Abstract
The enormous burden of diet-related chronic diseases has prompted interest in healthy food prescription programs. Yet, the impact of such programs remains unclear. The aim of this study was to conduct a systematic review of healthy food prescription programs and evaluate their impact on dietary behavior and cardiometabolic parameters by meta-analysis. A systematic search was carried out in Medline, Embase, Scopus, and Cochrane Central Register of Controlled Trials databases since their inception to 3 January, 2020 without language restriction. A systematic search of interventional studies investigating the effect of healthy food prescription on diet quality and/or cardiometabolic risk factors including BMI, systolic (SBP) and diastolic blood pressure (DBP), glycated hemoglobin (HbA1c), or blood lipids was carried out. Thirteen studies were identified for inclusion, most of which were quasi-experimental (pre/post) interventions without a control group (n = 9). Pooled estimates revealed a 22% (95% CI: 12, 32; n = 5 studies, n = 1039 participants; I2 = 97%) increase in fruit and vegetable consumption, corresponding to 0.8 higher daily servings (95% CI: 0.2, 1.4; I2 = 96%). BMI decreased by 0.6 kg/m2 (95% CI: 0.2, 1.1; I2 = 6.4%) and HbA1c by 0.8% (95% CI: 0.1, 1.6; I2 = 92%). No significant change was observed in other cardiometabolic parameters. These findings should be interpreted with caution in light of considerable heterogeneity, methodological limitations of the included studies, and moderate to very low certainty of evidence. Our results support the need for well-designed, large, randomized controlled trials in various settings to further establish the efficacy of healthy food prescription programs on diet quality and cardiometabolic health.
Keywords: food is medicine, chronic diseases, global burden of disease, food policy, nutrition, diet, food pharmacy, food insecurity, culinary medicine, public health
Statement of Significance: This is the first systematic review and meta-analysis to evaluate the impact of healthy food prescription programs on dietary behavior and cardiometabolic parameters.
Introduction
A poor-quality diet is a leading risk factor for noncommunicable diseases worldwide (1, 2), with 1 of every 5 deaths across the globe attributable to a suboptimal diet (3). Furthermore, diet-related diseases including obesity, diabetes, and cardiovascular disease place a tremendous financial burden on healthcare systems (4–6), with costs projected to rise over the coming decades (7, 8). Food insecurity, defined as lack of access to nutritionally adequate food, is associated with the greater consumption of inexpensive nutrient-poor foods (9–14), lower intake of fruit and vegetables and other healthy foods (15, 16), and higher risk of cardiometabolic diseases. Food insecurity is also linked to lower self-efficacy in managing chronic diseases owing to mental and financial strains, such as high costs of medications and other out-of-pocket healthcare expenses (17–20).
Based on the critical roles of poor diet quality and food insecurity in chronic disease, there is a growing interest in incorporating “food is medicine” interventions into healthcare systems to provide healthy foods as a treatment of vulnerable patients (21). One approach gaining momentum is “produce prescription,” whereby a physician or healthcare worker identifies patients, based on disease and/or food security criteria, eligible to receive free or discounted healthy produce. Eligibility criteria typically include a food insecurity or low-income criterion, and a diet-related health condition criterion such as the presence of diabetes, obesity, and/or hypertension. Patients are provided subsidized or free healthy foods, with uptake options including redemption of prescribed coupons at local food stores, or provision of fresh produce at the healthcare center or delivered to the home (22, 23).
Despite the rapidly growing interest in healthy food prescription programs by governments, payers, and healthcare providers, the impact of such programs on dietary behavior and cardiometabolic risk factors has not been systematically evaluated. Some individual studies have reported increases in participants’ awareness of healthy dietary behaviors (24, 25), with mixed findings for actual dietary behaviors and/or cardiometabolic risk profiles (26–28). The aim of the present work was to perform a systematic review and meta-analysis of interventional studies that evaluated healthy food “prescription” programs to gain insight into study designs, intervention types, and their effects on participants’ dietary behavior and cardiometabolic risk factors.
Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered on PROSPERO (CRD42020162553).
Search strategy
A systematic literature search up to 3 January, 2020 was conducted in Medline, Embase, Scopus, and Cochrane Central Register of Controlled Trials since their inception without language restriction using the following search terms: “fruit” and “vegetable” or “produce” or “food” adj3 “prescription” or “voucher” or “incentive” or “program” and “blood pressure” or “weight” or “body mass index” or “BMI” or “waist circumference” or “glucose” or “A1c” or “lipid” or “LDL” or “HDL” or “triglycerides” or “consum*” or “intake.” Reference lists of eligible studies were manually scanned to identify additional relevant publications.
Eligibility criteria, search strategy, and data extraction
Interventional studies that investigated the impact of healthy food prescription programs on dietary behavior and/or cardiometabolic risk factors including BMI, systolic (SBP) and diastolic blood pressure (DBP), glycated hemoglobin (HbA1c), or blood lipids in participants aged 18 y or older were eligible for inclusion. Our preliminary literature review revealed that patient populations that were often the target of healthy food prescription programs were those with type 2 diabetes mellitus, hypertension, and/or cardiovascular disease. We therefore wanted to assess how healthy food prescription interventions would impact well-established clinical markers of cardiometabolic disease risk that were most commonly reported by studies in the field. Included studies were either quasi-experimental (pre/post with or without an external control group) or randomized controlled trials (RCTs). In addition, healthy food prescription programs had to be integrated into the healthcare system, with patients identified and referred by a healthcare provider or other allied health staff member (e.g., dietitian). Studies involving pregnant or breastfeeding women, those investigating only financial or economic implications, and those only examining patient knowledge and attitudes, or ethical considerations were excluded. Observational studies, school-based food programs and government-led food security programs not linked to healthcare systems and workers and not administered with the primary purpose of tackling health outcomes were excluded. Qualitative studies that did not measure changes in dietary behavior or cardiometabolic risk factors were excluded, as were commentary or opinion pieces. Two reviewers (SB and either KT or DC) independently screened studies for eligibility and extracted the following data into prepiloted forms: period of data collection, study location and setting, study design, inclusion criteria, sample size, average participant age, proportion of female participants, duration of follow-up, program details, participation rate, effect size estimates, and data required to calculate variance of effect estimates (CIs, SEs, or P values). As studies were conducted in different countries and years, the value of the reported incentive offered to participants was converted into a standardized United States Dollar (USD)-equivalent amount adjusted for inflation to January 2020. We assessed the quality of RCTs using version 2 of Cochrane's Risk of Bias tool (RoB2) (29) and nonrandomized studies using Cochrane's Risk of Bias in Non-Randomised Studies-of Intervention (ROBINS-I) tool (30). Disagreements were resolved by consensus or via involvement of a third reviewer (JHYW). The overall certainty of evidence was evaluated using the Grades of Recommendation, Assessment, Development, and Evaluation Working Group (GRADE) framework (31).
Statistical analysis
The primary outcomes were changes in dietary behavior and cardiometabolic risk factors due to healthy food prescription programs, standardized as percent differences from either study baseline or compared with external control groups. For studies without a separate comparison group, we evaluated the pre/post difference. For RCTs (including parallel intervention and crossover studies) and studies with a separate comparison group, we evaluated the difference at the end of the study between the intervention and control group if only postintervention data were available, and the difference-in-difference (end-study differences between the treatment and control group accounting for baseline) if both baseline and follow-up data were available. The SEs of the percent differences in outcome data were calculated as described previously (32).
