Abstract
The development of a satisfactory means to reliably distinguish between the two closely related species Candida albicans and Candida dubliniensis in the clinical mycology laboratory has proved difficult because these two species are phenotypically so similar. In this study, we have detected homologues of the pH-regulated C. albicans PHR1 and PHR2 genes in C. dubliniensis. Restriction fragment length polymorphism analysis suggests that there are significant sequence differences between the genes of the two species. In order to exploit this apparent difference, oligonucleotide primers based on the coding sequence of the C. albicans PHR1 structural gene were designed and used in PCR experiments. Use of these primers with C. albicans template DNA from 17 strains yielded a predicted 1.6-kb product, while C. dubliniensis template DNA from 19 strains yielded no product. We therefore propose that PCR using these primers is a rapid and reliable means of distinguishing the two germ tube- and chlamydospore-producing species C. albicans and C. dubliniensis.
Opportunistic fungal infections have gained considerable importance during recent years, and oral candidosis is among the most common opportunistic infections encountered in human immunodeficiency virus-infected patients (13). Candida dubliniensis is a chlamydospore-positive, germ tube-positive species with many characteristics in common with Candida albicans. To date, C. dubliniensis isolates have been primarily recovered from the oral cavities of immunosuppressed individuals (20), although isolates have also been recovered from other anatomic sites (19). It has been shown to be a low-level constituent of the human oral flora and has the potential to cause oral candidosis (1, 18). Although C. albicans and C. dubliniensis are phenotypically very similar, they differ in their carbohydrate assimilation profiles, growth patterns at elevated temperatures, and intracellular β-glucosidase activities (1, 12, 14). In addition, C. dubliniensis possesses a very distinct genomic organization (1, 4, 19). In spite of these differences, rapid discrimination between C. albicans and C. dubliniensis in the clinical mycology laboratory is still problematic and usually only achieved effectively by using a combination of standard laboratory procedures (19). The incidence of C. dubliniensis and its role in disease have yet to be firmly established. In order to effectively address this situation, it is important to be able to differentiate this organism from C. albicans. For this reason, rapid techniques suitable for the accurate and reliable identification of C. dubliniensis in clinical laboratories are required. Because of the difficulty in using methods based on phenotypic parameters to distinguish efficiently between C. dubliniensis and C. albicans, we proposed to develop a rapid and reliable discriminatory method based on the use of molecular tools.
While analyzing the expression and genomic organization of genes regulated in response to the ambient pH in C. dubliniensis, we found that the genomic organization of the PHR1 and PHR2 genes differed between C. dubliniensis and C. albicans. In C. albicans, the expression of these functionally homologous genes is regulated by environmental pH and deletion analysis suggests that they play a role in morphogenesis and cell survival in vivo (2, 8, 15). The unique organization of these genes in C. dubliniensis suggested the opportunity to use these sequences as targets for species-specific oligonucleotides in PCR experiments to discriminate between C. dubliniensis and C. albicans.
Identification of PHR homologs in C. dubliniensis.
