000 and December 2019 at Nara Medical University Hospital. In the whole cohort (n = 392), platelet (PLT) count, serum levels of HA, 7S collagen, PIIINP, TIMP-1, Mac-2 binding protein glycosylation isomer (M2BPGi), N-terminal type III collagen propeptide (Pro-C3), fibrosis index based on 4 factors (FIB-4 index), aspartate aminotransferase-to-platelet ratio index (APRI), and enhances liver fibrosis (ELF) scores were determined in 102 patients (Fig. 1). Patients with PBC without histological confirmation or those without all 10 serum fibrosis indices were not included (n = 252). Patients were diagnosed based on the Japanese version of the clinical practice guidelines for PBC, which was developed in 2012 and revised by the Intractable Hepatobiliary Disease Study Group in 2017, with the support of the Ministry of Health, Labor, and Welfare of Japan.[20] Patients who met the following 3 criteria were included in the study:
Figure 1.
Flow chart of the study design. A retrospective review of medical records was undertaken for 392 patients with primary biliary cholangitis (PBC). In total, 227 patients with PBC who were not histologically confirmed or in whom not all 10 serum fibrosis indices, such as platelet (PLT) count, serum levels of HA, type IV collagen 7 second domain (7S collagen), procollagen type III amino-terminal peptide (PIIINP), metalloproteinase 1 (TIMP-1), Mac-2 binding protein glycosylation isomer (M2BPGi), N-terminal type III collagen propeptide (Pro-C3), fibrosis index based on 4 factors (FIB-4 index), aspartate aminotransferase-to-platelet ratio index (APRI), and enhanced liver fibrosis (ELF) scores, were not determined were excluded. The inclusion criteria were met by 102 out of 392 patients.
-
1.
positive for anti-mitochondrial antibody (AMA)/AMA-M2,
-
2.
elevation of serum biliary enzyme levels for >6 months, and
-
3.
typical histopathological feature of PBC observed on liver histology.
All patients were treatment naive when they underwent percutaneous liver biopsy. A total of 88/102 (86.3%) patients were treated with ursodeoxycholic acid (UDCA) 600 mg/day and the remaining 14/102 (13.7%) were treated with UDCA 900 mg/day when we did not obtain an optimal biochemical response to UDCA at a dose of 600 mg/day for 1 year. Liver-related death is defined as mortality related to hepatic events, including hepatocellular carcinoma, liver failure, and gastrointestinal bleeding. Exclusion criteria were autoimmune overlap syndrome,[22] use of immunosuppressive agents, and clinical findings suggestive of concomitant liver disease (i.e., hepatitis B virus and hepatitis C virus infection, and alcoholic liver disease) (n = 38). This study was conducted in accordance with the standards of the Declaration of Helsinki and an opt-out method was used to recruit patients for this study. The Ethics Committee of Nara Medical University Hospital approved this study (approval no. O11–118).
2.2. Clinical characteristics of patients with primarily biliary cholangitis
Gender, age, occurrence of clinical manifestations, survival, histological stage, observation period, biochemical tests, and fibrosis markers were recorded at baseline. Consequently, all patients included in the study, serologically and histologically diagnosed with PBC. Symptoms and complications of PBC include pruritus, jaundice, ascites, esophageal varices, and hepatocellular carcinoma.[20] Additionally, PLT count, serum levels of HA, 7S collagen, PIIINP, TIMP-1, M2BPGi, Pro-C3, and FIB-4 index, APRI, and ELF score were used as noninvasive biomarkers of liver fibrosis. The following formulas were used: FIB-4 index = (age × AST) / [(PLT count) × (ALT) 1/2]; APRI = [(sample AST / reference AST) × 100] / PLT count; and ELF score = 2.278 + [0.851 ln (HA) + 0.751 ln (PIIINP) + 0.394 ln (TIMP-1)]. HA, TIMP-1, and PIIINP levels were determined using chemiluminometric immunoassays conducted on the ADVIA Centaur XP Immunoassay System (Siemens Healthineers).[23] Serum 7S collagen was measured using radioimmunoassay kits (7S-RIA; Nippon DPC Corporation).[24] The Wisteria floribunda agglutinin (WFA)-positive Mac-2 bp assay was performed using an automated chemiluminescence enzyme immunoassay analyzer (HISCL-5000; Sysmex Corporation).[25] Pro-C3 level was measured using the UniQ PIIINP RIA assay (Orion Diagnostica Ltd.).[26]
2.3. Histological evaluation of liver tissues according to Scheuer and Nakanuma classifications
Liver specimens were obtained from all patients using an 18-G needle under ultrasound guidance. Tissue sections were stained with hematoxylin and eosin (H&E) and Mallory's azan stain, followed by assessment according to Scheuer[21] and Nakanuma[27] classifications. Briefly, Scheuer classification was used to classify patients into 4 stages. Subsequently, Nakanuma classification was used to reassess staging for bile duct loss (BDL) and fibrosis. BDL and fibrosis were individually scored and then subsequently summed up together (range for both scores, 0–3). A final score of 0 was classified as stage 1 (no or minimal disease progression), 1 or 2 as stage 2 (mild disease progression), 3 or 4 as stage 3 (moderate disease progression), and 5 or 6 as stage 4 (advanced disease progression). All liver specimens were confirmed with H&E staining to contain at least 15 portal tracts, including 11 full-portal triads composed of hepatic artery and portal vein branches and the bile duct.[28] Prof. Dr. Chiho Obayashi and Dr. Kohei Morita (Department of Diagnostic Pathology, Nara Medical University) independently reviewed all participants to validate histological characteristics of PBC. Interpretation discrepancies were solved by consensus.
