Abstract
The etiology of cancer type may vary significantly due to anatomy, embryology, and physiology of the cancer site. Although the association between potato consumption and colorectal cancer (CRC) was summarized in a 2018 meta-analysis of 5 cohort studies, to the best of our knowledge, no meta-analysis has evaluated potato consumption in relation to multiple cancer sites in adults. Medline/PubMed, ISI Web of Knowledge, Scopus, and the Cochrane Database of Systematic Reviews were searched for relevant publications through August 2020. We selected cohort or case-control studies conducted in adults that reported risk estimates (relative risk [RRs], HRs, and ORs) of potato intake for any cancer type. Random effects meta-analyses compared high and low intake categories. Twenty prospective cohort studies (total n = 785,348) including 19,882 incident cases, and 36 case-control studies (21,822 cases; 66,502 controls) were included. Among cohort studies, we did not find an association between high versus low intake of total potato (white and yellow) consumption and overall cancers: 1.04 (95% CI: 0.96, 1.11; tau2 = 0.005, n = 18). We found no relation between total potato consumption (high compared with low intake) and risk of CRC, pancreatic cancer, colon, gastric, breast, prostate, kidney, lung, or bladder cancer in cohort or case-control studies. We did not find an association between high versus low consumption of potato preparations (boiled/fried/mashed/roasted/baked) and risk of gastrointestinal-, sex-hormone-, or urinary-related cancers in cohort or case-control studies. Certainty of the evidence was low for total cancer, CRC, colon, rectal, renal, pancreatic, breast, prostate, and lung cancer and very low for gastric and bladder cancer. In conclusion, potato intake or potato preparations were not associated with multiple cancer sites when comparing high and low intake categories. This finding was consistent with the findings from the 2018 meta-analysis regarding potato intake and risk of CRC.
Keywords: potato, cancer, systematic review, meta-analysis, dose-response
Introduction
In recent years, the prevalence of cancer has dramatically increased in both developed and developing countries (1). In 2016, cancer was reported to be the second leading cause of death, responsible for one-sixth of the global mortality rate (2). Among important modifiable factors, much interest has been placed on diet in relation to cancer risk (3). Starchy foods and highly refined carbohydrates have been demonstrated to increase cancer risk in most studies ( 4).
As a non-cereal staple food consumed worldwide, an understanding of how potato consumption is associated with cancer is important (5). Potatoes are rich sources of fiber (resistant starch), essential nutrients (magnesium, potassium, vitamin C, and vitamin B-6), and phytochemicals (lutein and zeaxanthin) (6), which are negatively associated with carcinogenesis (7–11). However, the benefits of potato consumption have also been questioned due to its high content of starch and high glycemic index (GI) (12). A review study showed that high dietary GI is associated with increased risk of colorectal cancer (CRC) and prostate cancer, and a high dietary glycemic load (GL) is related to an increased risk of breast and endometrial cancers (13–15).
The biological effects and nutrient content of potatoes are affected by preparation and cooking methods. For example, boiled potatoes have a higher GI than other kinds of potato preparations. Long-term consumption of high GI or GL diets may lead to chronic hyperinsulinemia (13). Insulin is itself a mitogen and also increases the bioactivity of insulin-like growth factors (IGF-1) which can promote cancer by inhibiting apoptosis and stimulating cell proliferation (13). Fried potatoes are associated with an increased risk of cancer due to a higher content of trans fatty acids, salts, and acrylamide (16–18). Acrylamide exerts a mutagenic effect because of the capacity of glycidamide, its epoxide metabolite, to form DNA adducts (19). Some evidence indicates that trans fatty acids and salt intake induce chronic inflammation, which may be related to carcinogenesis (20, 21). Preparation methods are of particular interest given the global transition from the consumption of fresh potatoes to potato products like French fries, potato chips, boiled, mashed, and baked potatoes (22).
Findings regarding the association between potato consumption and cancer risk have been contradictory. Several studies have shown a significant association between total potato (white and yellow) intake or any kind of potato preparations and risk of multiple cancer sites (23–26). Although other research has found no significant associations between total potato (white and yellow) intake or any specific kind of potato intake and risk of cancer (27–32).
Several studies have indicated that high starch and carbohydrate intake may be associated with a variety of cancers including CRC, prostate, lung, breast, and endometrial (13–15). Therefore, it is necessary to investigate the overall relation between potato consumption and different types of cancers. In addition, the consumption of fried potatoes has been associated with different types of cancer (26, 33). To the best of our knowledge, the present study is the first to systematically study the association between potato consumption and risk of cancer at multiple sites. One meta-analysis investigated the association between potato consumption and risk of CRC (34), however, cancer itself was not a focus of this study (it only included 1 type of cancer, i.e. CRC) among a host of other disease outcomes. Further, the cohort studies pooled in that meta-analysis (34) included 1 study on sweet potatoes (35). We identified an additional 2 cohort studies (36, 37) that were not included in that meta-analysis. We know of no meta-analysis that has investigated associations between potato intake and risk of cancer at multiple sites. Therefore, we conducted a systematic review and meta-analysis to examine this relation using observational studies.
Methods
The current systematic review and meta-analysis was conducted using the Preferred Reporting Items of Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines (38).
Search strategy
Medline/PubMed, ISI Web of Knowledge, Scopus, and the Cochrane Database of Systematic Reviews were searched for studies on the association between potato consumption and risk of cancer published prior to August 2020. The query syntax was set using Medical Subject Headings (MeSH) and thesaurus search terms including: (“Potato*” OR “French fries”) AND (Cancer* OR Malignanc* OR Neoplas* OR Tumor* OR Carcinoma*). References retrieved from the studies as well as relevant reports were also hand-searched to reduce the likelihood of missing any publications. These steps were performed by 3 independent investigators (MDM, HM, MRA). Any disagreements were resolved through discussion or, if necessary, by a fourth investigator (LA). The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO)(CRD42020150160).
Inclusion criteria
Articles were included in the systematic review and meta-analysis if they: 1) had full texts written in English; 2) had cohort or case-control or pooled study designs; 3) were conducted with adults (aged ≥18 y); 4) defined exposure as total potato consumption, boiled, baked, roasted, mashed, or fried (potato chips or French fries); 5) reported either HRs, relative risks (RRs), or ORs with corresponding 95% CIs for the association between potato consumption and risk of cancer.
Exclusion criteria
Studies were eliminated if they were: 1) from unpublished data or gray literature, such as conference articles, editorials, theses, and patents; 2) animal, ecologic, cross-sectional studies, or randomized clinical trials (RCTs); 3) carried out among pregnant women or children; 4) did not report HRs, RRs, or ORs with corresponding 95% CIs; 5) examined nonrelevant outcomes; 6) analyzed potato consumption along with other food items; 7) did not provide the full text. In addition, studies on specific types of potatoes other than white or yellow potatoes (such as sweet potatoes) were excluded because of their different nutritional composition (39). In the case of multiple articles using the same dataset, the study with the largest sample size was included.
Data extraction
The following data were obtained from each study: first author's name, year of publication, study origin, cohort name, duration of follow-up, age range and gender of participants, study design, sample size and number of cases, type of potato preparation (boiled/fried/mashed/roasted/baked), methods applied for exposure assessment, outcomes, outcome evaluation methods, categories of potato intake, risk estimates and 95% CIs comparing the outcomes of interest in the highest category of potato consumption to the lowest category (maximally adjusted measures, if available), and potential confounders that were controlled in the study. We attempted to contact the corresponding author of the articles when they did not provide sufficient data (for risk estimates and/or 95% CIs) (40–47). However, we were unable to retrieve additional data through this method. If the study provided gender-specific associations, we pooled both risk estimates using fixed-effect models before entering them into the overall meta-analysis. These steps were carried out by 2 independent reviewers (MDM, MA). In the case of lack of consensus regarding study selection or data extraction, the principal investigator (LA) resolved the issue.
Risk of bias
Using the Risk Of Bias In Nonrandomized Studies (ROBINS-E) assessment tool (48), critical appraisal of the studies was done using 7 main domains (potential confounding, selection of participants, classification of exposure, departures from intended exposures, missing data, measurement of outcomes, and reporting bias). Studies were classified as having low, moderate, serious, or critical risk of bias (Supplementary Table 1). Quality assessment was performed by 2 authors (MA & AJ) independently, and a third party (LA) resolved any disagreements.
Certainty of evidence
Study quality was assessed using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) tool (49). Evaluation of the certainty of the evidence involves consideration of within-study risk of bias, the directness of the evidence, heterogeneity, precision of the effect or association estimates, and risk of publication bias in order to reach an overall certainty of the evidence rating of very low, low, moderate, or high for each outcome.
Informative statements to communicate the findings of the systematic review were provided using recommendations of the GRADE working group (50). According to the GRADE recommendations, communicating the findings of reviews are based on effect size and the certainty of the evidence. Informative statements are provided using key words or phrases including: will, probably, may, and we are uncertain.
Statistical analysis
Effect estimates were pooled using a random-effects model employing the metan command in STATA (51). To examine the weight of each study, the SE for the log RR/HR/OR of each study was considered as the estimated variance of the log RR, using inverse variance methods (52). Risk estimates with the largest number of adjustment potential confounders were entered into the meta-analysis. Between-study heterogeneity was explored using tau² as an absolute measure (with the metan command in STATA) (53). In addition, a subgroup analysis (including formal statistical tests to see if the differences between subgroups were significant) was conducted based on gender (male, female, both), case number, exposure assessment tool (FFQ, non-FFQ instrument), energy adjustment (yes, no), BMI adjustment (yes, no), country, study design (cohort, case-control), and CRC (colon, rectal) (using the metan command) (51).
In addition, we conducted dose-response analyses using the methods proposed by Greenland and Longnecker (54) and Orsini et al. (55). A 2-stage random-effects dose-response meta-analysis was conducted to examine likely nonlinear associations between potato intake and cancer (56). The number of patients with cancer, sample size, and risk estimates were extracted from studies with ≥3 quantitative exposure categories. If a study did not report the sample size in each category, we considered it to be similar across categories. In addition, the median or mean potato intake for each category was also extracted. In studies reporting the frequency of potato consumption, weekly grams of potato intake were calculated based on a serving size of 100 g (57). Nonlinear associations were examined by modeling exposure levels with the use of restricted cubic splines with 3 knots at the 10th, 50th, and 95th percentiles of the distribution (using the glst command in STATA) (58, 59). The null hypothesis was that the coefficient of the second spline was equal to zero.
Furthermore, a linear dose-response association between potato consumption and cancer risk was examined for each 100-g/wk increment in consumption using a 2-stage generalized least-squares trend estimation method (with glst and metan commands in STATA) (56, 54, 60). First, study-specific slope lines were obtained (using the glst command) and afterwards, these lines were combined to get an overall average slope (56). Study-specific slope lines were combined through a random-effects model (with the metan command) (51). In addition, potential publication bias was assessed by visual inspection of funnel plots (acquired from the metafunnel command) and also through using Egger's regression test (with the metabias command) (61, 62). Besides pooled analyses, we carried out sensitivity analyses (using the metaninf command in STATA to determine whether the overall estimates were affected by the effect size of a single study) (63). All statistical analyses were performed with STATA software, version 11.0 (STATA Corp.). All P values were 2-tailed and P values <0.05 were considered statistically significant.
Results
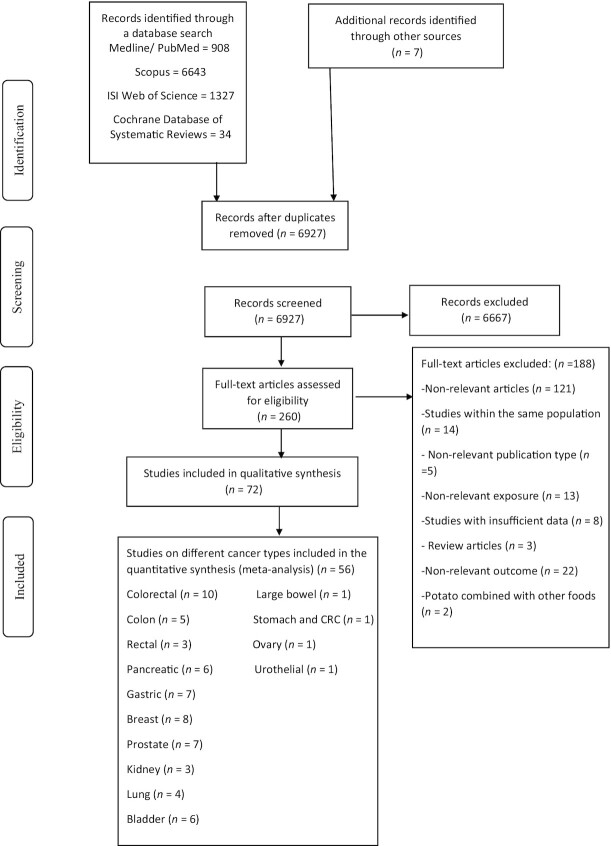
The systematic literature search resulted in 8912 articles (Figure 1). After reviewing the article titles and abstracts, 6667 publications were excluded. Among the 260 remaining publications, 188 were excluded because: the population was not relevant; it was an ecologic, cross-sectional, RCT study, or a review article; potato consumption was analyzed along with other food items or as part of a dietary pattern; >1 study was conducted on the same population; sweet potatoes were examined; or insufficient information was given (e.g. they did not report the risk estimates or 95% CIs) or nonrelevant outcomes were presented. Finally, 20 cohort studies, 48 case-control studies, and 4 pooled analyses were included in the systematic review. Out of those, 20 prospective cohort studies and 36 case-control studies were included in the meta-analysis.
FIGURE 1.
Flow diagram of study selection. CRC, colorectal cancer.
