Abstract
Emerging evidence shows an association between protein intake during infancy and later obesity risk, and that association may differ by protein sources. This systematic review summarized and evaluated prospective cohort studies assessing the long-term association of total protein intake and protein sources during infancy (from birth to 2 y) with subsequent obesity outcomes in childhood or adolescence. Literature searches were conducted in Embase, Medline, Scopus, and Web of Science. Sixteen studies that reported associations between total protein intake and/or protein intake from different sources from birth to 2 y and ≥1 obesity outcomes in childhood or adolescence from 9 cohorts were identified. Most studies (11/16) were rated as high quality. The most frequently reported association was total protein intake and BMI (up to 10 y) with 6 out of 7 cohorts showing significant positive associations. Similar associations were found for animal protein, but not for plant protein. Limited studies examined the association between protein intake (both total and sources) and body composition (body fat, fat mass, and fat-free mass) and revealed inconsistent findings. Overall, higher intakes of total and animal protein during infancy were associated with higher BMI in childhood and adolescence. Future studies investigating the contribution of protein sources in long-term obesity development are needed. This review was registered at PROSPERO as CRD42020166540.
Keywords: protein intake, protein sources, infancy, obesity, childhood
Statement of Significance: This is the first systematic review that summarized the evidence from the prospective cohort studies examining the association between intakes of total protein and protein from different sources from birth to 2 years and later obesity outcomes in childhood and adolescence.
Introduction
Childhood obesity is a major global public health concern (1, 2). The WHO estimated that 40 million children under the age of 5 y were overweight or obese in 2018 and that >340 million children and adolescents aged 5–19 y were overweight or obese in 2016 (1). Childhood obesity is a risk factor for obesity, diabetes, and cardiovascular disease in adulthood, with the WHO estimating that >1.9 billion adults over the age of 18 were overweight or obese in 2016 (1, 3). Thus, obesity prevention in early childhood is critical for combating overweight and obesity and obesity-related adverse health outcomes across the life span.
The first 1000 days, which span from conception through to 24 mo of age, represent a critical period for growth and development as well as the prevention of childhood obesity (4, 5). It is undisputed that protein is essential for healthy growth and development in children and that nutrition in early life determines obesity risk and associated health outcomes later in life (6–8). Emerging studies suggest that high protein intake in infancy may be associated with higher BMI or obesity risk in childhood and adolescence (7, 9, 10). The most commonly suggested mechanism is that excess protein intake during infancy may increase the secretion of insulin and insulin like growth factor I (IGF-I), thereby promoting rapid weight gain and, in turn, subsequent obesity development (7, 8, 11). Rapid weight gain during the first 2 y of life has been associated with a 3-fold increase in later obesity risk (12). Moreover, accumulating evidence demonstrates that protein intake from different sources (e.g., animal or plant) in early life may have different effects on subsequent obesity development (13–15).
No systematic review to date has focused on intakes of total protein and protein sources from birth to age 2 y and later obesity outcomes in childhood and adolescence. This information may be useful for the evaluation of current protein intake recommendations during infancy and for guiding health care professionals in providing dietary advice on optimal protein intake during infancy. Therefore, the aim of this systematic review was to comprehensively review and appraise studies that explored the long-term associations between intakes of total protein and protein from different sources during the first 2 y of life and subsequent obesity outcomes in childhood and adolescence.
Methods
This review was registered with PROSPERO (registration number CRD42020166540). Reporting of the review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
Eligibility criteria
Prospective cohort studies that assessed the association between total protein intake and/or protein intake from different sources from birth to 2 y and subsequent obesity outcomes in childhood and adolescence (up to 18 y) were eligible for inclusion. Studies were eligible if they included at least 1 obesity outcome, such as body weight (BW), BMI [SD score (SDS) or z score], overweight or obesity risk, or other body-composition measures (i.e., body fat, fat mass, fat-free mass, etc.). Studies were ineligible if they assessed protein intake beyond 2 y of age, or the follow-up assessment was undertaken after 18 y of age. This age limit was informed by the known differences in metabolic and physiological functions of protein in children and adults (16). For example, high protein intake has been promoted as beneficial for weight loss in adults due to its potential impact on satiety (17). For protein sources, studies that assessed intake of protein-containing foods per se, but not protein intake from that food source, were excluded. For example, studies that assessed the association between meat intake or infant formula of varying protein content and obesity outcomes were excluded (18, 19), whereas studies that examined protein intake from meat or infant formula with obesity outcomes were included. Reviews, cross-sectional studies, and commentaries were excluded. Studies that included children with endocrine/metabolic disorders or severe illness were excluded. Only human studies and studies published in English were eligible for inclusion.
Search strategy
Embase, Medline, Scopus, and Web of Science databases were systematically searched from inception to March 2020. Key search terms included “protein intake,” “protein sources,” “infancy,” “obesity,” and “body composition.” The full search strategy in Embase is provided in Supplemental Table 1. Additional articles were identified through searches in Google or screening of relevant study reference lists.
Study selection and extraction
Articles identified from the 4 databases were imported into Endnote X9 (Clarivate) and duplicates were removed. All titles and abstracts of the identified articles were initially screened against the eligibility criteria and ineligible articles were excluded. Full texts of the remaining articles were then retrieved and screened. Both titles/abstracts and full texts were assessed by 2 independent researchers. If conflicts over the inclusion of a study arose between the 2 researchers, a third researcher assessed the study and conflicts were resolved via discussion. The reasons for exclusion were recorded. Data extraction was conducted by 2 independent researchers (AS and H-JY) and checked for accuracy and completeness by a third researcher (MZ). Information on study sample characteristics, age at protein intake assessment, total protein intake, intake of protein sources, dietary assessment tool used, age at outcome assessment, obesity outcomes, confounders, and findings was extracted.
Results synthesis and meta-analysis
Findings from all included studies were summarized and synthesized into associations of total protein intake or individual protein source with each obesity outcome, respectively. Studies that evaluated potential linear or nonlinear relations or conducted subgroup analysis within the whole sample were also extracted. As most of the included studies reported B-coefficients or ORs for the associations between per-unit change in total protein or protein sources (e.g., animal and dairy) and obesity outcomes (continuous or categorical), meta-analyses to obtain a pooled estimate were conducted. For multiple studies that reported similar associations from the same cohort, results from 1 of the studies were included in the meta-analysis.
Risk-of-bias assessment
The quality of included studies was evaluated using the Scottish Intercollegiate Guidelines Network 50 (SIGN 50) methodology checklists for cohort studies (20). This tool was deemed the most suitable for assessing the quality of prospective cohort studies, taking into consideration selection bias, attrition rate, and assessment quality (21, 22). With this tool, study quality is evaluated via 5 aspects including research question, selection of subjects, assessment of outcomes, confounding, and statistical analysis (20). Two checklist items, “assessment of outcome is made blinded to exposure” and “recognition of exposure influencing outcome,” did not apply to our research question and were omitted. Two additional items on power size justification and source of funding adapted from the National Institute of Health Quality Assessment Tool for Observational Cohort and Cross-sectional Studies were added to the checklist to enhance the tool (23). Two reviewers (H-JY, MZ) conducted the quality assessment independently and resolved discrepancies through discussion. Studies were given a rating of high (majority of items met and results unlikely to change by further research), acceptable (most items met and conclusion may change in further studies), and low (most items not met and conclusion likely to change in further studies) (20).
