Abstract
Introduction:
Fatty liver disease (FLD) influences liver disease progression and liver cancer risk. We investigated the impact of FLD on liver disease severity in a large North American cohort with chronic hepatitis B (HBV).
Methods:
Liver biopsies from 420 HBsAg+ adults enrolled in the Hepatitis B Research Network and who were not on HBV therapy in the prior month were evaluated for inflammation and fibrosis. Steatohepatitis was based on steatosis, hepatocyte ballooning ± Mallory-Denk bodies and perisinusoidal fibrosis. Models evaluated factors associated with steatohepatitis, and the associations of steatohepatitis with fibrosis, and longitudinal ALT, AST and FIB-4.
Results:
The median age was 42 years, 62.5% were male and 79.5% were Asian. One hundred thirty-two (31.4%) patients had FLD [77 (18.3%) steatosis only, 55 (13.1%) steatohepatitis]. Older age, overweight/obesity, and diabetes were associated with steatohepatitis. Steatohepatitis (versus no FLD) was associated with 1.68 times higher risk of advanced fibrosis at baseline (95%CI,1.12-2.51) and there was indication of higher incident cirrhosis rate during follow-up. Steatohepatitis versus no FLD was also independently associated with, on average, 1.39 times higher ALT (P<0.01) and 1.25 times higher FIB-4 (P=0.04) across four years.
Discussion:
Coexisting steatosis occurred in nearly a third of adults (13% had steatohepatitis) with chronic HBV in this North American cohort who underwent liver biopsies. Steatohepatitis was associated with advanced fibrosis and higher biochemical measures of hepatic inflammation overtime. Therefore, additional to viral suppression, screening for and managing metabolic abnormalities, are important to prevent disease progression in HBV.
Keywords: nonalcoholic fatty liver disease, NASH, fibrosis, FIB-4, insulin resistance
Introduction
Chronic hepatitis B (HBV) infection affects more than 257 million individuals worldwide and is associated with a higher risk of cirrhosis and hepatocellular carcinoma (HCC).(1) In the United states, an estimated 0.8-2.2 million persons have chronic HBV.(2–4) Fatty liver disease (FLD) is also prevalent and significantly contribute to liver-related morbidity and mortality. (4–7) While hepatitis C (HCV) is a risk factor for diabetes and metabolic derangements (8–10), the relationship between lipid metabolism or hepatic steatosis in HBV is unclear, with studies showing conflicting results.(11) Nonetheless, studies mostly from Asia, have found a high prevalence of co-existing FLD among individuals with chronic HBV.(12–14) Coexisting metabolic derangement and FLD increase HCC (15–17) and cirrhosis risk (18, 19) in persons with HBV, suggesting an additive or synergistic effect. However, few studies to date have utilized histology to confirm the presence of steatosis or to examine whether there is a difference in the association between steatosis versus steatohepatitis on hepatic inflammation and fibrosis as well as clinical outcomes in HBV.(19)
The National Institute of Diabetes, Digestive, and Kidney Diseases-sponsored Hepatitis B Research Network (HBRN) was designed to investigate the pathogenesis, natural history, and therapy of chronic HBV in individuals living in North America who were monitored longitudinally. We utilized a large cohort with liver biopsies to describe the histologic features of coexisting FLD in the setting of chronic HBV in adults. Additionally, we determined the association of FLD with concurrent histological fibrosis, and disease progression using prospectively measured aspartate (AST) and alanine aminotransferase (ALT) levels and Fibrosis-4 (FIB-4), as surrogate measures, and HBV clinical and virologic outcomes on follow-up.
Participants and Methods:
Adult participants of the HBRN Cohort and Adult Immune Active Trial, from 21 centers in the US and in Toronto, Canada who had undergone a liver biopsy were included.(20) The Cohort study enrolled hepatitis B surface antigen (HBsAg)-positive adults 18 years or older who did not have a history of hepatic decompensation, HCC, solid organ or bone marrow transplantation or known human immunodeficiency virus (HIV), and who were not currently receiving HBV therapy. The Adult Immune Active Trial enrolled adults ≥18 years old, with HBV DNA ≥1000 IU/mL, and ALT >30 U/L in males, >20 U/L in females. For this report, we excluded: acute HBV, HCV, HDV, HIV coinfection, inadequate liver biopsy samples, and receipt of HBV therapy within 4 weeks prior to the biopsy.
Participants were enrolled from January 2011 to January 2018. The last follow-up assessment was completed in January 2020. Following enrollment, participants were evaluated at weeks 12 and 24, and every 24 weeks thereafter. For cross sectional analyses, data from the closest study assessment that was beyond 4 weeks of prior HBV medication use and was within 24 weeks of the liver biopsy were used. Alcohol use and HBsAg were only assessed annually and values within 48 weeks prior to the biopsy were used. For longitudinal analyses, participants with <1 assessment occurring at least 24 weeks following the liver biopsy and before randomization into the Adult Immune Active Trial, were excluded. Participants who started treatment following the biopsy as part of clinical care were included. All protocols were approved by the HBRN Steering Committee and the Institutional Review Boards (Research Ethics Board in Canada), and all participants provided written informed consent.
Assessment of liver histology
Liver biopsies were stained by a central laboratory and scored by the HBRN Pathology Committee without the pathologists’ knowledge of clinical and laboratory data. Adequate specimens (i.e., representative with a minimum of three portal tracts) were included. Using the Ishak scoring system, biopsies were scored for periportal, portal and lobular inflammation (range: 0-4) and fibrosis (range: 0-6).(21) Advanced fibrosis was defined as Ishak score ≥3. Perisinusoidal fibrosis was assessed as 0 for none, 1 for mild (visible only on trichrome) and 2 for moderate (evident on the H&E stain). Steatosis was graded as: none, minimal (<5% of hepatocytes), mild (5-33%), moderate (34-66)%, and severe (≥67%).(27) Steatohepatitis (see Figure 1) was based on presence of steatosis ≥5%, parenchymal inflammation, and hepatocyte ballooning with or without Mallory-Denk bodies and perisinusoidal fibrosis in an appropriate architectural pattern with consensus of the pathology committee.(22) FLD status was categorized as 1) steatohepatitis, 2) steatosis (defined as ≥5% steatosis without steatohepatitis), or 3) no FLD.
Figure 1.
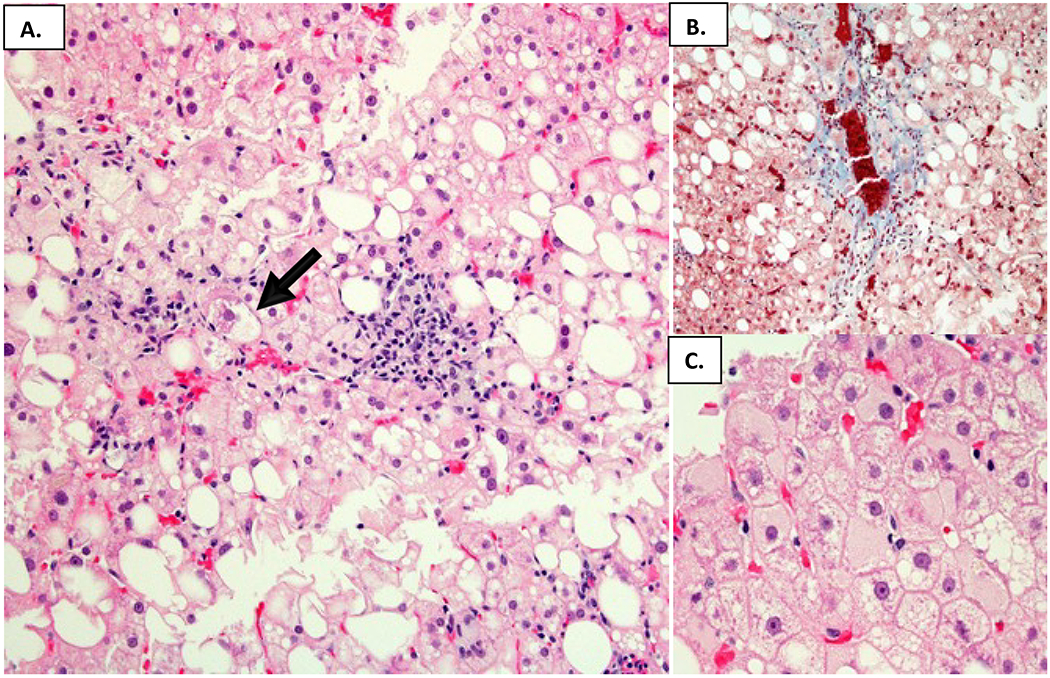
A representative liver biopsy showing typical features of steatohepatitis in HBV infected individuals. A. Steatosis with readily evident balloon hepatocytes and Mallory-Denk bodies depicted by the arrow (Hematoxylin and eosin). B. Zone 3 perisinusoidal fibrosis (Masson trichrome). C. Scattered ground glass cells (Hematoxylin and eosin).
