Abstract
Ultra-processed foods are industrially manufactured ready-to-eat or ready-to-heat formulations containing food additives and little or no whole foods, in contrast to processed foods, which are whole foods preserved by traditional techniques such as canning or pickling. Recent epidemiological studies suggest that higher consumption of ultra-processed food is associated with increased risk of cardiovascular disease (CVD). However, epidemiological evidence needs to be corroborated with criteria of biological plausibility. This review summarizes the current evidence on the putative biological mechanisms underlying the associations between ultra-processed foods and CVD. Research ranging from laboratory-based to prospective epidemiological studies and experimental evidence suggest that ultra-processed foods may affect cardiometabolic health through a myriad of mechanisms, beyond the traditionally recognized individual nutrients. Processing induces significant changes to the food matrix, for which ultra-processed foods may affect health outcomes differently than unrefined whole foods with similar nutritional composition. Notably, the highly degraded physical structure of ultra-processed foods may affect cardiometabolic health by influencing absorption kinetics, satiety, glycemic response, and the gut microbiota composition and function. Food additives and neo-formed contaminants produced during processing may also play a role in CVD risk. Key biological pathways include altered serum lipid concentrations, modified gut microbiota and host–microbiota interactions, obesity, inflammation, oxidative stress, dysglycemia, insulin resistance, and hypertension. Further research is warranted to clarify the proportional harm associated with the nutritional composition, food additives, physical structure, and other attributes of ultra-processed foods. Understanding how ultra-processing changes whole foods and through which pathways these foods affect health is a prerequisite for eliminating harmful processing techniques and ingredients.
Keywords: NOVA, cardiometabolic health, obesity, food additives, microbiome
Introduction
Cardiovascular diseases (CVDs) constitute the leading cause of mortality in the United States and globally, accounting for approximately one-third of all deaths (1, 2). Poor diet is the leading risk factor related to the overall CVD burden in the United States and is estimated to be associated with more than half of US deaths due to coronary heart disease (CHD) and stroke (3). Diet unequivocally plays a pivotal role in primary and secondary CVD prevention (4). Current research and practice are focusing on overall dietary patterns and quality, rather than single nutrients (4).
Food processing can profoundly influence diet quality; however, there is a broad spectrum of processing, ranging from minimal processing (e.g., frozen vegetables, dried fruits without added sugar or additives, pasteurized milk) to ultra-processing (e.g., carbonated soft drinks, fast foods, industrially produced breads, hot dogs) (Figure 1) (5). Ultra-processed foods, defined as industrially manufactured, ready-to-eat, or ready-to-heat formulations containing little whole foods (5), provide the majority of energy in the US diet (6). These foods do not only include so-called junk foods but also many foods that are marketed and perceived as healthy, such as flavored yogurts, reduced-calorie/low-fat products, breakfast cereals, and products “enriched” with beneficial nutrients (5).
FIGURE 1.
Food-processing spectrum as defined by the NOVA framework. NOVA (a name, not an acronym) classifies foods as minimally processed foods, processed culinary ingredients, processed foods, and ultra-processed foods based on the extent and purpose of industrial processing they have been submitted to (5). The figure provides examples of foods and the types of processing techniques used within each level.
While the evidence is still emerging, recent epidemiological studies suggest that higher consumption of ultra-processed foods is associated with increased risk of CVD. In the Framingham Offspring Study, after 18 y of follow-up, each additional daily serving of ultra-processed foods was associated with a 7% increase in the risk of incident CVD (7). Likewise, increased consumption of ultra-processed foods was associated with a 12% increased risk of CVD in the French NutriNet-Santé cohort study (8). Furthermore, there is convincing evidence from meta-analyses that specific ultra-processed products (processed meat, sugar-sweetened beverages) and nutrients that are abundant in ultra-processed foods (trans fats, sodium) increase the risk of CVD (9). Ultra-processed foods have also been linked to multiple CVD risk factors including obesity, hypertension, and metabolic syndrome in epidemiologic studies (6, 10) The current strength and level of evidence of these associations led the American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease to recommend avoiding trans fats and minimizing certain ultra-processed foods (processed red meats, foods high in refined carbohydrates such as refined grain products, and sweetened beverages) (11). Nevertheless, the potential health hazards associated with a broad range of ultra-processed foods, beyond the above-mentioned products, remain largely unrecognized by clinicians and public health professionals.
A major challenge for establishing evidence in cardiovascular nutrition is the paucity of large randomized clinical trials (RCTs) with hard cardiovascular endpoints. As a consequence, in establishing causality, evidence from well-conducted prospective observational studies needs to be corroborated with criteria of biological plausibility. Key to both the theory and practice of preventive cardiology is the understanding of mechanisms of action of various food ingredients and dietary patterns embodying the human “food-ome” (12). Therefore, the objective of this review is to summarize the available evidence regarding the putative biological mechanisms underlying the associations between intake of ultra-processed foods and the risk of cardiometabolic and atherosclerotic CVDs.
