Abstract
COVID-19 pandemic has changed the world dramatically since was first reported in Wuhan city, China [1]. Not only as a respiratory illness that could lead to fatal respiratory failure, but also some evidences suggest that it can propagate as a chronic disease associated with a variety of persistent post COVID-19 pathologies that affect patients’ life [2,3]. Pulmonary hypertension (PH) is one of the challenging diseases that may develop as a consequence of SARS-COV-2 infection in some COVID-19 survivors [4,5]. The vasopressor, proliferative, proinflammatory, and prothrombotic actions of endothelin [6] may be encountered in the COVID-19-induced PH pathology. And so, endothelin blockers may have an important role to restrict the development of serious PH outcomes with special precautions considering patients with significant hypoxemia.
Keywords: Endothelin, Fibrosis, Inflammation, Pulmonary hypertension, Post COVID syndrome, Thrombosis
Abbreviations
- AgII
angiotensin II
- ACE2
angiotensin converting enzyme
- AP-1
activator protein-1
- ARDS
acute respiratory distress syndrome
- ECs
Endothelial cells
- ET
endothelin
- edn-1
ET-1 gene
- ETAR
endothelin type A receptor
- ETBR
endothelin type B receptor
- IFNɣ
interferon-gamma
- IL-1b
interleukin-1b
- NFκB
nuclear factor kappa B
- NO
nitric oxide
- PASMCs
pulmonary arterial smooth muscle cells
- prostacyclin PGI2
prostacyclin
- PAH
pulmonary arterial hypertension
- PH
pulmonary hypertension
- PDEI5
phosphodiesterae type 5 inhibitor
- PPCS
persistent post-COVID syndrome
- RAAS
renin angiotensin aldosterone system
- ROS
reactive oxygen species
- TNFα
tumour necrosis factor-alpha
- TXA2
thromboxane A2
1. Introduction
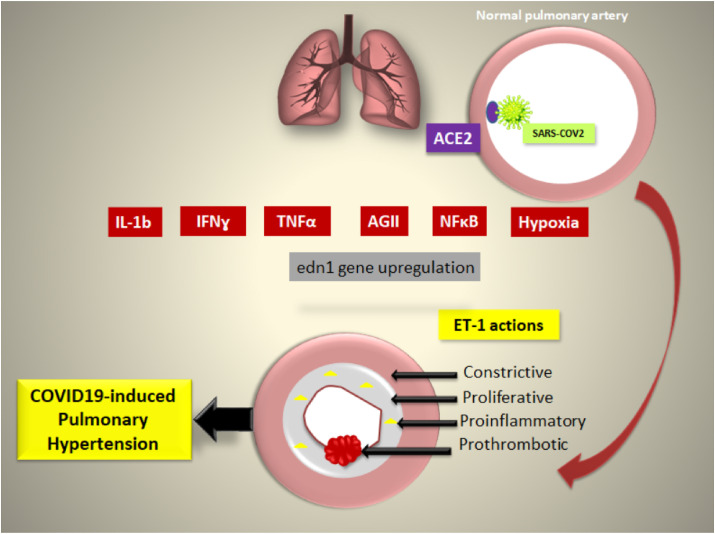
SARS-COV-2 gets access to the lung through binding to angiotensin converting enzyme 2 (ACE2), upon which SARS-COV2 enters the respiratory cells, replicates, and causes variable degrees of pulmonary affection according to the immunological state of the patients [[1], [7]]. Yet, sometimes dyspnea and fatigue reported in COVID-19 patients persist for weeks even after getting a negative COVID-19 PCR test, and still the degree of dyspnea is unmatched to the degree of lung damage determined by the conventional imaging techniques [8,9]. This dissociation between clinical signs and radiological findings may point-out to a latent microvascular pathology that significantly impaired the ventilation/perfusion (V/Q) balance and lead to the development of one of the persistent post-COVID syndrome (PPCS) pathologies. Many theories have discussed the possible underlying pathogenesis that result in the development of PPCS. Organ fibrosis is one of the widely accepted hypothesis for this longstanding illness [2,3] (see Fig. 1 ).
Fig. 1.