Assessment of clinical and laboratory data and data definitions
Detailed clinical and laboratory data definitions are provided in supplemental materials. Race/ethnicity and alcohol consumption (23) were by self-report. Body mass index (BMI) (24), diabetes, hypertension, and hyperlipidemia were recorded. Laboratory tests were measured and FIB-4 score was calculated.(25) The clinical and virologic outcomes including cirrhosis, hepatic decompensation, HCC, liver transplantation, death, and HBeAg and HBsAg loss in follow-up were adjudicated by an HBRN committee.
Statistical analysis
Descriptive statistics were used to summarize sample characteristics. Histology was compared by FLD status and by advanced fibrosis status, and demographic, clinical and virologic characteristics by FLD status using the χ2 test, the Fisher’s exact test, the Jonckheere-Terpstra trend test, or the Kruskal-Wallis test, as appropriate.
A multivariable log binomial regression model was used to test and estimate associations with steatohepatitis. Age, sex and HBV DNA were forced in the model. The following variables were also considered: race, alcohol use, race-adjusted BMI category, diabetes, hypertension, ALT, AST, HBV genotype, HBeAg status, and HBsAg level. Variables with P≥0.10 were removed using a stepwise variable selection method. A generalized linear model with the gamma link function (to address the skewed distribution of the outcome) was employed to evaluate the association between FLD and fibrosis scores. Additionally, log binomial regression was employed to evaluate the association between FLD status and advanced fibrosis. Both models controlled for age, sex, ALT, and HBV DNA.
Longitudinal analysis
A linear mixed model fit using maximum likelihood with a person-level random intercept was used to evaluate the adjusted association between FLD status and ALT through 192 weeks (3.7 years) of follow-up, with time since liver biopsy date as a continuous fixed effect. Sex, age at liver biopsy, alcohol use, and HBV DNA and current use of anti-HBV medication at each time point were forced in the model as fixed effects. To meet the normality assumption, the log2 scale was used and results are presented as mean ratios (factor by which the mean value of ALT differs for the comparator vs. the reference) rather than differences. Analysis was repeated replacing ALT with AST and with FIB-4. Since age is a component of FIB-4, it was omitted from the FIB-4 model.
Frequencies of death, HCC, hepatic decompensation and liver transplantation were too rare to evaluate in reference to FLD. Among adults without cirrhosis within 24 weeks of baseline, incident cirrhosis during follow-up was analyzed as incidence per 100 person-years of follow-up and compared by FLD status.
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). Reported P values are two-sided.
Results
Sample selection
Of 2030 potential participants, 421 met criteria for inclusion in the analysis sample (see Supplemental Figure for participant flow). Among participants, 198 (47.0%) underwent a biopsy for clinical indication, 91 (21.6%) for entry into the Adult Immune Active Trial, and 132 (31.4%) did not have reason recorded.
Participant characteristics
Table 1 provides clinical, virologic, and detailed histologic characteristics of participants. The median age was 42 (range 18-77) years, 62.5% were male and the majority (79.5%) were of Asian race. More than 60% were overweight or obese and nearly 9% were diabetic. Most participants were HBeAg negative with median log10 HBV DNA of 5.7 IU/mL.
Table 1.
Demographic, clinical, virologic and histopathologic characteristics of study participants with chronic HBV who underwent a liver biopsy
| Overall n=421a | |
|---|---|
| Demographic | |
| Age, median (25th: 75th) years | 42 (34:52) |
| Female, n (%) | 158 (37.5%) |
| Race, n (%) | n=419 |
| White | 43 (10.3%) |
| Black | 33 (7.9%) |
| Asian | 333 (79.5%) |
| Other | 10 (2.4%) |
| Clinical | |
| Weight status (race-specific), n (%) | n=368 |
| Underweight/Normal | 138 (37.5%) |
| Overweight | 150 (40.8%) |
| Obese | 80 (21.7%) |
| Diabetes, n (%) | 31 (8.8%) |
| Virologic | |
| HBeAg status, n (%) | n=367 |
| Positive | 143 (39.0%) n=375 |
| HBV DNA (log10 IU/mL), median (25th: 75th) | 5.7 (4.1: 7.7) |
| Steatosis severity, n (%) | |
| None (0%) | 78 (18.5%) |
| Minimal (<5%) | 219 (52.0%) |
| Mild (5-33%) | 79 (18.8%) |
| Moderate (34-66%) | 36 (8.6%) |
| Severe (≥67%) | 9 (2.1%) |
| Steatohepatitis, n (%) | 55 (13.1%) |
| HAI score, median (25th: 75th) | 5 (3: 7) |
| Ishak Fibrosis score, n (%) | |
| 0 | 93 (22.1%) |
| 1-2 | 236 (56.1%) |
| 3-4 | 62 (14.7%) |
| 5-6 | 30 (7.1%) |
| Perisinusoidal fibrosis grade, n (%) | n=420 |
| None | 307 (73.1%) |
| Mild | 82 (19.5%) |
| Moderate | 31 (7.4%) |
Abbreviations: HBcAg, quantitative hepatitis B core antigen; HBsAg, quantitative hepatitis B surface antigen; HBV, hepatitis B virus; HAI, hepatic activity index
Unless otherwise noted within the table.
On histology, 31.4% (95%CI, 26.9-35.8%; n=132) had FLD; 18.3% (95%CI, 61.5-92.6%; n=77) with steatosis only and 13.1% (95%CI, 41.5-68.6%; n=55) with steatohepatitis. Prevalence of advanced fibrosis (Ishak ≥3) was 21.8% and cirrhosis (Ishak ≥5) was present in 7.1% (Table 1).
Histologic characteristics by FLD status and advanced fibrosis status
Moderate or severe steatosis was more common in those with steatohepatitis (40% and 18.2%, respectively) than steatosis alone (9.1% and 5.2%, respectively). Among participants with steatohepatitis, a larger percentage of hepatocytes showed no immunostaining for either HBcAg or HBsAg 16.4% versus 5.6% and 7.8% in those with steatosis alone and no FLD, but the overall percent of HBcAg-positive, HBsAg-positive, or both HBcAg/HBsAg-positive hepatocytes was not significantly different among the three groups (P=0.09).
While the severity of portal (P=0.34), periportal (P=0.15), and lobular inflammation (P=0.18) were not statistically significantly different by FLD status, the presence of steatohepatitis versus no FLD or steatosis alone, was significantly associated with higher perisinusoidal fibrosis (Figure 2A; P<0.001 for both comparisons) and higher Ishak fibrosis scores (Figure 2B; P=0.002 for both comparisons). Moreover, while the grades of steatosis (moderate/severe steatosis 13.0% vs 10.0%, P=0.41) or the presence of any HBsAg-positive and/or HBcAg-positive immunostaining (95.7% vs 91.7%, P=0.20) did not differ in those with (n=92) vs without advanced (Ishak≥3) fibrosis (n=329), those with advanced fibrosis had higher frequency of features of steatohepatitis including hepatocyte ballooning (22.8% vs 8.8%, P <0.001) and Mallory-Denk bodies (20.7% vs 5.2%, P <0.001), along with higher grades (≥3) of portal (27.2% vs 4.0%, P <0.001), periportal (51.1% vs 12.5%, P <0.001) and lobular inflammation (39.1% vs 21.2%, P =0.02).
