Abstract
Rationale:
Peutz-Jeghers syndrome (PJS), a rare autosomal dominant disorder, is characterized by mucocutaneous pigmentations, hamartomatous polyps in the gastrointestinal tract, and a high risk of developing various malignancies. To the best of our knowledge, only 1 case of appendiceal carcinoid associated with PJS has been previously reported in the pediatric population.
Patient concerns:
We report a 7-year-old girl who was admitted for severe, intermittent abdominal pain and cramps, nausea, and vomiting. Multiple brown melanotic macules on the lips, buccal mucosa, and the tongue were noted.
Diagnosis:
A plain abdominal X-ray in a standing position revealed dilated intestinal loops with multiple air-fluid levels. A computed tomography scan of the abdomen showing a “coffee bean” appearance of the jejunal loop with a transition point to the duodenal loop. Axial-contrast-enhanced computed tomography scan of the abdomen showing dilated jejunum loops, filled with fluid with the swirled appearance of mesentery typical for volvulus. The diagnosis of PJS was based on clinical findings along with the histopathologic confirmation of the hamartomatous polyps.
Interventions:
An emergency laparotomy was performed, revealing a jejunojejunal intussusception starting 40 cm from the duodenojejunal flexure. Jejunotomy revealed that a lead-point intussusception was a necrotic hamartomatous polyp. After resecting the involved jejunal necrotic segment, including the polyp, end-to-end jejuno-jejunal anastomosis was performed. Further exploration revealed the presence of a jejunal mass 80 cm from the duodenojejunal flexure identified as another hamartomatous pedunculated polyp. The polyp was resected, and the enterotomy was then closed transversely. The grossly normal appendix was also removed.
Outcomes:
Clinical findings along with the histopathologically confirmed hamartomatous polyps were consistent with PJS. An appendiceal carcinoid (well-differentiated neuroendocrine tumor, European Neuroendocrine Tumor Society stage pT2) was incidentally detected during histological examination of the appendix. The patient and parents were counseled accordingly, focusing on active surveillance and control of symptoms. Two additional hamartomatous polyps (gastric and jejunal) were detected endoscopically and resected in the fourth postoperative week. A regular, 1-year follow-up and surveillance revealed no complications or recurrences.
Lessons:
Unusual neoplasms can occasionally be encountered in well-defined syndromes such as PJS. Therefore, active follow-up and surveillance are mandatory for all patients with PJS.
Keywords: appendix, carcinoid, children, hamartomas, intussusception, Peutz-Jeghers syndrome
1. Introduction
The Peutz-Jeghers syndrome (PJS) is an autosomal dominant disease (OMIM#175200) caused by a germline mutation of the serine/threonine kinase 11 (STK11) gene on chromosome 19p13.3.[1,2] PJS is characterized by mucocutaneous pigmentations, hamartomatous polyps in the gastrointestinal (GI) tract, and a significantly increased risk of various cancers, particularly in females.[1,2] The incidence of PJS is estimated to be between 1 in 50,000 to 1 in 200,000 live births.[3,4]
The diagnosis of PJS is made clinically when at least 2 of the following 3 diagnostic criteria are met: mucocutaneous lentiginosis, a family history of PJS, and at least 2 hamartomatous polyps of the GI tract.[3] Frequent complications in patients with PJS include bleeding, obstruction, and intussusception.[4]
Patients with PJS have an increased risk of various cancers and shortened life expectancy.[5] Most cancers originate from the GI tract, followed by breast, ovarian, cervical, lung, pancreatic, uterine, and testicular tumors.[3,5]
We herein present a case of a 7-year-old girl with appendiceal carcinoid as an incidental finding on laparotomy for jejunojejunal intussusception. Intussusception was caused by jejunal hamartomatous polyps, which were parts of the PJS spectrum. To the best of our knowledge, only 1 case of appendiceal carcinoid associated with PJS has been reported in the pediatric population so far.[6] Our patient is probably the youngest case reported so far.
2. Case report
A 7-year-old girl was admitted to our department for severe, intermittent abdominal pain and cramps, nausea, and vomiting. She appeared lethargic with generalized weakness but conscious. These symptoms were gradually intensified within 24 hours of the onset of pain.
