Graphical abstract
Keywords: Aerosol transmission, Personalized ventilation, Risk assessment model, Relative orientation, SARS-CoV-2
Abstract
In the context of COVID-19, new requirements are occurring in ventilation systems to mitigate airborne transmission risk in indoor environment. Personalized ventilation (PV) which directly delivers clean air to the occupant’s breathing zone is considered as a promising solution. To explore the potentials of PV in preventing the spread of infectious aerosols between closely ranged occupants, experiments were conducted with two breathing thermal manikins with three different relative orientations. Nebulized aerosols were used to mimic exhaled droplets transmitted between the occupants. Four risk assessment models were applied to evaluate the exposure or infection risk affected by PV with different operation modes. Results show that PV was effective in reducing the user’s infection risk compared with mixing ventilation alone. Relative orientations and operation modes of PV significantly affected its performance in airborne risk control. The infection risk of SARS-CoV-2 was reduced by 65% with PV of 9 L/s after an exposure duration of 2 h back-to-back as assessed by the dose–response model, indicating effective protection effect of PV against airborne transmission. While the side-by-side orientation was found to be the most critical condition for PV in airborne risk control as it would accelerate diffusion of infectious droplets in lateral diffusion to occupants by side. Optimal designs of PV for closely ranged occupants were hereby discussed. The four risk assessment models were compared and validated by experiments with PV, implying basically consistent rules of the predicted risk with PV among the four models. The relevance and applicability of these models were discussed to provide a basis for risk assessment with non-uniformly distributed pathogens indoor.
1. Introduction
Frequent outbreaks of respiratory infectious diseases in recent years have caused severe threats to human life and health. For example, the severe acute respiratory syndrome (SARS) epidemic in 2003, the H1N1 flu in 2009, and the Middle East Respiratory Syndrome (MERS) in 2013 all aroused the attention of people in prevention and control of the spread of these respiratory infectious diseases [1]. The novel coronavirus disease COVID-19 which was caused by the novel coronavirus SARS-CoV-2, exploded at the end of 2019, rapidly spread all over the world, and caused>228 million infections including over 4.6 million deaths as of September 14, 2021 [2]. These astonishing numbers of infections and deaths have highlighted the importance of using effective methods to prevent and control the spread of this contagious disease.
The COVID-19 is believed to be mainly transmitted through three routes: contact transmission, droplet transmission, and aerosol transmission [3]. World Health Organization [4] has proposed a series of measures for the public to prevent the probability of contact and droplet transmission, e.g. frequent hand washing, keeping a safe distance away (>1 m). However, the understanding of the airborne transmission route of COVID-19 is still insufficient. During the pandemic of COVID-19, many countries have reported clusters of infection in public transportations (e.g. airplanes, trains, buses, cruise ships, etc.), offices, restaurants, etc. [5], [6], [7], [8], [9], [10], [11], [12]. The clustered outbreaks in these public spaces may be mainly attributed to the relatively short distance between occupants of high density, durable exposure time, and poor air circulation [13], [14], [15]. Recently, more and more studies found a significant probability of airborne transmission route of COVID-19 in indoor environment [16], [17], [18], [19], [20], [21]. This highlights the importance of using ventilation as a measure to remove pathogenic aerosols and to mitigate airborne transmission risk in highly occupied space. It is also of great significance to develop novel ventilation strategies to meet the needs of airborne disease control on these specific occasions and to balance the energy consumption and large fresh air demand during the epidemic [22].
Two ventilation principles are mainly used to minimize airborne transmission risk in enclosed environments [23]. One way is to increase the overall ventilation rate to dilute the concentration of infectious particles [24], but the exact amount of sufficient ventilation rate for infection risk control is still unknown and at the same time the increased ventilation rate is realized at the expense of high energy consumption of HVAC systems [25], [26]. Another way is to increase the ventilation efficiency in the targeted area. Personalized ventilation (PV) that delivers clean air directly to the breathing zone of occupants is considered as a possible solution [27], [28].
PV has been widely used in vehicles (e.g. airplanes, coach buses, cars, etc.) to improve passengers’ thermal comfort. The performance of PV in regard to mitigating airborne transmission of infectious agents between occupants remains to be studied. The recent frequent outbreaks of respiratory diseases have attracted researchers' attentions to this efficient ventilation strategy in airborne infection risk control. For example, Niu et al. [29] reported a chair-based PV design with the PV nozzle placed just beneath the mouth at the microphone position, which could achieve up to 80% fresh air in the inhaled air with a supply flow rate of less than 3 L/s. Nielsen et al. [30] proposed a bed-integrated PV system with a textile pillow as the diffuser, which would provide a high degree of clean air protection (over 95%) against airborne cross infection. Pantelic et al. [31] investigated a desk amounted PV device and found it would reduce the user’s infection risk of influenza A and tuberculosis by 27% and 65%, respectively, considering different relative distances apart from the infected source (1–4 m). Melikov et al. [32] developed a novel PV air terminal device comprising a mobile unit attachable to a hospital bed which could increase the evacuation efficiency (ratio of tracer gas concentration at exhaust without PV to the tracer gas concentration at measured location with or without PV) from 0.23 (without PV) to 39 (with PV). Melikov and Dzhartov [33] mounted personalized ventilation and personalized exhaust (PV-PE) to seats in tandem and achieved a decrease by 42% of exhaled contaminant concentration with a PV flow rate of 10 L/s. Assaad et al. [34] evaluated the effect of different PV air terminal devices on direct inhalation exposure and found that the PV (10 L/s) could effectively enhance breathable air quality with a maximum exposure reduction of contaminant by 97% using the round movable panel. Xu et al. [35] indicated that PV could be a potential method for airborne infection control with a short distance, which reduced the bio-aerosol deposition on the face, the body and inhalation by a maximum of 98%, 85% and 100%, respectively.
However, the clean air delivery efficiency of PV was found to be affected by a number of factors, such as the PV configuration, PV flow rate, relative positioning to the occupants, background ventilation and so on [36], [37], [38]. With inappropriate assignment of PV to the user, its ability in airborne infection control may be decreased. For example, Xu et al. [38] found that PV for the protected occupant interacted with the infector’s exhalation flow with close proximity between occupants face-to-face (less than1 m), and the direct exposure of exhaled droplets from the infector was thereby elevated. Xu et al. [35] indicated that a larger PV flow rate performed better to prevent the inhalation of pathogen-laden droplets to the exposed occupant, but it was not the case for droplet deposition. There was another concern that PV may facilitate the dispersion of exhaled droplets when it was applied to the infected source alone [36], [39]. These uncertainties of PV restricted its further application in airborne transmission control, which should be clearly addressed.
Furthermore, there were no consistent risk assessment models applicable to PV to evaluate the airborne infection risk with non-uniformly distributed pathogens in occupied space in previous studies. Most of the infection risk assessment models applied in indoor environment were based on the assumption of a fully mixing condition of agents with room air. For example, the Wells-Riley model which has been extensively used in analyzing the quantitative association between ventilation and airborne infections in indoor premises, assumes well-mixed room air and a steady pathogen concentration [40], [41]. Similarly, the basic reproductive number (R 0) model [42] and the population infection model [43] were also established based on the assumption that ventilation and infectious pathogens were fully mixed and the spatial distribution differences of pathogens were not considered. To accurately evaluate the effectiveness of PV in airborne infection risk mitigation, the establishment of non-uniform risk assessment models applicable to PV should be the first step. It's also important to understand the relationships and differences between various risk assessment models, so as to provide a basis for choosing the right model for risk assessment under different airflow distribution patterns.
Thus, the objective of this study was twofold: (1) to explore the potential of PV in protecting against the airborne infection risk for occupants in a short distance; (2) to evaluate the applicability and effectiveness of different risk assessment models for the airborne infection risk assessment with PV. The findings of this study may contribute to the application and improvement of personalized ventilation devices in airborne infection risk control and may be helpful to provide a basis for risk model selection by using PV or other ventilation strategies which may create non-uniformly distributed infectious pathogens indoor.
2. Experimental method
2.1. Test room and ventilation
The experiment was conducted in a full-scale clean chamber under the cleanliness of ISO Class 7 standard [44]. The dimensions of the test chamber were 5 m (L) × 3.5 m (W) × 2.5 m (H). The experimental setup is shown in Fig. 1 . The ventilation system was designed with background mixing ventilation (MV) and personalized ventilation (PV), which was basically in line with the ventilation design of vehicles like airplanes or coach buses. The background ventilation was used to maintain the relative stability and thermal comfort of the background environment, and the personalized ventilation was set to meet the individual requirements of passengers by supplying fresh air to each passenger separately.
Fig. 1.
(a) Details of the indoor environment chamber and experimental setup; (b) the positioning of PV (PVs or PVt refers to PV being applied to the source manikin or the target manikin, respectively); (c) the real-life setup of back-to-back positioning with PV; (d) three different relative orientations. The dimensions are in meters (m).
Two square diffusers (0.57 m × 0.57 m) were located on the right wall (Fig. 1(a)). The conditioned air was supplied to the room from the upper diffuser with a temperature of 16.5 ± 1 °C and an airflow rate of 24.3 L/s (corresponding to an air change rate of 2 h−1), and was exhausted from the lower diffuser. The settings of temperature and airflow rate were basically complied with the bus regulation GB9673-1996 [45]. Two high-efficiency particulate air filters (HEPA, filtration efficiency of 99.5%) were respectively installed behind the inlet and exhaust diffusers to maintain the cleanness of the chamber, and the supplied air was considered as clean air free of particles. The room air was fully mixed with a measured average temperature of 25 ± 1 °C and relative humidity of 37 ± 3%, which was in line with the standard of a coach bus in cooling condition [45].