Figure 2.
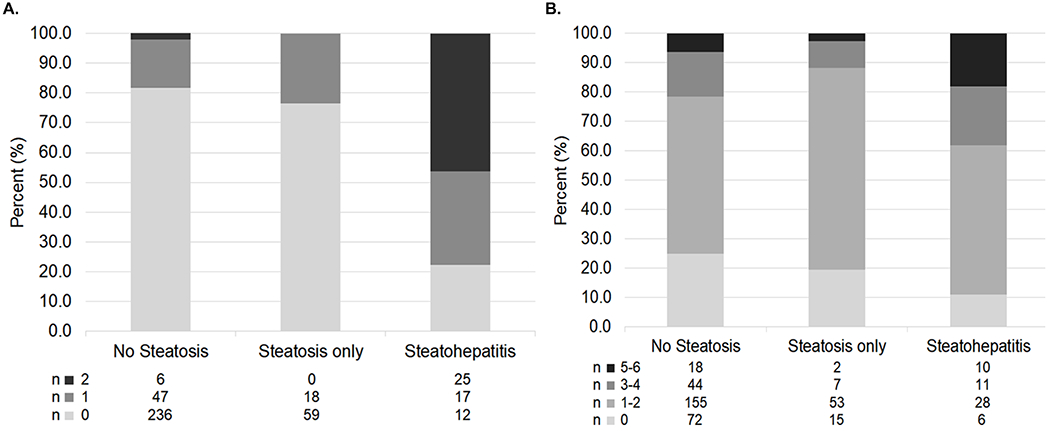
The severity of A. Perisinusoidal fibrosis (i.e., grade) and B. Ishak fibrosis (i.e., stage) by FLD status in study participants with chronic HBV.
Factors associated with steatohepatitis
Clinical and biochemical factors
Table 2 summarizes participant characteristics by FLD status. Participants with steatohepatitis were more likely to be older, obese and to have hypertension and diabetes compared to those with steatosis alone and those with no FLD (all P<0.001). A higher proportion with steatohepatitis appeared to have ALT >2x upper limit of normal compared to those with no FLD (71.1% vs 55.6%, P=0.058). The proportion of participants with steatohepatitis by age, sex, race, race-adjusted BMI category, and diabetes status is shown in Figure 3 and supporting data is provided in Supplemental Table 1.
Table 2:
Sociodemographic, clinical and virologic characteristics of study participants with chronic HBV by FLD status
| 1: No FLD | 2: Steatosis | 3: Steatohepatitis | Overall | Pairwise P-valuesb | |||
|---|---|---|---|---|---|---|---|
| n=289a | N=77a | n=55a | P-value b | 1 vs 2 | 1 vs 3 | 2 vs 3 | |
| Age, years, | <0.001 | 0.047 | <0.001 | 0.047 | |||
| Median (25th:75th) | 40 (32: 51) | 43 (35: 53) | 47 (42: 56) | ||||
| Female, n (%) | 117 (40.5%) | 24 (31.2%) | 17 (30.9%) | 0.18 | |||
| Race, n (%) | n=287 | 0.57 | |||||
| White | 27 (9.4%) | 10 (13.0%) | 6 (10.9%) | ||||
| Black | 26 (9.1%) | 4 (5.2%) | 3 (5.5%) | ||||
| Asian | 226 (78.7%) | 63 (81.8%) | 44 (80.0%) | ||||
| Other | 8 (2.8%) | 0 (0.0%) | 2 (3.6%) | ||||
| Past year alcohol use, n (%) | n=235 | n=68 | n=48 | 0.29c | |||
| None/minimal | 169 (71.9%) | 42 (61.8%) | 34 (70.8%) | ||||
| Low-risk | 48 (20.4%) | 21 (30.9%) | 8 (16.7%) | ||||
| At-risk | 18 (7.7%) | 5 (7.4%) | 6 (12.5%) | ||||
| BMI category (race-specific), n (%) | n=250 | n=67 | n=51 | <0.001 c | <0.001 | <0.001 | 0.02 |
| Underweight/Normal | 121 (48.4%) | 13 (19.4%) | 4 (7.8%) | ||||
| Overweight | 95 (38.0%) | 33 (49.3%) | 22 (43.1%) | ||||
| Obese | 34 (13.6%) | 21 (31.3%) | 25 (49.0%) | ||||
| Hyperlipidemia, n (%) | n=235 | n=67 | n=48 | 0.16 | |||
| Yes | 37 (15.7%) | 11 (16.4%) | 13 (27.1%) | ||||
| Hypertension, n (%) | n=236 | n=67 | n=48 | <0.001 | 0.003 | <0.001 | 0.03 |
| Yes | 42 (17.8%) | 24 (35.8%) | 27 (56.3%) | ||||
| Diabetes, n (%) | n=236 | n=67 | n=48 | <0.001 | 0.22 | <0.001 | 0.01 |
| Yes | 11 (4.7%) | 6 (9.0%) | 14 (29.2%) | ||||
| Chronic HBV phenotype, n (%) | n=239 | n=66 | n=50 | 0.08 | |||
| Immune tolerant | 0 (0.0%) | 1 (1.5%) | 0 (0.0%) | ||||
| HBeAg-positive | 90 (37.7%) | 25 (37.9%) | 11 (22.0%) | ||||
| HBeAg-negative | 83 (34.7%) | 22 (33.3%) | 25 (50.0%) | ||||
| Inactive carrier | 14 (5.9%) | 2 (3.0%) | 0 (0.0%) | ||||
| Indeterminant | 52 (21.8%) | 16 (24.2%) | 14 (28.0%) | ||||
| HBV Genotype, n (%) | n=260 | n=71 | n=50 | 0.76 | |||
| A | 39 (15.0%) | 9 (12.7%) | 8 (16.0%) | ||||
| B | 97 (37.3%) | 30 (42.3%) | 24 (48.0%) | ||||
| C | 96 (36.9%) | 28 (39.4%) | 15 (30.0%) | ||||
| D | 18 (6.9%) | 2 (2.8%) | 1 (2.0%) | ||||
| Other/multiple | 10 (3.8%) | 2 (2.8%) | 2 (4.0%) | ||||
| ALT (U/L) | n=252 | n=69 | n=52 | 0.53 | |||
| Median (25th:75th) | 60.5 (36: 98.5) | 58 (43: 86) | 67 (46: 93) | ||||
| ALT X ULN, n (%) | n=252 | n=69 | n=52 | 0.02 c | 0.45 | 0.058 | 0.45 |
| 0 to 1 | 29 (11.5%) | 3 (4.3%) | 1 (1.9%) | ||||
| >1 to 2 | 83 (32.9%) | 24 (34.8%) | 14 (26.9%) | ||||
| >2 | 140 (55.6%) | 42 (60.9%) | 37 (71.2%) | ||||
| AST (U/L) | n=230 | n=67 | n=48 | 0.29 | |||
| Median (25th:75th) | 40 (29: 65) | 41 (30: 55) | 51.5 (33: 63) | ||||
| AST X ULN, n (%) | n=230 | n=67 | n=48 | 0.27 c | |||
| 0 to 1 | 118 (51.3%) | 30 (44.8%) | 17 (35.4%) | ||||
| >1 to 2 | 72 (31.3%) | 32 (47.8%) | 24 (50.0%) | ||||
| >2 | 40 (17.4%) | 5 (7.5%) | 7 (14.6%) | ||||
| HBV DNA (log10 IU/mL) | n=253 | n=70 | n=52 | 0.16 | |||
| Median (25th:75th) | 5.7 (4.2: 7.7) | 6.0 (4.0: 8.1) | 5.3 (3.3: 6.7) | ||||
| HBeAg status, n (%) | n=246 | n=70 | n=51 | 0.049 | 0.79 | 0.057 | 0.057 |
| Positive | 101 (41.1%) | 30 (42.9%) | 12 (23.5%) | ||||
| Quantitative HBsAg (log10 IU/mL) | n=158 | n=50 | n=36 | 0.02 | 0.78 | 0.01 | 0.07 |
| Median (25th:75th) | 3.6 (3.0: 4.2) | 3.7 (2.8: 4.5) | 3.1 (2.8: 3.4) | ||||
Abbreviations: ALT, Alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DNA, deoxyribonucleic acid; FLD, fatty liver disease; HBV, Hepatitis B virus; HBeAg, quantitative hepatitis B e-antigen; HBsAg, quantitative hepatitis B surface antigen; ULD, upper limit of normal.