The possible implication of endothelin in the pathology of COVID-19-induced pulmonary hypertension. ACE2: angiotensin converting enzyme; AgII: angiotensin II; ET-1: Endothelin; IFNɣ: Interferon gamma; IL:Interleukin; NFκB: nuclear factor kappa ligand B; TNFα: Tumor necrosis factor-alpha.
An elevated level of endothelin (ET-1) has been correlated with different pathologies of PH. Yet, the role of ET-1 in the pathogenesis of pulmonary arterial hypertension (PAH) in which the pathology is confined to the pulmonary arteries, is well-established and has been linked to its proliferative, fibrotic, and prothrombotic potentials that lead to vascular remodeling [10]. In the era of COVID-19, the upregulation of ET-1 may contribute to form a pulmonary vascular pathology simulating PAH which can be managed accordingly using endothelin blockers, either selective ETAR antagonist (ambrisentan) or dual receptors blocker (bosentan or macietentan) that have been proved to refine the morbidity and mortality rates accompanying PAH [11].
In this review, we will discuss the possible association between COVID-19, the up-regulation of endothelin (ET-1), and the subsequent development of pulmonary/vascular fibrosis and PH. Additionally, we will address the possible beneficial outcomes of using endothelin blockers.
2. Pulmonary hypertension secondary to COVID-19
PH is a challenging disease that requires a special level of attention to diagnose and manage meanwhile managing COVID-19. PH is classified according to the pathophysiology and clinical findings into 5 groups: 1-pulmonary arterial hypertension (PAH), where the pathology is confined to the pulmonary vasculature, 2-PH secondary to left sided heart disease, 3-PH due to lung pathology, 4-thromboembolic-PH, and 5-multifactorial-PH [12].
The prevalence of PH in COVID-19 patients is still undefined, but COVID-19 as a systemic disease may propagate to cause different pathologies that could lead to more than one group from the PH categories.
In an interesting study carried out by Spagnolo et al. (2020) who analyzed different metrics derived from chest CT of confirmed COVID-19 patients, enlarged pulmonary artery diameter was associated with the worst clinical outcome and death [13]. The dilated pulmonary artery may refer to pulmonary arterial thrombosis or pulmonary hypertension (PH) that could have pre-existed or even developed during the course of COVID-19. This mandates preventing and early diagnosing of COVID-19-related PH.
Moreover, Van dongen et al. (2020) published a case report of a 60 year old COVID-19 patient who developed severe dyspnea and hypoxia 10 days after being discharged from hospital following COVID-19 infection. Transthoracic echocardiography (TTE) has proved PH while CT angiography, autoimmune laboratory evaluation and careful examination have excluded obstructive/macrovascular pulmonary embolism, hereditable PH and left ventricular cause of PH. Yet, they raised the concern of the possible development of post COVID-19 PH either as PAH or restrictive lung fibrosis [14].
As regards to lung fibrosis, the association between acute respiratory distress syndrome (ARDS) which occurs in about 5–8% of COVID-19 patients, and PH is well-known. The stages of ARDS (that involves exudative and proliferative phases which ends by tissue fibrosis), could explain the development of PH [4,15]. Furthermore, hypoxic vasoconstriction of the pulmonary vasculature, parenchymal lung damage, endothelial inflammation and injury, and thromboembolic disorders may aid in the development of pulmonary fibrosis and pulmonary hypertension [16,17]. Moreover, patients who are managed by mechanical ventilation are prone to the effect of the shear forces on the secretion of pro-fibrotic mediators: fibronectin, transforming growth factor beta (TGFβ), collagen I and collagen III, and the inhibition of the collagenase enzyme [2,18].
As regards to PAH, in a single-center, observational, cross-sectional study that included 200, non-ICU admitted, moderate to severe COVID-19 patients in Italy, Pagnesi et al. (2020) mentioned that the prevalence of pulmonary hypertension (PH) was 12% in the absence of clinical ARDS. According to Pagnesi et al. study, COVID-19 patients who developed PH were found to have signs of severe COVID-19 infection with poor clinical prognosis. Chest imaging and laboratory parameters revealed that PH versus non-PH patients had more lung damage and laboratory parameters abnormalities (e.g. lymphocytopenia and elevated D-Dimer) respectively and the echocardiography has proved signs of right ventricular dysfunction. Moreover, the oxygen saturation, the need for non-invasive mechanical ventilation, the need for ICU admission, and the mortality rate were much higher in patients with COVID-19 induced PH [5].
