Abstract
Numerous observational studies have investigated the role of the Dietary Inflammatory Index (DII®) in chronic disease risk. The aims of this umbrella review and integrated meta-analyses were to systematically synthesize the observational evidence reporting on the associations between the DII and health outcomes based on meta-analyses, and to assess the quality and strength of the evidence for each associated outcome. This umbrella review with integrated meta-analyses investigated the association between the DII and a range of health outcomes based on meta-analyses of observational data. A credibility assessment was conducted for each outcome using the following criteria: statistical heterogeneity, 95% prediction intervals, evidence for small-study effect and/or excess significance bias, as well as effect sizes and P values using calculated random effects meta-analyses. In total, 15 meta-analyses reporting on 38 chronic disease-related outcomes were included, incorporating a total population of 4,360,111 subjects. Outcomes (n = 38) were examined through various study designs including case-control (n = 8), cross-sectional (n = 5), prospective (n = 5), and combination (n = 20) study designs. Adherence to a pro-inflammatory dietary pattern had a significant positive association with 27 (71%) of the included health outcomes (P value < 0.05). Using the credibility assessment, Class I (Convincing) evidence was identified for myocardial infarction only, Class II (Highly suggestive) evidence was identified for increased risk of all-cause mortality, overall risk of incident cancer, and risk of incident site-specific cancers (colorectal, pancreatic, respiratory, and oral cancers) with increasing (more pro-inflammatory) DII score. Most outcomes (n = 31) presented Class III (Suggestive) or lower evidence (Weak or No association). Pro-inflammatory dietary patterns were nominally associated with an increased risk of many chronic disease outcomes. However, the strength of evidence for most outcomes was limited. Further prospective studies are required to improve the precision of the effect size.
Keywords: diet, inflammation, dietary inflammatory index, prevention, mental disorders, cancer, cardiovascular disease, non-communicable disorders, medicine
Introduction
Chronic low-grade inflammation is implicated in the pathogenesis of several chronic non-communicable diseases (1, 2). In particular, chronic systemic inflammation is associated with increased mortality from all causes, as well as with an increased risk of chronic disease including cancer, type 2 diabetes, neurodegenerative diseases, and cardiovascular disease (CVD) (3–8). Observational studies suggest that a range of pro-inflammatory markers including interleukin-6 (IL-6), IL-18, matrix metalloproteinase-9, soluble CD40 ligand, and tumor necrosis factor-α (TNF-α) are prospectively associated with coronary heart disease risk (9). In addition to physical chronic diseases, inflammation is implicated in a range of mental illnesses including depression, schizophrenia, and bipolar disorder (10–12). Elevated baseline C-reactive protein (CRP) levels predict de novo depression (13). Due to the substantial burden of chronic diseases on mortality and morbidity (14), studies that seek to understand and address the drivers of inflammation are of substantial scientific value and public health interest.
Diet is a key modifiable target for chronic disease risk reduction given that dietary factors remain the primary driver of the global burden of chronic disease (15, 16). Diet can affect chronic disease risk via multiple mechanisms of action, including modulation of the gut microbiome, oxidative stress, and energy balance (17, 18). Fundamental to these mechanisms of action is the potential pro- or anti-inflammatory properties of dietary patterns and individual dietary components. Increased adherence to healthy dietary patterns, as well as a higher consumption of nutrient-dense food groups, are associated with reduced inflammatory markers (19). For example, the Mediterranean dietary pattern—rich in fruits, vegetables, fatty fish, poultry, extra virgin olive oil, and whole grains—is associated with reductions in systemic inflammatory markers such as CRP (20). Intervention studies support causality: a meta-analysis of randomized controlled trials investigating the effect of a Mediterranean dietary pattern reported significant reductions in CRP and IL-6 as well as increased adiponectin (21). Furthermore, individual compounds within nutrient-dense foods including omega-3 fatty acids (22), fiber (23), and polyphenols (24) have demonstrated anti-inflammatory properties. In contrast, consumption of Western dietary patterns, characterized by low consumption of fruits and vegetables and high consumption of calorie-dense ultra-processed foods, are associated with increased levels of inflammatory markers (19).
The Dietary Inflammatory Index (DII®) provides a novel tool to further explore the mechanistic inflammatory contribution of various dietary components (25). Informed by an a priori literature-based method, the DII is based on 45 food parameters including individual nutrients (e.g., omega-3 fatty acids), compounds (e.g., flavonoids), and food items (e.g., garlic, ginger) that were identified within the literature as possessing either anti- or pro-inflammatory properties. The DII has now been validated in 29 studies with a range of inflammatory markers including CRP, IL-6, and TNF-α (26). A strategic advantage of the DII is that, in contrast to individual dietary compounds, the investigation of dietary patterns acknowledges the food matrix or the complex interactions of nutrients and compounds within foods and dietary patterns.
Since the development of the current DII in 2014 (25), over 450 studies have investigated the association between the DII and a diverse range of chronic disease-related outcomes, including all-cause mortality, depression, and intermediate risk factors for chronic disease such as elevated blood pressure or hypertension (26, 27). Due to the large number and diverse range of studies that have investigated the DII, there are now several meta-analyses that have synthesized these outcomes (28–36). However, no umbrella review has been conducted to assess the strength of association between the DII and these diverse chronic disease outcomes. The aim of this umbrella review was to aggregate and synthesize the results from meta-analyses of observational studies examining the association between the DII and any available health condition.
Methods
The study was reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (37) guidelines and was prospectively registered in an international registry of systematic reviews (PROSPERO registration no. CRD42020192991).
Literature search and selection criteria
All meta-analyses that examined the association between the DII and all available health outcomes using observational study designs (e.g., cross-sectional, prospective, case-control) were eligible for inclusion. There were no restrictions on the population or age group, with both healthy and clinical populations included. Eligible outcomes included those that were related to physical chronic diseases (e.g., CVD, cancer), mental illnesses (e.g., depression), and intermediate risk factors (e.g., hypertension).
Two independent authors (WM and JD) searched MEDLINE (via PubMed), PsycINFO (via Ovid), EMBASE (via Ovid), and the Cochrane databases (via Ovid), from journal inception dates to June 2020. Key search terms were related to the DII (DII OR “dietary inflammatory index” OR “inflammatory diet” OR “anti-inflammatory diet”) and the meta-analysis study design (“meta-analy*” OR metaanaly* OR “meta reg*” OR “metareg*”). Retrieved articles were independently screened in duplicate (WM and JK) to identify studies that potentially met the inclusion criteria. Any disagreement between authors over the eligibility of particular studies was resolved through discussion with a third reviewer (ML). In line with methods used in prior umbrella reviews (38–40), if two or more meta-analyses were available for the same disease outcome, the most recently updated and/or largest meta-analysis was included.
Data extraction
Duplicate extraction was conducted for data from the included studies for assessment of study quality and evidence synthesis. Data relating to study design, sample size, outcomes, and effect sizes were extracted. Where required, the study author of the original paper was contacted for further information on relevant data that were not reported.