Unless otherwise noted within the table.
Comparing all three groups. When p<0.05 pairwise comparisons were performed, with Holm’s adjustment for 3 comparisons.
From the Jonckheere Trend Test.
Figure 3.
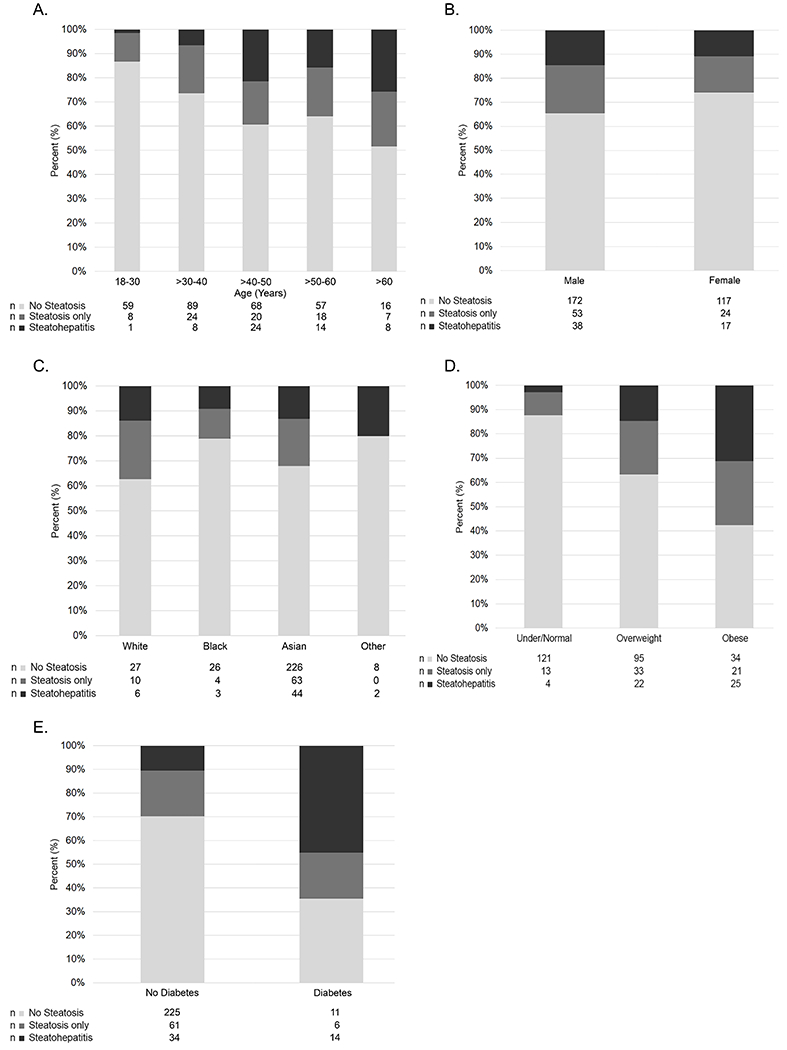
Distribution of FLD status by A. Age (years), B. Sex, C. Race, D. BMI category (race-specific), and E. Diabetes in study participants with chronic HBV.
Virologic factors
Participants with steatohepatitis had lower quantitative HBsAg levels than those with no FLD (median log10 3.1 vs 3.6 IU/mL, P=0.01) and appeared to have lower HBsAg levels than those with steatosis only (median log10 3.7 IU/mL, P=0.07). However, there were no significant differences in HBV DNA levels among the three groups with steatohepatitis, steatosis, and no FLD (median log10 5.3 vs 6.0 or 5.7 IU/mL, P =0.16) (Table 2). In addition, HBV genotype or phase of HBV infection were not significantly different by FLD categories.
On multivariable analysis with adjustment for sex (P=0.29) and HBV DNA level (P=0.18), older age [adjusted relative risk (aRR)=1.18, 95% CI, 1.04-1.35, per decade older; P=0.01], being overweight (aRR=2.55, 95% CI, 1.55-4.19) or obese (aRR=3.40, 95% CI, 2.04-5.68) versus normal weight (P<0.001), and having diabetes (aRR=1.41, 95% CI, 1.03-1.93; P=0.03) were independently associated with presence of steatohepatitis. There was also an indication that having hypertension increased risk of steatohepatitis (aRR=1.32, 95% CI, 0.96-1.81; P=0.09).
Relationship between presence of FLD and Ishak fibrosis
In evaluating select clinical and laboratory characteristics between participants with (n=92) and without (n=329) advanced fibrosis, those with advanced fibrosis were more likely to be male (72.8% vs 59.6%, P=0.02) and to have higher ALT (median 73 vs 56.5 U/L), AST (median 56 vs 36 U/L) and HBV DNA levels (median 6.5 vs 5.5 log10 IU/mL) (all P<0.001) compared to those without advanced fibrosis, but were not significantly different with respect to age (median 45 vs 42 years, P=0.11) and weight status (36.8% vs 37.7% normal weight; P=0.40).
After adjusting for age, sex, HBV DNA level and ALT level, in comparison to those with no FLD, the presence of steatohepatitis was associated with, on average, 1.33 (95% CI, 1.15-1.54) times higher Ishak fibrosis score or 1.68 times higher risk of advanced fibrosis (95% CI, 1.12-2.51) (Table 3). In addition, the presence of steatohepatitis was associated with, on average, 1.50 (95% CI, 1.25-1.80) times higher Ishak fibrosis score (P<0.001) or 2.89 times higher risk of advanced fibrosis (95% CI, 1.54-5.41) compared to those having steatosis alone.
Table 3.
Cross sectional associations between FLD status and fibrosis among study participants with chronic HBV.
| Fibrosis Score (0-6) | Advanced Fibrosis (Ishak≥3) | ||||
|---|---|---|---|---|---|
|
| |||||
| n | Adjusted Mean Ratioa (95%CI) N=348 |
P-value | Adjusted RR (95%CI) N=348 |
P-value | |
| FLD status (ref=neither) | 289 | <0.001 | 0.002 | ||
| Steatosis | 77 | 0.89 (0.77, 1.03) | 0.58 (0.33, 1.02) | ||
| Steatohepatitis | 55 | 1.33 (1.15, 1.54) | 1.68 (1.12, 2.51) | ||
| Age, per 10 years | 421 | 1.07 (1.01, 1.13) | 0.01 | 1.21 (1.05, 1.39) | 0.01 |
| Sex (ref=Female) | 158 | 0.04 | 0.07 | ||
| Male | 263 | 1.13 (1.01, 1.27) | 1.44 (0.97, 2.13) | ||
| HBV DNA, per log10 IU/mL | 375 | 1.06 (1.02, 1.09) | 0.002 | 1.16 (1.04, 1.28) | 0.005 |
| ALT, per log2 U/mL | 350 | 1.16 (1.09, 1.22) | <0.001 | 1.24 (1.08, 1.43) | 0.003 |
Abbreviations: ALT, Alanine aminotransferase; FLD, fatty liver disease.