Meta-analysis
Study-specific effect estimates were pooled using inverse-variance weighted random effects meta-analysis, according to the method of DerSimonian and Laird (33). Pooled effects are presented as percent difference (PD), indicating percentage change compared with baseline (for pre/post study design) or compared with a control group (for RCT or crossover study design), or as absolute change in the respective outcome measures. For the 1 study (34) that did not report measures of uncertainty (SD or SE) or statistics to derive these parameters, the SD was imputed from the pooled SD of the other studies included in the meta-analysis (35). Sensitivity analysis was performed by excluding this study from the meta-analysis to ascertain the impact of such an approach to the overall results. The I2 statistic was used to assess the heterogeneity of included studies, with values <25%, 25 to 50%, and >50% corresponding to low, moderate, and high degrees of heterogeneity, respectively (36). Publication bias was assessed by visual inspection of funnel plots and statistically using Egger's and Begg's tests. Too few studies were identified to allow stratified analyses and investigation of sources of heterogeneity by metaregression. Data are presented as mean ± SD or mean (95% CI) unless stated otherwise. All statistical analyses were conducted in STATA version 16 (Stata Corp), with 2-tailed α of 0.05.
Results
Study characteristics
Of 1074 studies identified by our search strategy, 13 met inclusion and exclusion criteria and were included for analysis (Figure 1 and Table 1) (24–27, 34, 37–44). Thirty-seven studies were excluded after reviewing the full texts (23, 28, 45–79). Most studies (n = 11) were conducted in the USA, 1 was conducted in France, and 1 in the UK. About half (n = 8) of the recruited participants experiencing food insecurity, and three-quarters (n = 9) of the patients had specific existing cardiometabolic conditions including overweight or obesity, hypertension, or type 2 diabetes mellitus. The most common study designs were pre/post intervention studies without a control group (n = 9) and pre/post intervention study with an externally matched control group (n = 1), followed by RCTs (n = 3). Most studies recruited middle-aged to older participants (mean age ranged between 45 and 60 y) and had a median sample size of 79 (range, n from 9 to 687). The median follow-up duration was 6 mo, and 3 studies had extended follow-up ranging from 12 to 18 mo. Amongst the 10 nonrandomized studies, 7 were deemed to have serious risk of bias and the remaining 3 were deemed to have critical risk of bias due to confounding (Supplemental Table 1). Of the 3 RCTs, 2 were deemed to have high risk of bias whereas the other was categorized as having some methodological concerns (Supplemental Table 2). The primary sources of funding were academic grants (n = 5), not-for-profit organizations (n = 3), government social support programs including food banks (n = 2), insurance companies (n = 2), and pharmaceutical companies (n = 1).
FIGURE 1.
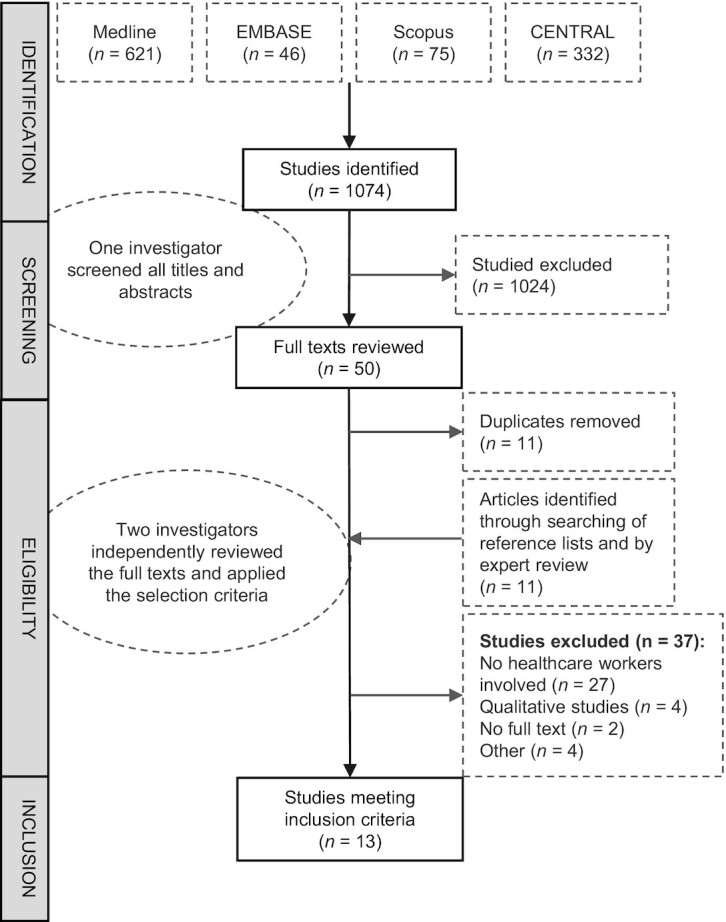
Flow diagram for the screening and inclusion of publications in the systematic review.
TABLE 1.
Characteristics of the healthy food prescription studies included in the systematic review1
| Study | Country | Setting | Design | Medical inclusion criteria | Food insecurity criteria | n | Age,2 y | % Female | Follow-up (mo) | RoB3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Bihan (37) | France | Healthcare center | RCT | None | Yes | 135 | 44.6 ± 8.1 | 56.3 | 3 | Some concerns |
| Buyuktuncer (24) | UK | Primary care clinics | Pre/post | None | No | 124 | ≥ 16 | 71.8 | 5 | Serious |
| Weinstein (38) | USA | Primary care or diabetes clinics | RCT | Diabetes mellitus, BMI >25 kg/m2 and HbA1c >7% | No | 79 | 55 ± 10.8 | 69 | 3 | High |
| Seligman (43) | USA | Primary care clinics | Pre/post | HbA1c ≥ 6.5% or previously diagnosed diabetes | Yes | 687 | 56.6 | 74 | 6 | Critical |
| Bryce (26) | USA | Healthcare center | Pre/post | Diabetes mellitus and HbA1c >6.5% | No | 65 | 52.5 ± 10.6 | 70.8 | 3.25 | Serious |
| Cavanagh (27) | USA | Healthcare center | Retrospective case-control | Diabetes mellitus, BMI >30 kg/m2 and/or hypertension | No | 108 | NR | NR | 18 | Serious |
| Feinberg (34) | USA | Healthcare center | Pre/post | Diabetes mellitus and HbA1c > = 8% | Yes | 95 | NR | NR | 18 | Critical |
| Izumi (39) | USA | Healthcare center | Pre/post | None | No | 9 | NR | 100 | 5.75 | Serious |
| Trapl (40) | USA | Healthcare center | Pre/post | Hypertension | Yes | 137 | 60.3 ± 10.9 | 71.1 | 6 | Serious |
| Emmert-Aronson (41) | USA | Healthcare center | Pre/post | Diabetes mellitus, prediabetes, cardiac disease, hypertension, dyslipidemia, obesity, anxiety, and/or depression | Yes | 49 | 59.1 ± 10.6 | 63.3 | 6 | Serious |
| Forbes (25) | USA | Healthcare center | Pre/post | At risk of chronic illness or metabolic disease, as determined by primary care physicians | Yes | 9 | NR | 55.6 | 1.5 | Serious |
| Orsega-Smith (42) | USA | Pediatrician clinics | Pre/post | Overweight | Yes | 41 | NR | NR | 12 | Serious |
| Ferrer (44) | USA | Primary care clinic | RCT | HbA1c >9% | Yes | 58 | 54 | 62 | 7 | High |
HbA1c, glycated hemoglobin; NR, not reported; RCT, randomized controlled trial; RoB, risk of bias.
2Values are mean ± SD.
3Detailed derivation of the overall risk of bias is provided in Supplemental Table 1.