Data analysis
We reanalyzed each meta-analysis dataset using a random effects model and reported effect sizes (relative risk, odds ratio, and weighted mean differences), with 95% confidence intervals (CI). In line with the methods of prior umbrella reviews (41), assuming the associations between the DII and health outcomes were linear, the lowest and highest categories—where the highest category indicates a more pro-inflammatory diet—were prioritized in the overall analyses. Additionally, the 95% prediction intervals were calculated for all random effect sizes, which provide the possible range in which the effect sizes of additional future studies is expected to fall (42). Statistical heterogeneity between studies was evaluated using the I2 statistic with a value ≥50% indicative of high heterogeneity and values >75% suggestive of very high heterogeneity. Evidence of a small-study effect was defined as a P value <0.10 using Egger's regression asymmetry test (43) and where the effect size of the largest individual study for each meta-analysis was more conservative than that of the overall summary effect for each outcome (44).
We conducted a test for excess significance for all outcomes (45), which evaluates whether the number of studies with nominally significant results (i.e., P value <0.05) within an included meta-analysis exceeds what would be expected based on the statistical power of the meta-analysis. As described elsewhere, the number of expected significant studies can be compared with the observed number of significant studies through a chi-square-based test (45). The larger the difference between observed and expected, the higher the degree of excess of significance bias.
Quality assessment of the meta-analyzed studies and evidence grading
The quality of all eligible meta-analyses was assessed using the AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews) quality assessment tool (46). In line with prior umbrella reviews (41, 47), and as summarized elsewhere (48, 49), the results of this umbrella review were classified as Convincing, Highly suggestive, Suggestive, Weak, or No evidence, as defined using the following criteria.
Convincing (Class I); where the number of cases is >1000, statistically significant using a P value of <1 × 10−6, I2 < 50%, 95% prediction interval excludes the null, the largest included individual study has a statistically significant effect (P ≤ 0.05), no small-study effects, and no excess significance bias
Highly suggestive (Class II); where the number of cases is >1000, statistically significant using a P value of <1 × 10−6, the largest included individual study has a statistically significant effect (P ≤ 0.05), and Class I criteria not met
Suggestive (Class III); where the number of cases is >1000, P value of <1 × 10−3, and Class I—II criteria not met
Weak (Class IV); statistically significant using a P value of ≤0.05 and Class I—III criteria not met
No evidence (Class V); no statistical significance using a P value of >0.05
Results
As shown in Figure 1, the systematic search identified 70 deduplicated articles. After applying the inclusion criteria, 15 meta-analyses of 38 distinct outcomes were included for review (28–36, 50–55).
FIGURE 1.
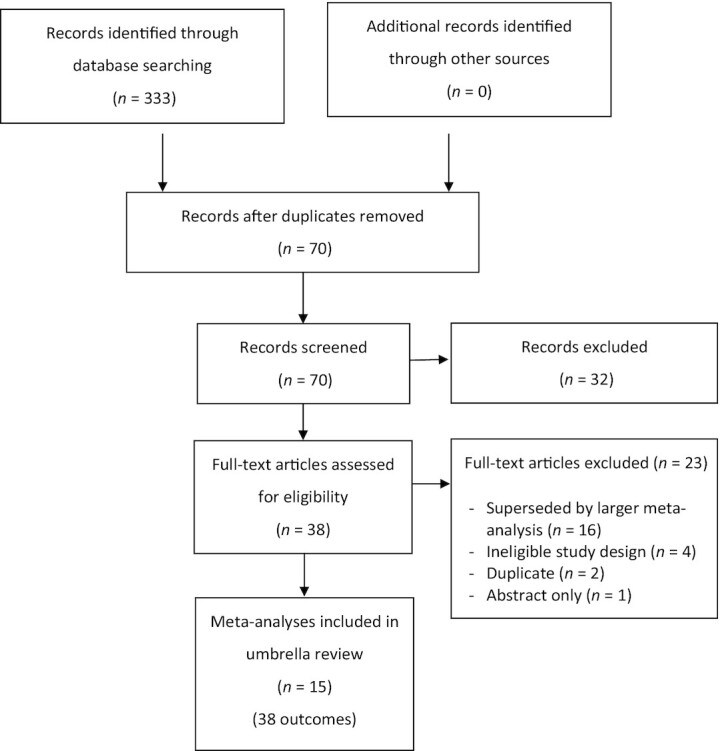
PRISMA flow chart of study selection.
Study characteristics
All meta-analyses were published within the last 5 years. The median number of studies included for each outcome was 6 (range: 2–44), the median number of participants was 36,592 (range: 1966–1,299,621), and the median number of cases (i.e., with the outcome of interest) was 2760 (range: 442–48,345). Outcomes predominantly included a combination of study designs (n = 20), with the remaining meta-analyses including only case-control (n = 8), cross-sectional (n = 5), and prospective (n = 5) study designs exclusively.
As displayed in Table 1, a range of outcomes were included for review: cancer (n = 16), metabolic risk markers (n = 11), CVDs (n = 6), all-cause and specific-cause mortality (n = 4), and depression (n = 1). The exposure variable for all analyzed outcomes was assessed by comparing the highest versus lowest categories (e.g., quartiles, tertiles) of adherence to a pro-inflammatory diet. Most outcomes (n = 30) were categorical variables, with the remaining 8 outcomes treated as continuous (HbA1c, fasting blood glucose, insulin, HOMA-IR, waist circumference, waist-to-hip ratio, systolic and diastolic blood pressure) (50).
TABLE 1.