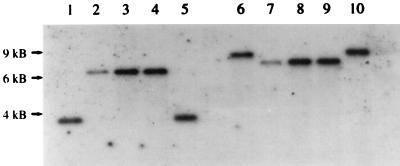
In order to investigate the presence of PHR1 and PHR2 homologous genes in C. dubliniensis, genomic DNA from reference strains and clinical isolates (Table 1) was isolated as previously described (16). DNA was digested with the restriction enzymes EcoRI and HindIII and subsequently used for Southern blot analysis. The membranes were hybridized by using an 800-bp ClaI fragment of the C. albicans PHR1 gene or a 1.6-kb HindIII fragment of the C. albicans PHR2 gene as a probe under stringent conditions (8, 15). Both probes hybridized with the digested C. dubliniensis DNA under these conditions (Fig. 1 and Table 1). However, the sizes of the hybridizing bands differed between the two species in experiments using EcoRI or HindIII (Fig. 1 and Table 1). These findings demonstrate that PHR1 and PHR2 homologous genes are present in the C. dubliniensis genome. The differences in the restriction fragment lengths point to an architecture of the PHR1-PHR2 genomic locus in C. dubliniensis different from that in C. albicans. In agreement with the observations of others who suggested that Candida stellatoidea is a variant or synonym of C. albicans, the C. stellatoidea reference strains tested were indistinguishable from C. albicans in this study (11) (Fig. 1 and Table 1). DNA from other chlamydospore-negative pathogenic or nonpathogenic species either did not hybridize at all or produced barely detectable signals of different sizes (Table 1). This may well be due to the existence of PHR homologous genes in these species. Indeed, using PCR with degenerate primers deduced from the C. albicans PHR1 and PHR2 sequences, we were able to amplify fragments with homologies to the PHR1 and PHR2 genes in the pathogenic yeast Candida glabrata (22). Furthermore, PHR homologous genes have also been described for Candida maltosa and Saccharomyces cerevisiae (9, 21). However, so far C. albicans is the only species demonstrating strong pH-dependent inverse expression of these genes.
TABLE 1.
Reference yeast strains, the sizes of restriction fragments in Southern blot analysis using PHR1 and PHR2 probes, and the sizes of PCR products in experiments using C. albicans PHR1-specific primers
| Species | Straina | Reference | Restriction fragment size (kb) by:
|
||||
|---|---|---|---|---|---|---|---|
| Southern blotting
|
PCR | ||||||
|
PHR1 probe
|
PHR2 probe
|
||||||
| EcoRI | HindIII | EcoRI | HindIII | ||||
| Candida albicans | SC5314 | Gillum et al. (5) | 3.7 | 8 | 3.9 | 1 | 1.6 |
| CA019 | This study | 3.7 | 8 | NDb | ND | 1.6 | |
| CA024 | This study | 3.7 | 8 | ND | ND | 1.6 | |
| 132A | Gallagher et al. (3) | 3.7 | 8 | ND | ND | 1.6 | |
| 3153 | Odds (10) | 3.7 | 8 | ND | ND | 1.6 | |
| Candida stellatoidea | ATCC 11006 | 3.7 | 8 | 3.9 | 1 | 1.6 | |
| ATCC 20408 | 3.7 | 8 | ND | ND | 1.6 | ||
| Candida dubliniensis | CBS 7987 | 6.5 | 7 | 20 | 0.8 | NSc | |
| CBS 7988 | 6.5 | 7 | 20 | 0.8 | NS | ||
| CD 33 | Sullivan et al. (20) | 6.5 | 7 | ND | ND | NS | |
| CD 38 | Sullivan et al. (20) | 6.5 | 7 | ND | ND | NS | |
| CM 1 | Sullivan et al. (20) | 6.5 | 7 | ND | ND | NS | |
| LP | This study | 6.5 | 7 | ND | ND | NS | |
| Candida glabrata | ATCC 90876 | NS | NS | 12 | 7.5 | 0.7 | |
| Candida tropicalis | CBS 94 | 5.5 | 14 | 7.5 | 13 | NS | |
| Candida parapsilosis | CBS 604 | 11 | 13 | NS | NS | NS | |
| Issatchenkia orientalis | CBS 673 | NS | NS | NS | NS | 2.1 | |
| Pichia guilliermondii | CBS 566 | NS | NS | NS | NS | NS | |
| Kluyveromyces marxianus | CBS 834 | NS | NS | NS | NS | 2.2 | |
| Candida maltosa | CBS 5611 | NS | NS | ND | ND | 2.2 | |
ATCC, American Type Culture Collection, Manassas, Va.; CBS, Centralbureau voor Schimmelcultures, Delft, The Netherlands.
ND, not determined.
NS, no signal detected.
FIG. 1.