Study characteristics
We included a total of 73 studies published between 1988 and 2019. Twenty studies, with a total of 785,348 participants and 19,882 incident cases, were prospective cohort studies (24, 27–29, 36, 37, 64–73,74–76). Forty-eight studies (23, 25, 26, 30–33, 66, 77–116) with 25,005 cases and 73,069 controls had case-control designs. Five studies (117–121) with 3,947,660 subjects, of which 35,760 had cancer were pooled analyses.
In most studies (n = 50), risk estimates were reported for both genders together, with only 2 studies providing gender-specific associations (72, 112). However, some studies were conducted with only females (n = 17) (24, 27, 28, 37, 66–68, 73, 76, 88, 95, 101, 104, 106, 113, 117, 121) or males (n = 8) (26, 29, 64, 69–71, 74, 110). The follow-up period in the cohort studies ranged from 0.75 to 22 y. Participant age ranged between 18 and 107 y. More detailed characteristics of the studies are presented in Tables 1 and 2.
TABLE 1.
Characteristics of cohort studies on the association of potato consumption on site-specific cancers in adults in the systematic review1
| First author (ref) | Cohort name | Country | Year | Age range (y) | Gender | Sample size (n) | Cases (n) | Follow-up (y) | Exposure | Exposure assessment | Outcome | Outcome assessment | Categorical or continuous | OR, RR, or HR (95%CI) | Adjustment2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Agnoli et al. (36) | European Prospective Investigation into Cancer and Nutrition (EPIC) | Italy | 2012 | M/F | 45,275 | 435 | 11.28 | Potatoes | FFQ (valid) | CRC | Databases of the regional cancer registries | T3 vs. T1 | HR: 1.12 (0.87–1.44) | — | |
| 2 | Asli et al. (24) | Norwegian Women and Cancer (NOWAC) | Norway | 2017 | 41–70 | F | 79788-79788-75474 | 275-637-100 | 12 | Potatoes | FFQ (valid) | Rectal cancer,colon cancer,CRC | Registry linkage | T3 vs. T1 | HR: 1.18(0.94, 1.48),HR: 1.63(1.15, 2.31),HR: 2.0 (1.07, 3.72) | 1,2,5,6,8,16 |
| 3 | Asli et al. (64) | HELGA | Norway | 2018 | 40–55 | M | 38, 766 | 121 | 12 | Potatoes | FFQ (valid) | PC | Registry linkage | T3 vs. T1 | HR: 1.01(0.56, 1.84) | 1,2,5,6,8,16 |
| 4 | Flood et al. (27) | Breast Cancer Detection Demonstration Project (BCDDP) | USA | 2002 | F | 45, 490 | 485 | 8.6 | Potatoes | FFQ (valid) | CRC | National Death Index | Q5 vs. Q1 | RR: 0.82 (0.65, 1.15) | 3,5,6,7,8,16,17,18,22,24,29,30 | |
| 5 | Michels et al. (65) | Nurses’ Health Study (NHS) and the Health Professionals’Follow-up Study (HPFS) | USA | 2000 | 30–75 | M/F | 136, 089 | 937-244 | 4.7 | Potatoes | FFQ (valid) | Colon,colon,rectal,rectal | Medical records | Q4 vs. Q1per 100-g/wk,Q4 vs. Q1per 100-g/wk | RR: 1.11 (0.86–1.43),RR: 0.93 (0.76–1.15),RR: 1.18 (0.69–2.00),RR: 0.94 (0.63–1.41) | 1,5,6,7,8,11,16,17,18,30,34,36,37 |
| 6 | Mucci et al. (66) | Swedish MammographyCohort | Sweden | 2005 | F | 61, 467 | 504-237 | 13.4 | Pan-fried potatoes,potato chips, and French fries | FFQ (valid) | Colon,rectal,colon,rectal cancer | Swedish Cancer Register | T3 vs. T1,T3 vs. T2,T3 vs. T3,T3 vs. T4 | RR: 1.1 (0.9–1.4),RR: 1.1 (0.8–1.6),RR: 0.9 (0.5–1.6),RR: 0.9 (0.4–2.0) | 1,3,6,15,16,20,21 | |
| 7 | Wilson et al. (69) | Health Professionals Follow-up Study (HPFS) | USA | 2008 | 40–75 | M | 47, 896 | 4174-5025-5025 | 10 | Potatoes,French fries,chips | FFQ (valid) | Prostate cancer | Medical records | Q5 vs. Q1,Q5 vs. Q1 | RR: 1.07 (0.96–1.19),RR: 0.88 (0.80–0.98),RR: 1.07 (0.98–1.17) | 1,2,5,6,7,8,11,12,16,17,18,22,28,30,38 |
| 8 | Wilson et al. (28) | Nurses’ Health Study II | USA | 2009 | 25–44 | F | 90, 628 | 1179 | 14 | Potatoes,baked-roasted-mashed,chips | FFQ (valid) | Breast cancer | Biennial follow-up questionnaires | Q5 vs. Q1 | RR: 0.98 (0.80, 1.19),RR: 0.97 (0.80, 1.17),RR: 0.98 (0.80, 1.19) | 5,6,7,8,14,16,17,21,31,32,39,44,46 |
| 9 | Verhoeven et al. (68) | Netherlands Cohort Study | Netherlands | 1997 | 55–69 | F | 62573 | 650 | 4.3 | Potatoes | FFQ | Breast cancer | Pathologically | Q5 vs. Q1 | RR: 1.14 (0.81, 1.62) | 1,11,16,17,31,32,35 |
| 10 | Sonestedt et al. (67) | Malmo¨ Diet and Cancer | Sweden | 2008 | 46–81 | F | 15, 773 | 544 | 10.3 | Boiled potatoes,French and deep-fried potatoes | FFQ | Breast cancer | Histologically | Q5 vs. Q1,Q5 vs. Q1 | HR: 0.91 (0.69–1.20),HR: 1.10 (0.88–1.40) | 1,3,5,7,8,16,17,32,35 |
| 11 | Drake et al. (71) | Malmo¨ Diet and Cancer cohort | Sweden | 2012 | 45–73 | M | 8128 | 817 | 15 | Potatoes | FFQ | Prostate cancer | Histologically | Q5 vs. Q1 | HR: 0.87 (0.69, 1.09) | 3,5,7,8,9,16,17,22 |
| 12 | Lee et al. (76) | Nurses’ Health Study (NHS) and the Health Professionals’Follow-up Study (HPFS) | USA | 2006 | 30–75 | M/F | 34, 146 | 113 | 20 | Potatoes | FFQ (valid) | Renal cancer | Medical records | ≥5/wk vs. <2/wk | RR: 1.05 (0.77–1.43) | 6,8,12,13,15,16,30 |
| 13 | Feskanich et al. (72) | Nurses’ Health Study (NHS) and the Health Professionals’Follow-up Study (HPFS) | USA | 2000 | 30–55,40–75 | F-M | 77283-47778 | 519-274 | 1210 | French fries | FFQ (valid) | Lung cancer | Participant report | Q5 vs. Q1 | RR: 1.09 (0.79–1.50),RR: 1.05 (0.67–1.64) | 1,8,16,17,30 |
| 14 | Lemarchand et al. (29) | AGRIculture and CANcer (AGRICAN) | France | 2016 | 50–75 | M | 81, 959 | 1672 | 0.75 | Potatoes | FFQ | Prostate cancer | ICD-O-3 | HR: 1.06 (0.94, 1.20) | 3,6,8,9 | |
| 15 | Diallo et al. (70) | Supplémentation en Vitamines et MinérauxAntioxydants (SU.VI.MAX) | France | 2016 | M | 3313 | 139 | 12.6 | Potatoes | 24-h dietary records | Prostate cancer | Biopsy | T3 vs. T1 | HR: 0.93 (0.56, 1.45) | 1,3,5,6,7,8,11,16,17,22 | |
| 16 | Kato et al. (73) | New York University Women's Health Study | USA | 1997 | 34–65 | F | 14, 727 | 100 | 12.22 | Potatoes | FFQ | CRC | Histologically | Q4 vs. Q1 | RR:1.05(0.62–1.79) | 1,3,6,9,16,20,22 |
| 17 | Lin et al. (37) | Women's Health Study (WHS) | USA | 2004 | >45 | F | 39, 876 | 223 | 10 | Potatoes | FFQ (valid) | CRC | Pathology reports | Q5 vs. Q1 | RR: 1.21 (0.76–1.92) | 1,6,7,8,11,16,17,18,30,34,36,37 |
| 18 | Stolzenberg-Solomo et al. (74) | α-Tocopherol, β-carotene Cancer Prevention | Finland | 2002 | 50–69 | M | 27, 111 | 163 | 12 | Potatoes | Dietary history questionnaire | PC | Finnish Cancer Registry | RR:1.02(0.63–1.64) | 1,8,16 | |
| 19 | Steinmetz et al. (76) | Iowa Women'sHealth Study | USA | 1994 | 55–69 | F | 41,837 | 212 | 5 | Potatoes | FFQ (valid) | Colon cancer | State Health Registry | Q4 vs. Q1 | RR:1.24(0.76–2.04) | 1,16 |
| 20 | Pols et al. (75) | Nambour Skin Cancer Study | Australia | 2011 | 501 | M/F | 1056 | 103 | 6 | Potatoes | FFQ | Basal cell cancer | Histologically | T3 vs. T1 | RR: 0.7 (0.4 –1.0) | 1,2,16,41,41,42,43 |
| 21 | Koushik et al. (117) | Pooling Project of Prospective Studies of Diet and Cancer | Multinational countries | 2005 | 27–93 | F | 560, 441 | 2068 | 7–22 | Potatoes | FFQ (valid) | Ovarian cancer | Histologically | Per 1 serving/d | RR: 0.97(0.81–1.15) | 6,7,8,16,31,32,34,36 |
| 22 | Koushik et al. (118) | Pooling Project of Prospective Studies of Diet and Cancer | Multinational countries | 2007 | 25–93 | M/F | 756, 217 | 5504 | 6–20 | Potatoes | FFQ (valid) | Colon cancer | Medical records | 1 serving/wk to <½serving/d | RR: 0.87 (0.75–1.02),RR: 0.89 (0.77–1.04),RR: 1.02 (0.86–1.21) | 3,5,6,8,11,16,17,18,29,30,34,39 |
| 23 | Koushik et al. (119) | Pooling Project of Prospective Studies of Diet and Cancer | Multinational countries | 2012 | 22–104 | M/F | 862, 584 | 2212 | 7–20 | Potatoes | FFQ (valid) | PC | Medical records | per 3 servings/wk | RR: 1.03(0.95–1.11) | 6,8,16,12,17 |
| 24 | Jung et al. (121) | Pooling Project of Prospective Studies of Diet and Cancer | Multinational countries | 2012 | 18–104 | F | 993, 466 | 4749-19, 749 | 11–20 | Potatoes | FFQ (valid) | Breast cancer(er+),breast cancer(er–) | Medical records | 1 serving/d | RR: 1.12(0.99–1.26),RR: 1.04(0.96–1.13) | 3,5,6,7,8,11,16,17,31,32,36,39 |
| 25 | Lee et al. (120) | Pooling Project of Prospective Studies of Diet and Cancer | Multinational countries | 2007 | 22–107 | M/F | 774, 952 | 1478 | 7–20 | Potatoes | FFQ (valid) | Renal cancer | Histologically | 1 serving/d | RR: 0.98 (0.75–1.27) | 1,6,8,13,16,17,32,44 |
CRC, colorectal cancer; F, females; ICD-O-3, International Classification of Diseases for Oncology, 3rd revision; M, males; PC, pancreatic cancer; RR, relative risk; T, tertile; Q4, Quartile; Q5, Quintile.
Adjustments: age (1), sex (2), education (3), race (4), height (4), BMI (5), physical activity (6), smoking (7), study center (8), year of interview (9), family history of cancer (10) and diabetes mellitus (11), hypertension (12), family income (13), number of meals per day (14), dietary intake of energy (15), alcohol (16), red meat (17), fruits and vegetables (18), fiber (19), fat (20), calcium (21), zinc (22), vitamin D (23), social class (24), employment in risky occupation (25), socioeconomic status (26), α-linolenic acid (27), nonsteroid anti-inflammatory drug (28), multivitamin supplements (29), age at menarche (30), parity (31), age at first pregnancy (32), hormone therapy (33), age at menopause (34), menopausal status (35), aspirin (36), prostate-specific antigen testing in previous period (37), oral contraceptive use (38), skin color (39), sunburn (40), elastosis (41), treatment allocation (42), age at first birth (43), ethnicity (44), glycemic load (45).
TABLE 2.