PV was used to provide clean air to the sedentary manikins in the test room with an adjustable flow rate of 3 L/s, 6 L/s, and 9 L/s, respectively. The setup of the PV system is shown in Fig. 2 . Recirculated room air was filtered by a customized HEPA (filtration efficiency of 99.5%) to maintain the cleanness and then supplied to the breathing zone of the thermal manikin with a downward direction of 23.6° and a relative distance of 0.36 m between the nozzle exit to the nose tip. The supplied air from PV was in isothermal condition with the ambient air. The configuration of PV was designed according to the optimal relative positioning to achieve a better thermal sensation and a higher ventilation efficiency [46]. Previous studies found that the flow pattern of the PV nozzle in aircraft cabins was similar to that of an ordinary circular nozzle [47], [48]. A circular nozzle with an exit diameter of 0.0508 m was applied to mimic the flow shape of a jet from the nozzles used in aircraft cabins or coaches. Two breathing thermal manikins were placed in the room to represent two adjacent passengers with different orientations, as shown in Fig. 1. PVs and PVt were installed in front of the source manikin and the target manikin, respectively. Different combinations of PVs and PVt are considered and listed in Table 1 .
Fig. 2.
Schematic of the setup of the personalized ventilation system (unit in m).
Table 1.
A list of experimental conditions.
| Case | Orientation of two manikins | PV use patterna | PV flow rate (L/s) | Separation distance between the two manikins (from mouth to mouth) (m) |
|---|---|---|---|---|
| 1 | 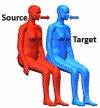 |
PVs | 3, 6, 9 | 0.65 |
| 2 | PVt | 3, 6, 9 | 0.65 | |
| 3 | PVs-t | 3, 6, 9 | 0.65 | |
| 4 | MV | 0 | 0.65 | |
| 5 |  |
PVs | 3, 6, 9 | 0.85 |
| 6 | PVt | 3, 6, 9 | 0.85 | |
| 7 | PVs-t | 3, 6, 9 | 0.85 | |
| 8 | MV | 0 | 0.85 | |
| 9 | 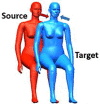 |
PVs | 3, 6, 9 | 0.86 |
| 10 | PVt | 3, 6, 9 | 0.86 | |
| 11 | PVs-t | 3, 6, 9 | 0.86 | |
| 12 | MV | 0 | 0.86 | |
PVs is PV applied to the source manikin only; PVt is PV applied to the target manikin only; PVs-t is PV applied to both the source and target manikin with the same flow rate; MV is the mixing ventilation used as the background ventilation alone without using PV.
2.2. Breathing thermal manikins
Two breathing thermal manikins (BTMs) placed in the middle of the chamber were used to represent the infected and exposed occupants in close proximity (Fig. 1). Manikin A represented the infected source, and manikin B was the exposed occupant. Three different relative orientations: side-by-side, back-to-back, and face-to-back were considered in experiments, which were designed according to the realistic relative positioning between adjacent passengers in vehicles. The distance between the two manikins (0. 65 m, side-by-side; 0.85 m, face-to-back; 0.86 m, back-to-back) corresponded to the normal distance for seat arrangements in coach buses or airplanes [45], [49], [50].
The heat release from the manikin’s body was approximately equivalent to a metabolic rate of 1.2 Met for a sedentary person with light work [51]. The surface temperatures of both manikins were measured with the thermocouples placed on the head, chest, and legs, respectively, with an average value of 30 °C. The exhalation minute volume of both manikins was 18.7 L/min. It was found that the breathing patterns of the occupants would affect the cross-infection risk and the highest exposure occurred when the infected person exhaled through the mouth [52], [53]. In all the experiments, both manikins exhaled through the mouth and inhaled through the nose with the same breathing frequency of 15 min−1, but in the opposite breathing cycle, which means the exposed manikin inhales while the source manikin exhales at the same time. This breathing pattern was considered as of higher infection risk than other breathing patterns [38], [52], [53]. The exhaled air was heated at a temperature of 32.3 ± 1 °C when leaving the mouth, which was set according to the measured exhalation temperature from real human subjects [49].
2.3. Aerosol generation and measurement
Several previous studies have used the Collision nebulizer to generate aerosols for virus assays (e.g. human influenza [54] or SARS-CoV-2 [55]). This study applied the Collison nebulizer (3-jet, BGI, Inc. Waltham, MA) and sodium chloride (NaCl) solution for atomization to mimic human exhaled droplets. Potter et al. [56] proved that human pulmonary secretion consists of water and 5.3% solids by weight. Nicas et al. [57] proposed that a droplet of respiratory fluid can be treated as a simple NaCl solution. Wan et al. [58] also adopted the NaCl solution as the simulated saliva. Edwards et al. [59], [60] used a nebulized 10% NaCl solution in distilled water to generate polydisperse droplets from human breathing. During the outbreak of COVID-19, there were numbers of asymptomatic infected cases whose exhaled breaths may also contain infections viruses and contribute to the SARS-CoV-2 transmission. It was found that the droplet existing in normal tidal exhalation had an approximate geometric mean diameter of 0.7 μm, or with a modal diameter less than 0.8 µm [61]. Liu et al. [62] measured the aerodynamic diameter (Dp) of SARS-CoV-2 aerosols, and found there were two main distribution ranges (Dp = 0.5 ∼ 1 μm and Dp > 2.5 μm) and part of the measurement space was dominated by Dp = 0.25–1 μm.
With a suitable composition of the solvent and precise atomization pressure control, the Collison nebulizer can provide aerosols with specific size distributions [31]. In our experiments, the solution was made by NaCl of 0.00338 g and the solvent of 100 cm3 made by a mixture of 50% distilled water and 50% isopropyl alcohol by volume. The atomization pressure was set as 10 psi = 0.069 MPa. The atomized aerosol diameter was found to be 0.7 μm (Geo. Mean) with a geometric standard deviation of 1.22–1.35, which basically corresponded to the size distribution of droplets in human exhaled breath [61], [63]. The droplets were mixed with the clean air and released from the mouth of the source manikin. The effects of sedimentation and resuspension of these droplets were not considered while passing through the inner airway of the manikin, as all the measurements were conducted after the droplets released from the manikin’s mouth.
Two measuring points (Fig. 1(b)) were placed at the mouth opening of the source manikin (Point 1) and 0.01 m below the nose of the exposed manikin (Point 2), respectively. An aerodynamic particle sizer (APS TSI model 3321) with 52 channels was used to measure the number and aerodynamic diameters of the particles with a sampling time interval of 1 s. The measurable aerodynamic particle size range of APS is 0.5–20 μm, and the smallest optical particle size that can be detected by APS is 0.37 μm. The efficiency of APS varies with particle size and increases from 30% at 0.5 μm to 100% at 0.9 μm [63], [64]. In order to prevent the settling loss of particles during the sampling process, a Teflon tube with good anti-stick properties was adopted to connect the APS. Restricted by the atomization pressure, the concentration of droplets released from the manikin was supposed to be higher than that from real human breathing. The initial concentration of droplets released from the manikin was thereby normalized with that obtained from human subjects [63] (number concentration of 0.092 cm−3) for further infection risk assessment. The room concentration under mixing ventilation would stabilize over time combined with the effect of aerosol release, HEPA filtration, and ventilation dilution, and all the measurements were conducted in a steady state to avoid the requirement for dynamic measurements with more APS probes.
3. Risk assessment models
The use of PV will create non-uniform air distribution in the breathing zone of human subjects. Models adaptable for risk assessment in non-uniform environment should be applied [65]. Here four different indicators or approaches: exposure risk index, intake fraction, dose–response model, and improved Wells-Riley model were applied for infection risk assessment with the effect of PV. The exposure risk index and intake fraction model cannot predict the infection risk of a specific disease but are able to evaluate the relative exposure level of the exhaled droplets. By adopting existing information of SARS-CoV-2 regarding its infectivity properties, the dose–response model and improved Wells-Riley model can be used to predict the infection risk of this contagious virus.
3.1. Exposure risk model
3.1.1. Exposure risk index (εbz)
The exposure risk index, ε bz, which reflects the efficiency of ventilation in certain points or regions in the room, has been widely used for contaminant exposure risk assessment in previous studies [33], [50], [66], [67], [68]. The εbz in the breathing zone of the exposed occupant can be defined as:
| (1) |
where C bz and C e are droplet concentrations in the breathing zone of the target manikin and the exhaust of the room, respectively; C PV is obtained at the PV outlet with a relatively low value as the PV air is filtered by a HEPA and is considered as clean air free of droplets. The best inhaled air quality will be achieved when εbz = 0 with all the inhaled air directly drawn from clean PV air. And ε bz = 1 indicates a fully mixing condition in the breathing zone of the exposed occupant and other parts of the room. It also happens with ε bz > 1, when C bz is larger than C e, indicating even worse air quality at the breathing zone of the exposed occupant than the background or at the exhaust. The ε bz depicts the mixing degree of exhaled contaminant from the source in the ventilated context, and a lower value indicates better performance of PV in exposure risk control.
