Abstract
Bifidobacterium bifidum OLB6378 (OLB6378) was selected as a strain that enhances the production of secretory immunoglobulin A (IgA) in vitro. This ability of non-live OLB6378 has been shown by a clinical trial in preterm infants. In the present study, we examined whether non-live OLB6378 also enhances the production of secretory IgA, even in full-term infants. One hundred full-term infants were allocated to receive formula with (BbF group, 49 infants) or without non-live OLB6378 (PF group, 51 infants). Breastfeeding was prioritised, so infant formula was used for infants with breastfeeding difficulties. The intervention was initiated by five days of age. The faecal IgA concentration and OLB6378 level were determined at one, two, four, and eight weeks of age. Faecal IgA in the BbF group (1.04 ± 0.47 mg/g of faeces, n=45) was significantly higher than that in the PF group (0.85 ± 0.42 mg/g of faeces, n=49) at four weeks of age (p=0.047). OLB6378 was not detected in faeces at any age. This indicated that production of secretory IgA in full-term infants may also be enhanced by non-live OLB6378 intake.
Keywords: newborn, infant formula, breastfeeding, probiotics
INTRODUCTION
Non-live Bifidobacterium is a safer and more convenient food material than live Bifidobacterium. Moreover, non-live Bifidobacterium cells stimulate the gut immune system in vitro and in vivo [1, 2]. However, those effects have not been studied sufficiently in a clinical setting, especially in infants. We selected Bifidobacterium bifidum OLB6378 (OLB6378), which enhances the production of secretory immunoglobulin A (IgA) [3], and confirmed the effect of non-live OLB6378 on enhancement of faecal IgA in low-birth-weight infants [4]. From the standpoint of trying to extend the versatility of non-live OLB6378, we hypothesised that non-live OLB6378 enhances the production of secretory IgA, even in full-term infants.
However, the effect observed in low-birth-weight infants may not be observed in full-term infants. Preterm infants have lower body weights, heights, head circumferences, muscle and fat cross-sectional areas, and bone mineral densities than term-born infants [5]. With regards to nutrient digestion and absorption, premature infants do not have levels of intestinal lactase activity and gastric protein digestion capacities comparable to those of mature infants [6,7,8]. They also have immature immune systems and higher levels of intestinal permeability than term infants [9, 10]. Moreover, low birth weight is negatively associated with exclusive breastfeeding [11]. Given these differences between preterm and full-term infants, we considered that the effects of non-live OLB6378 should be demonstrated again in full-term infants.
As a pilot study, we evaluated the effects of non-live OLB6378 administration on the enhancement of faecal IgA in full-term infants in this study.
MATERIALS AND METHODS
Study design and ethical approval
This was a double-blind, randomised, placebo-controlled study conducted among 100 newborn infants and their mothers. The protocol was approved by the Ethics Committee of Chiba University (Chiba, Japan; registration No. 2941) in compliance with the Declaration of Helsinki (June 2018). This study was registered in the University Hospital Medical Information Network (UMIN) Clinical Trail Registry (UMIN000033295).
Participants
Participants were recruited from pairs of infants (37–42 weeks of gestation; birthweight of 2,500 to 4,000 g) and their mothers at Miyake Women’s Clinic (Chiba, Japan) between October 2018 and March 2019. Written informed consent was obtained from each infant’s mother before enrolment in this study. The exclusion criteria were the presence of serious infections and major congenital malformations.
Randomisation and blinding
After pairs of infants and their mothers were enrolled, they were automatically randomised and divided into two groups, namely, the BbF group (infant formula containing non-live OLB6378) and PF group (placebo infant formula), by using the web system of Soiken Co., Ltd. (Tokyo, Japan) and minimisation for sex and delivery method. All subject data were anonymised, and the group allocations were concealed by Soiken Co., Ltd. The infant formula containing non-live OLB6378 and the placebo infant formula (blinded) were provided by Meiji Co., Ltd. (Tokyo, Japan), and the intervention was concealed by Meiji Co., Ltd.
Interventions and procedure
Breastfeeding was prioritised, and infant formula was used for infants when breastfeeding was impossible or when the amount of breast milk was insufficient. The intervention was initiated by five days of age and continued until two months of age. The test infant formula was prepared in the same manner as the placebo infant formula, which was commercially available. Briefly, 13.5 g of powdered formula was fully dissolved in a bottle by adding approximately 65 mL of cooled boiled water (above 70°C). The volume was then adjusted to 100 mL with cooled boiled water, and the entire bottle of formula was cooled until it was less than 40°C. Infants in the PF group received the Meiji Hohoemi infant formula (Meiji Co., Ltd., Tokyo, Japan) as a placebo, whereas those in the BbF group received the test infant formula, which was comprised of Meiji Hohoemi infant formula with a small amount of lactose replaced with non-live OLB6378 powder (37 mg of non-live OLB6378 concentrate containing >9.3 × 109 non-live cells per 100 g powdered infant formula). The amount of non-live OLB6378 (10 mg) in 200 mL of the infant formula was the same as that administered to low-birth-weight infants [4]. Non-live OLB6378 was prepared by heating (at 80°C for more than 5 min) a concentrated culture of B. bifidum OLB6378, which was cultivated using a hydrolysed milk protein medium. The stools of the infants were collected, and faecal IgA and Bifidobacterium were measured at one, two, four, and eight weeks of age. The amount of formula intake was recorded for three days before the stool was sampled. Breast milk samples were collected at two, four, and eight weeks of the infant’s age. The samples were stored at −20°C for several days immediately after sampling and then at −80°C until use.