Southern blot hybridization with the PHR1 gene probe. DNA from C. albicans SC5314 (lanes 1 and 6); C. dubliniensis CBS 7987 (lanes 2 and 7), CBS 7988 (lanes 3 and 8), and CD 33 (lanes 4 and 9); and C. stellatoidea ATCC 11006 (lanes 5 and 10) digested with EcoRI (lanes 1 to 5) or HindIII (lanes 6 to 10) was analyzed. The electrophoretic positions and sizes deduced from DNA standards are indicated on the left.
pH-dependent differential expression of the C. dubliniensis PHR homologs.
To determine whether the expression of the PHR homologous genes of C. dubliniensis resembles the pattern demonstrated for the C. albicans PHR1 and PHR2 genes, total RNA was isolated from the C. dubliniensis reference strains at pH 4 and pH 8 as previously described (6). Northern blot hybridization was performed with either the PHR1 or the PHR2 probe under stringent conditions. With each of the probes, a transcript with a size comparable to that of the PHR1 or PHR2 mRNA was detected in the C. dubliniensis reference strains (data not shown). Furthermore, the PHR homologous genes of C. dubliniensis exhibited a pH-dependent pattern of expression similar to that of PHR1 and PHR2. Thus, it seems likely that the pH-balanced system of the two functional homologues PHR1 and PHR2 of C. albicans is conserved in C. dubliniensis.
Amplification of PHR1 for differentiation between C. albicans and C. dubliniensis.
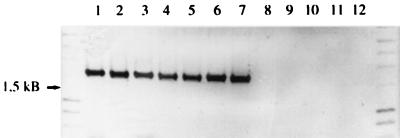
In order to investigate whether sequence differences between the PHR1 and PHR2 genes of C. albicans and their C. dubliniensis homologues might be useful in terms of differentiation between these two species, we performed PCR with primers derived from the PHR1 sequence of C. albicans. In addition to the strains listed in Table 1, 12 C. albicans and 13 C. dubliniensis fresh clinical isolates from the oral cavities of different human immunodeficiency virus-infected individuals from Ireland were subjected to PCR analysis. Identification of the strains was based on previously described phenotypic criteria (19). The sequence of the forward primer OK3 was 5′ ATG TAT TCA TTA ATC AAA TCA 3′. The sequence of the reverse primer OK4 was 5′ ATT TAA AAA ACA ACG GAC AT 3′. These primers are capable of amplifying the entire 1,644-bp open reading frame of PHR1. The PCR mixture (total volume, 100 μl) contained 100 ng of genomic template DNA, 0.2 mM each deoxynucleoside triphosphate, 1× AmpliTaq buffer, 1.5 mM MgCl2, 100 pmol of each primer, and 5 U of AmpliTaq polymerase (Perkin-Elmer, Weiterstadt, Germany). Amplification was performed after denaturation at 95°C for 5 min for 30 cycles with a 20-s denaturation at 95°C, primer annealing at 50°C for 60 s, and extension for 90 s at 72°C, followed by a final extension at 72°C for 10 min in a Trio Thermoblock system (Biometra, Göttingen, Germany). Each experiment included negative controls with all reagents except template DNA. As a positive control, plasmid pSMS-24 containing the complete PHR1 gene was used as the template DNA (15). Since assignment of isolates to C. dubliniensis is based on the missing amplification of the PHR1 homologue, proper adjustment of the controls is necessary. Products were separated on an 0.8% (wt/vol) agarose gel, stained with ethidium bromide, and compared to the positive control. Template DNA from all of the 17 C. albicans isolates and both C. stellatoidea strains led to the specific amplification of a fragment with the predicted size of approximately 1.6 kb that was identical to the positive control. When the same conditions were used with 19 C. dubliniensis isolates, no amplimers were detected (Fig. 2 and data not shown). In addition, the PCR test was applied to 109 oral isolates previously classified as C. albicans based on germ tube and chlamydospore formation. Differentiation between C. albicans and C. dubliniensis was then achieved in a blinded fashion based on either phenotypic characteristics or the PCR test. The results obtained by the two methods were shown to correlate (unpublished data). Identical results were obtained when colony material from the C. dubliniensis and C. albicans reference strains was used as a template for the PCR (data not shown). In addition, DNA from reference strains of the chlamydospore-negative species Candida tropicalis, Candida parapsilosis, C. glabrata, Kluyveromyces marxianus (Candida kefyr), Issatchenkia orientalis (Candida krusei), and Pichia guilliermondii (Candida guilliermondii) was isolated and used as a template in this PCR. Similar to the results obtained in the Southern blot analysis, no products were generated or there was amplification of fragments that clearly differed in size from the positive control (Table 1). This, however, does not interfere with the diagnostic value of the procedure since only chlamydospore-positive species are considered.
