Abstract
The field of nutrition has evolved from one focused primarily on discovery of the identities, metabolic functions, and requirements for essential nutrients to one focused on the application of that knowledge to the development and implementation of dietary recommendations to promote health and prevent disease. This evolution has produced a deeper appreciation of not only the roles of nutrients, but also factors affecting their functions in increasingly complex global health contexts. The intersection of nutrition with an increasingly more complex global health context necessitates a view of nutritional status as a biological variable (NABV), the study of which includes an appreciation that nutritional status is: 1) not limited to dietary exposure; 2) intimately and inextricably involved in all aspects of human health promotion, disease prevention, and treatment; and 3) both an input and an outcome of health and disease. This expanded view of nutrition will inform future research by facilitating considerations of the contexts and variability associated with the many interacting factors affecting and affected by nutritional status. It will also demand new tools to study multifactorial relations to the end of increasing precision and the development of evidence-based, safe, and effective standards of health care, dietary interventions, and public health programs.
Keywords: nutrients, nutrition, nutritional assessment, dietary interventions, biological variable
Introduction
The importance of food for health has been recognized for millennia. Hippocrates is said to have urged “Let food be thy medicine, and medicine be thy food.” However, despite advances in other areas of medicine and public health, significant gaps remain in our understanding of why and how food and nutrition affect health. Food and nutrition science are linked and sometimes conflated; however, they are distinct disciplines that have evolved, largely independently, with the advent of new knowledge and technology. Moreover, it is all too often the case that the research focus has been on food, a vehicle for nutrients and other bioactive components, rather than nutritional status, which reflects the biological sequences of events that occur after food is consumed. [The authors recognize that there are relevant intersections between food (processing, safety), nutritional status, and health (e.g., the intersection of poor nutritional status, mycotoxins/aflatoxins, and health), but for the purposes of this Perspective article the discussion is limited to the dichotomous relations between food intake and nutritional status. The term “nutrients” refers to macronutrients (carbohydrates, fats, and protein) and micronutrients (vitamins and minerals).] Although there is also often a tendency to take a linear view of the diet/food and disease relation (1), they are actually circular with a continual feedback such that these aspects of the public health continuum affect and are affected by each other.
This focus on food, its accessibility and availability (i.e., food security), and its associations with global targets such as stunting, wasting, or anemia has been critical in the global battle against hunger and malnutrition. However, this focus has had implications for our ability to expand our understanding of the role of nutritional status in health and disease and has limited our ability to make the case for the full integration of nutritional status into the clinical and global health responses. This limitation has manifested itself many times over the years and now again, in the public health response to the coronavirus disease 2019 (COVID-19) pandemic.
Confronted with the COVID-19 pandemic since the beginning of 2020, the global response has included a growing repository of published data reflecting ongoing efforts via the global research community to link current and future cohorts, surveillance, and research to gather data relevant to risk, disease course, or response to treatment. Yet again, nutrition is simply not included. All the known risk factors for susceptibility to COVD-19 (e.g., diabetes, obesity, cardiovascular disease) have strong nutritional connections. However, there is a paucity of data to address any aspect of nutritional status as either an input (i.e., risk factor, effector of susceptibility or pathogenesis) or an outcome (i.e., poor nutritional status as a result of illness or loss of taste and smell leading to poor response to treatment or other outcomes). In addition, the poorer outcomes that are overrepresented in underserved populations are clearly linked to poor nutrition. To date, the relation nutrition has to COVID-19 risk or outcomes has not been explored beyond the application of the linear logic described above, that is, attention to the impact of the disease on food insecurity and related global health targets (e.g., wasting, stunting) (2). As important as such statements are in highlighting the indirect effects of pandemics like COVID-19 on food access, availability, and related outcomes, they do not address why a deeper understanding of the biology of nutrition is integral to our ability to fully appreciate its role in the susceptibility to, pathogenesis of, and response to treatment of such diseases.
The case for integrating nutrition into the biomedical research agenda to improve our understanding of the biology and response to COVID-19 reflects a limited view of the science of nutrition. This is a pattern that has repeated itself in our response to myriad other complex public health challenges from HIV to Ebola to Zika, and now severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (see Text box 1). It raises 3 important questions: 1) why does this continue to happen? 2) why do we need to change this pattern? and 3) how can we make that happen? Furthermore, in the era of “precision medicine,” how can nutritional status and all it reflects in terms of the biological context of an individual's health be more effectively integrated into the clinical and public health armamentarium. [According to the Precision Medicine Initiative at NIH, precision medicine is “an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person” (https://medlineplus.gov/genetics/understanding/precisionmedicine/definition/).]
Box 1: PubMed keyword search
| HIV | 372,990 results |
| HIV and nutrition | 5745 results |
| HIV and malnutrition | 2417 results |
| HIV and food insecurity | 784 results |
| Nutritional status of HIV patients | 634 results |
| Nutritional assessment of HIV patients | 347 results |
| Ebola | 9769 results |
| Ebola and nutrition | 49 results |
| Ebola and malnutrition | 19 results |
| Ebola and food insecurity | 25 results |
| Nutritional status of Ebola patients | 1 result |
| Nutritional assessment of Ebola patients | 1 result |
| Zika | 8723 results |
| Zika and nutrition | 56 results |
| Zika and malnutrition | 4 results |
| Zika and food insecurity | 1 result |
| Nutritional status of Zika patients | 2 results |
| Nutritional assessment of Zika patients | 0 results |
| COVID-19 | 81,358 results |
| COVID-19 and nutrition | 1417 results |
| COVID-19 and food insecurity | 291 results |
| COVID-19 and malnutrition | 191 results |
| Nutritional status of COVID-19 patients | 67 results |
| Nutritional assessment of COVID-19 patients | 1 result |
If we were to distill this down to a core set of questions, they might include: “What is different about a given condition like COVID-19 that would demand a nutritional approach beyond the provision of a high-quality diet that provides all essential nutrients at currently recommended levels for otherwise healthy people?” Another related question is: “Are differences we might see in clinical outcomes due to poor intake or something inherent to the biology of the disease and/or its treatment that would require a nutritional intervention in addition to achieving dietary adequacy?”
The focus of this Perspective is on those factors that have the potential to enhance our ability to integrate the biology of nutrition more effectively into all aspects of health promotion and disease prevention.
We include a potential conceptual framework to expand our understanding of nutrition and its role in all aspects of human biology to inform efforts to improve health policy, programs, and standards of clinical care.
