Abstract
Natto is a traditional Japanese fermented soy product high in γ-polyglutamic acid (γ-PGA), whose beneficial effects have been reported. We prepared high-γ-PGA natto and compared the dietary influence on liver lipids and cecal microbiota in mice fed a diet containing it or a standard diet. The mice were served a 30% high-γ-PGA natto diet (PGA group) or standard diet (Con group) for 28 days. Liver lipids, fecal lipids, and fecal bile acids were quantified. Cecal microbiota were analyzed by PCR amplification of the V3 and V4 regions of 16S rRNA genes and sequenced using a MiSeq System. Additionally, the cecal short-chain fatty acid profile was assessed. The results revealed that the liver lipid and triglyceride contents were significantly lower (p<0.01) and amounts of bile acids and lipids in the feces were significantly higher in the PGA group than in the Con group. The cecal butyric acid concentration was observed to be significantly higher in the PGA group than in the Con group. Principal component analysis of the cecal microbiota revealed that the PGA and Con groups were distinct. The ratio of Firmicutes/Bacteroidetes was found to be significantly low in the PGA mice. The results revealed a significantly higher relative abundance of Lachnospiraceae (p<0.05) and significantly lower relative abundance of Coriobacteriaceae (p<0.01) in the PGA group. Analysis of the correlation between bacterial abundance and liver lipids, cecal short-chain fatty acids, fecal lipids, and fecal bile acids suggested that intestinal microbiota can be categorized into different types based on lipid metabolism. Hepatic lipid accumulation typically facilitates the onset of nonalcoholic fatty liver disease (NAFLD). Our findings suggest that high-γ-PGA natto is a beneficial dietary component for the prevention of NAFLD.
Keywords: γ-polyglutamic acid, natto, microbiota, mice
INTRODUCTION
Soy sauce and miso are traditional Japanese foods that are fermented from soy. To avoid bacterial contamination when making soy sauce and miso, a significant quantity of sodium chloride is commonly added. As a result, the final sodium chloride content has been reported to be high in soy sauce as well as miso [1]. Natto is also a traditional Japanese fermented soy product. As sodium chloride is not added when making natto, it has been considered to be an ideal fermented soy product [1]; natto is made by fermenting soybeans with Bacillus subtilis var. natto [2].
Several studies have reported the beneficial effects of natto [3]. Studies have shown that intake of natto triggers fibrinolytic activity in rats with hypercholesterolemia. Moreover, they have also found that natto decreases the total serum cholesterol levels in rats [4]. A study of healthy male volunteers showed that a natto-enriched meal significantly lowered blood glucose levels as compared to a control meal [5]. In another study, the effects of consumption of a single breakfast comprising natto and viscous vegetables or a similar control breakfast for 2 weeks were investigated in overweight individuals by evaluating their blood glucose level, lipid metabolism, and oxidative stress. The study reported significant decreases in the total and LDL-cholesterol levels in serum, which were assessed before and after the test meal period [6]. A cross-sectional study involving 652 eligible Japanese men revealed that higher consumption of fermented soy products and soy isoflavone is associated with reduced arterial stiffness [7]. Similarly, supplementation of the diet with 10% natto suppressed the increase in total serum cholesterol induced by a 1% cholesterol-enriched diet in rats [8]. In another study, the effects of intake of B. subtilis C-3102 for 8 days on the composition and metabolic activity of 25 healthy volunteers were investigated [9]. The study found that intake of the bacteria resulted in a significant decrease in the p-cresol concentration and a decrease in the abundance of Enterobacteriaceae [9].
Studies have shown that natto contains a significant quantity of γ-polyglutamic acid (γ-PGA) that is produced during the fermentation process by B. subtilis var. natto [10]. Recently, there has been considerable interest in the significance of γ-PGA. One study in mice revealed that a high-fat diet containing 3% γ-PGA significantly increased serum HDL-cholesterol levels while also decreasing serum triglyceride levels when compared to a normal high-fat diet [11]. Furthermore, the high molecular weight of γ-PGA has been suggested to act as a hypocholesterolemia agent in rats [12]. Oral administration of γ-PGA has been reported to upregulate serum interferon-β (IFN-β) expression levels without activating proinflammatory cytokines. Moreover, γ-PGA is considered a prophylactic antiviral agent against noroviruses [13].
The consumption of natto seems to contribute to the health maintenance of the Japanese people, as intake of natto has been reported to have beneficial effects [5,6,7,8]. Furthermore, γ-PGA has been reported to have a preventive effect against metabolic syndrome [11, 14]. It has also been reported to have an antibacterial effect [15]. Furthermore, it has been suggested that oral administration of γ-PGA increases the abundance of Lactobacillales while reducing the abundance of Clostridiales in the murine gut [16]. Natto affects lipid metabolism and gut microbiota. Therefore, we decided to investigate the effects of natto with an enhanced γ-PGA content on lipid metabolism and intestinal microbiota in mice.
We hypothesized that natto containing a high level of γ-PGA would affect liver lipids and that the effects would be mediated by the intestinal microbiota.
We prepared high-γ-PGA natto and investigated our hypothesis to study the influence of a high-γ-PGA natto diet on liver lipids and cecal microbiota in mice fed it compared to mice fed a standard diet.
MATERIALS AND METHODS
Materials
The high-γ-PGA natto was made by Takano Foods Co. Ltd (Ibaraki, Japan). The γ-PGA content of the natto was found to be 2.3%. The natto was freeze-dried and minced using a grinder. The milled natto was then filtered through a 24-mesh sieve.