FIG. 2.
Amplification products obtained following PCR with the primer set OK3-OK4 and Candida template DNA. Lanes 1 to 5, C. albicans SC5314, CA019, CA024, 132A, and 3153, respectively; lanes 6 and 7, C. stellatoidea ATCC 11006 and ATCC 20408, respectively; lanes 8 to 12, C. dubliniensis CBS 7987, CBS 7988, CD 33, CD 38, and LP, respectively. The 1.5-kb band deduced from DNA standards is indicated on the left.
Conclusion.
C. dubliniensis was only recently identified as a separate species, and since it is difficult to distinguish it from C. albicans in clinical samples there is still very little information available concerning its epidemiology and clinical significance. It has been shown that C. dubliniensis is able to readily develop fluconazole resistance under selective pressure (7). To determine whether this or other factors are implicated in the emergence of C. dubliniensis as a pathogen, and to measure what the true incidence of this species is in humans, novel methods for discriminating between C. dubliniensis and C. albicans are required. Until now, identification of C. dubliniensis was achieved by time-consuming investigation of a combination of potentially variable phenotypic characteristics (12, 17, 19). In this study, we describe for the first time the use of a structural gene, PHR1, as a target for a rapid and reliable PCR-based identification system with the potential to facilitate the analysis of the epidemiology of C. dubliniensis.
Acknowledgments
This work was supported by grant MU1212/2-1 from the Deutsche Forschungsgemeinschaft (to F.A.M.).
We thank W. A. Fonzi for plasmid pSMS-24. We are grateful to Uwe Gross and Michael Weig for critical reading of the manuscript.
REFERENCES
- 1.Coleman D C, Sullivan D J, Bennett D E, Moran G P, Barry H J, Shanley D B. Candidiasis: the emergence of a novel species, Candida dubliniensis. AIDS. 1997;11:557–567. doi: 10.1097/00002030-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 2.De Bernardis F, Mühlschlegel F A, Cassone A, Fonzi W A. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect Immun. 1998;66:3317–3325. doi: 10.1128/iai.66.7.3317-3325.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallagher P J, Bennett D E, Henman M C, Russel R J, Flint S R, Shanley D B, Coleman D C. Reduced azole susceptibility of Candida albicans from HIV-positive patients and a derivative exhibiting colony morphology variation. J Gen Microbiol. 1992;138:1901–1911. doi: 10.1099/00221287-138-9-1901. [DOI] [PubMed] [Google Scholar]
- 4.Gilfillan G D, Sullivan D J, Haynes K, Parkinson T, Coleman D C, Gow N A R. Candida dubliniensis: phylogeny and putative virulence factors. Microbiology. 1998;144:829–838. doi: 10.1099/00221287-144-4-829. [DOI] [PubMed] [Google Scholar]
- 5.Gillum A M, Tsay E Y H, Kirsch D R. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 6.Langford C J, Gallwitz D. Evidence for an intron-containing sequence required for the splicing of yeast RNA polymerase II transcripts. Cell. 1983;33:519–527. doi: 10.1016/0092-8674(83)90433-6. [DOI] [PubMed] [Google Scholar]