Physical examination was remarkable for epigastric and periumbilical tenderness and mild abdominal distention. In addition, multiple brown melanotic macules on the lips, buccal mucosa, and the tongue were noted (Fig. 1A and B). A plain abdominal X-ray in a standing position revealed dilated intestinal loops with multiple air-fluid levels. The abdominal ultrasonography findings suggested small bowel intussusception, but the presumed pathologic lead point for the intussusception was not found. Reformatted coronal computed tomography (CT) scan of the abdomen showing a “coffee bean” appearance of the jejunal loop with a transition point to the duodenal loop (Fig. 2A and C). Axial-contrast-enhanced CT scan of the abdomen showing dilated jejunum loops, filled with fluid with the swirled appearance of mesentery typical for volvulus (Fig. 2B). Reformatted sagittal CT scan showing dilated jejunal loop with mild postcontrast enhancing and swirled mesentery (Fig. 2D).
Figure 1.
A–B. The dark pigmented spots (melanocytic macules) affecting the lips, buccal mucosa, and the tongue in a 7-year-old girl.
Figure 2.
A–D. Reformatted coronal CT scan of the abdomen showing “coffee bean” appearance of the jejunal loop (red arrow) with transition point to duodenal loop (pointed red arrow) (A and C). Axial-contrast-enhanced CT scan of the abdomen showing dilated jejunum loops, filled with fluid (red arrows) with the swirled appearance of mesentery typical for volvulus (pointed red arrow) (B). Reformatted sagittal CT scan showing dilated jejunal loop with mild postcontrast enhancing (red arrow) and swirled mesentery (pointed red arrow) (D). CT = computed tomography.
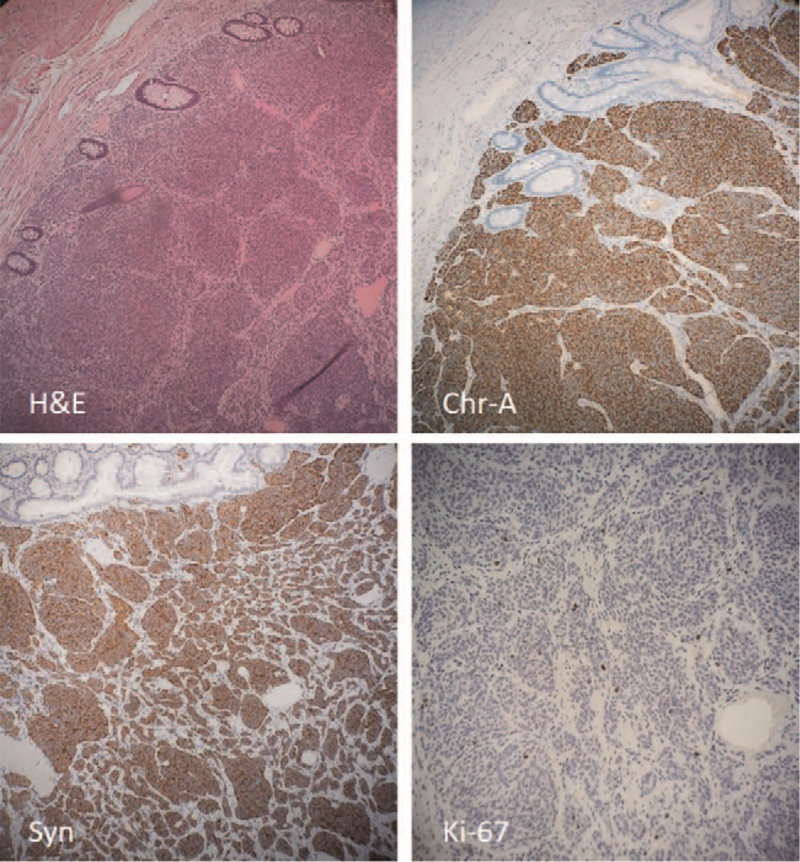
After urgent resuscitation and antibiotics, the patient was taken for emergency laparotomy. This revealed a jejunojejunal intussusception starting 40 cm from the duodenojejunal flexure (Fig. 3A). The gently manual reduction was made, but jejunal segmental necrosis was found. Jejunotomy revealed that a lead-point intussusception was a necrotic hamartomatous polyp (Fig. 3B). After resecting the involved jejunal necrotic segment (approximately 10 cm long), including the polyp (Fig. 3C), end-to-end jejuno-jejunal anastomosis was performed. Further exploration revealed the presence of a jejunal mass 80 cm from the duodenojejunal flexure identified as another hamartomatous pedunculated polyp (Fig. 3D). The polyp was resected, and the enterotomy was then closed transversely. The macroscopically normal appendix was also removed. The postoperative course was uneventful. Histologic findings confirmed the hamartomatous polyps. However, the histopathological examination of the appendix revealed an 8 × 6 mm large well-differentiated neuroendocrine tumor (carcinoid); the tumor cells infiltrated submucosa, muscularis propria, with minimal infiltration of the subserosa/mesoappendix (European Neuroendocrine Tumor Society stage pT2)[7] (Fig. 4A). The tumor cells showed low mitotic activity (<2 mitoses/2 mm2) and a low Ki-67 index (<1%) (Fig. 4D). The tumor cells were also diffusely positive for neuroendocrine markers Chromogranin-A (Fig. 4B) and Synaptophysin (Fig. 4C). These findings were consistent with a low-grade (G1) neuroendocrine tumor (carcinoid).
