Abstract
Background
The number of adults across the globe with significant depressive symptoms has grown substantially during the COVID-19 pandemic. The extant literature supports exercise as a potent behaviour that can significantly reduce depressive symptoms in clinical and non-clinical populations.
Objective
Using a suite of mobile applications, at-home exercise, including high intensity interval training (HIIT) and/or yoga, was completed to reduce depressive symptoms in the general population in the early months of the pandemic.
Methods
A 6-week, parallel, multiarm, pragmatic randomised controlled trial was completed with four groups: (1) HIIT, (2) Yoga, (3) HIIT+yoga, and (4) waitlist control (WLC). Low active, English-speaking, non-retired Canadians aged 18–64 years were included. Depressive symptoms were measured at baseline and weekly following randomisation.
Results
A total of 334 participants were randomised to one of four groups. No differences in depressive symptoms were evident at baseline. The results of latent growth modelling showed significant treatment effects in depressive symptoms for each active group compared with the WLC, with small effect sizes (ESs) in the community-based sample of participants. Treatment groups were not significantly different from each other. Effect sizes were very large (eg, week 6 ES range=−2.34 to −2.52) when restricting the analysis only to participants with high depressive symptoms at baseline.
Conclusions
At-home exercise is a potent behaviour to improve mental health in adults during the pandemic, especially in those with increased levels of depressive symptoms. Promotion of at-home exercise may be a global public health target with important personal, social and economic implications as the world emerges scathed by the pandemic.
Trial registration number
Keywords: depression, exercise, randomised controlled trial
Introduction
Early in the COVID-19 pandemic, fears of infection, economic hardship and global stay-at-home mandates were widely predicted to worsen mental health (ie, the disruption of psychological well-being), including depression, anxiety and general distress.1–3 The prediction has been borne out, with global rates of depression and anxiety reaching 28% and 26% during the pandemic, respectively.4 Few studies have investigated whether global rates of mental health concerns actually changed from pre-COVID-19 to the pandemic period. In a national poll completed 1 month into the pandemic, 10% and 23% of Canadians reported that they had high or some levels of depression, respectively, an increase from 4% and 15% reported prior to the pandemic.5 Twofold to threefold increases were also reported in the USA6 and the UK7 in the early months of the pandemic compared with before the pandemic.
Researchers and healthcare professionals promoted a wide range of approaches to maintain the mental health of all individuals during this pandemic, from actions individuals can take within their homes and outdoors, such as exercise, to assessment and treatment considerations that healthcare providers and institutions can implement.1–3 The WHO8 and global government agencies (eg, US Centers for Disease Control and Prevention9 and Public Health England)10 similarly recommended that the public engage in physical activity and exercise to attain and maintain mental health during the pandemic. These recommendations are supported by an extant literature providing compelling evidence for impactful prevention of11 and reductions in12 depressive symptoms in clinical and non-clinical populations following the adoption of physical activity programming. Many trials have also revealed the many neurobiological effects of long-term exercise in improving depressive symptoms.13 Yet, with the mandated closure of fitness centres and outdoor recreation sites (eg, local and state/provincial parks) at the start of the pandemic, opportunities for engaging in healthy behaviours remained limited to one’s home for the most part.
Our study tested whether completing exercise at home that required little physical space or equipment would lead to reductions in depressive symptoms among Canadian adults in the spring and summer of 2020. Depressive symptoms were chosen as the trial’s primary mental health outcome since loneliness/social isolation are strongly associated with depression14–16 and since loneliness/social isolation were particularly anticipated due to prevailing public health physical distancing mandates implemented during the early stages of the COVID-19 pandemic. Activities were completed with the use of a commercially available mobile application (app), with memberships provided free. Given that 88% of Canadians own smartphones17 and 94% of Canadians have home internet access, the use of a commercially available app that is both android and iOS compatible allowed us to test the effects of at-home exercise using app-based programs that are easily scalable to the public and widely implementable in the future, if effects are significant. The provision of free memberships and the minimal room or equipment required to complete the activities attended to some of the socioeconomic inequities that can result from at-home exercise during the pandemic, as highlighted by Sallis and colleagues.18
We partnered with a mobile application company, Down Dog (https://www.downdogapp.com), that has a suite of apps for a variety of activities that require little space or equipment. Two of the apps that were available for download at the start of the pandemic from Down Dog included whole body weight based high intensity interval training (HIIT) and yoga. Both HIIT19 and yoga20 have been shown to be effective in improving depressive symptoms. Participants in the active groups received access to either the HIIT or yoga application or access to both applications for 6 weeks.
The primary hypothesis of our pragmatic randomised clinical trial was that completing at-home HIIT and/or yoga with the use of a free mobile application will lead to significant declines in depressive symptoms in adults over a 6-week period compared with a waitlist control (WLC). We further explored whether the benefits were unique to or stronger for HIIT, yoga or their combination by comparing each group’s effects over time to one another. Finally, we tested whether the effects were more or less apparent in those with high depressive symptoms prerandomisation.
Methods
Trial design
The COvid-19 Pandemic and Exercise (COPE) trial was a parallel, multiarm, pragmatic randomised controlled trial, with participants allocated randomly to one of four treatment groups: (1) HIIT, (2) yoga, (3) HIIT+yoga or (4) WLC. Study protocol was registered at ClinicalTrials.gov and the Open Science Framework (https://osf.io/jbm63/).