Results
Study selection
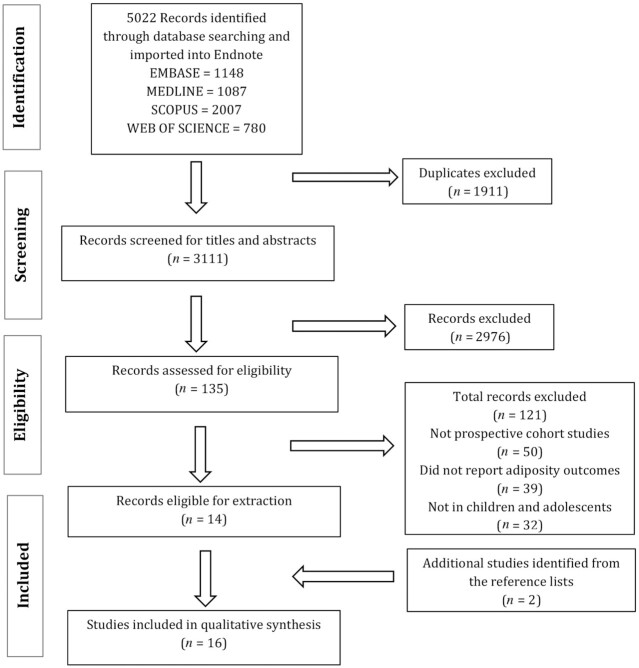
Figure 1 outlines the study selection process whereby 5022 studies were found in the 4 databases. Abstract and title assessment removed 2976 studies. Full-text assessment found 135 studies eligible for screening. Of the 135 screened texts, 50 articles were not prospective cohort studies, 39 studies did not assess obesity outcomes, and 32 studies had study populations that were not relevant to this review. Two additional articles were identified as relevant and included. The total number of eligible studies for inclusion was 16.
FIGURE 1.
Flowchart for the selection of prospective cohort studies examining the association between protein intake from birth to 2 y and obesity outcomes in childhood or adolescence.
Study characteristics
The 16 included studies reported results from 9 cohorts. The sample size ranged from 90 to 3573 participants (Table 1) (14, 24–38). Cohorts were predominantly from Northern or Western Europe (Denmark, Iceland, Germany, Netherland, the United Kingdom) followed by Italy and the United States. Most studies (n = 11) utilized data from the following 4 cohorts: the Second Longitudinal Icelandic Infant Study (n = 2) (34, 35), the Dortmund Nutritional and Anthropometric Longitudinal Design (DONALD) study (n = 3) (14, 26, 27), the Generation R study (n = 4) (24, 29, 36, 37), and the Gemini UK twin study (n = 2) (31, 32). These studies were considered separately in the current review as they assessed different associations between protein intake (total protein or protein sources) and obesity outcomes (different obesity outcomes at different follow-up time points) within the same cohort. Ten studies assessed protein intake before or at 1 y of age (24, 25, 28, 29, 33–38), and 3 studies assessed protein intake from 1 to 2 y of age (30–32). The remaining 3 studies examined protein intake both from birth to 1 y and 1 to 2 y of age (14, 26, 27). Five out of 16 studies assessed protein intake on >1 occasion (range: 2–24 mo across the first 2 y) (14, 25–27, 34). The numbers of studies that evaluated total protein intake only, protein sources only, and both were 7 (25–28, 31, 33, 34), 3 (30, 32, 38), and 6 (14, 24, 29, 34, 36, 37), respectively. The most assessed protein sources were animal protein and plant protein. Animal protein was further disaggregated into nondairy animal (meat/fish/eggs) protein or dairy protein. Two other studies also reported on milk or cereal proteins (14, 32).
TABLE 1.
Prospective cohort studies assessing the association between protein intakes from birth to 2 y and obesity outcomes in childhood or adolescence1
| First author, year (ref) | Sample characteristics, n, study country | Age at protein intake assessment, mo | Protein intake | Dietary assessment tool | Age at outcome assessment | Obesity outcomes | Adjusted confounders | Findings | Funding |
|---|---|---|---|---|---|---|---|---|---|
| Scaglioni, 2000 (33) | 147, Italy | 12 | Total protein (%E) | FFQ plus 24-h recall | 5 y | BMI | Child sex, weight, and length at birth and 12 mo; parental age | Children with BMI >90th percentile had higher total protein intake at 12 mo than those with BMI ≤90th percentile | N/D |
| Gunnarsdóttir, 2003 (25) | 90, Iceland | 2, 4, 6, 9, 12 | Total protein (%E) | 2-d WFR | 6 y | BMI | Total energy intake, intakes of fat and carbohydrate | Total protein at 2, 4, 9, 12 mo, but not 6 mo, and BMI in boys: + significantTotal protein and BMI in girls: NS | I |
| Hoppe, 2004 (28) | 100, Denmark | 9 | Total protein (g/d, g/kg BW) | 7-d FR | 10 y | BMI, weight, %BF | Child sex, parental height and weight, breastfeeding duration, BMI/weight/%BF at 9 mo | Total protein and weight: + significantTotal protein and BMI and %BF: NS | G |
| Günther, 2006 (26)2 | 313, Germany | 12, 18, 24 | Total protein (%E) | 3-d WFR | 5 y | BMI SDS | Total energy intake, maternal BMI, siblings, gestational age, breastfeeding status | Significant differences in BMI SDS by total protein intake tertiles from 12–24 mo were found in girls, not boys | G |
| Gunther, 2007 (27)2 | 203, Germany | 6, 12, 18–24 | Total protein groups (high-high, high-low, low-high, low-low) | 3-d WFR | 7 y | BMI SDS, %BF; overweight, overfatness | Child sex, total energy intake, maternal BMI, maternal education, gestational age, first birth, smoking, breastfeeding, siblings, and BMI SDS or %BF at 6 mo | All obesity outcomes were not statistically different by total protein intake groups at ages 6–12 moObesity measures by total protein intake groups at 12 and 18–24 mo | G |
| Günther, 2007 (14)2 | 203, Germany | 6, 12, 18–24 | Total protein and sources (%E and teriles) Animal: -Dairy -Meat Plant: -Cereal | 3-d WFR | 7 y | BMI SDS, %BF | Child sex, total energy intake, fat intake, siblings, firstborn status, maternal overweight, lnBF% BMI SDS at 6 mo | Total protein, animal, and dairy proteins at 12 mo and BMI SDS and %BF: + significantTotal protein, animal, and dairy proteins at 6, 18–24 mo and BMI SDS and %BF: NSLinear dose–response trend between total protein, animal, and dairy intake tertiles and BMI SDS and %BFPlant, meat, cereal proteins and BMI SDS and BF%: NS | G |
| Weijs, 2011 (38) | 120, Netherlands | 4–13 | Animal protein (total protein minus plant) tertiles (%E) | 2-d WFR | 8 y | BMI SDS, overweight (BMI SDS >1) | Child sex, age, body weight at 4–13 mo, breastfeeding status, maternal education, overweight, physical activity, and total energy intake | The highest animal protein tertile showed significant higher BMI SDS and risk of overweight compared with the lower 2 animal protein tertiles | G |
| Thorisdottir, 2013 (35)3 | 90 in 1995/1996; 170 in 2005; Iceland | 9, 12 | Total protein (%E) | 3-d WFR | 6 y | BMI | Child sex, total energy intake, birth weight, maternal education, breastfeeding duration | Total protein at 9 mo and BMI: NSTotal protein at 12 mo and BMI: + significant | N/D |
| Thorisdottir, 2014 (34)3 | 137, Iceland | 12 | Total protein and sources (%E) Animal: -Dairy -Meat/fish Plant | 2–3-d WFR | 6 y | BMI | Child sex, total energy intake, breastfeeding duration, maternal education and birth weight | Total protein and animal protein and BMI: + significantDairy and meat/fish protein, plant protein and BMI: NS | G |
| Braun, 2016 (24)4 | 3564, Netherlands | 13 | Total protein and sources (10 g/d) Animal: -Dairy -Nondairy animal Plant | FFQ | 14, 18, 24, 30, 36, 45 mo; 6, 9 y | BMI and weight SDS trajectories | Child sex, ethnicity, age, total energy intake, birth weight, breastfeeding, playing sports, household income, maternal BMI, education, folic acid use during pregnancy, smoking during pregnancy and diet score | Total protein, animal, dairy, nondairy animal protein and trajectories of weight SDS and BMI SDS: + significantPlant protein and trajectories of weight SDS and BMI SDS: NS | I |