Summary of included health outcomes and their associations with the Dietary Inflammatory Index within the general population
| Outcome | Study design included in MA | Level of comparison | Studies, n | Participants, n | Cases, n | Type of effect size metric | Effect size (95% CI) | 95% CI prediction intervals | P | I 2 | Largest study effect size (95% CI) | Publication bias | Small-study effect or excess significance bias | Evidence class |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | ||||||||||||||
| All-cause mortality (53) | Prospective | High versus low | 12 | 220,206 | 44,809 | RR | 1.235 (1.157, 1.318) | 1.01, 1.51 | 2.27 × 10−10 | 71.5% | 1.16 (1.1, 1.22) | Yes | Small-study effect | II |
| Cancer mortality (36) | Prospective | High versus low | 11 | 229,448 | 9497 | OR | 1.229 (1.067, 1.415) | 8.30 × 10−1, 1.82 | 4.27 × 10−3 | 54.1% | 1.33 (1.01, 1.76) | No | Neither | IV |
| CVD mortality (34) | Prospective | High versus low | 6 | 93,866 | 11,094 | OR | 1.374 (1.114, 1.696) | 7.00 × 10−1, 2.70 | 3.01 × 10−3 | 77.2% | 1.09 (1.01, 1.18) | Yes | Small-study effect | IV |
| CHD mortality (34) | Prospective | High versus low | 3 | 31,278 | 3,686 | RR | 1.634 (1.012, 2.636) | 1.00 × 10−2, 4.34 × 102 | 4.45 × 10−2 | 76.7% | 1.17 (1.05, 1.3) | Yes | Small-study effect | IV |
| Cancer risk | ||||||||||||||
| Overall cancer (30) | Case-control and Prospective | High versus low | 44 | 1,299,621 | 48,345 | RR | 1.599 (1.466, 1.745) | 1.01, 2.52 | 5.08 × 10−26 | 75.3% | 1.4 (1.28, 1.53) | Yes | Small-study effect | II |
| Colorectal cancer (30) | Case-control and Prospective | High versus low | 11 | 975,683 | 20,076 | RR | 1.426 (1.280, 1.589) | 1.03, 1.98 | 1.26 × 10−10 | 69.1% | 1.4 (1.28, 1.53) | No | Small-study effect | II |
| Prostate cancer (55) | Case-control and Prospective | High versus low | 10 | 52,943 | 5,326 | OR | 1.727 (1.341, 2.226) | 8.00 × 10−1, 3.74 | 2.35 × 10−5 | 78.2% | 1.07 (0.95, 1.2) | Yes | Small-study effect | III |
| Pancreatic cancer (29) | Case-control | High versus low | 2 | 3,551 | 1,143 | RR | 2.524 (1.941, 3.281) | Not estimable* | 4.73 × 10−12 | 0.0% | 2.48 (1.5, 4.1) | Not estimable* | No excess significance* | II |
| Respiratory cancer (pooled) (28) | Case-control | High versus low | 18 | 17,514 | 4,834 | OR | 2.274 (1.894, 2.729) | 1.24, 4.18 | 1.13 × 10−18 | 60.2% | 2.08 (1.47, 2.93) | Yes | Small-study effect | II |
| Esophageal cancer (28) | Case-control | High versus low | 5 | 4,645 | 1,310 | OR | 2.530 (1.738, 3.682) | 7.50 × 10−1, 8.85 | 1.25 × 10−6 | 71.7% | 1.71 (1.54, 1.9) | Yes | Small-study effect | III |
| Laryngeal cancer (28) | Case-control | High versus low | 3 | 2,805 | 997 | OR | 2.046 (0.848, 4.934) | 0.00, 9.08 × 104 | 1.11 × 10−1 | 85.6% | 3.3 (2.06, 5.28) | Yes | Neither | V |
| Oral cancer (28) | Case-control | High versus low | 3 | 4,785 | 1,366 | OR | 2.229 (1.735, 2.865) | 4.00 × 10−1, 1.13 × 101 | 3.72 × 10−10 | 0.0% | 2.08 (1.47, 2.93) | No | Neither | II |
| Pharyngeal cancer (28) | Case-control | High versus low | 7 | 5,279 | 1,161 | OR | 2.019 (1.544, 2.640) | 1.17, 3.48 | 2.81 × 10−7 | 20.3% | 1.64 (0.93, 2.89) | No | Neither | III |
| Lung cancer (52) | Prospective | High versus low | 3 | 149,929 | 2,453 | RR | 1.304 (1.130, 1.504) | 5.20 × 10−1, 3.29 | 2.71 × 10−4 | 0.0% | 1.28 (1.09, 1.51) | No | Neither | III |
| Breast cancer (32) | Case-control and Prospective | High versus low | 12 | 347,147 | 30,052 | RR | 1.335 (1.142, 1.560) | 7.60 × 10−01, 2.33 | 2.79 × 10−4 | 89.9% | 0.99 (0.91, 1.07) | Yes | Small-study effect | III |
| Ovarian cancer (32) | Case-control | High versus low | 4 | 7,982 | 3,104 | RR | 1.414 (1.214, 1.647) | 1.01, 1.98 | 8.57 × 10−6 | 0.0% | 1.47 (1.07, 2.01) | No | Neither | III |
| Gastric cancer (31) | Case-control and Prospective | High versus low | 3 | 2,118 | 700 | RR | 2.120 (1.411, 3.183) | 4.00 × 10−02, 1.17 × 102 | 2.93 × 10−4 | 42.7% | 1.63 (1.15, 2.29) | Yes | Small-study effect | IV |
| Endometrial cancer (32) | Case-control | High versus low | 2 | 1,966 | 751 | RR | 1.881 (0.803, 4.407) | Not estimable* | 1.46 × 10−1 | 68.6% | 1.34 (0.96, 1.87) | Not estimable* | No excess significance* | V |
| Kidney cancer (33) | Case-control and Prospective | High versus low | 2 | 36,118 | 1,030 | RR | 1.463 (1.157, 1.850) | Not estimable* | 1.49 × 10−3 | 0.0% | 1.52 (1.09, 2.13) | Not estimable* | No excess significance* | IV |
| Urothelial cancer (36) | Case-control and Prospective | High versus low | 2 | 42,869 | 1,069 | OR | 1.526 (0.972, 2.397) | Not estimable* | 6.63 × 10−2 | 65.2% | 1.24 (0.9, 1.7) | Not estimable* | No excess significance* | V |
| Cardiovascular disease risk | ||||||||||||||
| Hypertension (50) | Cross-sectional and Prospective | High versus low | 15 | 71,729 | 24,648 | OR | 1.133 (1.013, 1.266) | 8.00 × 10−1, 1.60 | 2.81 × 10−2 | 55.6% | 1.21 (1.02, 1.43) | No | Neither | IV |
| Cardiovascular (34) | Cross-sectional and Prospective | High versus low | 6 | 57,781 | 3,022 | OR | 1.345 (1.110, 1.631) | 8.40 × 10−1, 2.17 | 2.52 × 10−3 | 36.3% | 2.03 (1.06, 3.89) | No | Neither | IV |
| Myocardial infarction (34) | Case-control and Prospective | High versus low | 6 | 37,065 | 2,497 | RR | 1.717 (1.419, 2.077) | 1.31, 2.25 | 2.64 × 10−8 | 0.0% | 2.28 (1.09, 4.75) | Yes | Neither | I |
| IHD-CHD risk (34) | Cross-sectional and Prospective | High versus low | 3 | 23,962 | 875 | RR | 1.272 (0.874, 1.853) | 2.00 × 10−2, 7.83 × 101 | 2.09 × 10−1 | 62.2% | 0.96 (0.72, 1.28) | Yes | Small-study effect | V |
| Stroke (34) | Cross-sectional and Prospective | High versus low | 3 | 30,408 | 569 | RR | 1.099 (0.605, 1.999) | 0.00, 8.61 × 102 | 7.56 × 10−1 | 65.5% | 1.56 (1.21, 2.01) | No | Excess significance | V |
| Angina (34) | Cross-sectional and Prospective | High versus low | 2 | 23,436 | 442 | RR | 0.793 (0.561, 1.120) | Not estimable* | 1.88 × 10−1 | 0.0% | 0.83 (0.54, 1.28) | Not estimable* | No excess significance* | V |
| Mental health risk | ||||||||||||||
| Depression (35) | Cross-sectional and Prospective | High versus low | 15 | 55,490 | 4,884 | OR | 1.441 (1.225, 1.695) | 0.87 × 10−1, 2.40 | 1.02 × 10−6 | 58.8% | 1.46 (1.1, 1.94) | No | Neither | III |
| Metabolic risk markers | ||||||||||||||