Quantitative analyses of IgA
The quantification of faecal IgA was performed as described previously [4]. Breast milk was centrifuged (9,100 × g, 10 min, 4°C), and the whey in it was used for the quantification of IgA using a commercial ELISA kit (Human IgA ELISA Quantitation Set, Bethyl Laboratories, Inc., Montgomery, TX, USA).
Quantitative analyses of Bifidobacterium
For the quantification of Bifidobacterium, a real-time polymerase chain reaction (PCR) was performed with Bifidobacterium genus-specific primers, as described previously [12, 13]. B. bifidum OLB6378 was quantified using real-time PCR with strain-specific primers [14]. For the real-time PCR assay, bacterial genomic DNA was extracted from the stool samples using a commercial extraction kit (QuickGene DNA tissue kit, Kurabo, Osaka, Japan) as described previously [12, 15]. The detection limits of Bifidobacterium and B. bifidum OLB6378 were 104 and 107 cells/g of faeces, respectively.
Outcome measures
The primary outcome was defined as the concentration of faecal IgA. A previous study showed that the faecal IgA concentrations of preterm infants who received non-live OLB6378 were higher at one and two months of age [4]. Similarly, in this study, the faecal IgA levels at four and eight weeks of age were the focus of attention. The secondary outcome was defined as the amount of faecal Bifidobacterium.
In addition, exploratory subgroup analyses were performed. Infants were divided into the following three subgroups on the basis of the volume of infant formula intake recorded during the trial period: (1) always <200 mL/day (LM subgroup), (2) always ≥ 200 mL/day (HM subgroup), and (3) others (not always <200 mL/day or >200 mL/day; MM subgroup). In each subgroup, the PF and BbF groups were compared with exclusively breastfeeding infants (n=6), who were excluded from the primary outcome analyses in this study.
Sample size calculation
Whitehead et al. recommended using pilot trial sample sizes of 75, 25, 15, and 10 per treatment arm for standardised effect sizes that are extra small (≤0.1), small (0.2), medium (0.5), or large, respectively [16]. If the effect size of the faecal IgA of full-term infants was too small, it would be difficult to determine whether the intake of non-live OLB6378 had an effect on faecal IgA in full-term infants. Therefore, we aimed to include 30 infants in each group. To allow for up to 20% attrition and 20% exclusion, 100 infants were enrolled in this study (50 per group).
Statistical analyses
The data of exclusively breastfed infants were excluded from the outcome analyses. Between-group statistical significance for the faecal IgA concentration was determined using Student’s t-test and the Brunner–Munzel test. Between-group statistical significance for the faecal Bifidobacterium detection rate and cell counts was determined using Fisher’s exact test and the Mann–Whitney U-test. For all analyses, a p-value <0.05 was considered statistically significant.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) or R version 2.13 (The R Foundation, Vienna, Austria) for primary and secondary outcomes. Subgroup analyses and linear regression analysis were performed using IBM SPSS Statistics version 27 (IBM Corp., Armonk, NY, USA).
RESULTS
Background characteristics
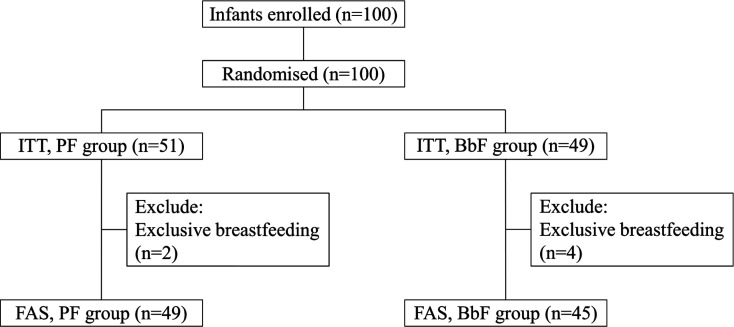
Following the protocol, 100 pairs of newborn infants and mothers were enrolled and randomly assigned to the PF group (n=51) or BbF group (n=49). During the recruitment period, 258 full-term infants were born in the clinic, and 39% of them were enrolled after obtaining informed consent from their mothers. The full analyses set (n=49 for the PF group; n=45 for the BbF group) was defined after excluding six infants who were exclusively breastfed (Fig. 1). Two infants in the BbF group discontinued participation in the study owing to a respiratory syncytial virus infection (18 and 33 days of age).
Fig. 1.
Study flowchart.
PF: placebo infant formula; BbF: infant formula containing non-live B. bifidum OLB6378;
FAS: Full Analysis Set.
In terms of the characteristics of the study population, gestational age was greater in the BbF group than in the PF group (Table 1). There were no significant differences in the other background characteristics. There were no side effects in either group in this study. There was no difference in adverse events between the two groups.
