Abstract
While sodium and potassium are individually important for blood pressure (BP) regulation, the relative contribution of sodium to potassium intake has not been sufficiently investigated. This study aimed to evaluate the association between urinary sodium to potassium ratio (UNa: K) and systolic and diastolic BP in adults. A systematic review (PROSPERO; CRD42016035296) was conducted and was reported according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Three scientific databases (MEDLINE, Scopus, Web of Science) were searched to March 2020 while reference lists of included articles were further hand-searched. Randomized controlled trials (RCT), cohort and cross-sectional studies that assessed 24-h urinary excretion in adults were included. Data from eligible studies were extracted and summarized. Random effects meta-analysis was conducted on RCT data to assess standardized mean differences (SMD) in systolic and diastolic BP according to 24-h UNa: K. Thirty-nine studies were included. Meta-analysis of 5 RCTs found a lower UNa: K ratio to be associated with a significantly greater reduction in systolic and diastolic BP compared with a higher UNa: K ratio [SMD: −1.09 (95% CI: −1.91, −0.28) mmHg and −1.42 (95% CI: −2.24, −0.59) mmHg, respectively]. Heterogeneity between RCTs was observed in systolic and diastolic BP (I2 = 97%, P < 0.0001 and I2 = 98%, P < 0.0001, respectively). The current body of evidence demonstrates that a lower 24-h UNa: K ratio is associated with lower BP in adults. Dietary strategies to achieve an increase in potassium while at the same time lowering sodium would be beneficial in lowering BP.
Keywords: hypertension, pre-hypertension, DASH diet, dietary patterns, sodium, potassium, public health, sodium-to-potassium ratio
Introduction
Hypertension and hypertension-related diseases such as stroke, renal dysfunction, and ischemic heart disease are major global health challenges. In addition, high blood pressure (BP) is one of the leading risk factors for cardiovascular disease globally (1). Despite the well-acknowledged relationship between a Western dietary pattern and lifestyle-related diseases (2), effective strategies to encourage the adoption of healthier dietary patterns have not been widely implemented. Dietary sodium and potassium intake are important in the etiology and pathogenesis of hypertension. Numerous studies, including the large ecological INTERSALT study, have demonstrated the influence and association of dietary sodium intake on BP (3). Two meta-analyses concluded that reduced dietary sodium intake resulted in reduced BP in both normotensive and hypertensive participants (4, 5) but there was a noticeably greater reduction in systolic BP (SBP) in hypertensive participants. Furthermore, 2 randomized double-blinded crossover clinical trials (RCTs) have demonstrated that restricting dietary sodium in pre-hypertensive individuals results in a gradual, significant decrease in BP (6, 7).
Conversely, an inverse association between BP and both dietary potassium intake and urinary potassium excretion has been shown in adults (3). Potassium supplementation has resulted in significant reductions in BP (8, 9); however, similar to sodium interventions, the benefits vary across the range of BP distribution.
The Dietary Approaches to Stop Hypertension (DASH) diet includes both low sodium and high potassium sources of food to collectively reduce SBP and diastolic BP (DBP) (10–12) within a whole-of-diet eating plan. The degree to which the DASH diet beneficially lowers BP varies between normotensive, pre-hypertensive, and hypertensive adults (13). Two short-term RCTs showed that the DASH diet in participants with hypertension had a greater reduction in BP than in their normotensive counterparts (11, 12).
It has been suggested that the ratio of sodium to potassium intake could be more important than the intake of either of these minerals alone (14–16). An analysis of the Japanese Nagahama study cohort reported that spot urine samples of sodium and potassium concentrations (n = 18,505) were positively correlated with BP, but that this association was steeper in older groups (17).
Given a lack of synthesis of the evidence to date, we conducted a systematic literature review to identify the association between urinary sodium to potassium ratio (UNa: K) and BP in adults.
Methods
This systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The study protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO), (http://www.crd.york.ac.uk/PROSPERO, registration number: CRD42016035296). The PICO (problem, intervention, comparison, outcome) question investigated was “Does a low versus high urinary sodium-to-potassium ratio result in BP reduction in adults?” Search terms and combinations relating to sodium to potassium ratio and hypertension were used, including: “sodium potassium ratio” OR “sodium to potassium ratio” OR “sodium-potassium ratio” OR “Na: K ratio” OR “sodium/potassium ratio” OR “Na/K ratio” OR “sodium: potassium ratio” AND “blood pressure” OR “hypertension.” In addition, hand-searching of reference lists of retrieved articles was undertaken. Three databases were searched; Scopus, Web of Science, and MEDLINE (Ovid), and all years of publication included until 30 March, 2020.
Types of studies eligible for inclusion were RCTs, cohort studies, and cross-sectional studies. Inclusion criteria were: 1) studies published in English; 2) conducted in adults over 18 years; 3) assessment of UNa: K using 24-h urine collections; and 4) reporting a primary outcome of measured office or ambulatory 24-h SBP and DBP.
Exclusion criteria included: 1) non-English articles; 2) animal studies; and 3) studies of potassium supplementation. All retrieved studies were exported to EndNote (EndNote X7, Thomson Reuters, 2014) and Excel (Microsoft Excel 2013, Redmont, WA) to identify any duplicates. Relevant studies were screened based on title and abstracts by 3 authors (RN; RI; RH), and full-text articles retrieved for further screening.
Assessment of level of evidence and quality rating
Data was extracted to summary tables, to include: the year of publishing; the country the study was conducted in; characteristics of study participants; urinary sodium and potassium; sodium to potassium ratio; study type; intervention method; change in SBP and DBP; regression coefficients for cross-sectional studies; quality rating and level of evidence. The level of evidence of all included studies was categorized according to Australian National Health and Medical Research Council (NHMRC) criteria, while the quality of the studies was assessed using the American Dietetic Association (ADA) Evidence Analysis Manual quality rating criteria checklist (18). The quality rating included questions relating to relevance and validity of the research studies in terms of study design, execution, statistical analysis, results, conclusions, and sponsorship. Data extraction and quality rating was conducted by two authors (RN, RI) and consensus reached with a third author (KC). In the case of missing data, of interest in the included studies, primary authors were contacted by email, with 1 follow-up email in the case of non-response.
Meta-analysis
Cochrane Review Manager Software, RevMan Version 5.3 (The Cochrane Collaboration, Copenhagen) was used to conduct a meta-analysis of identified RCTs. Random effects meta-analysis was conducted to calculate standardized mean differences (SMD) (with 95% CI) in change or final values for systolic and diastolic BP. Study groups were categorized as “lower sodium to potassium ratio” (experimental) and “higher sodium to potassium ratio” (control). In cases where there were more than 2 groups, study group data were pooled for analysis whereby data from groups that reduced Na: K ratio over the course of the study were compared with groups with increased Na: K ratio. Heterogeneity of the meta-analysis was determined using X2 and I2 tests with 75% considered as substantial heterogeneity (19).
Results
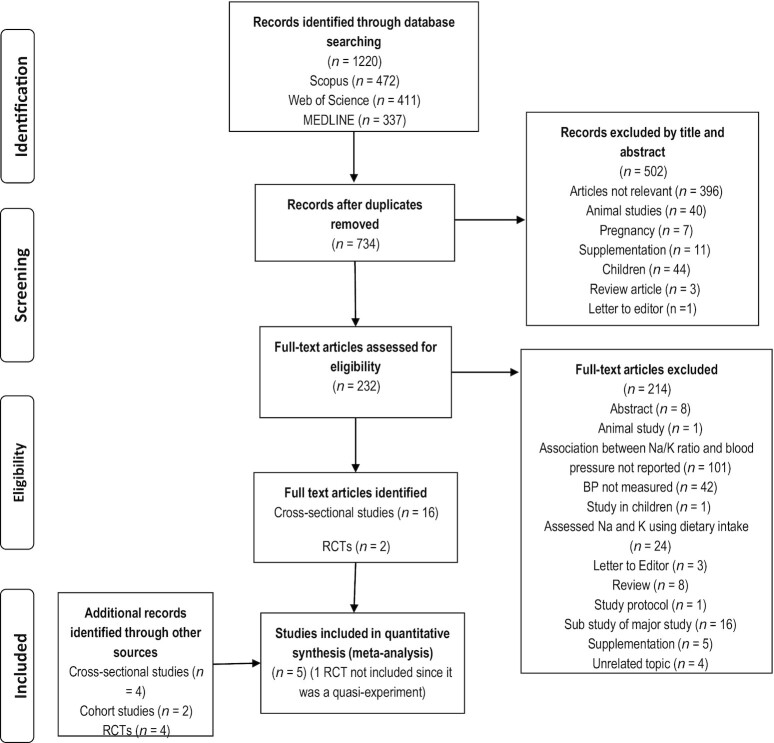
The literature search resulted in 734 articles after removal of duplicates, with 28 full papers included in this review (Figure 1). Articles included 6 RCTs (20–25) (only 5 of which were included in the meta-analysis since 1 was a quasi-experiment (24)), 2 cohort studies (14, 26), and 20 cross-sectional studies. All the 28 studies conducted 24-h urinary analysis at baseline, which is considered the “gold standard” measure for objectively assessing urinary sodium and potassium excretion (27) (Tables 1 and 2). Of these studies, 8 were conducted in China (15, 28–30, 29, 31, 32), 4 in the United States (21, 24, 25, 33), 2 in the United Kingdom (34, 35), and 3 in Australia (22, 36, 37, 38). One was a global study (39) while the remaining studies were from Indonesia (40), Switzerland (41), Iran (42), Korea (43), Mexico (44), South Africa (16), Canada (23), and Zaire (45).
FIGURE 1.
PRISMA flow chart for study selection process. RCT, randomized controlled trials.
TABLE 1.