We will herein make the case that the principles highlighted in the recent call for “sex as a biological variable” are equally compelling when applied to the study and integration of nutritional status in biomedical research, that is, “nutritional status as a biological variable (NABV).” [The NIH is committed to improving the health outcomes of men and women through support of rigorous science that advances fundamental knowledge about the nature and behavior of living systems. Sex and gender play a role in how health and disease processes differ across individuals, and consideration of these factors in research studies informs the development and testing of preventive and therapeutic interventions in both sexes. This notice focuses on NIH's expectation that scientists will account for the possible role of sex as a biological variable in vertebrate animal and human studies.” (see NIH Notice Number: NOT-OD-15-102, “Consideration of sex as a biological variable in NIH-funded research”; https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.html).] In this Perspective, we suggest that a new approach is needed to draw on both nutrition science and its translation to investigations of the impact of food/dietary patterns on health. This calls for rigorous studies addressing the nature and behavior of people, their foods, and their physical, biological, and social environments.
Current Status of Knowledge
Now only in its second century, the science of nutrition is relatively young (1). Guthrie (3) noted that it progressed through 2 eras. The first era, a “chemical-analytical” era, began at the end of the 19th century and involved the discovery and characterization of the vitamins and other essential dietary nutrients followed by the elucidation of their respective metabolic roles. This era relied on studies with animal models and livestock to elucidate the nature and implications of frank nutrient deficiencies. The second era involved the establishment of dietary standards. Prompted by the need for a wartime US food policy, this era commenced with the development of the first RDAs (4) in 1943, followed by the development of educational programs focused on nutrient needs. This era was also characterized by nutritional studies in clinical settings, first to address therapeutic needs during World War II and subsequently to address a variety of questions related to human health. These efforts, along with advances in the food, animal, and plant sciences, markedly reduced the shortages of total food and the prevalence of many nutritional deficiencies in most of the world.
Challenges
Although the efforts of those involved in these earlier eras (as reflected by the periodic process of revising the RDAs, now DRIs) and global technical guidance by authoritative agencies including the WHO continue, significant challenges remain in terms of our collective understanding of the importance of nutrition to both health and disease. Some of the key challenges facing the field of nutrition are highlighted in Text box 2.
Box 2: Drivers for a new era
Continued focus on nutrition as limited to exposure scenarios, i.e., too much or too little, resulting in an inconsistent or lack of integration of nutrition status as both an input and outcome of health and disease.
Lack of understanding of the functional implications of nutritional status or impact of interventions to change that status, particularly in an increasingly complex health context.
Incomplete appreciation of how nutrients interact within biological systems.
-
Challenges in our ability to avoid:
Unintended health consequences, or
Confused/confusing messages
In our opinion, the field of nutrition science has been stymied by an overemphasis on translation and a focus on single nutrients. This orientation has occurred in the absence of fundamental research to illuminate the role of nutrients within a complex biological milieu that most often consists of multiple macro- and micronutrients and bioactive substances in the diet interacting within biological systems. The field has also been limited with regard to both our ability to identify functional ramifications of nutrient status (i.e., what does it mean in terms of an individual's ability to maintain health?) and the tools needed to discern such impacts. Understanding the role of nutrition in human biology demands an appreciation of the nature of these interactions, as well as of the tools to assess those interactions. We hope the ability to address these challenges will be driven by new and innovative research and the application of a conceptual framework that will allow the adaptation of these new approaches to address a complex and continually changing global health context.
A Conceptual Framework
We propose that nutrition is best viewed as a broad concept describing the complex biology of what happens as food is acquired, consumed, and metabolized. Because nutritional status can both affect metabolism (and, thus, physiology) and be affected by metabolism, it is appropriately viewed as a biological variable with both cause and effect characteristics. Accordingly, we offer the term NABV as a framework capturing the complexity of that biology and integrating nutritional status as both an input and an outcome of health and disease. The NABV framework includes the following concepts:
Nutrition is the sum of the processes involved in taking in and utilizing food substances by which growth, repair, maintenance, and reproductive activities of the body or in any of its parts are accomplished. (The “processes of nutrition” include: ingestion, digestion, absorption, transport, metabolism, and elimination. A reciprocal relation exists between nutritional status and each of these processes such that each affects and is affected by the other.)
-
Nutritional status reflects the relative adequacy of nutrients to perform the various functions of life and has the following characteristics:
It is achieved as a result of the interaction of food and nutrients with the processes of nutrition
It can affect and/or be affected by these processes, which must be considered in understanding individual nutritional needs
It varies among individuals and can affect responses to medical and dietary treatments
That physiological functionality refers to the impact of nutritional status on maintenance of health and prevention of disease.
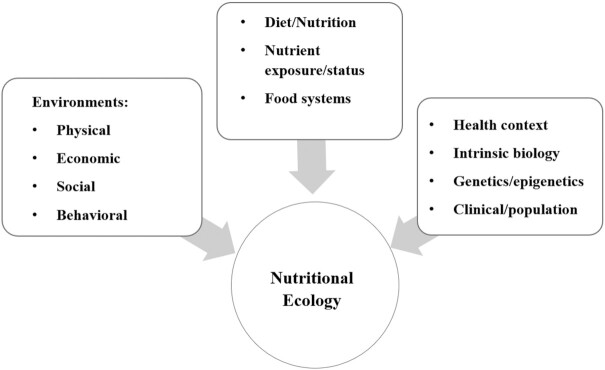
Informed by the expertise of the environmental science community, we can understand the above concepts as reflecting an “ecology,” that is, the interaction of a complex system, in this case the biology of nutritional status, with its environment. The NABV framework thus addresses the multiple features of the biological, physical, and sociopsychological environments that comprise the nutritional ecology related to health (Figure 1) (5). The NABV framework is, therefore, an ecological approach applicable at both “internal” or micro (via the understanding of the basic biology of nutrition) and “external” or macro (via translational activities for individuals and populations) levels of consideration. We feel that such applications can improve the scientific rigor of nutrition science research by accounting for sources of variability of both dependent and independent variables. As will be discussed in the following sections, the NABV framework is intended to support a new era in nutrition science—one that addresses the persistent and emerging challenges at the intersection of food, nutrition, and health.
FIGURE 1.
The “nutritional ecology” reflecting the interaction of a complex system, in this case the biology of nutritional status, with its biological, physical, and sociopsychological environments (5).