Potential Mechanisms Linking Ultra-processed Foods to CVD Risk
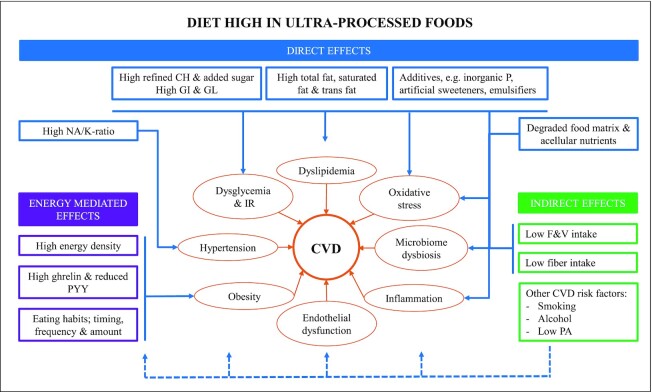
Processing can alter the nutritional (macro- and micronutrient content), physical (food structure), and chemical (presence of artificial sweeteners, additives, and neo-formed contaminants, glycemic index and load) characteristics of foods in ways that may alter their healthfulness. Food processing may also influence long-term dietary behaviors, satiety signaling, and food reward systems. The biological pathways through which ultra-processed foods influence cardiovascular health may involve complex mechanisms and synergies between many compounds and characteristics of ultra-processed foods, which are not yet fully understood (Figure 2). The underlying physio-pathological interrelations in atherogenesis and CVD progression are complex and involve multiple pathways. A constellation of factors such as metabolic, proinflammatory, prothrombotic, pro-oxidative, and endothelial dysfunction coexist and potentiate each other. A myriad of nuances exist. For instance, various levels of glucose metabolism alterations activate specific inflammatory patterns (13), while immune factors interact bilaterally with gut microbiota (14). Further, most cardiovascular risk factors are playing a role in triggering endothelial dysfunction and injury, as well as maintaining a prothrombotic, proinflammatory molecular environment (15). Through these factors and via a complex network of molecular feedback loop mechanisms, atherogenesis escalates and perpetuates (16), culminating with various cardiovascular events.
FIGURE 2.
Potential biological mechanisms underlying the association between ultra-processed foods and CVD. The diagram describes plausible biological pathways through which a diet high in ultra-processed foods may contribute to CVD. Key mechanisms include altered serum lipid concentrations, modified gut microbiota and host–microbiota interactions, obesity, inflammation, oxidative stress, dysglycemia, insulin resistance, and hypertension and hormonal imbalances. Arrows indicate stimulation of a pathway. CH, carbohydrates; CVD, cardiovascular disease; F&V, fruit and vegetables; GI, glycemic index; GL, glycemic load; IR, insulin resistance; NA/K-ratio, sodium-to-potassium ratio; P, phosphorus; PA, physical activity; PYY; peptide YY.
Excess weight and body adiposity
Higher consumption of ultra-processed foods has been associated with excess adiposity in epidemiological and experimental studies (6, 17–22). Notably, a recent RCT by Hall et al. (19) demonstrated that an ultra-processed diet increased ad libitum energy intake by ∼500 kcal/d and caused weight gain compared with a minimally processed diet. In the prospective Seguimiento University of Navarra study and the Brazilian Longitudinal Study of Adult Health, adult participants in the highest compared with the lowest quartile of ultra-processed food intake had a 26–29% higher risk of becoming overweight over ∼9 and 4 y of follow-up, respectively [HR (95% CI): 1.26 (1.10, 1.45) and 1.29 (1.08, 1.53), respectively] (17, 20). In 3 cross-sectional studies conducted in Brazil and the United States, participants with the highest compared with the lowest intake of ultra-processed foods (top vs. bottom quintile/quartile) had 31–48% higher odds of being overweight (6, 21), 41–97% higher odds of being obese (6, 21, 22), and 41–62% higher odds of abdominal obesity (defined as waist circumference ≥88/102 cm for men and women, respectively) (6, 21).
Ultra-processed foods may contribute to weight gain through their nutritional profile and by displacing low-calorie, nutritious, minimally processed foods from the diet (23). The high-intensity flavoring resulting from high levels of fat, salt, sugar, and artificial flavorings make ultra-processed products highly palatable, for which endogenous satiety mechanisms may be superseded (23). Ultra-processed foods are, on average, more hypercaloric and less satiating than minimally processed foods and culinary preparations based on minimally processed foods (23, 24). As human satiety mechanisms are more sensitive to volume than caloric content, foods with higher energy density may facilitate excessive energy intakes (25).