Key design features of the intervention programs
Primary care physicians were the exclusive referring healthcare providers in 5 studies; the remaining studies employed other members of the healthcare team, with or without primary care providers, to prescribe healthy food interventions (Table 2). Most studies (n = 9) utilized food subsidies as the means to provide access to healthy foods, although the amount subsidized varied widely from USD 14 to 189 per month, lasting from 1 to 6 mo. Four studies gave participants varying amounts of food supplies at no cost. For studies that provided subsidies, participants were able to redeem vouchers at various locations, most commonly at local supermarkets or farmers’ markets ( n = 7), although other studies chose less conventional settings such as mobile fresh food produce vans or food pantries located within a healthcare center (n = 4). The most commonly prescribed foods were fruits and/or vegetables (n = 10); 3 studies further incorporated other foods such as whole grains and lean proteins into their list of redeemable products. In addition to monetary incentives, most studies incorporated other intervention components into their programs such as dietary education classes (n = 9). Completion rate, calculated as the proportion of individuals who agreed to participate in the program and completed it, ranged from 15 to 100% with a median completion rate of 68%.
TABLE 2.
Key design features of the healthy produce prescription studies included in the systematic review
| Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Referrer | Type | Description | Duration | Foods included | Location | Additional components | Completion rate (%) |
| Bihan (37) | Dietitian | Subsidy | 17 to 67 USD1/mo2 | 3 mo | Fruits and vegetables | Supermarkets | Dietary advice | 45 |
| Buyuktuncer (24) | General practitioners, nurses, health visitors, and midwives | Subsidy | Discount of 3 USD for every 8 USD spent per transaction | 1 mo | Fruits and vegetables | Supermarket | Dietary advice and cooking sessions | 43.5 |
| Weinstein (38) | Primary care physicians | Subsidy | 30 USD/mo | 3 mo | Fruits and vegetables | Farmers' market | Dietary advice | 98.7 |
| Seligman (43) | Primary care physicians | Food provision | Prepacked boxes of food equivalent to 18 USD per box, every 1–2 wk | 6 mo | Whole grains, lean meats, beans, fruit, vegetables, milk, yogurt, cheese, and bread | Food bank | Dietary advice and recipes | 58 |
| Bryce (26) | Community health and social services | Subsidy | 14 USD/mo | 1 mo | Fruits and vegetables | Farmers' market | None | 29 |
| Cavanagh (27) | Nutritionist | Subsidy | 35 USD/mo | 6 mo | Fruits and vegetables | Mobile fresh produce van | None | 100 |
| Feinberg (34) | Primary care physicians | Food provision | Food supplies to make 10 meals/wk for the entire family | 18 mo | Fruits and vegetables, whole grains, and lean proteins | Food pantry in a clinical center | Diabetes education | NR3 |
| Izumi (39) | Community health worker | Subsidy | Subsidized membership cost of 95 USD per share per month of community supported agriculture program | 6 mo | Vegetables | Farmers' market | Dietary advice | 36 |
| Trapl (40) | Nonphysician healthcare providers | Subsidy | 44 USD/mo | 3 mo | Fruits and vegetables | Farmers' market | None | 61 |
| Emmert-Aronson (41) | Primary care physicians, dietitians, pharmacists, social workers, and medical assistants | Subsidy | 44 USD/mo | 4 mo | Vegetables | Food pantry in a clinical center | Physical activity, mindfulness meditation general health education, nutrition education, plant-based snacks, and group coaching | 100 |
| Forbes (25) | Primary care physicians | Subsidy | 189 USD/mo | 6 mo | Fruits and vegetables | Farmers' market | Dietary advice | 90 |
| Orsega-Smith (42) | Pediatricians | Food provision | 15–25 pounds/mo of fresh produce | 12 mo | Fruits and vegetables | Mobile fresh produce van | None | 100 |
| Ferrer (44) | Primary care physician | Food provision | 10 pounds of fresh produce biweekly | 6 | Fruits, vegetables, canned food, fish, chicken | Food pantry in clinical centre | Dietary advise and home visits by community health workers | 74 |
United States Dollar equivalent inflation adjusted to January 2020.
2The value of subsidy was adapted to family composition and depended on number of children and their caregivers.
3Not reported.
Effect of healthy food prescription on dietary outcomes
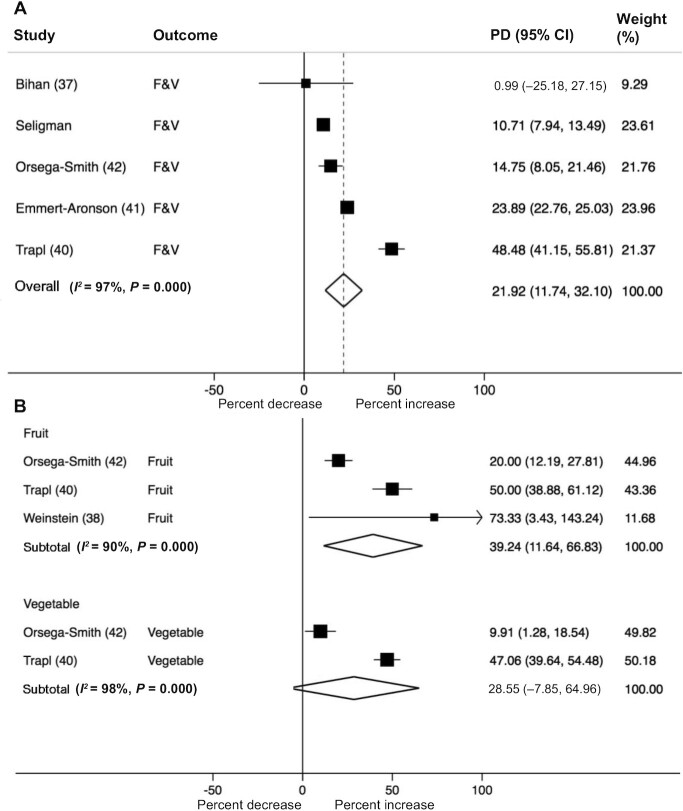
Nine studies reported dietary outcomes. Three did not report significant changes in dietary behavior following healthy food prescription programs whereas 6 reported an increase in fruit and/or vegetable intake as a result of the prescription program (Table 3). Raw pre- and postintervention data, where available, are provided in Supplemental Table 3. When results were pooled across studies, healthy food prescription programs increased daily combined fruit and vegetable intake by 22% (95% CI: 12, 32), and fruit intake by 39% (95% CI: 12, 67), with a similar but nonsignificant change in vegetable intake of 29% (95% CI: –8, 65) (Figure 2 and Table 4). This translated to an increase in combined fruit and vegetable intake of 0.8 servings/d (95% CI: 0.2, 1.4) and fruit consumption by 0.6 servings/d (95% CI: 0.3, 0.9), with a trend towards an increase in vegetable consumption by 0.5 servings/d (95% CI: −0.0, 1.1) (Table 4). There was no evidence of publication bias by Egger's and Begg's tests or by visual inspection of the funnel plots. A high level of heterogeneity was observed for each outcome (I2 >50%) but we were unable to meaningfully explore potential sources of heterogeneity due to the limited number of studies available. Excluding the study with imputed SD (34), based on pooled SD of other studies from the meta-analyses, had no substantial impact on the overall pooled estimates (data not shown). The detailed GRADE assessment for each studied outcome is presented in Supplemental Table 4. Based on the GRADE criteria, the overall certainty of evidence was rated “moderate” for treatment effect on fruit intake, “very low” for vegetable intake, and “low” for fruit and vegetable intake combined.
TABLE 3.
