Abstract
Background
Severe COVID-19 caused by SARS-CoV-2 should closely be cared because of the relatively high mortality rate. If SARS-CoV-2 could be cleared as soon as possible, the mortality rate might lower. In the present study, we analyzed factors which might be related to the clearance of SARS-CoV-2.
Methods
One hundred and twenty-eight severe COVID-19 cases were enrolled. All of them had been isolated and treated at Shenzhen Third People’s Hospital because they were positive for nucleic acid of SARS-CoV-2 tested by qRT-PCR. Their baseline clinical characteristics and antiviral regimens were collected and analyzed, respectively.
Results
Of the 128 enrolled severe COVID-19 cases, unfortunately 3 died. The mean viral duration of all patients was 23.5 (range 17−32) days. All patients achieved viral clearance during 9 weeks. 13.4% of patients achieved viral clearance within 2 weeks, and 63.0% of patients achieved viral clearance within 4 weeks. The combined regimens of three or more antiviral drugs, the use of invasive mechanical ventilation, and late admission might be related to the delay of viral clearance within 2 weeks. The use of arbidol, the use of invasive mechanical ventilation, and late admission might be related to the delay of viral clearance within 4 weeks. Patients often had a prolonged course of COVID-19 and hospitalization, and were more likely transferred to intensive care unit (ICU) for treatment, if they could not clear SARS-CoV-2 during 23 days.
Conclusion
Severe COVID-19 cases should be admitted to hospital as soon as possible. The combined regimens of three or more antiviral drugs might not be useful for viral clearance, and should be performed carefully and cautiously.
Keywords: Severe COVID-19, Combined regimens, Antiviral therapy, SARS-CoV-2
Introduction
The outbreak of corona virus disease 2019 (COVID-19) caused by severe acute respiratory syndrome corona virus-2 (SARS-CoV-2) has posed a serious impact on the health system worldwide. The number of confirmed COVID-19 cases and deaths dramatically increases in the world. The mortality rate of severe COVID-19 cases is relatively high because severe COVID-19 cases easily progress to life-threatening fatal clinical outcomes such as ARDS, respiratory failure, sepsis, septic shock, etc. It was reported that the median viral duration of survivors with SARS-CoV-2 infection is about 20.0 days (IQR 17.0–24.0). However, SARS-CoV-2 could be continuously detected in patients who unfortunately died of COVID-19 before they died [1]. Another study found that the viral clearance of patients in ICU is relatively slower compared with that of non-ICU patients [2]. For patients with COVID-19, the viral duration is closely related to the disease progression and the severity of the disease. Therefore, controlling SARS-CoV-2 replication and clearing it as soon as possible might improve the prognosis of patients with COVID-19.
Various antiviral drugs, such as interferon alpha, ribavirin, arbidol, chloroquine phosphate, lopinavir/ritonavir, etc., could be recommended to treat COVID-19 patients and might improve the prognosis of patients by the World Health Organization (WHO) and National Health Commission of China [[3], [4], [5]]. However, the use of these drugs is mostly based on data collected from treatment of SARS or MERS or the experimental results in vitro. Furthermore, their efficacy and safety still need further evaluating. For example, some researchers even believe that the use of lopinavir/ ritonavir could hinder viral clearance [6]. Others report that [7] the use of glucocorticoids might be related to the delay of viral clearance.
The viral duration varies with the severity of the disease. Therefore, there might be a difference in factors-related to viral clearance between mild and severe COVID-19 patients. In the present study, we enrolled 128 severe COVID-19 patients from Shenzhen Third People’s Hospital, and analyzed factors-related to the viral clearance.
Study design and participants
The retrospective study was approved by the Ethics Committee of Shenzhen Third People’s Hospital . All patients were isolated and treated at Shenzhen Third People’s Hospital which was the only designated hospital for treatment of COVID-19 cases in Shenzhen by local health authorities. Three hundred and ninety-five COVID-19 patients were included and selected. One hundred and twenty-eight patients with severe COVID-19 were enrolled (Fig. 1 ). All patients were positive for nucleic acid of SARS-COV-2 tested by qRT-PCR before they were admitted to Shenzhen Third People’s Hospital.
Fig. 1.
Enrollment and following-up of the study cohorts.
Definition
The definition of severe COVID-19, viral clearance, viral duration and discharge standards were according to Diagnosis and Treatment Guidelines of COVID-19 launched by National Health Commission of China. (http://www.nhc.gov.cn/xcs/zhengcwj/202003/4856d5b0458141fa9f376853224d41d7.shtml).
Data collection
Clinical data of the enrolled patients were extracted from the electronic medical records and collected by two doctors who were responsible for the following-up of COVID-19 cases in Shenzhen Third People’s Hospital.
