Abstract
Background
Recently, emerging evidence has suggested that atrial fibrillation (AF) has an epidemiological correlation with coronavirus disease 2019 (COVID-19). However, the clinical outcomes of AF in COVID-19 remain inconsistent and inconclusive. The aim of this study was to provide a comprehensive description of the impact of AF on the prognosis of patients with COVID-19 pneumonia.
Methods
Three electronic databases (PubMed, Embase, and Web of Science) were searched for eligible studies as of March 1, 2021. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were used to evaluate the associations between AF (preexisting and new-onset) and in-hospital mortality, post-discharge mortality, and ventilator use.
Results
A total of 36 individual studies were incorporated into our meta-analysis. The combined results revealed that preexisting AF was associated with increased in-hospital mortality (pooled OR: 2.07; 95% CI: 1.60–2.67; p < 0.001), post-discharge mortality (pooled OR: 2.69; 95% CI: 1.24–5.83; p < 0.05), and ventilator utilization (pooled OR: 4.53; 95% CI: 1.33–15.38; p < 0.05) in patients with COVID-19. In addition, our data demonstrated that new-onset AF during severe acute respiratory syndrome coronavirus 2 infection was significantly correlated with increased mortality (pooled OR: 2.38; 95% CI: 2.04–2.77; p < 0.001).
Conclusions
The presence of AF is correlated with adverse outcomes in patients with COVID-19 pneumonia, which deserves increased attention and should be managed appropriately to prevent adverse outcomes.
Keywords: COVID-19, Atrial fibrillation, Arrhythmia, Mortality, Meta-analysis
1. Introduction
Over the past year, coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has swept the globe with alarming morbidity and mortality [1]. While SARS-CoV-2 primarily infects the lungs, causing various pulmonary symptoms including cough and dyspnea with severe cases progressing to acute respiratory distress syndrome and death, it also affects multiple organs, particularly in the presence of complex cardiovascular comorbidities [2]. Published reports have detailed evidence that 20%–40% of hospitalized patients present with myocarditis, arrhythmias, acute coronary syndromes, fulminant heart failure, and cardiac death [[3], [4], [5], [6]]. Acute respiratory tract infections are recognized triggers of acute exacerbations of cardiovascular diseases, while the underlying cardiovascular diseases are often associated with comorbidities, which may result in elevated infection rates and mortality [2,7].
Atrial fibrillation (AF) is the most common sustained arrhythmia worldwide, sharing with COVID-19 a higher prevalence in populations with advanced age, cardiovascular risk factors, and comorbidities [8]. Approximately 20% of COVID-19 patients have been reported to have a history of AF, and new-onset AF represents a frequent complication, especially in those with severe cases [9]. Although there is emerging evidence suggesting an epidemiological association between AF and COVID-19, the understanding of clinical outcomes and prognosis of AF in COVID-19 is inconsistent and inconclusive. Some studies have indicated that AF, particularly new-onset, is an independent predictor of worse outcomes such as in-hospital mortality, mechanical ventilation, and cardiovascular death in patients with COVID-19 pneumonia [[9], [10], [11], [12], [13]], while others hold the view that the risk of mortality and mechanical ventilation are comparable with and without AF [[14], [15], [16]]. Given the ongoing controversial findings, it is necessary to perform a meta-analysis to systematically and comprehensively understand the impact of AF incidence on the outcomes of patients with COVID-19.
The present study aimed to thoroughly summarize and evaluate the effects of AF (delineated as preexisting and new-onset) on clinical outcomes (in-hospital mortality, post-discharge mortality, and ventilator use) in patients with COVID-19.
2. Material and methods
2.1. Search strategy
This meta-analysis was performed following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [17] (Supplementary Material 1). We systematically searched three electronic databases (PubMed, Embase, and Web of Science) for potential studies that investigated the outcomes of AF in COVID-19 from inception to March 1, 2021. The primary search terms were “atrial fibrillation” and “COVID-19” or “severe acute respiratory syndrome coronavirus 2”. The search strategy for each electronic database was designed by an experienced medical librarian (Hai-bo, Zhou), and all steps of the search and study selection process were carried out by two independent investigators (Ming-yue Chen and Fang-ping Xiao). We also searched pertinent publications' reference lists for additional studies. Duplicate results were removed. No restrictions were placed on the type of study design included, but only studies published in English were eligible for inclusion in the meta-analysis.
2.2. Inclusion and exclusion criteria
A preliminary screening of titles and abstracts was carried out by two independent investigators, followed by a detailed reading of the full text to further assess if the articles met the inclusion criteria. Inclusion criteria consisted of the following: (i) observational studies enrolling patients with AF with a diagnosis of COVID-19; (ii) studies containing specific outcomes of interest (in-hospital mortality, post-discharge mortality, and ventilator use); and (iii) studies published in English. Studies were excluded if they contained the following: (i) review articles, case reports, communications, non-research letters, and commentaries; (ii) studies with samples <20; (iii) repeated population studies; and (iv) insufficient data to extract or estimate odds ratios (ORs) and 95% confidence intervals (CIs) of relevant outcomes of interest.
2.3. Data extraction and quality assessment
All candidate studies were independently evaluated by two researchers, and any discrepancies were resolved by a third reviewer (Lin Kuai). For each eligible study, the following data items were recorded: first author, publication year, setting, study design, total number of cases and gender, average study population age, and relevant outcome data (in-hospital mortality, post-discharge mortality, and ventilator use). Quality assessments were measured by two independent investigators using the Newcastle-Ottawa Scale (NOS) [18]. The NOS consists of three parts: selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points). A study with a cumulative score of 6 or higher was considered high quality.