| Metabolic syndrome (54) | Case-control and Prospective | High versus low | 5 | 15,161 | 2,242 | RR | 1.006 (0.816, 1.242) | 5.80 × 10−1, 1.74 | 9.53 × 10−1 | 32.6% | 0.86 (0.6, 1.23) | No | Neither | V |
| HbA1c (50) | Cross-sectional | Continuous | 3 | 23,138 | — | WMD | 0.615 (0.266, 0.965) | −3.66, 4.89 | 5.60 × 10−4 | 87.5% | 0.4 (0.34, 0.46) | No | No small-study effect* | III |
| Fasting blood glucose (50) | Case-control and Prospective | Continuous | 15 | 93,739 | — | WMD | 1.083 (0.100, 2.065) | −2.38, 4.54 | 3.08 × 10−2 | 89.0% | 3.7 (0.04, 5.36) | No | No small-study effect* | IV |
| Insulin (50) | Cross-sectional | Continuous | 6 | 38,359 | — | WMD | 0.829 (0.169, 1.488) | −1.27, 2.93 | 1.38 × 10−2 | 86.5% | 2.47 (1.64, 3.3) | No | No small-study effect* | IV |
| HOMA-IR (50) | Cross-sectional | Continuous | 7 | 41,645 | — | WMD | 0.191 (0.021, 0.362) | −3.90 × 10−01, 7.70 × 10−01 | 2.80 × 10−2 | 93.2% | 0.88 (0.67, 1.09) | No | No small- study effect* | IV |
| Hyperglycemia (50) | Cross-sectional | High versus low | 11 | 30,424 | 4,883 | OR | 1.130 (0.948, 1.347) | 6.70 × 10−01, 1.91 | 1.73 × 10−1 | 60.7% | 1.09 (0.83, 1.44) | Yes | Small-study effect | V |
| Central obesity (51) | Cross-sectional | High versus low | 13 | 25,435 | 5,121 | OR | 1.162 (0.945, 1.429) | 6.00 × 10−01, 2.24 | 1.54 × 10−1 | 65.4% | 1.35 (0.94, 1.94) | No | Small-study effect | V |
| Waist circumference (51) | Case-control and Prospective | Continuous | 25 | 78,828 | — | WMD | 1.782 (0.722, 2.842) | −3.00, 6.56 | 9.82 × 10−4 | 100.0% | 3.7 (2.81, 4.59) | No | Neither | III |
| Waist-to-hip ratio (51) | Case-control and Prospective | Continuous | 11 | 16,685 | — | WMD | −0.005 (−0.039, 0.029) | −1.10 × 10−01, 1.00 × 10−01 | 7.59 × 10−1 | 87.1% | 0.0 (−0.01, 0.01) | No | No small- study effect* | V |
| Systolic blood pressure (50) | Case-control, Cohort, and Prospective | Continuous | 15 | 87,202 | — | WMD | 1.230 (0.283, 2.177) | −2.29, 4.76 | 1.09 × 10−2 | 91.5% | 5.4 (4.52, 6.28) | No | No small-study effect* | IV |
| Diastolic blood pressure (50) | Case-control and Prospective | Continuous | 12 | 79,871 | — | WMD | 0.009 (−0.686, 0.703) | −2.40, 2.42 | 9.81 × 10−1 | 91.6% | 1.7 (0.99, 2.41) | No | No small-study effect* | V |
Either tests for small-study effect, excess significance, or both, could not be conducted due to small sample size of included studies.
Evidence class criteria—class I (convincing): statistical significance at P < 10−6, >1000 cases (or >20,000 participants for continuous outcomes), the largest component study reported a significant effect (P < 0.05); the 95% prediction interval excluded the null, no large heterogeneity (I2 < 50%), no evidence of small-study effects (P > 0.10) and excess significance bias (P > 0.10); class II (highly suggestive): significance at P < 10−6, >1000 cases (or >20,000 participants for continuous outcomes), the largest component study reported a significant effect (P ≤ 0.05); class III (suggestive): statistical significance at P < 10−3, >1000 cases (or >20,000 participants for continuous outcomes); and class IV (weak): the remaining significant associations at P < 0.05.
Study results
Overall, 27 (71%) of the 38 outcomes reported statistically significant effect sizes using a random effects model (P value <0.05), with the following 7 outcomes surviving a more stringent P value (P < 1 × 10−6): incidence of myocardial infarction (34), oral cancer (28), respiratory cancer (28), pancreatic cancer (29), colorectal cancer (30), overall cancer (30), and all-cause mortality (53). In 27 (71%) meta-analyses, the largest included study was significant (Table 1). There was evidence of a small-study effect across 12 (31%) included outcomes (Supplemental Table 1). Heterogeneity was generally high with most outcomes (27 of 38; 71%) displaying an I2 value ≥50%. Seven outcomes (incidence of myocardial infarction (34), ovarian cancer (32), pharyngeal cancer (28), respiratory cancer (28), colorectal cancer (30), overall cancer (30), and all-cause mortality (53)) presented 95% prediction intervals excluding the null value. Evidence of excess significance was present for 1 outcome (stroke) from the 29 outcomes that were able to be assessed.
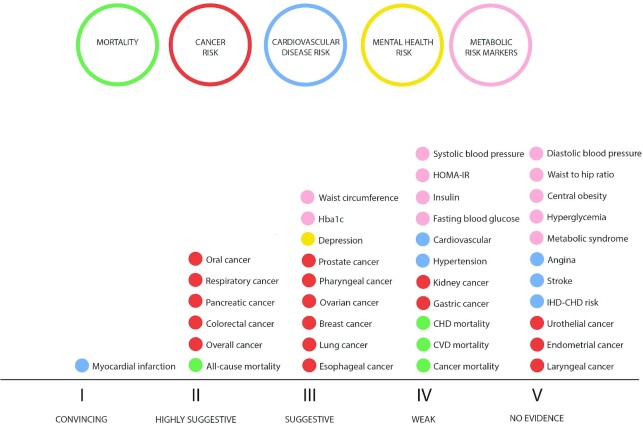
Credibility assessment
When the credibility assessment criteria was applied (Figure 2), 1 outcome presented convincing evidence (Class I): myocardial infarction (34). Six (16%) outcomes presented highly suggestive evidence [Class II: association between higher DII values and increased risk/presence of all-cause mortality (53), overall cancer (30), colorectal cancer (30), pancreatic cancer (29), respiratory cancers (28), oral cancer (28)], and 9 (24%) outcomes presented suggestive evidence [Class III: esophageal cancer (28), lung cancer (52), breast cancer (32), ovarian cancer (32), pharyngeal cancer (28), prostate cancer (55), depression (35), HbA1c (50), waist circumference (51)]. Eleven studies presented weak evidence (Class IV) and a further 11 presented no significant evidence for an association (P value >0.05; Table 1, Supplemental Table 1).
FIGURE 2.
Credibility assessment for each included outcome.
Quality assessment
The overall quality of included studies was moderate (median score: 16 of 32 using the AMSTAR tool), with limited reporting on a number of quality assessment items including details regarding excluded studies and sources of funding of the included studies (Supplemental Table 2).
Discussion
This is the first umbrella review to provide a comprehensive overview of the observational data assessing associations between the DII and all available health outcomes. This umbrella review comprised 15 meta-analyses of 38 outcomes in a total population of more than 4,360,111 participants. A pro-inflammatory dietary pattern was significantly associated with an increased risk for 27 (71%) of the included health outcomes. Convincing (Class I) evidence was presented for myocardial infarction only and highly suggestive (Class II) evidence was presented for all-cause mortality, overall cancer risk, and a range of site-specific cancers (colorectal cancer, pancreatic cancer, respiratory cancers, oral cancer).