Since the Ishak fibrosis score was skewed to the right, a generalized linear model with the gamma link function was employed. Thus, mean ratios are reported. A mean ratio of 1.33 for steatohepatitis versus no FLD indicates that someone with steatohepatitis has, on average, 1.33 times higher Ishak fibrosis score compared to someone without FLD.
FLD as a predictor of ALT, AST and FIB-4 levels over time
Over a median of 4.3 years (IQR: 2.2, 6.0) follow-up, participants had a median of 8.0 (IQR: 4.0-11.0) ALT and AST measures, and a median of 4.0 (IQR: 2.0-9.0) FIB-4 measurements. One third of measurements of ALT and AST (473 of 1374 observations) were while on treatment. Compared to those with no FLD, participants with steatohepatitis had higher values for these measurements at time of biopsy that persisted over time (Figure 4). With adjustment for age, sex, alcohol use, HBV DNA level and HBV treatment, in comparison to no FLD, having steatohepatitis was associated with, on average, 1.39 (95% CI, 1.20-1.62, P<0.001) times higher ALT, 1.16 (95% CI, 1.03-1.31, P =0.02) times higher AST, and 1.25 (95% CI, 1.00-1.56, P =0.04) times higher FIB-4 across four years of follow-up (Table 4). Additionally, compared to having steatosis alone, having steatohepatitis was associated with, on average, 1.24 (95% CI, 1.04-1.49, P =0.02) times higher ALT, and 1.17 (95% CI, 1.01-1.35, P =0.04) times higher AST, and 1.22 (95% CI, 0.92-1.60, P =0.10) times higher FIB-4; however, the difference in FIB-4 did not reach statistical significance. Comparison of participants with steatosis alone versus those with no FLD, did not show significant differences in ALT, AST or FIB-4 over time (Table 4). The proportion of participants with alcohol use, those who initiated HBV therapy, and the median HBV DNA levels by follow-up time point are shown in Supplemental Table 2. During follow-up, 102 (43.2%) participants started HBV therapy, of whom 97 received nucleos(t)ide analogues alone, 2 received nucleos(t)ide analogues in combination with pegylated interferon, and 3 received pegylated interferon alone. A sensitivity analysis with models testing associations between FLD status with ALT, AST and FIB-4, censoring assessments after participants started treatment (i.e., versus controlling for treatment) yielded similar results (data not shown). In addition, a sensitivity analysis with data extending to 288 weeks or 5.5 years of follow-up providing longer follow-up but with fewer participants beyond year 4, yielded similar estimates to those above (data not shown).
Figure 4.
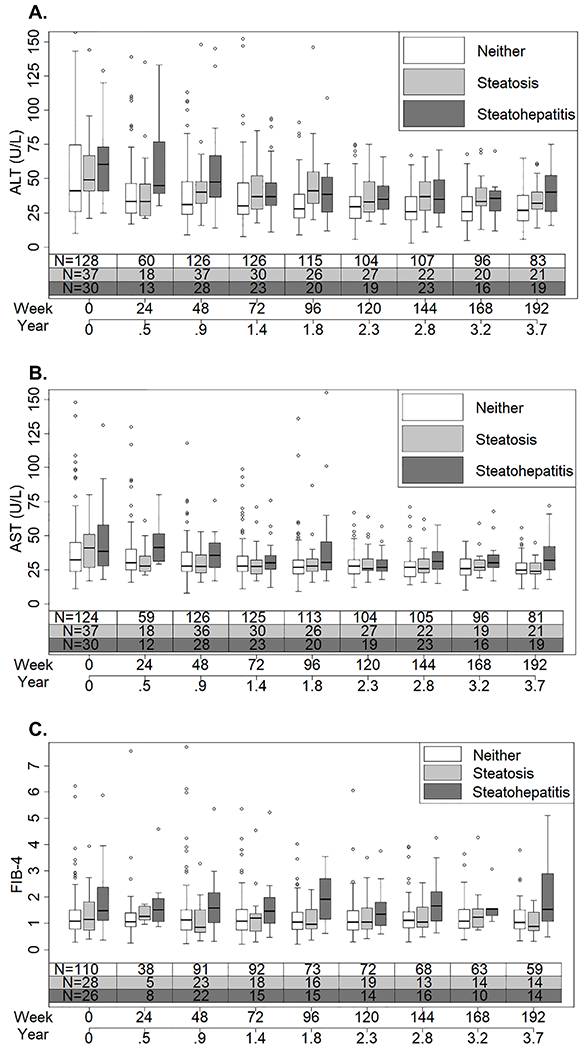
The distribution of A. ALT, B. AST, and C. FIB-4 over time by whether study participants with chronic HBV had steatohepatitis, steatosis, or neither at the time of biopsy.
Table 4.
Associations with ALT, AST and FIB-4, respectively, among study participants with chronic HBV over four years of follow-up.
| ALT (N=236) Adjusted Mean Ratio (95%CI)a |
P-value | AST (N=236) Adjusted Mean Ratio (95%CI)a |
P-value | FIB-4 (N=213) Adjusted Mean Ratio (95%CI)a |
P-value | |
|---|---|---|---|---|---|---|
| Fatty liver disease status | ||||||
| Steatosis vs neither | 1.12 (0.98, 1.29) | 0.10 | 0.99 (0.89, 1.11) | 0.93 | 1.03 (0.83, 1.27) | 0.92 |
| Steatohepatitis vs neither | 1.39 (1.20, 1.62) | <0.001 | 1.16 (1.03, 1.31) | 0.02 | 1.25 (1.00, 1.56) | 0.04 |
| Steatohepatitis vs steatosis | 1.24 (1.04, 1.49) | 0.02 | 1.17 (1.01, 1.35) | 0.04 | 1.22 (0.92, 1.60) | 0.10 |
| Age, per 10 years | 0.97 (0.92, 1.01) | 0.12 | 1.04 (1.00, 1.07) | 0.052 | - | |
| Sex (ref=Female) | <0.001 | <0.001 | 0.04 | |||
| Male | 1.48 (1.33, 1.64) | 1.23 (1.14, 1.34) | 1.18 (1.01, 1.38) | |||
| Past year alcohol use (ref=None/minimal) | 0.42 | 0.049 | 0.07 | |||
| Low-risk | 0.95 (0.86, 1.06) | 0.94 (0.87, 1.02) | 0.90 (0.81, 0.99) | |||
| At-risk | 1.06 (0.90, 1.25) | 1.11 (0.98, 1.27) | 1.00 (0.86, 1.17) | |||
| HBV DNA, per log10 IU/mL | 1.17 (1.15, 1.20) | <0.001 | 1.14 (1.12, 1.16) | <0.001 | 1.07 (1.05, 1.09) | <0.001 |
| Currently on HBV therapy (ref=No) | <0.001 | <0.001 | 0.001 | |||
| Yesa | 1.23 (1.10, 1.37) | 1.27 (1.16, 1.39) | 1.20 (1.07, 1.33) | |||
Abbreviations: ALT, Alanine aminotransferase; AST, aspartate aminotransferase; Fibrosis-4 index (FIB-4).
Each model was adjusted for time since biopsy (0-192 weeks) and the variables shown in the table. 473 of 1374 measurements of ALT and AST (i.e. observations) were while on treatment. To meet the normality assumption, the log 2 scale was used for the outcomes. Transformed results are presented as mean ratios (factor by which the average value of the outcome differs for the comparator vs. the reference) rather than differences. A mean ratio of 1.40 for steatohepatitis versus no FLD indicates that someone with steatohepatitis has, on average, 1.4 times higher ALT compared to someone without FLD.
Clinical and virologic events
Very few adverse clinical events were observed during follow-up with one death (due to renal failure), one HCC and one hepatic decompensation, each in a different participant, and no liver transplants. Sixteen (3.8%) participants had HBsAg loss, and among 166 HBeAg positive adults with follow-up data, 33 (19.9%) lost HBeAg. These outcomes were too rare to compare by FLD status.