The higher energy density and oro-sensory characteristics of ultra-processed foods (i.e., softer, less fibrous textures that are easy to chew) may also enable greater energy intakes in a shorter amount of time. Experimental studies suggest that increased eating rates can result in increased energy intakes, potentially due to delayed satiety signaling (26). Indeed, in the RCT by Hall et al. (19), average eating rate, measured as grams/minute and kilocalories/minute, was significantly higher during the ultra-processed diet compared with the minimally processed diet. Furthermore, higher concentrations of the appetite-suppressing hormone peptide YY were noted during the minimally processed diet compared with the ultra-processed diet (19).
Finally, the convenience, omnipresence, affordability, large portion sizes, and persuasive marketing of ultra-processed food may promote poor dietary habits, snacking, and overeating, potentially contributing to increased energy intake (27). Experimental studies indicate that visual cues and images of palatable foods, such as food advertisements, can activate brain regions that induce overeating and may encourage eating in the absence of hunger (28).
Glycemic response and related insulin dysregulation
Ultra-processed foods are the main source of total and added sugar in the US diet (29). Excessive sugar intakes, particularly in the form of sugar-sweetened beverages, are associated with multiple CVD risk factors, including overall and abdominal adiposity, hypertension, insulin resistance, type 2 diabetes, and dyslipidemia (30, 31). High-glycemic-index and high-glycemic-load diets are associated with increased risk of CHD and type 2 diabetes (9). The glycemic response to a particular food is determined by the quantity and quality of carbohydrates (source and digestibility); content of fat, protein, and fiber; food matrix structure (e.g., liquid or solid); and degree of processing (32). Experimental evidence supports that ultra-processed foods are, on average, more hyperglycemic than minimally and moderately processed foods (24). Hyperglycemia increases the risk of CVD by promoting weight gain, inflammation, oxidative stress, and endothelial dysfunction (32).
The physiological effects of low-calorie sweeteners are not well understood. Observational data from prospective studies suggest that regular consumption is associated with increased risk of type 2 diabetes (33). Animal and human experimental studies suggest that certain low-calorie sweeteners, such as sucralose, may alter glucose metabolism by influencing brain regulatory centers (34) and reduce insulin sensitivity by impairing gut–brain regulatory pathways (35).
Hypertension
Excessive dietary intake of sodium is associated with increased risk of hypertension, which is a major risk factor for CVD and stroke (9, 36). The mechanisms linking increased sodium intake and high blood pressure are complex and still not fully understood. Several pathways are implicated, including perturbed renal sodium homeostasis, extrarenal sodium handling, direct effects on vascular walls, and systemic and local neuro-hormonal pathways in conjunction with salt-sensitivity phenotypes (36, 37). Metabolic, hemodynamic, and inflammatory changes lead to volume expansion, water retention, endothelial stiffness, increased peripheral resistance, and subsequent increased blood pressure (36, 37). Epidemiological studies support that a higher sodium-to-potassium ratio (≥1.0) is associated with increased risk of CVD mortality, while higher potassium intakes are associated with lower risks (38). Commercially processed foods constitute the primary source of sodium in the US diet, while minimally processed foods such as milk, fruits, and vegetables are the primary dietary sources of potassium (39). A greater reliance on ultra-processed foods and lower intake of potassium-rich, minimally processed foods may therefore influence CVD risk by increasing sodium intake and altering the sodium-to-potassium ratio of the diet.
Gut microbiome
Diet is a key modulator of the gut microbiota composition and activity, with potential implications for host health (40). Alterations of the gut microbial ecosystem (changes in the relative abundance of specific microbial taxa or in gut bacterial diversity) and intestinal barrier dysfunction have been linked to excess adiposity, insulin resistance, type 2 diabetes, and CVD (40, 41). The underlying mechanisms are hypothesized to include increased bacterial production of atherogenic metabolites such as choline, trimethylamine N-oxide, and betaine; endotoxemia-induced low-grade systemic inflammation; modulation of the host immune system; and weight gain. Further mechanisms may involve increased calorie intake by the host, alterations in energy homeostasis, and hepatic lipid accumulation (40).