Animal experiment
Female Jcl:ICR mice (6 weeks old) were obtained from CLEA Japan Inc. (Tokyo, Japan). All mice were specific pathogen-free (SPF) and randomly categorized into two groups (n=7/group). They were sheltered individually in suspended stainless-steel cages with wire mesh bottoms under the following conditions: relative humidity of 65%, 12-hr periods of light and darkness, and a room temperature of 24 ± 0.5°C. They were fed AIN-93G diet for 7 days. Thereafter, the mice were fed a 30% high γ-PGA-affluent natto diet (PGA group) or control (standard AIN-93G) diet (Con group) for 28 days. The nutritional content of the natto was analyzed by Japan Food Research Laboratories according to the Analytical Manual for Standard Tables of Food Composition in Japan. The analysis revealed a high γ-PGA content in the natto and the following nutritional components: moisture, 2.4%; protein, 40.4%; lipids, 21.9%; ash, 5.8%; sugar, 11.2%; and dietary fiber, 18.3%. Accordingly, we modified the composition of the PGA and Con diets such that each had equivalent quantities of proteins, lipids, and carbohydrates. The AIN-93G diet was used as the diet for the Con group. The diet for the PGA group contained the following ingredients: tert-butylhydroquinone, 0.014 g/kg; choline bitartrate, 2.5 g/kg; L-cystine, 3 g/kg; vitamin mix (AIN-93), 10 g/kg; mineral mix (AIN-93G), 35 g/kg; sucrose, 64.9 g/kg; casein, 78.8 g/kg; soy bean oil, 4.3 g/kg; natto (high γ-PGA), 300 g/kg; α-cornstarch, 132 g/kg; and corn starch, 369.486 g/kg. Feces were collected from each mouse for 2 days immediately before dissection. Feces were dried in a lyophilizer (FD-1000, Tokyo Rikakikai Co., Ltd., Tokyo, Japan) for 24 hr, and the trap cooling temperature was maintained at −45°C. A combination anesthetic was prepared using medetomidine (0.3 mg/kg), midazolam (4.0 mg/kg), and butorphanol (5.0 mg/kg), and it was administered to all the mice via intraperitoneal injection. After anesthesia, the mice were sacrificed by exsanguination. Blood was collected from the heart into heparinized tubes during anesthesia. Plasma was separated from whole blood by centrifugation and stored at −80°C until further analysis. Liver, visceral fat, and cecal contents were also collected from each mouse. Cecal contents were stored at −80°C until further use for analyzing the short-chain fatty acid profile and DNA extraction. All animal procedures were approved by the Animal Care Committee of the Food Research Institute (Tsukuba, Japan; H30-028) and were in accordance with the “Guidelines for Animal Care and Experimentation” of the Food Research Institute, National Agriculture and Food Research Organization (Tsukuba, Japan).
Evaluation of cholesterol, triglyceride, and non-esterified fatty acid content in plasma
Cholesterol, triglyceride, and non-esterified fatty acid (NEFA) levels were analyzed using kits purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Total plasma cholesterol concentrations were evaluated using a cholesterol oxidase-based cholesterol E-test kit. Plasma triglyceride concentrations were determined using a triglyceride E-test kit, which is based on the glycerol-3-phosphate oxidase method. Plasma NEFA concentrations were evaluated using a NEFA C-test kit, which involves the acyl-coenzyme A (CoA) synthase, acyl-CoA oxidase, and peroxidase method.
Evaluation of lipid, triglyceride, and cholesterol content in liver
Liver lipids were extracted using the Bligh and Dyer method [17]. The extracted liver lipid was dissolved in 2-propanol containing 10% Triton X-100. Liver cholesterol and triglyceride concentrations were evaluated using methods similar to those used for determining plasma cholesterol and triglyceride levels.
Evaluation of fecal weight and fecal lipid extraction
Feces were collected for 2 days immediately before dissection and before the commencement of the experimental diet (preliminary breeding period). They were dried in a lyophilizer (FD-1000, Tokyo Rikakikai Co. Ltd., Tokyo, Japan) for 24 hr, and the trap cooling temperature was maintained at −45°C. Further, the lyophilized feces were weighed and then pulverized in a food mill (TML17, Tescom Co., Ltd., Tokyo, Japan) for 30 sec. Fecal lipids were extracted from the fecal powder according to the Bligh and Dyer method [17].
Evaluation of fecal bile acid content
Lyophilized fecal samples were weighed and pulverized before evaluating the bile acid content. The concentration of bile acid in feces was measured using a previously described method [18]. Fecal samples (50 mg) were suspended in a glass test tube containing 99.5% ethanol (2.5 mL), vortexed for 30 sec, incubated for 1 hr at 65°C, and centrifuged at 3,000 rpm for 10 min at 4°C. Supernatants were then transferred to a glass test tube. An equivalent volume (2.5 mL) of 99.5% ethanol was added to the sediment, and the procedure was repeated. Supernatants from both the extractions were pooled in the same glass test tube and dried at 65°C using N2 gas. After drying, 0.5 mL of 90% ethanol was added to the residue and vortexed for 30 sec. Total bile acid concentrations were evaluated using a total bile acid test (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) according to the manufacturer’s instructions.
Microbiota analysis
Cecal DNA was prepared using previously described methods [19]. An equivalent amount of cecal DNA (10 ng each) was used as a template, and the V3 and V4 regions of 16S rRNA genes were amplified using 341F (5′-CCTACGGGNGGCWGCAG-3′) [20] and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) [21] primers, which were linked to the Illumina overhang adapter sequences. An additional polymerase chain reaction (PCR) was performed to add barcodes to each sample. Amplicons were pooled in equal amounts, and paired-end (2 × 300 bp) sequencing was performed using a MiSeq System (Illumina Inc., San Diego, CA, USA) and MiSeq Reagent Kit v3 (Illumina Inc.) following quantification. QIIME2 2019.4.0 (https://qiime2.org) was used to analyze the sequences in demultiplexed format. Denoising of merged paired-end reads was performed using DADA2 [22]. Sequence variants, which were assigned as originating from chloroplasts and mitochondria, were removed from further analyses, and the Greengenes database [23] was used for each representative sequence to annotate the taxonomic information [24]. Alpha- and beta-diversities were analyzed by rarefying the feature table at a consistent sample depth of 65,000.
Evaluation of cecal short-chain fatty acid profiles
Cecal samples were diluted in distilled water, vortexed for 30 sec, and centrifuged. Supernatants were analyzed using a short-chain fatty acid analysis kit (YMC Co., Ltd., Kyoto, Japan) as previously described [25].