In the same context, and to exclude ARDS/post ARDS lung fibrosis, Tudoran et al. (2021) evaluated 91 patients previously hospitalized for mild to moderate COVID-19 without respiratory failure and with normal basal TTE that was performed during the hospitalization period. All patients have received anticoagulants during hospitalization and continued for 40 days after discharge. Two months after discharge and after ensuring patient recovery, TTE has revealed that 7.69% of the patients had PH and 10.28% had right ventricular dysfunction that could be explained in the view of endothelitis, endothelial dysfunction, and microthrombi of the pulmonary vessels following SARS-COV2 infection that progressed to PAH [19].
Furthermore, Calabrese et al. (2020) in a broad review that discussed the lessons gained from lung autopsies of COVID-19 victims found that vascular injury with both neutrophilic and lymphocytic infiltration together with endothelitis and fibrin thrombi were a landmark of SARS-COV-2 infection. Calabrese et al. examined seven lungs autopsies obtained from patients who died from COVID-19 and compared it with seven lungs of influenza victims. They found that COVID-19 lungs had distorted vasculature and sprouting angiogenesis. The alveolar capillaries microthrombi were 9 times more prevalent in COVID-19 than influenza. Additionally, four out of the seven COVID-19 lungs have shown pulmonary artery thrombi ranging from 1 mm to 2 mm in diameter [20].
Similarly, in a recent study by Suzuki et al. (2021) who compared pulmonary vessels samples from patients that died from SARS-COV-2 infection to other samples archived from patients who died of H1N1 or SARS-COV-1 infections, found a significant thickening in pulmonary vascular wall in COVID-19 samples in comparison with the other samples [21]. Suzuki et al. speculated the possible association between COVID-19 and the development of PAH [21].
3. Endothelin-1 in COVID-19 patients
Endothelial cells (ECs) can synthesize and release various endothelium-derived relaxation factors such as: nitric oxide (NO) and prostacyclin (PGI2), and other contractile factors including; endothelin (ET-1), thromboxane A2 (TXA2), reactive oxygen species (ROS), and angiotensin II (AgII), which play significant roles in the regulation of vascular tone [22].
ET-1, the most powerful vasopressor secreted from the vascular ECs, is secreted also from vascular smooth muscle cells, macrophages, endothelial lining of the airways, cardiac cells, brain neurons, and fibroblasts. ET-1 exerts its actions through binding to ET-type A (ETAR) and type B (ETBR) receptors [6].
3.1. Endothelial dysfunction and ET-1 upregulation
Angiotensin II, cytokines, free radicals, old age, and ethnic and racial differences are factors that can lead to endothelial dysfunction that is characterized by an increase in the production of endothelium derived contracting factors ET-1. Since patients with severe or critical COVID-19 frequently have underlying comorbidities (e.g. diabetes, hypertension, and cardiovascular diseases), they are more prone to have chronic endothelial dysfunction and abnormally high ET-1 level [[23], [24], [25]]. Moreover, ACE2; SARS-COV-2 receptors on the pulmonary vascular endothelium are occupied during viral entry and are susceptible to shedding alongside the replication of SARS-COV-2 that prompts endothelitis and endothelial dysfunction. Nevertheless, the hyper-inflammatory state reported in some COVID-19 patients can predispose to endothelial dysfunction with subsequent ET-1 overexpression [26].
3.2. Angiotensin II mediated ET-1 upregulation
The down regulation of ACE2 decreases the capacity of ACE2 to hydrolyze Ag II into Ag1-7 and leads to accumulation of supraphysiological levels of AgII. Moreover, ACE2 was found to regulate the sympathetic activity through reinforcing the inhibitory signals to the paraventricular neurons [27]. Thus, it is expected that the down regulation of ACE2 will potentiate the sympathetic signaling that stimulates the renin angiotensin aldosterone system (RAAS) to increase angiotensin II production.