Table 1. Characteristics of infants in the PF and BbF groups.
| PF group (n=49) | BbF group (n=45) | p-value | |
|---|---|---|---|
| Maternal characteristics | |||
| Age (years)1 | 31.7 ± 4.9 | 31.7 ± 5.6 | NS4 |
| Parity1 | 0.66 ± 0.77 | 0.69 ± 0.90 | NS4 |
| Infant characteristics | |||
| Gestational age (days)1 | 274.2 ± 5.8 | 276.6 ± 5.7 | 0.044 |
| Weight at birth (g)1 | 3,058.3 ± 335.1 | 3,120.1 ± 319.8 | NS4 |
| Height at birth (cm)1 | 48.1 ± 1.7 | 48.8 ± 1.3 | NS4 |
| Head circumference at birth (cm)1 | 32.7 ± 1.4 | 33.0 ± 1.5 | NS4 |
| Apgar score at 1 min1 | 8.6 ± 0.8 | 8.6 ± 1.3 | NS4 |
| Apgar score at 5 min1 | 9.6 ± 0.6 | 9.5 ± 0.8 | NS4 |
| Caesarean section2 | 6 (12.2) | 5 (11.1) | NS5 |
| Male sex2 | 22 (44.9) | 20 (44.4) | NS5 |
| Outcomes | |||
| Amount of bottle feeding (mL/day)3 | |||
| One week of age | 302 (189, 423) | 200 (120, 385) | NS6 |
| Two weeks of age | 360 (110, 510) | 213 (76, 375) | NS6 |
| Four weeks of age | 308 (56, 657) | 218 (69, 520) | NS6 |
| Eight weeks of age | 207 (0,722) | 117 (43, 510) | NS6 |
| Mortality2 | 0 (0) | 0 (0) | - |
| Serious adverse event2 | 0 (0) | 2 (4.4) | NS5 |
PF: placebo infant formula; BbF: infant formula containing non-live B. bifidum OLB6378; NS: not significant. 1Mean ± standard deviation, 2Number (%), 3Median and quartile (Q1, Q3), 4Student’s t-test, 5Fisher’s exact test, 6Mann-Whitney U-test. Serious adverse events included two cases of respiratory syncytial virus infection.
Outcomes
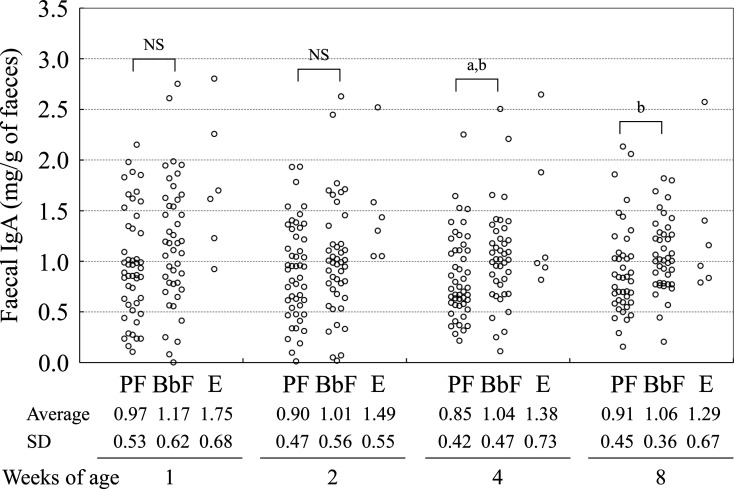
The faecal IgA concentration in the BbF group was significantly higher than that in the PF group at four weeks of age according to the t-test (Fig. 2). Linear regression after adjusting for gestational age and mother’s age revealed a significant partial correlation coefficient between the groups’ faecal IgA levels at four weeks of age (Table 2).
Fig. 2.
Transition of faecal IgA.
PF: placebo infant formula; BbF: infant formula containing non-live B. bifidum OLB6378; E: exclusive breastfeeding; NS: not significant (both Student’s t-test and Brunner–Munzel test); ap<0.05 (t-test); bp<0.05 (Brunner–Munzel test).
Table 2. Linear regression analysis of factors associated with faecal IgA at four weeks of age.
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| PRC (95% CI) | p-value | PRC (95% CI) | p-value | |
| Group (BbF group) | 188.3 (2.5, 374.0) | 0.047 | 191.8 (4.1, 379.4) | 0.04 |
| Sex (male) | 187.4 (1.6, 373.2) | 0.048 | 159.9 (−23.4, 343.3) | NS |
| Caesarean delivery | 168.4 (−129.4, 466.2) | NS | 171.9 (−143.6, 487.5) | NS |
| Gestational age | 5.5 (−10.7, 21.8) | NS | 0.5 (−15.6, 16.6) | NS |
| Weight at birth | −0.1 (−0.4, 0.2) | NS | −0.1 (−0.4, 0.2) | NS |
| Height at birth | 35.8 (−23.9, 95.5) | NS | 29.5 (−33.0, 91.9) | NS |
| Head circumference at birth | 7.5 (−55.6, 70.7) | NS | 3.9 (−52.7, 65.0) | NS |
| Apgar score at 5 min | 12.4 (−128.5, 153.3) | NS | 11.3 (−130.8, 153.5) | NS |
| Maternal age | 21.1 (2.8, 39.4) | 0.03 | 21.5 (3.4, 39.6) | 0.03 |
| Maternal history of allergy | 42.8 (−188.1, 273.8) | NS | −14.7 (−250.6, 221.2) | NS |
| Parity | 74.4 (−39.2, 187.9) | NS | −0.3 (−128.1, 127.5) | NS |
| Log (Bifidobacterium) at four weeks of age | 24.4 (−16.9, 65.7) | NS | 20.7 (−20.4, 61.9) | NS |
| IgA in breast milk at four weeks of age | −0.08 (−0.17, 0.01) | NS | −0.04 (−0.13, 0.06) | NS |
| Formula intake at four weeks of age | −0.30 (−0.69, 0.03) | NS | −0.22 (−0.54, 0.10) | NS |
BbF: infant formula containing non-live B. bifidum OLB6378; PRC: partial regression coefficient; CI: confidence interval. The multivariate was adjusted for group, maternal age, and gestational age.