A strength of the DII is its focus on dietary assessment that captures the composite effect of multiple dietary components, rather than a single nutrient or individual food item, where it is reductionistic and difficult to discern the effect from other co-occurring bioactive nutrients or their interactions. A further strength relates to the analysis of the association between health outcomes and a dietary pattern based on 1 consistent method, represented by the DII, as opposed to other dietary patterns (e.g., Mediterranean diet) where there are multiple post hoc and a priori methods of assessing a specific dietary pattern, which may reduce precision in the observed effect due to the variation in assessment methods (56).
There are a diverse range of bioactive compounds that may be responsible for the associations between the DII and the included health outcomes of the present review. Examples of dietary components that are incorporated in the DII and have demonstrated anti-inflammatory properties include phytochemicals such as polyphenols, omega-3 fatty acids, and dietary fiber (57). A higher dietary intake of polyphenols has been associated with reduced inflammatory markers with the proposed pathway via their antioxidant properties (24). Omega-3 fatty acids have been widely studied for their anti-inflammatory potential and include the modulation of eicosanoid and resolvin synthesis (58, 59). Anti- and pro-inflammatory effects of dietary compounds also appear to be mediated via the gut microbiome (60). Intake of dietary fibers, probiotic supplements, and fermented foods have been suggested to provide anti-inflammatory properties via the increase in anti-inflammatory short-chain fatty acids and other gut-derived metabolites (17, 61). In contrast, dietary components common to a Western-style dietary pattern such as trans- and saturated fatty acids may increase inflammation via mechanisms such as toll-like receptor 4 expression and modulation of the gut microbiome (62, 63).
Despite the majority (n = 27/38, 71%) of outcomes showing a significant (P < 0.05) positive association with adherence to a pro-inflammatory dietary pattern, only 1 outcome provided “convincing” (Class I) evidence and most outcomes presented Class III or lower evidence. This was largely attributed to the high level of statistical heterogeneity (n = 27/38, 71%, with I2 ≥ 50%), a 95% prediction interval that included the null (n = 31/38, 82%), and a P value greater than 10−6 (n = 30/38, 79%).
A possible explanation for the low credibility assessment and high levels of heterogeneity in many outcomes may be related to the type of populations included in each meta-analysis. For example, some prior meta-analyses suggested differential associations between the DII and health outcomes between men and women (29, 34). To illustrate, Shivappa et al. (34) reported that the DII was associated with CVD outcomes in women, but not men. To some extent, these observations may be explained by the limited number of studies that have assessed gender-specific differences. Furthermore, several outcomes had a limited number of included studies [e.g., 13 outcomes (34%) including n = 2–3 studies per analysis], thus limiting the power to detect a statistical association and, in some circumstances, preventing formal analysis of excess significance. An additional potential source of heterogeneity that is common to nutrition epidemiology relates to the complexity of assessing dietary intake. Variations in the dietary assessment tools used between studies to calculate DII as well as bias common to self-reported measures (e.g., social desirability) (64) may have introduced heterogeneity into the included outcomes.
Findings of the current umbrella review need to be interpreted with the following limitations in mind. First, as this study included only outcomes with available meta-analyses, additional outcomes where meta-analyses are currently unavailable could not be considered. For example, the DII has been associated with risk of multiple sclerosis in 2 prior studies (65, 66); however, these have not been the subject of any identified meta-analysis at this time. A related limitation of umbrella reviews in general is the use of existing meta-analyses, which are dependent on prior investigators’ decisions regarding the inclusion of individual studies and the analysis methods used including the type and extent of sensitivity analyses conducted. Second, as this umbrella review included observational data only, limitations common to this approach may also affect the results of this review, such as information bias and residual confounding. This is particularly pertinent to the current review as there were a limited number of meta-analyses that exclusively included prospective study designs, where information bias is reduced. Case-control and cross-sectional study designs were more common than prospective study designs and are associated with a higher potential for information bias and reverse causation. Subgroup analyses of included meta-analyses support this, with cross-sectional and case-control studies generally reporting a larger effect size than prospective studies (32, 35, 36). Future studies are encouraged to use prospective study designs to reduce the existing bias within the literature. Randomized controlled trials that provide an anti-inflammatory dietary intervention pattern consistent with lower DII scores would provide further evidence of directionality, as well as allowing for cause-effect inferences and reducing possible biases inherent to observational study designs. A related consideration is that poor diet quality is likely to cluster with other adverse health behaviors (e.g., smoking, alcohol consumption, sedentariness) that are also associated with the included chronic diseases outcomes. While many individual studies have adjusted for these risk factors, there is heterogeneity in the quality of the data and methods of adjustment. Consequently, problems with residual effects may persist. Finally, while this review assessed the strength of the evidence for each outcome according to a framework commonly used in umbrella reviews, this approach largely relies on statistical methods to determine evidence strength, which does not incorporate other factors such as the rigor of the included study designs, plausible underlying biological mechanisms, and effect sizes.
It also should be kept in mind that the literature on the DII is rapidly advancing. According to Clarivate Web of Science® there has been an increase in DII-focused articles of approximately 25% per year, on average (i.e., from 2014 to 2019 by year: 11, 32, 45, 78, 92, 104 articles). This indicates that the evidence will continue to accumulate for outcomes where an insufficient number of articles limited the possibility of meta-analysis. Also, existing topics on which a meta-analysis currently exists may have a sufficient increase in the number of qualifying articles to merit an additional meta-analysis. While expansion of the literature will, no doubt, contribute to the robustness of the evidence, it will be important to monitor other factors, including heterogeneity.
Notwithstanding the discussed limitations of the current literature, the evidence identified in this review provides further support for the role of improved diet quality as a protective factor against chronic disease risk and mortality. While this review suggests that higher adherence to an anti-inflammatory dietary pattern may be beneficial, other healthy dietary patterns such as the Mediterranean diet and government dietary guidelines are also strongly associated with an anti-inflammatory score using the DII (67, 68). These associations provide novel mechanistic evidence regarding the potential anti-inflammatory effect of these dietary patterns. In regard to the public health implications of these results, this suggests that diverse dietary patterns that incorporate factors related to the individual context (e.g., culture, food availability, taste preferences) may be associated with the same decrease in chronic disease risk observed in this review.
Conclusion
In summary, this umbrella review identified pro-inflammatory dietary patterns (reflected by a higher dietary inflammatory index) to be adversely associated with a range of chronic disease-related health outcomes. This provides further evidence for the role of anti-inflammatory dietary patterns in the prevention of chronic diseases, as well as inflammation as a mechanism of action in the genesis of adverse health outcomes. Further prospective evidence is required to explore this association in health outcomes where current studies are limited (e.g., pancreatic, endometrial, and urological cancers), to address the large degree of heterogeneity, and to explore potential subgroup populations that are particularly susceptible to diet-induced inflammation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors' responsibilities were as follows—WM led all aspects of the manuscript; JAD and JTK assisted with screening; MH, GLT, SC, EH, SBT, AW, ML, and HA provided data extraction; NV and LS provided data analysis; TS created the figures; AO, NS, JRH, LCB, MB, and FJ provided background expertise to the introduction, discussion, and interpretation of results; and all authors: read, contributed to, and approved the final manuscript.