| Pimpin, 2016 (31)5 | 2154, United Kingdom | 21 | Total protein (%E) | 3-d WFR | 36, 60 mo | BMI and weight trajectories, overweight (IOTF) | Child sex, age at diet entry, zygosity, ethnicity, feeding method, family SES, maternal BMI, rate of prior growth, birth weight, total energy intake | Total protein and trajectories of BMI and weight from 21–36 and 21–60 mo: + significantTotal protein and trajectories of BMI and weight from 21–36 and 21–60 mo: + significantTotal protein and overweight: NS | I |
| Voortman, 2016 (36)4 | 2911, Netherlands | 12 | Total protein and sources (10 g/d) Animal: -Dairy -Nondairy animal Plant | FFQ | 6 y | BMI SDS, FMI SDS, FFMI SDS | Child sex, total energy intake, maternal age, prepregnancy BMI, education level, smoking during pregnancy, household income, child ethnicity, birth weight, breastfeeding, total fat intake, diet quality score, screen time, pariticpation in sports, BMI at 13 mo | Total protein, animal protein and FMI SDS: + significant Total protein, animal protein and BMI SDS, FFMI SDS: NSPlant protein and BMI, FMI, FFMI SDS: NSDairy and nondairy animal and FMI SDS similar effect size | I, G |
| Voortman, 2016 (37)4 | 2965, Netherlands | 12 | Total protein and sources (10 g/d and tertile) Animal Plant | FFQ | 6 y | %BF, FMI | Maternal age, BMI, education, smoking during pregnancy, child ethnicity, birth weight z score, breastfeeding, total energy intake, fat intake, participation in sports, and screen time | Total protein and %BF: + significantLinear dose–response trend between total protein, animal, and dairy intake tertiles and %BF and FMISex-specific analyses revealed significant association in girls, but not in boys | I |
| Morgen, 2018 (30) | 7 y: 36,481; 11 y: 22,047; Denmark | 18 | Protein sourcesDairy (5 g/d)Meat/fish (2 g/d) | 4-d 24-h recall | 7, 11 y | BMI z score and overweight (IOTF) | Maternal pregnancy BMI, paternal BMI, maternal SES, maternal age at conception, parity, smoking during pregnancy, gestational weight gain | Dairy, meat/fish protein and BMI z score at 7 and 11 y: + significantDairy protein and overweight at 7 and 11 y: NSMeat/fish protein and overweight at 7 y: + significantMeat/fish protein and overweight at 11 y: NS | I |
| Pimpin, 2018 (32)5 | 1939, United Kingdom | 21 | Total protein and sourcesAnimal (meat, chicken fish, eggs) Dairy: -Milk Plant | 3-d 24-h recall | Quarterly 21–60 mo | Weight, BMI and overweight (IOTF) | Age at dietary assessment, child sex, birth weight, rate of prior weight gain, fat intake, total energy intake and height | Dairy and milk protein and weight, BMI, overweight: + significantAnimal, plant protein and weight, BMI, overweight: NS | I, G |
| Jen, 2019 (29)4 | 3573, Netherlands | 12 | Total protein and sources (5%E) Animal: -Dairy -Nondairy animal Plant | FFQ | Eight time points from 1 to 10 y and 6 to 10 y | BMI SDS, weight SDS trajectories from 1 to 10 y; FMI SDS, FFMI SDS trajectories from 6 to 10 y | Child sex, ethnicity, age at dietary measurement, total energy intake, birth weight, breastfeeding, diet quality, screen time, playing sports, household income, maternal BMI, maternal education, folic acid use during pregnancy, smoking during pregnancy | Total and animal protein and trajectories of weight SDS, BMI SDS and FMI SDS: + significantPlant protein and trajectories of BMI SDS and FMI SDS: NSNo association was found for any protein intake and FFMI SDS | I, G |
BW, body weight; FFMI, fat-free mass index; FMI, fat mass index; FR, food record; G, government; I, industry; IOTF, International Obesity Task Force; N/D, not described; ref, reference; SDS, SD score; SES, socioeconomic status; WFR, weighed food record; %BF, percentage of body fat; %E, percentage of energy; + significant, positive significant association (P < 0.05).
Second Longitudinal Icelandic Infant Study.
Dortmund Nutritional and Anthropometric Longitudinal Design study.
Generation R Study.
Gemini UK twin study.
Protein intake was assessed as grams per day, percentage of total energy intake (%E), or grams per kilogram of BW. Fifteen studies assessed total protein intake (14, 24–29, 31–38). Nine studies examined protein sources (14, 24, 29, 30, 32, 34, 36–38). Intakes of total protein and sources in each study are shown in Supplemental Table 2. Among studies that reported both animal and plant protein, total protein intake consisted of approximately two-thirds animal sources and one-third plant sources. Five studies collected dietary data using an FFQ (24, 29, 33, 36, 37). Eleven studies collected dietary data using a weighed food record (WFR), with most being conducted over 3 consecutive days (14, 25–28, 30–32, 34, 35, 38). BMI or BMI z score (SDS) were the most commonly reported obesity outcome followed by percentage of body fat (%BF), weight, overweight risk, fat mass index (FMI), and fat-free mass index (FFMI). Eleven studies included obesity outcomes at a single follow-up after 2 y of age, with a follow-up age ranging from 5 up to 11 y (14, 25–28, 33–38). Five studies assessed obesity outcomes on 2 or more occasions (24, 29–32). Of these, 4 assessed trajectories of obesity outcomes from protein intake assessment until 5 (31, 32), 9 (24), and 10 (29) y of age. The most commonly adjusted confounders were child sex, child obesity measures at study baseline or birth weight, breastfeeding status or duration, total energy intake, maternal education, and maternal BMI. Total energy intake is related to obesity, and protein intake also contributes to total energy intake. Controlling for total energy intake is essential to reveal the specific effect of protein intake on obesity independent of total energy intake (39). Most studies (13/16) adjusted for total energy intake and revealed significant relations between total protein or protein sources and obesity outcomes.
Risk of bias within studies
Of the 16 studies included in this review, 11 were deemed to be of high quality (14, 24, 26, 27, 29, 31, 32, 34–37), 2 were classified as acceptable (25, 28), and 3 received a low-quality rating (30, 33, 38) (Table 2). High-quality studies included a reliable assessment of protein intake by a validated dietary assessment method, objectively measured outcomes, adequately adjusted for important confounders (such as obesity measures at study baseline or birth weight and total energy intake and ≥1 maternal covariates), and reported CIs for the findings (14, 24, 26, 27, 29, 31, 32, 34–37). Studies that were determined to be of low quality failed to provide a reliable assessment of protein intake or obesity outcomes (self-reported) or did not adequately adjust for confounders (30, 33, 38).
TABLE 2.