3.1.2. Intake fraction (IF)
The intake fraction model (IF) is used to assess the ratio of contaminant inhaled by the exposed occupant to that exhaled by the infected occupant [69], [70], [71], [72]. The IF can be defined as:
| (2) |
where C in is the inhaled concentration of the exposed person (here C in = C bz, measured at P2); C ex is the exhaled concentration of the infected person (measured at P1); M in and M ex are the flow rates of inhaled flow of the exposed person and exhaled flow of the infected person, respectively; C in(t) and C ex(t) are the inhaled concentration of the exposed person and the exhaled concentration of the infected person at time t, respectively; t in and t ex are the exposure time of the exposed person and the respiratory duration of the infected person, respectively. The intake fraction normally varies between 0 and 1. The exposure level increases with a higher intake fraction value.
3.2. Infection risk models
3.2.1. Dose-response model
The dose–response model requires infectious dose data to construct the dose–response relationship [73]. It is also critical to obtain a realistic exposure level of airborne pathogens. The probability of airborne infection can be calculated by Eq. (3) which considers the spatial distribution of infectious droplets [74].
| (3) |
Here, P 1(t 0) is the probability of infection of the susceptible person within an exposure time of t 0. Since the infectivity of pathogens depends on the size of the aerosols carrying infectious agents [40], [75], the exposure level should be divided into different size bins in the dose–response model. m is the number of size bins. rj and βj are infectivity terms of the pathogen and deposition fraction of the infectious droplets of the jth size bin, respectively. c is the pathogen concentration in the respiratory fluid. p is the pulmonary ventilation rate of the susceptible person, and q is the breathing frequency of the infector. v(t)j is the volume density of expiratory droplets in the exhalation of the infector of the jth size bin, and f(t) is the viability time-function of the pathogen. In this study, the values of c and f(t) have been defined regarding the SARS-CoV-2 virus. Since the rj and βj of SARS-CoV-2 are still lacking, the parameters of influenza A were used instead [54]. The data related to the SARS-CoV-2 in the dose–response model is listed in Table 2 .
Table 2.
Data used for calculation of the infection risk of SARS-CoV-2 in the dose-responds model.
| Parameter | SARS-CoV-2 | Remarks |
|---|---|---|
| c | 1 × 105.25 TCID50a/mL | Obtained from the upper and lower respiratory tract in humans [55] |
| Respiratory deposition and infectious dose | ID50b = 1.8TCID50 r = 0.385c |
Infectious dose for aerosol ≤ 3 μm, the mean value of the range: 0.6–3.0; β = 0.6 [54] (for influenza A) |
| Viability of SARS-CoV-2 | f(t) = 0.01778×(0.5)- t/1.15 | Extrapolated from Fig. 1 in Doremalen et al. [55], 21 to 23 °C and 40% RH, the half-lives of SARS-CoV-2 approximately 1.1 to 1.2 h. |
| v(t)j | Assume final nucleus size represents 6% of the initial volume [57] | The calculation is given by Sze To et al. [74] |
TCID50 refers to the mean tissue-culture infectious dose, a unit to describe the quantity of virus.
ID50 refers to the median infective dose of the virus, which can be used to calculate the infectivity term r.
r = 0.385 and β = 0.6 for influenza A [54] are used for the calculation of SARS-CoV-2 intake dose with an aerosol aerodynamic diameter smaller than 3 μm.
The volume density (v(t)j) of the infector’s expiratory droplets can be derived from the mass concentration measured in the respiratory area of the exposed occupant [74]. Since the initial concentration of the droplets released from the source manikin in experiment was higher than that from real human subjects, the concentration of droplets measured at P1 from the source manikin should be normalized to the droplet concentration in real human breath of 0.092 cm−3 as measured by Morawska et al. [63].
3.2.2. Improved Wells-Riley model
The Wells-Riley model [40], [41] which has been extensively used in estimating the relationship between ventilation and airborne infection risk can be expressed by Eq. (4):
| (4) |
where PI is the probability of infection, C is the number of infections, S is the total number of susceptible persons, I is the number of infectors, q is quanta emission rate from the infected source (quanta/h), p is the pulmonary ventilation rate of a susceptible person (inhaled) (m3/h), Q is room ventilation rate with clean air (m3/h) and t is the exposure time interval (h). This model assumes a fully mixing condition of pathogens in room air and merely considers the ventilation removal effect on pathogen loss. In this study, an improved Wells-Riley model proposed by Buonanno et al. [77] was adopted to evaluate the infection risk of SARS-CoV-2 in indoor environment by considering both ventilation and other influencing factors on airborne transmission like the deposition and inactivation of the virus. Compared with the traditional Wells-Riley model (Eq. (4)), the improved model uses the concept of quantum concentration for risk assessment and provides the possibility to evaluate the effect of different airflow distribution patterns on pathogen distribution in the inhalation zone of the exposed occupant.
The viral load emitted by the infected person is defined as the quanta emission rate in the Wells-Riley model [40], [41], which can be obtained by a retrospective study of epidemic cases with Eq. (4). The quanta emission rates of three outbreak cases of COVID-19 [77], [78] in typical transportation environment are calculated and given in Table 3 .
Table 3.
Parameters of SARS-CoV-2 epidemic cases with the Wells-Riley model of Eq. (4).
| Case | Duration of stay | Number of infectors, I | Infected person | Total people, N | Ventilation rate, Q (m3/h) | Percentage of infection | Quanta emission ratea,q (h−1) |
|---|---|---|---|---|---|---|---|
| Bus in Hunan-1, China [77] | 2 h | 1 | 8 | 47 | 291 | 17% | 40.2 |
| Bus in Hunan-2, China [77] | 1 h | 1 | 2 | 12 | 160.7 | 16.7% | 54.4 |
| Vietnam flight [78] | 10 h | 1 | 16 | 217 | 2734.2 | 7.4% | 38.9 |
The quanta emission rate is obtained by inverse calucation with the Wells-Riley model in Eq. (4). The pulmonary ventilation rate p is supposed to be 0.54 m3/h.
The q can also be estimated with Eq. (5) on a basis of the viral load in the mouth, the type of respiratory activity (e.g. speaking, breathing), respiratory physiological parameters (e.g. inhalation rate), and activity level (e.g. resting, standing, light exercise) as proposed by Buonanno et al. [76].
| (5) |
Here, Cv is the viral load in the sputum (RNA copies/mL), Ci is defined as the ratio between one infectious quantum and the infectious dose expressed in viral RNA copies, Nd is the droplet number concentration (cm−3), and Vd(D) is the volume of a single droplet (mL) as a function of the droplet diameter (D). The q of SARS-CoV-2 estimated by Eq. (5) ranges from 10.5 quanta/h (at rest) to 33.9 quanta/h (light activity) for breathing and from 320 quanta/h (at rest) to 1030 quanta/h (light activity) for speaking. By overall considering the q obtained both from Eqs. (4), (5), an averaged value of 44.5 quanta/h of the three estimated cases in Table 3 is applied for infection risk of SARS-CoV-2 affected by PV with the improved Wells-Riley model, which can be expressed by Eqs. (6), (7).
| (6) |
Here, n(t) is defined as the quantum concentration (quanta/m3) of the indoor environment at time t, n 0 represents the initial concentration of quanta in the space, I is the number of infectors, and V is the volume of exposed space. IVRR (h−1) is the infectious virus removal rate in the exposed space, which is the sum of three factors: the air change rate (ACR) via ventilation, the droplet deposition on surfaces (k) and the viral inactivation (λ) [79].
| (7) |
The infection risk R at the total exposure time T is estimated by Eq. (7). Eq. (7) considers not only the ventilation removal effect, but also deposition and inactivation loss of airborne pathogens, which would be more realistic compared with the traditional Wells-Riley model [40], [41]. IR represents the inhalation rate of the exposed person. n(t) is obtained from Eq. (6). The detailed parameters in the improved Wells-Riley model for the calculation of infection risk of SARS-CoV-2 considering the effect of PV are listed in Table 4 .
Table 4.
Data used for calculation of the infection risk of SARS-CoV-2 with the improved Wells-Riley model.
| Parameter | SARS-CoV-2 | Remarks |
|---|---|---|
| q, quanta/h | 44.5 | Averaged value in Table 3. |
| Viral inactivation (λ), h−1 | 0.63 | The viral inactivation was evaluated on the basis of the SARS-CoV-2 half-life of 1.1 h [55]. |
| Particle deposition rate (k), h−1 | 8.47 × 10-5 | The deposition rate is evaluated as the ratio between the settling velocity of super-micrometric particles (v) of roughly 1.0 × 10-4 m/s as measured by Chatoutsidou and Lazaridis [80]. The height of the emission source (H) is 1.18 m (Fig. 1 (b)). |
| Air change rate (ACR), h−1 | 2 | The ACR is determined according to this experiment. |
| Inhalation rate (IR), m3/h | 0.54 | The inhalation rate represents a metabolic level of about 1.2 Met at sedentary condition. |
The infection risk of the three cases [77], [78] in Table 3 was calculated by the improved Wells-Riley model (Eq. (7)). Fig. 3 shows good agreement with the actual value by using the improved model. The maximum absolute deviation and relative deviation were 0.017 and 15%, respectively, indicating the improved Wells-Riley model being reasonable for SARS-CoV-2 risk assessment in enclosed indoor environment.
Fig. 3.
The predicted and actual probability of infection in existing cases.
The n(t) in the Wells-Riley model represents the average quantum concentration in the case of uniform mixing spatially. The use of PV will change the local pathogen distribution in the inhalation zone of the susceptible person. This study considers a proportional relationship between the quantum concentration and the aerosol concentration in the improved Wells-Riley model, which can be used to evaluate the infection risk of SARS-CoV-2 with the influence of different airflow patterns.