Characteristics of case-control studies on the association of potato consumption on site-specific cancers in adults in the systematic review1
| First author (ref) | Country | Year | Gender | Cases (n) | Controls (n) | Age - mean or range (cases) | Age - mean or range (controls) | Exposure | Exposure assessment | Outcome | Outcome assessment | Categorical or continuous | OR, RR, or HR (95%CI) | Adjustment2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Annema et al (78) | Australia | 2011 | M/F | 834 | 939 | 40–79 | 40–79 | Potatoes | FFQ (valid) | CRC | Australian Cancer Registry | Q4 vs. Q1 | OR: 1.14 (0.87,1.5) | 1,2,6,7,8,16,17,30,27 |
| 2 | Kampman et al. (96) | Netherland | 1995 | M/F | 232 | 259 | >75 | 75> | Potatoes | Dietary history | Colon cancer | Cancer registries | Q4 vs. Q1 | OR: 0.67 (0.37–1.22) | 1,2,11,16,17 |
| 3 | Polesel et al. (107) | Italy | 2013 | M/F | 198 | 594 | 18–76 | 18–76 | Potatoes | FFQ (valid) | Nasopharyngeal | Histologically | T3 vs. T1 | OR: 1.53 (0.84–2.76) | 1,2,3,8,9,10,17 |
| 4 | Rai et al. (109) | India | 2006 | M/F | 153 | 153 | 53.10 ± 12.27 | 50.49 ± 12.48 | Potatoes | FFQ | Gallbladder | Histologically | T3 vs. T1 | OR: 0.74 (0.25–2.19) | |
| 5 | Tayyem et al. (114) | Jordan | 2014 | M/F | 220 | 281 | >18 | >18 | Mashed potatoes,fried potatoes | FFQ (valid) | CRC | Q4 vs. Q1,Q4 vs. Q1 | OR: 1.12 (0.06–21.16),OR: 1.85 (0.54–6.35) | 3,7,8,16,44,19,32 | |
| 6 | Torkuz et al. (115) | Turkey | 2011 | M/F | 183 | 183 | 18–75 | 18–75 | Fried potatoes | FFQ | Nasopharyngeal cancer | Histologically | T3 vs. T1 | OR: 1.44 (0.91–2.28) | |
| 7 | Boeing te al. (81) | Germany | 1991 | M/F | 143 | 579 | 32–80 | 32–80 | Potatoes | FFQ | Stomach cancer | Histologically | T3 vs. T1 | RR: 0.81 (0.50, 1.32) | 1,2,9 |
| 8 | Bostetti et al. (82) | Italy | 2000 | M/F | 304 | 743 | 39–77 | 39–77 | Potatoes | FFQ | Esophageal cancer | Histologically | Q5 vs. Q1 | OR: 0.88 (0.53, 1.48) | 1,2,3,8,16,17 |
| 9 | Bravi et al. (85) | Italy | 2006 | M/F | 768 | 2078 | 22–79 | 22–79 | Potatoes | FFQ (valid) | Oral and pharyngeal cancer | Histologically | Q5 vs. Q1 | OR: 1.85 (1.19, 2.86), | 1,2,3,6,8,10,17 |
| 10 | Chan et al. (86) | USA | 2005 | M/F | 532 | 1701 | 21–85 | 21–85 | Potatoes | FFQ (valid) | PC | Histologically | Q4 vs. Q1 | OR: 1.4 (1.0–1.9) | 1,2,16 |
| 11 | Cornee et al. (30) | France | 1994 | M/F | 92 | 128 | 66.6 ± 10.4 | 66.6 ± 10.4 | Potatoes | Dietary history | Gastric cancer | Histologically | T3 vs. T1 | OR: 1.47 (0.72, 2.98) | 1,2,16,26 |
| 12 | Stefani et al. (87) | Uruguay | 2005 | M/F | 160-200 | 320-400 | 30–89,40–89 | 30–89, 40–89 | Potatoes | FFQ | Gastric cancer,esophagus cancer | Histologically,microscopically | Per 25 g/d | OR: 1.05 (0.93.1.18),OR: 1.05 (0.93.1.18) | 1,3,8,16,17,18,23 |
| 13 | Hoang et al. (92) | South Korea | 2016 | M/F | 415 | 830 | 53 | 53 | Potatoes | FFQ | Gastric cancer | Histologically | T3 vs. T1 | OR: 0.79 (0.57–1.09) | 3,7,8,11,14 |
| 14 | Deneo‐Pellegrini et al (89) | Uruguay | 1996 | M/F | 160 | 287 | 30–84 | 30–84 | Potatoes | FFQ | CRC | National Cancer Registry | T3 vs. T1 | RR: 0.74 (0.48–1.17) | 1,2,3,6,11,16,17 |
| 15 | Heck et al. (91) | France | 2008 | M/F | 513 | 713 | Potatoes | FFQ | Hypopharyngeal cancer | Pathological | Q4 vs. Q1 | OR: 0.48 (0.06, 3.86) | 1,2,3,4,17 | ||
| 16 | Ito et al. (95) | Japan | 2002 | F | 508 | 36, 490 | >30 | >30 | Potatoes | FFQ | Gastric cancer | Histologically | Q4 vs. Q1 | OR: 0.78 (0.53, 1.13) | 1,8,10,11 |
| 17 | Levi et al (97) | Switzerland | 1999 | M/F | 223 | 491 | <75 | <75 | Potatoes | FFQ (valid) | CRC | Histologically | T3 vs. T1 | OR: 1.41 (0.85, 2.33) | 1,2,3,4,6,7,16,17 |
| 18 | Bich NN et al. (80) | Japan | 2009 | M/F | 670 | 672 | Fried potatoes | FFQ | Stomach and CRC | Interview | T3 vs. T1 | OR: 0.88 (0.42–1.69) | 1,2 | ||
| 19 | Ping et al (105) | Sahara | 1998 | M/F | 100 | 265 | 40–84 | 40–84 | Potatoes | FFQ | CRC | Medical records | OR: 1.46 (0.90–2.37) | ||
| 20 | Steinmetz et al. (112) | Australia | 1993 | M/F | 121-99 | 241-197 | 63 | 63 | Mashed potatoes | FFQ | Colon cancer | Histologically | Q4 vs. Q1 | OR: 1.42 (0.72–2.80),OR: 1.37 (0.63–2.99) | 2,11,16,17,19,26,33 |
| 21 | Radosavljevic et al. (33) | Serbia | 2005 | M/F | 130 | 130 | 64.91 | 64.91 | Fried | FFQ | Bladder cancer | Clinical signs pathohistological finding | T3 vs. T1 | OR: 6.31 (2.91–13.70) | 8 |
| 22 | Pandey et al. (103) | India | 2002 | M/F | 64 | 101 | 45/95 | 45/95 | Potatoes | 30-d recall method | Gallbladder cancer | OR: 1.25 (0.19–10.3) | |||
| 23 | Mucci et al. (31) | Sweden | 2003 | M/F | 23-14-124-17 | 33-54-202-42 | 51–77 | 51–77 | French Fries,potato crisps,pan-fried potatoes,potatoes au gratin,total potatoes | FFQ | Bladder cancer | Population-based cancer registry | Q4 vs. Q1 | OR: 0.7 (0.4–1.1),OR: 0.9 (0.4–1.8),OR: 1.3 (0.7–2.4),OR: 0.5 (0.2–1.0),OR: 1.6 (0.7–3.5) | 1,2,6,8,16,17,18,19,21 |
| Sweden | 2003 | M/F | 36-16-49-15 | 33-54-202-42 | 51–77 | 51–77 | French fries-potato crisps- pan-fried potato-potato au gratin- total potato | FFQ | Kidney cancer | Population -based cancer registry | Q4 vs. Q1 | OR: 0.7 (0.3–1.6),OR: 0.7 (0.3–1.9),OR: 0.7 (0.4–1.4),OR: 1.1 (0.4–2.6),OR: 0.8 (0.3–2.2) | 1,2,6,8,16,17,18,19,21 | ||
| Sweden | 2003 | M/F | 58-48-226-59 | 33-54-202-42 | 51–77 | 51–77 | French fries-potato crisps- pan-fried potato-potato au gratin- total potato | FFQ | Large bowel cancer | Population-based cancer registry | Q4 vs. Q1 | OR: 0.8 (0.5–1.4),OR: 1.3 (0.8–2.1),OR: 0.8 (0.5–1.2)OR: 1.5 (0.8–2.7),OR: 1.2 (0.6–2.2) | 1,2,6,8,16,17,18,19,21 | ||
| 24 | Isa et al. (94) | China | 2013 | M/F | 487 | 469 | 60–79 | 60–79 | Potatoes | FFQ | Bladder cancer | Hospital | Q4 vs. Q1 | OR: 0.4 (0.2–0.9) | 1,2,8 |
| 25 | Balbi et al. (79) | Uruguay | 2001 | M/F | 144 | 576 | 40–89 | 40–89 | Potatoes | FFQ | Bladder cancer | Microscopically | T3 vs. T1 | OR: 0.38 (0.23, 0.64) | 1,2,3,6,8,16,17,25 |
| 26 | Stejneck et al. (111) | Sweden | 1990 | M/F | 418 | 511 | 79 | Fried potatoes | Questionnaire | Urothelial cancer | Histologically,cytologicallyregional cancer registry | Weekly vs. more seldom,T3 vs. T1 | RR: 1.6 (1.1, 2.6),RR: 1.8 (1.2–2.7) | 1,2,8 | |
| 27 | Demetriou et al. (88) | Cyprus | 2012 | F | 935 | 817 | 40–70 | Potatoes | FFQ | Breast cancer | Histologically | Per 100-g/wk | OR: 0.95 (0.87, 1.03) | 1,5,6,7,11,31,34 | |
| 28 | Mourouti et al. (101) | Greece | 2015 | F | 250 | 250 | 44–68 | 44–68 | Potatoes | FFQ (valid) | Breast cancer | Biopsy | OR: 1.23 (0.88, 1.68) | 1,6,7,8,11,31,34,35 | |
| 29 | Tajaddini et al. (113) | Iran | 2015 | F | 306 | 309 | 25–65 | 25–65 | Baked/boiled potato, fried potatoes | FFQ (valid) | Breast cancer | Histologically | T3 vs. T1 | OR: 0.45 (0.28–0.71),OR: 0.64 (0.39–1.05) | 1,6,16,32,36 |
| 30 | Pelucchi et al. (104) | Italy | 2003 | F | 2569-1031-1953 | 2588-2411-4154 | <79 | <79 | Fried/baked | FFQ (valid) | Breast cancer-ovarian cancer-large bowel cancer | Interview | T3 vs. T1 | OR: 0.9 (0.8–1.1),OR: 1.1 (0.9–1.3),OR: 0.8 (0.7–1.0) | 1,2,3,6,8,9,16,17 |
| 31 | Stott-Milleret et al. (26) | USA | 2013 | M | 1549 | 1492 | 35–74 | 35–74 | French fries-snack chips | FFQ | Prostate cancer | Population-based tumor registry | T3 vs. T1 | OR: 1.37 (1.11–1.69),OR: 1.08 (0.89–1.32) | 1,3,4,6,11,42 |
| 32 | Russnes et al. (110) | Norway | 2016 | M | 1499 | 1112 | 67/25 | 67/25 | Boiled potatoes | FFQ | Prostate cancer | Histologically, cytologically | T3 vs. T1 | OR: 1.12(0.87–1.42) | 1,3,6,8,9,16,22,23 |
| 33 | Plagens-Rotman et al. (106) | Poland | 2018 | F | 167 | 683 | 21–84 | 21–84 | French fries and chips | Questionnaire | Ovarian cancer | Histologically | Per 100-g/wk | OR: 2.06 (0.53–7.99) | 7 |
| 34 | Grieb et al. (90) | Georgia | 2006 | M/F | 333 | 333 | 64 | 64 | Fried potatoes | FFQ (valid) | Renal cancer | Interview | Q4 vs. Q1 | OR: 2.05 (1.19, 3.53) | 1,2,4,6,8,14 |
| 35 | Andreatta et al. (77) | Argentina | 2010 | M/F | 168 | 334 | 55 | 55 | Boiled Fried potatoes | FFQ (valid) | Urinary tract cancer | Histopathologically | T3 vs. T1 | OR: 0.47 (0.13–1.63) | 1,2,6,7,8,25,26 |
| 36 | Hue et al. (41) | China | 1997 | M/F | 227 | 227 | 53.2 | 53.2 | Potatoes | FFQ | Lung cancer | Histologically | Q4 vs. Q1 | OR: 0.8 (0.5–1.5) | 8,14 |
| 37 | Hue et al. (34) | Canada | 2002 | M/F | 161 | 483 | >20 | >20 | French fries/fried potatoes | FFQ | Lung cancer | Pathologically | Q4 vs. Q1 | OR: 1.7 (1.0–3.0) | 1,3,9,16,25 |
| 38 | Dosil-Diaz et al. (25) | Spain | 2008 | M/F | 295 | 322 | >35 | >35 | Potatoes | FFQ(valid) | Lung cancer | Histologically | T3 vs. T1 | OR: 0.08 (0.03–0.22) | 1,2,8,26 |
| 39 | Hu et al. (27) | China | 1988 | M/F | 241 | 241 | 25–80 | 25–80 | Potatoes | Interview | Stomach cancer | Histologically | - | OR: 1.54 (1.03–2.33) | 8,17 |
| 40 | Polesel et al. (108) | Italy | 2009 | M/F | 326 | 652 | 63 | 63 | Potatoes | FFQ (valid) | PC | Histologically | Q4 vs. Q1 | OR: 1.79 (1.12–2.86) | 1,2,3,6,8,9,10,12,16,17 |
| 41 | Lucenteforte et al. (99) | Italy | 2008 | M/F | 230 | 547 | 22–80 | 22–80 | Potatoes | FFQ | Stomach cancer | Histologically | Q5 vs. Q1 | OR: 2.04 (1.05–3.98) | 1,2,3,6,8,10,11,16 |
| 42 | Bosetti et al. (83) | Italy | 2002 | M/F | 527 | 1297 | 61 | 61 | Potatoes | FFQ (valid) | Laryngeal cancer | Histologically | OR: 1.86 (1.29–2.68) | 1,2,3,9,16,17 | |
| 43 | Lin et al. (98) | USA | 2009 | M/F | 844 | 888 | 64.4 | 64.9 | White potatoes | FFQ (valid) | Bladder cancer | Histologically | Q4 vs. Q1 | OR: 1.02 (0.75–1.39) | 1,2,8,16,17,45 |
| 44 | Ohba et al. (102) | Japan | 1996 | M/F | 141 | 282 | 64.4 | 64.4 | Potatoes | FFQ | PC | Pathologically | per 3 servings/wk | RR:1.06 (0.84–1.35) | 1,2,9 |
| 45 | Bravi et al. (84) | Italy | 2006 | M | 1369 | 1451 | 46–74 | - | Potatoes | FFQ (valid) | Prostate | Biopsy | Q4 vs. Q1 | OR: 0.9 (0.7–1.16) | 1,3,6,7,8,9,16,17 |
| 46 | Malagoli et al. (100) | Italy | 2019 | M/F | 380 | 719 | 55 | Potatoes | FFQ | Melanoma | Biopsy | T3 vs. T1 | OR: 0.83 (0.6–1.16) | 3,6,16 | |
| 47 | Maso et al. (122) | Italy | 2019 | M/F | 690 | 665 | 25–84 | 27–84 | Potatoes | FFQ | Bladder | Histologically | Q4 vs. Q1 | OR: 0.79 (0.54–1.13) | 1,2,3,8,9,12,16 |
| 48 | Shah et al. (116) | Malaysia | 2014 | M/F | 58 | 134 | 57.9 ± 12.79 | 57.6 ± 11.80 | Sauced or cooked potatoes | Dietary history | Gastric | Histologically | OR: 0.19 (0.05–0.68) | - | |
| Fried potato | OR: 0.56 (0.29–1.07) | - |
CRC, colorectal cancer; F, females; M, males; PC, pancreatic cancer; RR, relative risk, T, tertile; Q1, Quartile1/Quintile 1; Q4, Quartile; Q5, quintile.