In this study, B. bifidum OLB6378 was not detected in any infant faeces. The frequency of Bifidobacterium detection was higher in the BbF group than in the PF group at one, two, and four weeks of age, with the values tending to be higher at eight weeks of age (Table 3). However, no significant difference was observed in Bifidobacterium counts calculated from Bifidobacterium-positive samples.
Table 3. Prevalence and counts (log10 cells/g of faeces) of Bifidobacterium.
| Age, weeks | Prevalence, n (%) | Counts, median and quartile (Q1, Q3) | ||||
|---|---|---|---|---|---|---|
| PF group | BbF group | p-value1 | PF group | BbF group | p-value2 | |
| 1 | 27 (55.1) | 34 (75.6) | 0.03 | 9.14 (8.88, 9.55) | 9.12 (8.62, 9.83) | NS |
| 2 | 29 (59.2) | 39 (86.7) | <0.01 | 9.21 (8.86, 9.52) | 8.94 (8.19, 9.47) | NS |
| 4 | 32 (65.3) | 41 (91.1) | <0.01 | 9.42 (8.92, 9.65) | 9.14 (8.43, 9.42) | NS |
| 8 | 41 (83.7) | 43 (95.6) | NS | 9.17 (8.87, 9.55) | 9.15 (8.55, 9.39) | NS |
PF: placebo infant formula (n=49); BbF: infant formula containing non-live B. bifidum OLB6378 (n=45); NS: not significant. Counts, median and quartile (Q1, Q3), were calculated from Bifidobacterium-positive samples. 1Fisher’s exact test, 2Mann-Whitney U-test.
Effects of breastfeeding (bottle feeding)
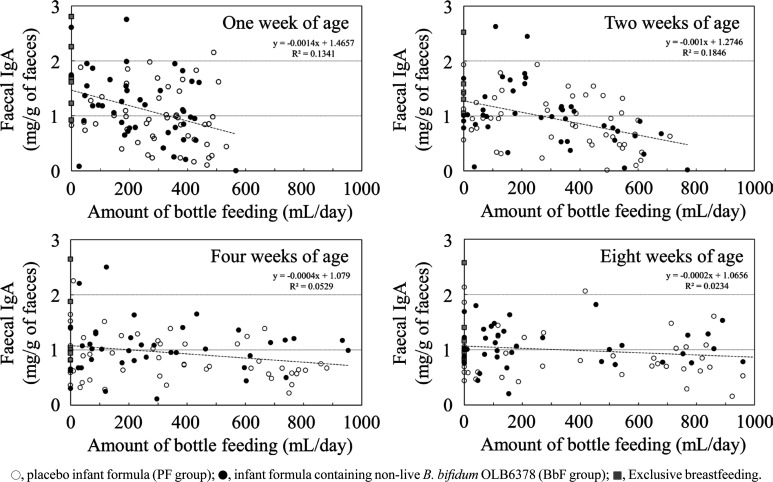
No difference in the IgA concentration of breast milk was observed between the PF and BbF groups (Table 4). A negative correlation between bottle feeding and faecal IgA was observed until two weeks of age (Fig. 3).
Table 4. Median and quartile (Q1, Q3) IgA concentrations (μg/mL) in breast milk.
| Age, weeks | PF group | BbF group | p-value1 |
|---|---|---|---|
| 2 | 426 (285, 716) | 395 (340, 558) | NS |
| 4 | 340 (232, 655) | 311 (248, 450) | NS |
| 8 | 270 (186, 381) | 265 (195, 383) | NS |
PF: placebo infant formula; BbF: infant formula containing non-live B. bifidum OLB6378; NS: not significant. 1Mann-Whitney U-test.
Fig. 3.
Relationship between formula intake and faecal IgA.
The Spearman’s rank correlation coefficients (p-value) at one, two, four, and eight weeks of age were –0.28 (<0.01), –0.38 (<0.01), –0.13 (0.22), and –0.08 (0.49), respectively.