4. Results
4.1. Exposure risk assessment
4.1.1. Exposure risk index (εbz)
Fig. 4 shows the exposure risk of droplets (ε bz) for the exposed occupant with different PV combinations and the three relative orientations. The ε bz of MV for all the three relative orientations was close to 1, indicating a relatively uniform mixing condition in the ventilated room with MV alone. It was found that the ε bz side-by-side was the highest among the three orientations, and the ε bz of PVs was also the highest among the three different combinations of PV supply. Especially the ε bz of PVs was all>1 with the side-by-side orientation, indicating an elevated exposure risk for the exposed occupant by applying PVs alone to the source manikin by side. The highest ε bz and flow variations were achieved of 1.60 ± 0.79 with PVs 3 L/s for the side-by-side orientation. The enhanced ε bz for PVs can be explained by the increased mixing of exhaled droplets with ambient air in the microenvironment around two manikins. When PV air was supplied to the source manikin, it interfered with the forward exhalation flow from the source, which would cause lateral dispersion of the exhaled droplets and a higher exposure level with the side-by-side orientation. The ε bz and its variations were lowered with the increase of PVs flow rate to 6 L/s or 9 L/s side-by-side as the dilution effect of the clean air from PVs became more significant. However, the exposure risk was still higher than that with MV alone.
Fig. 4.
Averaged exposure risk index with the standard deviation under different relative orientations and PV combinations.
The lowest ε bz occurred with PVs-t of 6 L/s back-to-back of merely 0.50. The ε bz of PVt and PVs-t with back-to-back and face-to-back orientations were all close to 0.53, regardless of the PV flow rate. Our previous study [81] indicated that the efficiency of clean air supply from PVt was dominated by the configuration of the PV nozzle rather than its supplied airflow volume. Fig. 5 illustrates the turbulence development of the nozzle jet, and the deterioration rate of the air quality from PV can be predicted by Eq. (8).
Fig. 5.
Schlieren image of the development process of a PV jet [81].
The theoretical expression of free jet concentration deterioration is shown in Eq. (8). Cx, C e and C 0 are the centerline concentration at distance × from the nozzle, the concentration at the exhaust of the room and in the outlet of PV nozzle, respectively. K c is a characteristic constant for the PV nozzle, and a is its exit area of the nozzle. When PV supplies clean air without infectious droplets, C 0 is then equal to C PV of zero. C bz is equivalent to Cx if the breathing zone of the occupant is aligned exactly right with the centerline of the PV airflow, just as the experimental setup in this study. Then Eq. (8) can be converted into the relationship between the contaminant concentration deterioration with ε bz.
| (8) |
As shown in Fig. 5 and Eq. (8), the ε bz is mainly determined by the characteristic constant K c of the nozzle, the nozzle exit area a and the relative distance between the nozzle exit and the breathing zone of the occupant. This explains why the ε bz is approximately equal for all the back-to-back or face-to-back cases with PVt or PVs-t. It can also be inferred that the clean air delivery efficiency of the PV nozzle is about 0.47, which agrees well with the tested efficiency of the same PV nozzle by our previous study [81]. For occupants placed side-by-side, the jet entrains polluted air from both the ambient air and lateral dispersed exhalation, the ε bz is hereby increased and cannot be predicted by Eq. (8) which is applicable to an approximate free jet.
4.1.2. Intake fraction (IF)
Fig. 6 shows the IF of the exposed occupant affected by PV with the three orientations. The IF under MV for all the three orientations was basically similar with a value around 0.01, with a negligible effect of direct exposure to the exhaled droplets from the source. The IF of the exposed occupant was significantly influenced by both the patterns of PV supply (e.g. PVs, PVt and PVs-t) and the relative orientations to the infectious source.
Fig. 6.
Intake fraction with PV for target manikin at different relative orientations: (a) side-by-side, (b) face-to-back and (c) back-to-back.
For all tested cases with PVt and PVs-t, the use of PV would lower the IF of the exposed occupant. The IF with PVs strongly depended on the relative orientations to the infectious source. The IF under PVs was significantly elevated with the side-by-side orientation compared with that with MV. The IF exceeded 0.015 with PVs of 3 L/s and decreased with a higher volume of PV, which was around 0.013 for both PVs of 6 L/s and 9 L/s. While the IF of PVs for back-to-back or face-to-back orientations was very close and similar to that with MV. The exposure risk of exhaled droplets was increased with the use of PVs for side-by-side orientation as it would interact with the exhaled flow from the source and promote the lateral diffusion of the exhalation flow to the person by side. An increased PVs volume would enhance the dilution effect of PV by supplying more fresh air to the source, leading to a lower IF for PVs 6 L/s and 9 L/s. The lateral diffusion of the droplets caused by PVs had no direct impact on the inhalation of the exposed occupant with back-to-face and back-to-back orientations. PVs diluted the exhaled flow from the source and accelerated its mixing with the ambient air for the two orientations.
The use of PVt or PVs-t would reduce the IF of the exposed occupant with similar effectiveness. Clean air was delivered to the inhalation zone of the exposed occupant with the use of PVt for all tested cases, which showed a protective effect against exhaled droplets for the PV user. However, the IF was not significantly affected by the flow volume of PVt especially for face-to-back or back-to-back cases. This could be explained by Eq. (8) as the droplet concentration in inhalation was determined by the nozzle’s efficiency itself. While the IF of PVt and PVs-t side-by-side was higher than the other two orientations by about 0.0025. The increased IF for the side-by-side orientation may be caused by the entrainment of exhaled droplets of PVt directly from the source with the development of the PVt flow due to the close distance between the two occupants of merely 0.65 m.
4.2. Infection risk assessment
4.2.1. Dose-response model
The dose–response model is used to estimate the infection risk of SARS-CoV-2 of the exposed person with an exposure duration of 2 h, as shown in Fig. 7 . The existing epidemiological data of SARS-CoV-2 in Table 2 was applied in this model to evaluate the effect of PV on infectious pathogen transmission.
Fig. 7.
Infection risk of SARS-CoV-2 assessed by the dose–response model over time in different relative orientations: (a) side-by-side, (b) face-to-back and(c) back-to-back.
It was found that the infection risk of SARS-CoV-2 with the three orientations was basically similar under MV. The infection risk with MV was less than 0.10 within 45 min and grew to about 0.56 with continuous exposure over 2 h. The use of PVt or PVs-t would mitigate the infection risk of SARS-CoV-2 by different degrees for the three relative orientations. The infection risk was significantly lowered to merely 0.02 over 45 min and 0.23 over 2 h with PVs-t 9 L/s back-to-back. For the side-by-side orientation, the infection risk over 2 h was 0.42 with PVs-t 9 L/s and 0.56 with PVt 9 L/s, respectively.
The back-to-back orientation performed the best in infection risk mitigation by applying PV to the exposure occupant, then was followed by the face-to-back orientation. The side-by-side orientation showed the poorest protection effect, which was in line with the evaluated results by the exposure risk index or intake fraction. Special attention should be paid to the side-by-side orientation with PVs alone, as the infection risk was significantly increased significantly to 0.81 with PVs 3 L/s, 0.70 with PVs 6 L/s and 0.71 with PVs 9 L/s for a durable exposure of 2 h, implying a high probability of infection of this disease.
4.2.2. Improved wells-riley model
Fig. 8 shows the infection risk of SARS-CoV-2 predicted by the improved Wells-Riley model for the three relative orientations under the effect of PV. The use of PVs side-by-side increased the infection risk significantly, and the highest infection risk was caused by PVs 3 L/s of 0.49 over 2 h. The infection risk of PVs side-by-side over 2 h was around 0.40 for both 6 L/s and 9 L/s. This is significantly lower than the infection risk predicted by the dose–response model.
Fig. 8.
Infection risk of SARS-CoV-2 assessed by the improved Wells-Riley model over time in different relative orientations: (a) side-by-side, (b) face-to-back and(c) back-to-back.
For the back-to-back and face-to-back orientation, the infection risk of SARS-CoV-2 with PVs was very close to that with MV. The evaluated infection risk for the exposed occupant didn’t vary much with the PV flow rates or the use of PVs, when the exposed occupant applied PV in back-to-back or face-to-back orientation. These results were basically consistent with the change laws of exposure risk evaluated by the exposure risk index in Fig. 4 or intake fraction in Fig. 6. The lowest infection risk was achieved by PVs-t 9 L/s in back-to-back orientation of approximately 0.18, which was slightly lower than the infection risk of SARS-CoV-2 predicted by the dose–response model of 0.23. The different growth rates of infection risk of SARS-CoV-2 between the two risk assessment models was caused by the different exponential terms in Eqs. (3), (7).
5. Discussion
5.1. Potentials of PV in risk mitigation
The risk reduction ratio ε r is defined as the ratio of reduced exposure risk or infection risk caused by PV (I PV, on) to the risk without PV (I PV, off), which is given by Eq. (9).
| (9) |
Fig. 9 shows the ε r obtained from the four risk assessment models considering different PV combinations and relative orientations between occupants. The ε r calculated by the averaged ε bz and IF is equivalent and they are depicted with the same columns in Fig. 9. The equivalence is caused by the same expressions of ε r (in average) in Eq. (9) by eliminating the effect of different denominators in Eqs. (1), (2).
Fig. 9.