Figure 3.
A-D. Intraoperative view of the jejunojejunal intussusception (A); Hamartomatous polyp was the leading point of intussusception (B); The resected jejunal segment, including the polyp (C); Another hamartomatous pedunculated polyp in the lower jejunum (D).
Figure 4.
A-D. Hematoxylin and eosin (H&E) slide revealing a well-differentiated neuroendocrine tumor (carcinoid, G1) infiltrating the appendiceal wall; neoplastic cells were positive for neuroendocrine markers: Chromogranin-A (Chr-A) and Synaptophysin (Syn); the proliferation rate of the neoplastic cells was low (<1%) as measured by Ki-67 (MIB-1 antibody).
The patient had a negative family history of PJS or other hereditary cancers, and both parents were healthy. Routine STK11 genetic testing was not available. Given that the clinical criteria for the diagnosis of PJS were fulfilled,[2,3,8,9] the counseling was provided,[2] focusing on active surveillance and control of symptoms.
In the fourth postoperative week, upper GI endoscopy and colonoscopy were done, revealing 2 additional hamartomatous polyps, first in the gastric fundus and the second in the jejunum. Endoscopic polypectomies were done, and hamartomatous polyps were histopathologically confirmed. The child is under regular follow-up and surveillance, and no recurrences or further complications have been found within a 1-year follow-up.
3. Discussion
This case report describes a rare association between the appendiceal carcinoid (well-differentiated neuroendocrine tumor) and PJS in a prepubertal girl. The patient had no family history of PJS, indicating a possible de novo STK11 mutation, which is present in ∼45% of affected individuals with a negative family history of PJS.[10] It is also well-documented that ∼50% of the patients with PJS have to undergo surgery by age 18 because of polyps-related complications, including abdominal pain, rectal bleeding, anemia, bowel obstruction, or intussusception.[11] Intussusception caused by intraluminal polyps in PJS is a rare cause of intestinal obstruction. It can potentially cause life-threatening complications. Unlike a classic clinical triad of intussusception (colicky/intermittent abdominal pain, vomiting, and red “currant jelly” stool), our patient did not have rectal bleeding. Since ultrasonography has a low sensitivity for certain causes of intussusception, such as intraluminal polyps,[12] the cause of intussusception in our case was explored by surgery. However, pre-operative CT in our patient showed evidence of small-bowel obstruction with focal intussusception.
Although the mechanism of carcinogenesis remains debatable, the patients with PJS have a 93% life-time-risk for various cancers,[13] predominantly of GI origins.[5] Utsunomiya et al[14] reported that benign complications of the polyps, such as bleeding and intussusception, predominate in the first 3 decades of life. In contrast, malignant complications become more common after that. The clinical presentation of our patient with polyp-induced intussusception confirmed this observation.
