Abstract
Ovarian dysfunction and lower circulating anti-Müllerian hormone (AMH) feature women living with HIV (WLWH). Because treated human immunodeficiency virus (HIV) infection is characterized by a pro-inflammatory/oxidative phenotype resulting in residual comorbidity, we sought to investigate possible associations between plasma AMH and markers of inflammation, immune activation/senescence/exhaustion, oxidative stress as well as comorbidities in a cohort of combined anti-retroviral therapy (cART)-treated WLWH versus age-matched HIV-uninfected, healthy women.
Eighty WLWH on effective cART aged 25 to 50 years and 66 age-matched healthy women were enrolled. We measured: plasma AMH, IL-6, reactive oxygen species modulator 1 (ROMO1) (ELISA); plasma tumor necrosis factor α, IL-10, soluble vascular cell adhesion molecule 1, osteopontin (Luminex); CD4/CD8 activation (CD38/CD69), apoptosis (CD95), exhaustion (PD1), maturation (CD45RA/CD45R0/CD127/CCR7), recent thymic emigrants (CD31/CD103) (flow cytometry). Mann Whitney and chi-squared tests were used. Univariate and multivariate logistic regression analyses were used to assess factors associated with low AMH (≤1 ng/mL).
Compared to healthy women, WLWH were more frequently non-Caucasian, drug/alcohol abusers, with history of late menarche, lower hormonal contraceptive use, with higher gravidity and lower parity. WLWH showed significantly lower AMH (P = .004) as well as higher ROMO1 (P = .0003) and tumor necrosis factor α (P < .0001). The multivariate analyses revealed ROMO1 (adjusted odds ratio [AOR]: 1.42, P = .03) and HIV infection (AOR: 8.1, P = .0001) as independently associated with low AMH. The logistic regression model with both HIV status and ROMO1 (a marker of oxidative stress) confirmed HIV as the only predictor of low AMH (AOR: 17, P = .0003).
Despite effective cART, WLWH showed lower AMH compared to age-matched peers, indicating pre-mature ovarian ageing. Both HIV and oxidative stress are independently associated with low AMH, emphasizing the impact of HIV-associated oxidative stress on reproductive aging.
Keywords: anti-Müllerian hormone, HIV infection, inflammation, ovarian reserve, oxidative stress, reproductive aging
1. Introduction
The majority of human immunodeficiency virus (HIV) infections among women occur early in reproductive life, supporting the need of better understanding the impact of HIV on reproductive functions, as well as the implications of reproductive aging on HIV course. Although ovarian dysfunction has been reported in women living with HIV (WLWH),[1] the mechanisms behind this phenomenon are still unclear. Several hypotheses have been proposed, including the direct effect of HIV itself, the effect of combined anti-retroviral therapy (cART), the effects of chronic inflammation/activation on the neuroendocrine axis and coexisting factors, such as smoking, body mass index (BMI), and substance abuse.[2]
A clear definition of ovarian reserve is still lacking; however, it is well known that its decline results in lower fertility.[3,4] Numerous tests have been developed to assess the ovarian reserve in order to evaluate the prognosis of spontaneous fertility. Among them, recent studies indicate that anti-Müllerian hormone (AMH) may offer the most accurate, simple, and non-invasive method for determining ovarian follicle reserve and reproductive aging,[5–7] since it can be measured in serum at any stage of the menstrual cycle.[8] The AMH is produced by the granulosa cells of pre-antral and small antral follicle pool,[9] and its mutations with reduced in vitro bioactivity have been linked to pre-mature ovarian insufficiency.[10]
In HIV infection, data on serum AMH levels are conflicting. While some authors reported that age, BMI, CD4 count, and viral load were independent contributors of lower levels of AMH in WLWH,[11,12] Scherzer et al[13] described higher levels of AMH in HIV-infected women, even after adjustment for CD4 counts and age. AMH levels in WLWH has also been linked to comorbidities. Indeed, the reduced ovarian reserve seems to be associated with increased risk of subclinical coronary atherosclerotic plaque even after controlling for cardiovascular diseases risk factors (including age) and immune activation, supporting the hypothesis of a role of ovarian reserve in contributing to cardiovascular diseases burden.[14] Besides, circulating 25-hydroxycholecalciferol was positively correlated with serum AMH levels, suggesting that circulating vitamin D is associated with ovarian reserve in late reproductive aged women.[15]
Whereas the occurrence of, or the lack of, altered patterns of reproductive aging in WLWH still needs to be fully elucidated, it is undoubted that hallmarks of HIV infection are the chronic low-grade inflammatory phenotype, coupled with significant immune remodeling and persisting oxidative stress that lead to pre-mature aging.[16,17]
Given that elevated levels of inflammatory cytokines play a critical role in damaging ovarian functions,[18] and that long-term moderate oxidative stress has been demonstrated to decrease the ovarian reproductive function by reducing follicle quality and progesterone production,[19] we hypothesized that the HIV-associated chronic inflammation and oxidative stress may negatively impact the ovarian reserve.
Thus, we decided to investigate whether AMH levels might be associated with systemic inflammation (plasma IL-6, tumor necrosis factor α [TNF-α], IL-10); markers of comorbidities, particularly bone and cardiovascular diseases (plasma osteopontin and soluble vascular cell adhesion molecule 1 [sVCAM-1], respectively); immune activation/senescence/exhaustion/maturation; and oxidative stress (plasma reactive oxygen species modulator 1 [ROMO1]) in a cohort of cART-treated HIV-infected women as compared to age-matched HIV-uninfected women.