Adjustments: age (1), sex (2), education (3), race (4), height (4), BMI (5), physical activity (6), smoking (7), study center (8), year of interview (9), family history of cancer (10) and diabetes mellitus (11), hypertension (12), family income (13), number of meals per day (14), dietary intake of energy (15), alcohol (16), red meat (17), fruit and vegetable (18), fiber (19), fat (20), calcium (21), zinc (22), vitamin D (23), social class (24), employment in risky occupation (25), socioeconomic status (26), α-linolenic acid (27), nonsteroid anti-inflammatory drug (28), multivitamin supplements (29), age at menarche (30), parity (31), age at first pregnancy (32), hormone therapy (33), age at menopause (34), menopausal status (35), aspirin (36), prostate-specific antigen testing in previous period (37), oral contraceptive use (38), skin color (39), sunburn (40), elastosis (41), treatment allocation (42), age at first birth (43), ethnicity (44), glycemic load (45).
In most studies, potato consumption was assessed using an FFQ. Fifty-eight studies reported the total quantity of potato consumption. Moreover, 14 studies reported the consumption of fried potatoes (31, 33, 66, 67, 77, 80, 90, 93, 104, 111, 113–116), 8 reported French fry consumption (26, 28, 31, 66, 67, 72, 93, 106), and 4 boiled/baked potato intake (67, 77, 110, 113).
Included in the systematic review, 10 studies reported results on CRC (24, 27, 36, 37, 73, 78, 89, 97, 105, 114), 8 studies were on breast cancer (28, 67, 68, 88, 101, 104, 113, 121), and 6 studies on pancreatic cancer (PC) (64, 74, 86, 102, 108, 119). We also included 7 studies on the analyses on prostate cancer (26, 29, 69–71, 84, 110), 7 on gastric cancer (23, 30, 81, 92, 95, 99, 116), 4 on kidney cancer (31, 76, 90, 120), 4 on lung cancer (25, 32, 72, 93), and 6 on bladder cancer (31, 33, 79, 94, 98, 122). Furthermore, 7 studies on colon cancer (24, 65, 66, 76, 96, 112, 118), 3 on rectal cancer (24, 65, 66), 3 on ovarian cancer (104, 106, 117), 2 on esophageal squamous cell cancer (82, 87), 2 on large bowel cancer (31, 104), 2 on gallbladder cancer (103, 109), 2 on nasopharyngeal cancer (107, 115), 2 on urothelial cancer (77, 111), and 6 on other cancer sites were evaluated in relation to potato consumption (75, 80, 83, 85, 91, 100). Thus, study-specific results are shown in Tables 1 and 2.
The results of the quality assessment are presented in Supplemental Table 1. The ROBINS-E tool indicated an overall low to moderate risk of bias and serious risk in some studies. In most studies, bias originated from exposure misclassification and from possible confounding.
Total potato (white and yellow) and risk of overall cancer in cohort studies
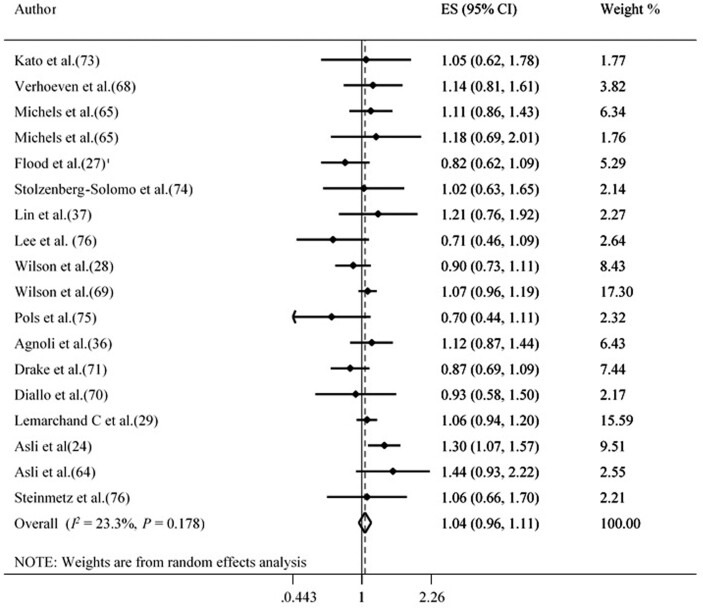
In cohort studies, participants with the highest total potato consumption (white and yellow) did not show significantly elevated risk of total cancers compared with participants in the low category (Summary Effect Estimate: 1.04 [95% CI: 0.96, 1.11; tau2 = 0.005, n = 18]) (Figure 2). There was no evidence of publication bias with Egger's regression test (P = 0.489) or when using a funnel plot (Supplemental Figure 1). Furthermore, sensitivity analyses showed that no individual study had a significant effect on the overall risk estimate (Supplemental Figure 2). Subgroup analysis showed total potato (white and yellow) intake significantly increase overall cancer in studies conducted in Europe (Table 3). A linear trend estimation indicated that a 100-g increment in total potato (white and yellow) intake was not associated with a higher risk of total cancer (pooled risk estimate: 1.00 [95% CI: 0.99, 1.00; tau2 < 0.001, n = 12]). However, some evidence of a nonlinear dose-response association was observed between total potato (white and yellow) consumption and risk of total cancer (P nonlinearity = 0.006, n = 12 studies) (Supplemental Figure 3).
FIGURE 2.
Forest plot derived from random-effects meta-analysis of studies investigating the association between high versus low total potato intake and total cancer in adults. ES, effect size.
TABLE 3.
Results of subgroup analysis for potato consumption and risk of site-specific cancer in adults1
| Group | Studies (n) | ES (95% CI) | P value | P-within subgroups heterogeneity | P-between subgroups heterogeneity | tau2 |
|---|---|---|---|---|---|---|
| Total cancer | ||||||
| total | 18 | 1.04 (0.96, 1.11) | 0.345 | 0.178 | — | 0.005 |
| Gender | ||||||
| Female | 7 | 1.05 (0.94, 1.18) | 0.311 | 0.113 | 0.706 | 0.017 |
| Male | 6 | 1.02 (0.95, 1.10) | 0.474 | 0.318 | 0.001 | |
| Both | 5 | 1.09 (0.94, 1.27) | 0.217 | 0.258 | 0.010 | |
| BMI adjustment | ||||||
| Yes | 11 | 0.99 (0.87, 1.11) | 0.881 | 0.333 | 0.327 | 0.002 |
| No | 7 | 1.06 (0.99, 1.13) | 0.069 | 0.129 | 0.021 | |
| Continent | ||||||
| America | 9 | 1.01 (0.93, 1.10) | 0.678 | 0.394 | 0.111 | 0.001 |
| Europe | 8 | 1.08 (1.004, 1.18) | 0.039 | 0.298 | 0.005 | |
| Australia | 1 | 0.70 (0.44, 1.10) | 0.127 | — | 0.127 | |
| Number of cases | ||||||
| <500 | 11 | 0.99 (0.87, 1.11) | 0.881 | 0.333 | 0.327 | 0.005 |
| ≥500 | 7 | 1.06 (0.99, 1.13) | 0.069 | 0.129 | 0.005 | |
| Colorectal cancer | ||||||
| Study design | ||||||
| Cohort | 5 | 1.09 (0.91, 1.31) | 0.310 | 0.132 | 0.99 | 0.017 |
| Case-control | 4 | 1.13 (0.86, 1.49) | 0.372 | 0.152 | 0.033 | |
| Gender | ||||||
| Female | 4 | 1.08 (0.83, 1.40) | 0.133 | 0.07 | 0.97 | 0.037 |
| Both | 5 | 1.12 (0.93, 1.35) | 0.102 | 0.259 | 0.010 | |
| Energy adjustment | ||||||
| Yes | 8 | 1.08 (0.94, 1.26) | 0.255 | 0.130 | 0.28 | 0.015 |
| No | 1 | 1.46 (0.90, 2.36) | 0.126 | — | <0.001 | |
| BMI adjustment | ||||||
| Yes | 6 | 1.03 (0.87, 1.22) | 0.703 | 0.187 | 0.04 | 0.014 |
| No | 3 | 1.29 (1.09, 1.52) | 0.003 | 0.658 | <0.001 | |
| Continent | ||||||
| America | 4 | 0.89 (0.73, 1.09) | 0.269 | 0.386 | 0.04 | 0.0006 |
| Europe | 3 | 1.24 (1.07, 1.44) | 0.003 | 0.575 | <0.001 | |
| Australia | 1 | 1.14 (0.86, 1.49) | 0.346 | — | <0.001 | |
| Africa | 1 | 1.46 (0.90, 2.36) | 0.126 | — | <0.001 | |
| Number of cases | ||||||
| <400 | 5 | 1.12 (0.87, 1.45) | 0.345 | 0.245 | 0.98 | 0.021 |
| ≥400 | 4 | 1.09 (0.91, 1.32) | 0.325 | 0.075 | 0.020 | |
| Pancreatic cancer | ||||||
| Study design | ||||||
| Cohort | 2 | 1.23 (0.87, 1.72) | 0.228 | 0.296 | 0.83 | 0.005 |
| Case-control | 3 | 1.23 (0.95, 1.60) | 0.110 | 0.147 | 0.025 | |
| Gender | ||||||
| Male | 1 | 1.02 (0.63, 1.64) | 0.935 | — | 0.49 | <0.001 |
| Both | 4 | 1.26 (1.02, 1.55) | 0.032 | 0.214 | 0.015 | |
| BMI adjustment | ||||||
| Yes | 2 | 1.59 (1.15, 2.19) | 0.004 | 0.505 | 0.04 | <0.001 |
| No | 3 | 1.09 (0.91, 1.30) | 0.315 | 0.791 | <0.001 | |
| Number of cases | ||||||
| <300 | 2 | 1.05 (0.85, 1.30) | 0.640 | 0.888 | 0.08 | <0.001 |
| ≥300 | 3 | 1.38 (1.10, 1.73) | 0.005 | 0.378 | <0.001 | |
| Continent | ||||||
| America | 1 | 1.20 (0.87, 1.65) | 0.266 | — | 0.33 | <0.001 |
| Europe | 3 | 1.38 (1.01, 1.89) | 0.040 | 0.253 | 0.020 | |
| Asia | 1 | 1.06 (0.83, 1.34) | 0.630 | — | <0.001 | |
| Breast cancer | ||||||
| Study design | ||||||
| Cohort | 2 | 0.95 (0.80, 1.14) | 0.646 | 0.253 | 0.98 | 23 |
| Case-control | 2 | 0.95 (0.82, 1.10) | 0.543 | 0.089 | 65 | |
| BMI adjustment | ||||||
| Yes | 3 | 0.93 (0.83, 1.05) | 0.290 | 0.211 | 0.29 | 35.7 |
| No | 1 | 1.14 (0.80, 1.61) | 0.459 | — | — | |
| Energy adjustment | ||||||
| Yes | 3 | 0.92 (0.82, 1.04) | 0.209 | 0.455 | 0.10 | 0 |
| No | 1 | 1.23 (0.89, 1.69) | 0.202 | — | — | |
| Number of cases | ||||||
| <1000 | 2 | 1.18 (0.93, 1.50) | 0.155 | 0.75 | 0.04 | 0 |
| ≥1000 | 2 | 0.90 (0.79, 1.02) | 0.103 | 0.99 | 0 | |
| Prostate cancer | ||||||
| Study design | ||||||
| Cohort | 4 | 1.03 (0.96, 1.12) | 0.318 | 0.405 | 0.28 | 0 |
| Case-control | 1 | 0.90 (0.69, 1.15) | 0.414 | — | — | |
| BMI adjustment | ||||||
| Yes | 4 | 1.04 (0.97, 1.12) | 0.246 | 0.612 | 0.13 | 0 |
| No | 1 | 0.87 (0.69, 1.09) | 0.233 | — | — | |
| Dietary assessment tool | ||||||
| FFQ | 4 | 1.02 (0.95, 1.10) | 0.436 | 0.274 | 0.67 | 22 |
| 24-h dietary records | 1 | 0.93 (0.57, 1.49) | 0.765 | — | — | |
| Number of cases | ||||||
| <1000 | 2 | 0.88 (0.71, 1.08) | 0.228 | 0.804 | 0.12 | 0 |
| ≥1000 | 3 | 1.04 (0.97, 1.13) | 0.221 | 0.456 | 0 | |
| Continent | ||||||
| America | 1 | 1.07 (0.96, 1.19) | 0.217 | — | 0.31 | — |
| Europe | 4 | 0.99 (0.90, 1.09) | 0.890 | 0.386 | 1.3 |
ES, effect size.