The causal relationship between PJS and appendiceal carcinoid remains unclear. Our comprehensive literature survey (PubMed/MEDLINE, Scopus, and Web of Science Core Collection) revealed only 1 case of appendiceal carcinoid in patients with PJS.[6] However, other GI neuroendocrine neoplasms (carcinoid of the rectum and neuroendocrine carcinoma of the duodenum) have been reported in adult patients with PJS[15,16] (Table 1). Also, other appendiceal malignancies (adenocarcinoma) have been found in patients with PJS.[17–20] In addition, Mojsilovic et al[21] reported a 3-generation family with PJS followed over 37 years. Among others, the authors found endocrine hyperplasia of chromogranin and serotonin-positive cells while some family members had clinically carcinoid-like symptoms[21] (Table 1). The link between the PJS and neuroendocrine neoplasms is not clear. However, these tumors have been closely linked to several related cancer syndromes such as neurofibromatosis type 1 and tuberous sclerosis complex.[22] Lodish et al[22] provided the hypothesis that the deregulation of the mammalian target of rapamycin pathway might be an underlying mechanism for specific tumors in these related syndromes.
Table 1.
Overview of the studies that reported neuroendocrine neoplasms of the gastrointestinal tract in patients with Peutz-Jeghers syndrome.
| Study (year) (reference) | Age (years) | Gender | Diagnosis | Anatomic location |
| Mojsilovic et al (2015)[21] | 21 (initially diagnosed with PJS at the age of 8) | Male | Neuroendocrine hyperplasia (Chr-A and serotonin positive cells) | Jejunum and ileum |
| Hofmann et al (2014)[6] | 21 | Male | Carcinoid | Appendix |
| Chen et al (2012)[16] | 42 | Male | Neuroendocrine carcinoma | Duodenum |
| Wada et al (1998)[15] | 8 | Male | Carcinoid | Rectum |
Chr-A = Chromogranin-A, PJS = Peutz-Jeghers syndrome.
The most common appendiceal tumors are mucinous neoplasms (e.g., low-grade appendiceal mucinous neoplasm and mucinous carcinomas), adenomas, serrated polyps, goblet cell tumors, neuroendocrine tumors, and colonic type carcinomas.[23] Among these tumors, appendiceal carcinoids, neoplasms derived from the subepithelial neuroendocrine cells of the appendix, are the most frequent tumors, accounting for up to 60% of all appendiceal tumors.[24] Although the appendix is the most common site for carcinoids in children,[25,26] these tumors are very rare, with the reported frequency of ∼0.2% to 0.4% of all pediatric appendectomies.[27,28] The clinical presentation of symptomatic cases is similar to that of acute appendicitis. However, appendiceal carcinoids are usually asymptomatic and are detected incidentally during surgery,[29] as recorded in our case. In addition, appendiceal carcinoids are associated with the most favorable survival rates compared with other neuroendocrine tumors.[30] The management algorithm for appendiceal carcinoids developed 3 decades ago has not changed significantly to date.[31,32] However, because of the rarity of carcinoid tumors in children, management guidelines have been challenging to generate.[33] Although extended resection and right colectomies are currently recommended for tumors >2 cm in adults, this treatment modality is inconsistent in the pediatric population due to reports that children with tumors ≥2 remained disease-free for 10 years after appendectomy alone.[34,35] For this reason, there are recommendations for less aggressive management of appendiceal carcinoids in children.[34] Given the tumor size in our patient (<1 cm), we also opted for a less aggressive surgical approach and active surveillance of the PJS patient.[2]
In conclusion, PJS is a rare inherited syndrome associated with various non-neoplastic and neoplastic complications, both of which require thorough surveillance and regular follow-up. Furthermore, GI carcinoids, including the appendiceal reported in our study, have rarely been described in PJC patients, which merits further research.
Author contributions
Conceptualization: Zlatan Zvizdic, Semir Vranic.
Data curation: Zlatan Zvizdic, Emir Milisic, Nermina Ibisevic, Irmina Sefic Pasic, Semir Vranic.
Formal analysis: Zlatan Zvizdic, Semir Vranic.
Investigation: Zlatan Zvizdic, Emir Milisic, Nermina Ibisevic, Irmina Sefic Pasic.
Writing – original draft: Zlatan Zvizdic, Semir Vranic.
Writing – review & editing: Zlatan Zvizdic, Semir Vranic.