Summary of changes in dietary and cardiometabolic outcomes in the healthy food prescription studies included in the systematic review1
| Changes in outcomes evaluated | |||||||
|---|---|---|---|---|---|---|---|
| Study | Design | Dietary behavior | BMI | BP | Plasma lipids | HbA1c | Details2 |
| Buyuktuncer (24) | Pre/post | ND | NR | NR | NR | NR | Fruit consumption (portions/d) did not differ between baseline (median 3; range 0 to 7) and follow-up (median 2.5; range 0 to 6). Vegetable consumption (portions/d) did not differ between baseline (median 2; range 0 to 7) and follow-up (median 2; range 0 to 4). |
| Izumi (39) | Pre/post | ND | NR | NR | NR | NR | Change in frequency of vegetable intake ≥2 cups/d = 25%. |
| Trapl (40) | Pre/post | + | NR | NR | NR | NR | Change in fruit intake = 0.8 (0.6 to 1.0) servings/d. Change in vegetable intake = 0.8 (0.6 to 1.0) servings/d. |
| Forbes (25) | Pre/post | + | NR | NR | NR | NR | Change in frequency of fruit intake ≥ 1/d = 25%. Change in frequency of dark green vegetables ≥ 1/wk = 25%. Change in frequency of orange-colored vegetables ≥1/wk = 50%. Change in frequency of other vegetables ≥ 1/wk = 25%. Statistical significance not tested |
| Orsega-Smith (42) | Pre/post | + | NR | NR | NR | NR | Change in fruit intake = 0.4 (0.1 to 0.7) servings/d. Change in vegetable intake = 0.2 (–0.1 to 0.6) servings/d. |
| Bihan (37) | RCT | ND | ND | ND | ND | NR | Change in fruit and vegetable intake = 0.12 (–0.42 to 0.66) servings/d. |
| Weinstein (38) | RCT | + | NR | ND | ND | ND | Change in fruit intake = 0.5 (0.1 to 0.9) servings/d. |
| Seligman (43) | Pre/post | + | NR | NR | NR | + | Change in fruit and vegetable intake = 0.3 servings/d. Change in HbA1c = –0.15%. |
| Emmert-Aronson (41) | Pre/post | + | + | + | NR | NR | Change in fruit and vegetable intake = 1.2 (1.1 to 1.4) servings/d. Change in SBP = –6.7 (–7.5 to –6.0) mmHg. Change in BMI = –0.4 (–0.9 to 0.1) kg/m2. |
| Ferrer (44) | RCT | NR | ND | NR | NR | + | Change in HbA1c = –1.4% (–2.7, –0.1). |
| Bryce (26) | Pre/post | NR | NR | ND | NR | + | Change in HbA1c = –0.7%. |
| Cavanagh (27) | Pre/post with externally matched controls | NR | + | NR | NR | NR | Change in BMI = –1.1 (–2.0 to –0.2) kg/m2. |
| Feinberg (34) | Pre/post | NR | NR | NR | + | + | Change in HbA1c = –2.1%. |
BP, blood pressure; HbA1c, glycated hemoglobin; ND, no difference; NR, not reported; RCT, randomized controlled trial; +, indicates beneficial change in the measured parameters.
Shows raw data corresponding to outcomes that showed a beneficial change (+). For RCTs and nonrandomized trials with pre- and postmeasurements in both treatment and control groups, we assessed group differences in change (mean, 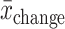 ; SEchange), using formulae [1] and [2]:
; SEchange), using formulae [1] and [2]:
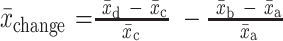 [1]
[1]
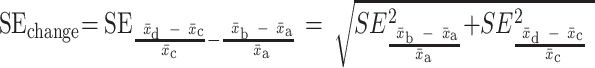 [2]
[2]
where 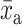 = control group mean at baseline,
= control group mean at baseline, 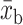 = control group mean at study end,
= control group mean at study end, 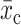 = intervention group mean at baseline, and
= intervention group mean at baseline, and 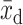 = intervention group mean at study end. For pre/post study designs without control groups, we assessed change over time with formulae [3] and [4]:
= intervention group mean at study end. For pre/post study designs without control groups, we assessed change over time with formulae [3] and [4]:
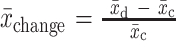 [3]
[3]
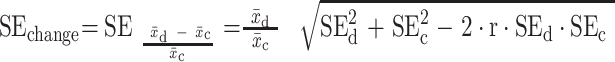 [4]
[4]
where r is the correlation coefficient within individuals, assumed to be 0.5 (80). For RCTs or nonrandomized trials with control group and measurements only at study end, we assessed group differences at the end of the study, using formulae [5] and [6]:
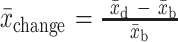 [5]
[5]
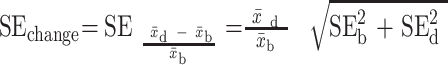 [6]
[6]
FIGURE 2.
Forest plot illustrating the change in fruit and/or vegetable intake per day (percentage difference, PD) following participation in healthy food prescription programs. Fruit and vegetable (F&V) intake was reported both as a composite variable (A) and separately (B). Data were pooled using random effects meta-analysis.
TABLE 4.
Pooled change in cardiometabolic outcomes as a result of healthy produce prescription programs1
| Percent change | Absolute change | Certainty of evidence (GRADE) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Studies (n) | Reference | Participants (n) | Estimate (95% CI) | P 2 | I2 (%) | Estimate (95% CI) | P 2 | I2 (%) | |
| Fruit and vegetable intake, servings/d3 | 5 | (37, 40–43) | 1039 | 22 (12, 32) | <0.001 | 97 | 0.79 (0.23, 1.35) | 0.004 | 96 | LOW4,5 ⊕⊕⊝⊝ |
| Fruit intake, servings/d | 3 | (38, 40, 42) | 257 | 39 (12, 67) | 0.005 | 90 | 0.59 (0.32, 0.87) | <0.001 | 59 | MODERATE6 ⊕⊕⊕⊝ |
| Vegetable intake, serving/d | 2 | (40, 42) | 178 | 29 (–8, 65) | 0.124 | 98 | 0.53 (–0.04, 1.10) | 0.068 | 87 | VERY LOW7,8,9 ⊕⊝⊝⊝ |
| SBP, mmHg | 4 | (26, 37, 38, 41) | 328 | –1.8 (–5.9, 2.3) | 0.383 | 86 | –2.39 (–7.77, 2.99) | 0.383 | 85 | VERY LOW10,11,12 ⊕⊝⊝⊝ |
| DBP, mmHg | 4 | (26, 37, 38, 41) | 328 | 0.0 (–1.2, 1.3) | 0.966 | 15 | 0.02 (–0.95, 0.99) | 0.964 | 13 | LOW10,12 ⊕⊕⊝⊝ |
| HbA1c, % | 5 | (26, 34, 38, 43, 44) | 1064 | –8.6 (–16.9, –0.35) | 0.041 | 99 | –0.81 (–1.56, –0.06) | 0.035 | 92 | VERY LOW10,13,14⊕⊝⊝⊝ |
| LDL, mM | 2 | (37, 38) | 214 | –1.1 (–12.4, 10.3) | 0.855 | 58 | –0.03 (–0.33, 0.27) | 0.856 | 50 | LOW15,16⊕⊕⊝⊝ |
| HDL, mM | 2 | (37, 38) | 214 | 2.9 (–4.9, 10.8) | 0.468 | 0 | 0.04 (–0.06, 0.14) | 0.463 | 0 | LOW15,16⊕⊕⊝⊝ |
| TG, mM | 2 | (37, 38) | 214 | 22.5 (–44.2, 89.2) | 0.509 | 52 | 0.23 (–0.44, 0.90) | 0.502 | 49 | LOW15,16⊕⊕⊝⊝ |
| BMI, kg/m2 | 3 | (27, 41, 44) | 215 | –1.6 (–2.8, –0.3) | 0.013 | 27 | –0.61 (–1.06, –0.16) | 0.008 | 6.4 | LOW17,18 ⊕⊕⊝⊝ |
DBP, diastolic blood pressure; GRADE, Grades of Recommendation, Assessment, Development, and Evaluation Working Group; HbA1c, glycated hemoglobin; RCT, randomized controlled trial; SBP, systolic blood pressure; TG, triglycerides.
P value of Z-test for significance of pooled change and 95% CI.
Where possible, fruit and vegetable intake (in servings/d) reported separately within a study was converted to combined fruit and vegetable intake by methods described previously (83) and meta-analyzed using the method described in the footnote of Table 3.