Statistical analysis
Data are expressed as the mean ± standard error (SE) or mean ± standard deviation (SD). All data were analyzed in Sigma Plot 11 (Systat Software, Inc., San Jose, CA, USA) using Student’s t-test or the Mann–Whitney rank-sum test. The significance level was set at p<0.05. Additionally, we performed a principal component analysis of the data using an online website of Gunma University (http://aoki2.si.gunma-u.ac.jp/BlackBox/BlackBox.html). We performed correlation analyses between the abundances of bacteria and liver lipids, cecal short-chain fatty acids, fecal lipids, fecal bile acids, visceral fat, and visceral fat/body weight (BW). Further, we prepared a heat map showing the correlation between the relative abundance of family bacteria or phylum bacteria and amount of liver lipids, liver cholesterol, liver triglycerides, fecal lipids, fecal bile acids, visceral fat, visceral fat/BW, cecal acetic acid, cecal butyric acid, cecal propionic acid, cecal valeric acid, cecal isovaleric acid, and cecal total short-chain fatty acids. In the heat map, correlation coefficients closer to −1, +1, and 0 are shown in darker blue colors, darker red colors, and no color, respectively.
RESULTS
General observations
No significant differences were observed in body weight, visceral fat, food consumption, and liver weight between the two groups of mice (Con vs. PGA: BW, 31.8 ± 0.7 g vs. 32.2 ± 0.8 g; visceral fat, 1.68 ± 0.18 g vs. 1.32 ± 0.30 g; food consumption, 4.2 ± 0.06 g vs. 4.0 ± 0.11 g; liver weight, 1.24 ± 0.03 g vs. 1.39 ± 0.06 g). Furthermore, cecal samples were found to weigh 0.15 ± 0.02 g and 0.43 ± 0.07 g in the Con and PGA groups, respectively, and the cecal content of the PGA group was significantly higher than that of the control group (p<0.01).
Analysis of plasma lipids
Plasma concentrations of total cholesterol, triglyceride, and NEFA are shown in Table 1. We did not find significant differences in the plasma concentrations of total cholesterol, triglyceride, and NEFA between the two groups.
Table 1. Concentrations of triglyceride (TG), total cholesterol (Chol), and non-esterified fatty acids (NEFA) in plasma.
| Chol (mg/dL) | TG (mg/dL) | NEFA (mEq/L) | |
|---|---|---|---|
| Con group | 63.0 ± 6.8 | 55.3 ± 7.7 | 0.37 ± 0.08 |
| PGA group | 65.0 ± 5.3 | 67.5 ± 5.9 | 0.42 ± 0.03 |
Con group: standard diet; PGA group: 30% high-γ-PGA natto diet.
Analysis of liver lipids
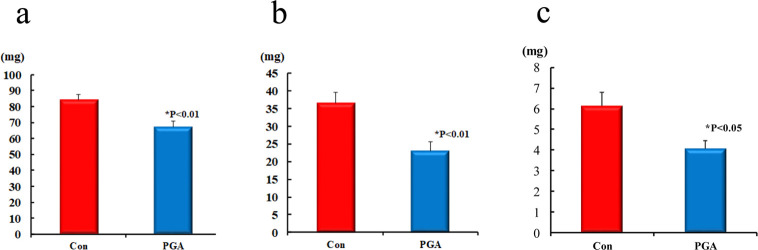
Figure 1 shows the lipid (Fig. 1-a), triglyceride (Fig. 1-b), and cholesterol (Fig. 1-c) contents in the liver. The liver lipid contents were found to be significantly lower in the PGA group compared to those in the Con group (p<0.01). The liver triglyceride contents were significantly lower in the PGA group mice compared to those of the Con group mice (p<0.01). Similarly, the liver cholesterol contents were found to be significantly lower in the PGA group mice than in the Con group mice (p<0.05).
Fig. 1.
Liver lipid contents (a), liver triglyceride contents (b), and liver cholesterol contents (c) in the control diet group (Con group) and high-γ-PGA natto diet group (PGA group).
Liver lipid contents were observed to be significantly lower in the PGA group than in the Con group (p<0.01). Liver triglyceride contents were observed to be significantly lower in the PGA group than in the Con group (p<0.01). Liver cholesterol contents were observed to be significantly lower in the PGA group than in the Con group (p<0.05). Values are expressed as the mean ± standard error (SE; n=7).
Fecal bile acid profile
The amounts of excreted bile acid in the feces are shown in Fig. 2. The amounts of excreted bile acid in the feces were found to be significantly higher in the PGA group (1.26 ± 0.09 μmol/day) than in the Con group (0.58 ± 0.02 μmol/day; p<0.001).
Fig. 2.
Amounts of bile acid excreted in feces in the Con and PGA groups. The amount of bile acid excreted in feces was significantly greater in the PGA group than in the Con group (p<0.001). Values are means ± SE (n=7).
Fecal lipids
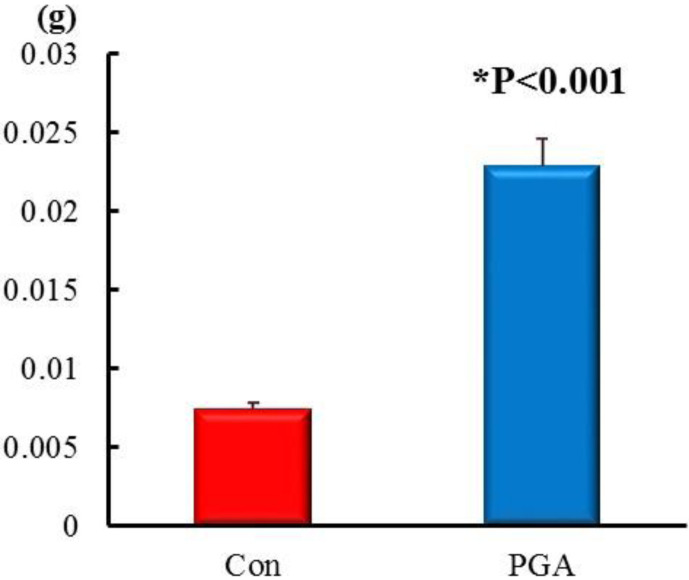
The amounts of lipids in lyophilized feces samples, which were collected on the final day of the experiment, were found to be significantly higher in the PGA group (0.022 ± 0.0017 g/day) than in the Con group (0.007 ± 0.0004 g/day; Fig. 3). These results suggested that a high-γ-PGA natto diet increases the fecal excretion of lipids.
Fig. 3.
