Key Points
SARS-CoV-2 vaccines are generally safe in patients with preexisting ITP but thrombocytopenia exacerbation may occur and requires monitoring.
Splenectomy and past use of 5 or more therapies predict higher risk of worsening thrombocytopenia in ITP patients post-SARS-CoV-2 vaccine.
Visual Abstract
Abstract
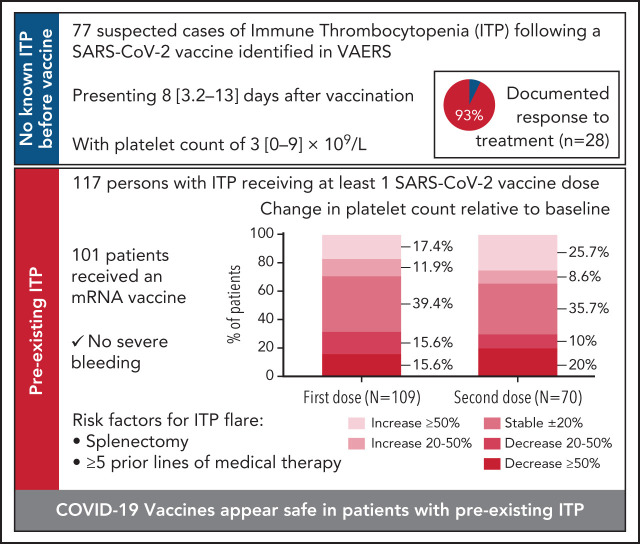
Cases of de novo immune thrombocytopenia (ITP), including a fatality, following SARS-CoV-2 vaccination in previously healthy recipients led to studying its impact in preexisting ITP. In this study, 4 data sources were analyzed: the Vaccine Adverse Events Reporting System (VAERS) for cases of de novo ITP; a 10-center retrospective study of adults with preexisting ITP receiving SARS-CoV-2 vaccination; and surveys distributed by the Platelet Disorder Support Association (PDSA) and the United Kingdom (UK) ITP Support Association. Seventy-seven de novo ITP cases were identified in VAERS, presenting with median platelet count of 3 [1-9] ×109/L approximately 1 week postvaccination. Of 28 patients with available data, 26 responded to treatment with corticosteroids and/or intravenous immunoglobulin (IVIG), and/or platelet transfusions. Among 117 patients with preexisting ITP who received a SARS-CoV-2 vaccine, 19 experienced an ITP exacerbation (any of: ≥50% decline in platelet count, nadir platelet count <30 × 109/L with >20% decrease from baseline, and/or use of rescue therapy) following the first dose and 14 of 70 after a second dose. Splenectomized persons and those who received 5 or more prior lines of therapy were at highest risk of ITP exacerbation. Fifteen patients received and responded to rescue treatment. In surveys of both 57 PDSA and 43 UK patients with ITP, prior splenectomy was associated with worsened thrombocytopenia. ITP may worsen in preexisting ITP or be identified de novo post–SARS-CoV2 vaccination; both situations responded well to treatment. Proactive monitoring of patients with known ITP, especially those postsplenectomy and with more refractory disease, is indicated.
Introduction
The COVID-19 pandemic led to urgent development and widespread use of SARS-CoV-2 vaccines.1,2 In January 2021, USA Today and The New York Times reported an otherwise-well 56-year-old physician diagnosed with de novo immune thrombocytopenia (ITP) 3 days after receiving the Pfizer-BioNTech (BNT162b2) COVID-19 vaccine who died 13 days later from intracranial hemorrhage refractory to administered treatments.3,4 This widely-publicized case led to interrogating the Vaccine Adverse Events Reporting System (VAERS) for other potential cases of de novo ITP.5,6
Discovery of additional cases of de novo ITP following SARS-CoV-2 vaccination generated considerable concern among patients with preexisting ITP and their health care providers. The desire for potentially life-saving vaccination was tempered by fear of ITP exacerbation and life-threatening bleeding. This report describes both additional discovery of de novo ITP cases and 3 independent series each describing outcomes of patients with preexisting ITP following SARS-CoV-2 vaccination.
Materials and methods
VAERS was reviewed to identify potential cases of de novo ITP following SARS-CoV-2 vaccination using search terms: immune thrombocytopenia, thrombocytopenia, decreased platelet count, immunoglobulin (IVIG) therapy, and platelet transfusion (last VAERS access 19 March, 2021). Duplicate entries, reports lacking platelet counts and details of treatments, platelet nadirs >50 × 109/L, presence of other active conditions including hematologic conditions, IVIG given for other indications, and mislabeled records were excluded. ITP was the presumptive diagnosis in cases of isolated thrombocytopenia <50 × 109/L refractory to platelet transfusion in the absence of alternative causes. Eight patients with de novo ITP contacted one of the authors (J.B.B.) and provided additional information; some of these patients may have been entered in VAERS. These patients were included to illustrate management of refractory ITP.
Data for patients with preexisting ITP were obtained via a 10-center retrospective study of adults with ITP who received a SARS-CoV-2 vaccine between December 2020 and March 2021 and who had a postvaccination platelet count; all but 1 center was in the United States. ITP was defined per American Society of Hematology and International Consensus guidelines.7,8 Deidentified clinical data were collected from electronic medical records, including patient demographics, duration of ITP, treatment history including past use of rituximab and/or splenectomy, SARS-CoV-2 vaccine type, bleeding symptoms, and platelet counts; all patients with postvaccination platelet counts were included. The data cutoff for both de novo and existing cases of ITP occurred prior to published reports of vaccine-induced thrombosis-thrombocytopenia syndrome (VITT-TTS)9 following adenoviral vaccines. Therefore testing for antiplatelet factor 4 antibodies had not been performed. The study was approved by the Institutional Review Board (IRB) of New-York-Presbyterian-Hospital Weill-Cornell-Medicine with waiver of informed consent.