Incident cirrhosis among those without baseline cirrhosis (N=406) was also uncommon, occurring at a rate of 0.49 per 100 person-years (95%CI, 0.26-0.95). However, there was an indication that incident cirrhosis might be more common among those with steatohepatitis (n=49; 1.47 per 100 person-years, 95% CI, 0.47-4.55) versus no FLD (n=283; 0.47 per 100 person-years, 95% CI, 0.21-1.04; P=0.12). Furthermore, in a sensitivity analysis, when follow-up data were censored at the initiation of HBV therapy, the incident cirrhosis rate was 0.71 (95%CI, 0.32, 1.58) per 100 person-years overall, 3.37 (95%CI 1.09, 10.44) per 100 person-years among those with steatohepatitis versus 0.51 (95%CI, (0.16, 1.57) per 100 person-years among those with no FLD (P=0.09). No one with steatosis only (n=74) had incident cirrhosis before or after censoring follow-up data for antiviral use.
Discussion:
In this multiethnic prospective adult cohort with chronic HBV who reside in North America and had undergone a liver biopsy, coexisting steatosis at ≥5% was present in nearly a third of the adults (31%) of whom 42% had steatohepatitis. The presence of steatohepatitis was cross-sectionally associated with advanced fibrosis independent of HBV DNA levels. Furthermore, presence of steatohepatitis was associated with higher AST and ALT and possibly higher FIB-4 levels throughout a study period of four years. A similar assessment of the HBRN pediatric cohort showed that prevalence of steatosis in HBV-infected children undergoing a liver biopsy was low at 4% and none had steatohepatitis (data not shown).
As expected, steatohepatitis was associated with known risk factors including older age, obesity, and diabetes. The prevalence of steatohepatitis of 13% in this study was higher than that estimated in the U.S. population at 1.5-6.5%.(26) In a recent study of 593 adult Chinese patients with HBV who underwent a liver biopsy prior to initiation of HBV therapy, 37.6% had NAFLD, defined as the presence of hepatic steatosis ≥5% with or without lobular inflammation and hepatocyte ballooning while 14% (n=83) had steatohepatitis; findings very similar to our study.(27) In that study, degrees of fibrosis did not differ in those with or without steatosis but comparisons between those with steatohepatitis and no steatosis or steatosis alone were not included.(27) In the current study, while steatosis was not associated with fibrosis, presence of steatohepatitis was associated with significantly higher degrees of fibrosis compared to no steatosis or steatosis alone. Thus, steatohepatitis signifies a more progressive liver injury that may have additive or synergistic effect on fibrosis in HBV liver disease.(28) Interestingly, 27% of adults in our cohort had perisinusoidal fibrosis but only about half of these met diagnostic criteria for steatohepatitis. The reason for this is unclear but may include prior steatohepatitis that has resolved, fibrosis related to another process, or perisinusoidal fibrosis as an unrecognized component of chronic HBV.
The relationship between steatosis and HBV disease remains controversial. Some studies from Asia have shown a lower prevalence of metabolic syndrome and steatosis in HBV (29–32), and even increasing steatosis severity with decreasing HBV replication.(33) Others using transient elastography have shown fibrosis progression with increasing steatosis severity, even when HBV is suppressed with therapy.(34) Recent data also suggest that baseline liver enzymes and their changes during follow-up are predictive of fibrosis progression or regression in the setting of FLD.(35) In this study, we show that independent of receipt of HBV therapy or HBV DNA levels, having steatohepatitis versus no FLD was associated with significantly higher FIB-4 and also higher AST and ALT levels throughout 4 years of follow-up. Moreover, the rate of incident cirrhosis appeared to be higher in those with steatohepatitis compared to those without FLD in follow-up. Although clinical outcomes of death, HCC and liver transplantation were infrequent in our contemporary prospective cohort, a recent retrospective study with longer follow-up showed an increased risk of adverse liver outcomes and all-cause mortality in those with HBV and steatohepatitis compared to those with HBV alone.(36)
The main limitation of our study is that because liver biopsies were not uniformly performed in all HBRN participants, the prevalence of FLD among patients with HBV in general could not be determined. However, it is noteworthy that the prevalence of FLD was similar among those who had a liver biopsy for clinical care versus as entry criteria into a treatment trial. In this study, we did not exclude at-risk alcohol use in order to fully capture the spectrum of FLD. Alcohol use may have contributed to FLD in a minority of participants but the proportion with at-risk drinking was only 8.8% at study entry and decreased to less than 5% during follow-up. Moreover, alcohol use was included as a covariate in the cross-sectional analysis and adjusted for as a time varying covariate in the longitudinal analysis. A major strength of this study is the detailed clinical, virologic, and histologic evaluation including blinded central reading of biopsy specimens, and the large number of biopsies provide robust data to examine the association between FLD and inflammation and fibrosis in HBV. While repeat liver biopsies were not performed, precluding our ability to evaluate the effect of steatohepatitis on fibrosis progression, repeated laboratory and clinical assessment allowed for meaningful evaluation of impact of steatohepatitis on biochemical measures of hepatic inflammation and fibrosis, and virologic and clinical outcomes during follow-up.
In summary, hepatic steatosis was common among adults with chronic HBV living in North America, who had undergone a liver biopsy and steatohepatitis was present in nearly one-sixth of our patients. Presence of steatohepatitis was associated with more advanced fibrosis and higher levels of AST, ALT and FIB-4 throughout follow-up, suggesting that steatohepatitis may also contribute to an increased rate of fibrosis progression over time. As obesity and diabetes were predominant risk factors for coexisting steatohepatitis, in the presence of these risks, clinicians may consider diagnostic workup for coexisting FLD such as noninvasive elastography or a liver biopsy especially among individuals with elevated liver enzymes and low or HBV DNA. Moreover, screening for and addressing metabolic abnormalities, in addition to viral suppression is important to prevent HBV disease progression and adverse outcomes.
Supplementary Material
Study Highlights:
WHAT IS KNOWN
Metabolic abnormalities are prevalent in individuals with chronic hepatitis B infection.
Metabolic abnormalities are risk factors for fatty liver disease and coexisting fatty liver disease is associated with increased risk of adverse outcomes in those with underlying liver disease.
WHAT IS NEW HERE
In this North American adult cohort with chronic hepatitis B infection who underwent a liver biopsy, coexisting steatosis was present in nearly a third of adults, of whom 42% had steatohepatitis.
Fatty liver disease in chronic hepatitis B was associated with known metabolic risk factors.
Presence of steatohepatitis but not steatosis alone was associated with baseline advanced hepatic fibrosis in chronic hepatitis B, and higher ALT, AST and FIB-4 levels during follow-up.
Acknowledgments
Financial support:
The HBRN was funded as a Cooperative Agreement between the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the following investigators: Lewis R. Roberts, MB, ChB, PhD (U01-DK082843), Anna Suk-Fong Lok, MD (U01-DK082863), Steven H. Belle, PhD, MScHyg (U01-DK082864), Kyong-Mi Chang, MD (U01-DK082866), Michael W. Fried, MD (U01-DK082867), Adrian M. Di Bisceglie, MD (U01-DK082871), William M. Lee, MD (U01-DK082872), Harry L. A. Janssen, MD, PhD (U01-DK082874), Daryl T-Y Lau, MD, MPH (U01-DK082919), Richard K. Sterling, MD, MSc (U01-DK082923), Steven-Huy B. Han, MD (U01-DK082927), Robert C. Carithers, MD (U01-DK082943), Mandana Khalili, MD (U01-DK082944), Kathleen B. Schwarz, MD (U01-DK082916), an interagency agreement with NIDDK: Lilia M. Ganova-Raeva, PhD (A-DK-3002-001) and support from the intramural program, NIDDK, NIH: Marc G. Ghany, MD and NCI, NIH: David E. Kleiner MD, PhD. Additional funding to support this study was provided to Kyong-Mi Chang, MD, the Immunology Center, (NIH/NIDDK Center of Molecular Studies in Digestive and Liver Diseases P30DK50306, NIH Public Health Service Research Grant M01-RR00040), Richard K. Sterling, MD, MSc (UL1TR000058, NCATS (National Center for Advancing Translational Sciences, NIH), Mandana Khalili, MD (CTSA Grant Number UL1TR000004), Michael W. Fried, MD (CTSA Grant Number UL1TR001111), Anna Suk-Fong Lok (CTSA Grant Number UL1RR024986, U54TR001959), and Kathleen B. Schwarz, MD (CTSA Grant Number UL1TR000423). Additional support was provided by Gilead Sciences, Inc. and Roche Molecular Systems via a CRADA through the NIDDK.