Quality assessment of studies assessing the association between protein intakes from birth to 2 y and obesity outcomes in childhood or adolescence1
| First author, year (ref) | Clearly focused question | Drop-out rate (<20%) | Comparison between participants and dropouts | Clearly defined outcome | Assessment of exposure reliable | Validity of outcome measure | Repeated exposure measure | Adequate adjustment for confounding | CI provided | Sample size justification or power description | Declaration of funding | Quality rating |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scaglioni, 2000 (33) | Y | Y | N | Y | Y | N | N | N | N | Y | N | Low |
| Gunnarsdóttir, 2003 (25) | Y | N | Y | Y | Y | Y | Y | N | N | N | Y | Acceptable |
| Hoppe, 2004 (28) | Y | N | N | Y | Y | Y | N | N | Y | Y | Y | Acceptable |
| Günther, 2006 (26) | Y | N/D | N | Y | Y | Y | Y | N | Y | N | Y | High |
| Günther, 2007 (27) | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | High |
| Günther, 2007 (14) | Y | N | N | Y | Y | Y | Y | Y | Y | N | Y | High |
| Weijs, 2011 (38) | Y | N | N | Y | N | N | N | Y | Y | N | Y | Low |
| Thorisdottir, 2013 (35) | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | High |
| Thorisdottir, 2014 (34) | Y | Y | Y | Y | Y | Y | N | Y | Y | N | Y | High |
| Braun, 2016 (24) | Y | N | Y | Y | Y | Y | N | Y | Y | N | Y | High |
| Pimpin, 2016 (31) | Y | N | N | Y | Y | Y | N | Y | Y | N | Y | High |
| Voortman, 2016 (36) | Y | N | N | Y | Y | Y | N | Y | Y | N | Y | High |
| Voortman, 2016 (37) | Y | N | N | Y | Y | Y | N | Y | Y | N | Y | High |
| Morgen, 2018 (30) | Y | N | N | Y | N | N | N | N | Y | N | Y | Low |
| Pimpin, 2018 (32) | Y | N | N | Y | Y | Y | N | Y | Y | Y | Y | High |
| Jen, 2019 (29) | Y | N | N | Y | Y | Y | N | Y | Y | N | Y | High |
N, no; N/D, not described; ref, reference; Y, yes.
Results of individual studies
The number of studies reporting associations between intakes of total protein or protein sources with each obesity outcome is shown in Table 3. Detailed findings for each study are presented in Supplemental Table 2.
TABLE 3.
Summary of studies reporting associations between intakes of total protein or protein sources and obesity outcomes1
| BMI | BMI z score (SDS) | %BF | Weight or weight SDS | Overweight risk | FMI or FFMI SDS | |
|---|---|---|---|---|---|---|
| Total protein | Scaglioni, 2000 (33) | Günther, 2006 (26) | Hoppe, 2004 (28)2 | Hoppe, 2004 (28) | Gunther, 2007 (27) | Voortman, 2016 (36) |
| Gunnarsdóttir, 2003 (25) | Gunther, 2007 (27) | Gunther, 2007 (27) | Pimpin, 2016 (31) | Pimpin, 2016 (31)2 | Voortman, 2016 (37) | |
| Hoppe, 2004 (28)2 | Günther, 2007 (14) | Günther, 2007 (14) | Braun, 2016 (24) | Jen, 2019 (29) | ||
| Thorisdottir, 2013 (35) | Braun, 2016 (24) | Voortman, 2016 (37) | Jen, 2019 (29) | |||
| Thorisdottir, 2014 (34) | Voortman, 2016 (36)2 | |||||
| Pimpin, 2016 (31) | Jen, 2018 (29) | |||||
| Animal protein (dairy and nondairy) | Thorisdottir, 2014 (34) | Günther, 2007 (14) | Voortman, 2016 (37) | Braun, 2016 (24) | Weijs, 2011 (38) | Jen, 2019 (29) |
| Weijs, 2011 (38) | Günther, 2007 (14) | Jen ,2019 (29) | Voortman, 2016 (36) | |||
| Braun, 2016 (24) | ||||||
| Voortman, 2016 (36)2 | ||||||
| Jen, 2019 (29) | ||||||
| Nondairy animal | Thorisdottir, 2014 (34)2 | Günther, 2007 (14)2 | Günther, 2007 (14)2 | Braun, 2016 (24) | Morgen, 2018 (30) | Jen, 2019 (29)2 |
| Pimpin, 2018 (32)2 | Braun, 2016 (24) | Pimpin, 2018 (32)2 | ||||
| Morgen, 2018 (30) | Jen, 2019 (29) | |||||
| Jen, 2019 (29) | ||||||
| Dairy protein | Thorisdottir, 2014 (34)2 | Günther, 2007 (14) | Günther, 2007 (14) | Braun, 2016 (24) | Pimpin, 2018 (32) | Jen, 2019 (29)2 |
| Pimpin, 2018 (32) | Braun, 2016 (24) | Pimpin, 2018 (32) | ||||
| Jen, 2019 (29) | Morgen, 2018 (30) | Jen, 2019 (29) | ||||
| Morgen, 2018 (30) | ||||||
| Plant protein2 | Thorisdottir, 2014 (34) | Günther, 2007 (14) | Günther, 2007 (14) | Braun, 2016 (24) | Pimpin, 2018 (32) | Jen, 2019 (29) |
| Pimpin, 2018 (32) | Braun, 2016 (24) | Voortman, 2016 (37) | Pimpin, 2018 (32) | Voortman, 2016 (36) | ||
| Voortman, 2016 (36) | Jen, 2019 (29) | |||||
| Jen, 2019 (29) |
FFMI, fat-free mass index; FMI, fat mass index; SDS, SD score; %BF, percentage of body fat.
Nonsignificant (P ≥ 0.05).
Total protein intake and BMI or BMI z score (SDS)
Twelve studies reported the relation between total protein intake and BMI (n = 6) (25, 28, 31, 33–35) or BMI z score (SDS) (n = 6) (14, 24, 26, 27, 29, 36) from 7 cohorts, with 10 studies reporting significant positive associations (P < 0.05) (Table 3) (14, 24–27, 29, 31, 34–36). Seven studies found higher total protein intake (g/d, %E, or g/kg BW) was associated with higher BMI or BMI z score (SDS) at 5 y (26, 33), 6 y (25, 34, 35), 9 y (24), and 10 y (29) (Table 1). The other 3 studies revealed higher intake of total protein in children with BMI ≥90th percentile versus <90th percentile or a higher BMI SDS in the high-protein-intake group compared with the low-protein-intake group (14, 27, 33). The remaining 2 studies reported no evidence of an association between total protein intake and BMI or BMI SDS at 6 y (28, 36). In summary, 6 out of 7 cohorts revealed some significant positive relation between total protein intake from birth to 2 y and subsequent BMI or BMI z score (SDS) up to 10 y.
Total protein intake and other obesity outcomes
Four studies reported the association between total protein intake (%E, g/d) and %BF from 3 cohorts (14, 27, 28, 37) (Table 3). Of these, 3 found significant positive associations (14, 27, 37). A consistent positive association was found in studies reporting on weight (28, 31) and weight SDS (24, 29). Two studies evaluating the relation between total protein intake and overweight risk reported inconsistent findings (27, 31). One study found that children with higher total protein intake were more likely to be at a higher risk of overweight at the age of 7 y (27). In contrast, the other study found that total protein intake (%E) was not associated with overweight risk either at 3 or 5 y of age (31). Three studies investigated FMI or FFMI at 6 or 10 y of age in the same cohort; a significant association was consistently found for total protein intake with FMI but not FFMI (29, 36, 37).
Protein sources and BMI or BMI z score (SDS)
Six studies examined the association between animal protein intake (total minus plant protein) and BMI (34) or BMI z score (SDS) from 3 cohorts (14, 24, 29, 36, 38). Of these, 5 reported significant positive associations (14, 24, 29, 34, 38) and 1 study reported no association (36). A total of 6 studies also assessed the relation between protein intake from nondairy animal sources (meat/fish/eggs) and BMI (32, 34) or BMI z score (SDS) (14, 24, 29, 30). Three found significant positive associations (24, 29, 30) and 3 found no associations (14, 32, 34). With respect to dairy protein, 4 out of 5 studies reported a positive significant association between dairy protein intake and BMI or BMI z score at 5 y (32), 7 y (14, 30), 9 y (24), and 10 y of age (29). For plant protein intake, all 6 studies reported no evidence of an association with BMI or BMI z score (14, 24, 29, 32, 34, 36).