Modification of the food matrix and the fat and fiber content of foods, and the inclusion of certain food additives during processing, may influence gut microbiota composition, function, and bacteria–host interactions (42). Dietary fibers (primarily soluble fiber), abundant in many unrefined plant foods, provide substrate for microbial fermentation and high-fiber diets are associated with increased microbial gene richness and microbial production of SCFA fermentation end-products, which have key functions in regulating host metabolism and immune system, and may attenuate inflammation (43–45). Western dietary patterns, characterized by low fiber intake and high consumption of sugar, fat, and animal protein, are associated with a distinct and less diverse microbiotic profile compared with diets rich in minimally processed plant foods (44). Importantly, low-fiber diets shift the gut microbial metabolism toward the utilization of proteins and host mucins, resulting in degradation of the intestinal mucus layer and increased susceptibility to chronic inflammatory diseases (44, 45). However, compared with diets based on unprocessed whole grains, high-fiber diets based on processed, extruded grains reduced bacterial diversity in animal models and led to less enrichment in beneficial butyrate-producing bacteria (46). Furthermore, evidence from animal and human studies supports that high-fat Western-type diets may promote low-grade systemic inflammation and metabolic disorders by increasing circulating concentrations of LPS derived from intestinal bacteria (metabolic endotoxemia) (47, 48). Animal studies also support that diets high in glucose or fructose may reduce gut microbiota diversity and increase intestinal permeability (49). In a human experimental study, endotoxemia increased after a meal of highly processed, high-fat, high-carbohydrate foods but not after an isocaloric meal including minimally processed whole grains, fruits, and nuts (50).
Changes to the physical structure of the food matrix during processing can alter nutrient bioaccessibility and absorption kinetics, with implications for gut microbiota composition, metabolism, and growth (42, 51). While plant-based, minimally processed foods have intact fibrous cell walls that provide substrate for fiber-degrading bacteria in the colon and ensure a slow release of nutrients along the digestive tract (52), nutrients in ultra-processed foods are largely acellular. Experimental studies suggest that the large share of acellular nutrients in ultra-processed foods leads to a high nutrient availability in the small intestine, which, in turn, promotes an inflammatory gut microbiota associated with cardiometabolic conditions (42, 51, 53).
Furthermore, accumulating evidence from animal and human studies suggests that consumption of low-calorie sweeteners may disrupt the diversity and balance of the gut microbiota and promote metabolic disorders and insulin resistance (54). Emulsifiers, used in industrial food processing, have in animal studies been shown to increase the proinflammatory potential of the microbiome by increasing microbiotic virulence factors (55).
Plasma lipid profile
Plasma lipid concentrations are influenced by the quantity and quality of dietary fats and carbohydrates, which, in turn, are influenced by food processing (56). There is scientific consensus that industrially produced trans fatty acids, present in partially hydrogenated vegetable oils in commercially manufactured foods, adversely affect blood lipoprotein profile and increase the risk of CHD (9, 56). As a result, the FDA has taken steps to remove partially hydrogenated vegetable oils from the US food supply (57). Evidence linking saturated fat to increased LDL cholesterol has led to longstanding recommendations to limit the intake of saturated fat (56). Major dietary sources of saturated fats include minimally processed foods, such as whole-fat dairy, processed culinary ingredients, such as cream and butter, but also ingredients primarily used in ultra-processed foods, such as palm oil and palm kernel oil (56). However, accumulating evidence supports that the health effects of saturated fat in the diet depend on the food source, and on the interacting effects of naturally occurring food components and contaminants generated from high-heat processing of oils (58). For example, it has been shown that processed coconut oil, but not virgin coconut oil, raises serum cholesterol concentrations in rats (59, 60). Additionally, intake of full-fat dairy and unprocessed meat does not seem to increase the risk of cardiometabolic diseases (58). As a result, scholars have recommended replacing dietary saturated fat targets with food-based guidelines for saturated fat intake that take into account the whole food matrix and degree of processing (58).
Furthermore, research suggests that the influence of carbohydrates on serum lipid concentrations is also determined by their source and processing level. Intake of sugar increases serum triglycerides, while consumption of whole grains lowers total and LDL cholesterol and, to a limited extent, triglycerides (61). Consumption of minimally processed whole grains, such as oatmeal, instead of ultra-processed, high-sugar, refined grain products may therefore reduce CVD risk by promoting healthy plasma lipid concentrations.
Endocrine pathways
Concern has been raised regarding the cumulative intake of phosphorus in the US diet due to the extensive use of inorganic phosphate salts as additives in industrial food processing (62). Inorganic phosphate is absorbed to a greater extent (80–100%) than organic phosphorus that occurs naturally in foods (40–60%) (62). Epidemiological studies assessing serum phosphate and experimental studies of excess dietary phosphorus further support an association with CVD (63). These effects are possibly mediated by the disruption of hormonal regulation of extracellular phosphate through increased secretion of parathyroid hormone and fibroblast growth hormone 23, both promoters of arterial calcification (63). Elevated tissue and serum phosphate concentrations are also linked to increased oxidative stress of the endothelial cells and impaired endothelial function (63).