Participants
Low active, English-speaking, Canadians aged 18–64 years, who were not retired at study entry and had access to the internet via a mobile device or computer were eligible to participate. Activity was assessed with the validated Stanford Leisure-Time Categorical Activity Item (L-CAT).21 Those who scored between 1 and 3 on the L-CAT were eligible to participate since these scores represent low activity as prescribed by the American College of Sports Medicine.22 Only individuals deemed capable of performing moderate intensity physical activity were eligible to participate, as assessed with the Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and, if necessary, further assessed with the Physical Activity Readiness Medical Examination (ePARmed-X+).23 Those hospitalised within the previous 3 months were not eligible, unless a note from their physician was provided stating their ability to participate.
Participants from across Canada were recruited through social media advertisements, including Facebook, Twitter and Instagram, reaching 21 406 unique viewers and 2731 engaged in the advertisements through shares, clicks or likes. The advertisements directed participants to the study website that provided a summary of the purpose of the study and inclusion/exclusion criteria. If interested, they were invited to email the study team to schedule an eligibility screening phone call. A study team member completed the screening using a scripted interview with Qualtrics, where eligibility screening data were stored. On agreement, eligible and interested participants received two links by email: (1) to the Qualtrics-based consent form for electronic signature and (2) to the PAR-Q+. Interested participants who were not cleared for exercise based on the PAR-Q+ or the ePARmed-X+ were required to receive clearance from their family physician or our team’s study physician. Following clearance, all participants completed our Qualtrics-based survey to assess the primary outcome: depressive symptoms.
Intervention
Participants randomised to one of the three treatment groups received a free 3-month membership to the mobile application version from Down Dog to access the applicable programs according to group assignment (see online supplemental materials – Methods section for details about the app). Participants in the treatment groups were asked to complete a minimum 4 weekly 20 min sessions for 6 weeks. This accumulation was based on global recommendations to complete 75 min of high/vigorous intensity activity per week (equating to 150 min of moderate intensity activity).24 Seventy-five minutes of high intensity activity (for the HIIT condition) was averaged up to 80 min to translate the guidelines to 4 weekly sessions of equal length for study purposes. To ensure equivalence of weekly accumulated activity across intervention conditions, we asked participants in the yoga and HIIT+yoga conditions to also engage in four 20 min sessions per week. WLC participants were asked to remain at the same activity level as during recruitment during the 6-week study and received the free 3-month membership to the yoga and HIIT apps after the 6 weeks. To ensure anonymity on the Down Dog platform, each participant received a participant ID that was preregistered by a study team member on the Down Dog platform. This also allowed us to track their weekly progress.
bjsports-2021-104379supp001.pdf (690.8KB, pdf)
Outcome
The primary outcome was depressive symptoms, measured weekly from baseline to the end of the sixth week of the trial with the 10-item Center for Epidemiological Studies – Depression Scale (CESD).25 Examples of items include, ‘I was bothered by things that usually don’t bother me’ and ‘I could not get going.’ Scores ranged between 0 (‘Rarely or none of the time (less than 1 day)’) and 3 (‘Most or all of the time (5–7 days)’). Sum scores were produced (potential range from 0 to 30 (sample range: 0 to 30)). A cut-off score of 10 or above is considered significant depressive symptoms in community samples.
Sample size
Using Optimal Design Software,26 367 participants were required to detect a small effect size (ES) δ=0.30 based on a two-level curvilinear growth model with power (1 - b) set at 0.80 and alpha set at 0.05 for a seven time points repeated measures design with four groups. A 25% attrition was expected over 6 weeks, thus a sample size of 490 was considered to be sufficient for the trial.
Randomisation and allocation
Sequence generation for randomisation was completed using Excel. Each member of the recruiting team received an Excel book with multiple blocks of randomised groupings. All blocks contained one of each potential grouping and a randomly assigned number generated by the data manager (second author). The treatment groups within each block were then sorted by their randomised number, allowing for unique configurations within each block and the grouping hidden on the Excel sheet. A member of the study team would unhide the result of the randomisation and allocated the participants to their group once they had completed the baseline surveys. The data manager and principal investigator (PI) (first author) remained blind to the participants’ allocations throughout the trial. The PI was blind to all randomisation until all data were prepared for analysis and initial primary analysis was completed.
Changes to the trial
On 7 August 2020, study recruitment was terminated for several reasons. First, interest in the study dropped substantially in late June, potentially due to the fact that fitness centres across Canada started to open, as did parks. Second, we wanted to keep the time frame of recruitment narrow to maintain similarity across participants in terms of impact of the pandemic. Finally, while we were very conservative with our original expected ES, a small-to-medium ES of δ=0.40 or a medium ES of 0.50 could have as easily been expected based on previous meta-analyses,12 27 requiring 209 and 134 participants, respectively, or 278 and 179, accounting for 25% attrition. More information on these changes can be accessed here, submitted 8 August 2020: https://osfio/a65vd/.
Statistical approach
Means and SD, or number and percentages, were calculated for all continuous or categorical sociodemographic variables, respectively. Analyses of variance or χ2 analyses were completed for the continuous and categorical factors, respectively, to compare group differences. Imputation, using random forest methods,28 was conducted for depression symptom score when the participant did not complete all items in the survey at any week (see online supplemental statistical approach for information on imputation procedures and survey items completion rates on online supplemental table S1). All descriptive statistics, multiple imputation and visualisations were run using R Statistical software (V.4.0.2).