Footnotes
Abbreviations: CT = computed tomography, GI = gastrointestinal, PJS = Peutz-Jeghers syndrome, STK11 = serine/threonine kinase 11.
How to cite this article: Zvizdic Z, Milisic E, Ibisevic N, Pasic IS, Vranic S. Appendiceal carcinoid in a pediatric patient with Peutz-Jeghers syndrome: a case report and comprehensive literature review. Medicine. 2021;100:39(e27389).
The Qatar National Library funded the publication of this article.
This study has been performed per the ethical standards of the Declaration of Helsinki (1964). The local institutional review board has the policy not to review case reports. Written informed consent was obtained from the patient's mother for anonymized patient information and images to be published in this article.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Daniell J, Plazzer JP, Perera A, Macrae F. An exploration of genotype-phenotype link between Peutz-Jeghers syndrome and STK11: a review. Fam Cancer 2018;17:421–7. [DOI] [PubMed] [Google Scholar]
- [2].Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut 2010;59:975–86. [DOI] [PubMed] [Google Scholar]
- [3].Giardiello FM, Trimbath JD. Peutz-Jeghers syndrome and management recommendations. Clin Gastroenterol Hepatol 2006;4:408–15. [DOI] [PubMed] [Google Scholar]
- [4].Kopacova M, Tacheci I, Rejchrt S, Bures J. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol 2009;15:5397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Riegert-Johnson DL, Westra W, Roberts M. High cancer risk and increased mortality in patients with Peutz-Jeghers syndrome. Gut 2012;61:322.author reply 322-323. [DOI] [PubMed] [Google Scholar]
- [6].Hofmann S, Barth TF, Kornmann M, Henne-Bruns D. Appendix carcinoid associated with the Peutz-Jeghers syndrome. Int J Surg Case Rep 2014;5:964–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pape UF, Niederle B, Costa F, et al. ENETS consensus guidelines for neuroendocrine neoplasms of the appendix (excluding goblet cell carcinomas). Neuroendocrinology 2016;103:144–52. [DOI] [PubMed] [Google Scholar]
- [8].Tomlinson IP, Houlston RS. Peutz-Jeghers syndrome. J Med Genet 1997;34:1007–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aaltonen LA, Jarvinen H, Gruber SB, Billaud M, Jass JR. Tumours of the small intestine: Peutz-Jeghers syndrome. World Health Organization Classification of Tumours: Pathology and Genetics. Tumours of the Digestive System. Lyon: IARC Press; 2000. [Google Scholar]
- [10].McGarrity TJ, Amos CI, Baker MJ. Peutz-Jeghers Syndrome. GeneReviews® [Internet]. February 2001. Available at: https://www.ncbi.nlm.nih.gov/books/NBK1266/. Updated July 14, 2016. [Google Scholar]
- [11].Gammon A, Jasperson K, Kohlmann W, Burt RW. Hamartomatous polyposis syndromes. Best Pract Res Clin Gastroenterol 2009;23:219–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Navarro O, Daneman A. Intussusception. Part 3: diagnosis and management of those with an identifiable or predisposing cause and those that reduce spontaneously. Pediatr Radiol 2004;34:305–12. quiz 369. [DOI] [PubMed] [Google Scholar]
- [13].Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000;119:1447–53. [DOI] [PubMed] [Google Scholar]
- [14].Utsunomiya J, Gocho H, Miyanaga T, Hamaguchi E, Kashimure A. Peutz-Jeghers syndrome: its natural course and management. Johns Hopkins Med J 1975;136:71–82. [PubMed] [Google Scholar]
- [15].Wada K, Asoh T, Imamura T, et al. Rectal carcinoid tumor associated with the Peutz-Jeghers syndrome. J Gastroenterol 1998;33:743–6. [DOI] [PubMed] [Google Scholar]
- [16].Chen XD, Yu YY, Yang L, Rui YY, Zhou ZG. Duodenal intussusception due to a giant neuroendocrine carcinoma in a patient with Peutz-Jeghers syndrome: case report and systematic review. Eur J Gastroenterol Hepatol 2012;24:722–6. [DOI] [PubMed] [Google Scholar]