Total potato (white and yellow) and risk of site-specific cancer
In the highest versus lowest analysis that combined colon, rectal, and CRC as an outcome (total CRC), total potato (white and yellow) consumption increased the risk of total CRC in cohort studies. Comparing the highest with lowest total potato (white and yellow) consumption categories, no significant association was found between total potato consumption and separate risk of CRC, colon, PC, breast, prostate, gastric, bladder, kidney, and lung cancer in cohort or case-control studies. The findings are reported in Table 4. Subgroup analyses revealed that the association between total potato (white and yellow) consumption and risk of CRC was significant in studies conducted in European countries (P = 0.003), and in studies in which BMI was not adjusted for (P = 0.003) (Table 3). Subgroup analyses indicated that the association between total potato (white and yellow) intake and PC was significant in studies conducted in Europe (P = 0.04), in those with a higher number of cases (P = 0.005), in studies that adjusted for BMI (P = 0.004) as covariates, and that included either gender (P = 0.032) (Table 3). Between-subgroup heterogeneity was observed for BMI adjustment and continent for CRC. For PC, we observed between-subgroup heterogeneity for BMI adjustment and number of cases. Regarding breast cancer, between-subgroup heterogeneity was observed for the number of cases (Table 3).
TABLE 4.
Findings of meta-analyses for the consumption of total potato and potato preparation with site-specific cancers in adults1
| Cancer type | Studies, n | Summary effect sizes (95% CI) | tau 2 |
|---|---|---|---|
| Total potato intake | |||
| Total CRC2 | |||
| Total | 13 | 1.09 (0.98, 1.22) | 0.008 |
| Cohort | 8 | 1.12 (1.004, 1.25) | 0.0006 |
| Case-control | 5 | 1.05 (0.79, 1.39) | 0.048 |
| CRC3 | |||
| Total | 9 | 1.11 (0.97, 1.28) | 0.015 |
| Cohort | 5 | 1.10 (0.92, 1.32) | 0.017 |
| Case-control | 4 | 1.13 (0.86, 1.49) | 0.033 |
| Colon cancer | |||
| Total | 4 | 1.09 (0.93, 1.28) | 0.0005 |
| Cohort | 3 | 1.13 (0.97, 1.33) | <0.0001 |
| Case-control | 1 | 0.67 (0.36, 1.21) | <0.0001 |
| Rectal cancer | |||
| Cohort | 2 | 1.48 (1.10, 1.98) | <0.0001 |
| Pancreatic cancer | |||
| Total | 5 | 1.21 (1.01, 1.45) | 0.008 |
| Cohort | 2 | 1.23 (0.87, 1.72) | 0.005 |
| Case-control | 3 | 1.23 (0.95, 1.60) | 0.025 |
| Gastric cancer | |||
| Case-control | 7 | 1.005 (0.69, 1.46) | 0.161 |
| Breast cancer | |||
| Total | 4 | 0.98 (0.85, 1.12) | 0.006 |
| Cohort | 2 | 0.97 (0.78, 1.21) | 0.006 |
| Case-control | 2 | 1.02 (0.75, 1.37) | 0.031 |
| Prostate cancer | |||
| Total | 5 | 1.03 (0.95, 1.10) | <0.0001 |
| Cohort | 4 | 1.04 (0.96, 1.12) | <0.0001 |
| Case-control | 1 | 0.90 (0.70, 1.16) | <0.0001 |
| Renal cancer | |||
| Total | 2 | 0.73 (0.48, 1.06) | <0.0001 |
| Cohort | 1 | 0.71 (0.46, 1.08) | <0.0001 |
| Case-control | 1 | 0.80 (0.29, 2.16) | <0.0001 |
| Bladder cancer | |||
| Case-control | 5 | 0.72 (0.46, 1.14) | 0.195 |
| Lung cancer | |||
| Case-control | 2 | 0.80 (0.49, 1.29) | <0.0001 |
| Fried potato intake | |||
| Gastrointestinal cancer | |||
| Total | 9 | 1.03 (0.89, 1.19) | <0.0001 |
| Cohort | 4 | 1.07 (0.90, 1.27) | <0.0001 |
| Case-control | 5 | 0.95 (0.74, 1.23) | <0.0001 |
| Sex-hormone-related cancer | |||
| Total | 6 | 1.01 (0.91, 1.11) | 0.007 |
| Cohort | 3 | 0.97 (0.90, 1.05) | 0.003 |
| Case-control | 3 | 1.15 (0.76, 1.75) | 0.084 |
| Urinary-related cancer | |||
| Case-control | 9 | 1.26 (0.82, 1.93) | 0.321 |
| Lung cancer | |||
| Total | 2 | 1.26 (0.81, 1.95) | 0.058 |
| Cohort | 1 | 1.07 (0.82, 1.39) | <0.0001 |
| Case-control | 1 | 1.70 (0.98, 2.94) | <0.0001 |
| Boiled/mashed/baked potato intake | |||
| Total | 4 | 0.87 (0.66, 1.14) | <0.0001 |
| Cohort | 2 | 0.95 (0.81, 1.11) | 0.054 |
| Case-control | 2 | 0.73 (0.30, 1.77) | 0.379 |
CRC, colorectal cancer.
2Total CRC included CRC, colon, and rectal cancers.
CRC included.
Estimation of a linear trend indicated that a 100-g/wk increment in total potato (white and yellow) intake was not associated with a higher risk of CRC (Summary Effect Estimate: 1.00 [95% CI: 0.99, 1.01; tau2 = 0.0001, n = 3]), PC (Summary Effect Estimate: 1.00 [95% CI: 0.99, 1.02; tau2 = 0.0001, n = 2] [1 cohort and 1 case-control study]), breast cancer (Summary Effect Estimate: 0.99 [95% CI: 0.97, 1.00; tau2 <0.001, n = 2]). However, evidence of a nonlinear dose-response association was observed between potato consumption (white and yellow) and risk of CRC (P nonlinearity = 0.019, n = 3 cohort studies) (Supplemental Figure 4).
No indication of publication bias was found with Egger's regression test or with a funnel plot (see Supplemental Figures 5–8) for CRC (P = 0.69), PC (P = 0.25), breast cancer (P = 0.07), or prostate cancer (P = 0.09).
In the meta-analysis comparing the high versus low intake categories, no individual study significantly affected the overall risk estimates for CRC, PC, breast, and prostate cancer (Supplemental Figures 9–12).
Potato preparations (fried versus boiled, mashed, baked, roasted) and risk of cancer
In the meta-analysis comparing high with low intake categories, no association was found between fried potato consumption and risk of gastrointestinal cancers, sex-hormone-related cancers, urinary-system-related cancers, and lung cancer in cohort or case-control studies. Participants in the highest boiled/mashed/baked/roasted potato intake categories did not show a significantly elevated risk of sex-hormone-related cancers compared with participants in the low category in cohort or case-control studies. The summary findings are reported in Table 4.
Certainty of evidence
The certainty of the evidence was assessed using GRADE. We found that studies on the associations of potato consumption with total cancer, CRC, colon, rectal, pancreatic, renal, lung, breast, and prostate cancers were of low quality, whereas studies on bladder and gastric cancers had very low quality. Furthermore, associations between fried potato consumption with gastrointestinal cancer, and sex-hormone-related cancer were low quality, whereas studies on urinary-related cancers and lung cancer had very low quality (Table 5).
TABLE 5.
GRADE evidence profile1
| Outcome | Studies n | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | ES (95% CI) | Assumed risk | Absolute risk (95% CI) | Certainty |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total potato | |||||||||||
| Total cancer | 18 | Cohort | Serious | Not serious | Not serious | Serious | None | 1.04 (0.96, 1.11) | 68 per 10000 | 2.72 per 10000(–7.48 to 2.75) | ⊕⊕◯◯Lowdue to risk of bias and imprecision |
| CRC | 9 | Cohort & case-control | Serious | Not serious | Not serious | Serious | None | 1.11 (0.97, 1.28) | 81 per 10000 | 8.9 per 10000(–22.68 to 2.43) | ⊕⊕◯◯Lowdue to risk of bias and imprecision |
| Colon cancer | 4 | Cohort & case-control | Serious | Not serious | Not serious | Serious | None | 1.09 (0.93, 1.28) | 68 per 10000 | 6.12 per 10000(–19.04 to 4.27) | ⊕⊕◯◯Lowdue to risk of bias and imprecision |
| Rectal cancer | 2 | Cohort | Serious | Not serious | Not serious | Serious | None | 1.47 (1.10, 1.98) | 17 per 10000 | 7.99 per 10000(–16.66 to –1.7) | ⊕⊕◯◯Lowdue to risk of bias and imprecision |
| Pancreatic cancer | 5 | Cohort & case-control | Serious | Not serious | Not serious | Serious | None | 1.21 (1.01, 1.45) | 19 per 10000 | 3.99 per 10000(–8.55 to –0.19) | ⊕⊕◯◯Lowdue to risk of bias and imprecision |
| Gastric cancer | 7 | Case-control | Serious | Serious | Not serious | Serious | None | 1.005 (0.69, 1.46) | 137 per 10000 | 0.68 per 10000(–63.02 to 42.47) | ⊕◯◯◯Very lowdue to risk of bias, imprecision, and inconsistency |
| Breast cancer | 4 | Cohort & case-control | Serious | Not serious | Not serious | Serious | None | 0.98 (0.85, 1.12) | 130 per 10000 | 2.6 per 10000(–15.6 to 19.5) | ⊕⊕◯◯Lowdue to risk of bias and imprecision |
| Prostate cancer | 5 | Cohort & case-control | Serious | Not serious | Not serious | Serious | None | 1.03 (0.95, 1.10) | 204 per 10000 | 6.12 per 10000(–20.4 to 10.2) | ⊕⊕◯◯Lowdue to risk of bias and imprecision |
| Renal cancer | 2 | Cohort & case-control | Serious | Not serious | Not serious | Serious | None | 0.73 (0.48, 1.06) | 24 per 10000 | 6.48 per 10000(–1.44 to 12.48) | ⊕⊕◯◯Lowdue to risk of bias and imprecision |
| Lung cancer | 2 | Cohort & case-control | Serious | Not serious | Not serious | Serious | None | 0.80 (0.49, 1.29) | 4781 per 10000 | 956 per 10000(–1386 to 2438) | ⊕⊕◯◯Lowdue to risk of bias and imprecision |
| Bladder cancer | 5 | Case-control | Serious | Serious | Not serious | Serious | None | 0.72 (0.46, 1.14) | 5094 per 10000 | 956 per 10000(–713 to 1426) | ⊕◯◯◯Very lowdue to risk of bias, imprecision, and inconsistency |
| Fried potato | |||||||||||
| Gastrointestinal cancers | 9 | Cohort & case-control | Serious | Not serious | Not serious | Serious | None | 1.03 (0.89, 1.19) | 81 per 10000 | 2.43 per 10000(–15.39 to 8.91) | ⊕⊕◯◯Lowdue to risk of bias and imprecision |
| Sex-hormone-related cancers | 6 | Cohort & case-control | Serious | Not serious | Not serious | Serious | None | 1.01 (0.91, 1.11) | 130 per 10000 | 1.3 per 10000(–14.3 to 11.7) | ⊕⊕◯◯Lowdue to risk of bias and imprecision |
| Urinary-related cancers | 9 | Cohort & case-control | Serious | Serious | Not serious | Serious | None | 1.26 (0.82, 1.93) | 3283 per 10000 | 853 per 10000(–3053 to 590) | ⊕◯◯◯Very lowdue to risk of bias, imprecision, and inconsistency |
| Lung cancer | 2 | Cohort & case-control | Serious | Serious | Not serious | Serious | None | 1.26 (0.86, 1.95) | 57 per 10000 | 14.82 per 10000(–54.15 to 7.98) | ⊕◯◯◯Very lowdue to risk of bias, imprecision, and inconsistency |
Symbols indicate the following strength of evidence: ⊕⊕⊕⊕, high (further research is very unlikely to change our confidence in the estimate of association); ⊕⊕⊕◯, moderate (further research is likely to have an important impact on our confidence in the estimate of association and may change the estimate); ⊕⊕◯◯, low (further research is very likely to have an important impact on our confidence in the estimate of association and is likely to change the estimate); and ⊕◯◯◯, very low (any estimate of association is very uncertain). CRC, colorectal cancer; ES, effect size; GRADE, Grading of Recommendations, Assessment, Development and Evaluation.
The following informative statements were based on the GRADE tool assessment: total potato (white and yellow) intake may not increase the risk of total cancer, CRC, colon, pancreatic, breast, prostate, lung, or renal cancers. Fried potato intake may not increase the risk of gastrointestinal cancers, and sex-hormone-related cancers. Total potato (white and yellow) intake may not increase the risk of gastric and bladder cancers but the evidence is very uncertain. Fried potato intake may not increase the risk of and urinary-related cancers and lung cancer but the evidence is very uncertain (Supplemental Table 2).
Discussion
The present meta-analysis examined the association between potato consumption and risk of site-specific cancers with data from 20 cohort and 36 case-control studies. Our findings showed a significant association between total potato (white and yellow) consumption and risk of total CRC. In addition, total potato (white and yellow) intake was not associated with a higher risk of total or other site-specific cancers. Furthermore, no association was found between potato preparations (fried/boiled, mashed, baked, roasted) and risk of other site-specific cancers. However, we observed a positive nonlinear association between potato consumption and the risk of total cancer and CRC based on a nonlinear analysis.