The accumulated AgII would further mediate the regulation of the transcriptional factor activator protein-1 (AP-1) that has been known to modulate genomic responses to proinflammatory and proliferative signals, and is one of the promoters of the ET-1 gene (edn1) activation. AP-1 can be stimulated also by thrombin which is elevated as a part of the thromboembolic disorder reported in COVID-19 [28].
Furthermore, edn1 gene transcription is known to be regulated also by: hypoxia, the transcription factor; nuclear factor kappa B (NFκB), tumour necrosis factor-alpha (TNFα), interferon-gamma (IFNɣ) and interleukin-1b (IL-1b), and all of these stimuli and cytokines are of great interest due to the role of their upregulation in the cytokine storm and the hyper inflammatory state observed in severe COVID-19 illness [26]. In the same context, edn1 gene variants and/or ETAR and ETBR gene variants causing high levels of circulating ET-1 might predispose to an increased risk of severe SARS CoV-2 infection [29].
Additionally, it was reported that SARS-CoV-2 cause pleiotropic alterations of glucose metabolism, leading to new-onset diabetes in some COVID-19 patients. Noticeably, glucose itself can stimulate NFκB activation and affecting the edn1 promoter with increased edn1 expression and subsequent ET-1 elevation in those patients [30].
So, in terms of the above mentioned data, it can be concluded that elevated circulating ET-1 levels or the presence of ET-1 gene variants are important biomarkers that can predict individuals liable to COVID-19-induced PH.
4. Endothelin-1 and pulmonary hypertension
In the lungs, ETAR is localized to pulmonary arterial smooth muscle cells (PASMC), whereas ETBR is found predominantly on the endothelium but is also present on PASMC. ET-1 acts through the ETAR receptors on the endothelial cells to stimulate vasoconstriction and proliferation [31]. Furthermore, ET-1 can: navigate an inappropriate apoptotic response, increases extracellular matrix deposition and perivascular fibrosis, stimulate a proinflammatory state, and possesses prothrombotic actions [32]. That is why ET-1 can arbitrate different forms of PH as it shares in pulmonary fibrosis, vascular remodeling, and thromboembolic events.
On the other hand, the stimulation of the ETB1 results in elaboration of pro-constrictory elements, such as thromboxane A2, leading to the speculation that pulmonary vasoconstriction in PAH may in fact be the result of activation of both receptor subtypes [33]. Yet, ETB2 can stimulate the release of NO and PGI2, leading to vasodilation that counteract the effects of ETAR and ultimately ET-1. Moreover, ETBRs have a protective function because of their role in local ET-1 clearance [34].
As regards to pulmonary fibrosis, ET-1 has an important role in the pathophysiological development of fibrosis. ET-1 has an intimate relation with TGFβ as both are upregulated simultaneously in many fibrotic disorders such as: systemic sclerosis, Takayasu arteritis, Buerger's disease, systemic lupus erythematousus, and pulmonary fibrosis [35]. TGFβ itself is a regulator and promoter of ET-1gene expression, which suggest that ET-1 is a mediator for the pro-fibrotic actions of TGFβ and evident by the ability of the dual ET-1 receptor antagonist; bosentan to block the pro-fibrotic actions of TGFβ. Moreover, bosentan was found to decrease the expression of NFκB which mediates the effects of ET-1 [36], as well as collagen I and fibronectin, which therefore would decrease the incidence of vasculopathy and fibrosis [37]. In concordance with this theory, the transgenic model of ET-1overexpression in mice resulted in pulmonary fibrosis suggesting a direct link between ET-1 and pulmonary fibrosis [38].
As regards to PAH, COVID-19 is believed to affect the pulmonary arteries inducing endothelial dysfunction and vascular remodeling through decreasing the clearance of AgII which itself can: 1-stimulate the upregulation of TGFβ and the connective tissue growth factor [39] and 2- upregulate ET-1 production. ET-1 may work directly or through the anti-endothelial cell antibodies which are released as early as the “endothelial injury phase”, to induce more endothelial injury with a sequential ET-1 over secretion and hence, fibrosis and vascular remodeling [40,41].