2. Methods
2.1. Patients
We consecutively enrolled 80 HIV-infected and 66 HIV-uninfected women attending the Clinic of Infectious Diseases and the Clinic of Obstetrics and Gynecology, ASST Santi Paolo e Carlo, Department of Health Sciences, University of Milan, Italy, for routine check-up examinations. HIV-infected subjects were on stable cART for at least 36 months, with undetectable plasma HIV-RNA viral load (<40 copies/mL) in at least 3 consecutive assessments and any CD4 count. Inclusion criteria for both HIV+ and HIV– women were: age ≤50 years, no menopause, no pregnancy.
All the enrolled patients provided written informed consent in accordance with the Declaration of Helsinki and approved by the Ethical Committee of our Institution (Comitato Etico, ASST Santi Paolo e Carlo, Milan, Italy).
Questionnaires were administered to all the study participants. Study interviews included queries regarding menstrual periods, obstetrical history, gynecological surgery, tobacco and drugs use, use of exogenous steroids or other medications, and medical conditions. For HIV-infected women, data on the viral infection were also collected.
2.2. Immunophenotype analyses
Lymphocyte surface phenotypes were evaluated by flow cytometry on fresh peripheral blood of HIV-infected women alone, using fluorochrome-labeled antibodies: L/D-BV510 (Miltenyi Biotech, Germany); CD4-APC-H7, CD8-PE-Cy7, CD38-PE, CD45R0-PerCPCy5.5, CD45RA-PerCPCy5.5, CD127-APC, CD31-FITC, CCR7-APC, CD103-PE, CD95-FITC, CD69-FITC, PD1-PE (BD Biosciences, Palo Alto, CA).
T-cell subsets were defined as: naive CCR7+ CD45RA+, central memory CCR7+CD45RA−, effector memory CCR7− CD45RA−, and terminally differentiated CCR7− CD45RA+ subsets. Besides, we measured: activation (CD45R0/CD38/CD69), apoptosis (CD95), exhaustion (PD-1), IL7R (CD127), and Recent Thymic Emigrants (CD31 or CD103) on both CD4 and CD8 T-cell subsets.
Briefly, 1 × 106 peripheral blood mononuclear Cells were stained with the appropriate antibodies for 20 minutes at 4°C in the dark, washed and then acquired using FACSVerse cytometer (BD Biosciences, Palo Alto, CA).
2.3. AMH quantification
On frozen plasma samples of both HIV-infected and uninfected women, we quantified the circulating levels of AMH (Human MIS/AMH, DuoSet, R&D Systems, Minneapolis, MN, catalog number: DY1737). Samples were thawed at room temperature and incubated at 56°C for 30 minutes. A 1000× dilution in phosphate-buffered saline was required to avoid inhibition of the reaction. Each plasma sample was measured in triplicate.
2.4. ROMO1 quantification
Plasma levels of ROMO1 in HIV-infected and uninfected women were measured in duplicate by ELISA assay (Human ROMO1 ELISA Kit, FineTest, Wuhan, Hubei, China, catalog number: EH2003), according to manufacturer's instructions. Fresh-frozen plasma samples were thawed at room temperature and then 2× diluted before running the assay.
2.5. IL-6 quantification
IL-6 levels on undiluted thawed plasma of HIV-infected and uninfected women were measured in duplicate by ELISA assay (Human IL-6 Immunoassay, R&D Systems, Minneapolis, MN, catalog number: HS600C), according to the manufacturer's instructions.
2.6. Multiplex assays
The relative content of IL-10, TNF-α, sVCAM-1, and osteopontin in thawed plasma of HIV-infected subjects and HIV-uninfected healthy women was quantified using Luminex Assay (R&D Systems, Minneapolis, MN, catalog number: FCSTM09-02 for IL-10 and TNF-α; LXSAHM-03 for sVCAM-1 and osteopontin). A 2× dilution factor was used. All samples were run in duplicate. The raw data were analyzed using Bio-Plex Manager software (Bio-Rad Laboratories, Hercules, CA, USA). Standard curves were generated from lyophilized standards provided with each kit. The concentration for each analyte in each sample was determined via interpolation from each corresponding standard curve.
2.7. Statistical analyses
All data were collected in a computerized database and analyzed by GraphPad 6.2 Prism (GraphPad Software Inc). Continuous variables were expressed as median and interquartile range, whereas categorical variables were expressed as absolute numbers and percentages. When HIV and controls groups were analyzed, we used non-parametric statistical tests, as appropriate.
Correlations analyses were examined using the non-parametric Spearman rank correlation test. To identify independent determinants of serum AMH levels, we performed univariate and multivariate linear regression analyses (SAS software 9.4, SAS Institute Inc., Cary, NC, USA). Based on literature, we defined low AMH as values ≤1 ng/mL.[20] Directed acyclic graphs (DAGs) were generated in order to have visual representation of causal assumptions and confounding factors, that may obscure the real effect. A 2-tailed P value less than .05 was considered statistically significant.
3. Results
3.1. Characteristics of HIV-infected and HIV-uninfected women
A total of 80 HIV-infected women and 66 age-matched seronegative women were included in the study. The baseline (enrollment) characteristics of the participants are listed in Table 1.