Amounts of fecal lipids in the Con and PGA groups (g/day). All feces were collected on the last two days of experimental dietary feeding. The amount of fecal lipids (g/day) was observed to be significantly higher in the PGA group than in the Con group (p<0.001). Values are expressed as the mean ± SE (n=7).
Analysis of cecal short-chain fatty acids
The short-chain fatty acid concentrations in the ceca of mice in the two dietary groups are shown in Fig. 4. Cecal total short-chain fatty acid concentrations tended to be higher in mice fed the PGA diet than in those fed the Con diet. Furthermore, the cecal butyric acid concentration was found to be significantly higher in the PGA group than in the Con group. These results suggested that a diet high in γ-PGA influences the production of cecal short-chain fatty acids in mice.
Fig. 4.
Concentrations of cecal short-chain fatty acids in the two dietary groups. Only the cecal butyric acid concentration (μmol/g cecal contents) showed a significant difference between the two dietary groups. The cecal butyric acid concentration was found to be significantly higher in the PGA group than in the Con group (p<0.05). Values are expressed as the mean ± SE (n=7).
Cecal microbiota
In our analysis of the phyla and families of intestinal microbiota (Table 2), we observed significant differences in the composition of the microbiota between the two dietary groups. At the phylum level, the relative abundance of Actinobacteria was found to be significantly higher in the Con group than in the PGA group. Similarly, the relative abundance of Bacteroidetes was found to be significantly higher in the PGA group than in the Con group. However, no significant difference was observed in the relative abundance of Firmicutes between the two groups. The ratio of the relative abundances of Firmicutes to Bacteroidetes was found to be significantly lower in the PGA group (Fig. 5). Furthermore, microbiota analysis at the family level revealed that the relative abundances of Lachnospiraceae (p<0.05), Mogibacteriaceae (p<0.01), Bacteroidales family S24-7 (p<0.01), and Bacillaceae (p<0.01) were significantly higher in the PGA group than in the Con group. On the other hand, the relative abundances of Bifidobacteriaceae (p<0.05) and Coriobacteriaceae (p<0.01) were found to be significantly lower in the PGA group than in the Con group.
Table 2. Relative abundance of cecal microbiota of mice of Con and PGA groups.
| Phylum / Family | Con | PGA | |
|---|---|---|---|
| Actinobacteria | 40.55 ± 6.37 | 15.70 ± 3.28** | |
| Bifidobacteriaceae | 34.41 ± 5.8 | 15.21 ± 3.28* | |
| Coriobacteriaceae | 6.13 ± 1.3 | 0.49 ± 0.09** | |
| Bacteroidetes | 7.60 ± 1.03 | 21.60 ± 1.98** | |
| Bacteroidaceae | 4.2 ± 0.75 | 5.57 ± 0.59 | |
| Rikenellaceae | 1.26 ± 0.25 | 0.84 ± 0.15 | |
| S24-7 | 1.23 ± 0.2 | 12.06 ± 1.05** | |
| Paraprevotellaceae | 0.91 ± 0.4 | 3.12 ± 0.79 | |
| Firmicutes | 51.59 ± 5.96 | 62.64 ± 2.65 | |
| Bacillaceae | 0 | 3.81 ± 0.38** | |
| Lactobacillaceae | 14.15 ± 1.93 | 9.19 ± 4.28 | |
| Streptococcaceae | 0.11 ± 0.04 | 0.02 ± 0.01 | |
| Turicibacteraceae | 0.68 ± 0.59 | 0 | |
| Christensenellaceae | 0.03 ± 0.01 | 0 | |
| Clostridiaceae | 3.11 ± 1.64 | 0 | |
| Dehalobacteriaceae | 0.01 ± 0.003 | 0.02 ± 0.01 | |
| Lachnospiraceae | 9.15 ± 1.87 | 15.18 ± 1.72* | |
| Peptococcaceae | 0.02 ± 0.01 | 0.03 ± 0.01 | |
| Ruminococcaceae | 3.82 ± 0.74 | 2.97 ± 0.3 | |
| Mogibacteriaceae | 0.11 ± 0.03 | 0.45 ± 0.05** | |
| Erysipelotrichaceae | 15.97 ± 2.83 | 15.15 ± 3.3 | |
| Proteobacteria | 0.26 ± 0.21 | 0.06 ± 0.01 | |
| Comamonadaceae | 0.04 ± 0.01 | 0.04 ± 0.003 | |
| Enterobacteriaceae | 0.21 ± 0.21 | 0.01 ± 0.01 | |
Con group: standard diet; PGA group: 30% high-γ-PGA natto diet.
**p<0.01 as compared with Con group, *p<0.05 as compared with Con group.
Values are expressed as mean ± SE (n=7).
Fig. 5.
Phylum level ratios of Firmicutes/Bacteroidetes of the mice in the Con and PGA groups.
The ratio of Firmicutes/Bacteroidetes was observed to be significantly lower in the PGA group (p<0.05). Values are expressed as the mean ± SE (n=7).
In comparisons of alpha diversity between the two groups, the Faith_pd value (mean ± SD) was significantly higher in the PGA group (13.2 ± 0.7) than in the Con group (11.5 ± 1.0) (p=0.018086). The observed number of operational taxonomic units (OTUs) (mean ± SD) was significantly higher in the PGA group (225.4 ± 21.6) than in the Con group (167.7 ± 25.5; p=0.008809).