2.4. Statistical analysis
The pooled OR along with the corresponding 95% CI was calculated to analyze the correlation between AF and poor outcomes (in-hospital mortality, post-discharge mortality, and ventilator use) in patients with COVID-19 pneumonia. Adjusted ORs were directly utilized if they were reported in the candidate papers; otherwise, unadjusted ORs were estimated via crude data provided in the studies. An OR > 1 and 95% CI that did not contain the value 1 indicated a worse prognosis in COVID-19 patients with AF. The heterogeneity of the studies was determined using Cochran's Q test and the Higgins I-squared statistic, with a Ph < 0.10 or I 2 > 50% indicating significant heterogeneity [19]. A random-effects model was applied if substantial heterogeneity was observed and a fixed-effects model was adopted in all other cases. Subgroup analysis, sensitivity analysis, and meta-regression were used to investigate and clarify the heterogeneity among the studies. The possibility of publication bias was assessed by Begg and Egger tests, and p-values <0.05 were considered statistically significant [20,21]. All statistical analyses were conducted using STATA version 16.0 (StataCorp, College Station, TX).
3. Results
3.1. Study characteristics
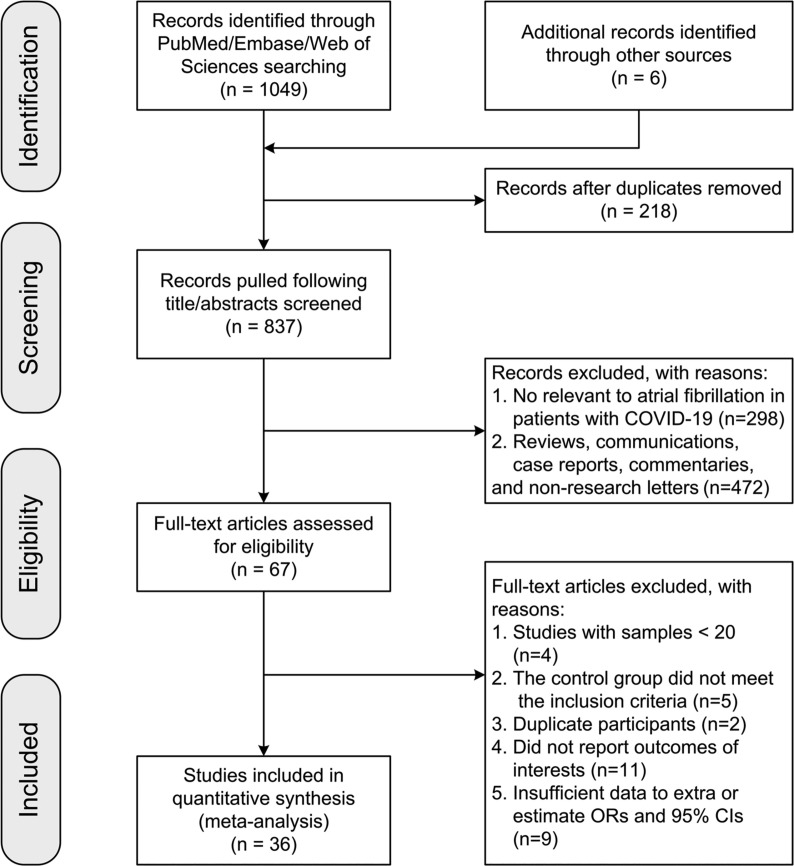
A total of 1055 papers were initially identified. After eliminating duplicates and carefully inspecting for inclusion and exclusion criteria, 36 studies published between 2020 and 2021 were included in our meta-analysis [[8], [9], [10], [11], [12], [13], [14], [15], [16],[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48]]. The flow diagram in Fig. 1 depicts the detailed study selection process. Of the 36 studies included, 12 were conducted in the United States [10,14,15,22,24,25,28,31,36,39,44,45], eight in Italy [8,9,16,32,38,42,43,48], four in Spain [13,23,34,46], four in the United Kingdom [26,35,40,41], three in Turkey [12,27,47], two in China [29,33], and one in Denmark [30], France [37], and Germany [11], respectively. Sixteen studies reported ORs and 95% CIs directly [9,14,15,22,23,26,28,29,31,34,[39], [40], [41], [42],44,48], while the ORs and 95% CIs of all others were estimated via the crude data provided in the article [8,[10], [11], [12], [13],16,24,25,27,30,32,33,[35], [36], [37], [38],43,[45], [46], [47]]. Fourteen of these were single-center studies [8,[12], [13], [14], [15],24,[31], [32], [33],36,40,[46], [47], [48]], and 22 were multicenter studies [[9], [10], [11],16,22,23,[25], [26], [27], [28], [29], [30],34,35,[37], [38], [39],[41], [42], [43], [44], [45]]. Thirty-two studies were retrospective [8,9,12,[14], [15], [16],[22], [23], [24], [25], [26], [27], [28], [29], [30], [31],34,[36], [37], [38], [39], [40], [41], [42], [43], [44]], and the remaining four studies were prospective [13,32,33,35]. In 23 studies, the NOS score was ≥6 [[8], [9], [10],[12], [13], [14], [15], [16],[22], [23], [24],[26], [27], [28], [29],31,32,35,[40], [41], [42],44,48], and in 13 the NOS score was <6 [11,25,30,33,34,[36], [37], [38], [39],43,[45], [46], [47]]. The primary characteristics of the 36 studies are presented in Table 1 .
Fig. 1.
Flowchart of study selection process. Abbreviations: COVID-19: coronavirus disease 2019; ORs: odds ratios; 95% CIs: 95% confidence intervals.