Statistics analysis
Data of continuous variables in this study were expressed by median (interquartile range) or mean ± SD, and data of categorical variables were expressed by absolute numbers (%). Mann–Whitney U test was used to compare differences between continuous variables. Chi-square test or Fisher exact test was used to compare differences between categorical variables. Logistic regression was used to analyze favorable factors-related to viral clearance. Differences were considered statistically significant at a P value < 0.05. All data were analyzed using SPSS v22.00 statistical analysis software (SPSS Inc., Chicago, IL).
Results
Baseline clinical characteristics
The baseline clinical characteristics of 128 severe COVID-19 patients were shown in Table 1 . The average age of them was 58 years (range 48−65), and the average BMI was 24.39 (range 22.27–26.65). Seventy-nine (61.7%) patients were male. Eleven (8.6%) patients had a history of smoking. Seven (5.5%) patients had underlying lung diseases, including COPD, emphysema, and chronic bronchitis. Fifty-three (41.4%) patients suffered from coronary heart disease, hypertension, and arrhythmia. Eight (6.3%) patients were taking ACEI/ ARB. Twenty-six (20.3%) patients suffered from other underlying diseases such as diabetes, thyroid disease, mental disease, hepatitis B, cytomegalovirus infection, and tumor.
Table 1.
Baseline characteristics of patients with severe COVID-19.
| Overall (n = 128) | Serious cases (n = 109) | Critical cases (n = 19) | P value | |
|---|---|---|---|---|
| Age, median (IQR), y | 58 (48−65) | 56 (47−64) | 65 (59−69) | 0.006a |
| Male sex | 79 (61.7%) | 64 (58.7%) | 15 (78.9%) | 0.094 |
| Underlying pulmonary diseases | 7 (5.5%) | 5 (4.6%) | 2 (10.5%) | 0.614 |
| Underlying cardiovascular diseases | 53 (41.4%) | 43 (39.4%) | 10 (52.6%) | 0.282 |
| Other underlying diseases | 26 (20.3%) | 21 (19.3%) | 5 (26.3%) | 0.692 |
| ACEI/ARB usage | 8 (6.3%) | 7 (6.4%) | 1 (5.3%) | 1.000 |
| Smoking history | 11 (8.6%) | 9 (8.3%) | 2 (10.5%) | 1.000 |
| BMI, median (IQR) | 24.39 (22.27−26.67) | 24.46 (22.55−26.82) | 23.31 (21.16−25.41) | 0.108 |
| Symptoms | ||||
| Fever | 50 (39.1%) | 43 (39.4%) | 7 (36.8%) | 0.830 |
| Respiratory symptoms | 105 (82%) | 89 (81.7%) | 16 (84.2%) | 1.000 |
| Cough | 44 (34.4%) | 41 (37.6%) | 3 (15.8%) | 0.065 |
| Expectoration | 58 (45.3%) | 49 (45%) | 9 (47.4%) | 0.845 |
| Sore throat | 19 (14.8%) | 15 (13.8%) | 4 (21.1%) | 0.635 |
| Rhinorrhoea | 14 (10.9%) | 13 (11.9%) | 1 (5.3%) | 0.645 |
| Chest tightness | 26 (20.3%) | 21 (19.3%) | 5 (26.3%) | 0.692 |
| Shortness of breath | 38 (29.7%) | 27 (24.8%) | 11 (57.9%) | 0.004a |
| Other symptoms | 60 (46.9%) | 48 (44%) | 12 (63.2%) | 0.123 |
| Myalgia | 31 (24.2%) | 27 (24.8%) | 4 (21.1%) | 0.953 |
| Fatigue | 44 (34.4%) | 36 (33%) | 8 (42.1%) | 0.442 |
| Headache | 13 (10.2%) | 9 (8.3%) | 4 (21.1%) | 0.196 |
| Nausea and vomiting | 9 (7%) | 6 (5.5%) | 3 (15.8%) | 0.258 |
| Diarrhea | 19 (14.8%) | 17 (15.6%) | 2 (10.5%) | 0.823 |
| Fever, cough, and shortness of breath | 39 (30.5%) | 28 (25.7%) | 11 (57.9%) | 0.005a |
| Respiratory failure | 9 (7%) | 4 (3.7%) | 5 (26.3%) | 0.002a |
| Laboratory findings | ||||
| WBC count, median (IQR), ×109/L | 4.6 (3.75−5.75) | 4.45 (3.52−5.58) | 5.39 (4.27−7.00) | 0.005a |
| NEUT count, median (IQR), ×109/L | 2.83 (2.17−4.20) | 2.75 (2.05−3.49) | 4.25 (2.81−6.31) | 0.001a |
| Lymphocyte count, median (IQR), ×109/L | 1.08 (0.85−1.34) | 1.11 (0.87−1.38) | 0.88 (0.52−1.21) | 0.021a |