Correlation analysis
We constructed a heat map illustrating the correlation between the relative abundances of bacterial phyla and the biochemical contents (quantity of liver lipids, liver cholesterol, liver triglyceride, fecal lipids, fecal bile acids, visceral fat, visceral fat/BW, cecal acetic acid, cecal butyric acid, cecal propionic acid, cecal valeric acid, cecal isovaleric acid, and cecal total short-chain fatty acids; Fig. 6). In this heatmap, the negative correlation becomes stronger as the blue intensity increases, while the positive correlation becomes stronger as the red intensity increases. We observed a trend indicating that the correlation of liver lipids, liver cholesterol, and liver triglyceride with the relative abundances of bacteria at the phylum level differed from that observed for fecal lipids and fecal bile acids with the relative abundances of bacteria at the phylum level (Fig. 6). We also constructed and a heat map illustrating the correlation between the relative abundances of bacterial families and the biochemical contents (quantity of liver lipids, liver cholesterol, liver triglyceride, fecal lipids, fecal bile acids, visceral fat, visceral fat/BW, cecal acetic acid, cecal butyric acid, cecal propionic acid, cecal valeric acid, cecal isovaleric acid, and cecal total short-chain fatty acids; Fig. 7). We also observed a trend indicating that the correlation of liver lipids, liver cholesterol, and liver triglyceride with the relative abundances of bacteria at the family level differed from that observed for fecal lipids and fecal bile acids with the relative abundances of bacteria at the family level (Fig. 7). The relative abundance of Lactobacillaceae showed positive correlation with hepatic total lipids and triglycerides (r=0.653; Fig. 8-a). The relative abundance of Lachnospiraceae was significantly higher in the PGA group and was negatively correlated with the concentration of liver triglycerides (r=−0.732; Fig. 8-b). The relative abundance of Coriobacteriaceae was positively correlated with the quantity of liver triglycerides (r=0.732; Fig. 8-c).
Fig. 6.
Heat map indicating the correlation between microbiota (phylum) and lipids, bile acids, visceral fat, and cecal short-chain fatty acids.
The heat map indicates the correlation between the relative abundances of bacterial phyla and liver lipids, liver cholesterol, liver triglyceride, fecal lipids, fecal bile acids, visceral fat, visceral fat/body weight (BW), and the concentrations of cecal acetic acid, cecal butyric acid, cecal propionic acid, cecal valeric acid, cecal isovaleric acid, and cecal total short-chain fatty acids. In this heat map, the negative correlation becomes stronger as the blue intensity increases, while the positive correlation becomes stronger as the red intensity increases.
Fig. 7.
Heat map indicating the correlation between microbiota (family) and lipids, bile acids, visceral fat, and cecal short-chain fatty acids.
The heat map indicates the correlation between the relative abundances of bacterial families and liver lipids, liver cholesterol, liver triglyceride, fecal lipids, fecal bile acids, visceral fat, visceral fat/body weight (BW), and the concentrations of cecal acetic acid, cecal butyric acid, cecal propionic acid, cecal valeric acid, cecal isovaleric acid, and cecal total short-chain fatty acids. In this heat map, the negative correlation becomes stronger as the blue intensity increases, while the positive correlation becomes stronger as the red intensity increases.
Fig. 8.
Correlation analysis between liver triglyceride content and relative abundance of Lactobacillaceae (a), Lachnospiraceae (b), and Coriobacteriaceae (c).
Principal component analysis
Principal component analysis was performed using data obtained on the relative abundances of Actinobacteria, Bacteroidetes, and Firmicutes and the concentrations of various biochemical contents (cecal acetic acid, cecal butyric acid, cecal propionic acid, cecal valeric acid, cecal isovaleric acid, liver lipids, liver cholesterol, liver triglyceride, fecal lipids, fecal bile acids, and visceral fat). The contribution rate was evaluated to be 45.0 for principal component 1, 20.9 for principal component 2, and 9.7 for principal component 3. Furthermore, a correlation diagram revealed that the distribution of the second principal component (y-axis) and first principal component (x-axis) corresponding to the principal component scores of the PGA and Con groups was clearly divided into two groups (Fig. 9).
Fig. 9.
Correlation diagram indicating the distribution of the second principal component (y axis) and first principal component (x axis).
The two dietary groups were observed to be clearly divided into two groups (circle, Con group; triangle, PGA group).
DISCUSSION
Natto is a traditional Japanese food derived from fermented soy, so it contains biochemical components of soy. Furthermore, dietary soy has been shown to influence the composition of the microbiota. Cross et al. [26] have reported that soy consumption may be linked to higher (p<0.05) relative abundances of multiple genera, including Prevotella, Lachnospiraceae, and Dorea, and lower relative abundances of Coprococcus, Clostridiaceae, Desulfovibrionaceae, Adlercreutzia, Bifidobacterium, and Peptostreptococcaceae. In this study, the relative abundance of Lachnospiraceae was found to be significantly higher in the PGA group mice than in the Con group mice. Furthermore, the relative abundance of Bifidobacteriaceae was found to be significantly lower in the PGA group mice than in the Con group mice. The PGA group mice were fed a fermented soybean diet. Consistent with the findings of Cross et al. [26], our study showed a higher relative abundance of Lachnospiraceae and a lower relative abundance of Bifidobacteriaceae in the cecal samples of the PGA group. Moreover, Cross et al. [26] showed that soy significantly alters the cecal microbial community of low-capacity running (LCR) rats, which were further found to have a lower Firmicutes/Bacteroidetes ratio. Similarly, we observed that the Firmicutes/Bacteroidetes ratio was significantly lower in the PGA group mice. This suggested that soy and fermented soy products influence the ratio of Firmicutes/Bacteroidetes in a similar manner. Further, our results showed that the relative abundance of Bacillaceae was significantly higher in the PGA group. Bacillus is used as a fermentation agent in the process of making natto. Therefore, a significant quantity of Bacillus was present in the PGA-containing natto diet; however, Bacillus was not present in the soybeans. Bacillaceae is a characteristic bacterium that is predominantly present in the microbiota of natto-fed mice. Our results showed that the relative abundance of Coriobacteriaceae was significantly lower in the PGA group than in the Con group.
Gut microbiota of mice are affected by γ-PGA. It has been reported that oral administration of γ-PGA increased the abundance of Lactobacillales and reduced the abundance of Clostridiales in the murine gut [16]. Furthermore, a diet containing 0.5% γ-PGA significantly increased the number of Lactobacillus in the ceca of mice [27]. These reports suggest that administration of γ-PGA to mice increases the number of Lactobacillales in the gut. However, consumption of the high-γ-PGA natto in the present study increased the abundance of Lachnospiraceae and Bacteroidales family S24-7, without increasing the abundance of Lactobacillaceae. The combination of γ-PGA and other components of natto may have contributed to the change in the intestinal microbiota in this study.
In comparisons of alpha diversity between the two groups, the Faith_pd value and observed OTUs were significantly higher in the PGA group than in the Con group. These results suggest that the addition of high-γ-PGA natto to the diet increases the diversity of the intestinal microbiota.