Downgraded by 1 for risk of bias: ≥4 studies non-RCTs; ≥3 studies with >10% loss to follow-up (risk of selection bias and attrition bias).
Downgraded by 1 for inconsistency: significant unexplained heterogeneity.
Downgraded by 1 for risk of bias: ≥2 studies non-RCTs; ≥1 study with >10% loss to follow-up (risk of selection and attrition bias).
Downgraded by 1 for risk of bias: ≥2 studies non-RCTs; 1 study with >10% loss to follow-up (risk of selection bias and attrition bias).
Downgraded by 1 for inconsistency: significant unexplained heterogeneity.
Downgraded by 1 for imprecision: does not meet optimal information size criterion.
Downgraded by 1 for risk of bias: ≥2 studies non-RCTs; ≥2 studies with >10% loss to follow-up (risk of selection bias and attrition bias).
Downgraded by 1 for inconsistency: significant unexplained heterogeneity.
Downgraded by 1 for imprecision: does not meet optimal information size criterion.
Downgraded by 1 for inconsistency: significant unexplained heterogeneity.
Downgraded by 1 for imprecision: does not meet optimal information size criterion.
Downgraded by 1 for risk of bias: ≥1 study with >10% loss to follow-up (risk of attrition bias).
Downgraded by 1 for imprecision: does not meet optimal information size criterion.
Downgraded by 1 for risk of bias: ≥2 studies non-RCTs (risk of selection bias).
Downgraded by 1 for imprecision: does not meet optimal information size criterion.
Effect of healthy food prescriptions on cardiometabolic outcomes
The effect of healthy food prescriptions on plasma lipids was evaluated in 2 studies, BMI in 3 studies, blood pressure in 4 studies, and HbA1c in 5 studies (Table 3 and Table 4). Pooled results identified a modest change in BMI of –1.6% (95% CI: –2.8, –0.3), which corresponded to an absolute change of –0.6 kg/m2 (95% CI: –1.1, –0.2). Likewise, a percent change in HbA1c of –8.6% (95% CI: –16.9, –0.4), corresponding to an absolute change of –0.8% (95% CI: –1.6, –0.1), was observed across studies. No significant treatment effects were observed for SBP, DBP, LDL, HDL, or triglycerides (Table 4). There was no evidence of publication bias by Egger's and Begg's tests or by visual inspection of the funnel plots. Based on the GRADE criteria, the certainty of evidence for treatment effects on cardiometabolic outcomes was rated “low” or “very low.”
Discussion
This systematic review and meta-analysis identified 13 healthy food prescription programs integrated into the healthcare system, which provided either monetary subsidies for or direct provision of fruits and vegetables as a treatment for patients, most often those experiencing food insecurity and/or with specific cardiometabolic conditions. The pooled findings suggest that these programs increase fruit and vegetable consumption and reduce BMI and HbA1c, without significant identified effects on other cardiometabolic risk factors. However, our systematic review also highlights that the findings are mostly based on nonrandomized study designs, with significant heterogeneity in the amount and duration of the food prescriptions, and with only a small number of studies evaluating cardiometabolic outcomes. Overall, these findings provide encouraging evidence that healthy food prescription programs may lead to improvements in diet quality and, even over a few months, BMI and HbA1c, with the magnitude of effect on HbA1c comparable to that achieved with commonly prescribed glucose lowering medications (81, 82). Our novel results strongly support the need for additional appropriately designed and adequately powered RCTs to test the impact of food prescription programs.
A key finding from this review is the heterogeneity in criteria pertaining to food security, household income, medical comorbidities, monetary value of subsidies or amount of fresh produce supplied, and duration of interventions. For some studies, the input of the healthcare professional(s) to the intervention and interaction with the healthcare system were not clearly described. Likewise, details of the nutrition education component of the programs were often not fully reported. Although it is possible that nutrition education acts to enhance the efficacy of food subsidies (84–86), there were insufficient data to tease out the independent or interactive effect of different components of the healthy food prescription interventions on dietary outcomes. Future studies are needed to evaluate the effectiveness of such programs in enhancing the nutritional knowledge of participants.
We also identified considerable methodological limitations and variable quality of the healthy food prescription studies. Most were quasi-experimental (nonrandomized) and did not have a control group, increasing the risk of bias and chance of overestimated findings, and precluding strong causal interpretations. Several studies were small, short-term pilot programs that were not powered to detect clinically meaningful changes in dietary or especially cardiometabolic outcomes, a limitation that could underestimate the positive impacts. These limitations are reflected in the serious or critical risk of bias identified in the nonrandomized studies and the high or concerning risk of bias in the randomized trials, and the moderate to very low strength of evidence for outcomes as determined by the GRADE assessment. These evaluations highlight the need for more rigorously designed and adequately powered RCTs to further evaluate the impact of healthy food prescription on dietary behavior and cardiometabolic outcomes. Future studies should also investigate potential sources of heterogeneity that were observed in our pooled estimates. Many of the studies did not simultaneously assess change in dietary behavior and cardiometabolic risk factors, making it difficult to ascertain whether a sufficiently large change in dietary behavior had occurred to alter the cardiometabolic parameters studied. Self-reporting of fruit and vegetable intake is subject to significant recall bias (which could overestimate effects) and measurement error (which could underestimate effects). All studies were conducted in high-income Western nations (particularly the USA), and therefore findings may not be applicable to other countries with different medical and social security systems. Finally, more food prescription studies in different countries with varying healthcare systems and dietary contexts are needed to further understand the impact of food prescription programs on diet and cardiometabolic health.
The focus of most healthy food prescriptions so far has been on fruit and vegetables. Other dietary components, including nuts, beans, whole grains, and fish are recognized as important for cardiometabolic health (87, 88), and the impact of including these dietary components remains to be evaluated. Of note, healthy food prescription programs could be more effective if they were combined with policies to address other barriers to healthy eating such as limited access to food stores, lack of cooking skills, and/or access to high-quality kitchens (80, 89–92, 93). We note that other “food is medicine” initiatives such as “medically tailored meal” programs are also being evaluated and seek to overcome these food security barriers by delivering preprepared meals to participants (76). The impact of “medically tailored meal” programs on dietary behavior and cardiometabolic outcomes also requires further evaluation.
The strengths of our investigation include its comprehensive search strategy and standardization of the reported change in dietary and cardiometabolic outcomes that enabled meta-analysis. The moderate to long durations of many of the included studies were comparable to prior evaluation of community-based nutrition intervention programs (94), and suggest healthy food prescription programs may enable sustained change in dietary behavior. Two-thirds of the individual studies, including 1 of the randomized trials, reported a positive effect on dietary outcomes over this time frame.
In conclusion, this systematic review and meta-analysis suggests that healthy food prescription programs may be beneficial in increasing consumption of fruit and vegetables and modestly reduce BMI and HbA1c. This investigation also identified substantial heterogeneity and varying methodological limitations of these studies. Our results support the need for future RCTs in a range of settings that are adequately designed and powered with appropriate comparison groups to assess robustly the efficacy of healthy food prescription programs on diet quality and cardiometabolic well-being.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ contributions were as follows—SB, MM, BN, and JHYW: contributed to research design; SB, DHC, and KT: conducted the research; SB: analyzed the data; SB, DHC, and KT: drafted the manuscript; all authors: provided critical review and feedback for the manuscript; SB: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
Sources of support: KT, BN, DC, MM, and JHYW are researchers within a National Health and Medical Research Council Centre for Research Excellence in reducing salt intake using food policy interventions (APP1117300). JHYW is supported by a University of New South Wales Scientia Fellowship. DM is supported by a NIH grant (2 R01 HL115189). DHC is supported by an Australian Government Research Training Scholarship.
Authors’ disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
MM and JHYW contributed equally to this project.