| Hemoglobin (Mean ± SD), g/dl | 138.45 ± 15.54 | 138.89 ± 15.15 | 135.95 ± 17.87 | 0.449 |
| CRP, median (IQR), mg/L | 40.6 (19.6−60.4) | 36.1 (12.23−58.08) | 55.30 (33.10−68.00) | 0.060 |
| hsCRP, median (IQR), mg/L | 25.25 (10.18−46.28) | 23.98 (9.51−40.79) | 34.28 (20.80−89.94) | 0.008a |
| IL-6, median (IQR), pg/mL | 19.85 (11.21−38.96) | 17.76 (10.48−30.77) | 42.98 (12.16−79.45) | 0.005a |
| PCT, median (IQR), μg/L | 0.057 (0.037−0.082) | 0.053 (0.034−0.074) | 0.085 (0.064−0.198) | 0.000a |
| ESR, median (IQR), mm/h | 41 (25−60) | 40 (25−58.75) | 52.00 (30.00−70.00) | 0.226 |
| Numbers of affected lobes | 6 (5−6) | 6 (5−6) | 6 (5−6) | 0.765 |
| Treatment | ||||
| Intravenous immunoglobulin therapy | 83 (64.8%) | 65 (59.6%) | 18 (94.7%) | 0.003a |
| Glucocorticoids usage | 77 (60.2%) | 60 (55%) | 17 (89.5%) | 0.005a |
| Interferon alpha atomized inhalation | 120 (93.8%) | 101 (92.7%) | 19 (100%) | 0.480 |
| Lopinavir/ritonavir | 115 (89.8%) | 96 (88.1%) | 19 (100%) | 0.239 |
| Favipiravir | 8 (6.3%) | 7 (6.4%) | 1 (5.3%) | 1.000 |
| Ribavirin | 54 (42.2%) | 43 (39.4%) | 11 (57.9%) | 0.133 |
| Oseltamivir | 26 (20.3%) | 21 (19.3%) | 5 (26.3%) | 0.692 |
| Arbidol | 40 (31.3%) | 32 (29.4%) | 8 (42.1%) | 0.269 |
| Combination of three or more antiviral drugs | 81 (63.3%) | 66 (60.6%) | 15 (78.9%) | 0.125 |
| Invasive mechanical ventilation | 58 (45.3%) | 39 (35.8%) | 19 (100%) | 0.000a |
| Treatment timing | ||||
| Time from symptoms to admission, median (IQR), d | 4 (2.25−7) | 4 (3−7) | 4 (2−10) | 0.546 |
| Outcomes | ||||
| Average virus duration, median (IQR), d | 23.5 (17−32) | 22 (17−30) | 34 (28−42) | 0.000a |
| Fever time, median (IQR), d | 7 (5−10) | 8 (5.25−10.75) | 6.5 (3−9) | 0.144 |
| Duration from illness onset to radiologic recovery, median (IQR), d | 8 (5−13) | 8 (5−12) | 16 (9−26) | 0.002a |
| Days of intensive care unit, median (IQR), d | 14.5 (10−16.75) | 10 (7.5−15.5) | 17 (14−30) | 0.012a |
| Disease course, median (IQR), d | 30.5 (23−38) | 28 (22−35) | 46 (37−53) | 0.000a |
| Hospital stay, median (IQR), d | 24 (19−33.75) | 22 (17.5−30.5) | 37 (32−46) | 0.000a |
| ARDS | 28 (21.9%) | 17 (15.6%) | 11 (57.9%) | 0.000a |
| Transfer to intensive care unit | 26 (20.3%) | 10 (9.2%) | 16 (84.2%) | 0.000a |
| Dead | 3 (2.3%) | 0 (0%) | 3 (15.8%) | 0.003a |
aP value indicated differences between non-severe patients and severe patients. P<0.05 was considered statistically significant.
Of the 128 patients, 105 (82%) patients had respiratory symptoms and 50 (39.1%) had fever. Nine (7%) patients progressed to respiratory failure during the course of COVID-19.
Although the counts of white blood cells (WBC), neutrophils, and lymphocytes of the 128 patients were in the normal range, they were close to the lower normal limit [WBC, 4.6 (3.75−5.75); neutrophils, 2.83 (2.17−4.20); lymphocytes 1.08 (0.85–1.34)]. Furthermore, the counts of CRP, hsCRP, IL-6, PCT and ESR also increased. All patients’ CT scans of chest showed pneumonia involved in an average of 6 (IQR: 5−6) lung lobes.
Eighty-three (64.8%) patients were intravenously administrated with human immunoglobulin. Seventy-seven (60.2%) patients were intravenously administrated with glucocorticoid. Eighty-one (63.3%) patients were treated with the combined regimens of three or more antiviral drugs, including interferon alpha inhalation, lopinavir/ ritonavir, favipiravir, ribavirin, oseltamivir and arbidol. Fifty-eight (45.3%) patients had to receive invasive mechanical ventilation (Fig. 1).