All randomised participants were included in the intent-to-treat analysis using Mplus (V.7.2).29 We adopted quadratic latent growth models29 based on the framework of structural equation models to account for non-linear trends in CESD scores over the 6 weeks. See online supplemental materials – statistical approach section for more details of our analytic approach and the equations of the conditional quadratic latent growth models.
To examine the treatment effects in depressive symptoms on the subpopulation with high depressive symptoms initially, we restricted the sample to participants with CESD scores of ≥1025 and, due to poor model fit when including the quadratic term, we used free time scores of the slope growth factor for non-linear trends (Muthén and Muthén, p. 124).29 The equations are similar to those used for the quadratic latent growth model, but only I and S were estimated, and S is freely estimated with specifying the first two time points to 0 and 1.
For all analyses, we computed effect sizes at each week using Feingold’s approach,30 equivalent to Cohen’s d (see online supplemental materials for additional details including Mplus code). Model fit indices used to ascertain model fit and fit statistics for the complete and restricted samples are included in the online supplemental materials - statistical approach section and online supplemental table S2, respectively. Maximum likelihood robust estimation was used for all the latent growth models because this type of estimator can easily handle outliers and missing data and provide more robust and accurate estimates. All codes are included in the online supplemental file 1.
Results
Participants
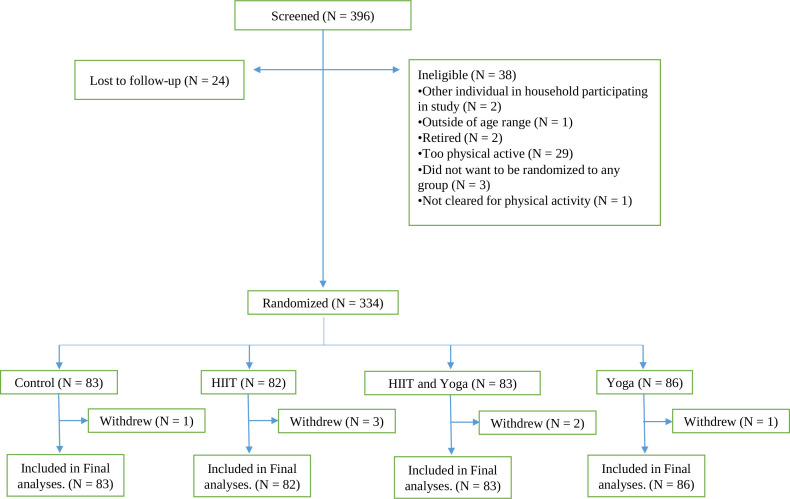
Three hundred and ninety-six individuals were screened for the COPE trial and, based on eligibility, 334 (84%) were enrolled between 27 May 2020 and 7 August 2020 (see figure 1, consort diagram). Descriptive statistics for the sociodemographic factors and depressive symptoms are presented in table 1. Treatment groups were not different from each other on any factor at baseline.
Figure 1.
Consort diagram.
Table 1.
Participant demographic information
| Variable | All n=334 |
WLC n=83 |
HIIT n=82 |
Yoga n=86 |
HIIT+yoga n=83 |
Baseline comparison |
| Age, years (SD) | 40.3 (12.4) | 41.0 (12.1) | 41.2 (12.7) | 37.8 (12.3) | 41.1 (12.6) | F(3, 329)=1.45, p=0.23 |
| CESD, score (SD) | 10.3 (5.78) | 10.4 (5.54) | 10.8 (6.23) | 10.4 (5.87) | 9.41 (5.43) | F(3, 330)=0.89, p=0.45 |
| Women, n (%) | 289 (87) | 74 (89) | 71 (87) | 72 (84) | 72 (87) | X 2 (3, n=334)=1.08, p=0.78 |
| Income, n (%)* | X 2 (15, n=334)=18.77, p=0.22 | |||||
| 0–40 000 | 30 (9) | 4 (5) | 11 (13) | 9 (10) | 6 (7) | |
| 40 001–80 000 | 68 (20) | 16 (19) | 12 (15) | 24 (28) | 16 (19) | |
| 80 001–120 000 | 66 (20) | 19 (23) | 14 (17) | 14 (16) | 19 (23) | |
| 120 001–160 000 | 51 (15) | 11 (13) | 12 (15) | 18 (21) | 10 (12) | |
| 160 000+ | 63 (19) | 20 (24) | 14 (17) | 11 (13) | 18 (22) | |
| No response | 56 (17) | 13 (16) | 19 (23) | 10 (12) | 14 (17) | |
| Employment status† | X 2 (12, n=334)=6.11, p=0.91 | |||||
| Full time | 177 (53) | 50 (60) | 40 (49) | 40 (47) | 47 (57) | |
| Part time | 44 (13) | 10 (12) | 13 (16) | 13 (15) | 8 (10) | |
| Not working | 48 (14) | 9 (11) | 14 (17) | 14 (16) | 11 (13) | |
| Student | 45 (13) | 10 (12) | 10 (12) | 14 (16) | 11 (13) | |
| Other | 20 (6) | 4 (5) | 5 (6) | 5 (6) | 6 (7) | |
| Education‡ | X 2 (12, n=334)=12.83, p=0.38 | |||||
| High school or less | 22 (7) | 3 (4) | 3 (4) | 10 (12) | 6 (7) | |