We did not observe a significant positive association between total potato (white and yellow) consumption and total cancer risk in cohort studies. However, nonlinear dose-response associations have been observed in prior cohort studies. Findings from case-control studies are subject to several methodological limitations (123). For example, they are prone to recall bias and selection bias, which can make it difficult to draw firm conclusions. Therefore, we did not perform analyses of total cancers for case-control studies. A systematic review and dose response meta-analysis of 2 prospective studies did not suggest a significant association between potato consumption and total cancer risk (124).
A significant positive association has been reported between potato intake and the risk of total cancer in European populations, who tend to consume high quantities of refined carbohydrates, bread, potatoes, pasta, and rice (125). High carbohydrate intake may be an index of a highly endogenous insulin environment, and insulin and the modulation of IGF-1 have been indicated to act as cancer-promoting agents for several types of cancers (13). Also, we found a significant marginal association between potato and risk of total cancers in studies not adjusted for BMI. Lack of adjustment for BMI may be an explanation for these significant positive associations between potato intake and risk of total cancer. Obesity, a major determinant of insulin resistance and hyperinsulinemia, has been related to cancer (126).
We found a significant association between potato consumption and risk of total CRC in cohort studies. A recent meta-analysis by Schwingshackl et al. did not find any association between potato consumption and risk of CRC (34). One study included in the review was restricted to a specific kind of potato (sweet potato) (35). Some research suggests that a typical Western dietary pattern comprised of high amounts of red meat, processed meat, potatoes, and refined carbohydrates is associated with a higher risk of CRC (127, 128). One case-control study showed tendencies toward a higher risk of colon cancer in participants who consumed high quantities of potato (112). Another study with a case-control design showed an association between potato consumption and increased risk of rectal cancer among whites, but not among African Americans (129). The etiology of colon and rectum cancer may vary significantly due to the anatomy, embryology, and physiology of the colon and the rectum (130). Our meta-analysis showed a significant association between total potato (white and yellow) consumption and rectal cancer but no relation with colon cancer. However, only a few studies have examined potato consumption in relation to colon or rectum cancer separately. Therefore, we may have had limited statistical power in this subgroup analysis. In addition, a pooled analysis in 2007 did not show a significant relation between potato consumption and risk of colon cancer (118).
We did not find a significant positive relation between total potato (white and yellow) consumption and risk of PC in cohort or case-control studies. This finding was consistent with a 2012 pooled analysis of 14 cohort studies on the association of total potato intake (per 606-g/wk increase in intake) with PC (119). Findings from that analysis revealed no significant linear association between each 606-g/wk increment in potato consumption and risk of PC (119). A meta-analysis of 10 cohort studies did not support an association between diets with a high GI, GL, total carbohydrates, or sucrose and PC risk (131).
We found no associations between total potato (white and yellow) intake and risk of other site-specific cancers such as breast, prostate, lung, gastric, kidney, and bladder cancer. With respect to specific kinds of potato preparation, we did not find an association between fried potatoes and risk of gastrointestinal-, sexual hormone-, and kidney-related cancers. We found no significant association between boiled/mashed/baked/roasted potato intake and sex-hormone-related cancers. In line with our findings, a systematic review and meta‐analysis of 32 epidemiological studies concluded that dietary acrylamide was not related to the risk of oral and pharyngeal, esophageal, stomach, colorectal, pancreatic, laryngeal, lung, breast, endometrial, ovarian, prostate, bladder, or lymphoid malignancies (132).
Potato consumption might induce both beneficial and harmful effects on health. Potatoes are a rich source of essential nutrients including starch, fiber, trace minerals (such as potassium), vitamins (such as vitamin C), and phytochemicals (lutein and zeaxanthin), which are necessary for the body to stay healthy (133). Large amounts of catalase enzyme are found in potatoes, which converts hydrogen peroxide into oxygen and water, and can prevent cell injury. On the other hand, potatoes have toxic compounds, such as α-solanine and α-chaconine which are known to induce toxicity. It is also noteworthy that the biological effects and nutrient content of potatoes may be impressed by preparation and cooking ways. Fried potatoes are typically high in dietary fats, in particular, trans fatty acids, salts, and acrylamide, which have been related to increased risk of cancer in some studies (16–18). Foodborne toxins such as acrylamide are formed when starchy foods such as potatoes and potato products are cooked at temperatures above 121°C. The highest concentrations of acrylamide are found in potato chips and French fries that are cooked at high temperatures. However, deep frying at 170°C is known to effectively lower the concentration of toxic compounds, whereas microwaving is only somewhat effective and freeze-drying or dehydration has little effect (133). One study showed that boiling, baking, and microwave potato preparation methods can reduce vitamin C, thiamin, riboflavin, niacin, folic acid, and vitamin B-6 (134). Additionally, boiled potato has a high GI compared with other kinds of potatoes, which is mainly due to the conversion of native starch granules into rapidly digestible starch (RDS) (135). Therefore, the amount of essential nutrients decreases with the potato processing, whereas its GI increases.
To the best of our knowledge, this is the first systematic review and meta-analysis to investigate the association between potato consumption and risk of cancer in multiple sites. Strengths of this study include a large sample size including different geographic regions with different dietary patterns. Our findings were stable and robust in sensitivity analyses. Moreover, findings were adjusted for a great number of confounding factors in the studies that were included and we found no evidence of publication bias. We used the GRADE tool to assess the certainty of the evidence. The certainty of the evidence was rated as low for the association between total potato (white and yellow) consumption and total cancer, CRC, colon, rectal, renal, pancreatic, breast, prostate, and lung cancers and was rated as very low for gastric and bladder cancers. Furthermore, the certainty of the evidence was rated as low for the association between fried potato consumption and gastrointestinal cancers, and sex-hormone-related cancers and was rated as very low for urinary-related cancers and lung cancer.
Besides these strengths, several limitations should be kept in mind when interpreting our findings. First, most of the studies that were included had case-control designs. Case-control studies are subject to recall bias, selection bias, and reverse causation bias. Second, data on different types of potatoes as well as potato preparation and processing methods were not available in most of the studies, which prevented us from performing a more accurate detailed analysis. Third, use of a self-administered questionnaire to assess potato consumption could result in measurement errors. Use of an FFQ for capturing variation in dietary intake, especially potato intake, in different regions might also lead to bias due to the fact that different types of potatoes are more commonly found in different geographical areas (which is not taken into account in the FFQ). Fourth, most of the studies did not adjust other food items when investigating the association between potato consumption and risk of cancer. Because our meta-analysis was conducted on observational studies, we cannot conclude the possible specific effects of residual confounding on the results of both each separate study and also the pooled estimates.
Considering the importance of potatoes as a food source in many parts of world, these findings warrant further investigation. Prospective cohort studies with consistent, improved methods of estimating intake are warranted to investigate the associations between specific kinds of potato and cancer.
Conclusion
Overall, we did not find a significant association between potato consumption and risk of site-specific cancers when comparing high and low categories or when using linear dose-response analyses. More cohort studies are needed to confirm the findings related to cancers with low or very low certainty evidence.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—MDM, LA, and MA: designed the study; MDA, HM, and MRA: independently carried out the literature search and screening of articles; MDM: analyzed the data; MDM, MA, and AJ: wrote the manuscript; HM: helped edit the writing; PJS: revised the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by Tehran University of Medical Sciences, Tehran, Iran (No.99-2-212-48517).
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–12 and Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: CRC, colorectal cancer; GI, glycemic index; GL, glycemic load; GRADE, Grades of Recommendation, Assessment, Development and Evaluation; IGF-1, insulin-like growth factor-1; PC, pancreatic cancer; RCT, randomized clinical trial; ROBINS-E, Risk Of Bias In Nonrandomized Studies; RR, relative risk.
Contributor Information
Manije Darooghegi Mofrad, Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
Hadis Mozaffari, Faculty of Land and Food Systems, University of British Columbia, Vancouver, Canada.
Mohammad Reza Askari, Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
Mohammad Reza Amini, Department of Clinical Nutrition, Faculty of Nutrition Sciences and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Alireza Jafari, Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
Pamela J Surkan, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Leila Azadbakht, Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran; Diabetes Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran; Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E86. [DOI] [PubMed] [Google Scholar]
- 2.Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, Aboyans V, Adetokunboh O, Afshin A, Agrawal A. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet North Am Ed. 2017;390(10100):1151–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barak Y, Fridman D. Impact of Mediterranean diet on cancer: focused literature review. Cancer Genomics-Proteomics. 2017;14(6):403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J, Pan G, Jiang H, Li W, Dong J, Zhang H, Ji X, Zhu Z. A meta-analysis between dietary carbohydrate intake and colorectal cancer risk: evidence from 17 observational studies. Biosci Rep. 2017;37(2):553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaheer K, Akhtar MH. Potato production, usage, and nutrition – a review. Crit Rev Food Sci Nutr. 2016;56(5):711–21. [DOI] [PubMed] [Google Scholar]
- 6.Camire ME, Kubow S, Donnelly DJ. Potatoes and human health. Crit Rev Food Sci Nutr. 2009;49(10):823–40. [DOI] [PubMed] [Google Scholar]
- 7.Park S, Ahn S, Shin Y, Yang Y, Yeom C. Vitamin C in cancer: a metabolomics perspective. Front Physiol. 2018;9:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng Y, Sun J, Yu J, Wang C, Su J. Dietary intakes of calcium, iron, magnesium, and potassium elements and the risk of colorectal cancer: a meta-analysis. Biol Trace Elem Res. 2019;189(2):325–35. [DOI] [PubMed] [Google Scholar]
- 9.Im HW, Suh B-S, Lee S-U, Kozukue N, Ohnisi-Kameyama M, Levin CE, Friedman M. Analysis of phenolic compounds by high-performance liquid chromatography and liquid chromatography/mass spectrometry in potato plant flowers, leaves, stems, and tubers and in home-processed potatoes. J Agric Food Chem. 2008;56(9):3341–9. [DOI] [PubMed] [Google Scholar]
- 10.Kaspar KL, Park JS, Brown CR, Mathison BD, Navarre DA, Chew BP. Pigmented potato consumption alters oxidative stress and inflammatory damage in men. J Nutr. 2011;141(1):108–11. [DOI] [PubMed] [Google Scholar]
- 11.Madiwale GP, Reddivari L, Stone M, Holm DG, Vanamala J. Combined effects of storage and processing on the bioactive compounds and pro-apoptotic properties of color-fleshed potatoes in human colon cancer cells. J Agric Food Chem. 2012;60(44):11088–96. [DOI] [PubMed] [Google Scholar]
- 12.Van Bakel M, Kaaks R, Feskens E, Rohrmann S, Welch A, Pala V, Avloniti K, Van Der Schouw Y, Du H, Halkjaer J. Dietary glycaemic index and glycaemic load in the European Prospective Investigation into Cancer and Nutrition. Eur J Clin Nutr. 2009;63(S4):S188. [DOI] [PubMed] [Google Scholar]