Table 1.
Study characteristics and quality assessment
| Study ID | Year | Setting | Study design | Patients with COVID-19 (M/F) | Age (median and range) (AF vs no AF) | ORs | NOS score (S C O) |
|---|---|---|---|---|---|---|---|
| Peltzer et al. [22] | 2020 | Multicenter, USA | Retrospective | 1053 (653/400) | 74.5 (± 13.0) vs 60.1 (± 17.0) | Adjusted | 3 2 3 |
| Rodilla et al. [23] | 2020 | Multicenter, Span | Retrospective | 12,226 (7018/5208) | 67.5 ± 16.1 (overall) | Adjusted | 3 2 1 |
| Inciardi et al. [8] | 2020 | Single center, Italy | Retrospective | 99 (80/19) | 67 ± 12 (overall) | Unadjusted | 3 0 3 |
| Russo et al. [16] | 2020 | Multicenter, Italy | Retrospective | 414 (253/161) | 65.5 (± 15.5) vs 73.7 (± 9.9) | Unadjusted | 3 1 3 |
| Bhatla et al. [24] | 2020 | Single center, USA | Retrospective | 700 (314/386) | N/A | Unadjusted | 3 0 3 |
| Yamada et al. [25] | 2020 | Multicenter, USA | Retrospective | 210 (N/A) | N/A | Unadjusted | 1 1 3 |
| Atkins et al. [26] | 2020 | Multicenter, UK | Retrospective | 507 (311/196) | 74.3 ± 4.5 (overall) | Adjusted | 2 2 3 |
| Quisi et al. [27] | 2020 | Multicenter, Turkey | Retrospective | 349 (153/196) | N/A | Unadjusted | 3 1 3 |
| Elias et al. [28] | 2020 | Multicenter, USA | Retrospective | 1258 (685/573) | 61.6 ± 18.4 (overall) | Adjusted | 2 2 3 |
| Wang et al. [29] | 2020 | Multicenter, China | Retrospective | 319 (152/167) | 64.97 ± 13.15 (overall) | Adjusted | 3 2 2 |
| Reilev et al. [30] | 2020 | Multicenter, Denmark | Retrospective | 11,122 (4693/6429) | N/A | Unadjusted | 3 1 1 |
| Gerwen et al. [15] | 2020 | Single center, USA | Retrospective | 3703 (2048/1655) | 56.8 ± 18.2 (overall) | Adjusted | 3 2 2 |
| Peterson et al. [14] | 2020 | Single center, USA | Retrospective | 355 (181/174) | 66.21 ± 14.21 (overall) | Adjusted | 2 2 3 |
| Mccullough et al. [31] | 2020 | Single center, USA | Retrospective | 754 (478/278) | 63.3 ± 16.0 (overall) | Adjusted | 3 2 2 |
| Ghio et al. [32] | 2020 | Single center, Italy | Prospective | 405 (278/127) | N/A | Unadjusted | 2 1 3 |
| Li et al. [33] | 2020 | Single center, China | Prospective | 113 (68/45) | 67.3 ± 14.1 (overall) | Unadjusted | 3 1 1 |
| Rodríguez-Molinero et al. [34] | 2020 | Multicenter, Span | Retrospective | 418 (238/180) | 65.4 ± 16.6 (overall) | Adjusted | 2 2 1 |
| Clift et al. [35] | 2020 | Multicenter, UK | Prospective | 6,083,102 (3,035,409/3047693) | N/A | Unadjusted | 3 1 2 |
| Shah et al. [36] | 2020 | Single center, USA | Retrospective | 487 (N/A) | 68.42 ± 16.70 (overall) | Unadjusted | 3 1 1 |
| Lano et al. [37] | 2020 | Multicenter, France | Retrospective | 122 (79/43) | 73.5 (64.2–81.2) | Unadjusted | 3 1 1 |
| Polverino et al. [38] | 2020 | Multicenter, Italy | Retrospective | 3179 (2171/1008) | 69.0 (57–78) | Unadjusted | 3 1 1 |
| Izurieta et al. [39] | 2020 | Multicenter, USA | Retrospective | 25,333,329 (N/A) | N/A | Adjusted | 2 2 1 |
| Gue et al. [40] | 2020 | Single center, UK | Retrospective | 316 (188/128) | N/A | Adjusted | 3 2 2 |
| Perez-Guzman et al. [41] | 2020 | Multicenter, UK | Retrospective | 614 (382/232) | 69 ± 25 (overall) | Adjusted | 3 2 1 |
| Canevelli et al. [42] | 2020 | Multicenter, Italy | Retrospective | 415 (219/196) | 84.3 ± 8.1 (overall) | Adjusted | 2 2 3 |
| Rossi et al. [43] | 2020 | Multicenter, Italy | Retrospective | 590 (399/191) | 76.2 (68.2–82.6, overall) | Unadjusted | 3 0 2 |
| Alvarez-Garcia et al. [44] | 2020 | Multicenter, USA | Retrospective | 6439 (3547/2892) | 63.5 ± 18 (overall) | Adjusted | 2 2 2 |
| Spinoni et al. [9] | 2021 | Multicenter, Italy | Retrospective | 637 (N/A) | N/A | Adjusted | 3 2 1 |
| Özdemir et al. [12] | 2021 | Single center, Turkey | Retrospective | 350 (194/156) | 76 (64–82) vs 51 (37–65) | Unadjusted | 4 1 2 |
| Sanz et al. [13] | 2021 | Single center, Spain | Prospective | 160 (96/64) | 75.9 (± 9.6) vs 64.9 (± 16.3) | Unadjusted | 4 1 1 |
| Mountantonakis et al. [10] | 2021 | Multicenter, USA | Retrospective | 2476 (1551/925) | 73.1 (± 13.5) vs 73.6 (± 13.3) | Unadjusted | 3 2 1 |
| Poterucha et al. [45] | 2021 | Multicenter, USA | Retrospective | 887 (N/A) | N/A | Unadjusted | 3 0 1 |
| García-Granj et al. [46] | 2021 | Single center, Span | Retrospective | 517 (290/227) | 81.6 (± 8.7) vs 66.5 (± 14.9) | Unadjusted | 3 1 1 |
| Kelesoglu et al. [47] | 2021 | Single center, Turkey | Retrospective | 658 (372/286) | 54 ± 14 (overall) | Unadjusted | 3 1 1 |
| Denegri et al. [48] | 2021 | Single center, Italy | Retrospective | 201 (N/A) | N/A | Adjusted | 3 2 1 |
| Zylla et al. [11] | 2021 | Multicenter, Germany | Retrospective | 166 (108/58) | 64.1 ± 16.7 (overall) | Unadjusted | 3 1 1 |
COVID-19: coronavirus disease 2019; M/F: Male/Female; AF: atrial fibrillation; OR: odds ratio; NOS: Newcastle-Ottawa scale; S: selection; C: comparability; O: outcome; N/A: not available.