| College, trade school or certificate | 52 (16) | 10 (12) | 12 (15) | 15 (17) | 15 (18) | |
| Bachelor or equivalent | 139 (42) | 37 (45) | 34 (41) | 36 (42) | 32 (39) | |
| Postgraduate/professional training | 118 (35) | 33 (40) | 31 (38) | 24 (28) | 30 (36) | |
| No response | 3 (1) | 0 (0) | 2 (2) | 1 (1) | 0 (0) | |
| Ethnoracial identification§§ | X2 (6, n=334)=5.37, p=0.50 | |||||
| White, n (%) | 198 (59) | 50 (60) | 54 (66) | 43 (50) | 51 (61) | |
| Asian, n (%) | 87 (26) | 22 (27) | 16 (20) | 28 (33) | 21 (25) | |
| Other/multiple selections/did not answer, n (%) | 49 (15) | 11 (13) | 12 (15) | 15 (17) | 11 (13) | |
| Marital status¶ | X 2 (9, n=334)=6.95, p=0.64 | |||||
| Married, n (%) | 187 (56) | 49 (59) | 48 (59) | 43 (50) | 47 (57) | |
| Separated/divorced/widowed, n (%) | 26 (8) | 7 (8) | 6 (7) | 5 (6) | 8 (10) | |
| Single, n (%) | 115 (34) | 27 (33) | 25 (30) | 36 (42) | 27 (33) | |
| Other/refused to/did not answer, n (%) | 6 (2) | 0 (0) | 3 (4) | 2 (2) | 1 (1) | |
| LCAT | X 2 (6, n=334)=10.22, p=0.12 | |||||
| 1, n (%) | 19 (6) | 2 (2) | 4 (5) | 8 (9) | 5 (6) | |
| 2, n (%) | 172 (51) | 36 (43) | 49 (60) | 46 (53) | 41 (49) | |
| 3, n (%) | 143 (43) | 45 (54) | 29 (35) | 32 (37) | 37 (45) | |
Note: data are presented for all participants (all) and separated by randomisation group (waitlist control (WLC); high intensity interval training (HIIT); yoga (yoga); combination (HIIT+yoga)). All group comparisons between continuous variables were complete using one-way analyses of variance; the comparisons between categorical variables were done using χ2 tests.
*For details of how the categories were formed, please see online supplemental table S3.
†For details of how the categories were formed, please see online supplemental table S4.
‡For details of how the categories were formed, please see online supplemental table S5.
§For details of how the categories were formed, please see online supplemental table S6.
¶For details of how the categories were formed, please see online supplemental table S7.
CESD, Centre for Epidemiological Studies – Depression Scale; LCAT, Stanford Leisure Time Activity Categorical Item.
Adherence results
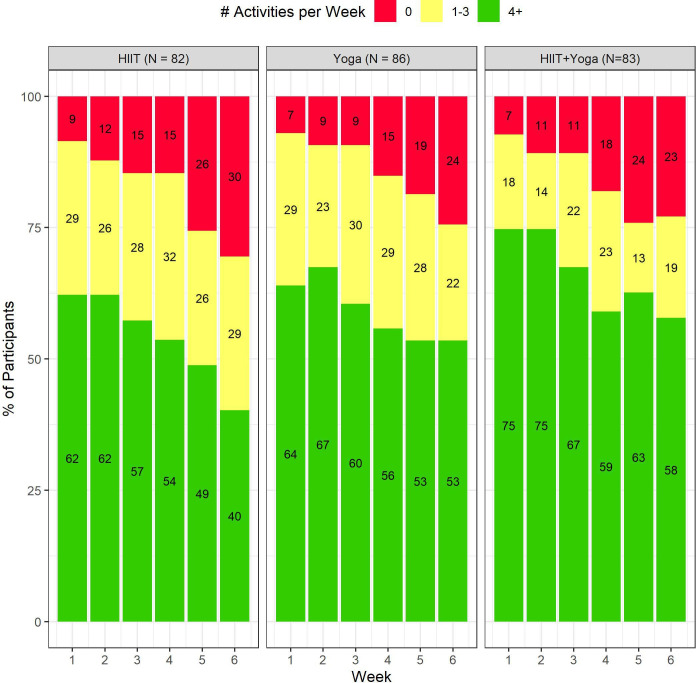
Sixty-two per cent, 64% and 75% of HIIT, yoga and HIIT+yoga participants, respectively, completed four or more sessions in the first week of the trial, with an additional 18%–29% completing between one and three workouts (figure 2). Adherence decreased during the study. While the majority of yoga and HIIT+yoga group participants were still meeting the requested four sessions per week by the end of the trial, only 40% in the HIIT group met these activity levels. Weekly completion rate for surveys by group are reported in online supplemental table S8 and weekly average minutes (and 95% CIs) of Down Dog activities completed per group are presented in online supplemental table S9. Participants in the combination group consistently engaged in more mean exercise on a weekly basis (between 71 min and 86 min throughout the 6 weeks) than those in the yoga arm (between 69 min and 78 min) or HIIT (between 46 min and 64 min). Waitlisted participants remained underactive throughout the study, as reported on the Godin Leisure Time Exercise Questionnaire31 (see online supplemental materials – Methods section for more details). Online supplemental table S10 displays weekly averages and SDs of MVPA in the WLC and active groups (outside usage of the apps).
Figure 2.
Exercise adherence rates by experimental condition.