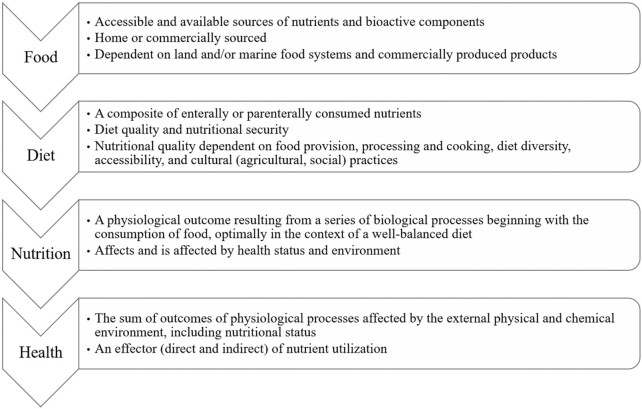
Figure 2 shows the diet/nutrition/health continuum in healthy, free-living people. Studying this continuum will call for assessments in 2 areas: the “how,” evaluating nutritional status, and the “why,” elucidating the functions of nutrients in biological systems.
FIGURE 2.
The diet/nutrition and health continuum.
The “How” Assessment
Elucidating the interrelations of diet, nutritional status, and health in humans will depend on the methods and designs of observational, preclinical, and clinical studies conducted to: 1) evaluate programs, policies, and guidance; 2) develop and support standards of clinical care; and 3) improve individual and population health outcomes. Methodologies for the clinical assessment of patients and the surveillance of populations should address the following core questions:
-
Are individuals/populations consuming nutrients at recommended levels, and if not, why?
Is the individual/population food insecure (i.e., are there access/availability issues)?
Is the individual/population adversely affected by “environmental” (climate, land/water resources, urban/rural/social/economic/political) factors?
Are the processes of nutrition (i.e., digestion, absorption, assimilation, and utilization of these nutrients) affected in individuals/populations?
-
Does an individual's unique biology (life stage, health) affect either 1 or 2 above? If so,
Is there a clear difference between established nutrient requirements (e.g., DRIs) and specific health/physiological condition–related needs?
What is the role of diet/nutrition in those conditions that would require special consideration or deviations beyond the provision of a balanced diet providing all the essential nutrients needed for growth, development, and health?
What is the best mode of nutrient delivery for the unique biological and environmental context?
The design of rigorous and reproducible studies to characterize the relations of diet/nutrition and health requires validated and reliable assessment tools appropriate for the questions being asked and the specifics of the health and environmental contexts. Such tools must address: nutrient exposure, bioavailable amounts of nutrients consumed; nutritional status in reference to accepted definitions for adequacy, marginal status, and deficiency; or nutrient function, the metabolic role(s) of specific nutrients; and the relevant functional outcome(s). Finally, it is critical to recognize that individuals are seldom deficient with respect to single nutrients, and the multiple nutrients in metabolic systems typically function in synergy. These facts should inform our efforts to develop more precise assessment methodologies and study designs.
Inferences from single measures can also be misleading. For example, reliance on dietary intake assessments implicitly assumes that what is being consumed is being utilized metabolically. Similarly, single measures of a given biomarker might not reflect the temporal dynamics in the levels of the relevant nutrients (i.e., at a point in time, with just 1 measure, how can one determine whether the variable is increasing or decreasing due to changes in dietary intake or reflects the impact of a given health condition on those measures?). Moreover, in the absence of intake data/history, how can one distinguish between a response to an intervention (as the result of the correction of a dietary inadequacy) compared with a response consequent to the amelioration of the unique biology/requirements associated with the presenting health issue (e.g., HIV, malaria, diabetes). Fundamentally, relying on a single measure, whether intake or biochemical, prevents one from making the distinction between a poor diet or the effects of the disease and as a consequence presents a significant challenge to our ability to improve precision of care in individuals and in populations.
Components of assessment
As highlighted above, dietary assessment is an integral part of our ability to interpret data intended to draw inferences about the role of nutrition in health. Although a more fulsome treatment of this important component of the nutritional assessment armamentarium is beyond the scope of this Perspective, the challenges of assessing dietary intake have been reviewed (6–8). Clearly, the ability to assess dietary intake, including the challenges of analysis and availability of accurate, timely food composition data, the reliance on subject recall rather than biologically relevant data that might be provided by such technologies as metabolomics, or controlling for various other sources of variability, is critical to drawing clearer conclusions about diet-health relations. The absence of data to address this critical component of nutritional assessment is illustrated by recent systematic reviews addressing needs for formal dietary guidelines for infants (9–12); their current value has been limited by the quality of the available evidence. The need to move this critical aspect of the field forward has been recently highlighted in the publication of the NIH Nutrition Research Strategic Plan (13), which includes a highly focused 10-y plan to address these issues as they pertain to the advancement of our understanding of diet and health relations.
In principle, useful biochemical assessments of nutrient status facilitate the sorting of individuals into 3 risk groups for nutritional adequacy, that is, adequate, marginal, or deficient status. But this can be difficult to achieve considering there are choices as to what to measure (i.e., what biomarker), in what biological milieu (e.g., whole blood, serum, urine, etc.), and what other factors to assess because they influence the response and/or significance of those biomarkers. These issues were considered in the Biomarkers of Nutrition for Development (BOND) project (14), which employed an ecological approach to provide evidence-informed advice to researchers (basic, clinical, demographic), clinicians, program developers/monitors, and policy-makers who rely on biomarkers to evaluate and make decisions about the role of nutrients in health. The BOND project included the examination of 6 nutrients (iron, zinc, iodine, vitamin A, vitamin B-12, and folate) of public health significance that also represented the spectrum of issues associated with the selection, use, and interpretation of biomarkers (14, 15–20). Each of the BOND reviews provided suggestions regarding the relative value of specific biomarkers for a given context (e.g., basic compared with clinical research, clinical assessment/care, population surveillance, program monitoring/evaluation) or to evaluate the strength of research to support policy/guidance.
In addition to the expert panel conclusions about the relative value of current and emerging biomarkers, 2 key issues emerged: 1) the need to recognize that nutrients often interact in ways relevant to the selection and interpretation of biomarkers; and 2) nutrient utilization, status, and function can affect and be affected by key biological/physiological systems and responses. This latter issue is exemplified by the reciprocal relations between nutrition and inflammation: each affect and are affected by each other (21). In addition to this reciprocity, we have come to appreciate the impact and need to account for the inflammatory response on the performance and interpretation of nutritional biomarkers (22).