The average time from onset of symptoms to admission was 4 (2.25−7) days, and the average viral duration was 23.5 (17−32) days. The average duration of fever was 7 (5−10) days. The average time from onset of symptoms to radiologic recovery was 8 (5−13) days. The average duration of hospitalization was 24 (19−33.75) days. The average course of the disease was 30.5 (23−38) days. Twenty-six (20.3%) patients were transferred to the Intensive Care Unit (ICU), and the average time of staying in the ICU was 14.5 (10−16.75) days. Twenty-eight (21.9%) patients had ARDS, and 3 (2.3%) patients unfortunately died.
Cumulative viral clearance rate at different time points
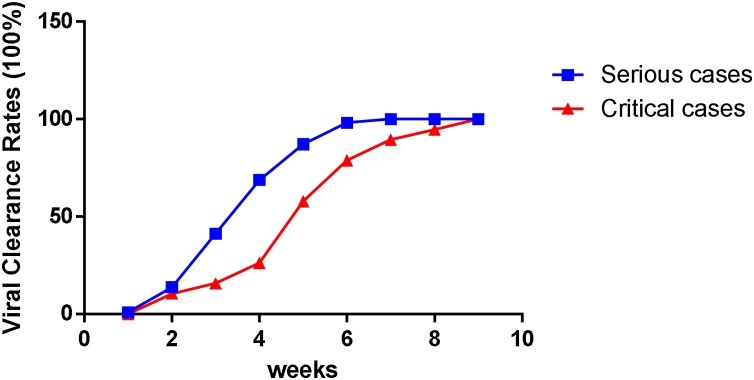
It took 9 weeks for all patients to achieve viral clearance. As shown in Table 2 , the viral clearance rates of severe COVID-19 patients within 1, 2, 3, 4, 5, 6, 7, 8, 9 weeks were 0.8, 13.3, 37.5, 62.5, 82.8, 95.3, 98.4, 99.2, 100%, respectively. It took 7 weeks or 9 weeks for serious cases or critical cases to completely achieve viral clearance, respectively. There were statistically significant differences in the viral clearance rate between serious cases and critical cases within 3, 4, 5, 6 and 7 weeks, respectively. However, there was not a statistically significant difference in the viral clearance rate between serious cases and critical cases at the other time points (Fig. 2 ).
Table 2.
Cumulative viral clearance rates at different time points.
| Time* | Cumulative virus clearance rate of overall patients (%) (n = 128) | Cumulative virus clearance rate of serious cases (%) (n = 109) | Cumulative virus clearance rate of critical cases (%) (n = 19) | P valuea |
|---|---|---|---|---|
| 1 weeks | 0.8 (1) | 0.9 (1) | 0 (0) | 1.000 |
| 2 weeks | 13.3 (17) | 13.8 (15) | 10.5 (2) | 0.701 |
| 3 weeks | 37.5 (48) | 41.3 (45) | 15.8 (3) | 0.041 |
| 4 weeks | 62.5 (80) | 68.8 (75) | 26.3 (5) | 0.001 |
| 5 weeks | 82.8 (106) | 87.2 (95) | 57.9 (11) | 0.002 |
| 6 weeks | 95.3 (122) | 98.2 (107) | 78.9 (15) | 0.004 |
| 7 weeks | 98.4 (126) | 100 (109) | 89.5 (17) | 0.021 |
| 8 weeks | 99.2 (127) | 100 (109) | 94.7 (18) | 0.148 |
| 9 weeks | 100 (128) | 100 (109) | 100 (19) |
From symptom.
P value indicated differences between non-severe patients and severe patients. P < 0.05 was considered statistically significant.
Fig. 2.
Cumulative viral clearance rates at different time points.
Then patients were divided into two groups according to age. Sixty-nine patients were included in Group A (age ≤ 60 years), and 59 patients in Group B (age > 60 years). Three patients in Group B did not survive from COVID-19. The recovery rate was 100% (69/69) in Group A, and 94.92% (56/59) in Group B (Fig. 3 ).
Fig. 3.
Recovery rates at different age groups.
The cumulative viral clearance rate of different aged subjects was shown in Fig. 4 . It took 7 weeks or 9 weeks for younger patients or old patients to completely achieve viral clearance.
Fig. 4.
Cumulative viral clearance rates at different time points.
Factors-related to viral clearance
In order to clarify factors-related to viral clearance in severe patients, logistics regression analysis was used to analyze factors-related to early viral clearance (within 2 weeks) and late viral clearance (within 4 weeks).
As shown in Table 3 , univariate logistics regression analysis showed that having underlying pulmonary diseases, the use of intravenous immunoglobulin therapy, the use of glucocorticoids, the combined regimens of three or more antiviral drugs, the use of invasive mechanical ventilation, and time from onset of symptoms to admission were related to viral clearance within 2 weeks. Then the above variables were included in the multivariate logistics regression analysis. The results showed that the combined regimens of three or more antiviral drugs (OR = 3.896, P = 0.033), the use of invasive mechanical ventilation (OR = 8.208, P = 0.013) were risk factors for early viral clearance, respectively. Time from onset of symptoms to admission was associated with viral clearance, and late admission was an unfavorable factor for early viral clearance (OR = 1.850, P = 0.003).