Protein sources and other obesity outcomes
Two studies assessed protein sources and %BF (14, 37). One study found that higher animal protein, but not plant protein, was associated with higher %BF at 7 y (14). When animal protein was further categorized into nondairy and dairy protein sources, a significant positive association with %BF was found for dairy protein only (14). Similarly, Voortman et al. (37) examined the association between animal versus plant protein and %BF at 6 y and found a significant positive association for animal protein but in girls only. Three studies assessing weight or weight SDS also revealed mixed findings (24, 29, 32). In the same cohort, both Braun et al. (24) and Jen et al. (29) found that, apart from plant protein, all animal, nondairy animal, and dairy source proteins were associated with higher weight SDS trajectories until 9 and 10 y, respectively. In contrast, Pimpin et al. (32) reported that dairy and milk protein, but not nondairy animal and plant proteins, predicted higher weight at 5 y. Intakes of animal protein (38), nondairy animal protein (30), dairy protein (32), and milk protein (32), but not plant protein (32), were found to increase the risk of overweight in later childhood or adolescence. Of the 2 studies that assessed FMI in the same cohort, a significant positive association was found for animal protein (29, 36), but not for dairy (29), nondairy animal (29), or plant (29, 36) protein sources.
Assessment of linear dose–response or nonlinear relation
Six studies examined the potential linear dose–response and nonlinear relation between protein intake or sources and obesity outcomes (14, 25, 26, 31, 37, 38). Three studies revealed a potential linear dose–response relation (14, 26, 37). In the DONALD cohort, a linear positive relation was found for intake tertiles of total protein (26) and animal protein (14) with BMI SDS at 7 y (P-trend < 0.05). In the Generation R Study, Voortman et al. (36) found a linear dose–response relation between total protein tertiles and %BF and FMI at 6 y. In comparison, a nonlinear threshold effect was found in 3 other studies. Gunnarsdóttir and Thorsdottir (25) found that BMI of the fourth total protein intake quartile was significantly higher than BMI of the first and second quartiles. Likewise, Weijs et al. (38) found that the highest animal protein intake tertile had a higher BMI SDS and overweight risk than the lower 2 tertiles. Pimpin et al. (31) also found that intake in the top 2 quintiles of total protein intake (16.3%E) was associated with higher BMI and weight compared with the lowest quartile.
Subgroup analysis
Three of the 16 studies reported sex-specific associations between total protein intake and BMI. Of these, 2 studies found significant positive associations between total protein intake and obesity outcomes in girls only (26, 37), whereas the third study found a significant positive association in boys only (25). Only 1 study reported subgroup analysis by child rapid growth in the first year (37). This study found that the associations between higher total protein intake at 1 y and higher BMI SDS and FMI at 6 y were stronger in children with catch-up growth than those with no catch-up growth (37).
Synthesis of results (meta-analysis)
A total of 12 studies that reported 1-unit change in protein intake or source and per-unit change in obesity outcome from 7 cohorts were considered for inclusion in meta-analyses (14, 24, 25, 28–32, 34–37). Suitability of pooling studies was limited by multiple factors, which included the small number of studies reporting similar associations. For total protein intake, the number of studies reporting each outcome was as follows: BMI (n = 5), BMI SDS (n = 4), %BF (n = 3), weight (n = 2), weight SDS (n = 2), overweight risk (n = 2), and FMI SDS (n = 3). Moreover, several studies reported results from the same cohort but with different follow-up endpoints (e.g., Generation R Study) (24, 29, 36, 37). Another factor was the heterogeneity in reporting of measurement units for exposure (e.g., g/d vs. %E) and variations in age at the protein intake assessment (e.g., 1 vs. multiple time points before 2 y of age). Furthermore, age at the outcome assessment (e.g., BMI at 1 follow-up vs. BMI trajectory from repeated follow-ups) also limits the feasibility for pooling of effects. Last, the instances of insufficient reporting of effects and their variability in some studies was also a crucial factor [e.g., results reported to a low level of precision (34) and “nonsignificant” (25) being reported with no effect sizes, 95% CI, or SE].
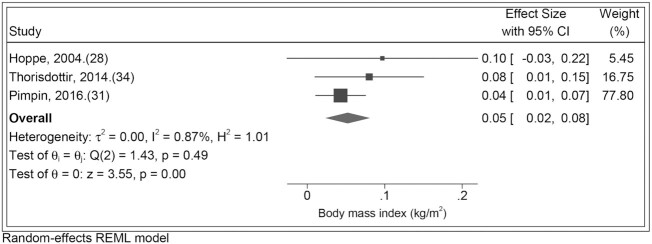
Of the considered meta-analyses, only those for total protein and BMI were deemed appropriate, with 4 of the 5 identified studies reporting on similar effects [estimated effect of protein intake (%E) at 12 or 21 mo on BMI at 5 or 6 y]. One study was omitted due to insufficient information as effects were reported for sexes separately and the result for girls was reported only as “NS” (nonsignificant P ≥ 0.05) (25). Among the 4 remaining studies, 2 included participants from the same cohort (but different participant subsets) and produced similar estimate effects; here only 1 study was chosen to be included on account of more precise reporting of effects (34, 35). Using results from 3 studies, a meta-analysis for the effect of total protein (%E) on BMI was conducted using a random-effects model, which gave a pooled effect estimate of 0.05 (95% CI: 0.02, 0.08) kg/m2 (Figure 2) (28, 31, 34). There was minimal heterogeneity in the effect estimates (I2 = 0.87%, chi-square test P < 0.49) (40). This pooled estimate was unsurprisingly similar to the effect estimate of 0.04 (95% CI: 0.01, 0.07) kg/m2 from the Pimpin et al. study (31), which had a weight of ∼80% in the meta-analysis due to a much larger sample size (and correspondingly narrower CI) than the other 2 studies included (28, 34).
FIGURE 2.
Study-specific and pooled estimates for the association between total protein intake (%E) from birth to 2 y and later BMI (kg/m2). REML, restricted maximum likelihood; %E, percentage of energy.
Discussion
This systematic review found that higher total protein intake during the first 2 y of life was associated with subsequent higher BMI or BMI SDS in later childhood and adolescence (from 3 to 10 y of age). Limited studies reported the association between total protein intake from birth to 2 y and other obesity outcomes (BW, %BF, overweight risk, FMI), and the totality of evidence remains inconclusive. For protein sources, higher intakes of animal protein, but not plant protein, from birth to 2 y were associated with higher BMI or BMI SDS. Within animal protein, evidence underpinning a positive association between dairy protein intake and BMI or BMI SDS appeared to be stronger than that for nondairy animal protein (meat/fish/eggs) intake. Examination of associations between protein sources during infancy and other obesity outcomes was limited, and findings were mixed.
The findings of the current systematic review extend the findings from 5 previously published reviews that also reported high protein intake in early life as a risk factor for later obesity outcomes (7, 8, 19, 41, 42). However, only 1 of these reviews adapted a systematic approach (41), and it summarized the literature until 2013 on protein intake from birth to 18 y and various long-term health outcomes, including obesity (43). The 4 other reviews were narrative, with only 2 that focused specifically on protein intake in the first 2 y of life and obesity outcomes (7, 8). The other 2 narrative reviews either reported on nutrient intakes (including protein) during infancy and later obesity risk or the effects of protein intake in the first 2 y of life on later health including obesity (9, 10). None of these reviews have provided in-depth evaluation of the literature on intakes of both total protein and protein from different sources on obesity outcomes including body composition.