Dr. Khalili was also partially supported by K24AA022523.
Author disclosures:
Mandana Khalili is a recipient of a research grant (to her institution) from Gilead Sciences Inc, and Intercept Pharmaceuticals and she has served as consultant for Gilead Sciences Inc. Raymond Chung, has research grants (to institution) from Gilead, AbbVie, BMS, Merck, Boehringer, Roche, and Janssen and from the MGH Research Scholars Program. Philip Rosenthal has research support from Gilead Sciences, Inc., AbbVie, Merck, Arrowhead and Retrophin and consults for Gilead, AbbVie, Retrophin, Albireo, Mirum, Audentes, and Dicerna. Mauricio Lisker-Melman serves on the speaker bureau for AbbVie, Gilead Sciences Inc, and SimplySpeaking. Richard Sterling has received research grants from Abbott, Abbvie, Gilead, and Roche and serves on the data safety and monitoring board for Pfizer and Baxter. Anna Lok has received research grant support (to the University of Michigan) from Bristol-Myers Squibb, Gilead and TARGET, and has served on advisory panels of Gilead and TARGET. Wendy King has received funding from Abbott. Marc G. Ghany, Atul K. Bhan, Rageshree Ramachandran, and David Kleiner do not have any disclosures relevant to this project.
Potential competing interests:
Mandana Khalili is a recipient of a research grant (to her institution) from Gilead Sciences Inc, and Intercept Pharmaceuticals and she has served as consultant for Gilead Sciences Inc. Raymond Chung, has research grants (to institution) from Gilead, AbbVie, BMS, Merck, Boehringer, Roche, and Janssen and from the MGH Research Scholars Program. Philip Rosenthal has research support from Gilead Sciences, Inc., AbbVie, Merck, Arrowhead and Retrophin and consults for Gilead, AbbVie, Retrophin, Albireo, Mirum, Audentes, and Dicerna. Mauricio Lisker-Melman serves on the speaker bureau for AbbVie, Gilead Sciences Inc, and SimplySpeaking. Richard Sterling has received research grants from Abbott, Abbvie, Gilead, and Roche and serves on the data safety and monitoring board for Pfizer and Baxter. Anna Lok has received research grant support (to the University of Michigan) from Bristol-Myers Squibb, Gilead and TARGET, and has served on advisory panels of Gilead and TARGET. Wendy King has received funding from Abbott. Marc Ghany, Atul K. Bhan, Rageshree Ramachandran, and David Kleiner do not have any disclosures relevant to this project.
List of Abbreviations:
- HBV
Hepatitis B virus
- HIV
human immunodeficiency virus
- BMI
body mass index
- FLD
fatty liver disease
- NAFLD
nonalcoholic FLD
- NASH
nonalcoholic steatohepatitis
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- HCV
hepatitis C virus
- RNA
ribonucleic acid
- US
United States
- DNA
Deoxyribonucleic acid
- HBRN
Hepatitis B Research Network
- HBsAg
hepatitis B surface antigen
- HBeAg
hepatitis B e antigen
- MS
metabolic syndrome
- ULN
upper limit of normal
Footnotes
The HBRN: Harvard Consortium: Daryl T-Y Lau, MD, MPH (Beth Israel Deaconess Medical Center, Boston, MA). Minnesota Alliance for Research in Chronic Hepatitis B Consortium: Lewis R. Roberts, MB, ChB, PhD (Mayo Clinic Rochester, Rochester, MN), Mohamed A. Hassan, MD (University of Minnesota, Minneapolis, MN), Sarah Jane Schwarzenberg, MD (Department of Pediatrics, University of Minnesota Masonic Children’s Hospital, Minneapolis, MN). Midwest Hepatitis B Consortium: Adrian M. Di Bisceglie, MD, (Saint Louis University School of Medicine, St Louis, MO), Jeffrey Teckman, MD (Department of Pediatrics, Cardinal Glennon Children’s Medical Center, Saint Louis University, St. Louis, MO). University of Toronto Consortium: Harry L. A. Janssen, MD, PhD (Toronto General Hospital, Toronto, Ontario), David K. Wong, MD (Toronto General Hospital, Toronto, Ontario), Joshua Juan, MD (Toronto General Hospital, Toronto, Ontario), Jordan Feld, MD, MPH (Toronto General Hospital, Toronto, Ontario), Colina Yim, NP, MN (Toronto General Hospital, Toronto, Ontario), Keyur Patel, MD (Toronto General Hospital, Toronto, Ontario), Simon C. Ling, MBChB (Department of Paediatrics, The Hospital for Sick Children, University of Toronto, Toronto, Ontario). HBV CRN North Texas Consortium: William M. Lee, MD (Division of Digestive and Liver Diseases, University of Texas Southwestern Medical Center at Dallas, Dallas, TX), Carol S. Murakami, MD (Division of Digestive and Liver Diseases, University of Texas Southwestern Medical Center at Dallas, Dallas, TX), Robert Perrillo, MD, (Baylor University Medical Center, Dallas, TX), Son Do, MD (University of Texas Southwestern, Dallas, TX), Norberto Rodriguez-Baez, MD (Department of Pediatrics, University of Texas Southwestern, Dallas, TX). Los Angeles Hepatitis B Consortium: Steven-Huy B. Han, MD (David Geffen School of Medicine, UCLA, Los Angeles, CA), Tram T. Tran, MD (Cedars Sinai Medical Center, Los Angeles, CA). San Francisco Hepatitis B Research Group Consortium: Norah A. Terrault, MD, MPH (University of California-San Francisco, San Francisco, CA and Keck Medicine at the University of Southern California, Los Angeles, CA), Stewart L. Cooper, MD (Division of General and Transplant Hepatology, California Pacific Medical Center, San Francisco, CA). Michigan Hawaii Consortium: Robert J. Fontana, MD (University of Michigan, Ann Arbor, MI), Naoky Tsai, MD (The Queen’s Medical Center, University of Hawaii, Honolulu, HI), Barak Younoszai, DO (The Queen’s Medical Center, University of Hawaii, Honolulu, HI). Chapel Hill, NC Consortium: Michael W. Fried, MD, (University of North Carolina at Chapel Hill, Chapel Hill, NC), Andrew Muir, M.D. (Duke University Medical Center, Durham, NC), Donna Evon, Ph.D. (University of North Carolina at Chapel Hill, Chapel Hill, NC), Jama M. Darling, MD (University of North Carolina at Chapel Hill, NC). PNW/Alaska Clinical Center Consortium: Robert C. Carithers, MD (University of Washington Medical Center, Seattle WA), Margaret Shuhart, M.D. (Harborview Medical Center, Seattle WA), Kris V. Kowdley, MD (Virginia Mason Medical Center, Seattle WA), Chia C. Wang, MD (Virginia Mason Medical Center, Seattle WA), Karen F. Murray, MD (Department of Pediatrics, University of Washington, Seattle, WA). Virginia Commonwealth University Medical Center: Velimir A. Luketic, MD (Virginia Commonwealth University Health System, Richmond, VA). Johns Hopkins University: Kathleen B. Schwarz, MD (Department of Pediatrics, Johns Hopkins Medical Institutions, Baltimore, MD). Liver Diseases Branch, NIDDK: T. Jake Liang, MD (National Institutes of Health, Bethesda, MD). Liver Disease Research Branch, NIDDK: Jay H. Hoofnagle, MD (National Institutes of Health, Bethesda, MD), Edward Doo, MD (National Institutes of Health, Bethesda, MD). Immunology Center: Kyong-Mi Chang, MD, (University of Pennsylvania Perelman School of Medicine, Philadelphia, PA), Jang-June Park, PhD (University of Pennsylvania Perelman School of Medicine, Philadelphia, PA). Data Coordinating Center: Steven H. Belle, PhD, MScHyg (Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA), Abdus Wahed, PhD (Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA).