2.4. Statistical analysis
All statistical analyzes were conducted using the GraphPad Prism version 9.0 for Windows (GraphPad Software, La Jolla, CA, USA). All serum fibrosis indices except PLT were abnormally distributed. Mann–Whitney test was used for non-normally distributed variables and Student t-test was used for normally distributed variables. Areas under the receiver operating characteristic (ROC) curves (AUCs) were used to evaluate the diagnostic value of fibrosis indices in order to identify patients with Scheuer stage III and those with Nakanuma fibrosis score of 2. Sensitivities, specificities, positive-predictive values (PPVs), negative-predictive values (NPVs), diagnostic accuracy (AC) with confidence intervals (CI 95%), and cut-off values of fibrosis indices were estimated from the ROC curves. The DeLongs nonparametric test was used to compare 2 AUC values of different fibrosis indices.[29] Complications and survival analyses were performed using the Kaplan–Meier method. The log-rank test was used to compare the incidence of complications and survival distributions between 2 or more groups. All tests were two-tailed, and a probability (P) value of <.05 was considered statistically significant.[30] The level of significance of Spearman's rank test was determined as P < .05 and r > 0.2, respectively.
3. Results
3.1. Baseline clinical characteristics of patients with different fibrosis stages
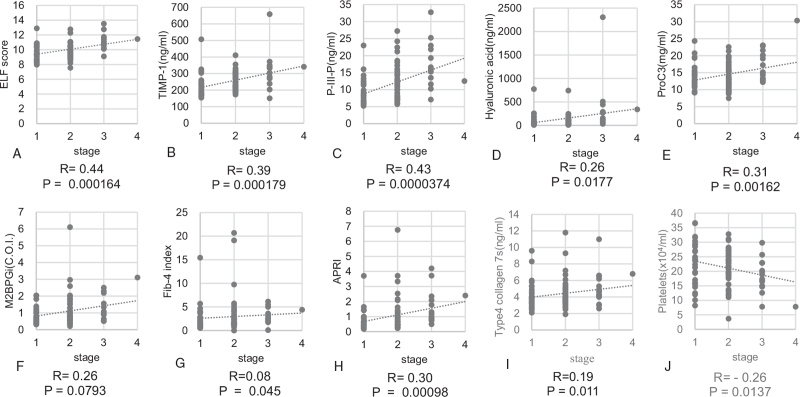
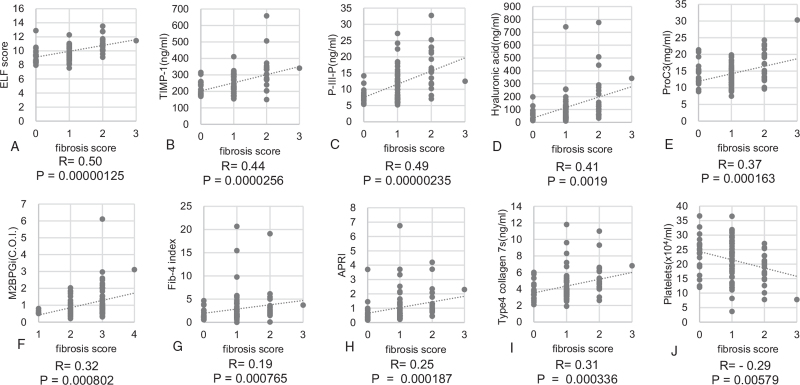
A total of 102 patients with histological findings compatible with PBC were investigated. Summary of demographics and baseline characteristics for 102 patients are shown in Table 1. Among them, 89 (87.3%) were women, and the median age was 61.0 ± 10.8 years at the time of histological diagnosis. Of 102 patients, 23 (22.5%) developed clinical complications, such as pruritus (11, 10.8%), ascites (6, 5.9%), esophageal varices (3, 2.9%), hepatocellular carcinoma (HCC) (2, 2.0%), and jaundice (1, 1.0%), during the study period. Six patients died of liver-related events, 2 and 4 of them had HCC and liver failure, respectively. The number of patients stratified as stage I, II, III, and IV according to the Scheuer classification was 33 (32.3%), 51 (50.0%), 17 (16.7%), and 1 (1.0%), respectively. The distribution of patients in stage 1, 2, 3, and 4 according to the Nakanuma classification were 6 (5.9%), 38 (37.3%), 53 (51.9%), and 5 (4.9%), respectively. The number of patients with fibrosis scores of 0, 1, 2, and 3 was 19 (18.6%), 60 (58.8%), 22 (21.6%), and 1 (1.0%), respectively. The distribution of BDL scores 0, 1, 2, and 3 was 9 (8.8%), 36 (35.3%), 39 (38.2%), and 18 (17.6%), respectively. The mean follow-up period was 4.5 ± 3.0 years. Mean values and standard deviation of the ELF score, TIMP-1 level, PIIINP level, HA level, Pro-C3 level, M2BPGi level, FIB-4 index, APRI, 7S collagen level, and PLT count were 10.0 ± 1.2, 253.5 ± 78.2, 11.8 ± 5.7, 116.4 ± 143.6, 14.3 ± 4.1, 1.07 ± 0.85, 2.93 ± 3.3, 1.48 ± 3.2, 4.4 ± 1.8, and 21.5 ± 6.7, respectively. Spearman's rank correlation coefficients between the Scheuer stage and ELF score, TIMP-1 level, PIIINP level, HA level, Pro-C3 level, M2BPGi level, FIB-4 index, APRI, 7S collagen level, and PLT count were 0.44, 0.39, 0.43, 0.26, 0.31, 0.26, 0.07, 0.27, 0.19, and −0.26, respectively (Fig. 2A–J). Those between the Nakanuma fibrosis score and ELF score, TIMP-1 level, PIIINP level, HA level, Pro-C3 level, M2BPGi level, FIB-4 index, APRI, 7S collagen level, and PLT count were 0.50, 0.44, 0.49, 0.41, 0.37, 0.31, 0.19, 0.22, 0.31, and − 0.29, respectively (Fig. 3A–J). All fibrosis biomarkers, except for the M2BPGi level, 7S collagen level, and Fib-4 index, were significantly correlated with the Scheuer stage, whereas all of them, except the Fib-4 index, correlated with the Nakanuma fibrosis score. ELF score had the highest correlation coefficient for liver fibrosis evaluated according to either the Scheuer or Nakanuma classification among 10 serum fibrosis indices.