A second source of ITP patient data were obtained from the Platelet Disorder Support Association’s (PDSA) IRB-approved ITP Natural History Registry (https://pdsa.org/images/COVID-19-survey-results.pdf). A third source came from the United Kingdom ITP Support Association, which performed a similar survey. Both surveys were posted online, and all responses were tabulated.
Continuous variables are described as means ± standard deviation (SD), or medians and interquartile ranges (IQR), and categorical variables as proportions. Groups were compared using a Student t test, Mann-Whitney test, χ2 or Fisher exact test as applicable. Relative risk (RR) and 95% confidence intervals (CI) were calculated to assess the strength of association. No multivariate analyses were performed. Missing information was handled by pairwise deletion; in certain cases, platelet counts were “adequate/normal” and thus could not be used for numerical analyses.
The lowest or highest postvaccination platelet count was used to characterize postvaccine change. A “stable platelet count” was defined as a postvaccination platelet count within 20% of the prevaccination level. An “exacerbation of ITP” was defined as the development of any 1 or more of the following: (1) ≥50% decline in platelet count from prevaccination baseline; (2) >20% decline from prevaccination baseline and platelet nadir <30 × 109/L; and/or (3) receipt of rescue therapy for ITP. No distinction was made among patients meeting 1, 2, or 3 criteria for the composite endpoint.
Results
De novo ITP: VAERS data
A total of 93 records in VAERS included a platelet count, or 'severe' or 'low' platelets and platelet-specific interventions. Of these, 16 were excluded (preexisting thrombocytopenia [n = 5], preexisting ITP [n = 10], thrombocytopenia resolved with platelet transfusion only [n = 1]), leaving 77 reports (Table 1). All received either the Pfizer-BioNTech (BNT162b2) (n = 37) or Moderna (mRNA-1273) (n = 40) vaccine. The mean age was 63 ± 20 years with 60% female. Premorbid autoimmune disease was reported in 32%.
Table 1.
Demographic and clinical characteristics of 77 individuals identified in VAERS without reported platelet disorders with suspected de novo ITP following SARS-CoV-2 immunization
| Characteristic | Mean ± SD, median [IQR], or n (%) | No available for analysis | |
|---|---|---|---|
| Age | 63 ± 19.7 | 76 | |
| Gender | |||
| ≤50 years old | Men | 3 (15) | 20 |
| Women | 17 (85) | ||
| >50 years old | Men | 28 (50) | 56 |
| Women | 28 (50) | ||
| Vaccine type | Moderna | 37 (48) | 77 |
| Pfizer-BioNTech | 40 (52) | ||
| No. of doses received prior to presentation | 1 2 |
51 (77.3) 15 (22.7) |
66 |
| Days to symptom onset | 8 [3.2-13] Range 0-38 |
74 | |
| Symptoms at presentation* | Mucocutaneous bleeding | 42 (73.7) | 57 |
| Genitourinary bleeding | 6 (10.5) | ||
| Gastrointestinal bleeding | 5 (6.9) | ||
| Central nervous system bleeding | 1 (1.8) | ||
| Bleeding reported but not specified | 2 (3.5) | ||
| No bleeding reported | 6 (10.5) | ||
| Platelet count at presentation | 3 [1-9] Range 0-47 |
73 | |
| ≤10 × 109/L | 58 (79.4) | ||
| History of autoimmune disease other than ITP | Any autoimmune disease | 22 (31.9) | 69 |
| Hypothyroidism† | 13 (18.8) | ||
| Rheumatologic | 4 (5.8) | ||
| Dermatologic | 2 (2.9) | ||
| Gastrointestinal | 1 (1.4) | ||
| Antiphospholipid syndrome‡ | 1 (1.4) | ||
| Multiple sclerosis | 1 (1.4) | ||
| Treatment | Any combination of steroids, IVIG, and platelet transfusion | 21 (44.7) | 46 |
| Steroids only | 16 (34) | ||
| Platelet transfusion only | 3 (8.5) | ||
| IVIG only | 1 (2.1) | ||
| TPO-RA, IVIG, +/− platelet transfusion, steroid | 3 (6.4) | ||
| Rituximab, steroids, IVIG, platelet transfusion | 1 (2.1) | ||
| Vincristine, IVIG, rituximab, TPO-RA, +/− platelet transfusion, steroid | 1 (2.1) | ||
| Response to therapy | Yes | 26 (92.9) | 28 |
| No | 2 (7.1) | ||
| Best known response | 30-50 × 109/L | 3 (11.5) | 26 |
| 50-100 × 109/L | 7 (26.9) | ||
| 100-150 × 109/L | 3 (11.5) | ||
| Normalization§ | 6 (23.1) | ||
| Improvementǁ | 7 (26.9) | ||
| Time to platelet count >30 × 109 cells/L | <3 d of treatment | 9 (81.8) | 11 |
More than 1 site of bleeding reported in some cases, excludes 1 patient with CNS bleeding whose thrombocytopenia resolved with platelet transfusion only and the index patient who did not present with but later developed CNS bleeding.
Includes 1 person with “antithyroglobulin antibody.”
Antiphospholipid syndrome in the same patient with other rheumatologic conditions.
Platelet count ≥150 x109/L or described as “platelets normalized.”
No platelet count provided but described as “improved” or “resolved.”
Of the 66 reports that specified first or second dose, 51 (77%) developed thrombocytopenia following dose 1 of a SARS-CoV-2 vaccine and 15 (23%) following dose 2. Median platelet count was 3 [1-9] ×109/L at a median of 8 [3-13] days following vaccination (n = 73). Seventy-four percent of patients presented with skin or oral mucosa bleeding. Six patients presented with genitourinary (GU) bleeding, 5 gastrointestinal (GI) bleeding, and 1 presented with central nervous system (CNS) bleeding. Additional information regarding bleeding was not available; however, the only death noted was the widely-reported “index” patient who developed CNS bleeding. No thrombotic events were reported in these patients.