Guarantor of the article: Mandana Khalili
Guarantor of the article: Dr. Khalili is the Guarantor of the article.
ClinicalTrials.gov Identifier: NCT01263587
References
- 1.Tang LSY, Covert E, Wilson E, et al. Chronic Hepatitis B Infection: A Review. JAMA 2018;319:1802–1813. [DOI] [PubMed] [Google Scholar]
- 2.Kowdley KV, Wang CC, Welch S, et al. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology 2012;56:422–33. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell T, Armstrong GL, Hu DJ, et al. The increasing burden of imported chronic hepatitis B--United States, 1974-2008. PLoS One 2011;6:e27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi ZM, Stepanova M, Younossi Y, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020;69:564–568. [DOI] [PubMed] [Google Scholar]
- 5.Khalili M, Lombardero M, Chung RT, et al. Diabetes and prediabetes in patients with hepatitis B residing in North America. Hepatology 2015;62:1364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khalili M, Shuhart MC, Lombardero M, et al. Relationship Between Metabolic Syndrome, Alanine Aminotransferase Levels, and Liver Disease Severity in a Multiethnic North American Cohort With Chronic Hepatitis B. Diabetes Care 2018;41:1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalili M, Lim JW, Bass N, et al. New onset diabetes mellitus after liver transplantation: the critical role of hepatitis C infection. Liver Transpl 2004;10:349–55. [DOI] [PubMed] [Google Scholar]
- 9.Mukhtar NA, Bacchetti P, Ayala CE, et al. Insulin sensitivity and variability in hepatitis C virus infection using direct measurement. Dig Dis Sci 2013;58:1141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serfaty L Metabolic Manifestations of Hepatitis C Virus: Diabetes Mellitus, Dyslipidemia. Clin Liver Dis 2017;21:475–486. [DOI] [PubMed] [Google Scholar]
- 11.Wang CC, Cheng PN, Kao JH. Systematic review: chronic viral hepatitis and metabolic derangement. Aliment Pharmacol Ther 2020;51:216–230. [DOI] [PubMed] [Google Scholar]
- 12.Charatcharoenwitthaya P, Pongpaibul A, Kaosombatwattana U, et al. The prevalence of steatohepatitis in chronic hepatitis B patients and its impact on disease severity and treatment response. Liver Int 2017;37:542–551. [DOI] [PubMed] [Google Scholar]
- 13.Zheng RD, Chen JN, Zhuang QY, et al. Clinical and virological characteristics of chronic hepatitis B patients with hepatic steatosis. Int J Med Sci 2013;10:641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang MM, Wang GS, Shen F, et al. Hepatic steatosis is highly prevalent in hepatitis B patients and negatively associated with virological factors. Dig Dis Sci 2014;59:2571–9. [DOI] [PubMed] [Google Scholar]
- 15.Ren H, Wang J, Gao Y, et al. Metabolic syndrome and liver-related events: a systematic review and meta-analysis. BMC Endocr Disord 2019;19:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu MW, Lin CL, Liu CJ, et al. Influence of Metabolic Risk Factors on Risk of Hepatocellular Carcinoma and Liver-Related Death in Men With Chronic Hepatitis B: A Large Cohort Study. Gastroenterology 2017;153:1006–1017 e5. [DOI] [PubMed] [Google Scholar]
- 17.Cheuk-Fung Yip T, Wai-Sun Wong V, Lik-Yuen Chan H, et al. Effects of Diabetes and Glycemic Control on Risk of Hepatocellular Carcinoma After Seroclearance of Hepatitis B Surface Antigen. Clin Gastroenterol Hepatol 2018;16:765–773 e2. [DOI] [PubMed] [Google Scholar]
- 18.Xu C, Chen J, Zhang PA. Relationship Between Diabetes Mellitus and Cirrhosis Risk in Chronic Hepatitis B Patients in Wuhan, China. Med Sci Monit 2019;25:8112–8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seto WK. Chronic hepatitis B and metabolic risk factors: A call for rigorous longitudinal studies. World J Gastroenterol 2019;25:282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghany MG, Perrillo R, Li R, et al. Characteristics of adults in the hepatitis B research network in North America reflect their country of origin and hepatitis B virus genotype. Clin Gastroenterol Hepatol 2015;13:183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696–9. [DOI] [PubMed] [Google Scholar]
- 22.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis 2012;32:3–13. [DOI] [PubMed] [Google Scholar]
- 23.NIAAA. What is ”low-risk“ drinking? National Institute on Alcohol Abuse and Alcoholism. http://rethinkingdrinking.niaaa.nih.gov/IsYourDrinkingPatternRisky/WhatsLowRiskDrinking.asp, Accessed July 20, 2020. [Google Scholar]
- 24.Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- 25.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 26.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 27.Shen F, Mi YQ, Xu L, et al. Moderate to severe hepatic steatosis leads to overestimation of liver stiffness measurement in chronic hepatitis B patients without significant fibrosis. Aliment Pharmacol Ther 2019;50:93–102. [DOI] [PubMed] [Google Scholar]
- 28.Yeh MM, Brunt EM. Pathological features of fatty liver disease. Gastroenterology 2014;147:754–64. [DOI] [PubMed] [Google Scholar]
- 29.Wong VW, Wong GL, Chu WC, et al. Hepatitis B virus infection and fatty liver in the general population. J Hepatol 2012;56:533–40. [DOI] [PubMed] [Google Scholar]
- 30.Joo EJ, Chang Y, Yeom JS, et al. Hepatitis B virus infection and decreased risk of nonalcoholic fatty liver disease: A cohort study. Hepatology 2017;65:828–835. [DOI] [PubMed] [Google Scholar]
- 31.Zhong GC, Wu YL, Hao FB, et al. Current but not past hepatitis B virus infection is associated with a decreased risk of nonalcoholic fatty liver disease in the Chinese population: A case-control study with propensity score analysis. J Viral Hepat 2018;25:842–852. [DOI] [PubMed] [Google Scholar]
- 32.Wang B, Li W, Fang H, et al. Hepatitis B virus infection is not associated with fatty liver disease: Evidence from a cohort study and functional analysis. Mol Med Rep 2019;19:320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hui RWH, Seto WK, Cheung KS, et al. Inverse relationship between hepatic steatosis and hepatitis B viremia: Results of a large case-control study. J Viral Hepat 2018;25:97–104. [DOI] [PubMed] [Google Scholar]
- 34.Seto WK, Hui RWH, Mak LY, et al. Association Between Hepatic Steatosis, Measured by Controlled Attenuation Parameter, and Fibrosis Burden in Chronic Hepatitis B. Clin Gastroenterol Hepatol 2018;16:575–583 e2. [DOI] [PubMed] [Google Scholar]
- 35.Kleiner DE, Brunt EM, Wilson LA, et al. Association of Histologic Disease Activity With Progression of Nonalcoholic Fatty Liver Disease. JAMA Netw Open 2019;2:e1912565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi HSJ, Brouwer WP, Zanjir WMR, et al. Nonalcoholic Steatohepatitis Is Associated With Liver-Related Outcomes and All-Cause Mortality in Chronic Hepatitis B. Hepatology 2020;71:539–548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