- 13.Sieri S, Krogh V. Dietary glycemic index, glycemic load and cancer: an overview of the literature. Nutr Metab Cardiovasc Dis. 2017;27(1):18–31. [DOI] [PubMed] [Google Scholar]
- 14.Sadeghi A, Sadeghi O, Khodadost M, Pirouzi A, Hosseini B, Saedisomeolia A. Dietary glycemic index and glycemic load and the risk of prostate cancer: an updated systematic review and dose-response meta-analysis. Nutr Cancer. 2020;72(1):5–14. [DOI] [PubMed] [Google Scholar]
- 15.Melkonian SC, Daniel CR, Ye Y, Pierzynski JA, Roth JA, Wu X. Glycemic index, glycemic load, and lung cancer risk in non-Hispanic whites. Cancer Epidemiol Biomarkers Prev. 2016;25(3):532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipworth L, Sonderman JS, Tarone RE, McLaughlin JK. Review of epidemiologic studies of dietary acrylamide intake and the risk of cancer. Eur J Cancer Prev. 2012;21(4):375–86. [DOI] [PubMed] [Google Scholar]
- 17.Ge S, Feng X, Shen L, Wei Z, Zhu Q, Sun J. Association between habitual dietary salt intake and risk of gastric cancer: a systematic review of observational studies. Gastroenterology Research and Practice. 2012;2012:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKelvey W, Greenland S, Chen MJ, Longnecker MP, Frankl HD, Lee ER, Haile RW. A case-control study of colorectal adenomatous polyps and consumption of foods containing partially hydrogenated oils. Cancer Epidemiol Biomarkers Prev. 1999;8(6):519–24. [PubMed] [Google Scholar]
- 19.Besaratinia A, Pfeifer GP. Genotoxicity of acrylamide and glycidamide. JNCI Journal of the National Cancer Institute. 2004;96(13):1023–9. [DOI] [PubMed] [Google Scholar]
- 20.WCRF/AICR . World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 21.Furihata C, Ohta H, Katsuyama T. Cause and effect between concentration-dependent tissue damage and temporary cell proliferation in rat stomach mucosa by NaCl, a stomach tumor promoter. Carcinogenesis. 1996;17(3):401–6. [DOI] [PubMed] [Google Scholar]
- 22.Tierno R, López A, Riga P, Arazuri S, Jarén C, Benedicto L, Ruiz de Galarreta JI. Phytochemicals determination and classification in purple and red fleshed potato tubers by analytical methods and near infrared spectroscopy. J Sci Food Agric. 2016;96(6):1888–99. [DOI] [PubMed] [Google Scholar]
- 23.Hu J, Zhang S, Jia E, Wang Q, Liu S, Liu Y, Wu Y, Cheng YJI. Diet and cancer of the stomach: a case-control study in China. Int J Cancer. 1988;41(3):331–5. [DOI] [PubMed] [Google Scholar]
- 24.Åsli LA, Olsen A, Braaten T, Lund E, Skeie GJN. Potato consumption and risk of colorectal cancer in the Norwegian women and cancer cohort. Nutr Cancer. 2017;69(4):564–72. [DOI] [PubMed] [Google Scholar]
- 25.Dosil-Díaz O, Ruano-Ravina A, Gestal-Otero JJ, Barros-Dios J. Consumption of fruit and vegetables and risk of lung cancer: a case-control study in Galicia, Spain. Nutrition. 2008;24(5):407–13. [DOI] [PubMed] [Google Scholar]
- 26.Stott-Miller M, Neuhouser ML, Stanford JP. Consumption of deep-fried foods and risk of prostate cancer. Prostate. 2013;73(9):960–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flood A, Velie EM, Chaterjee N, Subar AF, Thompson FE, Lacey Jr JV, Schairer C, Troisi R, Schatzkin A. Fruit and vegetable intakes and the risk of colorectal cancer in the Breast Cancer Detection Demonstration Project follow-up cohort. Am J Clin Nutr. 2002;75(5):936–43. [DOI] [PubMed] [Google Scholar]
- 28.Wilson KM, Mucci LA, Cho E, Hunter DJ, Chen WY, Willett W. Dietary acrylamide intake and risk of premenopausal breast cancer. Am J Epidemiol. 2009;169(8):954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemarchand C, Tual S, Boulanger M, Levêque-Morlais N, Perrier S, Clin B, Guizard A-V, Velten M, Rigaud E, Baldi IJSet al. Prostate cancer risk among French farmers in the AGRICAN cohort. Scand J Work Environ Health. 2016:144–52..42 [DOI] [PubMed] [Google Scholar]
- 30.Cornée J, Pobel D, Riboli E, Guyader M, Hémon BJE. A case-control study of gastric cancer and nutritional factors in Marseille, France. Eur J Epidemiol. 1995;11(1):55–65. [DOI] [PubMed] [Google Scholar]
- 31.Mucci L, Dickman P, Steineck G, Adami H, Augustsson KoC. Dietary acrylamide and cancer of the large bowel, kidney, and bladder: absence of an association in a population-based study in Sweden. Br J Cancer. 2003;88(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu J, Johnson KC, Mao Y, Xu T, Lin Q, Wang C, Zhao F, Wang G, Chen Y, Yang YJI. A case-control study of diet and lung cancer in Northeast China. Int J Cancer. 1997;71(6):924–31. [DOI] [PubMed] [Google Scholar]
- 33.Radosavljević V, Janković S, Marinković J, Dokić M. Diet and bladder cancer: a case-control study. Int Urol Nephrol. 2005;37(2):283–9. [DOI] [PubMed] [Google Scholar]
- 34.Schwingshackl L, Schwedhelm C, Hoffmann G, Boeing H. Potatoes and risk of chronic disease: a systematic review and dose-response meta-analysis. Eur J Nutr. 2019;58(6):2243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeh C-C, You S-L, Chen C-J, Sung F-C. Peanut consumption and reduced risk of colorectal cancer in women: a prospective study in Taiwan. WJG. 2006;12(2):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agnoli C, Grioni S, Sieri S, Palli D, Masala G, Sacerdote C, Vineis P, Tumino R, Giurdanella MC, Pala VJI. Italian Mediterranean Index and risk of colorectal cancer in the Italian section of the EPIC cohort. Int J Cancer. 2013;132(6):1404–11. [DOI] [PubMed] [Google Scholar]
- 37.Lin J, Zhang SM, Cook NR, Rexrode KM, Liu S, Manson JE, Lee I-M, Buring J. Dietary intakes of fruit, vegetables, and fiber, and risk of colorectal cancer in a prospective cohort of women (United States). Cancer Causes Control. 2005;16(3):225–33. [DOI] [PubMed] [Google Scholar]
- 38.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown C, Culley D, Yang C-P, Durst R, Wrolstad R. Variation of anthocyanin and carotenoid contents and associated antioxidant values in potato breeding lines. JASHS. 2005;130(2):174–80. [Google Scholar]
- 40.Benito E, Obrador A, Stiggelbout A, Bosch F, Mulet M, Munoz N, Kaldor J. A population-based case-control study of colorectal cancer in Majorca. I. Dietary factors. Int J Cancer. 1990;45(1):69–76. [DOI] [PubMed] [Google Scholar]
- 41.Bidoli E, Franceschi S, Talamini R, Barra S, La Vecchia C. Food consumption and cancer of the colon and rectum in north-eastern Italy. Int J Cancer. 1992;50(2):223–9. [DOI] [PubMed] [Google Scholar]
- 42.De Mesquita HB, Maisonneuve P, Runia S, Moerman C. Intake of foods and nutrients and cancer of the exocrine pancreas: a population-based case-control study in the Netherlands. Int J Cancer. 1991;48(4):540–9. [DOI] [PubMed] [Google Scholar]
- 43.La Vecchia C, Negri E, Decarli A, D'Avanzo B, Gallotti L, Gentile A, Fkanceschi S. A case-control study of diet and colo-rectal cancer in northern Italy. Int J Cancer. 1988;41(4):492–8. [DOI] [PubMed] [Google Scholar]
- 44.Levi F, La Vecchia C, Gulie C, Negri E. Dietary Factors and Breast Cancer Risk in Vaud, Switzerland. 1993. [DOI] [PubMed] [Google Scholar]
- 45.Macquart-Moulin G, Riboli E, Cornée J, Charnay B, Berthezene P, Day N. Case-control study on colorectal cancer and diet in Marseilles. Int J Cancer. 1986;38(2):183–91. [DOI] [PubMed] [Google Scholar]
- 46.Manousos O, Day N, Trichopoulos D, Gerovassilis F, Tzonou A, Polychronopoulou A. Diet and colorectal cancer: a case-control study in Greece. Int J Cancer. 1983;32(1):1–5. [DOI] [PubMed] [Google Scholar]
- 47.Wirfalt E, Mattisson I, Gullberg B, Olsson H, Berglund G. Fat from different foods show diverging relations with breast cancer risk in postmenopausal women. Nutr Cancer. 2005;53(2):135–43. [DOI] [PubMed] [Google Scholar]
- 48.Bero L, Chartres N, Diong J, Fabbri A, Ghersi D, Lam J, Lau A, McDonald S, Mintzes B, Sutton Pet al. The risk of bias in observational studies of exposures (ROBINS-E) tool: concerns arising from application to observational studies of exposures. Syst Rev. 2018;7(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. The GRADE Working Group; 2013; [Internet]. Available from: https://guidelinedevelopment org/handbook 2013. [Google Scholar]
- 50.Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, Brignardello-Petersen R, Carrasco-Labra A, De Beer H, Hultcrantz Met al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126–35. [DOI] [PubMed] [Google Scholar]
- 51.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 52.Marín-Martínez F, Sánchez-Meca J. Weighting by inverse variance or by sample size in random-effects meta-analysis. Educational and Psychological Measurement. 2010;70(1):56–73. [Google Scholar]
- 53.Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Syn Meth. 2017;8(1):5–18. [DOI] [PubMed] [Google Scholar]
- 54.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. [DOI] [PubMed] [Google Scholar]
- 55.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. The Stata Journal. 2006;6(1):40. [Google Scholar]
- 56.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. The Stata Journal. 2006;6(1):40–57. [Google Scholar]
- 57.Food, Agriculture Organization of the United N . Potato world: Production and Consumption. International Year of the Potato 2008. [Google Scholar]
- 58.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ (Clinical research ed). 2014;349:g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;; 4(3):218–28..4 [DOI] [PubMed] [Google Scholar]
- 61.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bradburn MJ. Updated and new commands for meta-analysis in STATA. Risk. 2004;4694(1):212.998. [Google Scholar]
- 63.Cro S, Morris TP, Kenward MG, Carpenter JR. Reference-based sensitivity analysis via multiple imputation for longitudinal trials with protocol deviation. The Stata Journal. 2016;16(2):443–63. [PMC free article] [PubMed] [Google Scholar]
- 64.Åsli LA, Braaten T, Olsen A, Tjønneland A, Overvad K, Nilsson LM, Renström F, Lund E, Skeie GoN. Potato consumption and risk of pancreatic cancer in the HELGA cohort. Br J Nutr. 2018;119(12):1408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michels KB, Giovannucci E, Joshipura KJ, Rosner BA, Stampfer MJ, Fuchs CS, Colditz GA, Speizer FE, Willett WC. Prospective study of fruit and vegetable consumption and incidence of colon and rectal cancers. J Natl Cancer Inst. 2000;92(21):1740–52. [DOI] [PubMed] [Google Scholar]
- 66.Mucci LA, Adami HO, Wolk AoC. Prospective study of dietary acrylamide and risk of colorectal cancer among women. Int J Cancer. 2006;118(1):169–73. [DOI] [PubMed] [Google Scholar]
- 67.Sonestedt E, Borgquist S, Ericson U, Gullberg B, Landberg G, Olsson H, Wirfält EJC. Plant foods and oestrogen receptor α- and β-defined breast cancer: observations from the Malmö Diet and Cancer cohort. Carcinogenesis. 2008;29(11):2203–9. [DOI] [PubMed] [Google Scholar]
- 68.Verhoeven DT, Assen N, Goldbohm RA, Dorant E, van't Veer P, Sturmans F, Hermus RJ, van den Brandt PA. Vitamins C and E, retinol, beta-carotene and dietary fibre in relation to breast cancer risk: a prospective cohort study. Br J Cancer. 1997;75(1):149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson KM, Giovannucci E, Stampfer MJ, Mucci L. Dietary acrylamide and risk of prostate cancer. Int J Cancer. 2012;131(2):479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diallo A, Deschasaux M, Galan P, Hercberg S, Zelek L, Latino-Martel P, Touvier MoN. Associations between fruit, vegetable and legume intakes and prostate cancer risk: results from the prospective Supplementation en Vitamines et Mineraux Antioxydants (SU. VI. MAX) cohort. Br J Nutr. 2016;115(9):1579–85. [DOI] [PubMed] [Google Scholar]
- 71.Drake I, Sonestedt E, Gullberg B, Ahlgren G, Bjartell A, Wallström P, Wirfält E. Dietary intakes of carbohydrates in relation to prostate cancer risk: a prospective study in the Malmö Diet and Cancer cohort. Am J Clin Nutr. 2012;96(6):1409–18. [DOI] [PubMed] [Google Scholar]
- 72.Feskanich D, Ziegler RG, Michaud DS, Giovannucci EL, Speizer FE, Willett WC, Colditz G. Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J Natl Cancer Inst. 2000;92(22):1812–23. [DOI] [PubMed] [Google Scholar]
- 73.Kato I, Akhmedkhanov A, Koenig K, Toniolo PG, Shore RE, Riboli E, Prospective Study of Diet and Female Colorectal Cancer: the New York University Women's Health Study. Nutr Cancer. 1997;28(3):276–81. [DOI] [PubMed] [Google Scholar]
- 74.Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, Virtamo J, Demetrius A. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol. 2002;155(9):783–92. [DOI] [PubMed] [Google Scholar]
- 75.van der Pols JC, Hughes MC, Ibiebele TI, Marks GC, Green AC. Food intake and risk of basal cell carcinoma in an 11-year prospective study of Australian adults. Eur J Clin Nutr. 2011;65(1):39–46. [DOI] [PubMed] [Google Scholar]
- 76.Steinmetz KA, Kushi LH, Bostick RM, Folsom AR, Potter J. Vegetables, fruit, and colon cancer in the Iowa women's health study. Am J Epidemiol. 1994;139(1):1–15. [DOI] [PubMed] [Google Scholar]
- 77.Andreatta MM, Navarro A, Munoz SE, Aballay L, Eynard AoCP. Dietary patterns and food groups are linked to the risk of urinary tract tumors in Argentina. Eur J Cancer Prev. 2010;19(6):478–84. [DOI] [PubMed] [Google Scholar]