Table 1.
Baseline characteristics of patients with primary biliary cholangitis.
| Variable | PBC patients (n = 102) |
| Gender (M/F) | 13/89 |
| Age (yr old)∗ | 61.0 ± 10.8 |
| Survival (Alive/dead) | 96/6 |
| Scheuer stage (I/II/III/IV) | 33/51/17/1 |
| Nakanuma stage (1/2/3/4) | 6/38/53/5 |
| Fibrosis score (0/1/2/3) | 19/60/22/1 |
| Bile duct loss score (0/1/2/3) | 9/36/39/18 |
| Platelet, (x109/l)∗ | 21.5 ± 4.8 |
| Albumin, (g/dl)∗ | 4.2 ± 0.59 |
| Aspartate transaminase, (IU/l)∗ | 71.5 ± 106.1 |
| Alanine aminotransferase, (IU/l)∗ | 68.4 ± 85.4 |
| Alkaline Phosphatase, (IU/l)∗ | 515.7 ± 329.5 |
| γ-glutamyltransferase, (IU/l)∗ | 222.3 ± 239.3 |
| Total bilirubin, (mg/dl)∗ | 0.92 ± 0.55 |
| Hyaluronic acid (ng/mL)∗ | 116.4 ± 143.6 |
| PIIINP (ng/mL)∗ | 11.8 ± 5.7 |
| TIMP-1 (ng/mL)∗ | 253.5 ± 78.2 |
| Type 4 collagen 7S (ng/mL)∗ | 4.4 ± 1.8 |
| M2BPGi (COI)∗ | 1.07 ± 0.85 |
| ProC3 (ng/ml)∗ | 14.3 ± 4.1 |
| FIB-4 index ∗ | 2.93 ± 3.3 |
| APRI∗ | 1.48 ± 3.2 |
| ELF score∗ | 10.0 ± 1.2 |
4C7S = 7S domain of type 4 collagen, APRI = the aspartate aminotransferase-to-platelet ratio index, ELF score = enhances liver fibrosis score, Fib-4 = the fibrosis index based on four factors, M2BPGi = Mac-2-binding protein glycosylation isomer, PBC = primarily biliary cholangitis, Pro-C3 = N-terminal type III collagen propeptide.
Median ± standard error of mean.
Figure 2.
Correlation among 10 liver fibrosis biomarkers and Scheuer stage in patients with primarily biliary cholangitis. (A) ELF score, (B) TIMP-1, (C) PIIINP, (D) Hyaluronic acid (E) Pro-C3 (F) M2BPGi, (G) FIB-4 index, (H) APRI, (I) 7S collagen, and (J) Platelet count. 7S collagen = type IV collagen 7 s domain, APRI = aspartate aminotransferase-to-platelet ratio index, ELF score = enhanced liver fibrosis score, FIB-4 index = fibrosis index based on four factors, M2BPGi = Mac-2 binding protein glycosylation isomer, PIIINP = procollagen type III amino-terminal peptide, Pro-C3 = N-terminal type III collagen propeptide, TIMP-1 = tissue inhibitor of metalloproteinases 1.
Figure 3.
Correlation among 10 liver fibrosis biomarkers and Nakanuma fibrosis score in patients with primarily biliary cholangitis. (A) ELF score, (B) TIMP-1, (C) PIIINP, (D) Hyaluronic acid (E) Pro-C3 (F) M2BPGi, (G) FIB-4 index, (H) APRI, (I) 7S collagen, and (J) Platelet count. 7S collagen = type IV collagen 7 s domain, APRI = aspartate aminotransferase-to-platelet ratio index, ELF score = enhanced liver fibrosis score, FIB-4 index = fibrosis index based on 4 factors, M2BPGi = Mac-2 binding protein glycosylation isomer, PIIINP = procollagen type III amino-terminal peptide, Pro-C3 = N-terminal type III collagen propeptide, TIMP-1 = tissue inhibitor of metalloproteinases 1.
3.2. Diagnostic performances of serum fibrosis biomarkers to identify significant liver fibrosis in patients with primarily biliary cholangitis
Nakanuma classification, a new histological staging and grading system score, is the most effective scoring system to evaluate all fibrosis degrees in PBC. The long-term prognosis of patients with PBC was previously found to be predicted only in Scheuer stage III and Nakanuma fibrosis score 2, both of which represent portal-bridging fibrosis.[31] The diagnostic sensitivity; specificity; PPV, NPV, and ELF score accuracy; TIMP-1, PIIINP, HA, Pro-C3, and M2BPGi levels; FIB-4 index; APRI; 7S collagen level; and PLT count for the differentiation of Scheuer stage ≥ III and Nakanuma fibrosis score of ≥2 in patients with PBC are shown in Tables 2 and 3, respectively. These findings indicated that the ELF score may predict poor prognosis in patients with PBC. The diagnostic accuracy of ELF score was compared to that of other fibrosis markers, including TIMP-1 level, PIIINP level, HA level, Pro-C3 level, M2BPGi level, FIB-4 index, APRI, 7S collagen level, and PLT count. Significant differences were observed in AUCs to identify Scheuer stage III between the ELF score and M2BPGi level (P = .046) and Nakanuma fibrosis score of 2 between the ELF score and PLT count, HA level, M2BPGi level, and FIB-4 index (P = .013, P = .016, P = .031, and P = .027, respectively).