Abbreviations used: DBP, diastolic blood pressure; GRADE, Grades of Recommendation, Assessment, Development, and Evaluation Working Group; HbA1c, glycated hemoglobin; RCT, randomized controlled trial; SBP, systolic blood pressure; USD, United States Dollar equivalent.
Contributor Information
Saiuj Bhat, School of Medicine, The University of Western Australia, Crawley, Australia.
Daisy H Coyle, The George Institute for Global Health, University of New South Wales, Sydney, Australia.
Kathy Trieu, The George Institute for Global Health, University of New South Wales, Sydney, Australia.
Bruce Neal, The George Institute for Global Health, University of New South Wales, Sydney, Australia; School of Public Health, Imperial College London, London, United Kingdom.
Dariush Mozaffarian, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Matti Marklund, The George Institute for Global Health, University of New South Wales, Sydney, Australia; Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA.
Jason H Y Wu, The George Institute for Global Health, University of New South Wales, Sydney, Australia.
References
- 1.He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet. 2006;367(9507):320–6. [DOI] [PubMed] [Google Scholar]
- 2.Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ. 2010;341:c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afshin A, Sur PJ, Fay KA, Cornaby L, Ferrara G, Salama JS, Mullany EC, Abate KH, Abbafati C, Abebe Zet al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393(10184):1958–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee CM, Colagiuri R, Magliano DJ, Cameron AJ, Shaw J, Zimmet P, Colagiuri S. The cost of diabetes in adults in Australia. Diabetes Res Clin Pract. 2013;99(3):385–90. [DOI] [PubMed] [Google Scholar]
- 5.Colagiuri S, Lee CM, Colagiuri R, Magliano D, Shaw JE, Zimmet PZ, Caterson ID. The cost of overweight and obesity in Australia. Med J Aust. 2010;192(5):260–4. [DOI] [PubMed] [Google Scholar]
- 6.Jardim TV, Mozaffarian D, Abrahams-Gessel S, Sy S, Lee Y, Liu J, Huang Y, Rehm C, Wilde P, Micha Ret al. Cardiometabolic disease costs associated with suboptimal diet in the United States: a cost analysis based on a microsimulation model. PLoS Med. 2019;16(12):e1002981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Barnighausen T, Davies J, Vollmer S. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care. 2018;41(5):963–70. [DOI] [PubMed] [Google Scholar]
- 8.Bloom DE, Cafiero ET, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, Feigl AB, Gaziano T, Mowafi M, Pandya Aet al. The Global Burden of Noncommunicable Diseases. Geneva: World Economic Forum; 2011. [Google Scholar]
- 9.Vozoris NT, Tarasuk VS. Household food insufficiency is associated with poorer health. J Nutr. 2003;133(1):120–6. [DOI] [PubMed] [Google Scholar]
- 10.Weigel MM, Armijos RX, Hall YP, Ramirez Y, Orozco R. The household food insecurity and health outcomes of U.S.-Mexico border migrant and seasonal farmworkers. J Immigr Minor Health. 2007;9(3):157–69. [DOI] [PubMed] [Google Scholar]
- 11.Che J, Chen J. Food insecurity in Canadian households. Health Rep. 2001;12(4):11–22. [PubMed] [Google Scholar]
- 12.Fitzgerald N, Hromi-Fiedler A, Segura-Perez S, Perez-Escamilla R. Food insecurity is related to increased risk of type 2 diabetes among Latinas. Ethn Dis. 2011;21(3):328–34. [PMC free article] [PubMed] [Google Scholar]
- 13.Seligman HK, Bindman AB, Vittinghoff E, Kanaya AM, Kushel MB. Food insecurity is associated with diabetes mellitus: results from the National Health Examination and Nutrition Examination Survey (NHANES) 1999–2002. J Gen Intern Med. 2007;22(7):1018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140(2):304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowman S. Low economic status is associated with suboptimal intakes of nutritious foods by adults in the National Health and Nutrition Examination Survey 1999–2002. Nutr Res. 2007;27(9):515–23. [Google Scholar]
- 16.Lallukka T, Pitkaniemi J, Rahkonen O, Roos E, Laaksonen M, Lahelma E. The association of income with fresh fruit and vegetable consumption at different levels of education. Eur J Clin Nutr. 2010;64(3):324–7. [DOI] [PubMed] [Google Scholar]
- 17.Lyles CR, Wolf MS, Schillinger D, Davis TC, Dewalt D, Dahlke AR, Curtis L, Seligman HK. Food insecurity in relation to changes in hemoglobin A1c, self-efficacy, and fruit/vegetable intake during a diabetes educational intervention. Diabetes Care. 2013;36(6):1448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vijayaraghavan M, Jacobs EA, Seligman H, Fernandez A. The association between housing instability, food insecurity, and diabetes self-efficacy in low-income adults. J Health Care Poor Underserved. 2011;22(4):1279–91. [DOI] [PubMed] [Google Scholar]
- 19.Seligman HK, Jacobs EA, Lopez A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363(1):6–9. [DOI] [PubMed] [Google Scholar]
- 21.Mozaffarian D, Mande J, Micha R. Food is medicine – the promise and challenges of integrating food and nutrition into health care. JAMA Intern Med. 2019;179(6):793–5. [DOI] [PubMed] [Google Scholar]
- 22.Sobal J, Bisogni CA. Constructing food choice decisions. Ann Behav Med. 2009;38(Suppl 1):S37–46. [DOI] [PubMed] [Google Scholar]
- 23.Herman DR, Harrison GG, Afifi AA, Jenks E. Effect of a targeted subsidy on intake of fruits and vegetables among low-income women in the Special Supplemental Nutrition Program for Women, Infants, and Children. Am J Public Health. 2008;98(1):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buyuktuncer Z, Kearney M, Ryan CL, Thurston M, Ellahi B. Fruit and vegetables on prescription: a brief intervention in primary care. J Hum Nutr Diet. 2014;27(Suppl 2):186–93. [DOI] [PubMed] [Google Scholar]
- 25.Forbes JM, Forbes CR, Lehman E, George DR. “Prevention produce”: integrating medical student mentorship into a fruit and vegetable prescription program for at-risk patients. Perm J. 2019;23:18–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryce R, Guajardo C, Ilarraza D, Milgrom N, Pike D, Savoie K, Valbuena F, Miller-Matero LR. Participation in a farmers' market fruit and vegetable prescription program at a federally qualified health center improves hemoglobin A1C in low income uncontrolled diabetics. Prev Med Rep. 2017;7:176–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavanagh M, Jurkowski J, Bozlak C, Hastings J, Klein A. Veggie Rx: an outcome evaluation of a healthy food incentive programme. Public Health Nutr. 2017;20(14):2636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comerford B, Doughty K, Njike V, Ayettey R, Weisel A, Katz D, Costales V. Impact of a fruit and vegetable prescription program on diet quality and cardio-metabolic risk factors in healthy adults in a worksite setting: a randomized controlled trial. Curr Dev Nutr. 2019;3(Supplement_1):nzz051. [Google Scholar]
- 29.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron Iet al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013; [Internet]. Available from:https://gdt.gradepro.org/app/handbook/handbook.html. [Google Scholar]
- 32.Shangguan S, Afshin A, Shulkin M, Ma W, Marsden D, Smith J, Saheb-Kashaf M, Shi P, Micha R, Imamura Fet al. A meta-analysis of food labeling effects on consumer diet behaviors and industry practices. Am J Prev Med. 2019;56(2):300–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 34.Feinberg A, Hess A, Passaretti M, Coolbaugh S, Lee T.. Prescribing food as a speciality drug. NEJM Catalyst. [Internet]. 2018; 3 December, 2019. Available from: https://catalyst.nejm.org/prescribing-fresh-food-farmacy/. [Google Scholar]