Summary of experimental studies exploring the association between sodium to potassium ratio and blood pressure in adults, using 24-h urine collections1
| Reference, year, country, study type | Subjects (n, age, sex, BMI2, BP3) | Intervention | Urinary Na4 and K4 | Na:K ratio5 | Change SBP and DBP (mmHg) | Conclusions, quality rating6, and level of evidence (NHMRC)7 |
|---|---|---|---|---|---|---|
| Wing et al. (24) 1984USAQuasi-experiment | Total n = 41, HTWeight intervention: n = 19Age: 56.53 ± 6.08ySBP: 155.74 ± 14.40 mmHgDBP: 93.47 ± 6.28 mmHgNa:K intervention:n = 22Age: 52.77 ± 9.84ySBP: 147.00 ± 20.24 mmHgDBP: 93.59 ± 8.86 mmHg | Weight control:5% reduction in body weightNo change in Na, K, or Na:K ratioNa:K interventionDecrease Na intake <70 mEq/dayIncrease K intake >100 mEq/day | Urinary Na:Weight intervention:Pre: 147.75 ± 69.27 mEq/dayPost: 139.87 ± 107.62 mEq/dayChange: −7.88 mEq/day Na:K intervention:Pre: 168.87 ± 96.01 mEq/dayPost: 107.72 ± 48.09 mEq/dayChange: −61.15 mEq/dayUrinary K: | Weight intervention:- Pre: 2.61- Post: 2.69- Change: +0.08Na:K intervention:- Pre: 2.50- Post: 1.52- Change: −0.98 | SBP:Weight intervention: -13.94 mmHg***Na:K intervention:-6.20 mmHg** DBP:Weight intervention:-7.71 mmHg**Na:K intervention: -4.1 mmHg** | Weight reduction reduced BP more than dietary modification aimed to lower |
| Na/K ratioØ, II | ||||||
| Weight intervention: | ||||||
| Pre: 59.09 ± 17.61 mEq/day | ||||||
| Post: 52.49 ± 22.33 mEq/day | ||||||
| Change: -6.60 mEq/day | ||||||
| Na:K intervention: | ||||||
| Pre: 76.11 ± 32.69 mEq/day | ||||||
| Post: 82.34 ± 35.22 mEq/day | ||||||
| Change: +6.23 mEq/day | ||||||
| Lin et al. (25) 2012 | Total n = 20, HT | Intervention: | Urinary Na: | Control: | SBP: | Lower Na/K ratio associated with lowered BPP, II |
| USA | Age: 44.3 ± 7.8y | DASH diet | Control: | Baseline: 2.76 ± 1.13 | Control: -0.9 ± 16.4 mmHg | |
| RCT | BMI: 33.9 ± 6.6 kg/m2 | Control: | Baseline: 122.6 ± 40.82 mmol/day | Change: 0.61 ± 1.20 | DASH: -9.6 ± 11.2* mmHg | |
| SBP: 144.2 ± 9.38 mmHg | American control diet | Change: 17.85 ± 65.93 mmol/day | DASH: | DBP | ||
| DBP: 88.5 ± 6.03 mmHg | DASH: | Baseline: 3.20 ± 0.69 | Control: 1.6 ± 11.6 mmHg | |||
| Baseline: 131.4 ± 42.83 mmol/day | Change: −.06 ± 2.91 | DASH: -8.6 ± 9.1 * mmHg | ||||
| Change: -1.5 ± 64.40 mmol/day | ||||||
| Urinary K: | ||||||
| Control: | ||||||
| Baseline: 46.6 ± 13.3 mmol/day | ||||||
| Change: -7.9 ± 16.4 mmol/day | ||||||
| DASH: | ||||||
| Baseline: 42.2 ± 14.7 mmol/day | ||||||
| Change: 25.3 ± 28.6 mmol/day | ||||||
| Dodson (20) 1983UKRCT | Total n = 53, HTModified diet:Age: 53.6 ± 8.5y | Modified diet:High dietary fibre (35-40 g/day)High unrefined carbohydrate (65% E) | Urinary Na:Modified diet:- Pre: 195 ± 78.1 mmol/day | Modified diet:- Pre: 2.68 ± 1.08- Post: 1.82 ± 1.09 | SBPModified diet: -5.3 mmHgControl: +4.4 mmHg | Lower Na/K ratio associated with lowered BPØ, II |
| SBP: 173.8 ± 23.8 mmHg | Low fat (15% E) | - Post: 136.7 ± 70.2 mmol/day | Control: | DBP | ||
| DBP: 98.4 ± 9.2 mmHg | Low Na (40-450 mmol/day) | Control: | - Pre: 2.96 ± 1.39 | Modified diet: -6.7 mmHg*** | ||
| Control: | Normal K (70-80 mmol/day) | - Pre: 180.2 ± 58.2 mmol/day | - Post: 2.84 ± 1.33 | Control: +2.3 mmHg | ||
| Age: 55.3 ± 6.7y | Control: | - Post: 162.6 ± 48.1 mmol/day | ||||
| SBP: 169.7 ± 18.2 mmHg | Normal Western diet | Urinary K: | ||||
| DBP: 94.4 ± 7.3 mmHg | Modified diet: | |||||
| - Pre: 83.5 ± 61.5 mmol/day | ||||||
| - Post: 80.1 ± 25.5 mmol/day | ||||||
| Control: | ||||||
| - Pre: 66.9±20 mmol/day | ||||||
| - Post: 68.2 ± 21.7 mmol/day | ||||||
| Sacks FM et al. (21) 2001USARCT | Total n = 412,NT and HTDASH diet: | Intervention:DASH dietControl: | Urinary Na:High sodium level:- DASH: 144±58 mmol/day | High sodium level:- DASH: 1.92- Control: 3.53 | SBP net change (95% CI)High: -5.9 (−8.0 to -3.7) mmHgIntermediate: -5.0 (-7.6 to -2.5) mmHg | Lower Na/K ratio associated with lowered BPP, II |
| Age: 47 ± 10y | Usual American diet | - Control: 141 ± 55 mmol/day | Intermediate sodium: | Low: -2.2 (-4.4 to -0.1) mmHg | ||
| BMI: 29±5 kg/m2 | Intermediate sodium: | - DASH: 1.32 | DBP: (net change) | |||
| SBP: 134 ± 10 mmHg | - DASH: 107±52 mmol/day | - Control: 2.59 | High: -2.9 (-4.3 to -1.5) mmHg | |||
| DBP: 86 ± 5 mmHg | - Control: 106±44 mmol/day | Low sodium level: | Intermediate: -2.5 (-4.1 to -0.8) mmHg | |||
| Control diet: | Low sodium level: | - DASH: 0.83 | Low: -1.0 (-2.5 to -0.4) mmHg | |||
| Age: 49 ± 10y | - DASH: 67±46 mmol/day | - Control: 1.52 | ||||
| BMI: 30±5 kg/m2 | - Control: 64±37 mmol/day | |||||
| SBP: 135 ± 10 mmHg | Urinary K: | |||||
| DBP: 86 ± 4 mmHg | High sodium level: | |||||
| - DASH: 75±27 mmol/day | ||||||
| - Control: 40 ± 14 mmol/day | ||||||
| Intermediate sodium: | ||||||
| - DASH: 81±31 mmol/day | ||||||
| - Control: 41±14 mmol/day | ||||||
| Low sodium level: | ||||||
| - DASH: 81±29 mmol/day | ||||||
| - Control: 42±14 mmol/day | ||||||
| Nowson et al. (22) | Total n = 212, | A: Normal diet | Urinary Na: | A: | SBP: | Lower Na/K ratio associated with lowered BPP, II |
| 1988 | HT | B: High potassium diet | A: | Pre: 1.4 | A: -3.8 ± 1.0 mmHg | |
| Australia | Age: NR | C: Low sodium diet | Pre: 107 mmol/day | Post: 1.5 | B: -7.7 ± 1.1 mmHg | |
| RCT | BMI: NR | D: Low sodium, | Post: 107 mmol/day | B: | C: -8.9 ± 1.0 mmHg | |
| SBP: NR | high potassium diet | B: | Pre: 1.2 | D: -7.9 ± 0.9 mmHg | ||
| DBP: Mean of 4 readings | Pre: 92 mmol/day | Post: 0.9 | DBP: | |||
| between 90 and 100 mmHg | Post: 99 mmol/day | C: | A: -1.6 ± 0.6 mmHg | |||
| C: | Pre: 1.3 | B: -4.7 ± 0.7 mmHg | ||||
| Pre: 97 mmol/day | Post: 0.7 | C: -5.8 ± 0.6 mmHg | ||||
| Post: 50 mmol/day | D: | D: -4.2 ± 0.7 mmHg | ||||
| D: | Pre: 1.2 | |||||
| Pre: 93 mmol/day | Post: 0.5 | |||||
| Post: 49 mmol/day | ||||||
| Urinary K: | ||||||
| A: | ||||||
| Pre: 84 mmol/day | ||||||
| Post: 75 mmol/day | ||||||
| B: | ||||||
| Pre: 82 mmol/day | ||||||
| Post: 114 mmol/day | ||||||
| C: | ||||||
| Pre: 79 mmol/day | ||||||
| Post: 72 mmol/day | ||||||
| D: | ||||||
| Pre: 81 mmol/day | ||||||
| Post: 104 mmol/day | ||||||
| Jenkins et al. (23) | Total n = 241 | Two diets: | Urinary Na: | Portfolio diet: | SBP: | Lower Na/K ratio associated with lowered BPP, II |
| 2015 | Age: >20–85 y | Portfolio diet | NR | Baseline: | Portfolio diet: | |
| Canada | Portfolio diet: Mean (95% CI) | per 1000 kcal diet | Urinary K: | 2.1 (95% CI: 1.9, 2.3) | -0.11 mmHg | |
| RCT | BMI: 27 (95% CI: 26-27) | 9.8g viscous fibres | NR | Change: | DASH: | |
| SBP: 120 (95% CI: 118-122) mmHg | 22.5g soy protein | 0 (95% CI: −0.2, 0.2) | -0.08 mmHg | |||
| DBP: 73 (95% CI: 72-75) mmHg | 0.94g plant sterols | Control DASH: | DBP: | |||
| Control DASH diet: | Control (DASH diet) | Baseline: | Portfolio diet: | |||
| Mean (95% CI) | 2.1 (95% CI: 1.8, 2.3) | -0.18*** mmHg | ||||
| BMI: 27 (95% CI: 26-28) | Change: | DASH: | ||||
| SBP: 118 (95% CI: 115–121) mmHg | 0 (95% CI: −0.3, 0.2) | -0.03 mmHg | ||||
| DBP: 72 (95% CI: 70–74) mmHg |
*P < 0.05. **P < 0.01. ***P < 0.001. †Not included in current meta-analysis because it is a quasi-experimental trial. BMI, body mass index; CI, 95% confidence interval; DBP, diastolic blood pressure; HT, hypertensive; Pre-HT, pre-hypertensive; NR: not reported; SBP, systolic blood pressure.