Subgroup analyses
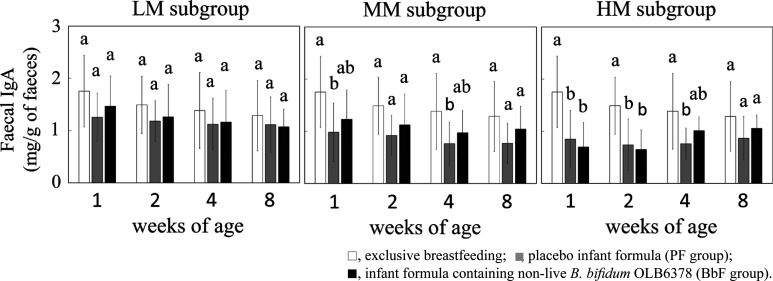
In the HM subgroup (low breastfeeding rate and high non-live OLB6378 intake subgroup), the exploratory subgroup analyses revealed that the faecal IgA levels in the PF group were lower than those of exclusively breastfed infants until four weeks of age (Fig. 4). In contrast, a difference was not observed between the BbF group and exclusively breastfed infants.
Fig. 4.
Subgroup analyses of faecal IgA.
Different letters indicate significant differences at each age (Tukey test).
DISCUSSION
In this randomised study, the faecal IgA concentration in the BbF group was significantly higher than that in the PF group at four weeks of age. Additionally, OLB6378 was not detected in faeces at any age. These results showed that intake of non-live OLB6378 potentially enhance faecal IgA in full-term infants. This is the first clinical study suggesting the effects of non-live Bifidobacterium on enhancement of secretory IgA in full-term infants, similar to the results in preterm infants [4].
For the evaluation of faecal IgA in the infants, it was necessary to consider the IgA from breast milk because breastfeeding was ethically prioritised during this study. In fact, the difference in the faecal IgA level at four weeks of age between the BbF and PF groups was not so large (1.04 mg/g vs. 0.85 mg/g). In addition, the faecal IgA level of the PF group at four weeks of age (0.85 mg/g) appeared to be lower than those at one (0.97 mg/g), two (0.90 mg/g), and eight (0.91 mg/g) weeks of age. This was possibly caused by IgA from breast milk because most of the infants were fed breast milk. Moreover, intake of non-live OLB6378 depends on the amount of bottle feeding, which is inversely proportional to the amount of breastfeeding, and this makes the discussion of this issue complicated. Maruyama et al. demonstrated that the faecal IgA level rapidly increased after the intake of colostrum [17]. Furthermore, Maruyama et al. speculated that the intestinal IgA level was markedly influenced by the intake of breast milk. In the current study, we also found a negative correlation between formula intake and faecal IgA at one and two weeks of age but not at four and eight weeks of age. This was most likely caused by the IgA concentration in the breast milk being high. The negative correlation can be explained by the high IgA concentration of the colostrum and the gradual decline in the concentration until 15 days of age [18].
Furthermore, considering the effect of the IgA in breast milk on the level of faecal IgA, we performed a subgroup analysis. Infants were divided on the basis of the volume of bottle feeding during this study. In this subgroup analysis, infants were simultaneously divided based on the amount of non-live OLB6378 ingested because the intake of non-live OLB6378 was dependent on the volume of bottle feeding. The faecal IgA level of the PF group in the HM subgroup (low breastfeeding rate and high non-live OLB6378 intake subgroup) was lower than that of the exclusively breastfed infants until four weeks of age, although no difference was observed in the LM subgroup (high breastfeeding rate and low non-live OLB6378 intake subgroup; Fig. 4). The impact of breast milk on the faecal IgA level was smaller in the HM subgroup than in the LM subgroup. In the HM subgroup, the faecal IgA level in the BbF group at four weeks of age was similar to that of the exclusively breastfed infants. In contrast, the faecal IgA in the PF group remained at a low level at four weeks of age. Koutras and Vigorita also showed that the faecal IgA of exclusively formula-fed infants remained completely undetectable in all neonates at two weeks of age, mostly undetectable or at very low levels at four weeks of age, and at very low levels at eight weeks of age [19]. In the same way, the faecal IgA levels might have been lower in the HM subgroup until four weeks of age. Therefore, at four weeks of age, the effects of non-live OLB6378 on the upregulation of pIgR and IgA production [3, 20] might have been observed in the HM subgroup. This suggested the possibility that the faecal IgA levels could have reached the levels of exclusive breastfeeding earlier in the BbF group than in the PF group.
This result was also supported by the linear regression analysis, which showed significant linear correlation between the intervention and increased IgA at four weeks of age. The analysis also showed that maternal age also independently affected the faecal IgA level. Several reports have suggested that older maternal age may be beneficial for early child health [21,22,23]. The social advantage associated with an older maternal age was considered one possible factor responsible for this [21, 22]. The present study might also suggest that older maternal age could possibly be a factor involved in the immunological development of infants.