Table 2.
Diagnostic accuracy of serum fibrosis markers for patients with PBC in Scheuer stage III.
| Variable | Cut-off | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) | P |
| ELF score | 10.0 | 92.3 (0.669–0.987) | 66.7 (0.583–0.810) | 0.81 (0.67–0.92) | 45.9 (0.295–0.631) | 96 (0.863–0.995) | 74.7 (0.643–0.834) | |
| TIMP-1 | 270 | 76.9 (0.462–0.950) | 72.2 (0.604–0.821) | 0.75 (0.59–0.92) | 33.3 (0.173–0.528) | 94.5 (0.849–0.989) | 72.9 (0.622–0.820) | .538 |
| P-III-P | 13.5 | 76.9 (0.462–0.950) | 74.6 (0.629–0.842) | 0.80 (0.69–0.94) | 35.7 (0.186–0.559) | 94.6 (0.851–0.989) | 75.0 (0.644–0.838) | .94 |
| HA | 125 | 53.3 (0.266–0.787) | 76.6 (0.656–0.855) | 0.67 (0.50–0.83) | 30.8 (0.143–0.518) | 89.4 (0.794–0.956) | 72.8 (0.626–0.816) | .059 |
| Pro-C3 | 12.5 | 94.4 (0.727–0.999) | 38.1 (0.277–0.493) | 0.74 (0.62–0.86) | 24.6 (0.151–0.365) | 97.0 (0.842 – 0.999) | 48.0 (0.380–0.582) | .821 |
| M2BPGi | 0.9 | 66.7 (0.349–0.901) | 61.4 (0.490–0.728) | 0.63 (0.45–0.81) | 22.9 (0.104–0.401) | 91.5 (0.796–0.976) | 62.2 (0.508–0.727) | .046 |
| Fib-4 index | 2.16 | 69.2 (0.386–0.909) | 57.9 (0.460–691) | 0.64 (0.48–0.80) | 22.0 (0.106–0.376) | 91.7 (0.800–0.977) | 59.6 (0.486–0.698) | .156 |
| APRI | 0.73 | 84.6 (0.546–0.981) | 59.2 (0.473–0.704) | 0.74 (0.62–0.85) | 26.2 (0.139–0.420) | 95.7 (0.855–0.995) | 62.9 (0.525–0.729) | .859 |
| 7S collagen | 4.1 | 73.3 (0.449–0.922) | 59.2 (0.473–0.704) | 0.65 (0.49–0.81) | 26.2 (0.139–0.420) | 91.8 (0.804–0.977) | 61.5 (0.508–0.716) | .647 |
| Platelet | 22.9 | 71.4 (0.419–0.916) | 51.9 (0.403–0.635) | 0.67 (0.53–0.82) | 21.3 (0.107–0.357) | 90.9 (0.783–0.975) | 54.9 (0.442–0.654) | .129 |
4C7S = 7S domain of type 4 collagen, APRI = the aspartate aminotransferase-to-platelet ratio index, ELF score = Enhances Liver Fibrosis score, Fib-4 = the fibrosis index based on four factors, M2BPGi = Mac-2-binding protein glycosylation isomer, PBC = primarily biliary cholangitis, Pro-C3 = N-terminal type III collagen propeptide.
Table 3.
Diagnostic accuracy of serum fibrosis markers for patients with PBC in Nakanuma fibrosis score 2.
| Variable | Cut-off | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) | P |
| ELF score | 10.0 | 89.5 (0.669–0.987) | 69.7 (0.571–0.804) | 0.84 (0.73–0.93) | 45.9 (0.295–0.631) | 95.8 (0.857–0.995) | 74.1 (0.635–0.830) | |
| TIMP-1 | 270 | 78.9 (0.544–0.939) | 77.3 (0.653–0.867) | 0.79 (0.66–0.93) | 50.0 (0.313–0.687) | 92.7 (0.824–0.980) | 77.6 (0.673–0.860) | .39 |
| P-III-P | 11.6 | 81.2 (0.604–0.966) | 59.2 (0.473–0.704) | 0.80 (0.71–0.92) | 34.0 (0.209–0.493) | 93.8 (0.828–0.987) | 64.2 (0.537–0.738) | .63 |
| HA | 125 | 57.1 (0.340–0.782) | 80.3 (0.691–0.888) | 0.75 (0.59–0.86) | 46.2 (0.266–0.666) | 86.4 (0.757–0.936) | 75.0 (0.649–0.834) | .016 |
| Pro-C3 | 17.1 | 54.5 (0.322–0.756) | 88.6 (0.795–0.947) | 0.75 (0.63–0.88) | 57.1 (0.340–0.782) | 87.5 (0.782–0.938) | 81.2 (0.722–0.883) | .36 |
| M2BPGi | 0.85 | 72.2 (0.465–0.903) | 62.5 (0.495–0.743) | 0.66 (0.52–0.80) | 35.1 (0.202–0.525) | 88.9 (0.759–0.963) | 64.6 (0.533–0.749) | .031 |
| Fib-4 index | 1.95 | 82.4 (0.566–0.962) | 54.8 (0.427–0.665) | 0.67 (0.54–0.79) | 29.8 (0.173–0.449) | 93 (0.809–0.985) | 60.0 (0.491–0.702) | .027 |
| APRI | 0.73 | 82.4 (0.566–0.962) | 61.1 (0.489–0.724) | 0.74 (0.63–0.86) | 33.3 (0.196–0.495) | 93.6 (0.825–0.987) | 65.2 (0.543–0.750) | .37 |
| 7S collagen | 4 | 90 (0.683–0.988) | 60.8 (0.488–0.720) | 0.74 (0.61–0.86) | 38.3 (0.245–0.536) | 95.7 (0.855–0.995) | 67.0 (0.566–0.764) | .52 |
| Platelet | 22.9 | 72.2 (0.465–0.903) | 53.4 (0.414–0.652) | 0.67 (0.55–0.80) | 27.7 (0.156–0.426) | 88.6 (0.754–0.962) | 57.1 (0.463–0.675) | .013 |
4C7S = 7S domain of type 4 collagen, APRI = the aspartate aminotransferase-to-platelet ratio index, ELF score = enhances liver fibrosis score, Fib-4 = the fibrosis index based on four factors, M2BPGi = Mac-2-binding protein glycosylation isomer, PBC = primarily biliary cholangitis, Pro-C3 = N-terminal type III collagen propeptide.