Body mass index is Mean ± SD unless otherwise indicated.
Blood pressure is Mean ± SD unless otherwise indicated.
Mean ± SD (all such values).
Mean ± SD values or changes.
Quality rating: P = Positive, Ø = Neutral, − = Negative.
NHMRC (National Health and Medical Research Council) rating is classified as I, III-1, III-2, III-3 or IV.
TABLE 2.
Summary of cross-sectional studies exploring associations between sodium to potassium ratio and blood pressure in adults using 24-h urinary collection1
| Reference, year, country, study type | Subjects (n, age, sex, BMI2, BP3) | Urinary Na and K4 | Na:K ratio | Regression coefficient (95% CI) (SBP and DBP)5 | Conclusions | Quality rating6 and level of evidence (NHMRC)7 |
|---|---|---|---|---|---|---|
| Jackson et al. (35) | Total n: 766 | Urinary Na | HT: 3.18 | SBP | Urinary Na:K associated with SBP | P, IV |
| 2018 | HT n: 235 | HT: 162.60 ± 3.04 mmol/day | Pre-HT: 2.90 | 1.72 [0.76-2.68]** | ||
| USA | Age: 52.3 ± 0.7y | Pre-HT: 154.51 ± 2.61 mmol/day | Optimal: 2.90 | DBP | ||
| Cross-sectional | BMI: 32.6 ± 1.0 kg/m2 | Optimal: 159.04 ± 3.19 mmol/day | 0.30 [−0.53 - 1.12] | |||
| Pre-HT n: 183 | Urinary K | |||||
| Age: 45.0 ± 1.2y | HT: 51.12 ± 1.10 mmol/day | |||||
| BMI: 31.2 ± 0.8 kg/m2 | Pre-HT: 53.35 ± 1.29 mmol/day | |||||
| Optimal n: 348 | Optimal: 55.24 ± 1.37 mmol/day | |||||
| Age: 37.0 ± 1.0y | ||||||
| BMI: 27.5 ± 0.5 kg/m2 | ||||||
| Stamler et al. (41) | Total including INTERMAP | Urinary Na | INTERMAP: 3.89 ± 2.11 | SBP | Urinary Na:K ratio associated with SBP and DBP | P, IV |
| 2018 | n: 4680; NT and HT | Total: 181.10 ± 72.42 mmol/day | INTERMAP: 2.43 [1.50-3.37]**** | |||
| Global (Japan, China, UK, USA) | Age: 49.17 ± 5.47y | US participants: 162.58 ± 59.37 mmol/day | US participants: 3.08 ± 1.23 | US participants: 2.46 [1.35- 3.58]**** | ||
| Cross-sectional | BMI: 26.37 ± 5.47 kg/m2 | Urinary K | DBP | |||
| SBP: 118.93 ± 14.69 mmHg | Total: 53.16 ± 20.02 mmol/day | INTERMAP: 0.92 [0.30- 1.54]** | ||||
| DBP: 73.84 ± 10.03 mm Hg | US participants: 57.65 ± 20.91 mmol/day | US participants: 1.20 (0.42-1.97)** | ||||
| US participants n: 2195 | ||||||
| Age: 49.14 ± 5.39y | ||||||
| BMI: 28.90 ± 5.92 kg/m2 | ||||||
| SBP: 118.60 ± 13.89 mmHg | ||||||
| DBP: 73.41 ± 9.68 mmHg | ||||||
| Farapti et al. (42) | Total n: 51 | Urinary Na | Total: 5.28 ± 1.68 | SBP | Urinary Na:K ratio associated with SBP not DBP | Ø,IV |
| 2017 | Female subjects | Total: 104.85 ± 59.3 mmol/day | NT: 4.74 ± 1.36 | 3.89 [1.18-6.6]* | ||
| Indonesia | Age: 56.98 ± 5.7y | NT: 94.6 ± 41.1 mmol/day | HT: 6.01 ± 1.89 | Excluding participants taking antihypertensive drugs: 4.89 [1.93-7.84]* | ||
| Cross-sectional | BMI: 25.96 ± 4.85 kg/m2 | HT: 120.5 ± 81.0 mmol/day | ||||
| SBP:132.25 ± 17.78 mmHg | Urinary K | |||||
| DBP: 83.63 ± 10.3 mmHg | Total: 20.5 ± 9.7 mmol/day | |||||
| NT n: 32 | NT: 21.2 ± 10.18 mmol/day | DBP | ||||
| Age: 57.19 ± 6.85y | HT:19.50 ± 9.08 mmol/day | 1.72 [−0.189-3.63] | ||||
| BMI: 24.26 ± 5.24 kg/m2 | ||||||
| SBP: 121.09 ± 9.89 mmHg | ||||||
| DBP: 77.03 ± 6.33 mmHg | ||||||
| HT n: 19 | ||||||
| Age: 57.16 ± 3.45y | ||||||
| BMI: 28.82 ± 1.36 mmHg | ||||||
| SBP: 151.05 ± 10.75 mmHg | ||||||
| DBP: 94.74 ± 4.24 mmHg | ||||||
| Glatz et al. (43)□ | Total n: 1336 | Urinary Na | NT: 2.35 (2.29; 2.62) | SBP (quintiles of BP) | Urinary Na:K ratio associated with SBP | P, IV |
| 2017 | German n: 709 | German: 160 ±66 mmol/day | HT: 2.52 (2.43; 2.62) | Q1 (n = 265) | ||
| Switzerland | Age: 48.8 ± 18y | French: 151 ± 71 mmol/day | 2.32 (2.21; 2.44) | |||
| Cross-sectional | BMI: 25.3 ± 4.3 kg/m2 | Italy: 158 ±72 mmol/day | Q2 (n = 262) | |||
| SBP: 126.1 ± 15.9 mmHg | Urinary K | 2.27 (2.16; 2.38) | ||||
| DBP: 75.5 ± 10.2 mmHg | German: 68.0 ± 24.8 mmol/day | Q3 (n = 268) | ||||
| French n: 428 | French: 65.7 ± 24.1 mmol/day | 2.36 (2.24; 2.47) | ||||
| Age: 47.8 ± 18.1y | Italy: 63.3 ± 27.3 mmol/day | Q4 (n = 269) | ||||
| BMI: 25.5 ± 5.1 kg/m2 | 2.48 (2.38; 2.59) | |||||
| SBP: 122.5 ± 14.9 mmHg | Q5 (n = 272) | |||||
| DBP: 74.2 ± 9.8 mmHg | 2.53 (2.42; 2.65) | |||||
| Italian n: 199 | DBP | |||||
| Age: 45.3 ± 18.4 y | NR8 | |||||
| BMI: 24.5 ± 4.2 kg/m2 | ||||||
| SBP: 120.5 ± 15 mmHg | ||||||
| DBP: 69.9 ± 9.9 mmHg | ||||||
| Mohammadifard et al. (44)⌂ | Total n: 796 | Urinary Na | Total: 3.08 | SBP | Urinary Na:K ratio is significantly positively correlated with Pre-HT | P, IV |
| 2017 | Males n: 349 | Total: 176.94 ± 71.97 mmol/day | NT: 3.13 | Odds ratios (95% CI) of Pre-HTQ1 (Quartile of urinary Na:K ratio) | ||
| Iran | Females n: 447 | NT: 177 ± 73 mmol/day | Pre-HT: 3.24 | |||
| Cross-sectional | Age: 38.9 ± 11.4y | Pre-HT: 176.8 ± 66.1 mmol/day | ||||
| BMI: 25.7 ± 4.4 kg/m2 | Urinary K | 1.00 (0.45-2.21) | ||||
| SBP: 112.0 ± 10.9 mmHg | Total: 57.5 ±42 mmol/day | Q2 1.31 (0.62-2.77) | ||||
| DBP: 70.8 ± 8.7 mmHg | NT: 56.5 ± 42.3 mmol/day | Q3 2.15 (1.08-4.55) | ||||
| NT n: 309 | Pre-HT: 54.5 ± 39.7 mmol/day | P = 0.029 | ||||
| Age: 37.8 ± 11.0y | DBP | |||||
| BMI: 25.4 ± 4.5 kg/m2 | N/A | |||||
| SBP: 108.9 ± 8.5 mmHg | ||||||
| DBP: 68.1 ± 6.5 mmHg | ||||||
| Pre-HT n: 40 | ||||||
| Age: 43.6 ± 11.7y | ||||||
| BMI: 28.4 ± 4.5 kg/m2 | ||||||
| SBP: 126.2 ± 9.2 mmHg | ||||||
| DBP: 83.5 ± 5.7 mmHg | ||||||
| Ndanuko et al. (39) | Total n: 328 | Urinary Na6 [mean (95% CI)] | 1.9 (1.5-2.4) | SBP | Urinary Na:K ratio associated with SBP | P, IV |
| 2017 | NT/HT | 139 (99.2-180) mmol/day | Correlation coefficient = 0.1, P = 0.02 | |||
| Australia | Male n: 27 | Urinary K [mean (95% CI) | ||||
| Cross-sectional | Females n: 73 | 74 (96.7-154.3)] mmol/day | DBP | |||
| Age: 43.6 ± 8y | NR | |||||
| BMI: 32.4 ± 4.2 kg/m2 | ||||||
| SBP: 124.9 ± 14.5 mmHg | ||||||
| DBP: 73.3 ± 9.9 mmHg | ||||||
| Rhee et al. (45) | Total n: 524 | Urinary Na | Total: 3.10 ± 1.22 | SBP | Urinary Na:K ratio associated with SBP | Ø, IV |
| 2017 | Age: 48.1 ± 9.8 y | Total: 159.8 ± 61.5 mmol/day | NT: 3.05 ± 1.21 | All subjects: | ||
| Korea | BMI: 23.9 ± 3.3 kg/m2 | NT: 154.8 ± 59.9 mmol/day | HT: 3.13 ± 1.25 | β coefficient = 1.278 | ||
| Cross-sectional | Casual SBP: 116.7 ± 12.9 mmHg | HT: 166.8 ± 63.1 mmol/day | (P = 0.006) | |||
| Casual DBP: 75.0 ± 9.7 mmHg | Urinary K | HTs: | ||||
| 24-h SBP: 116.6 ± 11.0 mmHg | Total: 55.9 ± 20.7 mmol/day | β coefficient = 1.446 | ||||
| 24-h DBP: 75.9 ± 9.8 mmHg | NT: | (P = 0.047) | ||||
| AoSBP: 110.7 ± 13.1 mmHg | 54.3 ± 18.8 mmol/day | DBP | ||||
| AoDBP: 76.6 ± 10.0 mmHg | HT:58.3 ± 23.0 mmol/day | NR | ||||
| NTs n: 305 | ||||||