Table 1.
Demographic and gynecological features of the study population.
| HW (n = 66) | WLWH (n = 80) | P | |
| Age, years (IQR)∗ | 37 (30–44) | 41 (34–44) | .225 |
| Ethnicity, (%)† | .0006 | ||
| White | 66 (100) | 64 (80) | |
| Black African/American | 0 | 12 (15) | |
| Hispanic | 0 | 4 (5) | |
| BMI (IQR)∗ | 22.2 (20.4–25.9) | 22.5 (20.7–25.7) | .589 |
| Current smoking, yes (%)† | 16 (24) | 39 (49) | .003 |
| Drug/alcohol abuse, yes (%)† | 0 | 16 (20) | <.0001 |
| HCV/HBV coinfection, yes (%)† | 0 | 5 (6) | .064 |
| Age at menarche, years (IQR)∗ | 12 (11–13) | 13 (11–14) | .073 |
| Hormonal contraceptive use | |||
| Current, yes (%)† | 19 (29) | 8 (10) | .005 |
| Ever, yes (%)† | 31 (52) | 39 (49) | .867 |
| Duration, years (IQR)∗ | 6 (2–10) | 3 (1–7.5) | .111 |
| Gravidity, yes (%)† | .001 | ||
| 0 | 31 (47) | 16 (20) | |
| 1 | 8 (12) | 17 (21) | |
| 2 | 15 (23) | 15 (19) | |
| 3+ | 12 (18) | 32 (40) | |
| Parity, yes (%)† | .005 | ||
| 0 | 32 (48) | 49 (61) | |
| 1 | 10 (15) | 16 (20) | |
| 2 | 21 (32) | 7 (9) | |
| 3+ | 3 (5) | 8 (10) | |
| Sterility, yes (%)† | 4 (5) | 8 (9) | .547 |
| Familiarity for POF, (%)† | .0001 | ||
| Yes | 3 (5) | 5 (6) | |
| No | 63 (95) | 57 (71.5) | |
| Unknown | 0 | 18 (22.5) | |
| History of irregular menses, yes (%)† | 13 (20) | 28 (35) | .044 |
| History of amenorrhea, yes (%)† | 2 (3) | 6 (7) | .294 |
| History of endometriosis, yes (%) | 2 (3) | 2 (2) | 1 |
| History of cysts, yes (%) | 3 (5) | 8 (10) | .346 |
| History of cystic enucleation, yes (%) | 2 (3) | 4 (5) | 1 |
| Thyroid pathology, yes (%) | 6 (9) | 6 (7) | .769 |
BMI = body mass index, HW = HIV-uninfected healthy women, POF = pre-mature ovarian failure, defined as menopause before age 40, WLWH = women living with HIV.
Data are presented as median (IQR: interquartile range), statistical analysis: Mann–Whitney U test.
Data are presented as absolute number (%), statistical analysis: chi-squared or Fisher exact test.
The racial distributions were slightly different in the 2 groups, with 15% of Black/African-American and 5% of Hispanic in the HIV-infected group (P = .0006, Table 1). Current smoking and drug/alcohol abuse were more common in HIV-infected women than in uninfected ones.
The number of pregnancies (gravidity) and history of irregular menses were higher in HIV-infected women (P = .001, P = .044, respectively, Table 1), while the number of births (parity) and the hormonal contraceptive use were less often reported by HIV-infected women (P = 0.005 both, Table 1).
Table 2 illustrates the HIV-related features. HIV-infected women were virologically suppressed, with a median CD4 current count and nadir of 592 (431–821) cells/μL and 260 (163–316) cells/μL respectively. The median time of HIV infection and cART duration were 14 (7–21) years and 6 (4–9) years, respectively. 32% of HIV+ women were on protease inhibitor-based regimen, while 38% on non-nucleoside reverse transcriptase inhibitors-based therapy.
Table 2.
HIV-related characteristics.
| WLWH (n = 80) | |
| Time since HIV diagnosis, years (IQR)∗ | 14 (7–21) |
| CD4 count, cells/μL (IQR)∗ | |
| Nadir | 260 (163–316) |
| At time of analyses | 592 (431–821) |
| CD8 T-cell count, cells/μL (IQR) | 696 (553–964) |
| CD4/CD8 ratio | 0.83 (0.61–1.16) |
| AIDS diagnosis†, yes (%) | 12 (15) |
| HIV-RNA, copies/mL (IQR) | 14.5 (11–30) |
| Time between HIV diagnosis and cART initiation, years (IQR) | 4 (0–12) |
| cART duration, years (IQR) | 6 (4–9) |
| Type of cART†, (%) | |
| 2NRTI+PI/r | 26 (32) |
| 2NRTI+NNRTI | 30 (38) |
| Others | 24 (30) |
cART = combination anti-retroviral therapy, HIV = human immunodeficiency virus, NNRTI = non-nucleoside reverse transcriptase inhibitors, NRTI = nucleoside reverse transcriptase inhibitors, PI/r = boosted protease inhibitors, WLWH = women living with HIV.
Data are presented as median and IQR (interquartile range).
Data are presented as absolute numbers (%).
3.2. Levels of soluble markers in HIV-infected and HIV-uninfected women
We first assessed circulating levels of AMH, ROMO1 (a marker of oxidative stress), inflammatory markers (IL-6, TNF-α, IL-10), and surrogate markers of cardiovascular and bone damage (i.e., sVCAM-1 and osteopontin, respectively) in the 2 groups.