3.3. Prognostic significance of enhances liver fibrosis score in primarily biliary cholangitis
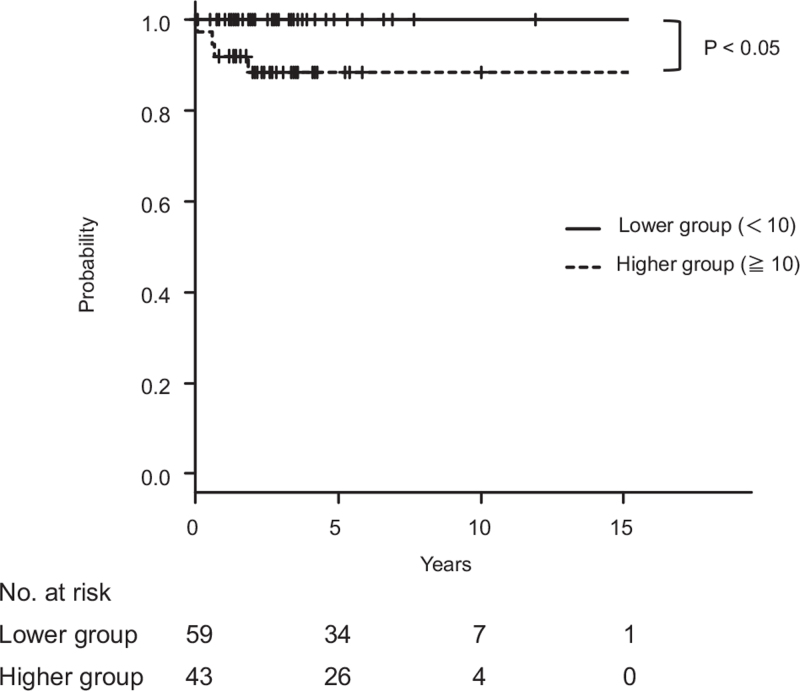
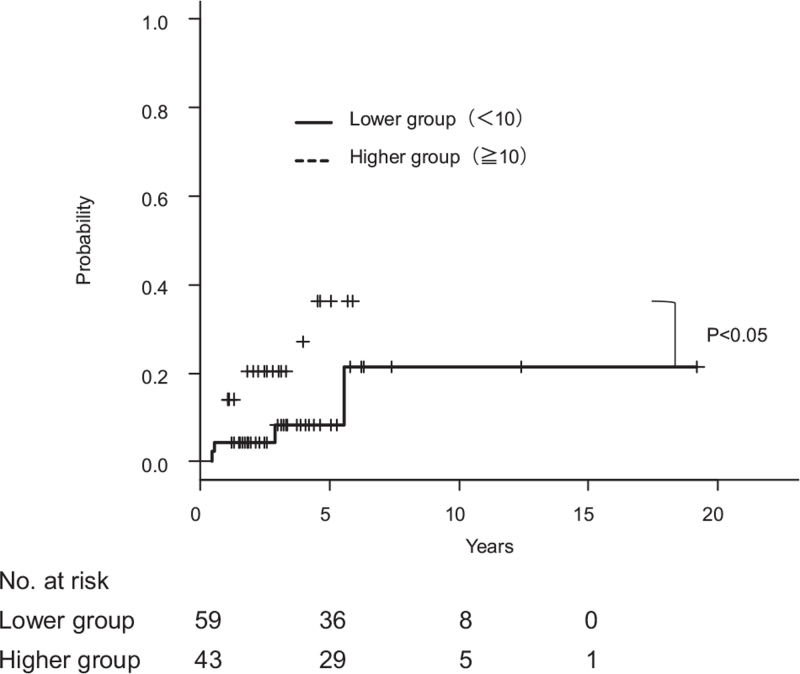
ELF score was the best predictor of Scheuer stage III and Nakanuma fibrosis score 2 with a cut-off value of 10.0 and AUCs of 0.81 and 084, respectively. The diagnostic performance of Kaplan–Meier analysis demonstrated that patients with PBC with an ELF score of ≥10.0 had higher incidence of clinical complications and worse survival than those with an ELF score of <10.0 (Figs. 4 and 5).
Figure 4.
Kaplan–Meier curves of liver-related survival according to ELF score in 102 patients with primary biliary cholangitis (PBC). Patients with PBC with an ELF score of ≥10.0 had worse prognosis than those with an ELF score of <10.0 (P < .05).
Figure 5.
Kaplan–Meier curves for the occurrence of liver-related events according to ELF score in 102 patients with primary biliary cholangitis (PBC). Patients with PBC with an ELF score of ≥10.0 developed clinical symptoms more frequently than those with an ELF score of <10.0 (P < .05).