| Age: 45.1 ± 9.6y | ||||||
| BMI: 23.1 ± 3.1 kg/m2 | ||||||
| Casual SBP: 110.9 ± 10.0 mmHg | ||||||
| Casual DBP: 70.4 ± 7.0 mmHg | ||||||
| 24-h SBP: 110.8 ± 7.0 mmHg | ||||||
| 24-h DBP: 70.3 ± 6.1 mmHg | ||||||
| AoSBP: 104.8 ± 10.1 mmHg | ||||||
| AoDBP: 72.0 ± 7.4 mmHg | ||||||
| HTs n: 219 | ||||||
| Age: 52.1 ± 8.5y | ||||||
| BMI: 25.1 ± 3.2 kg/m2 | ||||||
| Casual SBP: 124.8 ± 12.3 mmHg | ||||||
| Casual DBP: 81.5 ± 9.2 mmHg | ||||||
| 24-h SBP: 124.6 ± 10.6 mmHg | ||||||
| 24-h DBP: 83.7 ± 8.7 mmHg | ||||||
| AoSBP: 118.8 ± 12.5 mmHg | ||||||
| AoDBP: 83.1 ± 9.6 mmHg | ||||||
| Vallejo et al. (46) | Total n: 711 | Urinary Na | Male: 3.2 ± 1.2 | SBP | Urinary Na:K ratio not associated with SBP or DBP | P, IV |
| 2017 | NTs | Male: 162±64 mmol/day | Female: 2.9 ± 1.1 | −0.2 [−0.7, 0.5] | ||
| Mexico | Age: 37.4 ± 9.0y | Female: 125 ± 48.2 mmol/day | DBP | |||
| Cross-sectional | BMI: 27.1 ± 4.4 kg/m2 | Urinary K | 0.1 [−0.4, 0.6] | |||
| SBP: 106.2 ± 10.2 mmHg | Male: 54 ± 18.5 mmol/day | |||||
| DBP: 70.6 ± 7.9 mmHg | Female: 46.5 ± 16.4 mmol/day | |||||
| Male n: 228 | ||||||
| Age: 36.0 ± 8.9y | ||||||
| BMI: 27.2 ± 4.3 kg/m2 | ||||||
| SBP: 110.5 ± 9.8 mmHg | ||||||
| DBP: 73.4 ± 7.9 mmHg | ||||||
| Female n: 483 | ||||||
| Age: 38.1 ± 8.9y | ||||||
| BMI: 27.1 ± 4.5 kg/m2 | ||||||
| SBP: 104.2 ± 9.7 mmHg | ||||||
| DBP: 69.3 ± 7.6 mmHg | ||||||
| Ware et al. (16)9 | Total n: 2722 | Urinary Na | Low salt: 2.7 (2.3) | SBP | Urinary Na:K ratio not associated with SBP and DBP | Ø,IV |
| 2017 | NTs/HTs | Median (IQR) | Medium salt: 3.6 (2.1) | NR | ||
| South Africa | Low salt n: 164 | Low salt: 62.7 (28.8) mmol/day | High salt: 3.7 (2.4) | SBP | ||
| Cross-sectional | Data below are median (IQR) | Medium salt: 115.3 (37.3) mmol/day | NR | |||
| Age: 59 (18)y | High salt: 217 (118.7) mmol/day | |||||
| BMI: 28.0 (9.8) kg/m2 | Urinary K | |||||
| SBP: 132 (25) mmHg | Median (IQR) | |||||
| DBP: 81 (17) mmHg | Low salt: 22.2 (16.4) mmol/day | |||||
| Medium salt n: 195 | Medium salt: 33.0 (24.2) mmol/day | |||||
| Age: 57 (19)y | High salt: 67.0 (43.2) mmol/day | |||||
| BMI: 28.5 (8.1) kg/m2 | ||||||
| SBP: 127 (25) mmHg | ||||||
| DBP: 78 (16) mmHg | ||||||
| High salt n: 167 | ||||||
| Age: 49 (26)y | ||||||
| BMI: 30.4 (10.6) kg/m2 | ||||||
| SBP: 128 (24) mmHg | ||||||
| DBP: 80 (16) mmHg | ||||||
| Xu et al. (28) | Total n: 2281 | Urinary Na | 6.8 (1.5) | SBP | Urinary Na:Kratio associated with SBP and DBP | P, IV |
| 2017 | NTs/HTs | 166.9 ± 25.6 mmol/day | β coefficient (95% CI): | |||
| China | Age: 42.1 ± 13.4y | Urinary K | 0.97 [0.36 to 1.58]*** | |||
| Cross-sectional | BMI classification: | 25.3 ± 3.4 mmol/day | DBP | |||
| Normal n: 982 | β coefficient (95% CI): | |||||
| 43.1% (95% CI: 41.0,45.1) | 0.65 [0.26 to 1.04]*** | |||||
| Overweight n: 860 | ||||||
| 37.7% (95% CI: 35.7, 39.7) | ||||||
| Obese: (n = 439) | ||||||
| 19.2% (95% CI: 17.6, 20.9) | ||||||
| SBP: 131.4 ± 19.9 mmHg | ||||||
| DBP:83.6 ± 11.9 mmHg | ||||||
| Male n: 1135 | ||||||
| Age: 42.2 ± 13.5y | ||||||
| BMI: | ||||||
| Normal n: 480 | ||||||
| 42.3% (95% CI: 39.4, 45.2) | ||||||
| Overweight n: 458 | ||||||
| 40.4% (95% CI: 37.5, 43.2) | ||||||
| Obese n: 197 | ||||||
| 17.3% (95% CI: 15.2, 19.6) | ||||||
| SBP:134.6 ± 18.0 mmHg | ||||||
| DBP:85.9 ± 11.9 mmHg | ||||||
| Female: (n = 1146) | ||||||
| Age: 41.9 ± 13.4y | ||||||
| BMI classification | ||||||
| Normal n = 502 | ||||||
| 43.8% (95% CI: 40.9, 46.7) | ||||||
| Overweight n = 402 | ||||||
| 35.1% (95% CI: 32.3, 37.8) | ||||||
| Obese n: 242 | ||||||
| 21.1% (95% CI: 18.8, 23.5) | ||||||
| SBP: 128.2 ± 21.1 mmHg | ||||||
| DBP: 81.3 ± 11.5 mmHg | ||||||
| Yan et al. (29) | Total n: 1948 | Urinary Na | 6.8 | SBP | Urinary Na:K ratio associated with increased of SBP and DBP | P, IV |
| 2015 | HTs (23%) | 235.7 (SD NR) mmol//day | (SD NR) | β coefficient (95% CI): | ||
| China | Age: 41.4 ± 13.9y | Urinary K | 0.37 [0.26 to 0.49]*** | |||
| Cross-sectional | BMI: NR | 40.5 (SD NR) mmol/day | DBP | |||
| SBP: NR | β coefficient (95% CI): | |||||
| DBP: NR | 0.16 [0.03 to 0.29]* | |||||
| Huggins et al. (40) | Total n: 738 | Urinary Na | 1.99 ± 0.83 | SBP | Urinary Na:K ratio associated with increased SBP | P, IV |
| 2011 | HTs (43%) | 155.1 ± 63.1 mmol/day | β coefficient (95% CI): | |||
| Australia | Age: 64.0 ± 6.3y | Urinary K | 1.8 [0.2 to 3.4]* | |||
| Cross-sectional | Male n: 376 | 82.3 ± 27.9 mmol/day | DBP | |||
| BMI: 28.4 ± 4.0 kg/m2 | β coefficient (95% CI): | |||||
| SBP: 133.0 ± 13.1 mmHg | 0.40 [−0.69 to 1.48] | |||||
| DBP: 76.1 ± 9.2 mmHg | ||||||
| Female n: 407 | ||||||
| BMI: 28.0 ± 4.9 kg/m2 | ||||||
| SBP: 129.5 ± 16.8 mmHg | ||||||
| DBP: 66.6 ± 10.4 mmHg | ||||||
| Zhao et al. (15) | Total n: 839 | Urinary Na | North: 7.6 ± 2.4 | SBP | Urinary Na:K ratio associated with increased SBP and DBP | P, IV |
| 2004 | North n: 561 | North: 271±88 mmol/day | South: 3.7 ± 1.5 | Coefficient for difference between North and South (95% CI NR) 3.30*** | ||
| China | HTs = 22% | South: 139±57 mmol/day | ||||
| Cross-sectional | Age: 48.9 ± 5.8y | Urinary K | ||||
| BMI: 23.8 ± 3.5 kg/m2 | North: 37.1 ± 11.5 mmol/day | |||||
| SBP: 123.7 ± 18.4 mmHg | South: 40.6 ± 14.7 mmol/day | |||||
| DBP: 75.5 ± 10.4 mmHg | ||||||
| South n: 278 | ||||||
| HTs = 7% | ||||||
| Age: 49.1 ± 5.7y | ||||||
| BMI: 21.8 ± 2.6 kg/m2 | ||||||
| SBP: 116.3 ± 14.1 mmHg | ||||||
| DBP: 68.6 ± 8.0 mmHg | ||||||
| Xie et al. (30) | Total n: 353 | Urinary Na | Male: 6.1 ± 2.3 | SBP | Urinary Na:K ratio associated with increased SBP and DBP | P, IV |
| 2001 | NTs/HTs | Male: 152.9 ± 62.5 mmol/day | Female: 6.1 ± 2.6 | β coefficient (95% CI NR): | ||
| China | Male n: 191 | Female: 123.3 ± 59.3 mmol/day | Male: 1.167** | |||
| Cross-sectional | Age: 40.0 ± 16.5y | Urinary K | Female: 1.310** | |||
| BMI: 22.0 ± 2.3 kg/m2 | Male: 28.7 ± 17.2 mmol/day | DBP | ||||
| SBP: 116.9 ± 13.1 mmHg | Female: 23.7 ± 14.3 mmol/day | β coefficient (95% CI NR): | ||||
| DBP: 74.3 ± 8.9 mmHg | Male: 0.573* | |||||
| Female n: 162 | Female: NR | |||||
| Age: 36.7 ± 15.7y | ||||||
| BMI: 22.7 ± 2.8 kg/m2 | ||||||
| SBP: 117.6 ± 17.5 mmHg | ||||||
| DBP: 74.8 ± 11.1 mmHg | ||||||
| Tian et al. (31) | Total n: 663 | Urinary Na | Male: 6.6 ± 2.2 | SBP | Urinary Na:K ratio associated with increased SBP and DBP in females and both sexes combined | P, IV |
| 1995 | NTs/HTs | Male: 257.8 ± 86.0 mmol/day | Female: 6.1 ± 2.4 | β coefficient ± SE | ||
| China | Male n: 328 | Female: 249.2 ± 81.4 mmol/day | Male: 0.735 ± 0.402 | |||