The results of this study showed no side effects. Although there were two serious adverse events in the BbF group caused by respiratory syncytial virus (RSV)-related hospitalisation, there was no evidence of any causal relationship related to the intake of the non-live OLB6378. Very young infants, especially those six months old and younger, are at greatest risk for severe illness caused by RSV, so those infants were hospitalised. Both of these infants in the BbF group were discharged without any sequelae. With regard to live Bifidobacterium, several reports have shown that probiotic supplementation increased the cell counts of Bifidobacterium and amount of IgA in faeces [24,25,26]. However, it has also been reported that probiotic-supplemented infants had higher incidences of mucosal-associated illnesses in the first two years of life [27]. Moreover, Bifidobacterium bacteraemia and meningitis have been reported [28, 29], and the possibility of side effects caused by probiotic strains has also been reported in preterm infants [30]. Unlike live probiotics, non-live probiotics do not cause pathological conditions on their own, even though they stimulate the gastrointestinal immune system [31, 32]. Even non-live probiotics have reportedly enhanced the production of IgA in Peyer’s patch cells in vitro [33], in mice and post-weaning piglets in vivo [34, 35], and in preschool children in clinical trials [36]. Nevertheless, only one clinical trial using infant formula containing non-live Bifidobacterium has been conducted. Faecal IgA was also increased in preterm infants who consumed fermented formula, the manufacturing process of which includes a fermentation step with two probiotic strains, namely Bifidobacterium breve C50 and Streptococcus thermophilus 065, inactivated by heat at the end of the process [37]. This result was similar to that of our previous study on preterm infants who were administered non-live OLB6378 [9] and also supports the results of the present study.
Regarding the secondary outcome of this study, the detection rate of faecal Bifidobacterium (%) in the BbF group was higher than that in the PF group, even though non-live OLB6378 was administered to full-term infants in the BbF group (Table 3). Tsuji et al. reported that the detection rate of intestinal Bifidobacterium in healthy Japanese infants was 73% at seven days of age and 88% at one month of age [38]. In contrast, Sakata et al. and Penders et al. reported that the detection rate of Bifidobacterium was 100% at one month of age [39, 40]. The Bifidobacterium detection rate in this study was similar to that reported by Tsuji et al. [38] and that of the preterm infants that we reported previously (approximately 84% at four weeks of age). The Bifidobacterium detection rate could have appeared to be higher in the BbF group than in the PF group in this study because the detection rates were themselves objectively low. Unfortunately, we cannot explain why non-live OLB6378 intake increased the Bifidobacterium detection rate. Regarding this point, the effect of heat-killed Bifidobacterium longum BR-108 on mouse caecal microbiota is similar to that of viable BR-108, most likely because both heat-killed and viable BR-108 stimulate the gut immune system in similar manners [2]. We can only speculate that the Bifidobacterium detected in faeces was not the OLB6378 strain because the Bifidobacterium levels in the faeces (almost all were >108 cells/g of faeces) were higher than the detection limit for the OLB6378 strain (107 cells/g of faeces). Therefore, at minimum, the non-live cells of OLB6378 did not influence or only slightly influenced the numbers of Bifidobacterium in the faeces.
This study had some limitations. First, there was a difference in gestational age between the groups. However, it has been reported that there is a steady rise in the level of salivary IgA from birth to 18 months in both preterm and full-term children, with no significant difference between them [41]. Moreover, after adjusting for gestational and maternal ages, the linear regression analysis revealed a significant partial correlation coefficient between groups’ faecal IgA levels at four weeks of age (Table 2). Second, we did not investigate colostrum feeding. Therefore, the effects of colostrum on faecal IgA were not clear in this study. Finally, a power of 0.51 was insufficient for the faecal IgA at four weeks of age in this study (p=0.047, Cohen’s d=0.43). Therefore, a randomised study, in which the sample size is determined on the basis of the effect size of this pilot study, is needed in the future.
In conclusion, our results showed that the amount of intestinal IgA in full-term infants was increased by feeding them infant formula containing non-live OLB6378. Intake of non-live Bifidobacterium may stimulate IgA production in not only preterm infants but also full-term infants.
FUNDING
This study was funded by Meiji Co. Ltd., Tokyo.
CONFLICT OF INTEREST
Authors M. Terahara, Y. Nakamura, M. Tsuboi, and S. Jinno are employees of Meiji Co. Ltd., Tokyo, Japan. The other authors declare that they are free of any conflicts of interest. This study was funded by Meiji Co. Ltd., Tokyo.
Acknowledgments
We would like to thank all the infants and parents who participated in this study. We would also like to thank the staff at Miyake Women’s Clinic and Soiken Co., Ltd.