Various mechanisms have been proposed to explain how total protein intake in infancy may impact upon later obesity outcomes (44–47). The “early protein hypothesis” suggests that excess protein intake exceeding requirements may stimulate the secretion of insulin and IGF-I (7, 8, 27, 45, 46). High circulating concentrations of IGF-I in the body may stimulate adipogenesis via triggering both the differentiation and multiplication of preadipocytes (7, 8, 48, 49). In addition, high IGF-I may inhibit lipolysis (7, 8, 48), increasing long-term susceptibility for increased obesity (50). Furthermore, it has been suggested that excess protein intake can be converted into glucose via gluconeogenesis, which may lead to an increased amount of glucose stored as fat within the body and, in turn, promote body fat and weight gain (29).
With respect to the impact of protein sources on obesity outcomes, the differential effect of amino acids on insulin and IGF-I may provide an explanation (8, 24). Animal proteins, including meat and dairy proteins, are rich in leucine, arginine, and lysine, which have been linked to an increased secretion of IGF-I and the programming of preadipocytes (8, 24, 41, 51). Furthermore, amino acids in animal sources have also been shown to stimulate the mammalian target of rapamycin (mTOR), which can be linked to excessive adipogenesis (51, 52). In addition to the stimulation of IGF-I, it has been postulated that milk proteins may have insulinotropic properties that promote insulin production and insulin resistance (14). Moreover, proteins are rarely consumed in isolation but often with other macronutrients. Nondairy animal protein (i.e., meat) is often found in foods that are high in fat, which could potentially result in BW gain when consumed in high amounts (24). In contrast, plant protein has a lower leucine content than animal protein, which potentially may help explain the lack of an association between plant protein intake and obesity (24, 53). In contrast, plant protein sources are often found in foods that are high in fiber. Evidence has linked fiber to better satiety and a healthier gut microbiota, which may protect against the development of obesity (24, 54, 55).
This is the first review that systematically identified and assessed prospective cohort studies that examined the association between intakes of total protein and protein from different sources during infancy and obesity outcomes in childhood and adolescence. Most of the included studies were of high quality with a large sample size and long duration of follow-up (14, 24, 26, 27, 29, 31, 32, 34–37). Most studies utilized a validated FFQ and WFR to measure dietary intake (14, 24–38). Some studies assessed protein intake at multiple time points during infancy (14, 25–27, 34). Moreover, protein intake was assessed as both continuous and categorical variables to evaluate potential linear dose–responses versus nonlinear relations (14, 25, 26, 31, 37, 38), Several studies used a longitudinal design with repeated follow-ups that enabled the assessment of the association between protein intake and longitudinal obesity trajectories, increasing the reliability and robustness of result (24, 29–32). A range of obesity outcomes, such as FMI, FFMI, and %BF, rather than solely BMI, were examined (14, 24–37).
The included studies are subject to several important limitations that warrant discussion. The observational nature of all of the included studies cannot infer causal relations, although some studies attempted to adjust for a wide range of potential confounding factors such as child sex, child birth weight, maternal BMI, breastfeeding status, and total energy intake (14, 24–38). Given that obesity is a multifactorial health condition, unmeasured and residual confounding from other factors is possible. Comparison between participants and dropouts was made in some studies to address potential attrition bias (24, 25, 34). The 16 included studies reported findings from 9 cohorts, highlighting that further investigation in other population groups is warranted. Furthermore, studies were reported from high-income countries only, therefore limiting the ability to provide a comprehensive view of infant protein intake and obesity across global demographics (14, 24–38). The generalizability of the findings to low- or middle-income countries remains unclear. Lastly, assessment of obesity outcomes was self-reported in some studies (30, 33, 38). Reporting in a socially desirable way is common and may influence the results of these studies. It is noteworthy that there was large heterogeneity in study designs and measures across the included studies, making it hard to generalize the findings. There were also variations in age at both protein intake and outcome assessments as well as insufficient reporting of effects in some studies. Finally, limited studies explored the existence of a linear versus nonlinear relation between protein intake and obesity outcome, hindering the determination of an optimal intake level for protein intake during infancy.
The present systematic review provides valuable evidence for the association between protein intake during infancy and later obesity in childhood and adolescence. The findings could contribute to evaluation of recommendations for optimal protein intake for infants aged 0–24 mo. Due to the dearth of protein intake data during infancy and limited evaluation of the long-term effects of protein intake on later health, there is currently no standardized, global recommendation for protein intake during infancy (56–58). For infants aged 7–12 mo in Australia there is an Adequate Intake set at 14 g/d (57). In the United States there is an RDA of 11 g (58). The protein Reference Nutrient Intake in the United Kingdom from 7 to 12 mo ranges from 13.7 to 14.9 g/d (56). The evaluation and refinement of infancy protein intake recommendations would facilitate health care professionals to deliver evidence-based advice for protein intake during infancy and guide parents to make more healthful choices for their children in relation to the consumption of protein-containing foods. Consequently, this may lessen the development of obesity as well as the morbidity and mortality associated with overweight and obesity (59). In addition, knowledge on optimal protein intake during infancy for obesity prevention would inform the regulation of protein content in infant formulas or baby foods and facilitate the revision/development of food-based dietary guidelines for this age group (60, 61).
Although the existing research provides valuable insights into the significant influence of increased protein intake in infancy on BMI measures in childhood and adolescence, additional research is warranted. Rigorously designed prospective cohort studies and randomized controlled trials with long follow-up periods into later childhood and adolescence are needed to assess the long-term impact of protein intake in infancy and later obesity development. In addition, further studies should explore the potential linearity of the relation between infancy protein intake and obesity and how protein intake from different sources influences obesity development as well as the underlying mechanisms. To ensure the generalizability of findings to the wider population, additional studies in a diverse range of populations, ethnicities, and demographics are required.
In conclusion, consistent with existing narrative reviews, the present systematic review found that total protein intake in the first 2 y of life was associated with higher BMI in childhood and adolescence. Moreover, higher intake of animal protein, but not plant protein, during infancy was associated with higher BMI in childhood and adolescence. The totality of evidence for total protein intake and protein sources during infancy and other obesity outcomes remains inconclusive. Future research with rigorously designed studies in wider population groups is needed to further assess the long-term impact of protein intake in infancy and sources on later obesity development as well as the underlying mechanisms. Such information is fundamental for informing future evaluation of infant feeding guidelines and protein nutrient recommended intakes.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the contribution of Grace Parker, Amy Lilly, Kelly Bramble, Celeste Costanzo, and Jemima Tomlin for the literature search and study screening and extraction. The authors’ responsibilities were as follows—MZ and KJC: conceived the study; AS: conducted the literature search and performed study screening and extraction; MZ and H-JY: assisted with the study extraction; H-JY and MZ: assessed the study quality; GA: conducted the meta-analysis; AS and MZ: wrote the manuscript; AS, MZ, EAS-G,Q-QH, and KJC: interpreted the study findings; and all authors: critically reviewed and read and approved the final manuscript.
Notes
MZ is supported by the Australian National Health Medical Research Council Early Career Research Fellowship.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: BW, body weight; DONALD, Dortmund Nutritional and Anthropometric Longitudinal Design; FFMI, fat-free mass index; FMI, fat mass index; IGF-I, insulin like growth factor I; SDS, SD score; WFR, weighed food record; %BF, percentage of body fat; %E, percentage of total energy intake.
Contributor Information
Alexandra Stokes, School of Exercise and Nutrition eSciences, Deakin University, Geelong, Australia.
Karen J Campbell, Institute for Physical Activity and Nutrition, School of Exercise and Nutrition Sciences, Deakin University, Geelong, Australia.
Hong-Jie Yu, School of Health Sciences, Wuhan University, Wuhan, China.
Ewa A Szymlek-Gay, Institute for Physical Activity and Nutrition, School of Exercise and Nutrition Sciences, Deakin University, Geelong, Australia.
Gavin Abbott, Institute for Physical Activity and Nutrition, School of Exercise and Nutrition Sciences, Deakin University, Geelong, Australia.