Table 3.
Univariate and multivariate analysis of influencing factors of viral clearance within 2 weeks in severe COVID-19 patients.
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| Virus clearance within 2 weeks (n = 16) | No virus clearance within 2 weeks (n = 112) | P value* | OR | 95%CI | P value* | |
| Age | 0.159 | |||||
| <18 yr | 0 (0%) | 0 (0%) | ||||
| 18−60 yr | 6 (37.5%) | 63 (56.3%) | ||||
| >60 yr | 10 (62.5%) | 49 (43.8%) | ||||
| Sex, male | 8 (50%) | 71 (63.4%) | 0.303 | |||
| Underlying pulmonary diseases | 3 (18.8%) | 4 (3.6%) | 0.041 | NS | ||
| Underlying cardiovascular diseases | 9 (56.3%) | 44 (39.3%) | 0.198 | |||
| Other underlying diseases | 3 (18.8%) | 23 (20.5%) | 1.000 | |||
| ACEI/ARB usage | 0 (0%) | 8 (7.1%) | 0.581 | |||
| Smoking history | 0 (0%) | 11 (9.8%) | 0.404 | |||
| Obesity | 3 (18.8%) | 18 (16.2%) | 1.000 | |||
| Fever | 9 (56.3%) | 41 (36.6%) | 0.132 | |||
| Respiratory symptoms | 13 (81.3%) | 92 (82.1%) | 1.000 | |||
| Other symptoms | 5 (31.3%) | 55 (49.1%) | 0.181 | |||
| Diarrhea | 0 (0%) | 19 (17%) | 0.159 | |||
| Myalgia | 2 (12.5%) | 29 (25.9%) | 0.242 | |||
| Cough | 7 (43.8%) | 37 (33%) | 0.399 | |||
| Expectoration | 9 (56.3%) | 49 (43.8%) | 0.347 | |||
| Shortness of breath | 1 (6.3%) | 37 (33%) | 0.057 | |||
| Chest tightness | 4 (25%) | 22 (19.6%) | 0.868 | |||
| Headache | 1 (6.3%) | 12 (10.7%) | 0.912 | |||
| Sore throat | 0 (0%) | 19 (17%) | 0.159 | |||
| Rhinorrhoea | 3 (18.8%) | 11 (9.8%) | 0.521 | |||
| Nausea and vomiting | 2 (12.5%) | 7 (6.3%) | 0.695 | |||
| Fever, cough, and shortness of breath | 2 (12.5%) | 37 (33%) | 0.168 | |||
| Fatigue | 7 (43.8%) | 37 (33%) | 0.399 | |||
| Respiratory failure | 0 (0%) | 9 (8%) | 0.514 | |||
| WBC count, median (IQR), ×109/L | 5.17 (4.08–7.43) | 4.43 (3.53–5.65) | 0.061 | |||
| NEUT count, median (IQR), ×109/L | 3.52 (2.26–5.88) | 2.79 (2.16–3.81) | 0.118 | |||
| Lymphocyte count, median (IQR), ×109/L | 1.11 (0.84-1.43) | 1.07 (0.85-1.34) | 0.986 | |||
| Anemia | 2 (12.5%) | 2 (1.8%) | 0.076 | |||
| CRP, median (IQR), mg/L | 27.15 (14.45–151.9) | 42 (22.2–60.4) | 0.827 | |||
| hsCRP, median (IQR), mg/L | 25.82 (10.60–37.13) | 24.90 (10.18–46.96) | 0.971 | |||
| IL-6, median (IQR), pg/mL | 24.17 (8.12–38.37) | 19.54 (11.21–38.96) | 0.993 | |||
| PCT, median (IQR), μg/L | 0.05 (0.033–0.078) | 0.058 (0.038–0.084) | 0.637 | |||
| ESR, median (IQR), mm/h | 43.5 (15.5–57) | 41 (27–60) | 0.819 | |||
| Numbers of affected lobes | 5 (3.25–6) | 6 (5–6) | 0.063 | |||
| Intravenous immunoglobulin therapy | 5 (31.3%) | 78 (69.6%) | 0.003 | NS | ||
| Glucocorticoids usage | 5 (31.3%) | 72 (64.3%) | 0.012 | NS | ||
| Interferon alpha inhalation | 15 (93.8%) | 105 (93.8%) | 1.000 | |||
| lopinavir/ritonavir | 13 (81.3%) | 102 (91.1%) | 0.439 | |||
| Favipiravir | 2 (12.5%) | 6 (5.4%) | 0.581 | |||
| Ribavirin | 4 (25%) | 50 (44.6%) | 0.137 | |||
| Oseltamivir | 2 (12.5%) | 24 (21.4%) | 0.618 | |||
| Arbidol | 2 (12.5%) | 38 (33.9%) | 0.084 | |||
| Combination of three or more antiviral drugsa | 6 (37.5%) | 75 (67%) | 0.022 | 3.896 | 1.117−13.591 | 0.033 |
| Invasive mechanical ventilation | 2 (12.5%) | 56 (50%) | 0.005 | 8.208 | 1.562−43.129 | 0.013 |
| Time from symptoms to admission, median (IQR), d | 2 (1−3) | 4 (3−7) | 0.000 | 1.850 | 1.236−2.770 | 0.003 |
P value indicated differences between two group. P < 0.05 was considered statistically significant.