4. Discussion
Liver fibrosis progression has been shown to predict survival of patients with CLD including PBC.[31] However, repeated procedures are difficult to perform due to various limitations on the principle, sampling error, and cost.[32,33] Therefore, noninvasive methods of assessing liver fibrosis are required to overcome these limitations. A noninvasive approach to monitor fibrosis progression is clearly advantageous and accurately predicts clinical outcomes. Despite the identification of various promising biomarker candidates, efficient serum biomarkers of PBC have not yet been established. In this study, the ELF test can be used to effectively evaluate the liver fibrosis severity and to predict liver-related complications and mortality in patients with PBC. This is the first study to demonstrate that the ELF panel is an accurate noninvasive test for determining the severity of hepatic fibrosis according to 2 histological classifications and correctly identifies patients with liver-related complications and those with poor prognosis.
The ROC curve showed that the AUC and sensitivity values of ELF score were higher than that of PIIINP, but PIIINP had higher specificity than that of ELF score; this helped identify patients with Scheuer stage III. However, higher AUC, sensitivity, and specificity values of ELF compared with those of PIIINP helped identify patients with Nakanuma fibrosis score 2. Nakanuma fibrosis score is more useful than the Scheuer stage in terms of the estimation of liver fibrosis progression as Scheuer's classification is characterized by both fibrosis and bile duct changes, indicating that ELF score is more useful than PIIINP as a hepatic fibrosis marker in PBC. In case where liver biopsy is appropriate for diagnostic purposes, the Nakanuma classification should be employed for more accurate staging because a more recent staging system described by Nakanuma et al added individual scores for fibrosis, BDL, and severity of chronic cholestasis based on copper deposition in the liver to evaluate the histological stage of PBC. Therefore, the PBC prognosis has significantly improved over the last 2 decades due to the early diagnosis before the onset of clinical signs and manifestations. Inflamed and damaged intrahepatic bile ducts and ductular reaction were generally detected in early stage PBC. The Ishak system is based entirely on the extent of fibrosis and provides prognostic information in PBC, a condition characterized by the presence of typical histological features such as non-suppurative destructive cholangitis and ductopenia in its early stages. These data reinforce the fact that ELF score reflects liver fibrosis progression associated with bile duct damage.[3]
The ELF score of ≥10.0 was highly significantly associated with significant fibrosis in PBC and reliably identifies those highly at risk of experiencing these complications and death. The 2016 United Kingdom's National Institute for Health and Care Excellence (NICE) guidelines have shown that ELF of ≥10.51 could successfully diagnose advanced fibrosis in nonalcoholic fatty liver disease.[34] ELF score of ≥11.3 has been shown to be associated with a fivefold increased risk of developing a liver-related outcome in nonalcoholic steatohepatitis and compensated cirrhosis.[35] An ELF score of ≥7.3 indicates significant fibrosis in patients with chronic hepatitis type B requiring antiviral medications.[36] The ELF test of ≥9.1 is a promising noninvasive method to assess severe liver fibrosis in patients with chronic hepatitis C.[37] Differences in ELF score cut-off values in CLD remain unclear, but may be explained by fibrous expansion differences of portal areas among CLDs. Fredrich-Rust et al have shown that ELF cut-off for F4 is 9.89, whereas it was 10 in this cohort. The reason for this discrepancy remains unclear, but this could be partially explained by the fact that differences in the number of enrolled patients with PBC, and the histological classification used, determined the grade and stage of PBC.
The optimal cut-off value and diagnostic performance of respective serum fibrosis biomarkers might vary according to CLDs, and due to the prevalence of liver fibrosis stages in CLDs.[38,39] However, ELF score has been confirmed to predict significant liver fibrosis with a certain degree of accuracy. Nevertheless, optimization of cut-off values and evaluation of the diagnostic performance are prerequisite for the assessment of liver fibrosis in each clinical setting.[40] Furthermore, comparing AUCs among noninvasive methods based on liver histology as a reference remains controversial. Differences in AUCs of liver fibrosis markers do not always differentiate the quality difference among parameters. Furthermore, the inaccuracy of liver biopsy may be attributed to the misdiagnosis of various noninvasive techniques as histological features of PBC are heterogeneously distributed throughout the liver and sampling errors occur in percutaneous liver biopsy in PBC. These findings suggest that noninvasive composite blood biomarkers, including ELF score, are clinically useful in predicting fibrosis in PBC.
Nevertheless, our study results should be interpreted in light of several limitations. First, the small number of participants, especially of those with Scheuer stage 4 and Nakanuma fibrosis score of 3. Second, the histological progression of PBC may be relatively steady, regardless of whether the starting point is early or advanced disease. The number of patients with clinical manifestations is small. Third, comparison of ELF usefulness in different etiologies was restricted by a relatively low frequency of clinical symptom occurrence in PBC. Future studies are warranted to compare the need for magnetic resonance elastography and transient elastography as well as combinations of liver stiffness measurement and serum fibrosis indices. Noninvasive parameters should be assessed based on a large-scale prospective longitudinal study using surrogate end-points of changes in histological disease stage. Therefore, ELF panel can serve as a hepatic fibrosis marker and predictor of liver-related complications and mortality.
Acknowledgments
The authors would like to thank Siemens Healthcare Diagnostics Inc. (Tokyo, Japan), Hideyuki Fukushima, and Hiromichi Asano for ELF score measurement.
Author contributions
Conceptualization: Tadashi Namisaki, Hitoshi Yoshiji..
Data curation: Yuki Fujimoto, Takahiro Ozutsumi, Masanori Furukawa, Norihisa Nishimura, Yasuhiko Sawada, Koh Kitagawa.
Formal analysis: Ryuichi Noguchi.
Investigation: Takahiro Kubo, Fumimasa Tomooka, Soichi Takeda, Shinya Sato, Hideto Kawaratani, Kei Moriya.