Notes
WM is currently funded by an Alfred Deakin Postdoctoral Research Fellowship and a Multiple Sclerosis Research Australia Early Career Fellowship. WM has previously received funding from the Cancer Council Queensland and university grants/fellowships from La Trobe University, Deakin University, University of Queensland, and Bond University; received industry funding and has attended events funded by Cobram Estate Pty. Ltd; received travel funding from theNutrition Society of Australia; received consultancy funding from Nutrition Research Australia; and has received speakers’ honoraria from The Cancer Council Queensland and the Princess Alexandra Research Foundation. JK is supported through a Griffith University Postdoctoral Research Fellowship. MB is supported by a NHMRC Senior Principal Research Fellowship (1156072). MB has received grant/research support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 Milk Company, Meat and Livestock Board, Woolworths, Avant, and the Harry Windsor Foundation; has been a speaker for Astra Zeneca, Lundbeck, Merck, Pfizer; and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Lundbeck Merck, Pfizer and Servier—all unrelated to this work. EH is supported by a National Health and Medical Research Council Early Career Fellowship (APP1156909). MH is supported by an Australian Rotary Health PhD Scholarship and has received research support from the A2 Milk Company. SC is supported by a Deakin University Postgraduate Research (DUPR) Scholarship. ST is funded through the Mindgardens Alliance and is a contractor to Nutrition Research Australia. HA is supported by a Deakin University Postgraduate Industry Research Scholarship. AO is supported by a Future Leader Fellowship (#101160) from the Heart Foundation Australia and Wilson Foundation. AO has received research funding from the National Health & Medical Research Council, Australian Research Council, University of Melbourne, Deakin University, Sanofi, Meat and Livestock Australia, and Woolworths Limited; and honoraria from Novartis. The Food & Mood Centre has received funding from the Fernwood Foundation, the A2 Milk Company, and Be Fit Foods. AW is supported by an NHMRC Boosting Dementia Research Grant (GNT1171313). AW has received previous funding from the University of South Australia, the Nutrition Society of Australia, and the Pork Cooperative Research Centre, all of which are unrelated to this work. FJ has received: 1) competitive grant/research support from the Brain and Behaviour Research Institute, the National Health and Medical Research Council (NHMRC), Australian Rotary Health, the Geelong Medical Research Foundation, the Ian Potter Foundation, The University of Melbourne; 2) industry support for research from Meat and Livestock Australia, Woolworths Limited, the A2 Milk Company, Be Fit Foods; 3) philanthropic support from the Fernwood Foundation, Wilson Foundation, the JTM Foundation, the Serp Hills Foundation, the Roberts Family Foundation, the Waterloo Foundation; and 4) travel support and speakers’ honoraria from Sanofi-Synthelabo, Janssen Cilag, Servier, Pfizer, Health Ed, Network Nutrition, Angelini Farmaceutica, Eli Lilly, and Metagenics. FJ has written 2 books for commercial publication. ML is supported by a Deakin University Scholarship and has received research funding support from Be Fit Foods. LCB is supported by an NHMRC of Australia Emerging Leadership Investigator Grant (ID: 1172987) and a National Heart Foundation of Australia Post-Doctoral Research Fellowship (ID: 102498). LCB has received project funding from Edith Cowan University and the Department of Health Western Australia; and travel support from the Nutrition Society of Australia and The University of Western Australia.
Author disclosures: Dr James R. Hébert owns a controlling interest in Connecting Health Innovations LLC (CHI), a company that has licensed the right to his invention of the dietary inflammatory index (DII®) from the University of South Carolina in order to develop computer and smart phone applications for patient counselling and dietary intervention in clinical settings. Dr Nitin Shivappa is an employee of CHI. The subject matter of this paper will not have any direct bearing on that work, nor has that activity exerted any influence on this project.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AMSTAR, A Measurement Tool to Assess Systematic Reviews; CI, confidence interval; CRP, C-reactive protein; DII®, Dietary Inflammatory Index; IL, interleukin; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; TNF-α, tumor necrosis factor-α
Contributor Information
Wolfgang Marx, Deakin University, Institute for Mental and Physical Health and Clinical Translation (IMPACT), Food & Mood Centre, School of Medicine, Barwon Health, Geelong, Victoria, Australia.
Nicola Veronese, University of Palermo, Department of Internal Medicine, Geriatrics Section, Palermo, Italy.
Jaimon T Kelly, Centre of Applied Health Economics, Griffith University, Brisbane, Queensland, Australia; Menzies Health Institute Queensland, Griffith University, Gold Coast, Queensland, Australia.
Lee Smith, The Cambridge Centre for Sport and Exercise Sciences, Anglia Ruskin University, Cambridge, UK.
Meghan Hockey, Deakin University, Institute for Mental and Physical Health and Clinical Translation (IMPACT), Food & Mood Centre, School of Medicine, Barwon Health, Geelong, Victoria, Australia.
Sam Collins, Deakin University, Institute for Mental and Physical Health and Clinical Translation (IMPACT), Food & Mood Centre, School of Medicine, Barwon Health, Geelong, Victoria, Australia.
Gina L Trakman, Department of Dietetics, Nutrition, and Sport, La Trobe University, Melbourne, Victoria, Australia.
Erin Hoare, Deakin University, Institute for Mental and Physical Health and Clinical Translation (IMPACT), Food & Mood Centre, School of Medicine, Barwon Health, Geelong, Victoria, Australia.
Scott B Teasdale, School of Psychiatry, UNSW Sydney, Sydney, New South Wales, Australia.
Alexandra Wade, Alliance for Research in Exercise, Nutrition, and Activity, Allied Health and Human Performance, University of South Australia, Adelaide, South Australia, Australia.
Melissa Lane, Deakin University, Institute for Mental and Physical Health and Clinical Translation (IMPACT), Food & Mood Centre, School of Medicine, Barwon Health, Geelong, Victoria, Australia.
Hajara Aslam, Deakin University, Institute for Mental and Physical Health and Clinical Translation (IMPACT), Food & Mood Centre, School of Medicine, Barwon Health, Geelong, Victoria, Australia.
Jessica A Davis, Deakin University, Institute for Mental and Physical Health and Clinical Translation (IMPACT), Food & Mood Centre, School of Medicine, Barwon Health, Geelong, Victoria, Australia.
Adrienne O'Neil, Deakin University, Institute for Mental and Physical Health and Clinical Translation (IMPACT), Food & Mood Centre, School of Medicine, Barwon Health, Geelong, Victoria, Australia; Melbourne School of Population & Global Health, University of Melbourne, Melbourne, Victoria, Australia.
Nitin Shivappa, Department of Epidemiology and Biostatistics and Cancer Prevention and Control Program, Arnold School of Public Health, University of South Carolina, Columbia, South Carolina, USA; Department of Nutrition, Connecting Health Innovations LLC, Columbia, South Carolina, USA.
James R Hebert, Department of Epidemiology and Biostatistics and Cancer Prevention and Control Program, Arnold School of Public Health, University of South Carolina, Columbia, South Carolina, USA; Department of Nutrition, Connecting Health Innovations LLC, Columbia, South Carolina, USA.