The glucocorticoid used in this study is basically methylprednisolone, with a dose of 40−80 mg for a period of 1 week or less.
As shown in Table 4 , univariate logistics regression analysis showed that fever, PCT, the use of intravenous immunoglobulin therapy, the use of glucocorticoids, the use of arbidol, the use of invasive mechanical ventilation, and time from onset of symptoms to admission were related to viral clearance within 4weeks. Then the above variables were included in the multivariate logistics regression analysis. The results showed that the use of arbidor (OR = 3.338, P = 0.006), the use of invasive mechanical ventilation (OR = 3.820, P = 0.001) were risk factors for late viral clearance. Time from onset of symptoms to admission was associated with viral clearance, and late admission was a risk factor for late viral clearance (OR = 1.188, P = 0.003).
Table 4.
Univariate and multivariate analyses of influencing factors of viral clearance within 4 weeks in severe COVID-19 patients.
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| Virus clearance within 4 weeks (n = 79) | No virus clearance within 4 weeks (n = 49) | P value* | OR | 95%CI | P value* | |
| Age | 0.379 | |||||
| <18 yr | 0 (0%) | 0 (0%) | ||||
| 18−60 yr | 47 (57%) | 24 (49%) | ||||
| >60 yr | 34 (43%) | 25 (51%) | ||||
| Sex, male | 47 (59.5%) | 32 (65.3%) | 0.511 | |||
| Underlying pulmonary diseases | 4 (5.1%) | 3 (6.1%) | 1.000 | |||
| Cardiovascular diseases | 30 (38%) | 23 (46.9%) | 0.317 | |||
| Other underlying diseases | 17 (21.5%) | 9 (18.4%) | 0.667 | |||
| ACEI/ARB usage | 6 (7.6%) | 2 (4.1%) | 0.710 | |||
| Smoking history | 7 (8.9%) | 4 (8.2%) | 1.000 | |||
| Obesity | 14 (17.9%) | 7 (14.3%) | 0.589 | |||
| Fever | 37 (46.8%) | 13 (26.5%) | 0.022a | NS | ||
| Respiratory symptoms | 65 (82.3%) | 40 (81.6%) | 0.926 | |||
| Other symptoms | 39 (49.4%) | 21 (42.9%) | 0.473 | |||
| Diarrhea | 13 (16.5%) | 6 (12.2%) | 0.515 | |||
| Myalgia | 22 (27.8%) | 9 (18.4%) | 0.224 | |||
| Cough | 33 (41.8%) | 11 (22.4%) | 0.025a | |||
| Expectoration | 34 (43%) | 24 (49%) | 0.512 | |||
| Shortness of breath | 19 (24.1%) | 19 (38.8%) | 0.076 | |||
| Chest tightness | 17 (21.5%) | 9 (18.4%) | 0.667 | |||
| Headache | 6 (7.6%) | 7 (14.3%) | 0.359 | |||
| Sore throat | 13 (16.5%) | 6 (12.2%) | 0.515 | |||
| Rhinorrhoea | 9 (11.4%) | 5 (10.2%) | 0.834 | |||
| Nausea and vomiting | 6 (7.6%) | 3 (6.1%) | 1.000 | |||
| Fever, cough, and shortness of breath | 22 (27.8%) | 17 (34.7%) | 0.413 | |||
| Fatigue | 31 (39.2%) | 13 (26.5%) | 0.141 | |||
| Respiratory failure | 4 (5.1%) | 5 (10.2%) | 0.453 | |||
| WBC count, median (IQR), ×109/L | 4.41 (3.53–5.72) | 4.79 (3.88–5.88) | 0.433 | |||
| NEUT count, median (IQR), ×109/L | 2.76 (2.17−3.73) | 2.96 (2.19−4.34) | 0.417 | |||
| Lymphocyte count, median (IQR), ×109/L | 1.1 (0.83−1.48) | 1.06 (0.86−1.29) | 0.461 | |||
| CRP, median (IQR), mg/L | 36 (12.05−58.85) | 47 (26.73−64.90) | 0.231 | |||
| hsCRP, median (IQR), mg/L | 24.5 (10.34−47.26) | 28.72 (9.44−45.32) | 0.565 | |||
| IL-6, median (IQR), pg/mL | 18.33 (11.04−29.77) | 26.95 (11.10−54.42) | 0.167 | |||
| PCT, median (IQR), μg/L | 0.053 (0.032−0.073) | 0.063 (0.048−0.090) | 0.008a | NS | ||
| ESR, median (IQR), mm/h | 40 (22.5−60) | 44 (29−60) | 0.099 | |||
| Anemia | 2 (2.5%) | 2 (4.1%) | 0.637 | |||
| Numbers of affected lobes | 6 (4−6) | 6 (5−6) | 0.153 | |||
| Intravenous immunoglobulin therapy | 44 (55.7%) | 39 (79.6%) | 0.006a | NS | ||
| Glucocorticoids usage | 41 (51.9%) | 36 (73.5%) | 0.015a | NS | ||
| Interferon alpha atomized inhalation | 75 (94.9%) | 45 (91.8%) | 0.481 | |||
| lopinavir/ritonavir | 69 (87.3%) | 46 (93.9%) | 0.374 | |||
| Favipiravir | 6 (7.6%) | 2 (4.1%) | 0.710 | |||
| Ribavirin | 34 (43%) | 20 (40.8%) | 0.805 | |||
| Oseltamivir | 15 (19%) | 11 (22.4%) | 0.636 | |||
| Arbidol | 18 (22.8%) | 22 (44.9%) | 0.009a | 3.338 | 1.413–7.886 | 0.006a |