3.2. Preexisting AF and in-hospital mortality
Twenty studies investigated the impact of preexisting AF on in-hospital mortality in COVID-19 patients [8,[10], [11], [12],14,16,23,24,27,29,[32], [33], [34],[36], [37], [38], [39],41,44,46]. Given the significant heterogeneity (I 2 = 91.3%, pH < 0.001) among the included studies, a random-effects model was applied. Our results revealed that preexisting AF in patients with COVID-19 pneumonia was significantly correlated with increased in-hospital mortality, with a pooled OR of 2.07 (95% CI: 1.60–2.67; p < 0.001; Fig. 2 ). In subgroup analysis by study setting, the pooled ORs for in-hospital mortality were 2.15 (95% CI: 1.17–2.67; p < 0.05) for single-center studies and 2.01 (95% CI: 1.51–2.68; p < 0.001) for multicenter studies. After stratification by sample size (< 200 and ≥ 200 participants), the combined ORs were 3.50 (95% CI: 1.98–6.17; p < 0.01) and 1.95 (95% CI: 1.50–2.54; p < 0.0001), respectively. Subgroup analysis based on extraction method found pooled ORs of 1.34 (95% CI: 1.01–1.78; p < 0.05) with the direct method and 2.49 (95% CI: 1.99–3.12; p < 0.001) with the indirect method. In addition, the combined ORs of NOS < 6 and NOS ≥ 6 were 2.47 (95% CI: 1.41–4.33; p < 0.01) and 1.85 (95% CI: 1.37–2.51; p < 0 0.001), respectively. Further information on the heterogeneity of each subgroup and the values calculated by the fixed-effects model are summarized in Table 2 .
Fig. 2.
Meta-analysis of the association between preexisting atrial fibrillation and in-hospital mortality in COVID-19 pneumonia. Results are presented as pooled ORs with 95% CIs. Abbreviations: OR: odds ratio; 95% CI: 95% confidence interval.
Table 2.
Subgroup analysis of in-hospital mortality
| Analysis | N | R-OR (95% CI) | P | F-OR (95% CI) | P | I2 | Ph |
|---|---|---|---|---|---|---|---|
| In-hospital mortality | 20 | 2.07 (1.60, 2.67) | 0 | 1.10 (1.60, 2.67) | 0 | 91.3% | 0.000 |
| Subgroup 1: Single center | 7 | 2.15 (1.17, 2.67) | 0.014 | 2.15 (1.58, 2.92) | 0 | 70.2% | 0.003 |
| Multicenter | 13 | 2.01 (1.51, 2.68) | 0.000 | 1.09 (1.04, 1.13) | 0 | 93.3% | 0.000 |
| Subgroup 2: Sample size<200 | 4 | 3.50 (1.98, 6.17) | 0.003 | 3.50 (1.98, 6.17) | 0 | 0.1% | 0.391 |
| Sample size ≥ 200 | 16 | 1.95 (1.50, 2.54) | 0 | 1.10 (1.05, 1.14) | 0 | 92.4% | 0.000 |
| Subgroup 3: Univariate analysis | 13 | 2.49 (1.99, 3.12) | 0 | 2.19 (1.95, 2.46) | 0 | 46.9% | 0.032 |
| Multivariate analysis | 7 | 1.34 (1.01, 1.78) | 0.040 | 1.00 (0.96, 1.05) | 0.888 | 87.1% | 0.000 |
| Subgroup 4: NOS<6 | 8 | 2.47 (1.41, 4.33) | 0.002 | 1.02 (0.98, 1.07) | 0.353 | 93.1% | 0.000 |
| NOS ≥ 6 | 12 | 1.85 (1.37, 2.51) | 0 | 1.52 (1.38, 1.66) | 0 | 81.7% | 0.000 |
N: number of studies; R-OR: odds ratio calculated by random-effects model; F-OR: odds ratio calculated by fixed-effects model; 95% CI: 95% confidence interval; pH: P values of Q test for heterogeneity.