The reduction ratio of four predicts models of PV over 2 h with (a) 3 L/s; (b) 6 L/s; (c) 9 L/s. The red column indicates a negative value of the reduction ratio. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
It can be seen that from Fig. 9 that when PV is supplied to the exposed occupant, it always plays a positive role in protecting the occupant against airborne infection in all three orientations. But the effectiveness of PV in infection risk reduction varied with different orientations, indicating a significant impact of the relative orientations on PV’s performance. When the two occupants were placed back-to-back or face-to-back, both PVt and PVs-t were robust in protecting the exposed occupant from airborne infection and the ε r was mostly above 0.40 evaluated by the four different models. The protection efficiency depended on PV’s own clean air delivery efficiency if there was no direct entrainment of the exhaled air from the infectious source.
PV performed the best for the back-to-back orientation in mitigating the airborne infection risk between the two occupants compared with the other two orientations. Then was followed by the face-to-back orientation. A risk reduction ratio of 0.65 was achieved with PVs-t 9 L/s by using the dose–response model of a lasting exposure time of 2 h. PV used for the side-by-side occupants was found with the lowest ε r. In the case of PVs 3 L/s used for the infector alone side-by-side, the infection risk reduction ratio ε r was −0.81 as evaluated by the exposure risk index, implying the severe negative effect of PVs in airborne disease control side-by-side.
Some previous studies have also noticed the impact of the relative orientations between the occupants on the efficiency of PV in airborne transmission control. The problems and attentions of PV in airborne risk control are discussed in Table 5 , regarding five different orientations between the two occupants. Xu et al. [65] found that the PV jet would entrain droplets when it fell within the area accessible to the exhalation of the infector with a face-to-face orientation of relatively short distance, which would increase the infection risk by using PV. Li et al. [39] reported that the exposure risk slightly rose when only provided PV to the infector with a face-to-face orientation. Pantelic et al. [82] also found that the source from the back orientation had a lower intake fraction than the front and the side. Xu et al. [83] investigated the effect of different orientations on the personal exposure of a PV user to exhaled droplets and found that PV would decrease the exposure risk when the source was located at a relative back orientation to the infector. Previous studies conducted by Cermak and Melikov [84] and He et al. [85] also indicated that the highest exposure risk occurred when provided high volume PV airflow to the infector setting in tandem (the infector being at front). To summarize, for closely ranged occupants, the back-to-back and face-to-back (infector in the back) orientations are the most preferable for PV in mitigating airborne transmission risk.
Table 5.
Remarks on the application of PV in airborne transmission control for different orientation scenarios.
| Relative orientation | Remarks |
|---|---|
 |
|
 |
|
 |
|
 |
|
 |
|
In order to achieve a robust PV efficiency in mitigating airborne transmission between occupants, some recommendations for the application of PV in a highly occupied environment are discussed. The discussion is based on the experimental results in this study. As not all the seat arrangements in indoor environments or vehicles are the preferred orientation like back-to-back or face-to-back, Fig. 10 takes the side-by-side case as an example to illustrate some measures that may be useful to improve the potential efficiency of PV in airborne risk control.
Fig. 10.

Sketch of the measures that may be used to improve the ability of PV in mitigating airborne infection: ①use highly efficient PV ATDs, ②use personalized exhaust, and ③use partitions.
5.1.1. Highly efficient PV air terminal devices (ATDs)
The optimal performance for most of the ATDs has not exceeded 50–60% of clean air in inhalation [86]. In this study, the delivery efficiency of clean air for the present PV nozzle is about 47%. Some highly efficient ATDs providing over 90% of clean air should be developed to maximize the protective function of PV in airborne disease control.
5.1.2. Combined PV with personalized exhaust (PE)
PV used only by the infector may facilitate the transport of exhaled droplets, especially for the side-by-side orientation as discussed in Table 5. PE used by occupants may directly eliminate exhaled pathogens and be effective in infectious source removal [33], [87], [88], which may be a promising solution by combining the use of PV.
5.1.3. Set partitions between occupants
Partitions between the occupants are expected to block the transmission route between them [89], [90]. The penetration of the infectious exhalation flow to the exposed occupant face-to-face or face-to-back (infector at back) and lateral diffusion to the adjacent occupant side-by-side can be blocked by the partition between occupants with a sufficient height, which should be further validated.
5.2. Comparisons of the risk assessment models
All the four models used in this study can predict the exposure risk of non-uniformly distributed pathogens in the indoor environment due to the use of PV for the occupants. It can be seen from Fig. 9 that the basic rules of the predicted risk affected by PV are similar among the four models. This is because the core parameter used in the four models for risk prediction is the same, which is the droplet concentration in the breathing zone of the exposed occupant. The relevance and applicability of these four models are summarized in Table 6 .
Table 6.
Comparisons between the four risk assessment models.
| Exposure risk (εbz) | Intake fraction (IF) | Dose-response model | Wells-Riley model | |
|---|---|---|---|---|
| Applied transmission routes | Apply to the airborne transmission and droplets transmission route. | Apply to the airborne transmission and droplets transmission route. | Suitable for all transmission routes (airborne transmission, droplets transmission, contact transmission) [73]. | The Wells-Riley model can only be applied to the airborne transmission route [73]. |
| A quick assessment | Suitable | Suitable | Not suitable | Suitable |
| Time state | Real-time value (Transient) or time-averaged value (steady) | Time accumulative value or transient at a certain time | Time cumulative value | Time cumulative value |
| Spatial distribution of pathogens | Uniform or non-uniform | Uniform or non-uniform | Uniform or non-uniform | Uniform (Wells-Riley model [40]) Non-uniform (the improved Wells-Riley model [76]). |
| Infectivity of pathogens | Not considered. | Not considered. | The infectivity rj and viability f(t) of the pathogen and pathogen concentration in the respiratory fluid were considered in this model. | The infectivity term is implicitly included in the backward calculated quanta generation rate. The improved Wells-Riley model by Buonanno et al. [76] considered viral inactivation (IVRR). |
| Core parameter for risk assessment | The quantity concentration measured in the breathing zone of exposed occupants and the exhaust of the room. | The quantity concentration was measured in the source and the breathing zone of exposed occupants. | The mass concentration measured in the breathing zone of the exposed occupants and the does-response data was obtained from human infection experiments. | The quanta emission rate or measured quanta concentration in the breathing zone of the exposed occupants. |
The two indicators ε bz and IF can both predict the effect of PV on the exposure risk of exhaled contaminants from the source, just with different baselines. The exposure risk index ε bz demonstrates the relative contamination level of exhaled droplets in the breathing zone of the exposed occupant compared with the ambient environment. If the inhaled air of the exposed occupant is directly drawn from the initial core of the PV jet, ε bz will reach a minimum value of zero. The intake fraction IF, which illustrates the dilution effect of the exhaled droplets with respect to the source, is considered as a better exposure risk assessment model than ε bz to predict the exposure risk level [91]. As the increase of the air change rate may both dilute the contaminants in the inhalation and in the exhausts with the same proportion and results in a constant ε bz. In this study, it is found that ε bz and IF are both suitable for simple and fast predictions of the exposure risk of pathogens by experiments or simulations with tracer gas or tracer particles, but the infectivity of a specific disease agent is not considered.
Different from the traditional Wells-Riley model [40], [41], the improved Wells-Riley model [76] considered not only the ventilation removal but also the deposition and viability of the virus, which would be closer to the real situation. In addition, the traditional Wells-Riley model assumes a fully mixing condition of room air with pathogens without considering the patterns of air distribution. The improved Wells-Riley model adopts the concept of quantum concentration, which is applicable to describe non-uniformly distributed pathogen concentration affected by different airflow patterns. Both the models allowed quick assessment of the key parameter that is the quanta generation rate. They also allow quick prediction of the infection risk of a certain airborne disease especially at the beginning of the outbreak with the absence of accurate biological and infectivity data.
The dose–response model and the Wells-Riley model both can reflect the increasing trend of the infection risk over time and with the cumulative dose of viruses. Fig. 8 shows the infection risk increases exponentially over time as predicted by the Wells-Riley model, as the quantum concentration n(t) is supposed to be constant in the inhalation zone by the using average droplets concentration acquired from experiments. While the trend of infection risk is not growing in an exponential way with the dose–response model, as shown in Fig. 7. This is because the dose–response model considers both the viability of pathogens f(t) and the volume density of expiratory droplets in the exhalation of the infector v(t)j as a function of time, and the integral of f(t) and v(t)j with respect to time constitutes the exponential term as a changing value over time. The dose–response model considers several influencing factors that would avoid more implicit errors than the Wells-Riley model when performing a risk assessment. It provides a more complete risk assessment result. However, the key factors such as the r, c, β and f(t) are normally derived from a large number of human infectivity experiments and are sometimes difficult to acquire, especially at the beginning of the outbreak of a new disease. Take the ongoing pandemic of COVID-19 for example, the activity, viability and pathogenicity of SARS-CoV-2 are complicated, and settlement position and proportion in the human respiratory tract, the pathogenic mechanism of the virus as well as immunity characteristic of the human body are still difficult to determine. Thus, the determination of pathogen activity and pathogenicity from the source to the deposited respiratory tract is vital for the application of the dose–response model in the accurate risk assessment. The two infection risk models both provide further information for epidemiologists to understand the spread of the disease and can serve as useful tools for understanding the disease transmission mechanisms and developing corresponding infection control strategies.