Industrially manufactured foods are often sold in elaborate packaging; the packaging materials may contain endocrine-disrupting chemicals, such as bisphenol A (BPA). Limited epidemiological evidence suggests that greater exposure to BPA is associated with increased prevalence of major CVD risk factors, including diabetes, overall and abdominal obesity, and hypertension (64). BPA, which is structurally similar to 17β-estradiol, has been shown to promote insulin resistance, oxidative stress, inflammation, adipogenesis, and pancreatic B-cell dysfunction by binding to estrogen-related receptors (64). The detailed mechanisms of action remain poorly understood (64). BPA is widespread in the environment, but foods stored or reheated in BPA-lined containers are believed to constitute the primary source of human exposure (64). Although empirical evidence is limited, observational and intervention studies suggest that diets based on minimally processed and fresh foods are associated with lower urinary concentrations of BPA (65, 66). An analysis using data from the nationally representative NHANES 2013–2014 found that greater consumption of sugar-sweetened beverages, but not ultra-processed foods overall, was associated with higher urinary concentrations of BPA (65). Although, due to safety concerns, BPA is increasingly replaced by its structurally homologous compounds (e.g., bisphenol S and bisphenol F), these may have similar endocrine-disrupting effects (67).
Other mechanisms
Extensive heat treatment during processing and preparation leads to the formation of advanced glycation end-products (AGEs), which have been linked to increased oxidative stress and inflammation and may play a role in CVD etiology (68). AGE formation is particularly high in animal foods high in protein and fat, and increases with higher temperatures, greater cooking time, and the absence of moisture (68). Dry-heat processing and deep-frying of carbohydrate-rich foods (e.g., crackers, chips, cookies, and French fries) also accelerate AGE formation (68). A full understanding of the importance of dietary AGEs in CVD development and the causal nature of the AGE–CVD link remains to be determined (69).
Observational studies suggest that fruit and vegetable intake is inversely associated with CVD, CHD, stroke, and major CVD risk factors, including type 2 diabetes (9). Available evidence also supports a protective effect against CHD and stroke (9). The beneficial effect of fruits and vegetables is attributed to their high content of fiber, micronutrients, and phytochemicals, which may reduce CVD risk through multiple mechanisms, such as reduced oxidative stress, improved insulin sensitivity and serum lipid profile, and lower blood pressure (70). It is plausible that higher intakes of ultra-processed foods increase the risk of CVD and its intermediate risk factors by displacing less-processed, more nutritious foods such as fruit and vegetables from the diet. Conversely, part of the protective effect of high fruit and vegetable consumption may be due to reduced intakes of less-healthy ultra-processed foods.
Areas for Future Research
Research is warranted to further clarify the biological mechanisms through which ultra-processed foods may influence CVD risk, and the proportional harm associated with the nutritional composition, food additives, physical structure, and other attributes of ultra-processed foods. Understanding how ultra-processing changes whole foods and through which pathways these foods affect health is a prerequisite for eliminating harmful processing techniques and ingredients and identify “optimal” levels of processing. The effects of ultra-processed foods on the gut microbiota and microbiota–host interactions constitute an area of special scientific interest, given the accumulating evidence regarding the role of the gut microbiome in cardiometabolic health and diet–disease relations. Furthermore, it is imperative to elucidate the health effects of chronic exposures to the multiple food additives and substances present in ultra-processed foods and their packaging, as current regulations limiting the maximum levels of individual food additives do not take into account potential “cocktail effects” of combinations of compounds from various food sources.
Conclusions
Ultra-processing of foods may affect cardiometabolic health through a myriad of mechanisms, beyond the traditionally recognized individual nutrients. Research ranging from laboratory-based to prospective epidemiological studies and experimental evidence points to a network of mechanisms involving both direct and indirect effects. Ultra-processed foods not only bring poor-quality nutrients and ingredients, including refined carbohydrates and sugar, into the diet but also displace healthy whole foods, such as fruits and vegetables. Limited evidence supports that specific compounds, added or generated via aggressive processing, may also contribute to CVD development. Plausible biological pathways include increased energy intake, changes to the gut microbiome, alterations in the gut–brain satiety signaling, and hormonal effects. These chronic exposures may act as atherogenic initiators by targeting key atherogenic processes, such as dysglycemia, dyslipidemia, hypertension, obesity, inflammation, endothelial dysfunction, and oxidative stress.
Corroborated with other lines of evidence from prospective investigations, the current literature supports that consumption of ultra-processed food may be detrimental to cardiovascular health. A better understanding of the mechanisms underlying the ultra-processed food and CVD link will inform clinical practice and dietary guidance. Nutrition counseling is the cornerstone of preventive cardiology and should address ultra-processed foods, highlighting their pervasive metabolic effects, ubiquitous availability, and “hidden” sources in a variety of food formulations.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—FJ: conceptualized the review and the conducted literature searches; FJ, NP, and GV: contributed to the writing and reviewed the manuscript for important intellectual content; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this work.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: AGE, advanced glycation end-product; BPA, bisphenol A; CHD, coronary heart disease; CVD, cardiovascular disease; RCT, randomized clinical trial.
Contributor Information
Filippa Juul, Department of Epidemiology, School of Global Public Health, New York University, New York, NY, USA.