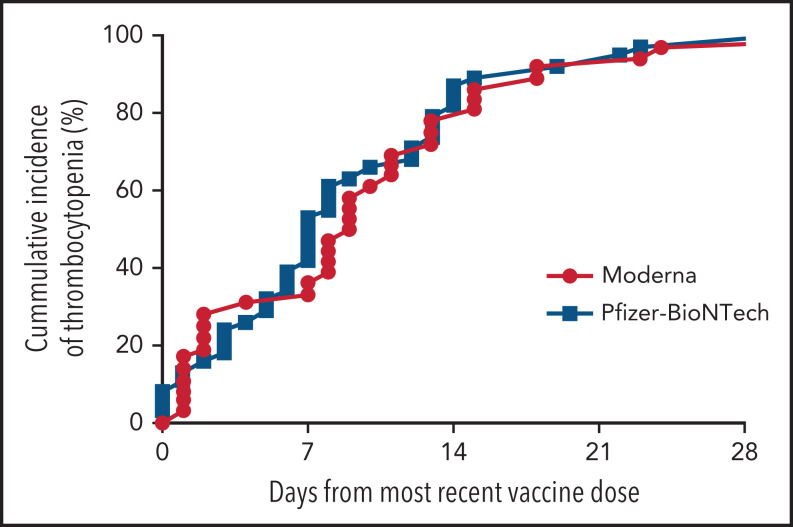
There was no significant difference in time to symptom onset (Figure 1) or platelet count at presentation (data not shown) between those who received the Moderna vs the Pfizer-BioNTech SARS-CoV-2 vaccine.
Figure 1.
Time to presentation with thrombocytopenia following the most recent dose of a SARS-CoV-2 vaccine. The graph compares vaccine type (Pfizer-BioNTech [n = 39] and Moderna [n = 37]) in persons without reported preexisting platelet disorders.
Among 46 records specifying treatment, all patients received IVIG and/or corticosteroids and/or platelet transfusions, and 5 received additional agents: thrombopoietin receptor agonists (TPO-RA), rituximab, or vincristine (Table 1). An increase in the platelet count to >30 x109/L was reported in 26 (93%) of 28 records with a follow-up platelet count. The 2 nonresponders included the “index” patient and a 63-year-old man with a platelet count of 1 × 109/L 11 days following Pfizer-BioNTech dose 1 with no response to ‘typical ITP therapies’ by day 4 (outcome unknown).
Additional information was available in 8 patients who contacted one of the authors (J.B.B.) because they were difficult to manage. It is unclear how many of these patients were reported to VAERS. Each case followed dose 1 of the Pfizer-BioNTech or Moderna SARS-CoV-2 vaccine and was distinguished by minimal response to platelet transfusion, corticosteroids, IVIG, and, in 7 of 8, rituximab.5 Initiation of additional treatment ranged from 3 to 13 days after diagnosis of ITP. Seven patients received romiplostim (3-7 μg/kg) and 1 eltrombopag (75 mg daily). Other agents included vincristine (1.5 mg IV push) in 6 patients, mycophenolate mofetil, dapsone, and cyclosporine (1 patient each). All 8 patients responded with improvement in platelet count. An 80-year-old woman on warfarin for antiphospholipid syndrome developed subarachnoid and subdural hemorrhages; corticosteroids, IVIG, platelet transfusion, and romiplostim increased her platelet count.
Preexisting ITP cohort: platelet counts before and after vaccination
Between December 2020 and March 2021, 117 patients with preexisting ITP from 10 centers were vaccinated for SARS-CoV-2 and had postvaccine platelet counts. The mean age was 63 ± 17 years, 62% were female, there was a median of 12 [4-23] years since diagnosis of ITP, and patients had received a median of 3 [2-4] prior medical treatments. Sixty-nine patients were receiving ITP treatment at the time of vaccination, 58 (84%) a TPO-RA. Twenty-seven had undergone splenectomy. Sixteen of 48 off-treatment patients had platelet counts ≥150 × 109/L (Table 2).
Table 2.
Demographic and clinical characteristics of 117 patients with preexisting ITP who received at ≥1 dose of a SARS-CoV-2 vaccine
| Characteristic | Mean ± SD, median [IQR], or n (%) | No. available for analysis |
|---|---|---|
| Age | 62.5 ± 16.9 | 116 |
| Gender | ||
| Male | 43 (37.8) | 117 |
| Female | 74 (62.2) | 74 (62.2) |
| Duration of ITP diagnosis (y) | 12 [4-23] | 97 |
| No. of previous ITP treatments | ||
| None | 6 (8.1) | 74 |
| Medical treatments* | 3 [2-4] | 75 |
| Rituximab | 41 (40.6) | 101 |
| Splenectomy | 25 (20.7) | 117 |
| Current ITP treatment | ||
| TPO-RA only | 47 (40.2) | 117 |
| Corticosteroid only | 4 (3.4) | |
| Other single-agent therapies† | 5 (4.3) | |
| TPO-RA + corticosteroid | 5 (4.3) | |
| TPO-RA + IVIG + mycophenolate | 3 (2.6) | |
| TPO-RA + ibrutinib | 2 (1.7) | |
| Corticosteroid + mycophenolate | 2 (1.7) | |
| TPO-RA + corticosteroid + mycophenolate | 1 (0.8) | |
| No current treatment and platelets <150 × 109/L | 32 (27.4) | |
| No current treatment and platelets ≥150 × 109/L | 16 (13.7) | |
| Comorbidities | ||
| Autoimmune hemolytic anemia | 10 (11.6) | 86 |
| Other autoimmune disease | 31 (36) | |
| Vaccine manufacturer | ||
| Moderna | 48 (42.1) | 114 |
| Pfizer-BioNTech | 53 (46.5) | |
| Janssen | 4 (3.5) | |
| Oxford-AstraZeneca | 9 (7.9) |
Only patients who have received treatments for ITP are included. Medical treatments includes rituximab.
Fostamatinib (n = 2), azathioprine (n = 1), dapsone (n = 1), and cyclosporine (n = 1).