To further explore potential sources of heterogeneity, a sensitivity analysis was subsequently conducted. We excluded each eligible study sequentially to evaluate the influence of individual studies on the overall effect estimates. The result of sensitivity analysis suggested that no single study materially impacted the pooled summary effect, which increased the credibility (Fig. 3 ). Additionally, meta-regression analysis showed that study setting (p > |z| = 0.961), cases (p > |z| = 0.318), and NOS scores (p > |z| = 0.475) did not significantly impact the heterogeneity of the pooled result, while the extraction methods (p > |z| = 0.022) may have significantly contributed to the heterogeneity. However, according to the results of subgroup analysis based on extraction method (i.e., for both direct and indirect extraction, the combined OR > 1 and 95% CI did not contain the value 1), we considered the result of our meta-analysis to be reliable and stable.
Fig. 3.
Sensitivity analysis of the relationship between preexisting atrial fibrillation and in-hospital mortality in COVID-19 pneumonia.
3.3. Preexisting AF and post-discharge mortality
Fourteen studies compared the post-discharge mortality of COVID-19 patients with and without preexisting AF [9,15,22,25,26,28,30,31,35,40,42,43,45,48]. The merged OR of post-discharge mortality was 2.69 (95% CI: 1.24–5.83; p < 0.05; Fig. 4 ), and this result showed significant heterogeneity (I 2 = 98.5%, pH < 0.001). To explore sources of heterogeneity, meta-regression was applied and the result demonstrated a significant effect of extraction method on the heterogeneity (p > |z| = 0.005), while the study setting (p > |z| = 0.493) and NOS scores (p > |z| = 0.267) were determined to be unimportant effect modifiers. After stratification by extraction method, the merged ORs were 1.60 (95% CI: 1.13–2.25; p < 0.01) with the direct method and 7.54 (95% CI: 3.18–17.88; p < 0.001) with the indirect method, suggesting the result was stable.
Fig. 4.
Meta-analysis of the association between preexisting atrial fibrillation and post-discharge mortality in COVID-19 pneumonia. Results are presented as pooled ORs with 95% CIs. Abbreviations: OR: odds ratio; 95% CI: 95% confidence interval.
3.4. Preexisting AF and ventilator use
Six studies reported data on ventilator use associated with preexisting AF in COVID-19 patients [11,12,15,29,45,46]. The combined OR of ventilator use across these studies was 4.53 (95% CI: 1.33–15.38; p < 0.05; Fig. 5 ), with clear heterogeneity (I 2 = 94.6%, pH < 0.001). Meta-regression analysis revealed that the extraction methods (p > |z| = 0.744), cases (p > |z| = 0.342), and NOS scores (p > |z| = 0.319) did not contribute to heterogeneity between the included studies, with only the study setting identified as a potential major source of heterogeneity (p > |z| = 0.000). Subgroup analysis based on study setting showed the pooled ORs for ventilator use were 1.52 (95% CI: 0.76–3.07; p > 0.05) for single-center and 14.48 (95% CI: 6.51–32.22; p < 0.001) for multicenter studies.
Fig. 5.
Meta-analysis of the association between preexisting atrial fibrillation and ventilator use in COVID-19 pneumonia. Results are presented as pooled ORs with 95% CIs. Abbreviations: OR: odds ratio; 95% CI: 95% confidence interval.
3.5. New-onset AF and mortality
Seven studies evaluated the impact of new-onset AF on mortality (including in-hospital and post-discharge mortality) in patients with COVID-19 pneumonia [9,10,13,16,22,45,47]. Owing to the fact that the heterogeneity among the studies was not significant (I 2 = 42.0%, pH = 0.111), a fixed-effects model was applied for statistical analysis. The pooled results suggested a meaningful correlation between new-onset AF and increased mortality in patients with COVID-19 pneumonia (pooled OR: 2.38; 95% CI: 2.04–2.77; p < 0.001; Fig. 6 ).
Fig. 6.
Meta-analysis of the association between new-onset atrial fibrillation and mortality (including in-hospital and post-discharge mortality) in COVID-19 pneumonia. Results are presented as pooled ORs with 95% CIs. Abbreviations: OR: odds ratio; 95% CI: 95% confidence interval.
3.6. Publication bias
Publication biases were assessed using Begg and Egger tests. For in-hospital mortality in patients with preexisting AF and COVID-19 pneumonia, we detected publication bias among the 20 included trials (Begg Pr > |z| = 0.770 and Egger Pr > |t| = 0.000). An Egger's publication bias plot is illustrated in Supplementary Fig. 1. The trim-and-fill method was performed to enroll missing studies, and the adjusted random-effects pooled OR of 1.794 (95% CIs: 1.411–2.280; Supplementary Fig. 2), was consistent with our primary analysis. For the meta-analysis of preexisting AF and post-discharge mortality in COVID-19 patients, although the Egger test indicated potential publication bias (Begg Pr > |z| = 0.443 and Egger Pr > |t| = 0.003), no study was imputed via the trim-and-fill method, and the adjusted random-effects pooled ORs remained 2.69 (95% CI: 1.24–5.83; p < 0.05). Furthermore, there was no evidence of publication bias for the analysis of preexisting AF and ventilator use (Begg Pr > |z| = 0.707 and Egger Pr > |t| = 0.166) or new-onset AF and mortality (Begg Pr > |z| = 1.000 and Egger Pr > |t| = 0.744).
4. Discussion
Since the beginning of the COVID-19 pandemic, a series of studies have reported data on the prognostic impact of AF on COVID-19 patients, though the results of these trials have remained inconsistent and inconclusive. Thus, we conducted this meta-analysis to synthesize all eligible studies into a more comprehensive understanding of the impact of AF (preexisting and new-onset) on the clinical outcomes of COVID-19 patients. The results of the current study revealed that preexisting AF is associated with increased in-hospital mortality (pooled OR: 2.07; 95% CI: 1.60–2.67; p < 0.001), post-discharge mortality (pooled OR: 2.69; 95% CI: 1.24–5.83; p < 0.05), and ventilator utilization (pooled OR: 4.53; 95% CI: 1.33–15.38; p < 0.05) in patients with COVID-19. In addition, our data demonstrated that new-onset AF in the context of COVID-19 pneumonia is correlated with increased mortality (pooled OR: 2.38; 95% CI: 2.04–2.77; p < 0.001).