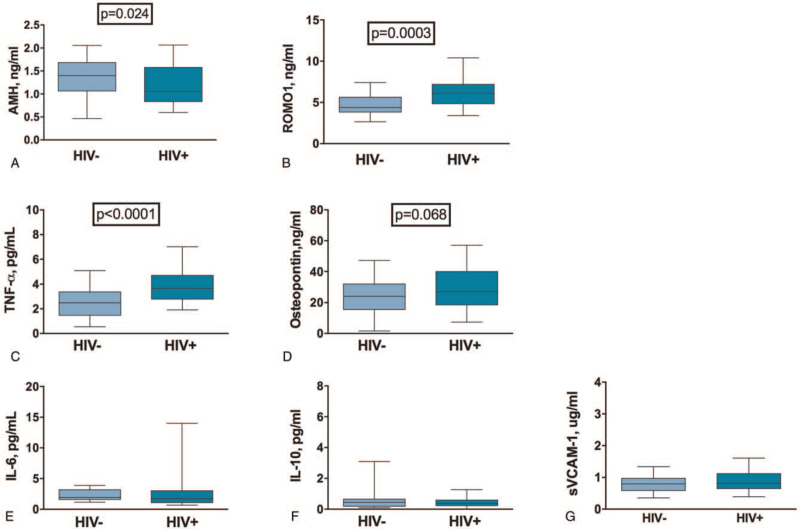
HIV-infected women showed significantly lower levels of AMH (1.40 vs 1.05 ng/mL, P = .024; Fig. 1A), and higher levels of ROMO1 (4.38 vs 6.11 ng/mL, P = .0003; Fig. 1B), TNF-α (2.48 vs 3.66 pg/mL, P < .0001; Fig. 1C) and a non-significant trend to higher osteopontin (24.14 vs 27.14 ng/mL, P = .068; Fig. 1D), as compared to healthy women. No differences in other soluble markers were observed (Fig. 1E–G).
Figure 1.
Circulating levels of AMH, ROMO1, pro-inflammatory and surrogate cardiovascular/bone damage markers. Data are presented as median and interquartile range (IQR). Mann–Whitney U test was used. (A) HIV-infected women showed significantly lower levels of AMH (1.40 [1.06–1.05] ng/mL vs 1.05 [0.83–1.58] ng/mL; P = .024). (B) HIV-infected women showed significantly higher levels of ROMO1 (4.38 ng/mL vs 6.11 ng/mL; P = .0003). (C) HIV-infected women showed significantly higher levels of TNF-α (2.48 [1.48–3.37] pg/mL vs 3.66 [2.79–4.71] pg/mL; P < .0001). (D) HIV-infected women showed a trend towards higher levels of osteopontin (24.14 [15.57–32.07] ng/mL vs 27.14 [18.53–39.98] ng/mL; P = .068). (E) HIV-infected and uninfected women showed similar levels of IL-6 (1.93 [1.61–3.18] pg/mL vs 1.72 [1.09–3.03] pg/mL; P = .152). (F) HIV-infected and uninfected women showed similar levels of IL-10 (0.45 [0.19–0.65] pg/mL vs 0.36 [0.23–0.59] pg/mL; P = .441). (G) HIV-infected and uninfected women showed similar levels of sVCAM-1 (0.79 [0.59–0.97] μg/mL vs 0.81 [0.65–1.12] μg/mL; P = .198). AMH = anti-Müllerian hormone, HIV = human immunodeficiency virus, ROMO1 = reactive oxygen species modulator 1, sVCAM-1 = soluble vascular cell adhesion molecule 1, TNF-α = tumor necrosis factor α.
3.3. Factors associated with low AMH
We next explored factors associated with low AMH levels (defined as AMH ≤1 ng/mL).[20]
By univariate analysis there was no association between AMH and the classical confounding factors known from the literature to affect AMH levels, such as age (odds ratio [OR]: 0.9, confidence interval [CI] 95% 0.9–1; P = .07), BMI (OR: 0.9, CI 95% 0.8–1; P = .7), smoking status (OR: 1, CI 95% 0.5–2; P = .9), and hormonal contraceptive use (OR: 0.3, CI 95% 0.1–1; P = .05).[21]
Among the inflammatory and surrogate markers of cardiovascular/bone damage, we decided to investigate only those significantly different between HIV-infected and uninfected women, finding no association between AMH and TNF-α (OR: 1.1, CI 95% 0.9–1.5; P = .2), as well as between AMH and osteopontin (OR: 0.9, CI 95% 0.9–1.1; P = .4).
Interestingly, the multivariate analysis revealed the oxidative stress and the HIV seropositive status as the only 2 factors associated with low circulating AMH. In particular, low levels of circulating AMH were associated with higher levels of plasma ROMO1 (OR: 1.4, CI 95% 1.07–1.95; P = .01), even after adjusting for age, BMI, and smoking status (adjusted odds ratio [AOR]: 1.42, CI 95% 1–1.9; P = .03).
Similarly, the HIV seropositive status was associated with low AMH (OR: 3.4 CI 95% 1.6–7; P = .001), even after adjusting for age, BMI, smoking status (AOR: 8.1, CI 95% 2.7–24, P = .0001).