The findings of the BOND project further reinforce the need to pursue new approaches to nutritional assessment that more fully integrate the biological context in the selection, interpretation, and deployment of biomarkers. These implications will be addressed in subsequent sections of this Perspective.
The “Why” Assessment
Another key aspect revealed by the BOND review process was the challenge of defining “function” as a critical component of nutritional assessment. Understanding and measuring the effects of nutritional status on metabolic and physiological functions supporting health have been a challenge in the selection, use, and interpretation of measures of nutritional status. Such effects range from specific functional responses to a change in nutritional status (i.e., deficiency or excess), to the capacity of an intervention to cause a desired outcome (e.g., improved growth, neurodevelopment, immune response). Such effects can be measured by assessing: direct impacts on a specific nutrient-dependent system (e.g., visual function in response to vitamin A, transketolase activation in response to thiamin, erythrocyte glutamic-pyruvate transaminase activity in response to vitamin B-6) or indirect and often nonspecific effects on functional outcomes (e.g., changes in growth, immune function, neurodevelopment). The interpretation of such measures calls for recognizing that the outcomes reflecting function might not be independently sensitive/specific to changes in nutrient status (e.g., neurodevelopment or anthropometry) and that traditional measures of program efficacy or public health impact might not be sensitive/specific to nutritional status in other contexts (e.g., growth or anemia).
Just as “nutrition-specific” and “nutrition-sensitive” interventions have become part of the public health vernacular, a similar approach is needed for nutritional status assessment. This calls for terminology that more clearly reflects expectations about a given measure:
“Biomarkers” reflect the amount of specific nutrients interpreted within a specific biological context, i.e., health or disease
“Bioindicators” reflect perturbations in biological systems (e.g., electroencephalography reflecting aberrations in neurophysiology, hemoglobin concentrations reflecting hematological status, C-reactive protein concentrations reflecting inflammation) but do not reflect specific nutrient status, that is, they cannot be expected to serve as a biomarker of particular nutrients
“Public health indicators” reflect perturbations in the external ecology (e.g., socioeconomic status, food insecurity, disability-adjusted life years, or stunting). These types of indicators are often used to suggest causality and thus are used as triggers for public health interventions
Importantly, both latter types of indicators (“bioindicators” and “public health indicators”) can be nutritionally sensitive without being nutrient-specific. As such, they cannot be reliably used to make inferences about diet/nutrition and health without supporting information (23).
Fundamentally, the objective of nutritional assessment is to determine what is good nutritional status in the context of health and disease and what constitutes “malnutrition.” These distinctions speak to expectations about the value of both the research tools employed and the targets (i.e., “public health indicators” for public health interventions). Refining our understanding of the role of nutrition in health calls for being clear about what is measured as well as its implications. As detailed below, this conundrum is exemplified by ongoing questions about how to define and respond to the intractable problems of global anemia and childhood stunting. For each, neither the proximal causes nor the most informative biomarkers are self-evident, thus compromising the ability to intervene. The complexity of both problems demands an ecological approach that includes elements of systems biology to understand the persistent problems and, ultimately, the translation of that understanding to sensitive and specific assessment methodologies and interventions. This requires going beyond single-nutrient or “too much or too little” approaches.
Applying New Technologies to Nutritional Science
Biology has been revolutionized by the application of “omics” techniques, that is, the simultaneous determination of multiple genes, proteins, or metabolites. These methods have changed research foci from specific functional pathways to physiological systems. Systems approaches in nutrition research are not new (24). “Nutriomics” (25, 26) approaches, for instance, have facilitated the study of the interactions between genes and nutrients to further our understanding of the nature of these interactions and their implications for health. Herein, we adapt the term “nutriomics” to refer to the study of patterns (i.e., “nutriomes”) of nutrient functions and interrelations. For example, the nutriome of inflammation as presented by van Erk et al. (27) shows that there are multiple nutrient-dependent points within this critical system. Further, it raises important questions: How do these nutrients interact to affect the function of the system? How sensitive is the system to fluctuations in particular nutrients? To what extent is the system affected by endogenous factors? What are the impacts of short- or long-term deprivation of particular nutrients?
Some of these types of questions have become the focus of our emerging understanding of the important intersection between nutrition and the human gut microbiome. The complexity and value of addressing this dynamic and complex set of intersecting systems and the relevance of the concepts presented in this article regarding the NABV approach was the subject of a recent systematic review (28). A further example of the value and complexity of a systems approach has been the evolving efforts to expand our understanding of neural networks and the human brain “connectome” (29, 30), an area of great potential in terms of the application of nutriomics.
Below, we will briefly describe 4 cases demonstrating the benefits of using the NABV framework in addressing persistent problems of high public health significance.
Human milk composition
The “Birth to 24 months (B-24)” project (31), which supported efforts to include infants and children from birth to 24 mo and pregnant women in future iterations of the Dietary Guidelines for Americans, identified several key knowledge gaps needed to fulfill this public health priority. The most prominent of these gaps concerns the limited understanding of the biology of human lactation and the nutritional ecology of human milk. Much of the focus to date has been on the individual components of human milk (32). This has resulted in an appreciation of the complexity of human milk and its many constituents related to the health/nutritional status of both infants and nursing mothers. The NABV framework prompts further research into the functions, regulation, and genetics of components of human milk including but not limited to the milk-fat globule (33), milk oligosaccharides (34), peptides (35), immune factors (36), microbiomes (those of the mother's breast, milk, and infant's oral cavity) (37, 38), and other cellular components (39). It also prompts questions about the interactions of these components within the milk matrix, across feedings and over time, that is, the chronobiology of human milk (40). In short, the NABV framework approaches human milk as a biological system—the understanding of which will inform improved infant feeding practices, standards of care, and public health guidance.
The value of gaining this type of new knowledge is clear in an examination of the impact of COVID-19 on infant feeding practices. At the outset of the COVID-19 pandemic, questions immediately arose regarding the safety and value of breastfeeding in terms of potential for vertical transmission of the SARS-CoV-2 virus and the value of breastfeeding in the context of SARS-CoV-2 infection. Answers to both questions were not readily available, which led to suggestions to deviate from existing policy regarding breastfeeding and use of human milk. It demanded urgent responses to how to sample and analyze human milk in the context of the infection, how to determine the immune response during lactation and its impact on human milk composition (nutritional and bioactive), and how best to communicate such new information to health care providers and the general public to allay the fears of a confused and fearful population (41).