- 35.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59(1):7–10. [DOI] [PubMed] [Google Scholar]
- 36.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: q statistic or I2 index?. Psychol Methods. 2006;11(2):193–206. [DOI] [PubMed] [Google Scholar]
- 37.Bihan H, Méjean C, Castetbon K, Faure H, Ducros V, Sedeaud A, Galan P, Le Clésiau H, Péneau S, Hercberg S. Impact of fruit and vegetable vouchers and dietary advice on fruit and vegetable intake in a low-income population. Eur J Clin Nutr. 2012;66(3):369–75. [DOI] [PubMed] [Google Scholar]
- 38.Weinstein E, Galindo RJ, Fried M, Rucker L, Davis NJ. Impact of a focused nutrition educational intervention coupled with improved access to fresh produce on purchasing behavior and consumption of fruits and vegetables in overweight patients with diabetes mellitus. Diabetes Educ. 2014;40(1):100–6. [DOI] [PubMed] [Google Scholar]
- 39.Izumi BT, Higgins CE, Baron A, Ness SJ, Allan B, Barth ET, Smith TM, Pranian K, Frank B. Feasibility of using a community-supported agriculture program to increase access to and intake of vegetables among federally qualified health center patients. J Nutr Educ Behav. 2018;50(3):289–96.e1. [DOI] [PubMed] [Google Scholar]
- 40.Trapl ES, Smith S, Joshi K, Osborne A, Benko M, Matos AT, Bolen S. Dietary impact of produce prescriptions for patients with hypertension. Prev Chronic Dis. 2018;15:E138–E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emmert-Aronson B, Grill KB, Trivedi Z, Markle EA, Chen S. Group medical visits 2.0: the open source wellness behavioral pharmacy model. J Altern Complement Med. 2019;25(10):1026–34. [DOI] [PubMed] [Google Scholar]
- 42.Orsega-Smith E, Slesinger N, Cotugna N. Local pediatricians partner with food bank to provide produce prescription program. J Hunger Environ Nutr. 2019;15(3):353–9. [Google Scholar]
- 43.Seligman HK, Lyles C, Marshall MB, Prendergast K, Smith MC, Headings A, Bradshaw G, Rosenmoss S, Waxman E. A pilot food bank intervention featuring diabetes-appropriate food improved glycemic control among clients in three states. Health Aff (Millwood). 2015;34(11):1956–63. [DOI] [PubMed] [Google Scholar]
- 44.Ferrer RL, Neira LM, De Leon Garcia GL, Cuellar K, Rodriguez J. Primary care and food bank collaboration to address food insecurity: a pilot randomized trial. Nutr Metab Insights. 2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghodsi D, Omidvar N, Eini-Zinab H, Rashidian A, Raghfar H. Impact of the National Food Supplementary Program for Children on household food security and maternal weight status in Iran. Int J Prev Med. 2016;7:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kennedy BM, Champagne CM, Ryan DH, Newton R Jr, Conish BK, Harsha DW, Levy EJ, Bogle ML, Lower Mississippi Delta Nutrition Intervention Research; Initiative. The “Rolling Store:” an economical and environmental approach to the prevention of weight gain in African American women. Ethn Dis. 2009;19(1):7–12. [PubMed] [Google Scholar]
- 47.Carrillo-Larco RM, Miranda JJ, Bernabé-Ortiz A. Impact of food assistance programs on obesity in mothers and children: a prospective cohort study in Peru. Am J Public Health. 2016;106(7):1301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ridberg RA, Bell JF, Merritt KE, Harris DM, Young HM, Tancredi DJ. Effect of a fruit and vegetable prescription program on children's fruit and vegetable consumption. Prev Chronic Dis. 2019;16:E73–E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedman DB, Freedman DA, Choi SK, Anadu EC, Brandt HM, Carvalho N, Hurley TG, Young VM, Hébert JR. Provider communication and role modeling related to patients' perceptions and use of a federally qualified health center-based farmers' market. Health Promot Pract. 2014;15(2):288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chrisinger A, Wetter A. Fruit and vegetable prescription program: design and evaluation of a program for families of varying socioeconomic status. J Nutr Educ Behav. 2016;48(7):S57. [Google Scholar]
- 51.AbuSabha R, Gargin M. Subscription to a fresh produce delivery program increases intake and variety of vegetables at no added cost to customers. J Hunger Environ Nutr. 2019;14(6):796–809. [Google Scholar]
- 52.An R, Patel D, Segal D, Sturm R. Eating better for less: a national discount program for healthy food purchases in South Africa. Am J Health Behav. 2013;37(1):56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basu S, Gardner CD, White JS, Rigdon J, Carroll MM, Akers M, Seligman HK. Effects of alternative food voucher delivery strategies on nutrition among low-income adults. Health Aff. 2019;38(4):577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buscail C, Margat A, Petit S, Gendreau J, Daval P, Lombrail P, Hercberg S, Latino-Martel P, Maurice A, Julia C. Fruits and vegetables at home (FLAM): a randomized controlled trial of the impact of fruits and vegetables vouchers in children from low-income families in an urban district of France. BMC Public Health. 2018;18(1):1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durward CM, Savoie-Roskos M, Atoloye A, Isabella P, Jewkes MD, Ralls B, Riggs K, LeBlanc H. Double Up Food Bucks participation is associated with increased fruit and vegetable consumption and food security among low-income adults. J Nutr Educ Behav. 2019;51(3):342–7. [DOI] [PubMed] [Google Scholar]
- 56.Freedman DA, Choi SK, Hurley T, Anadu E, Hébert JR. A farmers' market at a federally qualified health center improves fruit and vegetable intake among low-income diabetics. Prev Med. 2013;56(5):288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gardiner CK, Bryan AD. Monetary incentive interventions can enhance psychological factors related to fruit and vegetable consumption. Ann Behav Med. 2017;51(4):599–609. [DOI] [PubMed] [Google Scholar]
- 58.Gorham G, Dulin-Keita A, Risica PM, Mello J, Papandonatos G, Nunn A, Gorham S, Roberson M, Gans KM. Effectiveness of Fresh to You, a discount fresh fruit and vegetable market in low-income neighborhoods, on children's fruit and vegetable consumption, Rhode Island, 2010–2011. Prev Chronic Dis. 2015;12:E176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanson KL, Kolodinsky J, Wang W, Morgan EH, Pitts SBJ, Ammerman AS, Sitaker M, Seguin RA. Adults and children in low-income households that participate in cost-offset community supported agriculture have high fruit and vegetable consumption. Nutrients. 2017;9(7):726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kral TVE, Bannon AL, Moore RH. Effects of financial incentives for the purchase of healthy groceries on dietary intake and weight outcomes among older adults: a randomized pilot study. Appetite. 2016;100:110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leroy JL, Gadsden P, Rodríguez-Ramírez S, de Cossío TG. Cash and in-kind transfers in poor rural communities in Mexico increase household fruit, vegetable, and micronutrient consumption but also lead to excess energy consumption. J Nutr. 2010;140(3):612–17. [DOI] [PubMed] [Google Scholar]
- 62.Moran A, Thorndike A, Franckle R, Boulos R, Doran H, Fulay A, Greene J, Blue D, Block JP, Rimm EBet al. Financial incentives increase purchases of fruit and vegetables among lower-income households with children. Health Aff (Millwood). 2019;38(9):1557–66. [DOI] [PubMed] [Google Scholar]
- 63.Olsho LE, Klerman JA, Wilde PE, Bartlett S. Financial incentives increase fruit and vegetable intake among Supplemental Nutrition Assistance Program participants: a randomized controlled trial of the USDA Healthy Incentives Pilot. Am J Clin Nutr. 2016;104(2):423–35. [DOI] [PubMed] [Google Scholar]