5.3. Limitations
Experiments in this study were designed with a PV flow rate ranging from 3 L/s to 9 L/s with three relative orientations. The face-to-face and face-to-back (the infector at front) orientations discussed in Table 5 were not studied with present experiments. In practical applications, the PV terminal device may provide an adjustable flow rate varying from 3 L/s to even 20 L/s [86]. The protective effect of PV in mitigating infection risk with a higher flow rate was not validated. The relative alignment of PV to the occupant may also vary with different angles or distances compared with present experimental setup, which will affect the efficiency of PV in protecting the occupant against airborne infection. The influences of the motion of human body, the type of PV terminal devices, and the supplied air temperature from PV are still not clearly demonstrated and need further investigation. In the context of COVID-19 pandemic, accurate quantification of the activity and pathogenicity of SARS-CoV-2 is needed, which will be helpful to evaluate the airborne infection risk of the virus by using the infection risk models, in particular for the dose–response model.
6. Conclusion
This study explored the potentials of personalized ventilation (PV) in mitigating cross-infection risk between two closely ranged occupants in the indoor environment. Three typical relative orientations were studied, which were side-by-side, face-to-back, and back-to-back. The impact of different combinations of PV used for the infected source and/or the exposed occupant was also considered. To evaluate the effectiveness of PV in reducing the exposure or infection risk to the epidemic disease-COVID-19, four frequently-used risk assessment models were employed and compared. Some meaningful findings of this study were addressed as follows.
When PV airflow was directly supplied to the exposed occupant, it would always play apositive role in protecting the occupant against airborne transmission in all the tested scenarios, no matter it is combined with PV to the source or not. Relative orientations and operation modes of PV significantly affected its performance in airborne risk control. The lowest infection risk was achieved with PVs-t 9 L/s in back-to-back orientation, having a maximum risk reduction ratio of 0.65 evaluated by the dose–response model with a 2 h exposure duration.
The protective effect of PV for the exposed occupant was primarily dominated by the clean air delivery efficiency of PV itself. This efficiency was found to be approximately equal to 0.47 with PVt or PVs-t back-to-back and face-to-back, regardless of the varying PV flow rate. The PV efficiency was inferred to be determined by the both configuration of the PV nozzle and the relative alignment distance between the nozzle and the occupant.
PV airflow directly interacted with the exhaled airflow from the occupant and accelerated mixing and lateral diffusion of the exhaled droplets, making the side-by-side orientation the most critical condition for the use of PV. Especially when PVs was used with a low flow rate of merely 3 L/s and with an orientation side-by-side, the risk reduction ratio ε r reached a minimal level of −0.82 evaluated by the exposure risk index, implying severe negative effect of PVs in airborne risk control in this orientation. The dilution effect of PVs would be increased with higher flow volume, and a lower risk would be achieved in a side-by-side orientation but the risk was still higher than using mixing ventilation alone.
All the four risk assessment models in this study can be used to predict the exposure or infection risk of non-uniformly distributed pathogens in indoor environment with the use of PV. The predicted results were in basically consistent laws among the four models and the relevance and applicability of them were discussed to guide further selections of appropriate models for risk assessment. The two exposure risk models (ε bz and IF) were suitable for simple and fast prediction of the exposure risk of droplets through experiments or simulations, without considering the infectivity of a specific disease agent. The traditional and improved Wells-Riley model also allowed quick assessment of SARS-CoV-2 by knowing the quanta emission rate and other epidemiological data. The dose–response model may be the most accurate one among the four models but it also needs several aerodynamic and biological parameters of the infectious agent to avoid more implicit errors compared with the Wells-Riley model when performing a risk assessment.
Personalized ventilation was proved to be effective in mitigating airborne infection risk between occupants in short distance with a small amount of clean air supply, which can serve as a potential solution to minimize the airborne transmission risk between sedentary occupants in transportation or public space. As a possible solution to mitigate the infection risk of the ongoing pandemic of COVID-19 in an indoor environment, optimal PV design combined with the aid of accurate risk assessment models can serve as a useful tool in the ventilation strategy development and airborne infection risk control.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This project was financially supported by the National Natural Science Foundation of China (51808555, 51778520), the Natural Science Foundation of Shandong Province (ZR2019MEE060), the Opening Fund of State Key Laboratory of Green Building in Western China (LSKF202014), the Fundamental Research Funds for the Central Universities (XZY032020029) and the Danish Agency for Higher Education and Science International Network Programme (case no. 0192-00036B).
References
- 1.World Health Organization, Disease outbreaks. (Accessed 5 February 2020), 2020, https://www.who.int/emergencies/diseases/en/.
- 2.World Health Organization, Coronavirus (COVID-19) dashboard overview. (Accesses 20 September 2021), 2021, https://covid19.who.int/.
- 3.World Health Organization, Q&A: How is COVID-19 transmitted? What do we know about aerosol transmission? (Accessed 9 July 2020), 2020, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-how-is-covid-19-transmitted.
- 4.World Health Organization, Coronavirus disease (COVID-19) advice for the public, (Accessed 23 September 2020), 2020, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public.
- 5.Lu J., Gu J., Li K., Xu C., Su W., Lai Z., Zhou D., Yu C., Xu B., Yang Z. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China. Emerg. Infect. Dis. 2020;26:1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S.Y., Kim Y.-M., Yi S., Lee S., Na B.-J., Kim C.B., Kim J.-i., Kim H.S., Kim Y.B., Park Y., Huh I.S., Kim H.K., Yoon H.J., Jang H., Kim K., Chang Y., Kim I., Lee H., Gwack J., Kim S.S., Kim M., Kweon S., Choe Y.J., Park O.k., Park Y.J., Jeong E.K. Coronavirus disease outbreak in call center, South Korea. Emerg. Infect. Dis. 2020;26(8):1666–1670. doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamner L., Dubbel P., Capron I., Ross A., Jordan A., Lee J., Lynn J., Ball A., Narwal S., Russell S., Patrick D., Leibrand H. High SARS-CoV-2 attack rate following exposure at a choir practice - Skagit County, Washington, March 2020. MMWR. Mmwr-Morbid. Mortal. W. 2020;69(19):606–610. doi: 10.15585/mmwr.mm6919e6. [DOI] [PubMed] [Google Scholar]
- 8.Jang S., Han S.H., Rhee J.-Y. Cluster of coronavirus disease associated with fitness dance classes, South Korea. Emerg. Infect. Dis. 2020;26(8):1917–1920. doi: 10.3201/eid2608.200633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hijnen D., Marzano A.V., Eyerich K., GeurtsvanKessel C., Giménez-Arnau A.M., Joly P., Vestergaard C., Sticherling M., Schmidt E. SARS-CoV-2 transmission from presymptomatic meeting attendee, Germany. Emerg. Infect. Dis. 2020;26(8):1935–1937. doi: 10.3201/eid2608.201235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang S., Chang J.H., Oh B., Heo J. Possible aerosol transmission of COVID-19 associated with an outbreak in an apartment in Seoul, South Korea. Int. J. Infect. Dis. 2021;104:73–76. doi: 10.1016/j.ijid.2020.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller N., Kunze M., Steitz F., Saad N., Mühlemann B., Beheim-Schwarzbach J., Schneider J., Drosten C., Murajda L., Kochs S., Ruscher C., Walter J., Zeitlmann N., Corman V. Severe acute respiratory syndrome coronavirus 2 outbreak related to a nightclub, Germany. Emerg. Infect. Dis. 2020;27:645–648. doi: 10.3201/eid2702.204443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katelaris A., Wells J., Clark P., Norton S., Rockett R., Arnott A., Sintchenko V., Corbett S., Bag S. Epidemiologic evidence for airborne transmission of SARS-CoV-2 during church singing, Australia. Emerg. Infect. Dis. 2021;27:1677–1680. doi: 10.3201/eid2706.210465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X., Ou C., Yang H., Liu L., Song T., Kang M., Lin H., Hang J. Transmission of pathogen-laden expiratory droplets in a coach bus. J. Hazard. Mater. 2020;397:122609. doi: 10.1016/j.jhazmat.2020.122609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mesgarpour M., Abad J.M., Alizadeh R., Wongwises S., Doranehgard M.H., Ghaderi S., Karimi N. Prediction of the spread of Corona-virus carrying droplets in a bus - A computational based artificial intelligence approach. J. Hazard. Mater. 2021;413:125358. doi: 10.1016/j.jhazmat.2021.125358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu L., Nielsen P.V., Wang Y., Liu L. Measuring interpersonal transmission of expiratory droplet nuclei in close proximity. Indoor Built Environ. 2021;1420326X211029689 doi: 10.1177/1420326X211029689. [DOI] [Google Scholar]
- 16.Morawska L., Cao J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ong S.W., Tan Y.K., Chia P., Lee T., Ng O., Wong M., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Setti L., Passarini F., De Gennaro G., Barbieri P., Perrone M.G., Borelli M., Palmisani J., Di Gilio A., Torboli V., Fontana F., Clemente L., Pallavicini A., Ruscio M., Piscitelli P., Miani A. SARS-Cov-2 RNA found on particulate matter of Bergamo in Northern Italy: first evidence. Environ. Res. 2020;188:109754. doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morawska L., Tang J., Bahnfleth W., Bluyssen P., Boerstra A., Buonanno G., Cao J., Dancer S., Floto A., Franchimon F., Haworth C., Hogeling J., Isaxon C., Jimenez J., Kurnitski J., Li Y., Loomans M., Marks G., Marr L., Mazzarella L., Melikov A., Miller S.L., Milton D., Nazaroff W., Nielsen P., Noakes C., Peccia J., Querol X., Sekhar C., Seppänen O., Tanabe S., Tellier R., Tham K., Wargocki P., Wierzbicka A., Yao M. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142 doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J., Du G. COVID-19 may transmit through aerosol. Ir. J Med. Sci. 2020:1–2. doi: 10.1007/s11845-020-02218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fineberg H. Rapid expert consultation on the possibility of bioaerosol spread of SARS-CoV-2 for the COVID-19 pandemic (April 1, 2020) The National Academies Press N.R.C. ed; Washington, DC: 2020. [Google Scholar]
- 22.Yang B., Melikov A., Kabanshi A., Zhang C., Bauman F., Cao G., Awbi H., Wigö H., Niu J., Cheong K., Tham K., Sandberg M., Nielsen P., Kosonen R., Yao R., Kato S., Sekhar S., Schiavon S., Karimipanah T., Li X., Lin Z. A review of advanced air distribution methods – theory, practice, limitations and solutions. Energy Build. 2019;202 doi: 10.1016/j.enbuild.2019.109359. [DOI] [Google Scholar]
- 23.Nielsen P. Control of airborne infectious diseases in ventilated spaces. J. R. Soc. Interface. 2009;6:S747–S755. doi: 10.1098/rsif.2009.0228.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao B., Wu J. Particulate pollution in ventilated space: analysis of influencing factors. J. Hazard. Mater. 2009;163(1):454–462. doi: 10.1016/j.jhazmat.2008.06.118. [DOI] [PubMed] [Google Scholar]
- 25.Ding J., Yu C.W., Cao S.-J. HVAC systems for environmental control to minimize the COVID-19 infection. Indoor Built Environ. 2020;29(9):1195–1201. doi: 10.1177/1420326X20951968. [DOI] [Google Scholar]
- 26.Zheng W., Hu J., Wang Z., Li J., Fu Z., Li H., Jurasz J., Chou S., Yan J. COVID-19 impact on operation and energy consumption of heating, ventilation and air-conditioning (HVAC) systems. Adv. Appl. Eng. 2021;3:100040. doi: 10.1016/j.adapen.2021.100040. [DOI] [Google Scholar]
- 27.Xu C., Liu L. Personalized ventilation: one possible solution for airborne infection control in highly occupied space? Indoor Built Environ. 2018;27:873–876. doi: 10.1177/1420326X18777383. [DOI] [Google Scholar]
- 28.Gao N., Niu J. Personalized ventilation for commercial aircraft cabins. J. Aircraft. 2007;45:508–512. doi: 10.2514/1.30272. [DOI] [Google Scholar]
- 29.Niu J., Gao N., Ma P., Zuo H. Experimental study on a chair-based personalized ventilation system. Build. Environ. 2007;42(2):913–925. doi: 10.1016/j.buildenv.2005.10.011. [DOI] [Google Scholar]
- 30.Nielsen P.V., Jiang H., Polak M., Bed with integrated personalized ventilation for minimizing cross infection, In: Seppänen O, Säteri J, eds. Proceedings of Roomvent 2007. Helsinki: FINVAC ry. 2007. (2007) 218-228.