- 7.Moran G P, Sullivan D J, Henman M C, McCreary C E, Harrington B J, Shanley D B, Coleman D C. Antifungal drug susceptibilities of oral Candida dubliniensis isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected subjects and generation of stable fluconazole-resistant derivatives in vitro. Antimicrob Agents Chemother. 1997;41:617–623. doi: 10.1128/aac.41.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mühlschlegel F A, Fonzi W A. PHR2 of Candida albicans encodes a functional homolog of the pH-regulated gene PHR1 with an inverted pattern of pH-dependent expression. Mol Cell Biol. 1997;17:5960–5967. doi: 10.1128/mcb.17.10.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakazawa T, Horiuchi H, Ohta A, Takagi M. Isolation and characterization of EPD1, an essential gene for pseudohyphal growth of a dimorphic yeast, Candida maltosa. J Bacteriol. 1998;180:2079–2086. doi: 10.1128/jb.180.8.2079-2086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odds F C. The effect of growth medium on filament production in Candida albicans. Sabouraudia. 1974;12:112–119. doi: 10.1080/00362177485380151. [DOI] [PubMed] [Google Scholar]
- 11.Odds F C. Biological aspects of pathogenic Candida species. In: Odds F C, editor. Candida and candidosis. London, United Kingdom: Bailliere Tindall; 1988. pp. 7–15. [Google Scholar]
- 12.Pinjon E, Sullivan D, Salkin I, Shanley D, Coleman D. Simple, inexpensive, reliable method for differentiation of Candida dubliniensis from Candida albicans. J Clin Microbiol. 1998;36:2093–2095. doi: 10.1128/jcm.36.7.2093-2095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reef S E, Meyer K H. Opportunistic candidal infections in patients infected with human immunodeficiency virus: prevention issues and priorities. Clin Infect Dis. 1995;21(Suppl. 1):S99–S102. doi: 10.1093/clinids/21.supplement_1.s99. [DOI] [PubMed] [Google Scholar]
- 14.Salkin I F, Pruitt W R, Padhye A A, Sullivan D, Coleman D, Pincus D H. Distinctive carbohydrate assimilation profiles used to identify the first clinical isolates of Candida dubliniensis recovered in the United States. J Clin Microbiol. 1998;36:1467. doi: 10.1128/jcm.36.5.1467-1467.1998. . (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saporito-Irwin S M, Birse C E, Sypherd P S, Fonzi W A. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol Cell Biol. 1995;15:601–613. doi: 10.1128/mcb.15.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scherer S, Stevens D S. A Candida albicans dispersed, repeated gene family and its epidemiological applications. Proc Natl Acad Sci USA. 1988;85:1452–1456. doi: 10.1073/pnas.85.5.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoofs A, Odds F C, Colebunders R, Ieven M, Goossens H. Use of specialised isolation media for recognition and identification of Candida dubliniensis isolates from HIV-infected patients. Eur J Clin Microbiol Infect Dis. 1997;16:296–300. doi: 10.1007/BF01695634. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan D, Haynes K, Bille J, Boerlin P, Rodero L, Lloyd S, Henman M, Coleman D. Widespread geographic distribution of oral Candida dubliniensis strains in human immunodeficiency virus-infected individuals. J Clin Microbiol. 1997;35:960–964. doi: 10.1128/jcm.35.4.960-964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan D, Coleman D. Candida dubliniensis: characteristics and identification. J Clin Microbiol. 1998;36:329–334. doi: 10.1128/jcm.36.2.329-334.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan D J, Westerneng T J, Haynes K A, Bennett D E, Coleman D C. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141:1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 21.Vai M, Gatti E, Lacana E, Popolo L, Alberghina L. Isolation and deduced amino acid sequence of the gene encoding gp115, a yeast glycophospholipid-anchored protein containing a serine-rich region. J Biol Chem. 1991;266:12242–12248. [PubMed] [Google Scholar]
- 22.Weig, M., and F. Mühlschlegel. Unpublished data.