Platelet count following first dose
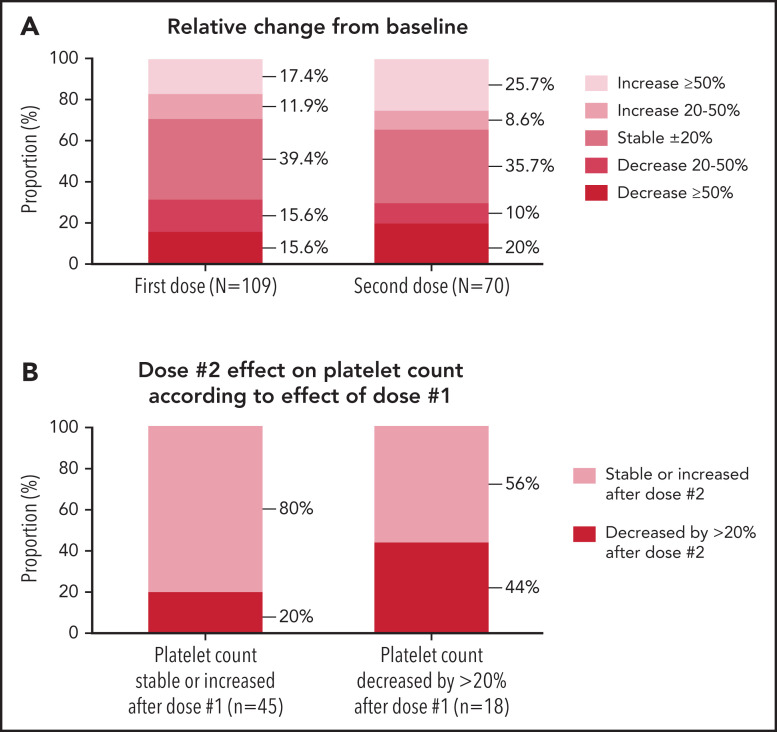
The median baseline platelet count in 109 patients was 101 [60–199] ×109/L assessed 14 [4-34] days prior to vaccination, and the median platelet count was unchanged at follow-up: 100 [50-195] ×109/L at 6 [4–9] days postvaccination (Table 3). Platelet counts rose in 32 (29%), remained stable (within 20% variation from baseline) in 43 (39%), and decreased in 34 patients (31%) (Figure 2A). Nineteen (17%) developed an exacerbation of ITP (Table 4) with 7 patients receiving rescue therapy. Rescue treatments included corticosteroids (n = 2), TPO-RA (n = 3), IVIG (n = 1), and a combination of IVIG, steroids, rituximab, and cyclosporine (n = 1) as well as increased dosing of ongoing ITP treatment (supplemental Figure 1, available on the Blood Web site). All responded to treatment with platelets >30 × 109/L or returned to prevaccination ranges within 2 to 4 weeks without major bleeding (supplemental Figure 1).
Table 3.
Platelet counts pre– and post–SARS-CoV-2 vaccination in patients with preexisting ITP
| Median [IQR], or n (%) | N | |
|---|---|---|
| Dose 1 | ||
| Platelet count prevaccination (×109/L) | 101 [60-199] | 109 |
| Timing of prevaccination platelet count(d)) | 14 [4.5-34] | 93 |
| Platelet count at first post-vaccine assessment (×109/L)* | 100 [50.5-195] | 109 |
| Timing of first postvaccination platelet count (d) | 6 [4-9] | 99 |
| Timing of platelet nadir† (d) | 6 [4.8-9.3] | 30 |
| Platelet count nadir† (×109/L) | 46.8 [27.8-93.3] | 34 |
| Platelet count 11-29 ×109/L | 7 (20.6) | 34 |
| Platelet count ≤10 ×109/L | 3 (8.8) | 34 |
| Dose 2 | ||
| Platelet count prevaccination (×109/L) | 101 [59.8-186] | 70 |
| Timing of prevaccination platelet count (d) | 11.5 [3-30] | 64 |
| Platelet count at first postvaccine assessment (×109/L) | 105.5 [52.8-202] | 70 |
| Timing of first postvaccination platelet count (d) | 5 [3-7.5] | 69 |
| Timing of platelet nadir† (d) | 5 [2.5-8.5] | 21 |
| Platelet count nadir† (×109/L) | 34 [10.5-116] | 21 |
| Platelet count 11-30 ×109/L | 5 (22.7) | 21 |
| Platelet count ≤10 ×109/L | 5 (23.8) | 21 |
Does not include 2 patients with “normal” platelet count.
Includes only patients with decrease >20% in platelet count.
Figure 2.
Relative change in platelet counts pre– and post–SARS-CoV-2 vaccination in patients with preexisting ITP. (A) Relative change in platelet counts from pre-vaccination levels following doses 1 and 2 of a SARS-CoV-2 vaccine. (B) Effect of dose #2 in platelet count according to effect observed after dose 1.
Table 4.