Georgeta Vaidean, School of Pharmacy and Health Sciences, Fairleigh Dickinson University, Florham Park, NJ, USA; Division of Cardiology, Lenox Hill Hospital, Northwell Health, New York, NY, USA.
Niyati Parekh, Public Health Nutrition Program, School of Global Public Health, New York University, New York, NY, USA; Department of Population Health, NYU Grossman School of Medicine, New York University, New York, NY, USA; Rory Meyers College of Nursing, New York University, New York, NY, USA.
References
- 1.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam Ket al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FNet al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. [DOI] [PubMed] [Google Scholar]
- 3.Micha R, Penalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. 2017;317(9):912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monteiro CA, Cannon G, Levy RB, Moubarac JC, Louzada ML, Rauber F, Khandpur N, Cediel G, Neri D, Martinez-Steele Eet al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22(5):936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juul F, Martinez-Steele E, Parekh N, Monteiro CA, Chang VW. Ultra-processed food consumption and excess weight among US adults. Br J Nutr. 2018;120(1):90–100. [DOI] [PubMed] [Google Scholar]
- 7.Juul F, Vaidean G, Lin Y, Deierlein A, Parekh N. Ultra-processed foods and incident cardiovascular disease in the Framingham Offspring Study. J Am Coll Cardiol. 2021;77(12):1520–31. [DOI] [PubMed] [Google Scholar]
- 8.Srour B, Fezeu LK, Kesse-Guyot E, Alles B, Mejean C, Andrianasolo RM, Chazelas E, Deschasaux M, Hercberg S, Galan Pet al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ. 2019;365:l1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micha R, Shulkin ML, Penalvo JL, Khatibzadeh S, Singh GM, Rao M, Fahimi S, Powles J, Mozaffarian D. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS One. 2017;12(4):e0175149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elizabeth L, Machado P, Zinocker M, Baker P, Lawrence M. Ultra-processed foods and health outcomes: a narrative review. Nutrients. 2020;12(7):1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JWet al. ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):e177–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman AM, Morris PB, Barnard N, Esselstyn CB, Ros E, Agatston A, Devries S, O'Keefe J, Miller M, Ornish Det al. Trending cardiovascular nutrition controversies. J Am Coll Cardiol. 2017;69(9):1172–87. [DOI] [PubMed] [Google Scholar]
- 13.Menini S, Iacobini C, Vitale M, Pugliese G. The inflammasome in chronic complications of diabetes and related metabolic disorders. Cells. 2020;9(8):1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeh CF, Chen YH, Liu SF, Kao HL, Wu MS, Yang KC, Wu WK. Mutual interplay of host immune system and gut microbiota in the immunopathology of atherosclerosis. Int J Mol Sci. 2020;21(22):8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mundi S, Massaro M, Scoditti E, Carluccio MA, van Hinsbergh VWM, Iruela-Arispe ML, De Caterina R. Endothelial permeability, LDL deposition, and cardiovascular risk factors—a review. Cardiovasc Res. 2018;114(1):35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poston RN. Atherosclerosis: integration of its pathogenesis as a self-perpetuating propagating inflammation: a review. Cardiovasc Endocrinol Metab. 2019;8(2):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendonca RD, Pimenta AM, Gea A, de la Fuente-Arrillaga C, Martinez-Gonzalez MA, Lopes AC, Bes-Rastrollo M. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr. 2016;104(5):1433–40. [DOI] [PubMed] [Google Scholar]
- 18.Poti JM, Braga B, Qin B. Ultra-processed food intake and obesity: what really matters for health—processing or nutrient content?. Curr Obes Rep. 2017;6(4):420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, Chung ST, Costa E, Courville A, Darcey Vet al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canhada SL, Luft VC, Giatti L, Duncan BB, Chor D, Fonseca M, Matos SMA, Molina M, Barreto SM, Levy RBet al. Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Public Health Nutr. 2020;23(6):1076–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva FM, Giatti L, de Figueiredo RC, Molina M, de Oliveira Cardoso L, Duncan BB, Barreto SM. Consumption of ultra-processed food and obesity: cross sectional results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) cohort (2008–2010). Public Health Nutr. 2018;21(12):2271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louzada ML, Baraldi LG, Steele EM, Martins AP, Canella DS, Moubarac JC, Levy RB, Cannon G, Afshin A, Imamura Fet al. Consumption of ultra-processed foods and obesity in Brazilian adolescents and adults. Prev Med. 2015;81:9–15. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig DS. Technology, diet, and the burden of chronic disease. JAMA. 2011;305(13):1352–3. [DOI] [PubMed] [Google Scholar]