The current meta-analysis incorporated four prospective studies and 32 retrospective studies, most of which reported poorer outcomes in COVID-19 patients presenting with AF than those without AF. A literature review published recently elaborated on the incidence, potential mechanisms, and clinical implications of AF in COVID-19 patients, suggesting that SARS-CoV-2 infection may increase susceptibility to AF and even worsen existing AF [49]. An earlier review summarized the possible mechanisms behind the association between AF and COVID-19 infection, detailing the contributions of myocardial microvascular pericytes, angiotensin, pulmonary hypertension, and regulatory T cells to COVID-19 [50]. Furthermore, Yang et al. performed a meta-analysis of studies published up to December 24, 2020, to explore the effect of AF on mortality in COVID-19, and the results supported a significant association between AF and increased mortality in patients with COVID-19 [51]. In 2021, a series of related papers have been published with clinical outcomes diversified beyond mortality. Our study incorporated these newly published studies and classified clinical outcomes into in-hospital mortality, post-discharge mortality, and ventilator use to explore their association with preexisting AF. Simultaneously, we reviewed studies that investigated new-onset AF during SARS-CoV-2 infection and demonstrated that new-onset AF was significantly associated with increased COVID-19 mortality, a finding that was not available in previous meta-analyses.
As one of the most frequent cardiac arrhythmias, the prevalence of AF in the general population is approximately 0.4% to 1.0% [52], while in COVID-19 patients, the prevalence is even higher. A recent study conducted in northern Italy on 99 patients hospitalized with COVID-19 reported a prevalence of AF of 19%, increasing to 36% in patients with other cardiac diseases and 42% in deaths [8]. Another study performed in New York hospitals showed that of 1258 hospitalized COVID-19 patients, 14.3% were complicated by preexisting AF, and 10.1% with no history of AF experienced new-onset AF after admission. [53]. According to a small study by Fumagalli et al., an estimated 75% of geriatric patients present with a history of AF [54]. Furthermore, Wang et al. found that AF was present in 22% of critically ill patients who required mechanical ventilation [55]. AF shares with COVID-19 the risk factors of advanced age, cardiovascular conditions, and comorbidities. It has also been reported that during hospitalization with pneumonia, the increase of serum inflammatory cytokines and presence of acute metabolic disorders can trigger AF, particularly new-onset AF [13]. The inflammatory cytokine storm also contributes to COVID-19 patients' deterioration and accelerates the progression to severe pneumonia, multiple organ failure, or death. The connection between COVID-19-associated myocardial injury and AF may be manifested in any combination of contributors including systemic coagulation disorder, inflammation, stress cardiomyopathy, hypoxemia, and direct viral heart injury, which indicates that the impact the presence of AF has on COVID-19 patients goes beyond the category of simple arrhythmia [56]. Based on the current results, the presence of AF appears to act as a marker of elevated infection rates, and to some extent, to predict a worse prognosis. However, given that most of the studies we included were retrospective and there was a risk of residual confusion, further causality needs to be established by reliable prospective cohort studies.
Several limitations must be acknowledged in our meta-analysis. First, the quality of the reported data was relatively low, with most of the studies being retrospective in design, making them more vulnerable to bias. Second, there was significant heterogeneity in the combined results of preexisting AF and ventilator use. Although meta-regression revealed study setting to be the source of heterogeneity, the 95% CI of the pooled effect estimate of single-center contained the value 1, which indicates that more prominent and reliable studies are needed to confirm their correlation. Third, subgroup analysis based on the extraction method found a pooled OR of 1.34 (95% CI: 1.01–1.78; p < 0.05) with multivariate analysis, indicating that the correlation between preexisting AF and in-hospital mortality was not very strong, for the number of enrolled studies was relative small, further studies are needed to confirm that AF is an independent risk factor. Fourth, too few published qualified studies to evaluate the correlation between new-onset AF and ventilator use, so we did not supply relevant parameters in the paper. Fifth, when the number of studies compiled in the meta-analyses is less than 10, the power of Begg and Egger tests is decreased substantially, which may lead to some undetected publication bias. Finally, only articles published in English were incorporated into our study, leaving the possibility that pertinent studies published in other languages were excluded.
5. Conclusion
Collectively, our study revealed that preexisting AF was associated with increased in-hospital mortality, post-discharge mortality, and ventilator use in COVID-19 patients, which suggests that preexisting AF predicts adverse prognosis to some extent. Moreover, we demonstrated that new-onset AF was significantly connected with increased mortality during SARS-CoV-2 infection. The current findings support a correlation between AF and adverse outcomes in patients with COVID-19 pneumonia; however, additional reliable studies are needed to further confirm that AF is an independent risk factor.
Availability of data and materials
Original data is available from the corresponding author on reasonable request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajem.2021.09.050.
Appendix A. Supplementary data
The following are the supplementary data related to this article.
Supplementary Fig. 1.
Egger's publication bias plot for in-hospital mortality.
Supplementary Fig. 2.
Filled funnel plots for publication bias test of in-hospital mortality.