The logistic regression model with both HIV status and oxidative stress variables, confirmed the HIV seropositive status as the only independent predictor of low AMH (AOR: 17, CI 95% 3.7–83; P = .0003).
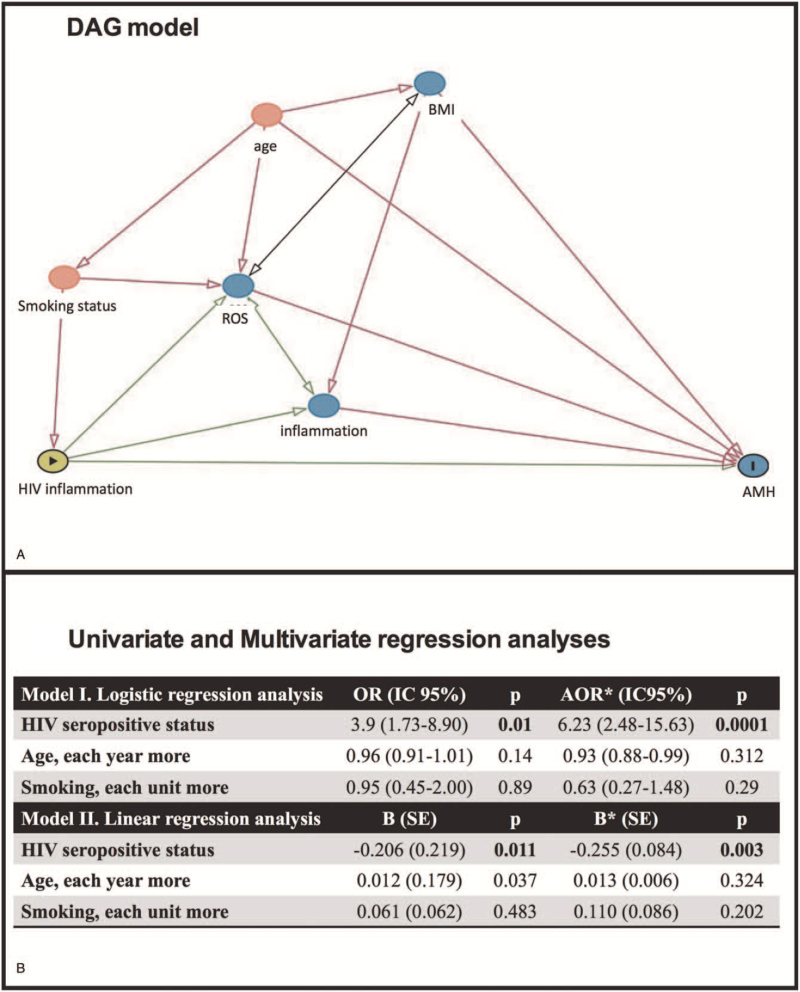
Given the number of confounders that might influence or mask the effect of HIV on AMH, we decided to generate a DAG, that helped us in identifying and clarifying the minimal sufficient adjustment for estimating the total effect of HIV infection on AMH levels.
The DAG showed the age and the smoking status alone as confounding factors (Fig. 2A). Thus, we generated a new regression model, confirming the HIV status as the only independent factor associated with low AMH (Fig. 2B).
Figure 2.
Predictors of low circulating AMH levels in HIV-infected and uninfected women. (A) Directed acyclic graph model was generated to have visual representation of confounding factors.  = exposure; I = outcome; green color = ancestor of exposure, blue color = ancestor of outcome, red color = ancestor of exposure and outcome; grey color = other variables; green arrow = causal path; red arrow = biasing path. (B) Univariate and multivariate regression analyses showed that HIV seropositive status was independently associated with low values of AMH. In the logistic model low AMH was defined as AMH ≤1 ng/mL. ∗mutually adjusted. AMH = anti-Müllerian hormone, BMI = body mass index, HIV = human immunodeficiency virus.
= exposure; I = outcome; green color = ancestor of exposure, blue color = ancestor of outcome, red color = ancestor of exposure and outcome; grey color = other variables; green arrow = causal path; red arrow = biasing path. (B) Univariate and multivariate regression analyses showed that HIV seropositive status was independently associated with low values of AMH. In the logistic model low AMH was defined as AMH ≤1 ng/mL. ∗mutually adjusted. AMH = anti-Müllerian hormone, BMI = body mass index, HIV = human immunodeficiency virus.
3.4. AMH and immune profile in HIV-infected women
Since the presence of HIV infection itself seems to negatively affect circulating levels of AMH, we focused on cART-treated women alone, trying to identify HIV-related factors that might be responsible for the low ovarian reserve in WLWH on suppressive cART.