- 24.Fardet A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: a preliminary study with 98 ready-to-eat foods. Food Funct. 2016;7(5):2338–46. [DOI] [PubMed] [Google Scholar]
- 25.Rolls BJ. Dietary energy density: applying behavioural science to weight management. Nutr Bull. 2017;42(3):246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson E, Almiron-Roig E, Rutters F, de Graaf C, Forde CG, Tudur Smith C, Nolan SJ, Jebb SA. A systematic review and meta-analysis examining the effect of eating rate on energy intake and hunger. Am J Clin Nutr. 2014;100(1):123–51. [DOI] [PubMed] [Google Scholar]
- 27.Moubarac JC, Martins AP, Claro RM, Levy RB, Cannon G, Monteiro CA. Consumption of ultra-processed foods and likely impact on human health: evidence from Canada. Public Health Nutr. 2013;16(12):2240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanhope KL, Goran MI, Bosy-Westphal A, King JC, Schmidt LA, Schwarz JM, Stice E, Sylvetsky AC, Turnbaugh PJ, Bray GAet al. Pathways and mechanisms linking dietary components to cardiometabolic disease: thinking beyond calories. Obes Rev. 2018;19(9):1205–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez Steele E, Baraldi LG, Louzada ML, Moubarac JC, Mozaffarian D, Monteiro CA. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open. 2016;6(3):e009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keller A, Heitmann BL, Olsen N. Sugar-sweetened beverages, vascular risk factors and events: a systematic literature review. Public Health Nutr. 2015;18(7):1145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Te Morenga LA, Howatson AJ, Jones RM, Mann J. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr. 2014;100(1):65–79. [DOI] [PubMed] [Google Scholar]
- 32.Blaak EE, Antoine JM, Benton D, Bjorck I, Bozzetto L, Brouns F, Diamant M, Dye L, Hulshof T, Holst JJet al. Impact of postprandial glycaemia on health and prevention of disease. Obes Rev. 2012;13(10):923–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azad MB, Abou-Setta AM, Chauhan BF, Rabbani R, Lys J, Copstein L, Mann A, Jeyaraman MM, Reid AE, Fiander Met al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. Can Med Assoc J. 2017;189(28):E929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan CB, Hashemi Z, Subhan FB. The impact of low and no-caloric sweeteners on glucose absorption, incretin secretion, and glucose tolerance. Appl Physiol Nutr Metab. 2017;42(8):793–801. [DOI] [PubMed] [Google Scholar]
- 35.Dalenberg JR, Patel BP, Denis R, Veldhuizen MG, Nakamura Y, Vinke PC, Luquet S, Small DM. Short-term consumption of sucralose with, but not without, carbohydrate impairs neural and metabolic sensitivity to sugar in humans. Cell Metab. 2020;31(3):493–502, e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He FJ, Tan M, Ma Y, MacGregor GA. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(6):632–47. [DOI] [PubMed] [Google Scholar]
- 37.Pilic L, Pedlar CR, Mavrommatis Y. Salt-sensitive hypertension: mechanisms and effects of dietary and other lifestyle factors. Nutr Rev. 2016;74(10):645–58. [DOI] [PubMed] [Google Scholar]
- 38.O'Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath Aet al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371(7):612–23. [DOI] [PubMed] [Google Scholar]
- 39.Woodruff RC, Zhao L, Ahuja JKC, Gillespie C, Goldman J, Harris DM, Jackson SL, Moshfegh A, Rhodes D, Sebastian RSet al. Top food category contributors to sodium and potassium intake—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2020;69(32):1064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boulange CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zinocker MK, Lindseth IA. The Western diet-microbiome-host interaction and its role in metabolic disease. Nutrients. 2018;10(3):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delzenne NM, Olivares M, Neyrinck AM, Beaumont M, Kjolbaek L, Larsen TM, Benitez-Paez A, Romani-Perez M, Garcia-Campayo V, Bosscher Det al. Nutritional interest of dietary fiber and prebiotics in obesity: lessons from the MyNewGut consortium. Clin Nutr. 2020;39(2):414–24. [DOI] [PubMed] [Google Scholar]
- 44.Makki K, Deehan EC, Walter J, Backhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–15. [DOI] [PubMed] [Google Scholar]
- 45.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014;20(5):779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moen B, Berget I, Rud I, Hole AS, Kjos NP, Sahlstrom S. Extrusion of barley and oat influence the fecal microbiota and SCFA profile of growing pigs. Food Funct. 2016;7(2):1024–32. [DOI] [PubMed] [Google Scholar]
- 47.Guerville M, Leroy A, Sinquin A, Laugerette F, Michalski MC, Boudry G. Western-diet consumption induces alteration of barrier function mechanisms in the ileum that correlates with metabolic endotoxemia in rats. Am J Physiol Endocrinol Metab. 2017;313(2):E107–E20. [DOI] [PubMed] [Google Scholar]