| Cross-sectional | Age: 43.6 ± 13.6 y | Urinary K | Female: 0.956 ± 0.380* | |||
| BMI: 23.4 ± 3.4 kg/m2 | Male: 42.4 ± 17.0 mmol/day | Total: 0.795 ± 0.277** | ||||
| SBP: 126 ± 18 mmHg | Female: 45.0 ± 18.2 mmol/day | DBP | ||||
| DBP: 81 ± 11 mmHg | β coefficient ± SE | |||||
| Female n: 335 | Male: 0.261 ± 0.253 | |||||
| Age: 43.5 ± 13.3y | Female: 0.561 ± 0.213** | |||||
| BMI: 23.6 ± 4.4 kg/m2 | Total: 0.400 ± 0.164* | |||||
| SBP: 121 ± 20 mmHg | ||||||
| DBP: 78 ± 11 mmHg | ||||||
| He et al. (32) | Total n: 419 | Urinary Na | High-mountain Yi: | SBP | Urinary Na:K ratio associated with increased SBP and DBP | Ø, IV |
| 1991 | NTs | High-mountain Yi: | 1.45 ± 0.92 | β coefficient 0.928**** | ||
| China | High-mountain Yi | 73.9 ± 50.3 mmol/day | Mountainside Yi: | (95% CI NR): | ||
| Cross-sectional | Age: 30.9 ± 11.5y | Mountainside Yi: | 3.45 ± 2.50 | DBP | ||
| BMI: 19.3 ± 2.2 kg/m2 | 117.9 ± 55.4 mmol/day | Country Yi: | β coefficient 0.645**** | |||
| SBP: 99.4 ± 7.8 mmHg | Country Yi: | 6.87 ± 3.79 | (95% CI NR): | |||
| DBP: 63.2 ± 7.4 mmHg | 159.4 ± 62.6 mmol/day | Country Han: | ||||
| Country Han: | 7.00 ± 2.23 | |||||
| Mountainside Yi | 186.0 ± 73.0 mmol/day | |||||
| Age: 36.4 ± 14.3y | Urinary K | |||||
| BMI: 19.4 ± 1.6 kg/m2 | High-mountain Yi: | |||||
| SBP: 101.8 ± 9.1 mmHg | 58.6 ± 31.0 mmol/day | |||||
| DBP: 62.2 ± 8.2 mmHg | Mountainside Yi: | |||||
| Country Yi | 48.5 ± 28.1 mmol/day | |||||
| Age: 39.3 ± 12.7y | Country Yi: | |||||
| BMI: 20.4 ± 2.1 kg/m2 | 28.3 ± 13.6 mmol/day | |||||
| SBP: 108.6 ± 9.2 mmHg | Country Han: | |||||
| DBP: 71.3 ± 8.2 mmHg | 29.0 ± 10.4 mmol/day | |||||
| Country Han | ||||||
| Age: 36.4 ± 12.1y | ||||||
| BMI: 20.8 ± 2.7 kg/m2 | ||||||
| SBP: 107.3 ± 12.1 mmHg | ||||||
| DBP: 69.6 ± 8.9 mmHg | ||||||
| Staessen et al. (37) | Total n: 301 Males | Urinary Na | Daytime: 2.4 ± 1.0 | SBP | Urinary Na:K ratio associated with increased SBP and DBP | Ø, IV |
| 1991 | NTs/HTs | Daytime: 120 ± 46 mmol/12h | Nighttime: 3.5 ± 1.6 | Whole day urine: | ||
| UK | Age: 45 ± 6y | Nighttime: 54 ± 29 mmol/12h | Whole day: 2.5 ± 10 | β coefficient ± SE | ||
| Cross-sectional | BMI: 24.1 ± 2.8 kg/m2 | Whole day: 174 ± 557 mmol/day | 2.110 ± 0.895* | |||
| SBP: 126 ± 16 mmHg | Urinary K | DBP | ||||
| DBP: 78 ± 11 mmHg | Daytime: 56 ± 20 mmol/12 hr | Whole day urine: | ||||
| Nighttime: 18 ±11 mmol/12h | β coefficient ± SE | |||||
| Whole day: 73±22 mmol/day | 2.084 ± 0.653** | |||||
| Kesteloot et al. (33) | Total n: 2008 | Urinary Na | M10 North: 6.56 ± 2.55 | SBP | Urinary Na:K ratio associated with increased SBP and DBP in northern China, while only with SBP in southern China | Ø, IV |
| 1987 | NTs/HTs | M North: 226.9 ± 88.8 mmol/day | M South: 6.73 ± 2.41 | β coefficient range: | ||
| China | Male n: 1002 | M South: 179.4 ± 72.9 mmol/day | F North: 5.92 ± 2.27 | 0.650 to 0.967* | ||
| Cross-sectional | North: | F11 North: 204.6 ± 82.1 mmol/day | F South: 6.15 ± 2.28 | DBP | ||
| Age: 40.4 ± 14.4 y | F South: 172.4 ± 70.6 mmol/day | β coefficient range: | ||||
| SBP: 125.7 ± 17.6 mmHg | Urinary K | 0.402 to 0.751 | ||||
| DBP: 83.1 ± 12.1 mmHg | M North: 37.5 ± 15.8 mmol/day | |||||
| South: | M South: 28.8 ± 13.0 mmol/day | |||||
| Age: 40.4 ± 14.4y | F North: 37.5 ± 17.1 mmol/day | |||||
| SBP: 118.1 ± 12.6 mmHg | F South: 29.7 ± 11.6 mmol/day | |||||
| DBP: 77.6 ± 9.8 mmHg | ||||||
| Female n: 1006 | ||||||
| North: | ||||||
| Age: 40.2 ± 14.4y | ||||||
| SBP: 122.0 ± 21.7 mmHg | ||||||
| DBP: 80.4 ± 14.7 mmHg | ||||||
| South: | ||||||
| Age: 40.5 ± 14.3y | ||||||
| SBP: 112.3 ± 14.3 mmHg | ||||||
| DBP: 75.0 ± 10.2 mmHg | ||||||
| Chan et al. (34) | Total n: 126 | Urinary Na | Males: 4.0 ± 1.7 | SBP | Non-significant association between urinary Na:K ratio with SBP and DBP (data not shown) | Ø, IV |
| 1998 | NTs/HTs | Male: 145.2 ± 48.7 mmol/day | Female: 3.5 ± 1.4 | NR | ||
| China | Males n: 42 | Female: 135.3 ± 45.8 mmol/day | DBP | |||
| Cross-sectional | Age: 41.8 ± 16.4y | Urinary K | NR | |||
| SBP: 113.4 ± 14.7 mmHg | Male: 40.4 ± 15.1 mmol/day | |||||
| DBP: 113.4 ± 14.7 mmHg | Female: 41.3 ± 14.3 mmol/day | |||||
| Female n: 84 | ||||||
| Age: 41.6 ± 10.9y | ||||||
| SBP: 106.0 ± 16.4 mmHg | ||||||
| DBP: 106.0 ± 16.4 mmHg | ||||||
| Kim et al. (51)9 | Total n: 740 | Urinary Na (median, 25th, 75th percentile) | 2.9 (2.2, 3.7) | SBP | Urinary Na:K associated with BP in older population | P, IV |
| 2019 | HTs = 40% | β coefficient (95% CI) | ||||
| South Korea | Data below is median (25th, 75th percentile) | 153.9 (116.3, 197.5) mmol/day | 0.0364 [−0.0308 to 0.1035] | |||
| Cross-sectional | Older group (≥55 years) | |||||
| Cross-sectional | Age: 48 (41, 56) y | Urinary K (median, 25th, 75th percentile) | 0.1325 [0.0031 to 0.2620]* | |||
| BMI: 23.5 (21.5, 25.6) kg/m2 | 54.9 (43.0, 69. 8) mmol/day | DBP | ||||
| HT n: 299 | β coefficient (95% CI) | |||||
| SBP: 115.3 (107.9, 122.8) mmHg | 0.0317 [−0.0322 to 0.0955] | |||||
| DBP: 75.5 (68.4, 82.2) mmHg | Older group (≥55 years) | |||||
| 0.1234 [0.0025 to 0.2444]* | ||||||
| Libianto et al. (38) | Total n: 116 | Urinary Na | 2.5 ± 1.1 | SBP | Urinary Na:K associated with SBP | P, IV |
| 2017 | NTs/HTs | 169±77 mmol/day | β coefficient (95% CI): | |||
| Australia | Age: 65 ± 12y | Urinary K | −2.373 [−4.447 to −0.299]* | |||
| Cross-sectional | BMI: 31±5 kg/m2 | 70±25 mmol/day | DBP | |||
| SBP: 130 ± 14 mHg | β coefficient (95% CI): | |||||
| DBP: NR | N/A | |||||
| M'buyamba-Kabangu | Total n: 416 | Urinary Na | 3.1 (0.3-13.3) | SBP | Urinary Na:K associated with both SBP and DBP | P, IV |
| et al. (47)9 | NTs/HTs | Mean 87 (range minimum- maximum 8 to 312) mmol/day | 1.02* (95% CI NR) | |||
| 1986 | Age: 32 ± 12y | DBP | ||||
| Zaire | SBP: 124 ± 20 mmHg | Urinary K | 0.68* (95% CI NR) | |||
| Cross-sectional | DBP: 72 ± 14 mmHg | Mean 33 (range minimum- maximum 3 to 132) mmol/day |
*P < 0.05. **P < 0.01.***P < 0.001. ****P < 0.0001. Electrolytes: □ Na: K ratio reported as adjusted (for age, BMI, sex and linguistic region) means (95% confidence limits); association of Na: K ratio with SBP reported by sex-specific quintile (Q) of systolic blood pressure P value < 0.001. ⌂ Quartiles (Q) based on urinary sodium to potassium ratio, SBP: P < 0.001, DBP: P = 0.003. AoSBP, aortic systolic blood pressure; AoDBP, aortic diastolic blood pressure; BMI, body mass index; DBP, diastolic blood pressure; HT, hypertensive; NR, not reported; NT, normotensive; Pre-HT, pre-hypertensive; SBP, systolic blood pressure.