We performed correlation analyses between the abundances of bacteria and different biochemical contents, which included liver lipids, cecal short-chain fatty acids, visceral fat, visceral fat/BW, fecal lipids, and fecal bile acids (Fig. 7). The abundances of Bacillaceae, Bacteroidaceae, Bacteroidales family S24-7, Mogibacteriaceae, and Lachnospiraceae were negatively correlated with liver lipids, liver cholesterol, and liver triglyceride, whereas they were positively correlated with fecal lipids and fecal bile acids. On the other hand, Bifidobacteriaceae, Christensenellaceae, Clostridiaceae Coriobacteriaceae, Lactobacillaceae, and Streptococcaceae were observed to be positively correlated with liver lipids, liver cholesterol, and liver triglyceride, whereas they were negatively correlated with fecal lipids and fecal bile acids. These results suggested that intestinal microbiota can be divided into different categories based on their relationships with lipid metabolism. Our results revealed that the relative abundances of Bifidobacteriaceae and Coriobacteriaceae were significantly lower in the PGA group. Conversely, the relative abundances of Bacillaceae, Bacteroidales family S24-7, Mogibacteriaceae, and Lachnospiraceae were found to be significantly higher in the PGA group. Liver lipid metabolism in the PGA group might have been affected by the presence of these bacteria. However, there have only been a few reports on the effect of intestinal bacteria on liver lipid metabolism, so further studies are required to clarify this point. Strong positive correlation was observed between the abundance of Bacteroidales family S24-7 and cecal butyric acid. Strong positive correlation was also observed between the abundance of Mogibacteriaceae and cecal butyric acid. The abundances of Bacteroidales family S24-7 (p<0.01) and Mogibacteriaceae (p<0.01) were significantly higher in the PGA group than in the Con group. The cecal butyric acid concentration was found to be significantly higher in the PGA group than in the Con group. These results suggested that the presence of high-γ-PGA natto in the diet influences the production of cecal butyric acids in mice by increasing the abundance of Bacteroidales family S24-7 and Mogibacteriaceae.
The amount of fecal lipids was found to be significantly higher in the PGA group than in the Con group. As there was no significant difference in the lipid contents of the diets and the amounts of food intake of the two groups, higher lipid levels in excreted feces might indicate lower lipid absorption by mice in the PGA group, which could be related to lower liver lipid levels. Increased lipid excretion might also influence the microbiota, resulting in an altered microbial composition in comparisons between the two dietary groups. Several studies have reported that dietary medium-chain fatty acids [28] and high-fat diet [29] alter the composition of the gut microbiota.
Mice fed a high-fat diet have been reported to show positive correlation between the detection of Lactobacillus gasseri and/or Lactobacillus taiwanensis DNA in feces and lipid droplets in the liver [30]. Further, it has been reported that the percentage of Lactobacillus spp. within the normal intestinal flora is higher in Tsumura, Suzuki, Obese Diabetes (TSOD) mice than in Tsumura, Suzuki, Non Obesity (TSNO) mice [31]. In this study, the relative abundance of Lactobacillaceae showed positive correlation with hepatic total lipids and triglycerides (Fig. 8-a). Higher abundance of Lactobacillaceae might be related to the accumulation of liver lipids.
On the other hand, it has been suggested that the relative abundance of Lachnospiraceae in the cecum is higher in TSNO mice than in TSOD mice [31]. Our results showed that the relative abundance of Lachnospiraceae was significantly higher in the PGA group and was negatively correlated with the concentration of liver triglycerides (r=−0.732; Fig. 8-b). The relative abundance of Lachnospiraceae might be related to the degree of obesity in the host. Moreover, mice fed a high-fat diet have been shown to have a lower relative abundance of Lachnospiraceae [32]. A significant negative correlation between the Lachnospiraceae family and body fat percentage has already been reported in mice [33]. Such reports suggest that decreased abundance of Lachnospiraceae might be related to enhanced lipid accumulation. Further, an inverse association between Lachnospiraceae and cardiovascular risk factors has been reported in adults with cardiovascular risk [34]. Thus, the higher relative abundance of Lachnospiraceae in the PGA group could have beneficial effects on host physiology.
In this study, we observed that the relative abundance of Coriobacteriaceae was significantly lower in the PGA group than in the Con group. Previously, it has been reported that Coriobacteriaceae is strictly associated with the hepatic concentrations of triglycerides, glucose, and glycogen in mice [35]. Consistent with this previously published study, our results showed that the relative abundance of Coriobacteriaceae was positively correlated with the liver triglyceride level (r=0.732; Fig. 8-c). Further, studies have reported that Eggerthella lenta belongs to the Coriobacteriaceae family, which has been shown to be related to the occurrence of type-2 diabetes [36]. Thus, the lower abundance of Coriobacteriaceae in the PGA group might be beneficial for preventing the occurrence of metabolic syndrome.
The present study revealed that the relative abundance of Bacteroidales family S24-7 was significantly higher in the PGA group than in the Con group and that the relative abundance of Bacteroides tended to be higher in the PGA group. Furthermore, significant negative correlation was observed between the relative abundances of Bacteroides (r=−0.541) and Bacteroidales family S24-7 (r=−0.631) and the liver cholesterol content. Similarly, significant negative correlation was observed between the relative abundance of Bacteroidales family S24-7 and the liver triglyceride content (r=−0.732). Recently, Bacteroides thetaiotaomicron-colonized gnotobiotic mice have been demonstrated to exhibit promotion of bile salt hydrolase (BSH) activity, which has been reported to alter bile acid metabolism, and lower liver and plasma lipid levels [37]. Bacteroides and Bacteroidales family S24-7 might be related to lipid and bile acid metabolism. Alterations in the bile acid profile have been shown to modulate the expression of lipid uptake and glucose metabolism-related genes [37]. Upregulation of bile acid in the lower gut could be one of the reasons for the alteration of the gut microbiota and lipid metabolism in the γ-PGA-fed mice.