Supplementary material 1
References
- 1.Lerner A.M., Robinson D.A., Yang L., Williams C.F., Newman L.M., Breen J.J., et al. Toward Understanding COVID-19 Recovery: National Institutes of Health Workshop on Postacute COVID-19. Ann Intern Med. 2021;174:999–1003. doi: 10.7326/M21-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inciardi R.M., Adamo M., Lupi L., Cani D.S., Di Pasquale M., Tomasoni D., et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spinoni E.G., Mennuni M., Rognoni A., Grisafi L., Colombo C., Lio V., et al. Contribution of Atrial Fibrillation to In-Hospital Mortality in Patients With COVID-19. Circ Arrhythm Electrophysiol. 2021;14 doi: 10.1161/CIRCEP.120.009375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mountantonakis S.E., Saleh M., Fishbein J., Gandomi A., Lesser M., Chelico J., et al. Atrial fibrillation is an independent predictor for in-hospital mortality in patients admitted with SARS-CoV-2 infection. Heart Rhythm. 2021;18:501–507. doi: 10.1016/j.hrthm.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zylla M.M., Merle U., Vey J.A., Korosoglou G., Hofmann E., Müller M., et al. Predictors and prognostic implications of cardiac arrhythmias in patients hospitalized for COVID-19. J Clin Med. 2021;10 doi: 10.3390/jcm10010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Özdemir İ.H., Özlek B., Çetin N. Permanent atrial fibrillation portends poor outcomes in hospitalized patients with COVID-19: a retrospective observational study. J Electrocardiol. 2021;65:113–120. doi: 10.1016/j.jelectrocard.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardo Sanz A., Salido Tahoces L., Ortega Pérez R., González Ferrer E., Sánchez R.Á., Zamorano Gómez J.L. New-onset atrial fibrillation during COVID-19 infection predicts poor prognosis. Cardiol J. 2021;28:34–40. doi: 10.5603/CJ.a2020.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson E., Lo K.B., DeJoy R., Salacup G., Pelayo J., Bhargav R., et al. The relationship between coronary artery disease and clinical outcomes in COVID-19: a single-center retrospective analysis. Coron Artery Dis. 2021;32:367–371. doi: 10.1097/MCA.0000000000000934. [DOI] [PubMed] [Google Scholar]
- 15.van Gerwen M., Alsen M., Little C., Barlow J., Genden E., Naymagon L., et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93:907–915. doi: 10.1002/jmv.26337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo V., Di Maio M., Attena E., Silverio A., Scudiero F., Celentani D., et al. Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with COVID-19: a multicenter observational study. Pharmacol Res. 2020;159:104965. doi: 10.1016/j.phrs.2020.104965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 22.Peltzer B., Manocha K.K., Ying X., Kirzner J., Ip J.E., Thomas G., et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J Cardiovasc Electrophysiol. 2020;31:3077–3085. doi: 10.1111/jce.14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodilla E., Saura A., Jiménez I., Mendizábal A., Pineda-Cantero A., Lorenzo-Hernández E., et al. Association of hypertension with all-cause mortality among hospitalized patients with COVID-19. J Clin Med. 2020;9 doi: 10.3390/jcm9103136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatla A., Mayer M.M., Adusumalli S., Hyman M.C., Oh E., Tierney A., et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada T., Mikami T., Chopra N., Miyashita H., Chernyavsky S., Miyashita S. Patients with chronic kidney disease have a poorer prognosis of coronavirus disease 2019 (COVID-19): an experience in New York City. Int Urol Nephrol. 2020;52:1405–1406. doi: 10.1007/s11255-020-02494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atkins J.L., Masoli J., Delgado J., Pilling L.C., Kuo C.L., Kuchel G.A., et al. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J Gerontol A Biol Sci Med Sci. 2020;75:2224–2230. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quisi A., Alıcı G., Harbalıoğlu H., Genç Ö., Er F., Allahverdiyev S., et al. The CHA2DS2-VASc score and in-hospital mortality in patients with COVID-19: A multicenter retrospective cohort study. Turk Kardiyol Dern Ars. 2020;48:656–663. doi: 10.5543/tkda.2020.03488. [DOI] [PubMed] [Google Scholar]
- 28.Elias P., Poterucha T.J., Jain S.S., Sayer G., Raikhelkar J., Fried J., et al. The Prognostic Value of Electrocardiogram at Presentation to Emergency Department in Patients With COVID-19. Mayo Clin Proc. 2020;95:2099–2109. doi: 10.1016/j.mayocp.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Chen L., Wang J., He X., Huang F., Chen J., et al. Electrocardiogram analysis of patients with different types of COVID-19. Ann Noninvasive Electrocardiol. 2020;25 doi: 10.1111/anec.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reilev M., Kristensen K.B., Pottegård A., Lund L.C., Hallas J., Ernst M.T., et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49:1468–1481. doi: 10.1093/ije/dyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCullough S.A., Goyal P., Krishnan U., Choi J.J., Safford M.M., Okin P.M. Electrocardiographic findings in coronavirus disease-19: insights on mortality and underlying myocardial processes. J Card Fail. 2020;26:626–632. doi: 10.1016/j.cardfail.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghio S., Baldi E., Vicentini A., Lenti M.V., Di Sabatino A., Di Matteo A., et al. Cardiac involvement at presentation in patients hospitalized with COVID-19 and their outcome in a tertiary referral hospital in Northern Italy. Intern Emerg Med. 2020;15:1457–1465. doi: 10.1007/s11739-020-02493-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L., Zhang S., He B., Chen X., Wang S., Zhao Q. Risk factors and electrocardiogram characteristics for mortality in critical inpatients with COVID-19. Clin Cardiol. 