Treatment groups versus WLC
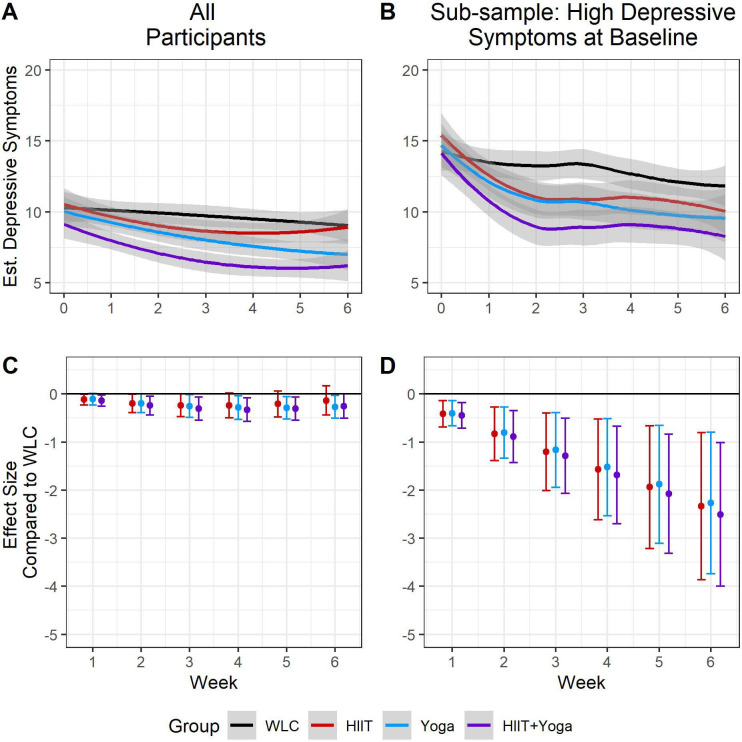
As seen in table 2A and figure 3A, the WLC participants had stable depressive symptoms throughout the 6 weeks (ie, non-significant S and Q). However, HIIT and HIIT+yoga significantly reduced in depressive symptoms over time in non-linear ways (figure 3a, online supplemental table S11 section A and online supplemental table S12 section A for HIIT and HIIT+yoga, respectively), whereas yoga reduced linearly over time (figure 3a, online supplemental table S13 section A).
Table 2.
Estimates for trajectories for WLC (A) and comparisons with active treatment groups (B1–3)
| Estimate | SE | 95% CI | |
| A. Estimates, SE and 95% CI for intercept (I), slope (S) and quadratic (Q) terms for WLC | |||
| I | 10.34 | 0.60 | 9.15 to 11.49 |
| S | −0.15 | 0.26 | −0.65 to 0.38 |
| Q | −0.01 | 0.04 | −0.09 to 0.07 |
| B. Estimates for differences between each group and WLC | |||
| B1. Differences in estimates for I | |||
| HIIT versus WLC | 0.26 | 0.87 | −1.46 to 1.97 |
| Yoga versus WLC | −0.34 | 0.86 | −2.02 to 1.34 |
| HIIT+yoga versus WLC | −1.27 | 0.83 | −2.89 to 0.35 |
| B2. Differences in estimates for S | |||
| HIIT versus WLC | −0.77 | 0.38 | −1.51 to −0.04 |
| Yoga versus WLC | −0.70 | 0.40 | −1.49 to 0.08 |
| HIIT+yoga versus WLC | −0.92 | 0.39 | −1.69 to −0.14 |
| B3. Differences in estimates for Q | |||
| HIIT versus WLC | 0.11 | 0.06 | 0.00 to 0.22 |
| Yoga versus WLC | 0.08 | 0.06 | −0.05 to 0.20 |
| HIIT+yoga versus WLC | 0.11 | 0.06 | −0.01 to 0.23 |
Results from the SEM model estimating intercept, slope and quadratic term for WLC (section A) and comparisons of these estimates with those of the three active groups (HIIT, yoga, HIIT+yoga; section B). Bold text denotes p<0.05.
HIIT, high intensity interval training; SEM, structural equation model; WLC, waitlist control.
Figure 3.
Trajectories and effect sizes for depressive symptoms over the course of the study. Note: figure 3A shows each group’s trajectories, in the full sample, while figure 3B show the trajectories for those with high (CESD score ≥10) levels of depressive symptoms. Figure 3C, D represent the effect sizes at each time point, for all participants and those with high levels of depressive symptoms, respectively. CESD, Center for Epidemiological Studies – Depression Scale; HIIT, high intensity interval training; WLC, waitlist control.
Treatment effect results revealed that baseline estimates for each group were not significantly different from that of the WLC (table 2, section B1; all p’s>0.05), whereas the growth rates over time (specifically the slopes) for HIIT and the HIIT+yoga were different from WLC (table 2, sections B2, B3). The ES for yoga (except week 1) and HIIT+yoga compared with WLC were significant with small effect sizes (range from −0.11 to −0.33), with ES getting larger over time. In the HIIT group, ES estimates were small and significant initially, though reduced in size by week 4 when they were no longer significant. See figure 3C and online supplemental table S14 for ES estimates.
Comparison of treatment groups
All three groups had similar trends in decreasing depressive symptoms over the course of the study, and effect size estimates for each week were not significantly different from each other (online supplemental tables S11−13).