ET-1 and thrombosis are linked at different levels. ET-1 enhances the endothelial release of Von Willebrand factor (vWF) that sequentially facilitates platelet adhesion [42]. In addition, ET-1 was found to promote thrombosis by enhancing adenosine di-phosphate (ADP)-mediated platelets aggregation [43]. Moreover, ET-1 can increase thrombin/antithrombin balance favoring a pro-thrombotic state. Furthermore, ET-1 injection in rabbits was found to produce a disseminated intravascular coagulation like disease. Interestingly, the thrombotic actions of angiotensin II was found to be blocked by an ETAR blocker; ABT-627. Senchenkova et al. (2010) explained this finding by the ability of ET-1 to up-regulate the production of plasma plasminogen activator-1 which impairs the fibrinolysis, and hence facilitates thrombus formation [44].
5. Endothelin blockers: possible role and precautions
Therapeutic strategies that modulate the activity of endothelin are, therefore, of interest to improve the functional status of patients with PAH. Currently, endothelin receptors antagonists (ERAs) have proven their efficacy with relatively few side effects and became an attractive option, either as a monotherapy or in combination therapy as first-line therapy in PAH patients with functional class II and III [45].
Despite the theoretical advantage of selective ETAR blockade, keeping the protective ETBR-ET-1 clearing actions, clinical efficacy appears greatly similar between bosentan, macientan, and ambrisentan [46]. However, ambrisentan has shown to exert a synergistic effect with the phosphodiesterase type 5 inhibitor (PDE5I); tadalafil and ambrisentan which is used nowadays as a first line drug either as monotherapy or combined therapy in treatment of PAH [47].
However, the use of endothelin blockers should be restricted to stable compensated non-hypoxic patients to avoid worsening of any V/Q mismatch. Moreover, ET-1 itself shares in the down regulation of ACE2, thus the use of endothelin blockade may be associated with an increase in the expression of ACE2 which could sequentially increase the susceptibility to SARS-COV-2 entry. Still, the increased ACE2 levels will decrease the level of angiotensin II, which has the advantage of blocking angiotensin II mediated pulmonary vasoconstriction as well as PSMCs proliferation and therefore improve PH prognosis and symptomatology. This warrants the need for more clinical studies in order to weight the risk/benefit ratio of using ERAs in selected compensated patients proved to have COVID-19 induced PH.
6. Conclusion
Moderate to severe COVID-19 disease could be considered as a risk factor to developing PH. Hence, sensitive parameters such as echocardiography are needed to be a part of routine clinical assessment in those patients to diagnose early COVID-19-induced PH. Endothelin can be upregulated in the course of COVID-19 disease and is accused with the development of COVID-19-induced PH. Yet, further research should be done to find more specific and accurate measures to predict patients at higher risk to develop post COVID-19 pulmonary hypertension and emphasize the possible role of endothelin blockers to prevent or ameliorate the pulmonary hypertension in those susceptible patients.
Data availability statement
Not applicable.
Funding statement
Not applicable.
Author contribution statement
Omnia Azmy Nabeh: conceptualization, Information collection, writing, supervision Lamiaa Matter: Information collection, writing, supervision, Mahmoud Khattab: Data collection, writing Esraa Menshawey: Data collection, writing.
Ethics approval statement
Not applicable.
Permission to reproduce material from other sources
Not applicable.
Declaration of competing interest
All authors have given final approval of the version to be published. The authors declare that all data were generated in-house and that no paper mill was used.