Body mass index is Mean ± SD unless otherwise indicated;
Blood pressure is Mean ± SD unless otherwise indicated;
Mean ± SD in mmol/day;
β coefficient (95% CI) unless specified;
Quality rating: P = Positive, Ø = Neutral, − = Negative;
NHMRC (National Health and Medical Research Council) rating is classified as I, III-1, III-2, III-3 OR IV;
NR: Not reported;
Subjects data and Na, K, Na: K ratio presented as median (IQR, interquartile range);
M: Male
F: Female
Methods to assess urinary Na: K concentrations were either the ion-specific electrode method (28–30, 29, 31–33, 35–37, 40, 41, 43) or emission flame photometry (30, 31). In the 24-h urinary studies, collections were deemed to be incomplete if urinary volumes were low (i.e., less than 400 mL (30), 500 mL (30, 36, 40), or 1L (37, 43) per day) and/or if 24-h urinary creatinine concentration was greater than 2 to 3 standard deviations beyond the sex-specific means (28, 32, 36, 37, 40, 41, 43). To further validate the urinary analyses, some studies also included a verbal questionnaire to inquire about the completeness of the 24-h urine samples collected or also included a dietary questionnaire (36, 40, 42). Study participants were classified as either normotensive or hypertensive and BMIs ranged from normal to obese (mean BMI 19.3–29.6 kg/m2).
The studies that performed 24-h urine collections specified measured brachial BP using a calibrated mercury or random zero sphygmomanometer (15, 20, 21, 24, 28–33, 40, 42, 46, 47) automated oscillometric device (14, 25, 26, 34, 36, 41) or a validated wrist-worn BP device (16). One study measured office BP, ambulatory BP, and aortic BP and reported all 3 (43).
All but 3 of the studies included in this review indicated that a higher Na: K ratio was associated with higher SBP and/or DBP as shown by regression coefficients, with a stronger correlation observed with SBP than with DBP. Three studies reported no association between Na: K ratio and SBP/DBP, even after the adjustments for covariates known to be associated with BP (32, 40, 44). One study that compared the use of 24-h, overnight and daytime urine collection with BP showed that 24-h urinary measures of Na: K ratio had stronger associations with BP compared with both overnight and daytime urine samples (35).
A summary of experimental studies (clinical trials) included in this review is shown in Table 1. Participants in these studies were aged 20 years or older and had BP indicative of pre-hypertension or were diagnosed as being hypertensive. Only 3 of the studies reported the BMI of participants, and these ranged from overweight to obese (27.2–33.9 kg/m2). Twenty-four-hour urine collection was included in all 5 of the RCTs. Most studies investigated the effect of decreasing sodium intake and/or increasing potassium intake on BP and reported the relationship according to the Na: K ratio. Common intervention diets used were either the DASH diet (20, 21, 25) or other diets (20, 22, 24, 34, 48) aimed to simultaneously reduce sodium intake and increase potassium intake, compared with a normal American or Western diet. For the purpose of our analyses, groups that had a lower Na: K ratio were compared with groups that had a higher Na: K ratio. For the cross-sectional studies shown in Table 2, participants’ ages ranged from 45 to 52 y with a BMI of between 24 and 32. The studies had both normotensive and hypertensive participants.
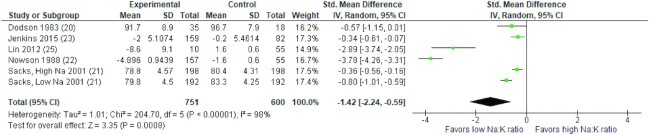
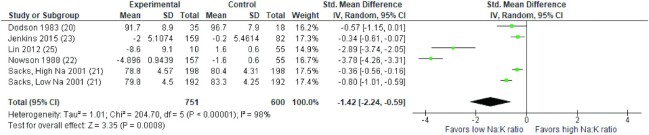
Results of the meta-analysis of 5 RCTs demonstrated that a diet with a lower Na: K ratio was associated with a significantly greater reduction in SBP and DBP compared with a higher Na: K ratio (SMD: −1.09 mmHg; 95% CI: −1.91, −0.28 mmHg and SMD: −1.42 mmHg; 95% CI: −2.24, −0.59 mmHg, respectively). High heterogeneity was observed for both SBP and DBP (I2= 97% and I2= 98%, P < 0.001, respectively). The forest plot of the meta-analysis for SBP and DBP is shown in Figures 2 and 3, respectively. As there were fewer than 10 studies included in the meta-analyses, funnel plots were not generated to investigate small study effects, in line with recommendations of the Cochrane Handbook (19).
FIGURE 2.
Forest plot of the association between different sodium to potassium ratios and systolic blood pressure (mmHg) in adults in 5 RCTs. Diamonds indicate weighted mean difference with 95% confidence intervals. RCT, randomized controlled trials.
FIGURE 3.
Forest plot of the association between different sodium to potassium ratios and diastolic blood pressure (mmHg) in adults in 5 RCTs. Diamonds indicate weighted mean difference with 95% confidence intervals. RCT, randomized controlled trials.
Discussion
This systematic review and meta-analysis has confirmed a positive association between increasing sodium to potassium ratio and both systolic and diastolic BP in adults. For the purpose of the review, an optimal Na: K was considered to be 1:1 or lower, as recommended by the WHO as a target for optimal health outcomes (49, 50). A meta-analysis of 5 RCTs resulted in a magnitude of reduction of 1.09 mmHg and 1.42 mmHg associated with a more favorable Na: K ratio, for systolic and diastolic BP, respectively. This effect can be considered both statistically and clinically significant. Appel et al. (11) demonstrated that lowering systolic and diastolic BP by 5.0 mmHg and 3.0 mmHg, respectively, would decrease the incidence of coronary heart disease and stroke by approximately 15% and 27%, respectively, in the US population. The dietary modifications included in the RCT interventions were based on the principles of the DASH diet, and our meta-analysis is consistent with a previously reported meta-analysis that described a reduction of 6.74 mmHg for SBP and 3.59 mmHg for DBP associated with adherence to a DASH diet (10).
In the experimental studies included in the meta-analysis, we found evidence of a greater reduction in BP associated with a greater magnitude of reduction in the urinary Na: K in participants with hypertension, compared with those with pre-hypertension. These findings are consistent with the study of Parfrey et al. (52), which found a larger reduction in BP in hypertensive groups, compared with normotensive counterparts following dietary modifications that lowered sodium and simultaneously increased potassium intake. A similar positive association was also observed in the cross-sectional studies included in this review. For example, Zhao et al. (15) concluded that lowering sodium to potassium ratio reduced BP in both pre-hypertensive and hypertensive individuals. However, Mohammadifard et al. (42) was able to show that the incidence of pre-hypertension had a greater association with UNa: K ratio than with either urinary sodium or urinary potassium alone. It is noteworthy that 4 other cross-sectional studies included in this review (30, 31, 53, 54) also reported a positive association between a higher sodium to potassium ratio and increased BP in normotensive populations. Finally, 1 study from China (30) reported that achieving an optimal sodium to potassium ratio could decrease systolic and diastolic BP by 6 mmHg and 3 mmHg, respectively, in normotensive individuals. Similarly, a study of normotensive individuals reported that a combination of low dietary sodium with high dietary potassium intake over a 2-wk period resulted in BP reduction (55). The evidence summarized in our current review indicates that a lowered UNa: K ratio is beneficial in both hypertensive and normotensive populations.
Biological plausibility exists regarding the combined effect of lowered sodium intake and increased potassium intake. The human body has developed numerous homeostatic mechanisms to maintain a tight sodium to potassium balance for fluid regulation and normal neuronal and muscular activity (56). Excessive dietary sodium intake resulting in deviations from the normal physiological range for prolonged periods leads to many pathophysiological conditions, including hypertension and cardiovascular disease (57). Conversely, the regulation of BP is affected by several physiological factors including blood volume, cardiac output, and peripheral resistance. Peripheral resistance is further influenced by the constant need to maintain blood viscosity homeostasis using sodium and potassium ions. Thus, changes to sodium intake will affect the renin-angiotensin-aldosterone system, which will consequently result in changes in plasma renin and aldosterone concentrations (4, 50, 58, 59). Maintaining a constantly high plasma sodium concentration due to high renin activity results in a prolonged high BP. However, a previous meta-analysis by Rhee et al. (59) found that while there was no significant correlation between plasma renin activity and 24-h urinary sodium, a longer duration of decreased sodium intake could reduce renin levels, thereby resulting in lowered BP.
Equally important, a deficit in plasma potassium results in sodium retention in the kidneys and a cellular potassium deficit, which affects vascular smooth muscle contraction and peripheral vascular resistance. This indirectly results in higher BP through the sodium–calcium exchanger type 1 mechanism (60). Conversely, an increased potassium intake could have antihypertensive effects by promoting endothelium vasodilation through stimulating the sodium pump and opening potassium channels. It is puzzling, therefore, why the majority of investigations on the effect of sodium reduction on blood pressure have focused on measuring and reporting urinary sodium excretion without considering its relative excretion to urinary potassium. Our systematic review of cross-sectional and cohort studies indicate that 24-h UNa: K ratio was more significantly associated with the changes of blood pressure than either urinary sodium or urinary potassium alone (26, 30, 37, 40, 41), as well as with either reported dietary sodium or dietary potassium alone (40, 42).
Practical ways to reduce sodium intake while increasing potassium intake include increasing intake of fruits, vegetables, low-fat dairy, whole grains, poultry, fish, and nuts (prepared with little or no salt) (10, 61) as well as reducing intake of processed foods high in sodium. These are characteristics of the DASH diet, known to be effective in lowering systolic and diastolic BP (10). However, the DASH diet is not the only food-based strategy to obtain BP reductions (62). Other beneficial dietary patterns include the Nordic and Mediterranean diets (62).