2020;43:1624–1630. doi: 10.1002/clc.23492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodríguez-Molinero A., Gálvez-Barrón C., Miñarro A., Macho O., López G.F., Robles M.T., et al. Association between COVID-19 prognosis and disease presentation, comorbidities and chronic treatment of hospitalized patients. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clift A.K., Coupland C., Keogh R.H., Diaz-Ordaz K., Williamson E., Harrison E.M., et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah C., Grando D.J., Rainess R.A., Ayad L., Gobran E., Benson P., et al. Factors associated with increased mortality in hospitalized COVID-19 patients. Ann Med Surg (Lond) 2020;60:308–313. doi: 10.1016/j.amsu.2020.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lano G., Braconnier A., Bataille S., Cavaille G., Moussi-Frances J., Gondouin B., et al. Risk factors for severity of COVID-19 in chronic dialysis patients from a multicentre French cohort. Clin Kidney J. 2020;13:878–888. doi: 10.1093/ckj/sfaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polverino F., Stern D.A., Ruocco G., Balestro E., Bassetti M., Candelli M., et al. Comorbidities, cardiovascular therapies, and COVID-19 mortality: a nationwide, italian observational study (ItaliCO) Front Cardiovasc Med. 2020;7:585866. doi: 10.3389/fcvm.2020.585866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izurieta H.S., Graham D.J., Jiao Y., Hu M., Lu Y., Wu Y., et al. Natural history of coronavirus disease 2019: risk factors for hospitalizations and deaths among >26 Million US medicare beneficiaries. J Infect Dis. 2021;223:945–956. doi: 10.1093/infdis/jiaa767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gue Y.X., Tennyson M., Gao J., Ren S., Kanji R., Gorog D.A. Development of a novel risk score to predict mortality in patients admitted to hospital with COVID-19. Sci Rep. 2020;10:21379. doi: 10.1038/s41598-020-78505-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez-Guzman P.N., Daunt A., Mukherjee S., Crook P., Forlano R., Kont M.D., et al. Clinical characteristics and predictors of outcomes of hospitalized patients with COVID-19 in a multi-ethnic London NHS Trust: a retrospective cohort study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canevelli M., Palmieri L., Raparelli V., Lo Noce C., Colaizzo E., Tiple D., et al. Prevalence and clinical correlates of dementia among COVID-19-related deaths in Italy. Alzheimers Dement (Amst) 2020;12 doi: 10.1002/dad2.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossi L., Malagoli A., Biagi A., Zanni A., Sticozzi C., Comastri G., et al. Renin-angiotensin system inhibitors and mortality in patients with COVID-19. Infection. 2021;49:287–294. doi: 10.1007/s15010-020-01550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvarez-Garcia J., Lee S., Gupta A., Cagliostro M., Joshi A.A., Rivas-Lasarte M., et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol. 2020;76:2334–2348. doi: 10.1016/j.jacc.2020.09.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poterucha T.J., Elias P., Jain S.S., Sayer G., Redfors B., Burkhoff D., et al. Admission cardiac diagnostic testing with electrocardiography and troponin measurement prognosticates increased 30-day mortality in COVID-19. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.018476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.García-Granja P.E., Veras C., Aparisi Á., Amat-Santos I.J., Catalá P., Marcos M., et al. Atrial fibrillation in patients with SARS-CoV-2 infection. Med Clin (Barc) 2021;157:58–63. doi: 10.1016/j.medcli.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelesoglu S., Yilmaz Y., Ozkan E., Calapkorur B., Gok M., Dursun Z.B., et al. New onset atrial fibrilation and risk faktors in COVID-19. J Electrocardiol. 2021;65:76–81. doi: 10.1016/j.jelectrocard.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denegri A., Pezzuto G., Arienzo M., Morelli M., Savorani F., et al. Clinical and electrocardiographic characteristics at admission of COVID-19/SARS-CoV2 pneumonia infection. Intern Emerg Med. 2021:1–6. doi: 10.1007/s11739-020-02578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gawałko M., Kapłon-Cieślicka A., Hohl M., Dobrev D., Linz D. COVID-19 associated atrial fibrillation: Incidence, putative mechanisms and potential clinical implications. Int J Cardiol Heart Vasc. 2020;30:100631. doi: 10.1016/j.ijcha.2020.100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone E., Kiat H., McLachlan C.S. Atrial fibrillation in COVID-19: A review of possible mechanisms. FASEB J. 2020;34:11347–11354. doi: 10.1096/fj.202001613. [DOI] [PubMed] [Google Scholar]
- 51.Yang H., Liang X., Xu J., Hou H., Wang Y. Meta-analysis of atrial fibrillation in patients with COVID-19. Am J Cardiol. 2021;144:152–156. doi: 10.1016/j.amjcard.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Go A.S., Hylek E.M., Phillips K.A., Chang Y., Henault L.E., Selby J.V., et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 53.Abrams M.P., Wan E.Y., Waase M.P., Morrow J.P., Dizon J.M., Yarmohammadi H., et al. Clinical and cardiac characteristics of COVID-19 mortalities in a diverse New York City Cohort. J Cardiovasc Electrophysiol. 2020;31:3086–3096. doi: 10.1111/jce.14772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fumagalli S., Salani B., Gabbani L., Mossello E., Ungar A. Covid-19 cases in a no-Covid-19 geriatric acute care setting. A sporadic occurrence. Eur J Intern Med. 2020;77:141–142. doi: 10.1016/j.ejim.2020.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atri D., Siddiqi H.K., Lang J.P., Nauffal V., Morrow D.A., Bohula E.A. COVID-19 for the cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Data Availability Statement
Original data is available from the corresponding author on reasonable request.