High depression group
When restricted to those participants with prerandomisation baseline ≥10 CESD, (n=173; mean=14.8, SD=3.98), all treatment groups had significantly greater reductions in depressive symptoms over time compared with the WLC (online supplemental table S15, section B). Rate of decrease in depressive symptoms for each group, in descending strength, was −3.39 (HIIT+yoga), −3.24 (HIIT), −3.18 (yoga) and −1.18 (WLC) (see figure 3B for trends). Within the first week, ES for each treatment group compared with WLC were significant and of small size (ES range −0.41 to −0.44) and continued to grow over the course of the study to very large when the trial was completed (ES range −2.34 to −2.51). See figure 3D and online supplemental table S13 for ES results.
Discussion
Significant treatment effects in depressive symptoms were observed for participants randomised to complete HIIT or yoga, or a combination of the two, at-home using a suite of mobile applications over a 6-week period compared with WLC participants. While WLC participants’ depressive symptoms remained steady throughout the 6-week period, those in the three active arms had significant declines, as hypothesised. ES at each week were small for all three active groups, with the greatest effects in the combination group. Effects were very large when the sample was restricted to those with high depressive symptoms prior to randomisation, again with the apparent largest effects in the combined HIIT+yoga group. These differences could be attributed to the higher number of HIIT+yoga participants who completed at least four weekly workouts throughout the study, and the fact that those in the combined group completed the most minutes of activity every week. Our results highlight that providing variety to participants in pragmatic clinical trials can lead to the greatest adherence with the largest effects. This is supported by experimental research that demonstrates that those who engage in greater varieties of exercises are most likely to report more sustained physical activity.32 Our study reveals an impactful health behaviour in which adults, especially those with significant depressive symptoms, can engage that can potentially offer relief from the burden of the pandemic.
Prior to the COVID-19 pandemic, percentages of adults across the globe who were already insufficiently active were troublesome.33 Prior to COVID-19 in Canada, only 18% of adults were physically active at recommended levels when measured with accelerometers.34 Evidence suggests that these trends worsened during the early months of the pandemic, globally35 and within Canada.36 This is not surprising given the mandated stay-at-home orders that were implemented across all of Canada and most of the globe in the early months of the pandemic (see ref 37 for a review of the timeline of restrictions in Canada during the first wave). A global call for action18 prioritised at-risk groups (eg, frontline, low-paid, healthcare system workers, laid off adults and older adults) as intervention targets for physical activity programming to reduce risk and severity of infection with COVID-19. Not included in the call for action were those at wider risk for mental health issues, and recent studies are directing attention to individuals in different countries who are at risk for pandemic-related depression. In the UK, for example, adults aged 18–34 years, women and adults with children had the greatest increases in depression during the early months of COVID-19.7 Similar elevated risk to women and young adults was identified in the USA, as was to those who self-identified as Hispanic, had lower education levels, were not married or had more life stressors resulting from the pandemic (eg, financial/employment loss and COVID-19 related death of a family member).6 In our study, the majority of participants were women (87%), nearly half were 18–39 years of age (47%) and had children at home (42%). Yet, a large majority would not be considered to have met significant economic or employment challenges, as many were employed. The extent to which exercise programming can specifically benefit adults with economic challenges remains unclear.
There are several limitations to the current study. First, while our study sought to recruit equal numbers of women and men, men did not participate at the same rate as women, likely a result of yoga appealing mostly to women.38 Second, we partnered with a company with commercially available mobile apps, which allowed us to evaluate the efficacy of app-delivered programs that can be delivered at scale. While potential scalability makes our findings important, the study was not designed to control the types, difficulty or lengths of activities. Thus, it is impossible to quantify the frequency, intensity, type or time spent in specific exercises for prescriptive and clinical purposes. A final limitation is all participants were asked to complete a minimum of four sessions/week at 20 min/session, so that the total weekly HIIT would meet global recommendations for vigorous exercise.24 Yet, 80 weekly minutes of moderate activity, such as yoga, do not reach the recommended 150 min of moderate intensity activity. While global recommendations are set at 75 vigorous or 150 moderate intensity minutes of physical activity per week,24 there is considerable evidence suggesting that weekly physical activity even below recommended threshold levels is effective for many health issues,39 including depression.40
By 2030, the World Economic Forum projected that mental illness will account for US$6 trillion of the annual global economic burden, accounting for more than half the burden from all non-communicable diseases.41 With the increasing prevalence of global citizens with COVID-19 related depressive symptoms, the personal, social and economic burden can be expected to be even more far-reaching and devastating. The results from the current trial suggest that health officials should continue to promote exercise to the public,1 directing such promotion especially to those experiencing significant depressive symptoms, with the provision of low-cost or free exercise-based mobile applications to use at home as part of healthcare systems’ initiatives. Free eHealth mobile applications are effective in improving medication adherence and clinic attendance for non-communicable physical diseases, leading to substantial cost-effectiveness.42 Perhaps, then, the free provision of exercise-based mobile applications, supported by healthcare systems, could be one method to help reduce the emerging global mental health crisis and its eventual economic burden.
What are the findings?
This pragmatic randomised controlled trial provides evidence suggesting that at-home app-based exercise in various forms (high intensity interval training or yoga or their combination) can significantly improve depressive symptoms over a 6-week period in adults during the pandemic. Effects were strongest for those who were provided opportunities for both high intensity interval training and yoga. When the sample was restricted to only those with high baseline depressive symptoms, the weekly effects were very large.
How might it impact on clinical practice in the future?