- 31.Pantelic J., Sze To G.N., Tham K., Chao C., Khoo Y. Personalized ventilation as a control measure for airborne transmissible disease spread. J. R. Soc. Interface. 2009;6:S715–S726. doi: 10.1098/rsif.2009.0311.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melikov A., Bolashikov Z., Georgiev E., Novel ventilation strategy for reducing the risk of airborne cross infection in hospital rooms, Proceedings of Indoor Air 2011, June 5-10, Austin, TX, Paper 1037 (2011).
- 33.Melikov A., Dzhartov V. Advanced air distribution for minimizing airborne cross-infection in aircraft cabins. HVAC&R Res. 2013;19:926–933. doi: 10.1080/10789669.2013.818468. [DOI] [Google Scholar]
- 34.Assaad D.A., Ghali K., Ghaddar N., Katramiz E., Ghani S. Evaluation of different personalized ventilation air terminal devices: Inhalation vs. clothing-mediated exposures. Build. Environ. 2021;192:107637. doi: 10.1016/j.buildenv.2021.107637. [DOI] [Google Scholar]
- 35.Xu J.C., Wang C.T., Fu S.C., Chan K.C., Chao C.Y.H. Short-range bioaerosol deposition and inhalation of cough droplets and performance of personalized ventilation. Aerosol Sci. Tech. 2021;55(4):474–485. doi: 10.1080/02786826.2020.1870922. [DOI] [Google Scholar]
- 36.Bolashikov Z.D., Melikov A.K. Methods for air cleaning and protection of building occupants from airborne pathogens. Build. Environ. 2009;44(7):1378–1385. doi: 10.1016/j.buildenv.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katramiz E., Assaad D.A., Ghaddar N., Ghali K. The effect of human breathing on the effectiveness of intermittent personalized ventilation coupled with mixing ventilation. Build. Environ. 2020;174 doi: 10.1016/j.buildenv.2020.106755. [DOI] [Google Scholar]
- 38.Xu C., Wei X., Liu L., Su L., Liu W., Wang Y., Nielsen P. Effects of personalized ventilation interventions on airborne infection risk and transmission between occupants. Build. Environ. 2020;180 doi: 10.1016/j.buildenv.2020.107008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X., Niu J., Gao N. Co-occupant's exposure to exhaled pollutants with two types of personalized ventilation strategies under mixing and displacement ventilation systems. Indoor air. 2013;23(2):162–171. doi: 10.1111/ina.12005. [DOI] [PubMed] [Google Scholar]
- 40.Wells W. Airborne Contagion and Air Hygiene. (1955) 117-122. Cambridge, MA: Cambridge University Press, 10.2105/AJPH.45.11.1495-C, 1955. 10.2105/AJPH.45.11.1495-C. [DOI]
- 41.Riley E., Murphy G., Riley R. Airborne spread of measles in a suburban elementary school. Am. J. Epidemiol. 1978;107(5):421–432. doi: 10.1093/oxfordjournals.aje.a112560. [DOI] [PubMed] [Google Scholar]
- 42.Rudnick S., Milton D. Risk of indoor airborne infection transmission estimated from carbon dioxide concentration. Indoor Air. 2003;13(3):237–245. doi: 10.1034/J.1600-0668.2003.00189.x. [DOI] [PubMed] [Google Scholar]
- 43.Chick S.E., Koopman J.S., Soorapanth S., Brown M.E. Infection transmission system models for microbial risk assessment. Sci. Total. Environ. 2001;274:197–207. doi: 10.1016/S0048-9697(01)00749-5. [DOI] [PubMed] [Google Scholar]
- 44.ISO 14644-1:2015 (Edition 2) Cleanrooms and associated controlled environments. Publication date: 2015-12.
- 45.GB 9673-1996. Hygienic standard for public means of transportation. General administration of quality supervision, inspection and quarantine of the People's Republic of China, 1996.
- 46.Zhang T., Yin S., Wang S. An under-aisle air distribution system facilitating humidification of commercial aircraft cabins. Build. Environ. 2010;45(4):907–915. doi: 10.1016/j.buildenv.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo Y., Jiang N., Yao S., Dai S., Liu J. Turbulence measurements of a personal airflow outlet jet in aircraft cabin. Build. Environ. 2014;82:608–617. doi: 10.1016/j.buildenv.2014.10.007. [DOI] [Google Scholar]
- 48.Fang Z., Liu H., Li B., Baldwin A., Wang J., Xia K. Experimental investigation of personal air supply nozzle use in aircraft cabins. Appl. Ergon. 2015;47:193–202. doi: 10.1016/j.apergo.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 49.GB 7258-2017. Technical specifications for safety of power-driven vehicles operating on roads. General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, 2017.