Methodology: Koji Murata, Kosuke Kaji, Takemi Akahane, Akira Mitoro.
Resources: Hiroaki Takaya, Yuki Tsuji, Junya Suzuki, Akihiko Shibamoto, Masahide Enomoto, Koji Ishida, Hiroyuki Ogawa.
Validation: Satoshi Iwai, Hirotetsu Takagi.
Writing – original draft: Yukihisa Fujinaga.
Writing – review & editing: Tadashi Namisaki, Hitoshi Yoshiji.
Footnotes
Abbreviations: APRI = the aspartate aminotransferase-to-platelet ratio index, ELF score = enhances liver fibrosis score, Fib-4 = the fibrosis index based on four factors, M2BPGi = Mac-2-binding protein glycosylation isomer, PBC = primarily biliary cholangitis, PIIINP = procollagen type III amino-terminal peptide, PLT = platelet, Pro-C3 = N-terminal type III collagen propeptide, TIMP-1 = metalloproteinase 1, UDCA = ursodeoxycholic acid.
How to cite this article: Fujinaga Y, Namisaki T, Takaya H, Tsuji Y, Suzuki J, Shibamoto A, Kubo T, Iwai S, Tomooka F, Takeda S, Fujimoto Y, Enomoto M, Murata K, Ishida K, Ogawa H, Takagi H, Ozutsumi T, Furukawa M, Nishimura N, Sawada Y, Kitagawa K, Sato S, Kaji K, Kawaratani H, Moriya K, Noguchi R, Akahane T, Mitoro A, Yoshiji H. Enhanced liver fibrosis score as a surrogate of liver-related complications and mortality in primary biliary cholangitis. Medicine. 2021;100:39(e27403).
Raw data were generated at Department of Gastroenterology, Nara Medical University. Derived data supporting the findings of this study are available from the corresponding author [T.N] on request.
Informed consent was obtained from all participants before the initiation of the study. This study was approved by the Human Ethics Review Committee of Nara University Hospital. (Approval No. O11–118) and was conducted in accordance with the Declaration of Helsinki.
All patients gave their written informed consent to participate in the study.
The authors have no conflicts of interests to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Kawata K, Joshita S, Shimoda S, et al. The ursodeoxycholic acid response score predicts pathological features in primary biliary cholangitis. Hepatol Res 2020;51:80–9. [DOI] [PubMed] [Google Scholar]
- [2].Hayashi M, Abe K, Fujita M, Okai K, Takahashi A, Ohira H. Changes in serum levels of leucine-rich alpha2-glycoprotein predict prognosis in primary biliary cholangitis. Hepatol Res 2019;49:385–93. [DOI] [PubMed] [Google Scholar]
- [3].Namisaki T, Fujinaga Y, Moriya K, Yoshiji H. The association of histological progression with biochemical response to ursodeoxycholic acid in primary biliary cholangitis. Hepatol Res 2020;51:31–8. [DOI] [PubMed] [Google Scholar]
- [4].Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hayashi M, Abe K, Fujita M, Takahashi A, Hashimoto Y, Ohira A. Serum Gas6 and Axl as non-invasive biomarkers of advanced histological stage in primary biliary cholangitis. Hepatol Res 2020;50:1337–46. [DOI] [PubMed] [Google Scholar]
- [6].Takamura M, Matsuda Y, Kimura N, et al. Changes in disease characteristics of primary biliary cholangitis: an observational retrospective study from 1982 to 2016. Hepatol Res 2020;51:166–75. [DOI] [PubMed] [Google Scholar]
- [7].McHutchison J, Poynard T, Afdhal N. International fibrosis group meeting participants. Fibrosis as an end point for clinical trials in liver disease: a report of the international fibrosis group. Clin Gastroenterol Hepatol 2006;4:1214–20. [DOI] [PubMed] [Google Scholar]
- [8].Corpechot C, Carrat F, Poujol-Robert A, et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology 2012;56:198–208. [DOI] [PubMed] [Google Scholar]
- [9].Plebani M, Giacomini A, Floreani A, et al. Biochemical markers of hepatic fibrosis in primary biliary cirrhosis. Ric Clin Lab 1990;20:269–74. [DOI] [PubMed] [Google Scholar]
- [10].Voumvouraki A, Koulentaki M, Notas G, Sfakianaki O, Kouroumalis E. Serum surrogate markers of liver fibrosis in primary biliary cirrhosis. Eur J Intern Med 2011;22:77–83. [DOI] [PubMed] [Google Scholar]
- [11].Farkkila M, Rautiainen H, Karkkainen P, Karvonen AL, Nurmi H, Niemela O. Serological markers for monitoring disease progression in noncirrhotic primary biliary cirrhosis on ursodeoxycholic acid therapy. Liver Int 2008;28:787–97. [DOI] [PubMed] [Google Scholar]
- [12].Zhang HC, Hu RF, Zhu T, Tong L, Zhang QQ. Primary biliary cirrhosis degree assessment by acoustic radiation force impulse imaging and hepatic fibrosis indicators. World J Gastroenterol 2016;22:5276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Umemura T, Joshita S, Sekiguchi T, et al. Serum wisteria floribunda agglutinin-positive Mac-2-binding protein level predicts liver fibrosis and prognosis in primary biliary cirrhosis. Am J Gastroenterol 2015;110:857–64. [DOI] [PubMed] [Google Scholar]
- [14].Joshita S, Umemura T, Usami Y, et al. Serum autotaxin is a useful disease progression marker in patients with primary biliary cholangitis. Sci Rep 2018;8:8159.doi: 10.1038/s41598-018-26531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nishikawa H, Enomoto H, Iwata Y, et al. Impact of serum Wisteria floribunda agglutinin positive Mac-2-binding protein and serum interferon-gamma-inducible protein-10 in primary biliary cirrhosis. Hepatol Res 2016;46:575–83. [DOI] [PubMed] [Google Scholar]