Patient characteristics and incidence of ITP exacerbation following SARS-CoV-2 vaccination
| First vaccine dose | Second vaccine dose | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Platelet count decrease ≥50%, n (%) | Platelet count decrease >20% and nadir <30 × 109/L, n (%) | Use of recue therapy, n (%) | ITP exacerbation,* n (%) | n | Platelet count decrease ≥50%, n (%) | Platelet count decrease >20% and nadir <30 × 109/L, n (%) | Use of rescue therapy, n (%) | ITP exacerbation,* n (%) | |
| All patients | 111 | 16 (14.4) | 10 (9) | 7 (6.3) | 19 (17.1) | 70 | 14 (20) | 10 (14.3) | 9 (12.9) | 14 (20) |
| Splenectomy | 25 | 11 (44) | 8 (32) | 6 (24) | 12 (48) | 19 | 7 (36.8) | 4 (21) | 4 (21) | 7 (36.8) |
| No splenectomy | 86 | 5 (5.8) | 2 (2.3) | 1 (1.2) | 7 (8.1) | 51 | 7 (13.7) | 6 (11.8) | 5 (9.8) | 7 (13.7) |
| Relative Risk [95% CI] |
1.8 [1.3-2.8] |
1.4 [1.02-2.2] |
||||||||
| 0-4 prior medical therapies | 54 | 2 (3.7) | 2 (3.7) | 3 (5.6) | 3 (5.6) | 43 | 7 (16.3) | 6 (14) | 6 (14) | 7 (16.3) |
| ≥5 prior medical therapies | 16 | 8 (50) | 4 (25) | 3 (18.8) | 9 (56.3) | 12 | 5 (41.7) | 2 (16.7) | 1 (8.3) | 5 (41.7) |
| Relative Risk [95% CI] |
2.2 [1.4-4.1] |
1.4 [0.9-2.6] |
||||||||
| Prior rituximab use† | 39 | 9 (23.1) | 4 (10.3) | 3 (7.7) | 10 (25.6) | 26 | 9 (34.6) | 5 (19.2) | 5 (19.2) | 9 (34.6) |
| No prior rituximab use† | 56 | 4 (7.1) | 4 (7.1) | 4 (7.1) | 6 (10.7) | 34 | 5 (14.7) | 5 (14.7) | 4 (11.8) | 5(14.7) |
| Relative Risk [95% CI] |
1.2 [0.9-1.6] |
1.3 [0.9-1.9] |
||||||||
| On current therapy for ITP | 67 | 11 (16.4) | 6 (9) | 4 (6) | 14 (20.9) | 40 | 11 (27.5) | 7 (17.5) | 6 (15) | 11 (27.5) |
| No current therapy, prevaccine platelet count <150 × 109/L | 30 | 4 (13.3) | 3(10) | 2 (6.7) | 4 (13.3) | 16 | 0 | 0 | 0 | 0 |
| No current therapy, prevaccine platelet count ≥150 × 109/L | 14 | 1 (7.1) | 1 (7.1) | 1 (7.1) | 1 (7.1) | 7 | 1 (12.5) | 1 (12.5) | 1 (12.5) | 1 (12.5) |
| Any concurrent autoimmune disease | 34 | 5 (14.7) | 3 (8.8) | 3 (8.8) | 6 (17.6) | 25 | 5 (20) | 4 (10) | 3 (12) | 5 (20) |
| No concurrent autoimmune disease | 47 | 4 (8.5) | 3 (6.4) | 2 (4.3) | 6 (12.8) | 30 | 6 (20) | 3 (16) | 3 (10) | 6 (20) |
| Relative Risk [95% CI] |
1.06 [0.8-1.4] |
1 [0.7-1.4] |
||||||||
Defined as development of any 1 or more of the following: (1) ≥50% decline in platelet count from prevaccination baseline; (2) >20% decline from prevaccination baseline and platelet nadir <30 ×109/L; and/or (3) receipt of rescue therapy for ITP.
Prior rituximab use was specifically solicited in data collection as well as all prior treatment history.
Platelet count following second dose
The median platelet count prior to the second vaccination was 101 [60-186] ×109/L (n = 70) assessed 12 [3-30] days prior to vaccination. At a median of 5 [3-8] days postvaccination, the median platelet count was 106 [53-202] ×109/L (Table 3). Platelets rose in 24 (34%), remained stable in 25 (36%), and declined in 21 (30%) (Figure 2A). Fourteen (20%) patients developed an exacerbation of ITP. The 9 patients receiving rescue treatment responded with platelets >30 x109/L or returned to prevaccination ranges (supplemental Figure 1).
Risk factors for ITP exacerbation
Patients with prior splenectomy had a significantly higher risk of exacerbation after dose 1 (12/25, 48%) compared with nonsplenectomized patients (7/86, 8%, RR 1.8 [1.3-2.8]) (Table 4). After dose 2, 7/19 (37%) splenectomized patients developed an exacerbation compared with 7/51 (14%) nonsplenectomized patients (RR 1.4 [1.02-2.2]). Patients treated with ≥5 prior lines of therapy also had a significantly higher risk of exacerbation after dose 1 (9/16, 56%) compared with patients with 0 to 4 prior lines of therapy (3/54, 6%, RR 2.2 [1.4-4.1]). After dose 2, 5/12 (42%) individuals who received ≥5 lines of therapy developed an ITP exacerbation, whereas for 0 to 4 lines of therapy, only 7/43 (16%) patients developed ITP exacerbation (RR 1.4 [0.9-2.6]).
Prior use of ≥5 lines of medical treatment was more common among splenectomized patients (59% vs 11% in nonsplenectomized group). The incidence of ITP exacerbation after dose 1 was highest among splenectomized patients with ≥5 lines of therapy (6/10, 60%) compared with 1/47 (2%) who had not undergone splenectomy and had received ≤4 prior treatments (Table 5).
Table 5.
Incidence of ITP exacerbation* after sample stratification by history of splenectomy and number of prior medical therapies or history of rituximab use
| First vaccine dose | Second vaccine dose | |||||
|---|---|---|---|---|---|---|
| n | ITP exacerbation (n) | ITP exacerbation (%) | n | ITP exacerbation (n) | ITP exacerbation (%) | |
| Splenectomy, 0-4 prior therapies | 7 | 2 | 28.6 | 6 | 1 | 16.7 |
| Splenectomy, ≥5 prior therapies | 10 | 6 | 60 | 9 | 4 | 44.4 |
| No splenectomy, 0-4 prior therapies | 47 | 1 | 2.1 | 37 | 6 | 16.2 |
| No splenectomy, ≥5 prior therapies | 6 | 3 | 50 | 3 | 1 | 33.3 |
| Splenectomy, no prior rituximab | 6 | 3 | 50 | 5 | 2 | 40 |
| Splenectomy + prior rituximab | 17 | 8 | 47.1 | 13 | 5 | 38.5 |
| No splenectomy, no prior rituximab | 50 | 3 | 6 | 28 | 3 | 10.7 |
| No splenectomy + prior rituximab | 22 | 2 | 9.1 | 14 | 4 | 28.6 |
Defined as development of any 1 or more of the following: (a) ≥50% decline in platelet count from prevaccination baseline; (b) >20% decline from prevaccination baseline and platelet nadir <30 ×109/L; and/or (c) receipt of rescue therapy for ITP.