| Combination of three or more antiviral drugsa | 48 (60.8%) | 33 (67.3%) | 0.452 | |||
| Invasive mechanical ventilation | 26 (32.9%) | 32 (65.3%) | 0.000a | 3.820 | 1.700−8.583 | 0.001a |
| Time from symptoms to admission, median (IQR), d | 4 (2−6) | 5 (3−9.5) | 0.015a | 1.188 | 1.06−1.33 | 0.003a |
Abbreviations: BMI, body mass index; WBC, white blood cell; NEUT, Neutrophils; NS, not significant.
P value indicated differences between two group. P < 0.05 was considered statistically significant.
Comparison of clinical outcomes of COVID-19 patients with different viral duration
According to the average viral duration, severe COVID-19 patients were divided into two groups: patients with long viral duration (>23 days) and patients with short viral duration (≤23 days). As shown in Table 5 , compared with patients with short viral duration, patients with long viral duration had longer hospitalization and longer course of the disease. Furthermore, patients with long viral duration were more likely transferred to ICU for treatment [22 (33.8%) VS 4 (6.3%)].
Table 5.
Comparison of treatment outcomes between groups of different virus duration.
| ≤23 days (n = 63) | >23days (n = 65) | P value | |
|---|---|---|---|
| Days of fever, median (IQR), d | 7 (5−10) | 8 (6−11) | 0.198 |
| Duration of radiologic recovery, median (IQR), d | 8 (6−12) | 9 (5−15) | 0.265 |
| Days of ICU, median (IQR), d | 13 (10−14.5) | 15 (10−17) | 0.439 |
| Days of hospitalization, median (IQR), d | 19 (15−22) | 32 (25.5−38) | 0.000 |
| Disease course, median (IQR), d | 23 (19−28) | 38 (32.5−45) | 0.000 |
| ARDS | 13 (20.6%) | 15 (23.1%) | 0.738 |
| Transfer to intensive care unit | 4 (6.3%) | 22 (33.8%) | 0.000 |
| Dead | 0 (0%) | 3 (4.6%) | 0.244 |
Discussion
Some studies reported [[6], [7], [8]] that late admission is a risk factor for viral clearance of patients with COVID-19. In the present study, we found that for severe COVID-19 patients, late admission was also a risk factor for viral clearance. So patients with severe COVID-19 should be treated with antiviral therapy as soon as possible. However, it was still unclear which of regimens such as antiviral therapy or adjuvant therapy could accelerate the viral clearance of COVID-19 patients.
Arbidol, an antiviral drug to influenza virus, could specifically inhibit the fusion of virus to the host cell membrane, and the synthesis of viral RNA [9]. Previous studies also found that arbidol could inhibit the replication of SARS-CoV [10]. As a result, arbidol is recommended to try to treat COVID-19 by National Health Commission of China. Recently, a study [11] found that the viral clearance rate of patients treated with arbidol (16 patients) is 100% after 2 weeks of treatment course, while the viral clearance rate of those treated with lopinavir/ ritonavir (34 patients) is only 55.9%. However, another study [12] found that the median viral duration of patients treated with arbidol is 18 days, while the viral duration of the control group is 16 days. So arbidol could not helpfully accelerate viral clearance. This was in accordance with our results. Our results found that arbidol was a negative factor-related to viral clearance for patients with severe COVID-19. However, the sample size of our study was relatively small which could lead to different results. We think that the role of abidor in viral clearance of patients with severe COVID-19 should be further evaluated.
In the present study, we also found that the use of interferon alpha inhalation, lopinavir/ritonavir, favipiravir, ribavirin, and oseltamivir could not helpfully accelerate viral clearance. Previous studies also reported that the drugs above could not have an obvious effect on improving the clinical outcome of COVID-19 cases [13,14]. As a result, it should be carefully evaluated to use the antiviral drugs above to treat COVID-19 cases.