- 64.Savoie-Roskos M, Durward C, Jeweks M, LeBlanc H. Reducing food insecurity and improving fruit and vegetable intake among farmers' market incentive program participants. J Nutr Educ Behav. 2016;48(1):70–6.e1. [DOI] [PubMed] [Google Scholar]
- 65.Wright L, Arce KS, Himmelgreen D, Epps JB. Farm2Fork: use of the health belief model to increase fresh fruit and vegetable intake among food pantry participants. J Hunger Environ Nutr. 2019;14(1–2):252–61. [Google Scholar]
- 66.Schroeter C, Corder T, Brookes B, Reller V. An incentive-based health program using MyPlate: a pilot study analyzing college students' dietary intake behavior. J Am College Health. 2019:1–8. [DOI] [PubMed] [Google Scholar]
- 67.Molitor F, Sugerman SB, Sciortino S. Fruit and vegetable, fat, and sugar-sweetened beverage intake among low-income mothers living in neighborhoods with Supplemental Nutrition Assistance Program-Education. J Nutr Educ Behav. 2016;48(10):683–90.e1. [DOI] [PubMed] [Google Scholar]
- 68.Pellegrino S, Bost A, McGonigle M, Rosen L, Peterson-Kosecki A, Colon-Ramos U, Robien K.. Fruit and vegetable intake among participants in a District of Columbia farmers' market incentive programme. Public Health Nutr. 2018;21(3):601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scantlebury RJ, Moody A, Oyebode O, Mindell JS.. Has the UK Healthy Start voucher scheme been associated with an increased fruit and vegetable intake among target families? Analysis of Health Survey for England data, 2001–2014. J Epidemiol Community Health. 2018;72(7):623–9. [DOI] [PubMed] [Google Scholar]
- 70.Blickenderfer Z. Vegetable Prescription Programs: A New Take on Holistic Health. University of Pennsylvania; 2016. Available from ScholarlyCommons: http://repository.upenn.edu/senior_seminar/19. [Google Scholar]
- 71.Aiyer JN, Raber M, Bello RS, Brewster A, Caballero E, Chennisi C, Durand C, Galindez M, Oestman K, Saifuddin Met al. A pilot food prescription program promotes produce intake and decreases food insecurity. Transl Behav Med. 2019;9(5):922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.George DR, Manglani M, Minnehan K, Chacon A, Gundersen A, Dellasega C, Kraschnewski JL. Examining feasibility of mentoring families at a farmers' market and community garden. Am J Health Educ. 2016;47(2):94–8. [Google Scholar]
- 73.Slagel N, Newman T, Sanville L, Dallas J, Thurman S, Cummings P, Cotto-Rivera E, Thompson J, Lee JS. The effects of a fruit and vegetable prescription program (FVRx)® for low-income individuals on fruit and vegetable intake and food purchasing practices. J Nutr Educ Behav. 2018;50(7):S103. [Google Scholar]
- 74.Ramírez-Luzuriaga MJ, Unar-Munguía M, Rodríguez-Ramírez S, Rivera JA, González de Cosío T. A food transfer program without a formal education component modifies complementary feeding practices in poor rural Mexican communities. J Nutr. 2016;146(1):107–13. [DOI] [PubMed] [Google Scholar]
- 75.Tonguet-Papucci A, Houngbe F, Huybregts L, Ait-Aissa M, Altare C, Kolsteren P, Huneau JF. Unconditional seasonal cash transfer increases intake of high-nutritional-value foods in young Burkinabe children: results of 24-hour dietary recall surveys within the Moderate Acute Malnutrition Out (MAM'Out) randomized controlled trial. J Nutr. 2017;147(7):1418–25. [DOI] [PubMed] [Google Scholar]
- 76.Berkowitz SA, Delahanty LM, Terranova J, Steiner B, Ruazol MP, Singh R, Shahid NN, Wexler DJ. Medically tailored meal delivery for diabetes patients with food insecurity: a randomized cross-over trial. J Gen Intern Med. 2019;34(3):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goddu AP, Roberson TS, Raffel KE, Chin MH, Peek ME. Food Rx: a community-university partnership to prescribe healthy eating on the South Side of Chicago. J Prev Interv Community. 2015;43(2):148–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joshi K, Smith S, Bolen SD, Osborne A, Benko M, Trapl ES. Implementing a produce prescription program for hypertensive patients in safety net clinics. Health Promot Pract. 2018;20(1):94–104. [DOI] [PubMed] [Google Scholar]
- 79.Black AP, Vally H, Morris PS, Daniel M, Esterman AJ, Smith FE, O'Dea K. Health outcomes of a subsidised fruit and vegetable program for Aboriginal children in northern New South Wales. Med J Aust. 2013;199(1):46–50. [DOI] [PubMed] [Google Scholar]
- 80.Reicks M, Randall JL, Haynes BJ. Factors affecting consumption of fruits and vegetables by low-income families. J Am Diet Assoc. 1994;94(11):1309–11. [DOI] [PubMed] [Google Scholar]
- 81.Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16(5):457–66. [DOI] [PubMed] [Google Scholar]
- 82.Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes Care. 2010;33(8):1859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murray J, Fenton G, Honey S, Bara AC, Hill KM, House A. A qualitative synthesis of factors influencing maintenance of lifestyle behaviour change in individuals with high cardiovascular risk. BMC Cardiovasc Disord. 2013;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yeh MC, Ickes SB, Lowenstein LM, Shuval K, Ammerman AS, Farris R, Katz DL. Understanding barriers and facilitators of fruit and vegetable consumption among a diverse multi-ethnic population in the USA. Health Promot Int. 2008;23(1):42–51. [DOI] [PubMed] [Google Scholar]
- 85.Pomerleau J, Lock K, Knai C, McKee M. Interventions designed to increase adult fruit and vegetable intake can be effective: a systematic review of the literature. J Nutr. 2005;135(10):2486–95. [DOI] [PubMed] [Google Scholar]
- 86.Welsh EM, Jeffery RW, Levy RL, Langer SL, Flood AP, Jaeb MA, Laqua PS. Measuring perceived barriers to healthful eating in obese, treatment-seeking adults. J Nutr Educ Behav. 2012;44(6):507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu R, Vandermeer BW, Shamliyan TA, O'Neil ME, Yazdi F, Fox SH, Morton SC. Handling Continuous Outcomes in Quantitative Synthesis. Methods Guide for Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2013. Contract No.: AHRQ Publication No. 13-EHC103-EF. [PubMed] [Google Scholar]
- 88.Jilcott Pitts SB, Wu Q, Demarest CL, Dixon CE, Dortche CJ, Bullock SL, McGuirt J, Ward R, Ammerman AS. Farmers' market shopping and dietary behaviours among Supplemental Nutrition Assistance Program participants. Public Health Nutr. 2015;18(13):2407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Robles B, Montes CE, Nobari TZ, Wang MC, Kuo T. Dietary behaviors among public health center clients with electronic benefit transfer access at farmers' markets. J Acad Nutr Diet. 2017;117(1):58–68. [DOI] [PubMed] [Google Scholar]
- 90.Kropf ML, Holben DH, Holcomb JP Jr, Anderson H. Food security status and produce intake and behaviors of Special Supplemental Nutrition Program for Women, Infants, and Children and Farmers' Market Nutrition Program participants. J Am Diet Assoc. 2007;107(11):1903–8. [DOI] [PubMed] [Google Scholar]
- 91.Afshin A, Micha R, Khatibzadeh S, Mozaffarian D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100(1):278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353:i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perez-Lizaur AB, Kaufer-Horwitz M, Plazas M. Environmental and personal correlates of fruit and vegetable consumption in low income, urban Mexican children. J Hum Nutr Diet. 2008;21(1):63–71. [DOI] [PubMed] [Google Scholar]
- 94.Lappalainen R, Saba A, Holm L, Mykkanen H, Gibney MJ, Moles A. Difficulties in trying to eat healthier: descriptive analysis of perceived barriers for healthy eating. Eur J Clin Nutr. 1997;51(Suppl 2):S36–40. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.