REFERENCES
- 1.Yasui H, Nagaoka N, Mike A, Hayakawa K, Ohwaki M. 1992. Detection of Bifidobacterium strains that induce large quantities of IgA. Microb Ecol Health Dis 5: 155–162. [Google Scholar]
- 2.Makioka Y, Tsukahara T, Ijichi T, Inoue R. 2018. Oral supplementation of Bifidobacterium longum strain BR-108 alters cecal microbiota by stimulating gut immune system in mice irrespectively of viability. Biosci Biotechnol Biochem 82: 1180–1187. [DOI] [PubMed] [Google Scholar]
- 3.Terahara M. 2017. Challenge of development of probiotics for neonates. Milk Science 66: 247–253 (in Japanese). [Google Scholar]
- 4.Tanaka K, Tsukahara T, Yanagi T, Nakahara S, Furukawa O, Tsutsui H, Koshida S. 2017. Bifidobacterium bifidum OLB6378 simultaneously enhances systemic and mucosal humoral immunity in low birth weight infants: a non-randomized study. Nutrients 9: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad I, Nemet D, Eliakim A, Koeppel R, Grochow D, Coussens M, Gallitto S, Rich J, Pontello A, Leu SY, Cooper DM, Waffarn F. 2010. Body composition and its components in preterm and term newborns: a cross-sectional, multimodal investigation. Am J Hum Biol 22: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mobassaleh M, Montgomery RK, Biller JA, Grand RJ. 1985. Development of carbohydrate absorption in the fetus and neonate. Pediatrics 75: 160–166. [PubMed] [Google Scholar]
- 7.Kien CL, Heitlinger LA, Li BU, Murray RD. 1989. Digestion, absorption, and fermentation of carbohydrates. Semin Perinatol 13: 78–87. [PubMed] [Google Scholar]
- 8.Demers-Mathieu V, Qu Y, Underwood MA, Borghese R, Dallas DC. 2018. Premature infants have lower gastric digestion capacity for human milk proteins than term infants. J Pediatr Gastroenterol Nutr 66: 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melville JM, Moss TJM. 2013. The immune consequences of preterm birth. Front Neurosci 7: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver LT, Laker MF, Nelson R. 1984. Intestinal permeability in the newborn. Arch Dis Child 59: 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneko A, Kaneita Y, Yokoyama E, Miyake T, Harano S, Suzuki K, Ibuka E, Tsutsui T, Yamamoto Y, Ohida T. 2006. Factors associated with exclusive breast-feeding in Japan: for activities to support child-rearing with breast-feeding. J Epidemiol 16: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka K, Nakamura Y, Terahara M, Yanagi T, Nakahara S, Furukawa O, Tsutsui H, Inoue R, Tsukahara T, Koshida S. 2019. Poor Bifidobacterial colonization is associated with late provision of colostrum and improved with probiotic supplementation in low birth weight infants. Nutrients 11: 839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gueimonde M, Tölkkö S, Korpimäki T, Salminen S. 2004. New real-time quantitative PCR procedure for quantification of bifidobacteria in human fecal samples. Appl Environ Microbiol 70: 4165–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toshimitsu T, Nakamura M, Ikegami S, Terahara M, Itou H. 2013. Strain-specific identification of Bifidobacterium bifidum OLB6378 by PCR. Biosci Biotechnol Biochem 77: 572–576. [DOI] [PubMed] [Google Scholar]
- 15.Tsukahara T, Inoue R, Nakayama K, Inatomi T. 2018. Inclusion of Bacillus amyloliquefaciens strain TOA5001 in the diet of broilers suppresses the symptoms of coccidiosis by modulating intestinal microbiota. Anim Sci J 89: 679–687. [DOI] [PubMed] [Google Scholar]
- 16.Whitehead AL, Julious SA, Cooper CL, Campbell MJ. 2016. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res 25: 1057–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maruyama K, Hida M, Kohgo T, Fukunaga Y. 2009. Changes in salivary and fecal secretory IgA in infants under different feeding regimens. Pediatr Int 51: 342–345. [DOI] [PubMed] [Google Scholar]
- 18.Bridgman SL, Konya T, Azad MB, Sears MR, Becker AB, Turvey SE, Mandhane PJ, Subbarao P, Scott JA, Field CJ, Kozyrskyj AL, CHILD Study Investigators.2016. Infant gut immunity: a preliminary study of IgA associations with breastfeeding. J Dev Orig Health Dis 7: 68–72. [DOI] [PubMed] [Google Scholar]
- 19.Koutras AK, Vigorita VJ. 1989. Fecal secretory immunoglobulin A in breast milk versus formula feeding in early infancy. J Pediatr Gastroenterol Nutr 9: 58–61. [PubMed] [Google Scholar]
- 20.Nakamura Y, Terahara M, Iwamoto T, Yamada K, Asano M, Kakuta S, Iwakura Y, Totsuka M. 2012. Upregulation of polymeric immunoglobulin receptor expression by the heat-inactivated potential probiotic Bifidobacterium bifidum OLB6378 in a mouse intestinal explant model. Scand J Immunol 75: 176–183. [DOI] [PubMed] [Google Scholar]