In the current review there were many differences between studies, such as the number of participants, gender, age, study intervention, and duration of the intervention. Four cross-sectional studies in our review found different results for males and females (30, 29, 54, 63), which could be related to confounding effects of higher alcohol consumption in males and higher BMI in females. In comparison to cited studies that investigated 24-h urinary collections (30, 29), results between genders were inconsistent with results from those studies that collected spot urinary collections (54, 63). For example, 2 studies found a stronger association between blood pressure and UNa: K ratio in males, using casual urinary collections (54, 63) whereas a stronger association was found in females by Tian et al. (29) in their study, which collected 24-h urinary samples. Although 24-h urine was estimated in studies using casual urinary collections, all studies found an association and/or a correlation between UNa: K ratio and BP.
BMI is a factor known to affect BP, and Jackson et al. found a significant association between UNa: K ratio and BP only in obese participants with BMI ≥ 30 (2.28; 95% CI 0.60, 3.96, P < 0.05), but not in participants with a BMI <25 (0.66; −1.89, 3.20) (29). A cross-sectional study from China included in this review that compared normal weight and overweight/obese participants found a stronger association between higher sodium to potassium ratio and hypertension in the overweight/obese subjects (29). Conversely, other large-scale studies such as INTERSALT and INTERMAP samples found no mediating effect of BMI on the association between urinary sodium, potassium, and BP in Chinese sub-groups (3, 31).
The impact of UNa: K ratio in the context of low salt intakes warrants further exploration. For example, if populations shift their salt intakes to meet the WHO global target of <5g/d, but dietary potassium intake remains low, this may blunt the predicted BP reductions. The study by Farapti et al. (40) suggested that normotensive and hypertensive participants included in their study had low sodium concentrations, accompanied by even lower potassium intakes than recommended resulting in a positive association between UNa: K ratio and BP. A South African study by Ware et al. (16) reported a greater regression slope between age and SBP and DBP observed in those with a UNa: K ratio above 2, while associations were only evident with BP for sodium excretion at levels equivalent to ≥9g/d of salt.
A number of limitations need to be considered in the interpretation of this review. First, only 5 studies were included in the meta-analysis. The results of the meta-analysis indicated considerable heterogeneity. This may have been in part the result of variation between study characteristics, such as sample size and the dietary interventions used. Due to the small number of studies eligible for inclusion in the meta-analysis, it was not considered appropriate to further explore heterogeneity via sub-group analyses or meta-regression. There is an identified need for further RCTs on this topic. Second, other factors that affect BP measurements may not have been adequately controlled for in all studies. These include the use of diuretics, having a full bladder when taking BP readings, caffeine intake, exercise intensity, and alcohol consumption. It is important to note that causality cannot be inferred from data obtained from the included cross-sectional studies, and these were included in the review to indicate totality of evidence rather than draw firm conclusions from the studies. Additionally, many high-quality studies that have reported both 24-h urinary sodium and potassium excretion concentrations would not have been included in this review if the ratio of Na: K was not reported. Finally, the search strategy was restricted to published articles, which may have resulted in a publication bias. However, due to the small number of studies in the meta-analysis, we were unable to explore this formally using tests of funnel plot asymmetry.
Conclusions
This systematic review and meta-analysis has identified an association between lower sodium to potassium ratio and reduced BP in adults. This effect is evident for individuals with BP levels indicative of pre-hypertension and in those that are hypertensive. The quality of evidence for normotensives is too low to draw conclusions. The ratio of urinary sodium to potassium excretion appears to be a better predictor of BP than measurement of sodium or potassium excretion alone. However, further well-designed studies are required to identify the optimal sodium to potassium ratio in populations that have varying cuisines and to investigate the effect of different dietary patterns that contribute to the intake of these two cations.
ACKNOWLEDGEMENTS
All authors: read and approved the final manuscript.
Notes
Author disclosures: The authors declare no conflicts of interest.
Abbreviations used: ADA, American Dietetic Association; BP, blood pressure; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; NHMRC, National Health and Medical Research Council; RCT, randomized controlled trials; SBP, systolic blood pressure; SMD, standardized mean differences; UNa: K, urinary sodium to potassium ratio.
Contributor Information
Rhoda N Ndanuko, The George Institute for Global Health, University of New South Wales, Sydney, NSW, Australia.
Rukayat Ibrahim, School of Medicine, University of Wollongong, Wollongong, NSW, Australia; University of Surrey, Guildford, United Kingdom.
Retno A Hapsari, School of Medicine, University of Wollongong, Wollongong, NSW, Australia.
Elizabeth P Neale, School of Medicine, University of Wollongong, Wollongong, NSW, Australia.
David Raubenheimer, Charles Perkins Centre, The University of Sydney, Sydney, NSW, Australia.
Karen E Charlton, School of Medicine, University of Wollongong, Wollongong, NSW, Australia.
References
- 1.Benziger CP, Roth GA, Moran AE. The Global Burden of Disease Study and the preventable burden of NCD. Global Heart. 2016;11(4):393–7. [DOI] [PubMed] [Google Scholar]
- 2.Hosseini Z, Whiting SJ, Vatanparast H. Current evidence on the association of the metabolic syndrome and dietary patterns in a global perspective. Nutr Res Rev. 2016;29(2):152–62. [DOI] [PubMed] [Google Scholar]
- 3.Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion.. Intersalt Cooperative Research Group. BMJ. 1988;297:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He FJ, Li J, MacGregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346(apr03 3):f1325. [DOI] [PubMed] [Google Scholar]
- 5.Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013;346(apr03 3):f1326–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macgregor G, Best F, Cam J, Markandu N, Elder D, Sagnella G, Squires M. Double-blind randomised crossover trial of moderate sodium restriction in essential hypertension. Lancet North Am Ed. 1982;319(8268):351–5. [DOI] [PubMed] [Google Scholar]
- 7.Macgregor GA, Sagnella GA, Markandu ND, Singer DRJ, Cappuccio FP. Double-blind study of three sodium intakes and long-term effects of sodium restriction in essential hypertension. Lancet North Am Ed. 1989:334(8674):1244–7. [DOI] [PubMed] [Google Scholar]
- 8.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ. Effects of oral potassium on blood pressure: meta-analysis of randomized controlled clinical trials. JAMA. 1997;277(20):1624–32. [DOI] [PubMed] [Google Scholar]
- 9.Gu D, He J, Wu X, Duan X, Whelton PK. Effect of potassium supplementation on blood pressure in Chinese: a randomized, placebo-controlled trial. J Hypertens. 2001;19(7):1325–31. [DOI] [PubMed] [Google Scholar]
- 10.Saneei P, Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24(12):1253–61. [DOI] [PubMed] [Google Scholar]
- 11.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MMet al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336(16):1117–24. [DOI] [PubMed] [Google Scholar]
- 12.Lopes HF, Martin KL, Nashar K, Morrow JD, Goodfriend TL, Egan BM. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension. 2003;41(3):422–30. [DOI] [PubMed] [Google Scholar]
- 13.Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr. 2015;113(1):1–15. [DOI] [PubMed] [Google Scholar]
- 14.Yin L, Deng G, Mente A, Sun Y, Liu X, Zhang X, Wang X, Wang Y, Bo J, Chen Het al. Association patterns of urinary sodium, potassium, and their ratio with blood pressure across various levels of salt-diet regions in China. Sci Rep. 2018;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao L, Stamler J, Yan LL, Zhou B, Wu Y, Liu K, Daviglus ML, Dennis BH, Elliott P, Ueshima Het al. Blood pressure differences between northern and southern Chinese: role of dietary factors the International Study on Macronutrients and Blood Pressure. Hypertension. 2004;43(6):1332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ware LJ, Charlton K, Schutte AE, Cockeran M, Naidoo N, Kowal P. Associations between dietary salt, potassium and blood pressure in South African adults: WHO SAGE Wave 2 Salt & Tobacco. Nutrition, Metabolism & Cardiovascular Diseases. 2017;27:784–91. [DOI] [PubMed] [Google Scholar]
- 17.Higo Y, Nagashima S, Tabara Y, Setoh K, Kawaguchi T, Takahashi Y, Kosugi S, Nakayama T, Matsuda F, Wakamura T. Association of the spot urine sodium-to-potassium ratio with blood pressure is independent of urinary Na and K levels: The Nagahama Study. Hypertens Res. 2019;42(10):1624–30. [DOI] [PubMed] [Google Scholar]
- 18.Academy of Nutrition and Dietetics. Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process. January 2012. Chicago: Academy of Nutrition and Dietetics; 2012. [Google Scholar]
- 19.Higgins JPT, Green Seds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [Updated March 2011]. London: The Cochrane Collaboration;2011. [Google Scholar]
- 20.Dodson PM, Beevers M, Fletcher RF, Pacy PJ, Bal P, Taylor KG. The effects of a high fibre, low fat and low sodium dietary regime on diabetic hypertensive patients of different ethnic groups. Postgrad Med J. 1983;59(696):641–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller Iii ER, Simons-Morton DGet al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10. [DOI] [PubMed] [Google Scholar]