There was no difference in age, gender, vaccine type (data not shown), or history of autoimmune disease (Table 4) between those who did and did not develop an ITP exacerbation.
Comparison of platelet responses following dose 1 and dose 2
Sixty-three patients had platelet counts available after both doses of a SARS-CoV-2 vaccine. We investigated if the response to dose 1 could predict the effect of dose 2 on platelet count. Of the patients who had stable or increased platelet counts after dose 1, 80% percent had stable or increased counts after dose 2. Among patients whose platelets decreased by >20% after dose 1, the effect of dose 2 was less consistent: only 44% had a decrease >20% in platelet counts (Figure 2B; supplemental Figure 2). Of 5 patients who received rescue treatment following dose 1, 4 did not receive rescue after dose 2.
PDSA natural history registry survey of patients with ITP
Of 122 individuals with preexisting ITP who completed the PDSA survey, 57 received a SARS-CoV-2 vaccine and had postvaccination platelet counts. The survey did not differentiate between first and second vaccine doses. Forty-four of 57 (75%) respondents were women (mean age 51 years). Vaccine type was Moderna (29), Pfizer-BioNTech (23), Oxford-AstraZeneca (ChAdOx1 nCoV-19) (4), and Janssen (1). Nineteen individuals (33%) reported decreased postvaccination platelet counts, including 2 with platelets <10 × 109/L, 1 with mucocutaneous bleeding (supplemental Table 1). Participants who were postsplenectomy had a higher risk of a postvaccination platelet decline >100 × 109/L (RR 1.8 [1.3-3.4]). Participants in remission had a lower incidence of platelet count decline >100 × 109/L (RR 0.7 [0.5-0.9]) compared with those with active ITP.
United Kingdom ITP support association survey
Of 311 participants, only 43 (32 female) reported postvaccination platelet counts, of which 11 were higher, 18 were stable, and 14 were lower than before vaccination. Vaccine type was Pfizer-BioNTech (24) and Oxford-AstraZeneca (19). Among the 14 participants reporting decreased platelets, the prevaccine median platelet count was 78 [58-173] × 109/L falling to 12 [10-68] × 109/L postvaccine; 13 decreased by ≥50%, and 10 reached platelet nadirs <30 × 109/L (7 Oxford-AstraZeneca, 3 Pfizer-BioNTech). Splenectomy was again associated with a higher risk of a ≥50% decrease in platelet count postvaccination (RR 2.3 [1.1-7.9]).
Discussion
This report describes the effects of SARS-CoV-2 vaccination on platelet counts of patients without known (de novo) and with preexisting ITP. The study does not include patients with VITT-TTS, nor does it include patients with inherited thrombocytopenia. This is the largest report to date both of patients with apparent de novo ITP identified in VAERS and also of postvaccination platelet counts in patients with preexisting ITP. We report findings of ITP development or exacerbation secondary to the first large-scale administration of mRNA vaccines.
In de novo ITP patients identified from VAERS, the median time to presentation was 8 days, similar to a recent report of thrombocytopenia after the Oxford-AstraZeneca vaccine.10 There is evidence dating back to the 1960s, primarily in children, that attenuated live viral vaccines cause ITP perhaps via direct effect on megakaryocytes.11 In contrast, the best study of killed vaccines in adults did not identify an increased incidence of ITP postvaccination.12 While mRNA vaccines are novel, they are thought to represent a new form of “killed” vaccines; there is no reason to suspect they directly impact megakaryocytes. A recent report of a national registry from Scotland suggested that the Pfizer-BioNTech vaccine did not result in an increased incidence of ITP, while results with the Oxford-AstraZeneca vaccine were equivocal.13 Preexisting undiagnosed asymptomatic ITP with postvaccination exacerbation could be one explanation for development of ITP within days postvaccination. This would also be consistent with the failure to demonstrate an increased postvaccination incidence of ITP since these cases were evolving at the time of vaccination. Other etiologies for occurrence of de novo ITP include molecular mimicry and underlying predisposition to autoimmunity; these might represent cases not presenting until at least 1 week postvaccination.14 No data are available on the etiology or incidence of de novo ITP in this report. This study also did not identify predictive factors for de novo ITP; although, 32% of cases had preexisting autoimmune disease, which may be higher than expected in the adult US population.15,16 Long-term outcomes of postvaccine cases of ITP could not be assessed.
It is encouraging to note that among VAERS reports with available information, almost 90% of patients responded to first-line ITP treatment: steroids and/or IVIG and/or platelet transfusions. In 8 patients who were difficult to treat, the addition of a TPO-RA and single-dose vincristine led to good responses. Vincristine appeared to accelerate response compared with the expected 7 to 14 days with TPO–RAs. Reasons not to use anti-CD20 treatment (eg, rituximab) for suspected postvaccination ITP include a 1- to 8-week time to response, negation of the recent vaccination, and inability to effectively revaccinate for months.17,18
Details regarding the 8 patients unresponsive to first-line therapy were sent to an author featured in a publication of one of these cases in the lay press.19 As such, these refractory cases likely represent a small fraction of de novo cases of ITP rather than an influx of refractory cases postvaccination. Exactly why certain de novo cases of ITP, either postvaccination or idiopathic, are refractory is not well understood.
The findings of de novo ITP postvaccination led to the examination of vaccine effects in patients with preexisting ITP. Surprisingly, postvaccination changes in platelet counts in this group were approximately evenly divided among those that increased, remained stable, or decreased in all 3 data sources analyzed. We do not have a parallel comparison group for the vaccinated ITP patients, but the postvaccination platelet fluctuations were prominent in both directions, with changes far exceeding those seen in the placebo arms of numerous ITP studies.20-23
In the multicenter cohort of ITP patients, approximately 1 in 5 developed an ITP exacerbation after vaccination. Rescue treatment, whether increased dosing of ongoing treatment and/or addition of new ITP treatment, was universally effective. No major bleeding occurred.