Sodium butyrate has been shown to protect the intestinal barrier function [38]. We observed a positive correlation between butyric acid and the relative abundance of Bacteroidales family S24-7. Thus, Bacteroidales family S24-7 might be associated with the integrity of the gut barrier function. It has been reported that many bacteria belonging to the Lachnospiraceae family can produce butyric acid [39]. In our study, the relative abundances of Bacteroidales family S24-7 and Lachnospiraceae were found to be significantly higher in the PGA group. Moreover, the cecal butyrate concentration was observed to be significantly elevated in the PGA group. Thus, intake of natto might be beneficial for strengthening the intestinal barrier function through enhanced production of butyrate. It has been reported that the abundance of Bacteroidales family S24-7 was high in CD obesity-prone rats fed resistant starch [40]. A resistant starch diet has been reported to increase butyrate production by intestinal microbiota [41]. The relationship between butyric acid and Bacteroidales family S24-7 requires further study.
Principal component analysis (Fig. 9) based on the correlation diagram between first and second principal components revealed that the two dietary groups were distributed in independent regions. Thus, the data suggest that each of these two groups have different characteristics with no intersecting regions. Principal component 1 was defined as decrease of the relative abundance of Actinobacteria and increase of the relative abundance of Firmicutes, and principal component 2 was defined as upregulation of the cecal acetic acid concentration and downregulation of liver lipids. Thus, the principal component analysis inferred that the two dietary groups were clearly divided into two regions due to the differences in their cecal microbiota compositions and liver lipid levels.
In a previous study of rats fed natto, it was reported that hepatic cholesterol was markedly increased by cholesterol loading and that natto did not have an inhibitory effect on this [8]. In our study, the liver cholesterol contents were found to be significantly lower in the PGA group mice than in the Con group mice (p<0.05). Fortification of γ-PGA content in natto may be effective for improving lipid metabolism in the liver.
Accumulation of liver lipids has been reported to facilitate the onset of nonalcoholic fatty liver disease (NAFLD). NAFLD represents a spectrum of liver diseases that are caused by the accumulation of ectopic fat in the liver, which cannot be explained by alcohol consumption [42]. NAFLD is strongly associated with obesity, type 2 diabetes mellitus (T2DM), and metabolic syndrome [42].
In this study, fecal lipids and fecal bile acids were found to be significantly higher in the PGA group than in the Con group. Liver lipids were found to be significantly lower in the PGA group than in the Con group. Thus, these results suggest that natto with a high γ-PGA concentration would be a beneficial dietary component for the prevention of NAFLD.
Acknowledgments
This work was supported by a grant for a commissioned project study entitled “Research Development for Discovering of regional agricultural products and foods having a beneficial impact on health” from the Ministry of Agriculture, Fishery and Forestry, Japan (JPJ005336). The amplicon analysis of the 16S rRNA gene was supported by the Advanced Genomics Breeding Section of the Institute of Crop Science, NARO (NICS; Project ID: 18A17).
REFERENCES
- 1.Hesseltine CW, Wang HWAL. 1967. Traditional fermented foods. Biotechnol Bioeng 9: 275–288. [Google Scholar]
- 2.Hara T, Ueda S. 1982. Regulation of polyglutamate production in Bacillus subtilis (natto): transformation of high PGA productivity. Agric Biol Chem 46: 2275–2281. [Google Scholar]
- 3.Nagata C, Wada K, Tamura T, Konishi K, Goto Y, Koda S, Kawachi T, Tsuji M, Nakamura K. 2017. Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: the Takayama study. Am J Clin Nutr 105: 426–431. [DOI] [PubMed] [Google Scholar]
- 4.Park KJ, Kang JI, Kim TS, Yeo IH. 2012. The antithrombotic and fibrinolytic effect of natto in hypercholesterolemia rats. Prev Nutr Food Sci 17: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishikawa A, Kishi M, Yamagami K. 2009. Effect of intake of natto and soybeans on postprandial blood glucose levels in healthy adults. Journal of Urban Living and Health Association 53: 257–260. [Google Scholar]
- 6.Taniguchi-Fukatsu A, Yamanaka-Okumura H, Naniwa-Kuroki Y, Nishida Y, Yamamoto H, Taketani Y, Takeda E. 2012. Natto and viscous vegetables in a Japanese-style breakfast improved insulin sensitivity, lipid metabolism and oxidative stress in overweight subjects with impaired glucose tolerance. Br J Nutr 107: 1184–1191. [DOI] [PubMed] [Google Scholar]
- 7.Uemura H, Katsuura-Kamano S, Nakamoto M, Yamaguchi M, Fujioka M, Iwasaki Y, Arisawa K. 2018. Inverse association between soy food consumption, especially fermented soy products intake and soy isoflavone, and arterial stiffness in Japanese men. Sci Rep 8: 9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuji K, Tsuji E. 1986. Effect of natto-feeding on cholesterol levels of rats. Jpn J Nutr 44: 41–44. [Google Scholar]
- 9.Suzuki H, Watabe J, Takeuchi H, Tadano Y, Masuda S, Maruta K. 2004. Effects of Bacillus subtilis C-3102 intakes on the composition and metabolic activity of fecal microflora of humans. J Intestinal Microb 18: 93–99. [Google Scholar]
- 10.Kanno A, Takamatsu H. 1995. Determination of γ-polyglutamic acid in “Natto” using cetyltrimethylammonium bromide. Nippon Shokuhin Kagaku Kogaku Kaishi 42: 878–886. [Google Scholar]
- 11.Lee EH, Son WC, Lee SE, Kim BH. 2013. Anti-obesity effects of poly-γ-glutamic acid with or without isoflavones on high-fat diet induced obese mice. Biosci Biotechnol Biochem 77: 1694–1702. [DOI] [PubMed] [Google Scholar]
- 12.Park JH, Choi JC, Sung MH, Kang JH, Chang MJ. 2011. High molecular weight poly-gamma-glutamic acid regulates lipid metabolism in rats fed a high-fat diet and humans. J Microbiol Biotechnol 21: 766–775. [DOI] [PubMed] [Google Scholar]