- [17].Kurihara K, Suganuma T. Appendiceal cancer leading to intussusception detected incidentally during follow-up for Peutz-Jeghers syndrome. Clin J Gastroenterol 2020;13:1136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Miyahara M, Saito T, Etoh K, et al. Appendiceal intussusception due to an appendiceal malignant polyp--an association in a patient with Peutz-Jeghers syndrome: report of a case. Surg Today 1995;25:834–7. [DOI] [PubMed] [Google Scholar]
- [19].Takahashi M, Sawada T, Fukuda T, Furugori T, Kuwano H. Complete appendiceal intussusception induced by primary appendiceal adenocarcinoma in tubular adenoma: a case report. Jpn J Clin Oncol 2003;33:413–5. [DOI] [PubMed] [Google Scholar]
- [20].Matsushita K, Kagawa Y, Kato T, et al. A case of appendiceal intussusception induced by appendiceal carcinoma. Gan To Kagaku Ryoho 2014;41:1628–30. [PubMed] [Google Scholar]
- [21].Mojsilovic M, Katic V, Ilic I, et al. A thirty-seven-year follow-up of Peutz-Jeghers syndrome across three generations. Acta Fac Medicae Naiss 2015;32:221–6. [Google Scholar]
- [22].Lodish MB, Stratakis CA. Endocrine tumours in neurofibromatosis type 1, tuberous sclerosis and related syndromes. Best Pract Res Clin Endocrinol Metab 2010;24:439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ruoff C, Hanna L, Zhi W, Shahzad G, Gotlieb V, Saif MW. Cancers of the appendix: review of the literatures. ISRN Oncol 2011;2011:728579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Connor SJ, Hanna GB, Frizelle FA. Appendiceal tumors: retrospective clinicopathologic analysis of appendiceal tumors from 7,970 appendectomies. Dis Colon Rectum 1998;41:75–80. [DOI] [PubMed] [Google Scholar]
- [25].Neves GR, Chapchap P, Sredni ST, Viana CR, Mendes WL. Childhood carcinoid tumors: description of a case series in a Brazilian cancer center. Sao Paulo Med J 2006;124:21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zvizdić Z, Đuran A, Karavdic K, Jakic A, Milisic E. Carcinoid tumors of the appendix vermiform in children-ten year analysis of 1503 appendectomies. BH Surgery 2011;1:100–3. [Google Scholar]
- [27].Doede T, Foss HD, Waldschmidt J. Carcinoid tumors of the appendix in children–epidemiology, clinical aspects and procedure. Eur J Pediatr Surg 2000;10:372–7. [DOI] [PubMed] [Google Scholar]
- [28].Akova F, Aydin E, Nur Eray Y, et al. Long-term outcomes in pediatric appendiceal carcinoids: Turkey experience. Eur J Pediatr 2018;177:1845–50. [DOI] [PubMed] [Google Scholar]
- [29].Moris D, Tsilimigras DI, Vagios S, et al. Neuroendocrine neoplasms of the appendix: a review of the literature. Anticancer Res 2018;38:601–11. [DOI] [PubMed] [Google Scholar]
- [30].Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Moertel CG, Weiland LH, Nagorney DM, Dockerty MB. Carcinoid tumor of the appendix: treatment and prognosis. N Engl J Med 1987;317:1699–701. [DOI] [PubMed] [Google Scholar]
- [32].Bamboat ZM, Berger DL. Is right hemicolectomy for 2.0-cm appendiceal carcinoids justified? Arch Surg 2006;141:349–52. discussion 352. [DOI] [PubMed] [Google Scholar]
- [33].Kim SS, Kays DW, Larson SD, Islam S. Appendiceal carcinoids in children--management and outcomes. J Surg Res 2014;192:250–3. [DOI] [PubMed] [Google Scholar]
- [34].Assadi M, Kubiak R, Kaiser G. Appendiceal carcinoid tumors in children: does size matter? Med Pediatr Oncol 2002;38:65–6. [DOI] [PubMed] [Google Scholar]
- [35].Cernaianu G, Tannapfel A, Nounla J, Gonzalez-Vasquez R, Wiesel T, Trobs RB. Appendiceal carcinoid tumor with lymph node metastasis in a child: case report and review of the literature. J Pediatr Surg 2010;45:e1–5. [DOI] [PubMed] [Google Scholar]