An increased risk of developing ITP exacerbation in splenectomized patients was independently seen in each of the 3 data sources. Whether having undergone splenectomy, even if successful, represents more refractory ITP or if the absence of the spleen in some way influences (worsens) the vaccination effect on the platelet count is unclear. In the multicenter cohort, a significantly higher proportion of patients developing ITP exacerbation were postsplenectomy, had a longer duration of ITP, and/or had received ≥5 treatments for ITP. These categories overlap considerably, albeit not completely; all suggest that the worse the ITP, the more likely there is to be a thrombocytopenic effect of vaccination. No patient with normal platelet counts off-treatment and who had not undergone splenectomy developed an exacerbation of ITP. Furthermore, only 1 patient who had a past history of neither splenectomy nor having received ≥5 treatments had an exacerbation. Thus, the patient at greatest risk of a substantial decrease in platelets postvaccination seems to be one whose treatment history has demonstrated the need for more aggressive ITP therapies.
A critical question was whether platelet response to the first vaccine dose predicted platelet response to the second dose (for 2-dose immunization schedules). Most patients who did not develop ITP exacerbation after the first vaccine dose also did well with the second dose. Over half of those who experienced a decrease in their platelet counts after the first dose had stable or increased platelet counts after the second dose; only 1 patient received ITP-directed therapy after both vaccine doses. This suggests that ITP patients who are eligible to receive additional vaccine doses, especially if they tolerated the first dose well, may safely do so. A decrease in platelet count with a prior vaccine dose does not guarantee the same effect with a subsequent dose. No major bleeding was seen in any patient. Whether this is also the case after booster doses will require further study.
Limitations of this study include its retrospective design and reliance on information of undefined completeness from diverse sources. Since participation in VAERS and the PDSA and UK surveys was voluntary, there might have been an increased number of adverse events and/or poor outcomes driving the choice to report. Since asymptomatic ITP patients may have chosen not to obtain pre and postvaccination platelet counts, the exclusion of these patients may have resulted in an overestimation of platelet decrease postvaccination. The majority of patients received the Moderna and Pfizer-BioNTech vaccines, limiting the ability to assess potential differences in changes with platelet counts following the adenovirus-based Janssen and Oxford-AstraZeneca SARS-CoV-2 vaccines. Incomplete data resulted in different numbers of patients available for different analyses. In a few patients with preexisting ITP, the prevaccination platelet count was obtained months prior to vaccination; however, 75% of prevaccination counts were within 1 month of vaccination. Although the timing and number of postvaccine counts were not fixed, most patients obtained counts 5 to 7 days after vaccination. As data were deidentified, there may have been overlap between the multicenter and PDSA survey cases. Despite these limitations, overall, there was consistency across each of the 3 ITP cohorts, including similar percentages with increased, stable, and decreased postvaccination platelet counts as well as the adverse influence of previous splenectomy.
Our data support and expand upon a reports of postvaccination ITP,24,25 that the incidence of severe thrombocytopenia and bleeding is low, and patients can be managed with standard, and occasionally, intensified approaches to therapy. These data strongly support the safety of the SARS-CoV-2 vaccines, both acutely in the rare patients developing ITP de novo and in patients with preexisting ITP. Therapy was effective in essentially all who developed clinically meaningful thrombocytopenia. Obtaining prevaccination counts followed by weekly monitoring of the platelet count in consultation with a hematologist after each vaccination may be warranted in most ITP patients, especially those postsplenectomy or who had received 5 or more prior treatment regimens.
In summary, this report provides a factual basis to encourage SARS-CoV-2 vaccination for patients with ITP by describing the relatively infrequent adverse outcomes and their reversibility with treatment. It also encourages receipt of both doses of 2-dose vaccines, which appears to be particularly important as SARS-CoV-2 variants emerge.
Supplementary Material
The online version of this article contains a data supplement.
Footnotes
Presented in abstract form at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 13 December 2021.
For access to deidentified individual subject data of any of the datasets, please contact eul7001@med.cornell.edu.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.-J.L., J.D., E.I., C.K., A.C.N., and J.B.B. contributed to the design of the study, participated in patient enrollment and treatment, data collection, and assembly of data; E.-J.L., M.B.-M., H.A., A.C., J.D., T.G., E.I., C. Kessler, C. Kruse, A.D.L., A.I.L., H.A.L., A.C.N., A.E.R., M.D.T., J.T., D.J.K., E.C., and J.B.B. performed research; E.-J.L., M.B.-M., D.B.C., and J.B.B. analyzed data and wrote the manuscript; and all authors participated in the manuscript writing and provided their reviews and feedback with editing during the development of the manuscript and provided final approval for the manuscript prior to submission.