Lauren C Blekkenhorst, School of Medical and Health Sciences, Edith Cowan University, Joondalup, Western Australia, Australia; Medical School, The University of Western Australia, Perth, Western Australia, Australia.
Michael Berk, Deakin University, Institute for Mental and Physical Health and Clinical Translation (IMPACT), Food & Mood Centre, School of Medicine, Barwon Health, Geelong, Victoria, Australia.
Toby Segasby, Deakin University, Institute for Mental and Physical Health and Clinical Translation (IMPACT), Food & Mood Centre, School of Medicine, Barwon Health, Geelong, Victoria, Australia.
Felice Jacka, Deakin University, Institute for Mental and Physical Health and Clinical Translation (IMPACT), Food & Mood Centre, School of Medicine, Barwon Health, Geelong, Victoria, Australia; Black Dog Institute, Sydney, New South Wales, Australia; James Cook University, Townsville, Queensland, Australia; Centre for Adolescent Health, Murdoch Children's Research Institute, VIC, Australia.
References
- 1.Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Doré J, Franceschi C, Lehtinen MJ, Recker T, Salvioli S. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev. 2017;40:95–119. [DOI] [PubMed] [Google Scholar]
- 2.Pawelec G, Goldeck D, Derhovanessian E. Inflammation, ageing and chronic disease. Curr Opin Immunol. 2014;29:23–8. [DOI] [PubMed] [Google Scholar]
- 3.Bonaccio M, Di Castelnuovo A, Pounis G, De Curtis A, Costanzo S, Persichillo M, Cerletti C, Donati MB, De Gaetano G, Iacoviello L. A score of low-grade inflammation and risk of mortality: prospective findings from the Moli-sani Study. Haematologica. 2016;101(11):1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proctor MJ, McMillan DC, Horgan PG, Fletcher CD, Talwar D, Morrison DS. Systemic inflammation predicts all-cause mortality: a Glasgow inflammation outcome study. PLoS One. 2015;10(3):e0116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calle MC, Fernandez ML. Inflammation and type 2 diabetes. Diabetes Metab. 2012;38(3):183–91. [DOI] [PubMed] [Google Scholar]
- 6.Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Pariggiano I, Bianchi R, Crisci M, D'Acierno L, Giordano R. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep. 2014;16(9):435. [DOI] [PubMed] [Google Scholar]
- 7.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amor S, Peferoen LA, Vogel DY, Breur M, van der Valk P, Baker D, van Noort JM. Inflammation in neurodegenerative diseases–an update. Immunology. 2014;142(2):151–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaptoge S, Seshasai SRK, Gao P, Freitag DF, Butterworth AS, Borglykke A, Di Angelantonio E, Gudnason V, Rumley A, Lowe GD. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35(9):578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berk M, Williams LJ, Jacka FN, O'Neil A, Pasco JA, Moylan S, Allen NB, Stuart AL, Hayley AC, Byrne MLet al. So depression is an inflammatory disease, but where does the inflammation come from?. BMC Medicine. 2013;11(1):200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller N. Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr Bull. 2018;44(5):973–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedetti F, Aggio V, Pratesi ML, Greco G, Furlan R. Neuroinflammation in bipolar depression. Front Psychiatry. 2020;11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasco JA, Nicholson GC, Williams LJ, Jacka FN, Henry MJ, Kotowicz MA, Schneider HG, Leonard BE, Berk M. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry. 2010;197(5):372–7. [DOI] [PubMed] [Google Scholar]
- 14.Roth GA, Abate D, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, Abdollahpour I, GBD 2017 Causes of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):173–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afshin A, Sur PJ, Fay KA, Cornaby L, Ferrara G, Salama JS, Mullany EC, Abate KH, Abbafati C, Abebe Z. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet North Am Ed. 2019;393(10184):1958–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier T, Gräfe K, Senn F, Sur P, Stangl GI, Dawczynski C, März W, Kleber ME, Lorkowski S. Cardiovascular mortality attributable to dietary risk factors in 51 countries in the WHO European Region from 1990 to 2016: a systematic analysis of the Global Burden of Disease Study. Eur J Epidemiol. 2019;34(1):37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cryan JF, O'Riordan KJ, Cowan CS, Sandhu KV, Bastiaanssen TF, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. [DOI] [PubMed] [Google Scholar]
- 18.Tosti V, Bertozzi B, Fontana L. Health benefits of the Mediterranean diet: metabolic and molecular mechanisms. J Gerontol: Series A. 2018;73(3):318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80(4):1029–35. [DOI] [PubMed] [Google Scholar]
- 20.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J Am Coll Cardiol. 2004;44(1):152–8. [DOI] [PubMed] [Google Scholar]
- 21.Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis. 2014;24(9):929–39. [DOI] [PubMed] [Google Scholar]
- 22.Reinders I, Virtanen J, Brouwer I, Tuomainen T. Association of serum n-3 polyunsaturated fatty acids with C-reactive protein in men. Eur J Clin Nutr. 2012;66(6):736–41. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y, Griffith JA, Chasan-Taber L, Olendzki BC, Jackson E, Stanek EJ III, Li W, Pagoto SL, Hafner AR, Ockene IS. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006;83(4):760–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harms LM, Scalbert A, Zamora-Ros R, Rinaldi S, Jenab M, Murphy N, Achaintre D, Tjønneland A, Olsen A, Overvad K. Plasma polyphenols associated with lower high-sensitivity C-reactive protein concentrations: a cross-sectional study within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Br J Nutr. 2020;123(2):198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips CM, Chen L-W, Heude B, Bernard JY, Harvey NC, Duijts L, Mensink-Bout SM, Polanska K, Mancano G, Suderman M. Dietary inflammatory index and non-communicable disease risk: a narrative review. Nutrients. 2019;11(8):1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hébert JR, Shivappa N, Wirth MD, Hussey JR, Hurley TG. Perspective: the Dietary Inflammatory Index (DII)—lessons learned, improvements made, and future directions. Adv Nutr. 2019;10(2):185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hua R, Liang G, Yang F. Meta-analysis of the association between dietary inflammatory index (DII) and upper aerodigestive tract cancer risk. Medicine (Baltimore). 2020;99(17):e19879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayedi A, Emadi A, Shab-Bidar S. Dietary inflammatory index and site-specific cancer risk: a systematic review and dose-response meta-analysis. Adv Nutr. 2018;9(4):388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li D, Hao X, Li J, Wu Z, Chen S, Lin J, Li X, Dong Y, Na Z, Zhang Y. Dose-response relation between dietary inflammatory index and human cancer risk: evidence from 44 epidemiologic studies involving 1,082,092 participants. Am J Clin Nutr. 2018;107(3):371–88. [DOI] [PubMed] [Google Scholar]