- 21.Kato T, Yorifuji T, Yamakawa M, Inoue S, Doi H, Eboshida A, Kawachi I. 2017. Association of maternal age with child health: a Japanese longitudinal study. PLoS One 12: e0172544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutcliffe AG, Barnes J, Belsky J, Gardiner J, Melhuish E. 2012. The health and development of children born to older mothers in the United Kingdom: observational study using longitudinal cohort data. BMJ 345: e5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YN, Choi DW, Kim DS, Park EC, Kwon JY. 2021. Maternal age and risk of early neonatal mortality: a national cohort study. Sci Rep 11: 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radke M, Picaud JC, Loui A, Cambonie G, Faas D, Lafeber HN, de Groot N, Pecquet SS, Steenhout PG, Hascoet JM. 2017. Starter formula enriched in prebiotics and probiotics ensures normal growth of infants and promotes gut health: a randomized clinical trial. Pediatr Res 81: 622–631. [DOI] [PubMed] [Google Scholar]
- 25.Holscher HD, Czerkies LA, Cekola P, Litov R, Benbow M, Santema S, Alexander DD, Perez V, Sun S, Saavedra JM, Tappenden KA. 2012. Bifidobacterium lactis Bb12 enhances intestinal antibody response in formula-fed infants: a randomized, double-blind, controlled trial. JPEN J Parenter Enteral Nutr 36 Suppl: 106S–117S. [DOI] [PubMed] [Google Scholar]
- 26.Bazanella M, Maier TV, Clavel T, Lagkouvardos I, Lucio M, Maldonado-Gòmez MX, Autran C, Walter J, Bode L, Schmitt-Kopplin P, Haller D. 2017. Randomized controlled trial on the impact of early-life intervention with bifidobacteria on the healthy infant fecal microbiota and metabolome. Am J Clin Nutr 106: 1274–1286. [DOI] [PubMed] [Google Scholar]
- 27.Quin C, Estaki M, Vollman DMJ, Barnett JA, Gill SK, Gibson DL. 2018. Probiotic supplementation and associated infant gut microbiome and health: a cautionary retrospective clinical comparison. Sci Rep 8: 8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esaiassen E, Hjerde E, Cavanagh JP, Simonsen GS, Klingenberg C, Norwegian Study Group on Invasive Bifidobacterial Infections.2017. Norwegian Study Group on Invasive Bifidobacterial Infections. Bifidobacterium bacteremia: clinical characteristics and a genomic approach to assess pathogenicity. J Clin Microbiol 55: 2234–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakazawa T, Kaneko K, Takahashi H, Inoue S. 1996. Neonatal meningitis caused by Bifidobacterium breve. Brain Dev 18: 160–162. [DOI] [PubMed] [Google Scholar]
- 30.Sato S, Uchida T, Kuwana S, Sasaki K, Watanabe T, Saito J, Kawaji T. 2016. Bacteremia induced by Bifidobacterium breve in a newborn with cloacal exstrophy. Pediatr Int (Roma) 58: 1226–1228. [DOI] [PubMed] [Google Scholar]
- 31.Adams CA. 2010. The probiotic paradox: live and dead cells are biological response modifiers. Nutr Res Rev 23: 37–46. [DOI] [PubMed] [Google Scholar]
- 32.Kawashima T, Ikari N, Kouchi T, Kowatari Y, Kubota Y, Shimojo N, Tsuji NM. 2018. The molecular mechanism for activating IgA production by Pediococcus acidilactici K15 and the clinical impact in a randomized trial. Sci Rep 8: 5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SH, Jeung W, Choi ID, Jeong JW, Lee DE, Huh CS, Kim GB, Hong SS, Shim JJ, Lee JL, Sim JH, Ahn YT. 2016. Lactic acid bacteria improves Peyer’s patch cell-mediated immunoglobulin A and tight-junction expression in a destructed gut microbial environment. J Microbiol Biotechnol 26: 1035–1045. [DOI] [PubMed] [Google Scholar]
- 34.Hiramatsu Y, Hosono A, Takahashi K, Kaminogawa S. 2007. Bifidobacterium components have immunomodulatory characteristics dependent on the method of preparation. Cytotechnology 55: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuruta T, Inoue R, Tsukahara T, Matsubara N, Hamasaki M, Ushida K. 2009. A cell preparation of Enterococcus faecalis strain EC-12 stimulates the luminal immunoglobulin A secretion in juvenile calves. Anim Sci J 80: 206–211. [DOI] [PubMed] [Google Scholar]
- 36.Hishiki H, Kawashima T, Tsuji NM, Ikari N, Takemura R, Kido H, Shimojo N. 2020. A double-blind, randomized, placebo-controlled trial of heat-killed Pediococcus acidilactici K15 for prevention of respiratory tract infections among preschool children. Nutrients 12: 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campeotto F, Suau A, Kapel N, Magne F, Viallon V, Ferraris L, Waligora-Dupriet AJ, Soulaines P, Leroux B, Kalach N, Dupont C, Butel MJ. 2011. A fermented formula in pre-term infants: clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory IgA. Br J Nutr 105: 1843–1851. [DOI] [PubMed] [Google Scholar]
- 38.Tsuji H, Oozeer R, Matsuda K, Matsuki T, Ohta T, Nomoto K, Tanaka R, Kawashima M, Kawashima K, Nagata S, Yamashiro Y. 2012. Molecular monitoring of the development of intestinal microbiota in Japanese infants. Benef Microbes 3: 113–125. [DOI] [PubMed] [Google Scholar]
- 39.Sakata S, Tonooka T, Ishizeki S, Takada M, Sakamoto M, Fukuyama M, Benno Y. 2005. Culture-independent analysis of fecal microbiota in infants, with special reference to Bifidobacterium species. FEMS Microbiol Lett 243: 417–423. [DOI] [PubMed] [Google Scholar]
- 40.Penders J, Vink C, Driessen C, London N, Thijs C, Stobberingh EE. 2005. Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol Lett 243: 141–147. [DOI] [PubMed] [Google Scholar]
- 41.Wan AKL, Seow WK, Purdie DM, Bird PS, Walsh LJ, Tudehope DI. 2003. Immunoglobulins in saliva of preterm and full-term infants. Oral Microbiol Immunol 18: 72–78. [DOI] [PubMed] [Google Scholar]