Conflict-of-interest disclosures: E.-J.L. is a consultant for Principia Biopharma Inc. M.B.-M. spouse is employed at Kadmon, Inc and previously at Jounce Therapeutics. H.A.L. receives research funding to his institution from Agios, Dova, and Amgen and is a consultant for Agios, Dova, Argenx, Sobi, Novartis, Moderna, and Rigel. A.C.N. has served as a consultant for Synergy; has received authorship royalties from UpToDate; and his institution has received research support on his behalf from Alexion, Bayer, Novartis, Novo Nordisk, Pfizer, Sanofi, Spark, and Takeda. J.D. has not personally received any payment but reports that PDSA received grants, honorarium, and/or consultancy fees from Amgen, Argenx, CSL Behring, Novartis, Pfizer, Principia, Rigel, and UCB. T.G. receives research funding from Rigel Corporation and Principia, is a consultant for Amgen, Dova, Novartis, Principia, and Cellphire, had travel/accommodations and expenses paid by Amgen, Dova, and Cellphire, and received honoraria from Amgen, Novartis, Sanofi, and Dova. A.K. has not personally received any payment but reports that PDSA received grants, honorarium, and/or consultancy fees from Amgen, Argenx, CSL Behring, Novartis, Pfizer, Principia, Rigel, and UCB. C. Kessler has received research funding from Octapharma, Genentech, Takeda, and Bayer, and is part of the board of directors or advisory committees for Octapharma, Genentech, Takeda, Bayer, Novo Nordisk, Pfizer, and CSL Behring. C.K. has not personally received any payment but reports that PDSA received grants, honorarium, and/or consultancy fees from Amgen, Argenx, CSL Behring, Novartis, Pfizer, Principia, Rigel, and UCB. A.D.L. receives research funding to his institution from BioMarin, Sangamo, Pfizer, is a consultant for Merck, and is on the Advisory Board of BioMarin, Dova, CSL, Catalyst, and HEMA Biologics. H.A.L. receives research support from Sanofi/Genzyme, Novartis, and Argenx, and consulting fees from Novartis, Dova, Amgen, Moderna and Pfizer. A.C.N. is a consultant for Amgen, Angle, Argenx, Dova, Grifols, Novartis, Pfizer, and Shionogi, received funding from Amgen, Novartis, and Rigel; received honoraria directly from Amgen, Angle, argenx, Dova, Novartis, Rigel, and Shionogi; and paid expert testimony from Argenx and Rigel. M.D.T. receives research support from Grifols, Novo Nordisk, Pfizer, Principia, Spark Therapeutics, Takeda, UCB; speaker bureau from Amgen, Dova, Grifols, Octapharma, Sobi, Takeda, UCB; and is a consultant/Advisory Board consultant for Amgen, BioMarin, Dova, Genentech, Octapharma, Principia, Sobi, Spark Therapeutics, Takeda and UCB. J.T. received speaker honoraria from Amgen and Novartis. D.J.K. receives research funding to institution from Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, Bristol Myers Squibb (BMS), Immunovant, Kezar, Principia, Protalex, Rigel, Takeda (Bioverativ), and UCB; and serves as a consultant for Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, BioCryst, Bristol Myers Squibb (BMS), Caremark, CRICO, Daiichi Sankyo, Dova, Genzyme, Immunovant, Incyte, Kyowa-Kirin, Merck Sharp Dohme, Momenta, Novartis, Pfizer, Platelet Disorder Support Association, Principia, Protalex, Protalix, Rigel, Sanofi, Genzyme, Shionogi, Shire, Takeda (Bioverativ), UCB, UpToDate, Zafgen. D.B.C. has received relevant research support from Alexion and Aplagon, served as a consultant or as a member of a data safety monitoring board for Rigel, Dova, CSL Behring, Principia and Arch Oncology. J.B.B. is a consultant and is on advisory boards of Amgen, Novartis, Dova, Rigel, UCB, Argenx, Janssens, Regeneron, RallyBio, Sanofi, Pfizer, and has received honoraria from UpToDate. The remaining authors declare no competing financial interests.
Correspondence: Eun-Ju Lee, Department of Medicine, Division of Hematology, New York Presbyterian Hospital/Weill Cornell Medicine, 1305 York Ave, 7th Floor, New York, NY 10065; e-mail: eul7001@med.cornell.edu.
REFERENCES
- 1.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020; 383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weintraub K. Death of Florida doctor after receiving COVID-19 vaccine under investigation. USA Today. Published 6 January, 2021.
- 4.Grady DMP. Doctor’s death after covid vaccine is being investigated. New York Times. Published 12 January 2021.
- 5.Lee EJ, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96(5):534-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welsh KJ, Baumblatt J, Chege W, Goud R, Nair N. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2021;39(25):3329-3332. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393. [DOI] [PubMed] [Google Scholar]
- 8.Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202-2211. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hippisley-Cox J, Patone M, Mei XW, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oski FA, Naiman JL. Effect of live measles vaccine on the platelet count. N Engl J Med. 1966;275(7):352-356. [DOI] [PubMed] [Google Scholar]
- 12.Grimaldi-Bensouda L, Michel M, Aubrun E, et al. ; PGRx Immune Thrombocytopenia Study Group. . A case-control study to assess the risk of immune thrombocytopenia associated with vaccines. Blood. 2012;120(25):4938-4944. [DOI] [PubMed] [Google Scholar]
- 13.Simpson CR, Shi T, Vasileiou E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nature Med. 2021;27(7):1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Nardi MA, Borkowsky W, Li Z, Karpatkin S. Role of molecular mimicry of hepatitis C virus protein with platelet GPIIIa in hepatitis C-related immunologic thrombocytopenia. Blood. 2009;113(17):4086-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84(3):223-243. [DOI] [PubMed] [Google Scholar]
- 16.McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42(2):252-265. [DOI] [PubMed] [Google Scholar]
- 17.Cooper N, Stasi R, Cunningham-Rundles S, et al. The efficacy and safety of B-cell depletion with anti-CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. Br J Haematol. 2004;125(2):232-239. [DOI] [PubMed] [Google Scholar]
- 18.Nazi I, Kelton JG, Larché M, et al. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood. 2013;122(11):1946-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grady D. A few covid vaccine recipients developed a rare blood disorder. New York Times. Published 8 February 2021.
- 20.Kuter DJ,Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371(9610): 395-403. [DOI] [PubMed] [Google Scholar]
- 21.Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237-2247. [DOI] [PubMed] [Google Scholar]
- 22.Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo- controlled trial. Lancet. 2009;373(9664): 641-648. [DOI] [PubMed] [Google Scholar]
- 23.Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93(7):921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuter DJ. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. Br J Haematol. 2021;195(3):365-37010.1111/bjh.17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crickx E, Moulis G, Ebbo M, et al. Safety of anti-SARS-CoV-2 vaccination for patients with immune thrombocytopenia. Br J Haematol. 2021;195(5):703-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.