- 22.Nowson CA, Morgan TO. Change in blood pressure in relation to change in nutrients effected by manipulation of dietary sodium and potassium. Clin Exp Pharmacol Physiol. 1988;15(3);225–42. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins DJA, Jones PJ, Frohlich J, Lamarche B, Ireland C, Nishi SK, Srichaikul K, Galange P, Pellini C, Faulkner Det al. The effect of a dietary portfolio compared to a DASH-type diet on blood pressure. Nutr Metab Cardiovasc Dis. 2015;25(12);1132–9. [DOI] [PubMed] [Google Scholar]
- 24.Wing RR, Caggiula AW, Nowalk MP, Koeske R, Lee S, Langford H. Dietary approaches to the reduction of blood pressure: the independence of weight and sodium/potassium interventions. Prev Med. 1984;13(3):233–44. [DOI] [PubMed] [Google Scholar]
- 25.Lin PH, Allen JD, Li YJ, Yu M, Lien LF, Svetkey LP. Blood pressure-lowering mechanisms of the DASH dietary pattern. J Nutr Metab. 2012;2012:472396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mente A, O'Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di Cet al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371(7):601–11. [DOI] [PubMed] [Google Scholar]
- 27.Elliott P, Brown I. Sodium intakes around the world. Background document prepared for the Forum and Technical meeting on Reducing Salt Intake in Populations (Paris 5–7 October, 2006). Geneva: World Health Organization; 2007. [Google Scholar]
- 28.Xu J, Chen X, Ge Z, Liang H, Yan L, Guo X, Zhang Y, Wang L, Ma J. Associations of usual 24-hour sodium and potassium intakes with blood pressure and risk of hypertension among adults in China's Shandong and Jiangsu Provinces. Kidney Blood Press Res. 2017;42(1):188–200. [DOI] [PubMed] [Google Scholar]
- 29.Yan L, Bi Z, Tang J, Wang L, Yang Q, Guo X, Cogswell ME, Zhang X, Hong Y, Engelgau Met al. Relationships between blood pressure and 24-hour urinary excretion of sodium and potassium by body mass index status in Chinese adults. J Clin Hypertens. 2015;17(12):916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie J, Liu L, Kesteloot H. Blood pressure and urinary cations in a low-fat intake Chinese population sample. Acta Cardiol. 2001;56(3):163–8. [DOI] [PubMed] [Google Scholar]
- 31.Tian HG, Nan Y, Shao RC, Dong QN, Hu G, Pietinen P, Nissinen A. Associations between blood pressure and dietary intake and urinary excretion of electrolytes in a Chinese population. J Hypertens. 1995;13(1):49–56. [PubMed] [Google Scholar]
- 32.He J, Tell GS, Tang YC, Mo PS, He GQ. Relation of electrolytes to blood pressure in men. The Yi People Study. Hypertension. 1991;17(3):378–85. [DOI] [PubMed] [Google Scholar]
- 33.Kesteloot H, Huang DX, Li YL, Geboers J, Joossens JV. The relationship between cations and blood pressure in the People's Republic of China. Hypertension. 1987;9(6):654–9. [DOI] [PubMed] [Google Scholar]
- 34.Chan TY, Chan AY, Lau JT, Critchley JA. Sodium and potassium intakes and blood pressure in Chinese adults in Hong Kong: a comparison with southern China. Asia Pac J Clin Nutr. 1998;7(1):33–6. [PubMed] [Google Scholar]
- 35.Jackson SL, Cogswell ME, Zhao LX, Terry AL, Wang CY, Wright J, King SMC, Bowman B, Chen TC, Merritt Ret al. Association between urinary sodium and potassium excretion and blood pressure among adults in the United States National Health and Nutrition Examination Survey, 2018. Circulation. 2018;137(3):237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Little P, Kelly J, Barnett J, Dorward M, Margetts B, Warm D. Randomised controlled factorial trial of dietary advice for patients with a single high blood pressure reading in primary care. BMJ. 2004;328(7447):1054–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staessen J, Broughton PMG, Fletcher AE, Markowe HLJ, Marmot MG, Rose G, Semmence A, Shipley MJ, Bulpitt CJ. The assessment of the relationship between blood pressure and sodium intake using whole-day, daytime and overnight urine collections. J Hypertens. 1991;9(11):1035–40. [DOI] [PubMed] [Google Scholar]
- 38.Libianto R, Moran J, O'Callaghan C, Baqar S, Chen AX, Baker ST, Clarke M, MacIsaac RJ, Jerums G, Ekinci EI. Relationship between urinary sodium-to-potassium ratio and ambulatory blood pressure in patients with diabetes mellitus. Clin Exp Pharmacol Physiol. 2018;45(1):94–7. [DOI] [PubMed] [Google Scholar]
- 39.Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, O'Donnell KM, Batterham MJ. Relationship between sodium and potassium intake and blood pressure in a sample of overweight adults. Nutrition. 2017;33:285–90. [DOI] [PubMed] [Google Scholar]
- 40.Huggins CE, O'Reilly S, Brinkman M, Hodge A, Giles GG, English DR, Nowson CA. Relationship of urinary sodium and sodium-to-potassium ratio to blood pressure in older adults in Australia. Med J Aust. 2011;195(3):128–32. [DOI] [PubMed] [Google Scholar]
- 41.Stamler J, Chan Q, Daviglus ML, Dyer AR, Van Horn L, Garside DB, Miura K, Wu Y, Ueshima H, Zhao Let al. Relation of dietary sodium (salt) to blood pressure and its possible modulation by other dietary factors: The INTERMAP Study. Hypertension. 2018;71(4):631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farapti F, Nadhiroh SR, Sayogo S, Mardiana N. Urinary and dietary sodium to potassium ratio as a useful marker for estimating blood pressure among older women in Indonesian urban coastal areas. Med J Nutrition Metab. 2017;10(2):113–22. [Google Scholar]
- 43.Glatz N, Chappuis A, Conen D, Erne P, Péchère-Bertschi A, Guessous I, Forni VE, Gabutti L, Muggli F, Gallino Aet al. Associations of sodium, potassium and protein intake with blood pressure and hypertension in Switzerland. Swiss Med Wkly. 2017;147:w14411. [DOI] [PubMed] [Google Scholar]
- 44.Mohammadifard N, Khaledifar A, Khosravi A, Nouri F, Pourmoghadas A, Feizi A, Esmaillzadeh A, Sarrafzadegan N. Dietary sodium and potassium intake and their association with blood pressure in a non-hypertensive Iranian adult population: Isfahan salt study. Nutr Diet. 2017;74(3);275–82. [DOI] [PubMed] [Google Scholar]
- 45.Rhee MY, Shin SJ, Gu N, Nah DY, Kim BK, Hong KS, Cho EJ, Sung KC, Lee SY, Kim KI. Relationship between 24-h urine sodium/potassium ratio and central aortic systolic blood pressure in hypertensive patients. Hypertens Res. 2017;40(4):405–10. [DOI] [PubMed] [Google Scholar]
- 46.Vallejo M, Colin-Ramirez E, Mancia SR, Rosado RC, Madero M, Vazquez OI, Vargas-Barron J. Assessment of sodium and potassium intake by 24 h urinary excretion in a healthy Mexican cohort. Arch Med Res. 2017;48(2):195–202. [DOI] [PubMed] [Google Scholar]
- 47.M'Buyamba-kabangu JR, Fagard R, Lijnen P, Mbuy RW, Staessen J, Amery A. Blood pressure and urinary cations in urban Bantu of Zaire. Am J Epidemiol. 1986;124(6):957–68. [DOI] [PubMed] [Google Scholar]
- 48.Chien KL, Hsu HC, Chen PC, Su TC, Chang WT, Chen MF, Lee YT. Urinary sodium and potassium excretion and risk of hypertension in Chinese: report from a community-based cohort study in Taiwan. J Hypertens. 2008;26(9):1750–6. [DOI] [PubMed] [Google Scholar]
- 49.Kwok TCY, Chan TYK, Woo J. Relationships of urinary sodium/potassium excretion and calcium intake to blood pressure and prevalence of hypertension among older Chinese vegetarians. Eur J Clin Nutr. 2003;57(2):299–304. [DOI] [PubMed] [Google Scholar]
- 50.Cook NR, Kumanyika SK, Cutler JA. Effect of change in sodium excretion on change in blood pressure corrected for measurement error. The Trials of Hypertension Prevention, Phase I. Am J Epidemiol. 1998;148(5):431–44. [DOI] [PubMed] [Google Scholar]
- 51.Kim MK, Kwon M, Rhee MY, Kim KI, Nah DY, Kim SW, Gu N, Sung KC, Hong K S, Cho EJet al. Dose-response association of 24-hour urine sodium and sodium to potassium ratio with nighttime blood pressure at older ages. Eur J Prev Cardiol. 2019;26:952–60. [DOI] [PubMed] [Google Scholar]
- 52. WHO. Guideline: Sodium intake for adults and children. 2012, Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 53. WHO/FAO. Diet, Nutrition and the Prevention of Chronic Diseases. Geneva: World Health Organization; 2003. [PubMed] [Google Scholar]
- 54.Parfrey PS, Wright P, Goodwin FJ, Vandenburg MJ, Holly JMP, Evans SJW, Ledingham JM. Blood pressure and hormonal changes following alteration in dietary sodium and potassium in mild essential hypertension. Lancet North Am Ed. 1981;317(8211):59–63. [DOI] [PubMed] [Google Scholar]
- 55.Klag MJ, He J, Coresh J, Whelton PK, Chen JY, Mo JP, Qian MC, Mo PS, He GQ. The contribution of urinary cations to the blood pressure differences associated with migration. Am J Epidemiol. 1995;142(3):295–303. [DOI] [PubMed] [Google Scholar]
- 56.Lisheng L, Jinxiang X, Weiqing F. Urinary cations and blood pressure: a collaborative study of 16 districts in China. J Hypertens. Suppl1988;6(4):S587–90. [PubMed] [Google Scholar]
- 57.Skrabal F, Aubock J, Hortnagl H. Low sodium/high potassium diet for prevention of hypertension: probable mechanisms of action. Lancet North Am Ed. 1981;318(8252):895–900. [DOI] [PubMed] [Google Scholar]
- 58.McDonough AA, Youn JH. Potassium homeostasis: the knowns, the unknowns, and the health benefits. Physiology. 2017;32(2);100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson AT, Edwards DG, Farquhar WB. The influence of dietary salt beyond blood pressure. Curr Hypertens Rep. 2019;21(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2017;4:CD004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rhee OJ, Rhee MY, Oh SW, Shin SJ, Gu N, Nah DY, Kim SW, Lee JH. Effect of sodium intake on renin level: analysis of general population and meta-analysis of randomized controlled trials. Int J Cardiol. 2016;215:120–6. [DOI] [PubMed] [Google Scholar]
- 62.Adrogué HJ, Madias NE. Mechanisms of disease sodium and potassium in the pathogenesis of hypertension. N Engl J Med. 2007;356(19):1966–78. [DOI] [PubMed] [Google Scholar]
- 63.Moore TJ, Vollmer WM, Appel LJ, Sacks FM, Svetkey LP, Vogt TM, Conlin PR, Simons-Morton DG, Carter-Edwards L, Harsha DW. Effect of dietary patterns on ambulatory blood pressure results from the Dietary Approaches to Stop Hypertension (DASH) trial. Hypertension. 1999;34(3):472–7. [DOI] [PubMed] [Google Scholar]