- 50.Sze To G.N., Wan M.P., Chao C.Y.H., Fang L., Melikov A. Experimental study of dispersion and deposition of expiratory aerosols in aircraft cabins and impact on infectious disease transmission. Aerosol Sci. Technol. 2009;43(5):466–485. doi: 10.1080/02786820902736658. [DOI] [Google Scholar]
- 51.Xu C., Nielsen P.V., Gong G., Liu L., Jensen R.L. Measuring the exhaled breath of a manikin and human subjects. Indoor Air. 2015;25(2):188–197. doi: 10.1111/ina.2015.25.issue-210.1111/ina.12129. [DOI] [PubMed] [Google Scholar]
- 52.Villafruela J.M., Olmedo I., José J.S. Influence of human breathing modes on airborne cross infection risk. Build. Environ. 2016;106:340–351. doi: 10.1016/j.buildenv.2016.07.005. [DOI] [Google Scholar]
- 53.Ai Z.T., Melikov A.K. Airborne spread of expiratory droplet nuclei between the occupants of indoor environments: a review. Indoor Air. 2018;28(4):500–524. doi: 10.1111/ina.2018.28.issue-410.1111/ina.12465. [DOI] [PubMed] [Google Scholar]
- 54.Alford R., Kasel J., Gerone P., Knight V. Human influenza resulting from aerosol inhalation. Proc. Soc. Exp. Biol. Med. 1966;122:800–804. doi: 10.3181/00379727-122-31255. [DOI] [PubMed] [Google Scholar]
- 55.Doremalen N.V., Bushmaker T., Morris D.H., Holbrook M., Gamble A., Williamson B.N., Tamin A., Harcourt J., Thornburg N., Gerber S., Lloyd-Smith J., Wit E.D., Munster V. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Potter J.L., Matthews L., Lemm J., Spector S. Human pulmonary secretions in health and disease. Ann. Ny. Acad. Sci. 1963;106(2):692–697. doi: 10.1111/j.1749-6632.1963.tb16677.x. [DOI] [PubMed] [Google Scholar]
- 57.Nicas M., Nazaroff W.W., Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J. Occup. Environ. Hyg. 2005;2(3):143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wan M., Chao C., Ng Y.D., Sze To G.N., Yu W.C. Dispersion of expiratory droplets in a general hospital ward with ceiling mixing type mechanical ventilation system. Aerosol. Sci. Technol. 2007;41:244–258. doi: 10.1080/02786820601146985. [DOI] [Google Scholar]
- 59.Edwards N.J., Widrick R., Potember R., Gerschefske M. Quantifying respiratory airborne particle dispersion control through improvised reusable masks: The physics of non-pharmaceutical interventions for reducing SARS-CoV-2 (COVID-19) airborne transmission. J. Nanomater. 2020;1(3):116–128. doi: 10.33696/Nanotechnol.1.015. [DOI] [Google Scholar]
- 60.Edwards N.J., Widrick R., Wilmes J., Breisch B., Gerschefske M., Sullivan J., Potember R., Espinoza-Calvio A. Reducing COVID-19 airborne transmission risks on public transportation buses: an empirical study on aerosol dispersion and control. medRxiv. 2021 doi: 10.1101/2021.02.25.21252220. [DOI] [Google Scholar]
- 61.Papineni R., Rosenthal F. The size distribution of droplets in the exhaled breath of healthy human subjects. J. Aerosol. Med. 1997;10(2):105–116. doi: 10.1089/JAM.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y., Ning Z., Chen Y.u., Guo M., Liu Y., Gali N.K., Sun L.i., Duan Y., Cai J., Westerdahl D., Liu X., Xu K.e., Ho K.-F., Kan H., Fu Q., Lan K.e. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 63.Morawska L., Johnson G.R., Ristovski Z.D., Hargreaves M., Mengersen K., Corbett S., Chao C.Y.H., Li Y., Katoshevski D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009;40(3):256–269. doi: 10.1016/j.jaerosci.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Armendariz A.J., Leith D. Concentration measurement and counting efficiency for the aerodynamic particle sizer 3320. J. Aerosol Sci. 2002;33(1):133–148. doi: 10.1016/S0021-8502(01)00152-5. [DOI] [Google Scholar]
- 65.Xu C., Liu W., Liu L.i., Cao S., Ren Y. Non-uniform risk assessment methods for personalized ventilation on prevention and control of COVID-19. Chinese Sci. Bull. 2021;66:465–474. doi: 10.1360/TB-2020-0830. [DOI] [Google Scholar]
- 66.Olmedo I., Nielsen P., Adana M.R., Jensen R.L., Grzelecki P. Distribution of exhaled contaminants and personal exposure in a room using three different air distribution strategies. Indoor Air. 2012;22(1):64–76. doi: 10.1111/j.1600-0668.2011.00736.x. [DOI] [PubMed] [Google Scholar]
- 67.Liu L., Li Y., Nielsen P.V., Wei J., Jensen R.L. Short-range airborne transmission of expiratory droplets between two people. Indoor Air. 2017;27(2):452–462. doi: 10.1111/ina.12314. [DOI] [PubMed] [Google Scholar]
- 68.Gao N.P., Niu J. Modeling particle dispersion and deposition in indoor environments. Atmos. Environ. 2007;41:3862–3876. doi: 10.1016/j.atmosenv.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nazaroff W.W. Indoor particle dynamics. Indoor Air. 2004;14(s7):175–183. doi: 10.1111/ina.2004.14.issue-s710.1111/j.1600-0668.2004.00286.x. [DOI] [PubMed] [Google Scholar]
- 70.Bennett D., McKone T., Evans J.S., Nazaroff W., Margni M., Jolliet O., Smith K.R. Defining intake fraction. Environ. Eng. Sci. 2002;36(9):207A–211A. doi: 10.1021/es0222770. [DOI] [PubMed] [Google Scholar]
- 71.Berlanga F., Adana M.R., Olmedo I., Villafruela J.M., José J.S., Castro F. Experimental evaluation of thermal comfort, ventilation performance indices and exposure to airborne contaminant in an airborne infection isolation room equipped with a displacement air distribution system. Energy Build. 2018;158:209–221. doi: 10.1016/j.enbuild.2017.09.100. [DOI] [Google Scholar]
- 72.Zhang B., Guo G., Zhu C., Ji Z., Lin C. Transport and trajectory of cough-induced bimodal aerosol in an air-conditioned space. Indoor Built Environ. 2020 doi: 10.1177/1420326X20941166. [DOI] [Google Scholar]
- 73.Sze To G.N., Chao C. Review and comparison between the Wells-Riley and dose‐response approaches to risk assessment of infectious respiratory diseases. Indoor Air. 2010;20:2–16. doi: 10.1111/j.1600-0668.2009.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sze To G.N., Wan M., Chao C., Wei F., Yu S.C., Kwan J. A methodology for estimating airborne virus exposures in indoor environments using the spatial distribution of expiratory aerosols and virus viability characteristics. Indoor Air. 2008;18:425–438. doi: 10.1111/j.1600-0668.2008.00544.x. [DOI] [PubMed] [Google Scholar]
- 75.Day W., Berendt R. Experimental tularemia in Macaca mulatta: relationship of aerosol particle size to the infectivity of airborne Pasteurella tularensis. Infect. Immun. 1972;51:77–82. doi: 10.1016/j.culher.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buonanno G., Stabile L., Morawska L. Estimation of airborne viral emission: Quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 2020;141:105794. doi: 10.1016/j.envint.2020.105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luo K., Lei Z., Hai Z., Xiao S., Rui J., Yang H., Jing X., Wang H., Xie Z., Luo P., Li W., Li Q., Tan H., Xu Z., Yang Y., Hu S., Chen T. Transmission of SARS-CoV-2 in Public transportation vehicles: a case study in hunan province. China. Open. Forum. Infect. Di. 2020;7 doi: 10.1093/ofid/ofaa430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khanh N.C., Thai P.Q., Quach H., Thi N.H., Dinh P.C., Duong T., Mai L., ] NghiaTú T.A., Quang L., Quang T.D., Nguyen T., Vogt F., Anh D.D. Transmission of SARS-CoV-2 during long-haul flight. Emerg Infect Dis. 2020;26:2617–2624. doi: 10.3201/eid2611.203299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang W., Marr L.C. Dynamics of airborne influenza A viruses indoors and dependence on Humidity. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0021481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chatoutsidou S.E., Lazaridis M. Assessment of the impact of particulate dry deposition on soiling of indoor cultural heritage objects found in churches and museums/libraries. J. Cult. Herit. 2019;39:221–228. doi: 10.1016/j.culher.2019.02.017. [DOI] [Google Scholar]
- 81.Xu C., Nielsen P., Liu L., Jensen R.L., Gong G. Impacts of airflow interactions with thermal boundary layer on performance of personalized ventilation. Build. Environ. 2018:13531–13541. doi: 10.1016/j.buildenv.2018.02.048. [DOI] [Google Scholar]
- 82.Pantelic J., Tham K., Licina D. Effectiveness of a personalized ventilation system in reducing personal exposure against directly released simulated cough droplets. Indoor Air. 2015;25(6):683–693. doi: 10.1111/ina.12187. [DOI] [PubMed] [Google Scholar]
- 83.Xu J., Fu S., Chao C. Performance of airflow distance from personalized ventilation on personal exposure to airborne droplets from different orientations. Indoor Built Environ. 2020 doi: 10.1177/1420326x20951245. [DOI] [Google Scholar]
- 84.Cermak R., Melikov A. Protection of occupants from exhaled infectious agents and floor material emissions in rooms with personalized and underfloor ventilation. HVAC&R Res. 2007;13:23–38. doi: 10.1080/10789669.2007.10390942. [DOI] [Google Scholar]
- 85.He Q., Niu J., Gao N., Zhu T., Wu J. CFD study of exhaled droplet transmission between occupants under different ventilation strategies in a typical office room. Build. Environ. 2011;46(2):397–408. doi: 10.1016/j.buildenv.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Melikov A.K. Personalized ventilation. Indoor Air. 2004;14(s7):157–167. doi: 10.1111/j.1600-0668.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 87.Zheng X., Qian H., Liu L. Numerical study on a new personalized ventilation system application in cross infection prevention (in Chinese) J. Cent. South. Univ. (Nat Sci Ed) 2011;42:3905–3911. doi: 10.1016/S1003-9953(10)60145-4. [DOI] [Google Scholar]
- 88.Yang J., Sekhar S., Cheong K., Raphael B. Performance evaluation of a novel personalized ventilation-personalized exhaust system for airborne infection control. Indoor Air. 2015;25(2):176–187. doi: 10.1111/ina.12127. [DOI] [PubMed] [Google Scholar]
- 89.Mirzaie M., Lakzian E., Khan A., Warkiani M., Mahian O., Ahmadi G. COVID-19 spread in a classroom equipped with partition – A CFD approach. J. Hazard. Mater. 2021;420:126587. doi: 10.1016/j.jhazmat.2021.126587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ren C., Xi C., Wang J., Feng Z., Nasiri F., Cao S., Haghighat F. Mitigating COVID-19 infection disease transmission in indoor environment using physical barriers. Sustain. Cities. Soc. 2021;74:103175. doi: 10.1016/j.scs.2021.103175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Olmedo I., Berlanga F., Villafruela J.M., Adana M.R. Experimental variation of the personal exposure in a hospital room influenced by wall heat gains. Build. Environ. 2019;154:252–262. doi: 10.1016/J.BUILDENV.2019.03.008. [DOI] [Google Scholar]