- 78.Annema N, Heyworth JS, McNaughton SA, Iacopetta B, Fritschi L. Fruit and vegetable consumption and the risk of proximal colon, distal colon, and rectal cancers in a case-control study in Western Australia. J Am Diet Assoc. 2011;111(10):1479–90. [DOI] [PubMed] [Google Scholar]
- 79.Balbi J, Larrinaga M, De SE, Mendilaharsu M, Ronco A, Boffetta P, Brennan PJE. Foods and risk of bladder cancer: a case-control study in Uruguay. Eur J Cancer Prev. 2001;10(5):453–8. [DOI] [PubMed] [Google Scholar]
- 80.Bich NN, Van Hieu N, Van Quyet H, Do Duc LoCP. Cooking temperature, heat-generated-carcinogens, and the risk of stomach and colorectal cancers. Eur J Cancer Prev. 2009;10:83–6. [PubMed] [Google Scholar]
- 81.Boeing H, Frentzel-Beyme R, Berger M, Berndt V, Göres W, Körner M, Lohmeier R, Menarcher A, Männl H, Meinhardt MJI. Case-control study on stomach cancer in Germany. Int J Cancer. 1991;47(6):858–64. [DOI] [PubMed] [Google Scholar]
- 82.Bosetti C, La Vecchia C, Talamini R, Simonato L, Zambon P, Negri E, Trichopoulos D, Lagiou P, Bardini R, Franceschi SoC. Food groups and risk of squamous cell esophageal cancer in northern Italy. Int J Cancer. 2000;87(2):289–94. [PubMed] [Google Scholar]
- 83.Bosetti C, Talamini R, Levi F, Negri E, Franceschi S, Airoldi L, La Vecchia C. Fried foods: a risk factor for laryngeal cancer?. Br J Cancer. 2002;87(11):1230–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bravi F, Bosetti C, Dal Maso L, Talamini R, Montella M, Negri E, Ramazzotti V, Franceschi S, La Vecchia CJU. Food groups and risk of benign prostatic hyperplasia. Urology. 2006;67(1):73–9. [DOI] [PubMed] [Google Scholar]
- 85.Bravi F, Bosetti C, Filomeno M, Levi F, Garavello W, Galimberti S, Negri E, La Vecchia C. Foods, nutrients and the risk of oral and pharyngeal cancer. Br J Cancer. 2013;109(11):2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chan JM, Wang F, Holly E, Biomarkers P. Vegetable and fruit intake and pancreatic cancer in a population-based case-control study in the San Francisco Bay area. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2093–7. [DOI] [PubMed] [Google Scholar]
- 87.De Stefani E, Boffetta P, Deneo-Pellegrini H, Ronco AL, Correa P, Mendilaharsu M. The role of vegetable and fruit consumption in the aetiology of squamous cell carcinoma of the oesophagus: a case-control study in Uruguay. Int J Cancer. 2005;116(1):130–5. [DOI] [PubMed] [Google Scholar]
- 88.Demetriou CA, Hadjisavvas A, Loizidou MA, Loucaides G, Neophytou I, Sieri S, Kakouri E, Middleton N, Vineis P, Kyriacou KJBc. The Mediterranean dietary pattern and breast cancer risk in Greek-Cypriot women: a case-control study. BMC Cancer. 2012;12(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deneo-Pellegrini H, De Stefani E,Ronco A,. Vegetables, Fruits, and Risk of Colorectal Cancer: A case-control study from Uruguay. Nutr Cancer. 1996;25(3):297–304. [DOI] [PubMed] [Google Scholar]
- 90.Grieb SMD, Theis RP, Burr D, Benardot D, Siddiqui T, Asal N. Food groups and renal cell carcinoma: results from a case-control study. J Am Diet Assoc. 2009;109(4):656–67. [DOI] [PubMed] [Google Scholar]
- 91.Heck JE, Sapkota A, Vendhan G, Roychowdhury S, Dikshit RP, Jetly DH, Brennan P, Boffetta P, Hashibe M. Dietary risk factors for hypopharyngeal cancer in India. Cancer Causes Control. 2008;19(10):1329. [DOI] [PubMed] [Google Scholar]
- 92.Hoang BV, Lee J, Choi IJ, Kim Y-W, Ryu KW, Kim JJW. Effect of dietary vitamin C on gastric cancer risk in the Korean population. WJG. 2016;22(27):6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu J, Mao Y, Dryer D, White K, Canadian Cancer Epidemiology Research Group . Risk factors for lung cancer among Canadian women who have never smoked. Cancer Detect Prev. 2002;26(2):129–38. [DOI] [PubMed] [Google Scholar]
- 94.Isa F, Xie L-P, Hu Z, Zhong Z, Hemelt M, Reulen RC, Wong Y, Tam P-C, Yang K, Chai Cet al. Dietary consumption and diet diversity and risk of developing bladder cancer: results from the South and East China case-control study. Cancer Causes Control. 2013;24(5):885–95. [DOI] [PubMed] [Google Scholar]
- 95.Ito LS, Inoue M, Tajima K, Yamamura Y, Kodera Y, Hirose K, Takezaki T, Hamajima N, Kuroishi T, Tominaga SJA. Dietary factors and the risk of gastric cancer among Japanese women: a comparison between the differentiated and non-differentiated subtypes. Ann Epidemiol. 2003;13(1):24–31. [DOI] [PubMed] [Google Scholar]
- 96.Kampman E, Verhoeven D, Sloots L, van't Veer P. Vegetable and animal products as determinants of colon cancer risk in Dutch men and women. Cancer Causes Control. 1995;6(3):225–34. [DOI] [PubMed] [Google Scholar]
- 97.Levi F, Pasche C, La Vecchia C, Lucchini F, Franceschi SJB. Food groups and colorectal cancer risk. Br J Cancer. 1999;79(7–8):1283–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin J, Kamat A, Gu J, Chen M, Dinney CP, Forman MR, Wu X, Biomarkers P. Dietary intake of vegetables and fruits and the modification effects of GSTM1 and NAT2 genotypes on bladder cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(7):2090–7. [DOI] [PubMed] [Google Scholar]
- 99.Lucenteforte E, Scita V, Bosetti C, Bertuccio P, Negri E, La Vecchia C. Food groups and alcoholic beverages and the risk of stomach cancer: a case-control study in Italy. Nutr Cancer. 2008;60(5):577–84. [DOI] [PubMed] [Google Scholar]
- 100.Malagoli C, Malavolti M, Farnetani F, Longo C, Filippini T, Pellacani G, Vinceti M. Food and beverage consumption and melanoma risk: a population-based case-control study in Northern Italy. Nutrients. 2019;11(9):2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mourouti N, Kontogianni MD, Papavagelis C, Plytzanopoulou P, Vassilakou T, Malamos N, Linos A, Panagiotakos D. Adherence to the Mediterranean diet is associated with lower likelihood of breast cancer: a case-control study. Nutr Cancer. 2014;66(5):810–7. [DOI] [PubMed] [Google Scholar]
- 102.Ohba S, Nishi M, Miyake HJI. Eating habits and pancreas cancer. Int J Pancreatol. 1996;20(1):37–42. [DOI] [PubMed] [Google Scholar]
- 103.Pandey M, Shukla VoCP. Diet and gallbladder cancer: a case-control study. Eur J Cancer Prev. 2002;11(4):365–8. [DOI] [PubMed] [Google Scholar]
- 104.Pelucchi C, Franceschi S, Levi F, Trichopoulos D, Bosetti C, Negri E, La Vecchia CoC. Fried potatoes and human cancer. Int J Cancer. 2003;105(4):558–60. [DOI] [PubMed] [Google Scholar]
- 105.Ping Y, Ogushi Y, Okada Y, Haruki Y, Okazaki I, Ogawa T. Lifestyle and colorectal cancer: a case-control study. Environ Health Prev Med. 1998;3(3):146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Plagens-Rotman K, Chmaj-Wierzchowska K, Pięta B, Bojar I. Modifiable lifestyle factors and ovarian cancer incidence in women. Ann Agric Environ Med. 2018;25(1):36–40. [DOI] [PubMed] [Google Scholar]
- 107.Polesel J, Serraino D, Negri E, Barzan L, Vaccher E, Montella M, Zucchetto A, Garavello W, Franceschi S, La Vecchia Cet al. Consumption of fruit, vegetables, and other food groups and the risk of nasopharyngeal carcinoma. Cancer Causes Control. 2013;24(6):1157–65. [DOI] [PubMed] [Google Scholar]
- 108.Polesel J, Talamini R, Negri E, Bosetti C, Boz G, Lucenteforte E, Franceschi S, Serraino D, La Vecchia C. Dietary habits and risk of pancreatic cancer: an Italian case-control study. Cancer Causes Control. 2010;21(4):493–500. [DOI] [PubMed] [Google Scholar]
- 109.Rai A, Mohapatra SC, Shukla HS. Correlates between vegetable consumption and gallbladder cancer. Eur J Cancer Prev. 2006;15(2):134–7. [DOI] [PubMed] [Google Scholar]
- 110.Russnes KM, Möller E, Wilson KM, Carlsen M, Blomhoff R, Smeland S, Adami H-O, Grönberg H, Mucci LA, Bälter K. Total antioxidant intake and prostate cancer in the Cancer of the Prostate in Sweden (CAPS) study. A case control study. BMC Cancer. 2016;16:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steineck G, Hagman U, Gerhardsson M, Norell S. Vitamin A supplements, fried foods, fat and urothelial cancer. A case-referent study in Stockholm in 1985–87. Int J Cancer. 1990;45(6):1006–11. [DOI] [PubMed] [Google Scholar]
- 112.Steinmetz KA, Potter JoC. Food-group consumption and colon cancer in the Adelaide case-control study. I. Vegetables and fruit. Int J Cancer. 1993;53(5):711–9. [DOI] [PubMed] [Google Scholar]
- 113.Tajaddini A, Pourzand A, Sanaat Z, Pirouzpanah S. Dietary resistant starch contained foods and breast cancer risk: a case-control study in northwest of Iran. Asian Pac J Cancer Prev. 2015;16(10):4185–92. [DOI] [PubMed] [Google Scholar]
- 114.Tayyem RF, Shehadah I, Abu-Mweis SS, Bawadi HA, Bani-Hani KE, Al-Jaberi T, Al-Nusairr M, Heath D. Fruit and vegetable intake among Jordanians: results from a case-control study of colorectal cancer. Cancer Control. 2014;21(4):350–60. [DOI] [PubMed] [Google Scholar]
- 115.Turkoz FP, Celenkoglu G, Dogu GG, Kalender ME, Coskun U, Alkis N, Ozkan M, Turk HM, Arslan U. Risk factors of nasopharyngeal carcinoma in Turkey – an epidemiological survey of the Anatolian Society of Medical Oncology. Asian Pac J Cancer Prev. 2011;12(11):3017–21. [PubMed] [Google Scholar]
- 116.Shah S, Ghazi H. Tobacco chewing and risk of gastric cancer: a case-control study in Yemen. EMHJ-Eastern Mediterranean Health Journal. 2016;22(10):719–26. [DOI] [PubMed] [Google Scholar]
- 117.Koushik A, Hunter DJ, Spiegelman D, Anderson KE, Arslan AA, Beeson WL, Van Den Brandt PA, Buring JE, Cerhan JR, Colditz Get al. Fruits and vegetables and ovarian cancer risk in a pooled analysis of 12 cohort studies. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2160–7. [DOI] [PubMed] [Google Scholar]
- 118.Koushik A, Hunter DJ, Spiegelman D, Beeson WL, van den Brandt PA, Buring JE, Calle EE, Cho E, Fraser GE, Freudenheim Jet al. Fruits, vegetables, and colon cancer risk in a pooled analysis of 14 cohort studies. J Natl Cancer Inst. 2007;99(19):1471–83. [DOI] [PubMed] [Google Scholar]
- 119.Koushik A, Spiegelman D, Albanes D, Anderson KE, Bernstein L, Van Den Brandt PA, Bergkvist L, English DR, Freudenheim JL, Fuchs C. Intake of fruits and vegetables and risk of pancreatic cancer in a pooled analysis of 14 cohort studies. Am J Epidemiol. 2012;176(5):373–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee JE, Männistö S, Spiegelman D, Hunter DJ, Bernstein L, van den Brandt PA, Buring JE, Cho E, English DR, Flood Aet al. Intakes of fruit, vegetables, and carotenoids and renal cell cancer risk: a pooled analysis of 13 prospective studies. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jung S, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, van den Brandt PA, Buring JE, Cerhan JR, Gaudet MM, Giles G. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst. 2013;105(3):219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Di Maso M, Turati F, Bosetti C, Montella M, Libra M, Negri E, Ferraroni M, La Vecchia C, Serraino D, Polesel J. Food consumption, meat cooking methods and diet diversity and the risk of bladder cancer. Cancer Epidemiol. 2019;63:101595. [DOI] [PubMed] [Google Scholar]
- 123.Willett W. Nutritional Epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- 124.Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality – a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46(3):1029–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wirfält E, McTaggart A, Pala V, Gullberg B, Frasca G, Panico S, Bueno-de-Mesquita H, Peeters P, Engeset D, Skeie G. Food sources of carbohydrates in a European cohort of adults. Public Health Nutr. 2002;5(6b):1197–215. [DOI] [PubMed] [Google Scholar]
- 126.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1531–43. [PubMed] [Google Scholar]
- 127.Miller PE, Lesko SM, Muscat JE, Lazarus P, Hartman TJ. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2010;62(4):413–24. [DOI] [PubMed] [Google Scholar]
- 128.Randi G, Edefonti V, Ferraroni M, La Vecchia C, Decarli A. Dietary patterns and the risk of colorectal cancer and adenomas. Nutr Rev. 2010;68(7):389–408. [DOI] [PubMed] [Google Scholar]
- 129.Williams CD, Satia JA, Adair LS, Stevens J, Galanko J, Keku TO, Sandler RS. Dietary patterns, food groups, and rectal cancer risk in Whites and African-Americans. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li F-y. Colorectal cancer, one entity or three. J Zhejiang Univ Sci B. 2009;10(3):219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aune D, Chan DS, Vieira AR, Navarro Rosenblatt DA, Vieira R, Greenwood DC, Cade JE, Burley VJ, Norat T. Dietary fructose, carbohydrates, glycemic indices and pancreatic cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol. 2012;23(10):2536–46. [DOI] [PubMed] [Google Scholar]
- 132.Pelucchi C, Bosetti C, Galeone C, La Vecchia C. Dietary acrylamide and cancer risk: an updated meta-analysis. Int J Cancer. 2015;136(12):2912–22. [DOI] [PubMed] [Google Scholar]
- 133.Gupta U, Gupta S. The important role of potatoes, an underrated vegetable food crop in human health and nutrition. Current Nutrition & Food Science. 2019;15(1):11–9. [Google Scholar]
- 134.Augustin J, Johnson S, Teitzel C, True R, Hogan J, Toma R, Shaw R, Deutsch R. Changes in the nutrient composition of potatoes during home preparation: II. Vitamins. American Potato Journal. 1978;55(12):653–62. [Google Scholar]
- 135.García-Alonso A, Goni I. Effect of processing on potato starch: in vitro availability and glycaemic index. Nahrung. 2000;44(1):19–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.