Although new guidance has emerged, along with answers to several core questions (42–47), the immediacy of the issues surrounding SARS-CoV-2 transmission has outweighed the need to address the role of nutrition in this context. Other important questions include the role of nutrition on SARS-CoV-2 vaccine response and the impact of SARS-CoV-2 infection on maternal nutrition, lactation performance, and human milk composition. Of additional concern are the implications of exposure to SARS-CoV-2 for infant health and development irrespective of whether the child is infected with the virus or not. Answers to such questions will demand an ecological approach that fully integrates all the aspects of the NABV concepts presented herein.
Drug–nutrient interactions
Responses to xenobiotic agents (e.g., therapeutic or recreational drugs, environmental toxins, etc.) can vary according to nutritional status, which can affect both the pharmacokinetics (absorption, distribution, metabolism, localization in tissues, biotransformation, and excretion) and pharmacodynamics (relations of drug concentration and action) of drug metabolism. Both aspects of drug metabolism can be affected by the biology of nutrition. Whereas food intake patterns and nutritional status are known to be capable of affecting drug pharmacokinetics (48), relatively little attention has been paid to the roles of nutrients in drug metabolism beyond effects on mixed function oxidases involved in drug metabolism and pharmacodynamics (49). The NABV framework would prompt researchable questions addressing the interrelations of multiple nutrients in systems regulating drug metabolism (i.e., the nutriome of pharmacology), implications of multiple nutrient deficiencies, effects of xenobiotics on nutrient utilization and function, and the interactions of drug, nutritional status, and the gut microbiome. It would also prompt inquiries into the physiological responses to disease (e.g., the impact of the acute-phase response on nutrient function) and the relation of nutritional status to drug metabolism during development.
Again, the evolving COVID-19 pandemic provides a compelling example of why we need to integrate the NABV approach more fully into our understanding and evaluation of this complex system. As highlighted in the recent review by Bhandari et al. (50), much has been learned and added to our treatment arsenal for COVID-19. In addition, we now have the benefit of myriad new vaccines that will require a functioning immune system to achieve immunity. Given what we already have learned about the impact of the pandemic on global food security, we can assume that there will be an increasing number of vulnerable populations that will become malnourished. How pre-existing and new malnutrition affect not only the susceptibility to SARS-CoV-2 infection but also the response to interventions (both treatment and vaccination) are compelling issues that warrant close attention.
By promoting ecological approaches in these research areas, the NABV framework would call for the consideration of a wider array of variables, which is likely to enhance the rigor and reproducibility of such studies to the end of meeting the goals of precision medicine through the development of more sensitive and specific interventions informed by more inclusive nutrition assessment tools.
Anemia
Reduction of the global prevalence of anemia is a target for many public health efforts. The presumption by many is that anemia is synonymous with nutritional iron deficiency (NID). In fact, there is a common view that 50% of anemia is caused by NID (51); however, it has recently been estimated that perhaps as little as 25% or 37% of anemia in school-aged children and women of reproductive age, respectively, is due to NID (52). Moreover, ∼40% of the world's women are anemic (53). This limits their work and learning capacities, predisposes them to infection, and places pregnant women at increased risk of poor birth outcomes and dying during delivery.
Both anemia and NID are significant global health concerns, but they are not the same. Although NID remains the major micronutrient deficiency worldwide, the conflation of NID with anemia impacts our ability to address anemia from a public health perspective and obfuscates the realities that: 1) anemia is a multifactorial disease reflecting the influence of both the internal (e.g., genetics, inflammation, etc.) and external (e.g., nutrition, sanitation, infection, altitude, etc.) ecologies; 2) we need better tools to make diagnoses leading to targeted treatment; and 3) hemoglobin concentration has value as a biomarker of hematology, but it is not a biomarker of NID and should not be used as a public health trigger for interventions targeting NID. Fundamentally, if correcting “anemia” is the public health target, we need answers to critical questions, including:
What are the contributions of various factors (nutritional, physiological, and genetic) to the etiology of anemia?
If the cause of anemia is nutritional, what other micronutrients—iron, folate, vitamin B-12, vitamin B-6, etc.—are responsible?
How do we assess anemia and/or measure hemoglobin concentrations (venous or capillary sampling?) (54) and interpret those results in the absence of relevant biomarkers of nutrient status or the consideration of other potential causes?
As with other infections, anemia has been implicated as an outcome of SARS-CoV-2 infection. And, as with other infections, the association of COVID-19 with anemia is considered most prominently in the context of the food insecurity scenario, that is, COVID-19 causes food insecurity, malnutrition, and NID (55). There are many reasons to assume that SARS-CoV-2 infection might lead to anemia, not the least of which is that it clearly causes a profound inflammatory response that could result in a functional (nonnutritional) iron deficiency and concomitant adverse functional outcomes (56, 57). In light of the complexity of this potential relation, the ability to make a plausible case for either etiology, assessment, or treatment dictates a comprehensive and integrated approach as outlined by the NABV framework.
Using a more ecological view, the NABV framework employed in any particular location would prompt a more precise approach to determining the role of nutrition in the pathogenesis and prevalence of anemia and would afford an opportunity to consider the specific environmental context and its implications for intervention (24).
Stunting
Impaired child growth characterized by stunting (low height-for-age z-score) affects >20% of children younger than 5 y. Although stunting is a global phenomenon, it is more prevalent in resource-poor communities, particularly in sub-Saharan Africa and Southeast Asia (58). Although stunting is seen as a result of nutritional deficits with myriad concomitant factors (e.g., intrauterine growth restriction, chronic infection, poor maternal health, genetics, and other environmental factors), its physiological bases are not well understood and raise questions about its utility as a public health target (59, 60). The physiology of stunting is complex, involving both lean and fat mass, and children with stunting are at risk of poor outcomes, including an increased risk of obesity (61).
As with the previous examples, stunting as a global health indicator has been highlighted as a potential outcome of COVID-19–driven food insecurity (62). Again, employing the linear logic of the disease leading to food insecurity, malnutrition, and then the usual complications of poor nutrition does not allow for a closer examination of the biological connection between the risk of, prevention or treatment of, and outcome of the infection. Is there a plausible connection between the infection and the outcome, that is, stunting? If so, what role does nutrition play, and how can we improve the precision of both the assessment and interventions to address these scenarios?