References
- 1.Yang J., Chen X., Deng X., Chen Z., Gong H., Yan H., Wu Q., Shi H., Lai S., Ajelli M., Viboud C. Disease burden and clinical severity of the first pandemic wave of COVID-19 in Wuhan, China. Nat. Commun. 2020 Oct 27;11(1) doi: 10.1038/s41467-020-19238-2. 1-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg P., Arora U., Kumar A., Wig N. The “post‐COVID” syndrome: how deep is the damage? J. Med. Virol. 2021 Feb;93(2):673–674. doi: 10.1002/jmv.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan F., Ye T., Sun P., Gui S., Liang B., Li L., Zheng D., Wang J., Hesketh R.L., Yang L., Zheng C. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020 Jun;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calcaianu G., Calcaianu M., Gschwend A., Canuet M., Meziani F., Kessler R. Hemodynamic profile of pulmonary hypertension (PH) in ARDS. Pulm. Circ. 2018 Jan;8(1) doi: 10.1177/2045893217753415. 2045893217753415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagnesi M., Baldetti L., Beneduce A., Calvo F., Gramegna M., Pazzanese V., Ingallina G., Napolano A., Finazzi R., Ruggeri A., Ajello S. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart. 2020 Sep 1;106(17):1324–1331. doi: 10.1136/heartjnl-2020-317355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aubert J.D., Juillerat-Jeanneret L. Endothelin-receptor antagonists beyond pulmonary arterial hypertension: cancer and fibrosis. J. Med. Chem. 2016 Sep 22;59(18):8168–8188. doi: 10.1021/acs.jmedchem.5b01781. [DOI] [PubMed] [Google Scholar]
- 7.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020 Jun 1;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., Fan Y., Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020 Apr 1;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020 May 1;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupuis J., Cernacek P., Tardif J.C., Stewart D.J., Gosselin G., Dyrda I., Bonan R., Crépeau J. Reduced pulmonary clearance of endothelin-1 in pulmonary hypertension. Am. Heart J. 1998 Apr 1;135(4):614–620. doi: 10.1016/s0002-8703(98)70276-5. [DOI] [PubMed] [Google Scholar]
- 11.Shao D., Park J.E., Wort S.J. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacol. Res. 2011 Jun 1;63(6):504–511. doi: 10.1016/j.phrs.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Simonneau G., Gatzoulis M.A., Adatia I., Celermajer D., Denton C., Ghofrani A., Gomez Sanchez M.A., Krishna Kumar R., Landzberg M., Machado R.F., Olschewski H. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2013 Dec 24;62(25S):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 13.Spagnolo P., Cozzi A., Foà R.A., Spinazzola A., Monfardini L., Bnà C., Alì M., Schiaffino S., Sardanelli F. CT-derived pulmonary vascular metrics and clinical outcome in COVID-19 patients. Quantitative imaging in medicine and surgery. 2020 Jun;10(6):1325. doi: 10.21037/qims-20-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Dongen C.M., Janssen M.T., van der Horst R.P., van Kraaij D.J., Peeters R.H., van den Toorn L.M., Mostard R.L. Unusually rapid development of pulmonary hypertension and right ventricular failure after COVID-19 pneumonia. Eur. J. Case Rep. Intern. Med. 2020;7(7) doi: 10.12890/2020_001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araz O. Current pharmacological approach to ARDS: the place of bosentan. Euras. J. Med. 2020 Feb;52(1):81. doi: 10.5152/eurasianjmed.2020.19218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walkey A.J., Summer R., Ho V., Alkana P. Acute respiratory distress syndrome: epidemiology and management approaches. Clin. Epidemiol. 2012;4:159. doi: 10.2147/CLEP.S28800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnham E.L., Janssen W.J., Riches D.W., Moss M., Downey G.P. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur. Respir. J. 2014 Jan 1;43(1):276–285. doi: 10.1183/09031936.00196412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kouzbari K., Hossan M.R., Arrizabalaga J.H., Varshney R., Simmons A.D., Gostynska S., Nollert M.U., Ahamed J. Oscillatory shear potentiates latent tgf-β1 activation more than steady shear as demonstrated by a novel force generator. Sci. Rep. 2019 Apr 15;9(1):1–8. doi: 10.1038/s41598-019-42302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tudoran C., Tudoran M., Lazureanu V.E., Marinescu A.R., Pop G.N., Pescariu A.S., Enache A., Cut T.G. Evidence of pulmonary hypertension after SARS-CoV-2 infection in subjects without previous significant cardiovascular pathology. J. Clin. Med. 2021 Jan;10(2):199. doi: 10.3390/jcm10020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calabrese F., Pezzuto F., Fortarezza F., Hofman P., Kern I., Panizo A., von der Thüsen J., Timofeev S., Gorkiewicz G., Lunardi F. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Arch. 2020 Jul 9:1–4. doi: 10.1007/s00428-020-02886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki Y.J., Nikolaienko S.I., Shults N.V., Gychka S.G. COVID-19 patients may become predisposed to pulmonary arterial hypertension. Med. Hypotheses. 2021 Feb 1;147:110483. doi: 10.1016/j.mehy.2021.110483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godo S., Shimokawa H. Endothelial functions. Arterioscler. Thromb. Vasc. Biol. 2017 Sep;37(9):e108–e114. doi: 10.1161/ATVBAHA.117.309813. [DOI] [PubMed] [Google Scholar]
- 23.Bermejo-Martin J.F., Almansa R., Torres A., González-Rivera M., Kelvin D.J. COVID-19 as a cardiovascular disease: the potential role of chronic endothelial dysfunction. Cardiovasc. Res. 2020 Aug 1;116(10):e132–e133. doi: 10.1093/cvr/cvaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bermejo-Martin J.F., Martín-Fernandez M., López-Mestanza C., Duque P., Almansa R. Shared features of endothelial dysfunction between sepsis and its preceding risk factors (aging and chronic disease) J. Clin. Med. 2018 Nov;7(11):400. doi: 10.3390/jcm7110400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhaun N., Webb D.J. Endothelins in cardiovascular biology and therapeutics. Nat. Rev. Cardiol. 2019 Aug;16(8):491–502. doi: 10.1038/s41569-019-0176-3. [DOI] [PubMed] [Google Scholar]
- 26.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020 Jun;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukerjee S., Gao H., Xu J., Sato R., Zsombok A., Lazartigues E. ACE2 and ADAM17 interaction regulates the activity of presympathetic neurons. Hypertension. 2019 Nov;74(5):1181–1191. doi: 10.1161/HYPERTENSIONAHA.119.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai T., Hirata Y., Emori T., Yanagisawa M., Masaki T., Marumo F. Induction of endothelin-1 gene by angiotensin and vasopressin in endothelial cells. Hypertension. 1992 Jun;19(6_pt_2):753–757. doi: 10.1161/01.hyp.19.6.753. [DOI] [PubMed] [Google Scholar]
- 29.Stow L.R., Jacobs M.E., Wingo C.S., Cain B.D. Endothelin‐1 gene regulation. Faseb. J. 2011 Jan;25(1):16–28. doi: 10.1096/fj.10-161612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubino F., Amiel S.A., Zimmet P., Alberti G., Bornstein S., Eckel R.H., Mingrone G., Boehm B., Cooper M.E., Chai Z., Del Prato S. New-onset diabetes in covid-19. N. Engl. J. Med. 2020 Aug 20;383(8):789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davie N., Haleen S.J., Upton P.D., Polak J.M., Yacoub M.H., Morrell N.W., Wharton J. ET(A) and ET(B) receptors modulate the proliferation of human pulmonary artery smooth muscle cells. Am. J. Respir. Crit. Care Med. 2002;165:398–405. doi: 10.1164/ajrccm.165.3.2104059. [DOI] [PubMed] [Google Scholar]
- 32.Giaid A., Yanagisawa M., Langleben D., Michel R.P., Levy R., Shennib H., Kimura S., Masaki T., Duguid W.P., Stewart D.J. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1993 Jun 17;328(24):1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 33.Seo B., Oemar B.S., Siebenmann R., von Segesser L., Lüscher T.F. Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation. 1994 Mar;89(3):1203–1208. doi: 10.1161/01.cir.89.3.1203. [DOI] [PubMed] [Google Scholar]