- 13.Lee W, Kim M, Lee SH, Jung HG, Oh JW. 2018. Prophylactic efficacy of orally administered Bacillus poly-γ-glutamic acid, a non-LPS TLR4 ligand, against norovirus infection in mice. Sci Rep 8: 8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeon YH, Kwak MS, Sung MH, Kim SH, Kim MH, Chang MJ. 2013. High-molecular-weight poly-gamma-glutamate protects against hypertriglyceridemic effects of a high-fructose diet in rat. J Microbiol Biotechnol 23: 785–793. [DOI] [PubMed] [Google Scholar]
- 15.Lee NR, Go TH, Lee SM, Jeong SY, Park GT, Hong CO, Son HJ. 2014. In vitro evaluation of new functional properties of poly-γ-glutamic acid produced by Bacillus subtilis D7. Saudi J Biol Sci 21: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin HE, Choi JC, Lim YT, Sung MH. 2017. Prebiotic effects of poly-gamma-glutamate on bacterial flora in murine gut. J Microbiol Biotechnol 27: 412–415. [DOI] [PubMed] [Google Scholar]
- 17.Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 18.Tamura M, Hori S, Hoshi C, Nakagawa H. 2012. Effects of rice bran oil on the intestinal microbiota and metabolism of isoflavones in adult mice. Int J Mol Sci 13: 10336–10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamura M, Nakagawa H, Hori S, Suzuki T, Hirayama K. 2019. Plasma quercetin metabolites are affected by intestinal microbiota of human microbiota-associated mice fed with a quercetin-containing diet. J Clin Biochem Nutr 65: 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO. 2013. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy R, Lappin DF, Dixon PM, Buijs MJ, Zaura E, Crielaard W, O’Donnell L, Bennett D, Brandt BW, Riggio MP. 2016. The microbiome associated with equine periodontitis and oral health. Vet Res (Faisalabad) 47: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13: 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. 2012. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72: 5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura M, Hori S, Inose A, Kobori M. 2020. Effects of γ-polyglutamic acid on blood glucose and caecal short chain fatty acids in adult male mice. Food Nutr Sci 11: 8–22. [Google Scholar]
- 26.Cross TL, Zidon TM, Welly RJ, Park YM, Britton SL, Koch LG, Rottinghaus GE, de Godoy MRC, Padilla J, Swanson KS, Vieira-Potter VJ. 2017. Soy improves cardiometabolic health and cecal microbiota in female low-fit rats. Sci Rep 7: 9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura M, Hoshi C, Kimura Y, Suzuki T, Yamamoto-Maeda M. 2018. Effects of γ-polyglutamic acid on the cecal microbiota and visceral fat in KK-Ay/TaJcl male mice. Food Sci Technol Res 24: 151–157. [Google Scholar]
- 28.Zentek J, Buchheit-Renko S, Männer K, Pieper R, Vahjen W. 2012. Intestinal concentrations of free and encapsulated dietary medium-chain fatty acids and effects on gastric microbial ecology and bacterial metabolic products in the digestive tract of piglets. Arch Anim Nutr 66: 14–26. [DOI] [PubMed] [Google Scholar]
- 29.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. 2009. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 137: 1716–24.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng H, Liu J, Jackson MI, Zhao FQ, Yan L, Combs GF., Jr2013. Fatty liver accompanies an increase in Lactobacillus species in the hind gut of C57BL/6 mice fed a high-fat diet. J Nutr 143: 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horie M, Miura T, Hirakata S, Hosoyama A, Sugino S, Umeno A, Murotomi K, Yoshida Y, Koike T. 2017. Comparative analysis of the intestinal flora in type 2 diabetes and nondiabetic mice. Exp Anim 66: 405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martins LMS, Perez MM, Pereira CA, Costa FRC, Dias MS, Tostes RC, Ramos SG, de Zoete MR, Ryffel B, Silva JS, Carlos D. 2018. Interleukin-23 promotes intestinal T helper type17 immunity and ameliorates obesity-associated metabolic syndrome in a murine high-fat diet model. Immunology 154: 624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins B, Hoffman J, Martinez K, Grace M, Lila MA, Cockrell C, Nadimpalli A, Chang E, Chuang CC, Zhong W, Mackert J, Shen W, Cooney P, Hopkins R, McIntosh M. 2016. A polyphenol-rich fraction obtained from table grapes decreases adiposity, insulin resistance and markers of inflammation and impacts gut microbiota in high-fat-fed mice. J Nutr Biochem 31: 150–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tindall AM, McLimans CJ, Petersen KS, Kris-Etherton PM, Lamendella R. 2020. Walnuts and vegetable oils containing oleic acid differentially affect the gut microbiota and associations with cardiovascular risk factors: follow-up of a randomized, controlled, feeding trial in adults at risk for cardiovascular disease. J Nutr 150: 806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Claus SP, Ellero SL, Berger B, Krause L, Bruttin A, Molina J, Paris A, Want EJ, de Waziers I, Cloarec O, Richards SE, Wang Y, Dumas ME, Ross A, Rezzi S, Kochhar S, Van Bladeren P, Lindon JC, Holmes E, Nicholson JK. 2011. Colonization-induced host-gut microbial metabolic interaction. MBio 2: e00271–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. 2012. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490: 55–60. [DOI] [PubMed] [Google Scholar]
- 37.Yao L, Seaton SC, Ndousse-Fetter S, Adhikari AA, DiBenedetto N, Mina AI, Banks AS, Bry L, Devlin AS. 2018. A selective gut bacterial bile salt hydrolase alters host metabolism. eLife 7: e37182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han X, Song H, Wang Y, Sheng Y, Chen J. 2015. Sodium butyrate protects the intestinal barrier function in peritonitic mice. Int J Clin Exp Med 8: 4000–4007. [PMC free article] [PubMed] [Google Scholar]
- 39.Vital M, Karch A, Pieper DH. 2017. Colonic butyrate-producing communities in humans: an overview using omics data. mSystems 2: 00130–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obanda D, Page R, Guice J, Raggio AM, Husseneder C, Marx B, Stout RW, Welsh DA, Taylor CM, Luo M, Blanchard EE, Bendiks Z, Coulon D, Keenan MJ. 2018. CD obesity-prone rats, but not obesity-resistant rats, robustly ferment resistant starch without increased weight or fat accretion. Obesity (Silver Spring) 26: 570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coppola S, Avagliano C, Calignano A, Berni Canani R. 2021. The protective role of butyrate against obesity and obesity-related diseases. Molecules 26: 682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. 2014. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol 20: 1724–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]