Stunting is yet another compelling example of the need to account for both the external and internal ecologies to improve precision in surveillance and treatment. In addressing stunting, the NABV framework would prompt a more integrated approach for the evaluation of its physiological, nutritional, and environmental basis, thereby improving the precision of assessment and treatment.
Tying it all together
Global challenges like COVID-19 reflect a continuum of events from risk to outcome to treatment. This continuum is circular rather than linear and provides the opportunity for continual input throughout the cycle to inform each stage leading to improved precision of assessment and intervention. This continuum reflects the ecology of COVID-19 (or any other disease) and reinforces the need for a more comprehensive and integrated approach. With specific regard to the cases discussed above, each is a significant public health challenge in its own right and has become a focal point of interest in the context of COVID-19. Unfortunately, although there has been increasing attention paid to each of the cases addressed, the primary challenges remain: 1) we must address the current lack of evidence sufficient to support policy and standards of care; and 2) we must cultivate a knowledge-based view of the role of nutritional status in developing such policies and standards. These constitute significant challenges to addressing the pandemic and developing useful messaging for a fearful community.
Conclusions
An important goal of biomedical research is to achieve a level of precision in health promotion, disease prevention, and treatment. The core premise is that context matters, and one size does not fit all. The NABV framework is an ecological approach to conceptualizing complex problems in which nutritional status is involved as either a cause, an effect, or (more likely) both cause and effect. Such problems include those that have been persistent in public health, including stunting and anemia.
The NABV framework calls for a clearer understanding of the multiple nutrients interacting within systems—the nutriome. This understanding requires:
Consideration of a wider array of end points to account for both food/nutrient exposure as well as impacts on nutrient status and function to improve the validity and reliability of methods of assessment
A deeper understanding of the intersections of biological systems, for example, anemia, iron interventions, and the microbiome (63)
Improved understanding and exploitation of the linkages of nutrient biomarkers and bioindicators reflecting the functions of multiple metabolic/physiological systems of interest
Improved collection and analyses of large data sets made interpretable at the point of care
Viewing nutritional status as a biological variable and integrating the various relations among nutrient-related measures and their effects on health and disease highlights the complexity and importance of nutrition science. The NABV approach will also help facilitate achieving the goals of precision medicine, because nutritional status is highly individualized, even in persons with similar food habits, thus affecting physiological function, immune competence, and drug efficacy in idiosyncratic ways.
The earlier eras of nutrition science revealed the various nutrients, both essential (i.e., those that must be provided via a dietary/exogenous source) and nonessential (i.e., those that can be endogenously produced), that play indispensable roles in metabolic and physiological systems. Subsequent research on the functional effects of single nutrients along with population-based epidemiology have been used to infer the public health implications of common dietary patterns. These efforts have focused largely on the presumed causal character of nutrient functions and have not always appreciated that nutrient functions can be related to functions of other nutrients and metabolites. Studies of dietary patterns have also been limited by their attention to either the internal (plausible biological mechanisms, validity of nutritional assessment methods) and external (accuracy of exposure measures) ecologies, weakening apparent cause–effect inferences. As a result, the advancement of our understanding of the actual role of nutrition in the support of health promotion and disease prevention has been limited.
The NABV framework offers the potential to transcend those limitations. It is an ecological approach facilitated by systems-based nutriomics methods that are now feasible because of advances in analytical and data processing methodologies. Methods are now available to consider the large arrays of factors interacting to influence the functions of nutrients, and these afford unprecedented opportunities to develop evidence-informed public health programs/policies. Standards of care can now be based on integrated, multidisciplinary approaches that include an understanding of nutrition science and human biology and that adhere to the principles of rigor and reproducibility. The challenges are daunting, but we should not be daunted by them. It will take a concerted effort, but the return will be well worth it.
ACKNOWLEDGEMENTS
We acknowledge the role of BioCentric, Inc. staff in editing and formatting the manuscript in accordance with the journal style.
The authors’ responsibilities were as follows—DJR: oversaw the compilation and revisions of the review with input of all authors throughout the process; DJR, ALS, AAB, GFC: edited all sections of the manuscript and had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: GFC has no relevant financial or personal relationships to declare. He is a part-time employee of Tufts University, Boston, MA, and a consultant to the Cincinnati Children's Hospital and Research Center, Cincinnati, OH, and to Clemson University, Clemson, SC. ALS is an employee of the Academy of Nutrition and Dietetics, serves on the Scientific Advisory Board for the American Council on Exercise, and for Nephroceuticals, Inc., and is on the Executive Committee for the International Society of Renal Nutrition and Metabolism. DJR and AAB report no conflicts of interest.
The contents of this article represent the authors’ views and do not constitute an official position of the National Institutes of Health or the US Government.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Abbreviations used: BOND, Biomarkers of Nutrition for Development; COVID-19, coronavirus disease 2019; NABV, nutritional status as a biological variable; NID, nutritional iron deficiency; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Contributor Information
Daniel J Raiten, Pediatric Growth and Nutrition Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
Gerald F Combs, Jr, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
Alison L Steiber, Academy of Nutrition and Dietetics, Cleveland, OH, USA.