At-home exercise during the COVID-19 pandemic proved to be an impactful and affordable health behaviour in which adults, especially those with high depressive symptoms, can engage to bolster their mental health. In light of the long-term mental health consequences of COVID-19 with which many adults are expected to struggle, even after a return to normal, promoting and supporting programming in communities at the individual level will emerge as a necessary clinical and health policy initiative.
Acknowledgments
We would like to acknowledge Anaïs Charbonneau, Abby Cheung, Sarah de Faye, Christina Whang, Carina Wong, Tommy Yang and Rachel Zhang for their contribution to recruitment and screening of participants and to Anaïs Charbonneau, Sarah de Faye and Rachel Zhang for their contribution to data processing.
Footnotes
Twitter: @BeauchampDr
Correction notice: This article has been corrected since it published Online First. The funding statement has been updated.
Contributors: Study was conceptualised and designed by EP, MRB and NM. Implementation was completed by NM, BH, NG and JW. MSK (physician) and SH (exercise physiologist) reviewed the files of participants who did not meet clearance using our online screening tool. YL developed the analytic plan, and YL and BH completed data analyses. The first draft was completed by EP; all tables and figures were prepared by BH and reviewed by all other authors for comments.
Funding: Funding for this research was provided by the Canada Research Chairs Program and Canadian Institutes of Health Research Grant # PJT 169211.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. All of the individual participant data collected during the trial after de-identification, and reported in the manuscript, will be available at https://osf.io/g9xqp following publication. All of the protocols, statistical analysis plan, informed consent form, analytic code, will be available 4 months following trial's end. .
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Study protocol was approved by the University of British Columbia’s Behavioral Review Ethics Board (#H20-01497).
References
- 1. Holmes EA, O'Connor RC, Perry VH, et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry 2020;7:547–60. 10.1016/S2215-0366(20)30168-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pfefferbaum B, North CS. Mental health and the Covid-19 pandemic. N Engl J Med 2020;383:510–2. 10.1056/NEJMp2008017 [DOI] [PubMed] [Google Scholar]
- 3. Galea S, Merchant RM, Lurie N. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention. JAMA Intern Med 2020;180:817–8. 10.1001/jamainternmed.2020.1562 [DOI] [PubMed] [Google Scholar]
- 4. Nochaiwong S, Ruengorn C, Thavorn K, et al. Global prevalence of mental health issues among the general population during the coronavirus disease-2019 pandemic: a systematic review and meta-analysis. Sci Rep 2021;11:10173. 10.1038/s41598-021-89700-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mental Health Research Canada . Mental health during COVID-19 outbreak wave 1, 2020. Available: https://static1.squarespace.com/static/5f31a311d93d0f2e28aaf04a/t/5f86800c5757437eb2b17f08/1602650133129/Full+Report+of+Findings+of+Survey+-+FINAL.pdf [Accessed 23 Jun 2021].
- 6. Ettman CK, Abdalla SM, Cohen GH, et al. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open 2020;3:e2019686. 10.1001/jamanetworkopen.2020.19686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pierce M, Hope H, Ford T, et al. Mental health before and during the COVID-19 pandemic: a longitudinal probability sample survey of the UK population. Lancet Psychiatry 2020;7:883–92. 10.1016/S2215-0366(20)30308-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . Coronavirus disease (COVID-19): staying active, 2020. Available: https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-staying-active [Accessed 23 Jan 2021].
- 9. Centers for Disease Control and Prevention (CDC) . COVID-19 coping with stress.
- 10. Public Health England . Guidance for the public on the mental health and wellbeing aspects of coronavirus (COVID-19), 2020. Available: https://www.gov.uk/government/publications/covid-19-guidance-for-the-public-on-mental-health-and-wellbeing/guidance-for-the-public-on-the-mental-health-and-wellbeing-aspects-of-coronavirus-covid-19 [Accessed 21 Jan 2021].
- 11. Schuch FB, Vancampfort D, Firth J, et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry 2018;175:631–48. 10.1176/appi.ajp.2018.17111194 [DOI] [PubMed] [Google Scholar]
- 12. Ashdown-Franks G, Firth J, Carney R, et al. Exercise as medicine for mental and substance use disorders: a Meta-review of the benefits for neuropsychiatric and cognitive outcomes. Sports Med 2020;50:151–70. 10.1007/s40279-019-01187-6 [DOI] [PubMed] [Google Scholar]
- 13. Schuch FB, Deslandes AC, Stubbs B, et al. Neurobiological effects of exercise on major depressive disorder: a systematic review. Neurosci Biobehav Rev 2016;61:1–11. 10.1016/j.neubiorev.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 14. Beutel ME, Klein EM, Brähler E, et al. Loneliness in the general population: prevalence, determinants and relations to mental health. BMC Psychiatry 2017;17. 10.1186/s12888-017-1262-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mushtaq R, Shoib S, Shah T. Psychiatric disorders and physical health ? A review on the psychological aspects of loneliness. J Clin Diagnostic Res 2014;8:WE01–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erzen E, Çikrikci Özkan, Ö Çikrikci. The effect of loneliness on depression: a meta-analysis. Int J Soc Psychiatry 2018;64:427–35. 10.1177/0020764018776349 [DOI] [PubMed] [Google Scholar]
- 17. Statistics Canada . Smartphone use and smartphone habits by gender and age group, inactive, 2021. Available: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=2210011501 [Accessed 23 Jun 2021].