Qi-Qiang He, School of Health Sciences, Wuhan University, Wuhan, China.
Miaobing Zheng, Institute for Physical Activity and Nutrition, School of Exercise and Nutrition Sciences, Deakin University, Geelong, Australia.
References
- 1.World Health Organization . Obesity and overweight. [Internet]2020. [cited 10 Sep 2020]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [Google Scholar]
- 2.Lu L, Xun P, Wan Y, He K, Cai W. Long-term association between dairy consumption and risk of childhood obesity: a systematic review and meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2016;70(4):414–23. [DOI] [PubMed] [Google Scholar]
- 3.Brands B, Demmelmair H, Koletzko B; EarlyNutrition Project.. How growth due to infant nutrition influences obesity and later disease risk. Acta Paediatr. 2014;103(6):578–85. [DOI] [PubMed] [Google Scholar]
- 4.Lucas A. Programming by early nutrition: an experimental approach. J Nutr. 1998;128(2):401S–6S. [DOI] [PubMed] [Google Scholar]
- 5.Pietrobelli A, Agosti M. Nutrition in the first 1000 days: ten practices to minimize obesity emerging from published science. Int J Environ Res Public Health. 2017;14(12):1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Health and Medical Research Council . Australian Dietary Guidelines. [Internet]2013. [cited 16 Sep 2020]. Available from: https://www.eatforhealth.gov.au/guidelines. [Google Scholar]
- 7.Tang M. Protein intake during the first two years of life and its association with growth and risk of overweight. Int J Environ Res Public Health. 2018;15(8):1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lind MV, Larnkjær A, Mølgaard C, Michaelsen KF. Dietary protein intake and quality in early life: impact on growth and obesity. Curr Opin Clin Nutr Metab Care. 2017;20(1):71–6. [DOI] [PubMed] [Google Scholar]
- 9.Rolland-Cachera MF, Akrout M, Péneau S. Nutrient intakes in early life and risk of obesity. Int J Environ Res Public Health. 2016;13(6):564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michaelsen KF, Greer FR. Protein needs early in life and long-term health. Am J Clin Nutr. 2014;99(3):718S–22S. [DOI] [PubMed] [Google Scholar]
- 11.Weng SF, Redsell SA, Nathan D, Swift JA, Yang M, Glazebrook C. Estimating overweight risk in childhood from predictors during infancy. Pediatrics. 2013;132(2):e414–21. [DOI] [PubMed] [Google Scholar]
- 12.Zheng M, Lamb KE, Grimes C, Laws R, Bolton K, Ong KK, Campbell K. Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta-analysis of evidence. Obes Rev. 2018;19(3):321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appleton J, Russell CG, Laws R, Fowler C, Campbell K, Denney-Wilson E. Infant formula feeding practices associated with rapid weight gain: a systematic review. Matern Child Nutr. 2018;14(3):e12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Günther AL, Remer T, Kroke A, Buyken AE. Early protein intake and later obesity risk: which protein sources at which time points throughout infancy and childhood are important for body mass index and body fat percentage at 7 y of age?. Am J Clin Nutr. 2007;86(6):1765–72. [DOI] [PubMed] [Google Scholar]
- 15.Garden FL, Marks GB, Almqvist C, Simpson JM, Webb KL. Infant and early childhood dietary predictors of overweight at age 8 years in the CAPS population. Eur J Clin Nutr. 2011;65(4):454–62. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . Protein and amino acid requirements in human nutrition: report of a Joint FAO/WHO/UNU Expert Consultation. [Internet]. 2007. [cited 17 Sep 2020]. Available from: https://apps.who.int/iris/bitstream/handle/10665/43411/WHO_TRS_935_eng.pdf?ua=1. [PubMed] [Google Scholar]
- 17.Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein—its role in satiety, energetics, weight loss and health. Br J Nutr. 2012;108(Suppl 2):S105–S12. [DOI] [PubMed] [Google Scholar]
- 18.Abrams SA, Hawthorne KM, Pammi M. A systematic review of controlled trials of lower-protein or energy-containing infant formulas for use by healthy full-term infants. Adv Nutr. 2015;6(2):178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patro-Gołąb B, Zalewski BM, Kouwenhoven SM, Karaś J, Koletzko B, Bernard van Goudoever J, Szajewska H. Protein concentration in milk formula, growth, and later risk of obesity: a systematic review. J Nutr. 2016;146(3):551–64. [DOI] [PubMed] [Google Scholar]
- 20.Scottish Intercollegiate Guidelines Network . Methodology checklist 3: cohort studies. In: SIGN 50: a guideline developers’ handbook. Edinburgh (UK): The Network; 2012. [Google Scholar]
- 21.Bai A, Shukla V, Bak G, Wells G. Quality assessment tool project report. Ottawa (Canada): Canadian Agency for Drugs and Technologies in Health; 2012. [Google Scholar]
- 22.West S, King V, Carey TS, Lohr KN, McKoy N, Sutton SF, Lux L. Systems to rate the strength of scientific evidence. Rockville (MD): Agency for Healthcare Research and Quality; 2002. [PMC free article] [PubMed] [Google Scholar]
- 23.National Heart, Lung, and Blood Institute . Study quality assessment tools. [Internet]. 2020.; [cited 16 Sep 2020]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. [Google Scholar]
- 24.Braun KV, Erler NS, Kiefte-de Jong JC, Jaddoe VW, van den Hooven EH, Franco OH, Voortman T. Dietary intake of protein in early childhood is associated with growth trajectories between 1 and 9 years of age. J Nutr. 2016;146(11):2361–7. [DOI] [PubMed] [Google Scholar]
- 25.Gunnarsdóttir I, Thorsdottir I. Relationship between growth and feeding in infancy and body mass index at the age of 6 years. Int J Obes. 2003;27(12):1523–7. [DOI] [PubMed] [Google Scholar]
- 26.Günther A, Buyken A, Kroke A.. The influence of habitual protein intake in early childhood on BMI and age at adiposity rebound: results from the DONALD study. Int J Obes. 2006;30(7):1072–9. [DOI] [PubMed] [Google Scholar]
- 27.Gunther AL, Buyken AE, Kroke A. Protein intake during the period of complementary feeding and early childhood and the association with body mass index and percentage body fat at 7 y of age. Am J Clin Nutr. 2007;85(6):1626–33. [DOI] [PubMed] [Google Scholar]
- 28.Hoppe C, Mølgaard C, Thomsen BL, Juul A, Michaelsen KF. Protein intake at 9 mo of age is associated with body size but not with body fat in 10-y-old Danish children. Am J Clin Nutr. 2004;79(3):494–501. [DOI] [PubMed] [Google Scholar]
- 29.Jen V, Braun KV, Karagounis LG, Nguyen AN, Jaddoe VW, Schoufour JD, Franco OH, Voortman T. Longitudinal association of dietary protein intake in infancy and adiposity throughout childhood. Clin Nutr. 2019;38(3):1296–302. [DOI] [PubMed] [Google Scholar]
- 30.Morgen CS, Ängquist L, Baker JL, Andersen A-MN, Sørensen TI, Michaelsen KF. Breastfeeding and complementary feeding in relation to body mass index and overweight at ages 7 and 11 y: a path analysis within the Danish National Birth Cohort. Am J Clin Nutr. 2018;107(3):313–22. [DOI] [PubMed] [Google Scholar]
- 31.Pimpin L, Jebb S, Johnson L, Wardle J, Ambrosini GL. Dietary protein intake is associated with body mass index and weight up to 5 y of age in a prospective cohort of twins, 2. Am J Clin Nutr. 2016;103(2):389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pimpin L, Jebb SA, Johnson L, Llewellyn C, Ambrosini GL. Sources and pattern of protein intake and risk of overweight or obesity in young UK twins. Br J Nutr. 2018;120(7):820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scaglioni S, Agostoni C, De Notaris R, Radaelli G, Radice N, Valenti M, Giovannini M, Riva E. Early macronutrient intake and overweight at five years of age. Int J Obes. 2000;24(6):777–81. [DOI] [PubMed] [Google Scholar]