Andrew A Bremer, Pediatric Growth and Nutrition Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
References
- 1.Mozaffarian D, Rosenberg I, Uauy R. History of modern nutrition science–implications for current research, dietary guidelines, and food policy. BMJ. 2018;361:k2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Headey D, Heidkamp R, Osendarp S, Ruel M, Scott N, Black R, Shekar M, Bouis H, Flory A, Haddad Let al. Impacts of COVID-19 on childhood malnutrition and nutrition-related mortality. Lancet. 2020;396:519–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guthrie HA. Introductory nutrition. 6th ed. St Louis: Times Mirror/Mosby College Pub; 1986. [Google Scholar]
- 4.National Research Council Food and Nutrition Board . Recommended dietary allowances. Nutr Rev. 1943;1:164–8. [Google Scholar]
- 5.Raiten DJ, Combs GF. Nutrition ecology: an integrated approach to address the intersection of climate/environmental change, food systems, nutrition and health. In: Fan S, Yosef S, Pandya-Lorch Reditors. Agriculture for improved nutrition: seizing the momentum. Boston: CAB International; 2019. p. 68–80. [Google Scholar]
- 6.O'Gorman A, Brennan L. The role of metabolomics in determination of new dietary biomarkers. Proc Nutr Soc. 2017;76:295–302. [DOI] [PubMed] [Google Scholar]
- 7.Frongillo EA, Baranowski T, Subar AF, Tooze JA, Kirkpatrick SI. Establishing validity and cross-context equivalence of measures and indicators. J Acad Nutr Diet. 2019;119:1817–30. [DOI] [PubMed] [Google Scholar]
- 8.Dao MC, Subar AF, Warthon-Medina M, Cade JE, Burrows T, Golley RK, Forouhi NG, Pearce M, Holmes BA. Dietary assessment toolkits: an overview. Public Health Nutr. 2019;22:404–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoody EE, Spahn JM, Casavale KO. The Pregnancy and Birth to 24 Months Project: a series of systematic reviews on diet and health. Am J Clin Nutr. 2019;109(Suppl 1):685S–97S. [DOI] [PubMed] [Google Scholar]
- 10.Raghavan R, Dreibelbis C, Kingshipp BL, Wong YP, Abrams B, Gernand AD, Rasmussen KM, Siega-Riz AM, Stang J, Casavale KOet al. Dietary patterns before and during pregnancy and birth outcomes: a systematic review. Am J Clin Nutr. 2019;109(Suppl 1):729S–56S. [DOI] [PubMed] [Google Scholar]
- 11.Raghavan R, Dreibelbis C, Kingshipp BL, Wong YP, Abrams B, Gernand AD, Rasmussen KM, Siega-Riz AM, Stang J, Casavale KOet al. Dietary patterns before and during pregnancy and maternal outcomes: a systematic review. Am J Clin Nutr. 2019;109(Suppl 1):705S–28S. [DOI] [PubMed] [Google Scholar]
- 12.Obbagy JE, Spahn JM, Wong YP, Psota TL, Spill MK, Dreibelbis C, Gungor DE, Nadaud P, Raghavan R, Callahan EHet al. Systematic review methods for the Pregnancy and Birth to 24 Months Project. Am J Clin Nutr. 2019;109(Suppl 1):698S–704S. [DOI] [PubMed] [Google Scholar]
- 13.Rodgers GP, Collins FS. Precision nutrition—the answer to “what to eat to stay healthy.” JAMA. 2020;324:735–6. [DOI] [PubMed] [Google Scholar]
- 14.Raiten DJ, Combs GF Jr. Directions in nutritional assessment. Biomarkers and bioindicators: providing clarity in the face of complexity. Sight Life Mag. 2015;29:39–44. [Google Scholar]
- 15.Rohner F, Zimmermann M, Jooste P, Pandav C, Caldwell K, Raghavan R, Raiten DJ. Biomarkers of nutrition for development—iodine review. J Nutr. 2014;144:1322S–42S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey LB, Stover P, McNulty H, Fenech M, Gregory J, Mills J, Pfeiffer CM, Fazili Z, Zhang M, Ueland PMet al. Biomarkers of nutrition for development—folate review. J Nutr. 2015;145:1636S–80S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King JC, Brown KH, Gibson RS, Krebs NF, Lowe NM, Siekmann JH, Raiten DJ. Biomarkers of nutrition for development (BOND)—zinc review. J Nutr. 2015;146:858S–85S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of nutrition for development (BOND)—vitamin A review. J Nutr. 2016;146:1816S–48S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch S, Pfeiffer CM, Georgieff MK, Brittenham G, Fairweather-Tait S, Hurrell RF, McArdle HJ, Raiten DJ. Biomarkers of nutrition for development (BOND)—iron review. J Nutr. 2018;148(Suppl 1):1001S–67S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen LH, Miller JW, de Groot L, Rosenberg IH, Smith DA, Refsum H, Raiten DJ. Biomarkers of nutrition for development (BOND): vitamin B-12 review. J Nutr. 2018;148(Suppl 4):1995S–2027S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raiten DJ, Ashour FA, Ross AC, Meydani SN, Dawson HD, Stephensen CB, Brabin BJ, Suchdev PS, van Ommen B, the INSPIRE Consultative Group. Inflammation and nutritional science for programs/policies and interpretation of research evidence (INSPIRE). J Nutr. 2015;145:1039S–108S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suchdev P, Namaste S, Aaron GJ, Raiten DJ, Brown KL, Flores-Ayala RC. Overview of the biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Adv Nutr. 2016;7:349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waterlow JC. Classification and definition of protein-calorie malnutrition. BMJ. 1972;3:566–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Ommen B, Fairweather-Tait S, Freidig A, Kardinaal A, Scalbert A, Wopereis S. A network biology model of micronutrient related health. Br J Nutr. 2008;99(S3):S72–80. [DOI] [PubMed] [Google Scholar]
- 25.Fenech MF. Nutriomes and personalised nutrition for DNA damage prevention, telomere integrity maintenance and cancer growth control. Cancer Treat Res. 2014;159:427–41. [DOI] [PubMed] [Google Scholar]
- 26.Fenech MF. Nutriomes and nutrient arrays – the key to personalised nutrition for DNA damage prevention and cancer growth control. Genome Integr. 2010;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Erk MJ, Wopereis S, Rubingh C, van Vliet T, Verheij E, Cnubben NH, Pedersen TL, Newman JW, Smilde AK, van der Greef Jet al. Insight in modulation of inflammation in response to diclofenac intervention: a human intervention study. BMC Med Genet. 2010;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shanahan ER, McMaster JJ, Staudacher HM. Conducting research on diet-microbiome interactions: a review of current challenges, essential methodological principles, and recommendations for best practice in study design. J Hum Nutr Diet. 2021;1–14. [DOI] [PubMed] [Google Scholar]
- 29.Heckman EL, Doe CQ. Establishment and maintenance of neural circuit architecture. J Neurosci. 2021;41:1119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zwilling CE, Talukdar T, Zamroziewicz MK, Barbey AK. Nutrient biomarker patterns, cognitive function, and fMRI measures of network efficiency in the aging brain. Neuroimage. 2019;188:239–51. [DOI] [PubMed] [Google Scholar]
- 31.Raiten DJ, Raghavan R, Porter A, Obbagy JE, Spahn JM. Executive summary: evaluating the evidence base to support the inclusion of infants and children from birth to 24 mo of age in the Dietary Guidelines for Americans—“the B-24 Project.”. Am J Clin Nutr. 2014;99:663S–91S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X, Jackson RT, Khan SA, Ahuja J, Pehrsson PR. Human milk nutrient composition in the United States: current knowledge, challenges, and research needs. Curr Dev Nutr. 2018;2:nzy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee H, Padhi E, Hasegawa Y, Larke J, Parenti M, Wang A, Hernell O, Lönnerdal B, Slupsky C. Compositional dynamics of the milk fat globule and its role in infant development. Front Pediatr. 2018;6:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thurl S, Munzert M, Boehm G, Matthews C, Stahl B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr Rev. 2017;75:920–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demmelmair H, Prell C, Timby N, Lönnerdal B. Benefits of lactoferrin, osteopontin and milk fat globule membranes for infants. Nutrients. 2017;9:817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen SD, Beverly RL, Dallas DC. Milk proteins are predigested within the human mammary gland. J Mammary Gland Biol Neoplasia. 2017;22:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Togo A, Dufour JC, Lagier JC, Dubourg G, Raoult D, Million M. Repertoire of human breast and milk microbiota: a systematic review. Future Microbiol. 2019;14:623–41. [DOI] [PubMed] [Google Scholar]
- 38.Latuga MS, Stuebe A, Seed PC. A review of the source and function of microbiota in breast milk. Semin Reprod Med. 2014;32:68–73. [DOI] [PubMed] [Google Scholar]
- 39.Witkowska-Zimny M, Kaminska-El-Hassan E. Cells of human breast milk. Cell Mol Biol Lett. 2017;22:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hahn-Holbrook J, Saxbe D, Bixby C, Steele C, Glynn L. Human milk as “chrononutrition”: implications for child health and development. Pediatr Res. 2019;85:936–42. [DOI] [PubMed] [Google Scholar]
- 41.Spatz DL, Froh EB. Birth and breastfeeding in the hospital setting during the COVID-19 pandemic. MCN. Am J Matern Child Nurs. 2021;46:30–5. [DOI] [PubMed] [Google Scholar]
- 42.AAP News . AAP issues guidance on breastfeeding during COVID-19 pandemic. [Internet]. American Academy of Pediatrics; April 23, 2020 [cited January 14, 2021] Available from:https://www.aappublications.org/news/2020/04/23/covid19breastfeeding042320.