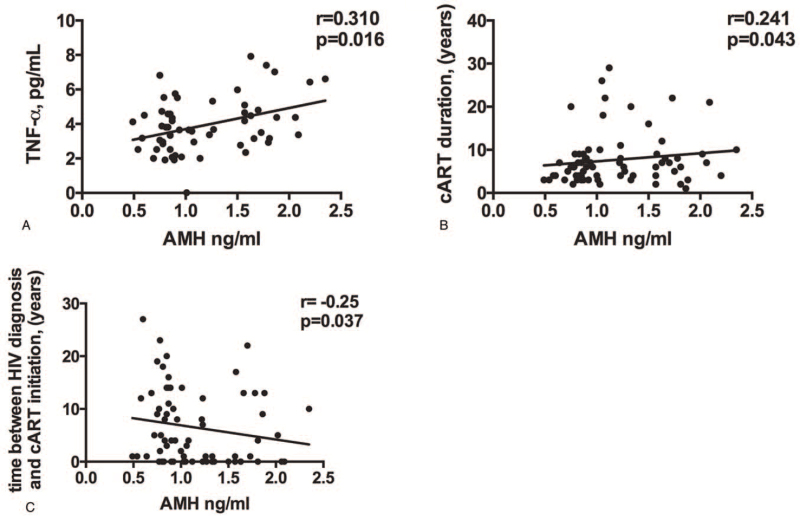
While we failed to find any association between plasma AMH and T-cell immune activation, senescence and exhaustion (data not shown), we did find a modest association between AMH and plasma TNF-α (r = 0.310; P = .016; Fig. 3A), as well as a positive correlation with cART duration (r = 0.241; P = .043; Fig. 3B) and a negative correlation with time between HIV infection diagnosis and cART initiation (r = –0.25; P = .037; Fig. 3C).
Figure 3.
Factors associated with low circulating AMH levels in WLWH. (A) Circulating AMH levels positively associated with residual levels of TNF-α (r = 0.310; P = .016). (B) Plasma AMH levels positively correlate with cART duration (r = 0.241; P = .043). (C) AMH levels negatively correlate with time between HIV diagnosis and cART initiation (r = –0.25; P = .037). AMH = anti-Müllerian hormone, cART = combined anti-retroviral therapy, HIV = human immunodeficiency virus, TNF-α = tumor necrosis factor α, WLWH = women living with HIV.
4. Discussion
The advent of cART has dramatically improved the life expectancy of people living with HIV.[22] Nevertheless, this prolonged survival is associated with an increased prevalence of comorbidities, due to chronic proinflammatory state.[23] An increasing number of studies showed that inflammation is associated with the occurrence of aging; this phenomenon known as inflamm-aging comprises different mechanisms: oxidative stress, inflammation, cytokines, DNA damage, autophagy, and non-enzymatic glycation.[24]
Data to specifically assess how exactly the inflamm-aging process affects WLWH, however, are conflicting and inconclusive. To date, it is still unclear the reason why WLWH show a reduced ovarian reserve and how this reduction is associated with fertility dysfunction and pre-mature menopause.
In the attempt to clarify the interplay between the HIV infection and the ovarian reserve, we decided to investigate the possible associations between AMH levels and markers of inflammation, immune activation/senescence/exhaustion, oxidative stress, and comorbidities in cART-treated WLWH.
In line with some previous observations,[11,12] our cohort of cART-treated WLWH showed lower levels of circulating AMH compared to age-matched HIV-uninfected peers, indicating pre-mature ovarian ageing. Interestingly, when we explored the possible factors associated with this finding, HIV infection per se and oxidative stress were the only 2 factors independently associated with low AMH. The association between AMH and oxidative stress is not surprising, given that high levels of reactive oxygen species (ROS) notoriously lead to a gradual decline in follicle quantity and quality.[19] Nonetheless, this is the first time that high levels of circulating ROMO1 (a marker of oxidative stress) have been reported to associate with low levels of AMH in cART-treated WLWH, emphasizing the role of oxidative stress on reproductive aging.
ROMO1 is a mitochondrial inner membrane non-selective cation channel with redox sensor functions involved in the regulation of mitochondrial dynamics and intra-cellular ROS production.[25] Upregulation of ROMO-1 – which is reflected in heightened plasma ROMO1 levels –, with subsequent increased release of endogenous ROS, has been described in several pathologic conditions characterized by high oxidative stress and inflammation, such as cancer, idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease, and obstructive sleep apnea syndrome.[26,27,28] Given the direct correlation between circulating ROMO1 and ROS production, plasma ROMO1 levels can be used as a surrogate marker of oxidative stress.
Remarkably, however, when applying a logistic regression model accounting for both HIV status and oxidative stress variables, HIV seropositivity was the only independent predictor of low AMH, suggesting that, despite full HIV viremia suppression by cART, HIV still exerts a strong and direct negative impact on ovarian reserve.
We therefore decided to investigate whether the increased risk of lower AMH in our cohort of WLWH may be mediated by circulating activated/senescent/exhausted T lymphocytes, given that the interactions between leukocytes, ovarian follicles, and inflammatory responses are crucial for ovulation.[29] We did not find any evidence of a possible association between AMH and immune activation/senescence/exhaustion. Similarly, we failed to find a link between low AMH and markers of bone and cardiovascular diseases. The lack of association with residual HIV-driven hyperactivated/senescent/exhausted immune profile, as well as co-morbidities markers, suggests that other factors should be taken into consideration, such as tissue HIV reservoirs, coinfections, and microbial translocation.
Interestingly, we found a positive correlation between AMH and TNF-α in cART-treated WLWH, supporting the role of certain cytokines in modulating the functions of granulosa cells, possibly through a direct effect on serum reproductive hormones.[30,31]
Finally, we observed a weakly positive correlation between AMH and cART duration in WLWH as well as a negative correlation with time between HIV infection diagnosis and cART initiation, likely supporting the protective role of anti-retroviral therapy in preserving the ovarian reserve, possibly through partially restoring the CD4 T-cell compartment and/or abating viral replication.