- [16].Osman KT, Maselli DB, Idilman IS, et al. Liver stiffness measured by either magnetic resonance or transient elastography is associated with liver fibrosis and is an independent predictor of outcomes among patients with primary biliary cholangitis. J Clin Gastroenterol 2020;55:449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Friedrich-Rust M, Rosenberg W, Parkes J, Herrmann E, Zeuzem S, Sarrazin C. Comparison of ELF, FibroTest and FibroScan for the non-invasive assessment of liver fibrosis. BMC Gastroenterol 2010;10:103.doi: 10.1186/1471-230X-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mayo MJ, Parkes J, Adams-Huet B, et al. Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology 2008;48:1549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kakuda Y, Harada K, Sawada-Kitamura S, et al. Evaluation of a new histologic staging and grading system for primary biliary cirrhosis in comparison with classical systems. Hum Pathol 2013;44:1107–17. [DOI] [PubMed] [Google Scholar]
- [20].Working Subgroup for Clinical Practice Guidelines for Primary Biliary C.Guidelines for the management of primary biliary cirrhosis: The Intractable Hepatobiliary Disease Study Group supported by the Ministry of Health, Labour and Welfare of Japan. Hepatol Res 2014;44 Suppl S1:71–90. [DOI] [PubMed] [Google Scholar]
- [21].Scheuer P. Primary biliary cirrhosis. Proc R Soc Med 1967;60:1257–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chazouilleres O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology 1998;28:296–301. [DOI] [PubMed] [Google Scholar]
- [23].Casals G, Fernandez-Varo G, Melgar-Lesmes P, et al. Factors involved in extracellular matrix turnover in human derived cardiomyocytes. Cell Physiol Biochem 2013;32:1125–36. [DOI] [PubMed] [Google Scholar]
- [24].Hogemann B, Voss B, Pott G, Rauterberg J, Gerlach U. 7 S collagen: a method for the measurement of serum concentrations in man. Clin Chim Acta 1984;144:01–10. [DOI] [PubMed] [Google Scholar]
- [25].Jekarl DW, Choi H, Lee S, et al. Diagnosis of liver fibrosis with wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA-M2BP) among chronic hepatitis B patients. Ann Lab Med 2018;38:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nielsen MJ, Nedergaard AF, Sun S, et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res 2013;5:303–15. [PMC free article] [PubMed] [Google Scholar]
- [27].Nakanuma Y, Zen Y, Harada K, et al. Application of a new histological staging and grading system for primary biliary cirrhosis to liver biopsy specimens: interobserver agreement. Pathol Int 2010;60:167–74. [DOI] [PubMed] [Google Scholar]
- [28].Cholongitas E, Senzolo M, Standish R, et al. A systematic review of the quality of liver biopsy specimens. Am J Clin Pathol 2006;125:710–21. [DOI] [PubMed] [Google Scholar]
- [29].DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- [30].Carbone M, Sharp SJ, Flack S, et al. The UK-PBC risk scores: derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology 2016;63:930–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Namisaki T, Moriya K, Noguchi R, et al. Liver fibrosis progression predicts survival in patients with primary biliary cirrhosis. Hepatol Res 2017;47:E178–86. [DOI] [PubMed] [Google Scholar]
- [32].Fujinaga Y, Namisaki T, Moriya K, et al. Identification of clinical risk factors for histological progression of primary biliary cholangitis. Hepatol Res 2019;49:1015–25. [DOI] [PubMed] [Google Scholar]
- [33].Sekiguchi T, Umemura T, Fujimori N, et al. Serum cell death biomarkers for prediction of liver fibrosis and poor prognosis in primary biliary cirrhosis. PLoS One 2015;10:e0131658.doi: 10.1371/journal.pone.0131658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Glen J, Floros L, Day C, Pryke R. Guideline Development Group. Non-alcoholic fatty liver disease (NAFLD): summary of NICE guidance. BMJ 2016;354:i4428.doi: 10.1136/bmj.i4428. [DOI] [PubMed] [Google Scholar]
- [35].Are VS, Vuppalanchi R, Vilar-Gomez E, Chalasani N. Enhanced liver fibrosis score can be used to predict liver-related events in patients with nonalcoholic steatohepatitis and compensated cirrhosis. Clin Gastroenterol Hepatol 2021;19:1292–3.e3. [DOI] [PubMed] [Google Scholar]
- [36].Trembling PM, Lampertico P, Parkes J, et al. Performance of Enhanced Liver Fibrosis test and comparison with transient elastography in the identification of liver fibrosis in patients with chronic hepatitis B infection. J Viral Hepat 2014;21:430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Parkes J, Guha IN, Roderick P, et al. Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepat 2011;18:23–31. [DOI] [PubMed] [Google Scholar]
- [38].Inadomi C, Takahashi H, Ogawa Y, et al. Accuracy of the Enhanced Liver Fibrosis test, and combination of the Enhanced Liver Fibrosis and non-invasive tests for the diagnosis of advanced liver fibrosis in patients with non-alcoholic fatty liver disease. Hepatol Res 2020;50:682–92. [DOI] [PubMed] [Google Scholar]
- [39].Anstee QM, Lawitz EJ, Alkhouri N, et al. Noninvasive tests accurately identify advanced fibrosis due to NASH: baseline data from the STELLAR trials. Hepatology 2019;70:1521–30. [DOI] [PubMed] [Google Scholar]
- [40].Mehta SH, Lau B, Afdhal NH, Thomas DL. Exceeding the limits of liver histology markers. J Hepatol 2009;50:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]