- 43.American College of Obstetricians & Gynecologists . Coronavirus (COVID-19), pregnancy, and breastfeeding: a message for patients. [Internet]. Updated December 22, 2020 [cited January 14, 2021]. Available from: https://www.acog.org/womens-health/faqs/coronavirus-covid-19-pregnancy-and-breastfeeding.
- 44.McGuire MK, Seppo A, Goga A, Buonsenso D, Collado MC, Donovan SM, Müller JA, Ofman G, Monroy-Valle M, O'Connor DLet al. Best practices for human milk collection for COVID-19 research. Breastfeed Med. 2021;16(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pace RM, Williams JE, Järvinen KM, Belfort MB, Pace CD, Lackey KA, Gogel AC, Nguyen-Contant P, Kanagaiah P, Fitzgerald Tet al. COVID-19 and human milk: SARS-CoV-2, antibodies, and neutralizing capacity. medRxiv. 2021;12(1):e03192–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lackey KA, Pace RM, Williams JE, Bode L, Donovan SM, Järvinen KM, Seppo AE, Raiten DJ, Meehan CL, McGuire MAet al. SARS-CoV-2 and human milk: what is the evidence?. Matern Child Nutr. 2020;16:e13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rollins N, Minckas N, Jehan F, Lodha R, Raiten D, Thorne C, Van de Perre P, Ververs M, Walker N, Bahl Ret al. A public health approach for deciding policy on infant feeding and mother-infant contact in the context of COVID-19. Lancet Glob Health. 2021;9:e552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Won CS, Oberlies NH, Paine MF. Mechanisms underlying food-drug interactions: inhibition of intestinal metabolism and transport. Pharmacol Ther. 2012;136:186–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raiten DJ. Nutrition and pharmacology: general principles and implications for HIV. Am J Clin Nutr. 2011;94:1697S–702S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhandari R, Khanna G, Kuhad A. Pharmacological insight into potential therapeutic agents for the deadly Covid-19 pandemic. Eur J Pharmacol. 2021;890:173643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907–16. [DOI] [PubMed] [Google Scholar]
- 52.Petry N, Olofin I, Hurrell RF, Boy E, Wirth JP, Moursi M, Donahue Angel M, Rohner F. The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: a systematic analysis of national surveys. Nutrients. 2016;8:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Development Initiatives . Global Nutrition Report: shining a light to spur action on nutrition. Bristol (UK): Development Initiatives; 2018. [Google Scholar]
- 54.Neufeld L, Larson L, Kurpad A, Mburu S, Martorell R, Brown K. Hemoglobin concentration and anemia diagnosis in venous and capillary blood: biological basis and policy implications. Ann N Y Acad Sci. 2019;1450:172–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kulkarni R, Rajput U, Dawre R, Sonkawade N, Pawar S, Sonteke S, Varvatte B, Aathira KC, Gadekar K, Varma Set al. Severe malnutrition and anemia are associated with severe COVID in infants. J Trop Pediatr Epub. 2021:67:fmaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bellmann-Weiler R, Lanser L, Barket R, Rangger L, Schapfl A, Schaber M, Fritsche G, Wöll E, Weiss G. Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID-19 infection. J Clin Med. 2020;9:2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sonnweber T, Boehm A, Sahanic S, Pizzini A, Aichner M, Sonnweber B, Kurz K, Koppelstätter S, Haschka D, Petzer Vet al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients' performance: a prospective observational cohort study. Respir Res. 2020;21:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Onis M, Blossner M, Borghi E. Prevalence and trends of stunting among preschool children, 1990–2020. Public Health Nutr. 2012;15:142–8. [DOI] [PubMed] [Google Scholar]
- 59.Raiten DJ, Bremer AA. Exploring the nutritional ecology of stunting: new approaches to an old problem. Nutrients. 2020;12:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leroy JL, Frongillo EA. Perspective: what does stunting really mean? A critical review of the evidence. Adv Nutr. 2019;10:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Briend A, Khara T, Dolan C. Wasting and stunting—similarities and differences: policy and programmatic implications. Food Nutr Bull. 2015;36(1 Suppl 1):S15–23. [DOI] [PubMed] [Google Scholar]
- 62.Akseer N, Kandru G, Keats EC, Bhutta ZA. COVID-19 pandemic and mitigation strategies: implications for maternal and child health and nutrition. Am J Clin Nutr. 2020;112:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paganini D, Zimmermann MB. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: a review. Am J Clin Nutr. 2017;106(Suppl 6):1688S–93S. [DOI] [PMC free article] [PubMed] [Google Scholar]