- 34.Ergul A. Endothelin‐1 and endothelin receptor antagonists as potential cardiovascular therapeutic agents. Pharmacotherapy. 2002 Jan;22(1):54–65. doi: 10.1592/phco.22.1.54.33505. [DOI] [PubMed] [Google Scholar]
- 35.Manitsopoulos N., Nikitopoulou I., Maniatis N.A., Magkou C., Kotanidou A., Orfanos S.E. Highly selective endothelin-1 receptor A inhibition prevents bleomycin-induced pulmonary inflammation and fibrosis in mice. Respiration. 2018;95(2):122–136. doi: 10.1159/000481201. [DOI] [PubMed] [Google Scholar]
- 36.Muller D.N., Mervaala E.M., Schmidt F., Park J.K., Dechend R., Genersch E., Breu V., Löffler B.M., Ganten D., Schneider W., Haller H. Effect of bosentan on NF-κB, inflammation, and tissue factor in angiotensin II–induced end-organ damage. Hypertension. 2000 Aug;36(2):282–290. doi: 10.1161/01.hyp.36.2.282. [DOI] [PubMed] [Google Scholar]
- 37.Cosenzi A., Bernobich E., Trevisan R., Milutinovic N., Borri A., Bellini G. Nephroprotective effect of bosentan in diabetic rats. J. Cardiovasc. Pharmacol. 2003 Dec 1;42(6):752–756. doi: 10.1097/00005344-200312000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Hocher B., Schwarz A., Fagan K.A., Thone-Reineke C., El-Hag K., Kusserow H., Elitok S., Bauer C., Neumayer H.H., Rodman D.M., Theuring F. Pulmonary fibrosis and chronic lung inflammation in ET-1 transgenic mice. Am. J. Respir. Cell Mol. Biol. 2000 Jul 1;23(1):19–26. doi: 10.1165/ajrcmb.23.1.4030. [DOI] [PubMed] [Google Scholar]
- 39.Zuo W, Zhao X, Chen YG. SARS coronavirus and lung fibrosis. InMolecular Biology of the SARS-Coronavirus 2010 (pp. 247-258). Springer, Berlin, Heidelberg.
- 40.Yamamoto T., Geshi Y., Kuno S., Kase N., Mori H. Anti-endothelial cell antibody in preeclampsia: clinical findings and serum cytotoxicity to endothelial cell. Jpn. J. Clin. Immunol. 1998 Dec 31;21(5):191–197. doi: 10.2177/jsci.21.191. [DOI] [PubMed] [Google Scholar]
- 41.Filep J.G., Bodolay E., Sipka S., Gyimesi E., Csipö I., Szegedi G. Plasma endothelin correlates with antiendothelial antibodies in patients with mixed connective tissue disease. Circulation. 1995 Nov 15;92(10):2969–2974. doi: 10.1161/01.cir.92.10.2969. [DOI] [PubMed] [Google Scholar]
- 42.Halim A., Kanayama N., El Maradny E., Maehara K., Masahiko H., Terao T. Endothelin-1 increased immunoreactive von Willebrand factor in endothelial cells and induced micro thrombosis in rats. Thromb. Res. 1994 Oct 1;76(1):71–78. doi: 10.1016/0049-3848(94)90208-9. [DOI] [PubMed] [Google Scholar]
- 43.Ohlstein E.H., Storer B., Nambi P., Given M., Lippton H. Endothelin and platelet function. Thromb. Res. 1990 Mar 15;57(6):967–974. doi: 10.1016/0049-3848(90)90163-7. [DOI] [PubMed] [Google Scholar]
- 44.Senchenkova E.Y., Russell J., Almeida-Paula L.D., Harding J.W., Granger D.N. Angiotensin II–mediated microvascular thrombosis. Hypertension. 2010 Dec 1;56(6):1089–1095. doi: 10.1161/HYPERTENSIONAHA.110.158220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaumais M.C., Guignabert C., Savale L., Jaïs X., Boucly A., Montani D., Simonneau G., Humbert M., Sitbon O. Clinical pharmacology of endothelin receptor antagonists used in the treatment of pulmonary arterial hypertension. Am. J. Cardiovasc. Drugs. 2015 Feb;15(1):13–26. doi: 10.1007/s40256-014-0095-y. [DOI] [PubMed] [Google Scholar]
- 46.Humbert M., Lau E.M., Montani D., Jaïs X., Sitbon O., Simonneau G. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation. 2014 Dec 9;130(24):2189–2208. doi: 10.1161/CIRCULATIONAHA.114.006974. [DOI] [PubMed] [Google Scholar]
- 47.Mazzuca M.Q., Khalil R.A. Vascular endothelin receptor type B: structure, function and dysregulation in vascular disease. Biochem. Pharmacol. 2012 Jul 15;84(2):147–162. doi: 10.1016/j.bcp.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.