- 48.Lopez-Moreno J, Garcia-Carpintero S, Jimenez-Lucena R, Haro C, Rangel-Zuniga OA, Blanco-Rojo R, Yubero-Serrano EM, Tinahones FJ, Delgado-Lista J, Perez-Martinez Pet al. Effect of dietary lipids on endotoxemia influences postprandial inflammatory response. J Agric Food Chem. 2017;65(35):7756–63. [DOI] [PubMed] [Google Scholar]
- 49.Do MH, Lee E, Oh MJ, Kim Y, Park HY. High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients. 2018;10(6):761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32(12):2281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spreadbury I. Comparison with ancestral diets suggests dense acellular carbohydrates promote an inflammatory microbiota, and may be the primary dietary cause of leptin resistance and obesity. Diabetes Metab Syndr Obes. 2012;5:175–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grundy MM, Lapsley K, Ellis PR. A review of the impact of processing on nutrient bioaccessibility and digestion of almonds. Int J Food Sci Technol. 2016;51(9):1937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Penttinen R, Kinnula H, Lipponen A, Bamford JK, Sundberg LR. High nutrient concentration can induce virulence factor expression and cause higher virulence in an environmentally transmitted pathogen. Microb Ecol. 2016;72(4):955–64. [DOI] [PubMed] [Google Scholar]
- 54.Nettleton JE, Reimer RA, Shearer J. Reshaping the gut microbiota: impact of low calorie sweeteners and the link to insulin resistance?. Physiol Behav. 2016;164(Pt B):488–93. [DOI] [PubMed] [Google Scholar]
- 55.Chassaing B, Van de Wiele T, De Bodt J, Marzorati M, Gewirtz AT. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66(8):1414–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JGet al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136(3):e1–23. [DOI] [PubMed] [Google Scholar]
- 57.FDA . Final determination regarding partially hydrogenated oils (removing trans fats). [Internet]. USDA, Food and Drug Administration; 2015; [updated January 4, 2018; accessed 2020 Nov 10]. Available from: https://www.fda.gov/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/ucm449162.htm. [Google Scholar]
- 58.Astrup A, Magkos F, Bier DM, Brenna JT, de Oliveira Otto MC, Hill JO, King JC, Mente A, Ordovas JM, Volek JSet al. Saturated fats and health: a reassessment and proposal for food-based recommendations: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(7):844–57. [DOI] [PubMed] [Google Scholar]
- 59.Arunima S, Rajamohan T. Influence of virgin coconut oil-enriched diet on the transcriptional regulation of fatty acid synthesis and oxidation in rats—a comparative study. Br J Nutr. 2014;111(10):1782–90. [DOI] [PubMed] [Google Scholar]
- 60.Brenna JT, Kothapalli KS. Commentary on “Influence of virgin coconut oil-enriched diet on the transcriptional regulation of fatty acid synthesis and oxidation in rats—a comparative study” by Sakunthala Arunima and Thankappan Rajamohan. Br J Nutr. 2014;112(9):1425–6. [DOI] [PubMed] [Google Scholar]
- 61.Hollaender PL, Ross AB, Kristensen M. Whole-grain and blood lipid changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr. 2015;102(3):556–72. [DOI] [PubMed] [Google Scholar]
- 62.Calvo MS, Moshfegh AJ, Tucker KL. Assessing the health impact of phosphorus in the food supply: issues and considerations. Adv Nutr. 2014;5(1):104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calvo MS, Uribarri J. Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am J Clin Nutr. 2013;98(1):6–15. [DOI] [PubMed] [Google Scholar]
- 64.Ranciere F, Lyons JG, Loh VH, Botton J, Galloway T, Wang T, Shaw JE, Magliano DJ. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ Health. 2015;14:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buckley JP, Kim H, Wong E, Rebholz CM. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ Int. 2019;131:105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng CY, Tsai EM, Kao TH, Lai TC, Liang SS, Chiu CC, Wang TN. Canned food intake and urinary bisphenol A concentrations: a randomized crossover intervention study. Environ Sci Pollut Res Int. 2019;26(27):27999–8009. [DOI] [PubMed] [Google Scholar]
- 67.Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000–2014. Environ Sci Technol. 2015;49(19):11834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poulsen MW, Hedegaard RV, Andersen JM, de Courten B, Bugel S, Nielsen J, Skibsted LH, Dragsted LO. Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol. 2013;60:10–37. [DOI] [PubMed] [Google Scholar]
- 69.Chaudhuri J, Bains Y, Guha S, Kahn A, Hall D, Bose N, Gugliucci A, Kapahi P. The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell Metab. 2018;28(3):337–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alissa EM, Ferns GA. Dietary fruits and vegetables and cardiovascular diseases risk. Crit Rev Food Sci Nutr. 2017;57(9):1950–62. [DOI] [PubMed] [Google Scholar]