- 31.Liang Y, Jiao H, Qu L, Liu H. Positive association between dietary inflammatory index and gastric cancer risk: a systematic review and meta-analysis. Nutr Cancer. 2020;72(8):1290–6. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z-Y, Gao X-P, Zhu S, Liu Y-H, Wang L-J, Jing C-X, Zeng F-F. Dietary inflammatory index and risk of gynecological cancers: a systematic review and meta-analysis of observational studies. J Gynecol Oncol. 2018;30(3):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu D-L, Ren Z-J, Zhang Q, Ren P-W, Yang B, Liu L-R, Dong Q. Meta-analysis of the association between the inflammatory potential of diet and urologic cancer risk. PLoS One. 2018;13(10):e0204845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shivappa N, Godos J, Hébert JR, Wirth MD, Piuri G, Speciani AF, Grosso G. Dietary inflammatory index and cardiovascular risk and mortality—a meta-analysis. Nutrients. 2018;10(2):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tolkien K, Bradburn S, Murgatroyd C. An anti-inflammatory diet as a potential intervention for depressive disorders: a systematic review and meta-analysis. Clin Nutr. 2019;38(5):2045–52. [DOI] [PubMed] [Google Scholar]
- 36.Zahedi H, Djalalinia S, Asayesh H, Mansourian M, Abdar ZE, Gorabi AM, Ansari H, Noroozi M, Qorbani M. A higher dietary inflammatory index score is associated with a higher risk of incidence and mortality of cancer: a comprehensive systematic review and meta-analysis. Int J Prev Med. 2020;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith L, Luchini C, Demurtas J, Soysal P, Stubbs B, Hamer M, Nottegar A, Lawlor RT, Lopez-Sanchez GF, Firth J. Telomere length and health outcomes: an umbrella review of systematic reviews and meta-analyses of observational studies. Ageing Res Rev. 2019;51:1–10. [DOI] [PubMed] [Google Scholar]
- 39.Veronese N, Demurtas J, Thompson T, Solmi M, Pesolillo G, Celotto S, Barnini T, Stubbs B, Maggi S, Pilotto A. Effect of low-dose aspirin on health outcomes: an umbrella review of systematic reviews and meta-analyses. Br J Clin Pharmacol. 2020;86(8):1465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grabovac I, Veronese N, Stefanac S, Haider S, Jackson SE, Koyanagi A, Meilinger M, Stubbs B, Firth J, Soysal P. Human immunodeficiency virus infection and diverse physical health outcomes: an umbrella review of meta-analyses of observational studies. Clin Infect Dis. 2020;70(9):1809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veronese N, Solmi M, Caruso MG, Giannelli G, Osella AR, Evangelou E, Maggi S, Fontana L, Stubbs B, Tzoulaki I. Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses. Am J Clin Nutr. 2018;107(3):436–44. [DOI] [PubMed] [Google Scholar]
- 42.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14(3):263–73. [DOI] [PubMed] [Google Scholar]
- 45.Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. 2007;4(3):245–53. [DOI] [PubMed] [Google Scholar]
- 46.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JP. Environmental risk factors and Parkinson's disease: an umbrella review of meta-analyses. Parkinsonism Relat Disord. 2016;23:1–9. [DOI] [PubMed] [Google Scholar]
- 48.Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. Can Med Assoc J. 2009;181(8):488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–40. [DOI] [PubMed] [Google Scholar]
- 50.Farhangi MA, Nikniaz L, Nikniaz Z, Dehghan P. Dietary inflammatory index potentially increases blood pressure and markers of glucose homeostasis among adults: findings from an updated systematic review and meta-analysis. Public Health Nutr. 2020;23(8):1362–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farhangi MA, Vajdi M. The association between dietary inflammatory index and risk of central obesity in adults: an updated systematic review and meta-analysis. Int J Vitam Nutr Res. 2020;90(5–6):535–52. [DOI] [PubMed] [Google Scholar]
- 52.Fowler ME, Akinyemiju TF. Meta-analysis of the association between dietary inflammatory index (DII) and cancer outcomes. Int J Cancer. 2017;141(11):2215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia-Arellano A, Martínez-González MA, Ramallal R, Salas-Salvadó J, Hébert JR, Corella D, Shivappa N, Forga L, Schröder H, Muñoz-Bravo C. Dietary inflammatory index and all-cause mortality in large cohorts: The SUN and PREDIMED studies. Clin Nutr. 2019;38(3):1221–31. [DOI] [PubMed] [Google Scholar]
- 54.Namazi N, Larijani B, Azadbakht L. Dietary inflammatory index and its association with the risk of cardiovascular diseases, metabolic syndrome, and mortality: a systematic review and meta-analysis. Horm Metab Res. 2018;50(5):345–58. [DOI] [PubMed] [Google Scholar]
- 55.Zhu Y, Li Q, Xu X. Dietary inflammatory index and the risk of prostate cancer: a dose-response meta-analysis. Eur J Clin Nutr. 2020;74(7):1001–8. [DOI] [PubMed] [Google Scholar]
- 56.Radd-Vagenas S, Kouris-Blazos A, Singh MF, Flood VM. Evolution of Mediterranean diets and cuisine: concepts and definitions. Asia Pac J Clin Nutr. 2017;26(5):749–63. [DOI] [PubMed] [Google Scholar]
- 57.De Mello V, Schwab U, Kolehmainen M, Koenig W, Siloaho M, Poutanen K, Mykkänen H, Uusitupa M. A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: the Sysdimet study. Diabetologia. 2011;54(11):2755. [DOI] [PubMed] [Google Scholar]
- 58.Souza PR, Marques RM, Gomez EA, Colas RA, De Matteis R, Zak A, Patel M, Collier DJ, Dalli J. Enriched marine oil supplements increase peripheral blood specialized pro-resolving mediators concentrations and reprogram host immune responses: a randomized double-blind placebo-controlled study. Circ Res. 2020;126(1):75–90. [DOI] [PubMed] [Google Scholar]
- 59.Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2(3):355–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blander JM, Longman RS, Iliev ID, Sonnenberg GF, Artis D. Regulation of inflammation by microbiota interactions with the host. Nat Immunol. 2017;18(8):851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–15. [DOI] [PubMed] [Google Scholar]
- 62.Fritsche KL. The science of fatty acids and inflammation. Adv Nutr. 2015;6(3):293S–301S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29(2):359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol. 1995;24(2):389–98. [DOI] [PubMed] [Google Scholar]
- 65.da Costa Silva BY, de Carvalho Sampaio HA, Shivappa N, Hebert JR, da Silva Albuquerque L, Carioca AAF, D'Almeida JAC, Maia CSC, de Melo MLP. Dietary Inflammatory Index and clinical course of multiple sclerosis. Eur J Clin Nutr. 2019;73(7):979–88. [DOI] [PubMed] [Google Scholar]
- 66.Shivappa N, Hebert JR, Behrooz M, Rashidkhani B. Dietary inflammatory index and risk of multiple sclerosis in a case-control study from Iran. Neuroepidemiology. 2016;47(1):26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mayr HL, Thomas CJ, Tierney AC, Kucianski T, George ES, Ruiz-Canela M, Hebert JR, Shivappa N, Itsiopoulos C. Randomization to 6-month Mediterranean diet compared with a low-fat diet leads to improvement in Dietary Inflammatory Index scores in patients with coronary heart disease: the AUSMED Heart Trial. Nutr Res. 2018;55:94–107. [DOI] [PubMed] [Google Scholar]
- 68.Wirth MD, Hébert JR, Shivappa N, Hand GA, Hurley TG, Drenowatz C, McMahon D, Shook RP, Blair SN. Anti-inflammatory Dietary Inflammatory Index scores are associated with healthier scores on other dietary indices. Nutr Res. 2016;36(3):214–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.