COVID-19 is a novel emerging disease, and there are not effective regimens to cure it worldwide to date. Thus, we tried to use the combined regimens of different antiviral drugs to treat patients with severe COVID-19 patients at the early epidemic of COVID-19. Because we thought that different antiviral drugs could have different mechanisms of action, the combined regimens of multiple antiviral drugs might produce synergistic effects which could helpfully improve clinical prognosis of COVID-19 cases. Unfortunately, we found that the combined regimens of three or more antiviral drugs could not promote viral clearance. Furthermore, it was reported that the combined regimens of a variety of antiviral drugs might increase the side-effects of drugs on patients [15]. As a result, it should be evaluated carefully and cautiously to use the combined regimens of several antiviral drugs to treat severe COVID-19 patients.
We also found that the use of invasive mechanical ventilation was an independent risk factor for viral clearance which was in accordance with the results of Chen et al. [6]. Patients treated with invasive mechanical ventilation had usually prolonged hospitalization which could cause them re-infection, and the delay of viral clearance.
In the present study, we also found that compared with patients with short viral duration, patients with long viral duration had a longer hospitalization and longer disease course, and were more likely to be transferred to ICU for treatment. Therefore, it was necessary to find a favorable regimen to achieve viral clearance as soon as possible in order to shorten hospitalization, the disease course and hinder the disease progression.
Our study also had some limitations. Firstly, the present study was a retrospective study and lacked an effective control group. Secondly, the present study did not determine which combined regimens of antiviral drugs could result in the delay of viral clearance. To clarify the role of antiviral drugs in viral clearance, more randomized and controlled prospective studies are required in the future.
Funding
This present study was funded by the National Clinical Research Center for Infectious Diseases, F unds for the construction of key medical disciplines in Shenzhen, and Shenzhen Key Medical Discipline Construction Fund (No. SZXK076), and the Sanming Project of Medicine in Shenzhen(SZSM201612014), and Sino-German Centerfor Research Promotion (SGC)'s Rapid Response Funding Call for Bilateral Collaborative Proposals between China and Germany in COVID-19 Related Research (No. C-0032).
Competing interests
None declared.
Ethical approval
The retrospective study was approved by the Ethics Committee of Shenzhen Third People’s Hospital.
Consent for publication
The work described has not been submitted elsewhere for publication, and all the authors listed have approved the manuscript that is enclosed. There is no ethical/legal conflict involved in the manuscript.
Availability of data and materials
If the data used to support the findings of this study are requested, please contact Corresponding authors Prof. Xingfei Pan and Prof. Fang Wang.
Authors’ contributions
Xuan Li, Xingfei Pan, Fang Wang contributed to the conception of the study; Liyang Zhou, Xingfei Pan contributed significantly to analysis and manuscript preparation; Xuan Li, Liyang Zhou, Fang Wang, Lina Zhang performed the data analyses and wrote the manuscript; Shuo Zheng, Fang Huang collected the data and helped perform the analysis with constructive discussions; Liyang Zhou carefully revised the manuscript.
Acknowledgements
Not applicable.
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80(5):e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical management of 2019 nCoV guidelines by National Health Commission of the People Republic of China. Available at: http://www.nhc.gov.cn/yzygj/s7652m/202001/7450028ab6084101ae8110f0aaf81271.shtml.
- 4.WHO. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected.
- 5.Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X., Zhang Y., Zhu B., Zeng J., Hong W., He X. medRxiv; 2020. Associations of clinical characteristics and antiviral drugs with viral RNA clearance in patients with COVID-19 in Guangzhou, China: a retrospective cohort study. [DOI] [Google Scholar]
- 7.Ling Y., Xu S.B., Lin Y.X., Tian D., Zhu Z.Q., Dai F.H. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133(9):1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu K., Chen Y., Yuan J., Yi P., Ding C., Wu W. Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2020;71(15):799–806. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vankadari N. Arbidol: a potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein. Int J Antimicrob Agents. 2020;56(2) doi: 10.1016/j.ijantimicag.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khamitov R.A., Loginova S., Shchukina V.N., Borisevich S.V., Maksimov V.A., Shuster A.M. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr Virusol. 2008;53(4):9–13. [PubMed] [Google Scholar]
- 11.Zhu Z., Lu Z., Xu T., Chen C., Yang G., Zha T. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. 2020;81(1):e21–e23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lian N., Xie H., Lin S., Huang J., Zhao J., Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. 2020;26(7):917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 14.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak — an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palleria C., Di Paolo A., Giofrè C., Caglioti C., Leuzzi G., Siniscalchi A. Pharmacokinetic drug-drug interaction and their implication in clinical management. J Res Med Sci. 2013;18(7):601–610. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
If the data used to support the findings of this study are requested, please contact Corresponding authors Prof. Xingfei Pan and Prof. Fang Wang.