Several limitations need to be acknowledged. First of all, the limited sample size. Secondly, the lack of information on polycystic ovarian syndrome and the lack of matching for ethnicity, drug/alcohol abuse, smoking habits, all factors known to affect AMH levels. Thirdly, the absence of ovarian biopsies, that might have shed light onto the pathogenesis of reduced ovarian reserve in WLWH, allowing us to directly study the proper function of granulosa cells, the HIV reservoirs, the CD4 quantity and quality, the integrity of tight junctions possibly linked to microbial translocation. Lastly, the absence of controls such as cART-untreated WLWH and/or patients at different stages of disease, that might have allowed to discern whether the heightened oxidative stress observed in WLWH is a direct consequence of HIV viral activity or else a side effect of certain cART regimens; however, we believe that the higher oxidative stress in our cohort is likely a combination of both factors.
In conclusion, to our knowledge this is the first study investigating the role of inflammation, oxidative stress, immune activation/senescence/exhaustion, and comorbidities markers on ovarian reserve in cART-treated WLWH, as compared to age-matched peers. Despite the correlative nature of the study, the findings of an association between low circulating AMH, high plasma levels of ROMO1, and HIV infection above all other factors indicate that the HIV-associated oxidative stress play a pivotal role in promoting reproductive aging. Further studies are still needed to understand the pathogenesis of ovarian dysfunction and the possible role of cART in WLWH.
Acknowledgments
We thank all the patients who participated in the study and the staff of the Clinic of Infectious Diseases and Tropical Medicine and of the Clinic of Obstetrics and Gynecology at ASST Santi Paolo e Carlo who cared for the patients.
We are particularly thankful to Professor Alessandro Cozzi-Lepri for his precious help and support in statistical analyses.
Presented in part at 9th IAS 2017, July 23 to 26, 2017, Paris, France. Poster #WEPEB0567.
Author contributions
EM performed lab experiments, analyzed and interpreted the data, designed the figures and wrote the manuscript. CT, MAu, VB and MAl contributed to interpreting the data and wrote the manuscript. VS administered and collected the gynecological questionnaires. ESC performed lab experiments. LG performed the statistical analyses. ADM and AMM contribute to data interpretation and reviewed the manuscript. MR conceived and supervised the study. GM conceived and supervised the study and finalized the draft of the manuscript.
Conceptualization: Esther Merlini, Giulia Marchetti.
Data curation: Valentina Sacchi.
Formal analysis: Esther Merlini, Lidia Gazzola.
Investigation: Esther Merlini, Elvira Stefania Cannizzo.
Methodology: Lidia Gazzola.
Supervision: Antonella d’Arminio Monforte, Anna Maria Marconi, Marina Ravizza, Giulia Marchetti.
Validation: Camilla Tincati, Matteo Augello, Valeria Bono, Marina Allegrini, Giulia Marchetti.
Writing – original draft: Esther Merlini.
Writing – review & editing: Camilla Tincati, Matteo Augello, Valeria Bono, Marina Allegrini, Antonella d’Arminio Monforte, Anna Maria Marconi, Marina Ravizza, Giulia Marchetti.
Footnotes
Abbreviations: AMH = anti-Müllerian hormone, AOR = adjusted odds ratio, BMI = body mass index, cART = combined anti-retroviral therapy, CI = confidence interval, DAG = directed acyclic graph, HIV = human immunodeficiency virus, OR = odds ratio, ROMO1 = reactive oxygen species modulator 1, ROS = reactive oxygen species, sVCAM-1 = soluble vascular cell adhesion molecule 1, TNF-α = tumor necrosis factor α, WLWH = women living with HIV.
How to cite this article: Merlini E, Tincati C, Sacchi V, Augello M, Bono V, Cannizzo ES, Allegrini M, Gazzola L, Monforte Ad, Marconi AM, Ravizza M, Marchetti G. Predictors of low ovarian reserve in cART-treated women living with HIV. Medicine. 2021;100:39(e27157).