- 18. Sallis JF, Adlakha D, Oyeyemi A, et al. An international physical activity and public health research agenda to inform coronavirus disease-2019 policies and practices. J Sport Health Sci 2020;9:328–34. 10.1016/j.jshs.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shepherd SO, Wilson OJ, Taylor AS, et al. Low-Volume high-intensity interval training in a Gym setting improves Cardio-Metabolic and psychological health. PLoS One 2015;10:e0139056. 10.1371/journal.pone.0139056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brinsley J, Schuch F, Lederman O, et al. Effects of yoga on depressive symptoms in people with mental disorders: a systematic review and meta-analysis. Br J Sports Med 2021;55:992–1000. 10.1136/bjsports-2019-101242 [DOI] [PubMed] [Google Scholar]
- 21. Kiernan M, Schoffman DE, Lee K, et al. The Stanford leisure-time activity categorical item (L-Cat): a single categorical item sensitive to physical activity changes in overweight/obese women. Int J Obes 2013;37:1597–602. 10.1038/ijo.2013.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American College of Sports Medicine . Guidelines for exercise testing and prescription. 10th Edn. Philadelphia: Wolters Kluwer, 2018. https://www.acsm.org/read-research/books/acsms-guidelines-for-exercise-testing-and-prescription [Google Scholar]
- 23. Bredin SSD, Gledhill N, Jamnik VK, et al. PAR-Q+ and ePARmed-X+: new risk stratification and physical activity clearance strategy for physicians and patients alike. Can Fam Physician 2013;59:273–7. [PMC free article] [PubMed] [Google Scholar]
- 24. Bull FC, Al-Ansari SS, Biddle S, et al. World Health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451–62. 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: evaluation of a short form of the CES-D. Am J Prev Med 1994;10:77–84. 10.1016/S0749-3797(18)30622-6 [DOI] [PubMed] [Google Scholar]
- 26. Raudenbush SW, Spybrook J, Congdon R. Optimal design plus empirical evidence (version 3.0) 2011, 2011. Available: http://www.wtgrantfoundation.org/resources/optimal-design
- 27. Schuch FB, Vancampfort D, Richards J, et al. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res 2016;77:42–51. 10.1016/j.jpsychires.2016.02.023 [DOI] [PubMed] [Google Scholar]
- 28. Mayer M. MissRanger: Fast Imputation of Missing Values. R Package Version 2.1.0, 2019. Available: https://cran.r-project.org/package=missRanger%0A
- 29. Muthén LK, Muthén BO. Mplus User’s Guide. 7th edn. Los Angeles: CA, 2012. [Google Scholar]
- 30. Feingold A. New approaches for estimation of effect sizes and their confidence intervals for treatment effects from randomized controlled trials. Quant Method Psychol 2019;15:96–111. 10.20982/tqmp.15.2.p096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci 1985;10:141–6. [PubMed] [Google Scholar]
- 32. Sylvester BD, Standage M, McEwan D, et al. Variety support and exercise adherence behavior: experimental and mediating effects. J Behav Med 2016;39:214–24. 10.1007/s10865-015-9688-4 [DOI] [PubMed] [Google Scholar]
- 33. Guthold R, Stevens GA, Riley LM, et al. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health 2018;6:e1077–86. 10.1016/S2214-109X(18)30357-7 [DOI] [PubMed] [Google Scholar]
- 34. Statistics Canada . The daily — Canadian health measures survey: activity monitor data, 2017. Available: https://www150.statcan.gc.ca/n1/daily-quotidien/170419/dq170419e-eng.htm [Accessed 23 Jun 2021].
- 35. Stockwell S, Trott M, Tully M, et al. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: a systematic review. BMJ Open Sport Exerc Med 2021;7:e000960. 10.1136/bmjsem-2020-000960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lesser IA, Nienhuis CP. The impact of COVID-19 on physical activity behavior and well-being of Canadians. Int J Environ Res Public Health 2020;17. 10.3390/ijerph17113899. [Epub ahead of print: 31 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Canadian Medical Association Journal News . COVID-19: A timeline of Canada’s first-wave response – CMAJ News. Available: https://cmajnews.com/2020/06/12/coronavirus-1095847/ [Accessed 23 Jun 2021].
- 38. Park CL, Braun T, Siegel T. Who practices yoga? A systematic review of demographic, health-related, and psychosocial factors associated with yoga practice. J Behav Med 2015;38:460–71. 10.1007/s10865-015-9618-5 [DOI] [PubMed] [Google Scholar]
- 39. Warburton DER, Bredin SSD. Reflections on physical activity and health: what should we recommend? Can J Cardiol 2016;32:495–504. 10.1016/j.cjca.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 40. Ravindran AV, Balneaves LG, Faulkner G, et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 5. complementary and alternative medicine treatments. Can J Psychiatry 2016;61:576–87. 10.1177/0706743716660290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bloom DE, Cafiero ET, Jané-Llopis E. The global economic burden of noncommunicable diseases, 2012. [Google Scholar]
- 42. Iribarren SJ, Cato K, Falzon L, et al. What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PLoS One 2017;12:e0170581. 10.1371/journal.pone.0170581 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjsports-2021-104379supp001.pdf (690.8KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. All of the individual participant data collected during the trial after de-identification, and reported in the manuscript, will be available at https://osf.io/g9xqp following publication. All of the protocols, statistical analysis plan, informed consent form, analytic code, will be available 4 months following trial's end. .