- 34.Thorisdottir B, Gunnarsdottir I, Palsson GI, Halldorsson TI, Thorsdottir I. Animal protein intake at 12 months is associated with growth factors at the age of six. Acta Paediatr. 2014;103(5):512–17. [DOI] [PubMed] [Google Scholar]
- 35.Thorisdottir B, Gunnarsdottir I, Thorisdottir AV, Palsson GI, Halldorsson TI, Thorsdottir I. Nutrient intake in infancy and body mass index at six years in two population-based cohorts recruited before and after revision of infant dietary recommendations. Ann Nutr Metab. 2013;63(1-2):145–51. [DOI] [PubMed] [Google Scholar]
- 36.Voortman T, Braun K, Kiefte-de Jong J, Jaddoe V, Franco O, Van Den Hooven E. Protein intake in early childhood and body composition at the age of 6 years: the Generation R Study. Int J Obes. 2016;40(6):1018–25. [DOI] [PubMed] [Google Scholar]
- 37.Voortman T, van den Hooven EH, Tielemans MJ, Hofman A, Kiefte-de Jong JC, Jaddoe VW, Franco OH. Protein intake in early childhood and cardiometabolic health at school age: the Generation R Study. Eur J Nutr. 2016;55(6):2117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weijs PJ, Kool LM, van Baar NM, van der Zee SC. High beverage sugar as well as high animal protein intake at infancy may increase overweight risk at 8 years: a prospective longitudinal pilot study. Nutr J. 2011;10(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willett W. Nutritional epidemiology. Oxford (UK): Oxford University Press; 2012. [Google Scholar]
- 40.Ryan R; Cochrane Consumers and Communication Review Group . Cochrane Consumers and Communicaton Group: meta-analysis; La Trobe University, Melbourne [Internet]. Published 1 December 2016. [cited 20 Oct 2020]. Available from: http://cccrg.cochrane.org/sites/cccrg.cochrane.org/files/public/uploads/meta-analysis_revised_december_1st_1_2016.pdf.
- 41.Luque V, Closa-Monasterolo R, Escribano J, Ferré N.. Early programming by protein intake: the effect of protein on adiposity development and the growth and functionality of vital organs. Nutr Metab Insights. 2016;8(Supp1 1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patro-Gołąb B, Zalewski BM, Kołodziej M, Kouwenhoven S, Poston L, Godfrey KM, Koletzko B, van Goudoever JB, Szajewska H. Nutritional interventions or exposures in infants and children aged up to 3 years and their effects on subsequent risk of overweight, obesity and body fat: a systematic review of systematic reviews. Obes Rev. 2016;17(12):1245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hörnell A, Lagström H, Lande B, Thorsdottir I. Protein intake from 0 to 18 years of age and its relation to health: a systematic literature review for the 5th Nordic Nutrition Recommendations. Food Nutr Res. 2013;57(1):21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koletzko B, Demmelmair H, Grote V, Prell C, Weber M. High protein intake in young children and increased weight gain and obesity risk. Oxford (UK): Oxford University Press; 2016. [DOI] [PubMed] [Google Scholar]
- 45.Kong AP, Choi KC, Wong GW, Ko GT, Ho CS, Chan MH, Ozaki R, Ma RC, Lau JT, Chan JC. Serum concentrations of insulin-like growth factor-I, insulin-like growth factor binding protein-3 and cardiovascular risk factors in adolescents. Ann Clin Biochem. 2011;48(3):263–9. [DOI] [PubMed] [Google Scholar]
- 46.Michaelsen KF, Larnkjaer A, Molgaard C. Early diet, insulin-like growth factor-1, growth and later obesity. World Rev Nutr Diet. 2013;106:113–18. [DOI] [PubMed] [Google Scholar]
- 47.Ong KK, Elmlinger M, Jones R, Emmett P, Holly J, Ranke MB, Dunger DB; ALSPAC Study Team. . Growth hormone binding protein levels in children are associated with birth weight, postnatal weight gain, and insulin secretion. Metabolism. 2007;56(10):1412–17. [DOI] [PubMed] [Google Scholar]
- 48.Koletzko B, Broekaert I, Demmelmair H, Franke J, Hannibal I, Oberle D, Schiess S, Baumann BT, Verwied-Jorky S; E.U. Childhood Obesity Project . Protein intake in the first year of life: a risk factor for later obesity?. Adv Exp Med Biol. 2005;569:69–79. [DOI] [PubMed] [Google Scholar]
- 49.Putet G, Labaune J-M, Mace K, Steenhout P, Grathwohl D, Raverot V, Morel Y, Picaud J-C. Effect of dietary protein on plasma insulin-like growth factor-1, growth, and body composition in healthy term infants: a randomised, double-blind, controlled trial (Early Protein and Obesity in Childhood (EPOCH) study). Br J Nutr. 2016;115(2):271–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madsen AL, Larnkjær A, Mølgaard C, Michaelsen KF. IGF-I and IGFBP-3 in healthy 9 month old infants from the SKOT cohort: breastfeeding, diet, and later obesity. Growth Horm IGF Res. 2011;21(4):199–204. [DOI] [PubMed] [Google Scholar]
- 51.Laursen MF, Andersen LB, Michaelsen KF, Mølgaard C, Trolle E, Bahl MI, Licht TR. Infant gut microbiota development is driven by transition to family foods independent of maternal obesity. Msphere. 2016;1(1):e00069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Curr Biol. 2009;19(22):R1046–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melnik BC. The potential mechanistic link between allergy and obesity development and infant formula feeding. Allergy Asthma Clin Immunol. 2014;10(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization . Infant and young child feeding: model chapter for textbooks for medical students and allied health professionals. Geneva (Switzerland): World Health Organization; 2009. [PubMed] [Google Scholar]
- 55.McCarty M. Vegan proteins may reduce risk of cancer, obesity, and cardiovascular disease by promoting increased glucagon activity. Med Hypotheses. 1999;53(6):459–85. [DOI] [PubMed] [Google Scholar]
- 56.British Nutrition Foundation . Nutrition requirement. [Internet]. 2016; [updated October 2016; cited 18 Sep 2020]. Available from: https://www.nutrition.org.uk/attachments/article/234/Nutrition%20Requirements_Revised%20Oct%202016.pdf. [Google Scholar]
- 57.National Health and Medical Research Council . Nutrient Reference Values for Australia and New Zealand: protein. [Internet]. 2006; [updated 9 April 2014; cited 18 Sep 2020]. Available from: https://www.nrv.gov.au/nutrients/protein. [Google Scholar]
- 58.US Department of Agriculture . Infant nutrition and feeding guide. [Internet]. 2019; [cited 18 Sep 2020]. Available from: https://wicworks.fns.usda.gov/resources/infant-nutrition-and-feeding-guide. [Google Scholar]
- 59.National Health and Medical Research Council . Eat for health educator guide. [Internet]. 2013; [updated February 2013; cited 17 Sep 2020]. Available from: https://www.eatforhealth.gov.au/sites/default/files/content/The%20Guidelines/n55b_educator_guide_140321_1.pdf. [Google Scholar]
- 60.National Health and Medical Research Council . Eat for health infant feeding guidelines: summary. Canberra (Australia): National Health and Medical Research Council; 2012. [Google Scholar]
- 61.Lung T, Baur LA, Bauman A, Hayes A. Can reducing childhood obesity solve the obesity crisis in Australia?. Obesity. 2020;28(5):857–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.