This work was partially supported by Fondazione di Comunità Milano, grant number FON_NAZ20ADARM_01.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Yalamanchi S, Dobs A, Greenblatt RM. Gonadal function and reproductive health in women with human immunodeficiency virus infection. Endocrinol Metab Clin North Am 2014;43:731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nguyen RHN, Gange SJ, Wabwire-Mangen F, et al. Reduced fertility among HIV-infected women associated with viral load in Rakai District, Uganda. Int J STD AIDS 2006;17:842–6. [DOI] [PubMed] [Google Scholar]
- [3].Gurtcheff SE, Klein NA. Diminished ovarian reserve and infertility. Clin Obstet Gynecol 2011;54:666–74. [DOI] [PubMed] [Google Scholar]
- [4].Yücel B, Kelekci S, Demirel E. Decline in ovarian reserve may be an undiagnosed reason for unexplained infertility: a cohort study. Arch Med Sci 2018;14:527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Visser JA, de Jong FH, Laven JSE, Themmen APN. Anti-Müllerian hormone: a new marker for ovarian function. Reproduction 2006;131:01–9. [DOI] [PubMed] [Google Scholar]
- [6].Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-müllerian hormone as a marker of ovarian reserve. Aust New Zeal J Obstet Gynaecol 2005;45:20–4. [DOI] [PubMed] [Google Scholar]
- [7].Van Rooij IAJ, Den Tonkelaar I, Broekmans FJM, et al. Anti-müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause 2004;11:601–6. [DOI] [PubMed] [Google Scholar]
- [8].Pankhurst MW, Chong YH. Variation in circulating antimüllerian hormone precursor during the periovulatory and acute postovulatory phases of the human ovarian cycle. Fertil Steril 2016;106: 1238-1243.e2. [DOI] [PubMed] [Google Scholar]
- [9].Zec I, Tislaric-Medenjak D, Bukovec Megla Z, Kucak I. Anti-Müllerian hormone: a unique biochemical marker of gonadal development and fertility in humans. Biochem Medica 2011;21:219–30. [DOI] [PubMed] [Google Scholar]
- [10].Alvaro Mercadal B, Imbert R, Demeestere I, et al. AMH mutations with reduced in vitro bioactivity are related to premature ovarian insufficiency. Hum Reprod 2015;30:1196–202. [DOI] [PubMed] [Google Scholar]
- [11].Santulli P, De Villardi D, Gayet V, et al. Decreased ovarian reserve in HIV-infected women. AIDS 2016;30:1083–8. [DOI] [PubMed] [Google Scholar]
- [12].Wessman M, Korsholm A-S, Bentzen JG, et al. Anti-müllerian hormone levels are reduced in women living with human immunodeficiency virus compared to control women: a case–control study from Copenhagen, Denmark. J Virus Erad 2018;4:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Scherzer R, Bacchetti P, Messerlian G, et al. Impact of CD4+ lymphocytes and HIV infection on anti-Müllerian hormone levels in a large cohort of HIV-infected and HIV-uninfected women. Am J Reprod Immunol 2015;73:273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Looby SE, Fitch KV, Srinivasa S, et al. Reduced ovarian reserve relates to monocyte activation and subclinical coronary atherosclerotic plaque in women with HIV. AIDS 2016;30:383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Merhi ZO, Seifer DB, Weedon J, et al. Circulating vitamin D correlates with serum antiMüllerian hormone levels in late-reproductive-aged women: women's Interagency HIV Study. Fertil Steril 2012;98:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Leng SX, Margolick JB. Aging, sex, inflammation, frailty, and CMV and HIV infections. Cell Immunol 2020;348:104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aberg JA. Aging, inflammation, and HIV infection. Top Antivir Med 2012;20:101–5. [PMC free article] [PubMed] [Google Scholar]
- [18].Huang Y, Hu C, Ye H, et al. Inflamm-aging: a new mechanism affecting premature ovarian insufficiency. J Immunol Res 2019;2019:8069898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shi L, Zhang J, Lai Z, et al. Long-term moderate oxidative stress decreased ovarian reproductive function by reducing follicle quality and progesterone production. PLoS One 2016;11:e0162194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ficicioglu C, Cenksoy PO, Yildirim G, Kaspar C. Which cut-off value of serum anti-Müllerian hormone level can predict poor ovarian reserve, poor ovarian response to stimulation and in vitro fertilization success? A prospective data analysis. Gynecol Endocrinol 2014;30:372–6. [DOI] [PubMed] [Google Scholar]
- [21].Karkanaki A, Vosnakis C, Panidis D. The clinical significance of anti-müllerian hormone evaluation in gynecological endocrinology. Hormones 2011;10:95–103. [DOI] [PubMed] [Google Scholar]
- [22].Marcus JL, Chao CR, Leyden WA, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016;73:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011;53:1120–6. [DOI] [PubMed] [Google Scholar]
- [24].Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000;908:244–54. [DOI] [PubMed] [Google Scholar]
- [25].Lee GY, You DG, Lee HR, Hwang SW, Justin Lee C, Yoo YD. Romo1 is a mitochondrial nonselective cation channel with viroporin-like characteristics. J Cell Biol 2018;217:2059–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ye L, Mao S, Fang S, Zhang J, Tan Y, Gu W. Increased serum Romo1 was correlated with lung function, inflammation, and oxidative stress in chronic obstructive pulmonary disease. Inflammation 2019;42:1555–60. [DOI] [PubMed] [Google Scholar]
- [27].Amini MA, Talebi SS, Karimi J. Reactive oxygen species modulator 1 (ROMO1), a new potential target for cancer diagnosis and treatment. Chonnam Med J 2019;55:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ye L, Qian Y, Li Q, et al. Serum Romo1 is significantly associated with disease severity in patients with obstructive sleep apnea syndrome. Sleep Breath 2018;22:743–8. [DOI] [PubMed] [Google Scholar]
- [29].Skinner MK, Schmidt M, Savenkova MI, Sadler-Riggleman I, Nilsson EE. Regulation of granulosa and theca cell transcriptomes during ovarian antral follicle development. Mol Reprod Dev 2008;75:1457–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Machelon V, Nomé F, Durand-Gasselin I, Emilie D. Tumor necrosis factor-α induces interleukin-6 mRNA and protein in human granulosa luteinizing cells via protein tyrosine kinase without involving ceramide. Mol Cell Endocrinol 1997;126:173–84. [DOI] [PubMed] [Google Scholar]
- [31].Duffy DM, Ko C, Jo M, Brannstrom M, Curry TE. Ovulation: parallels with inflammatory processes. Endocr Rev 2019;40:369–416. [DOI] [PMC free article] [PubMed] [Google Scholar]