Abstract
Purpose:
Ocular biomechanical properties are important in understanding glaucoma pathogenesis but the affected tissues are unclear. In this study, we compared corneal wave speed (a measure of corneal elasticity) and ocular rigidity coefficient between glaucomatous and normal eyes.
Methods:
Twenty glaucomatous eyes from 10 patients and 20 normal eyes from 13 controls, matched for age, intraocular pressure (IOP), and axial length were included. Ocular rigidity was calculated based on the difference in supine IOP by pneumatonometry with and without a 10-g weight. Corneal wave speed was determined by ultrasound surface wave elastography. A small, 0.1 s harmonic vibration at 100 Hz was generated through the closed eyelids. Wave propagation was captured by an ultrasound transducer, and wave speed was determined from the phase change with distance. Comparisons were performed using generalized estimating equation models.
Results:
There were no significant differences in corneal wave speed between glaucomatous and normal eyes (2.16 ± 0.25 m/s vs 2.07 ± 0.16 m/s, P= 0.17). However, ocular rigidity was significantly lower in glaucomatous eyes (0.0218 ± 0.0033 μL−1 vs 0.0252 ± 0.0050 μL−1, P= 0.01). Corneal wave speed was not correlated with age and IOP in either group (P≥ 0.23) but was correlated with ocular rigidity (R=0.48, P=0.02) and inversely correlated with axial length (R=−0.53, P=0.01) in glaucomatous eyes.
Conclusion:
Glaucomatous eyes tend to have lower ocular rigidity than healthy eyes with similar age, IOP, and axial length. However, the lack of a difference in corneal wave speed suggests that corneal tissue may not be significantly affected, and scleral changes likely plays a more important role in glaucoma.
Keywords: Ocular biomechanical properties, Ultrasound elastography, Corneal wave speed, Glaucoma, Ocular rigidity
Precis:
Ocular biomechanics were compared between treated glaucoma patients and healthy subjects matched for age, IOP, and axial length. There was no difference in corneal wave propagation speed, but ocular rigidity was lower in glaucomatous eyes.
Introduction
Glaucoma is a progressive optic neuropathy and is the second most common cause of blindness in the world, with over 2.5 million people affected in the United States alone.1 Intraocular pressure (IOP) is the primary risk factor in glaucoma, and lowering of IOP is currently the only effective treatment for glaucoma. However, up to 50% of glaucoma patients have statistically normal IOP when diagnosed,2 and a significant proportion of patients develop progressive vision loss despite therapy to reduce IOP.3 Conversely, most patients with elevated IOP do not develop glaucoma.4 These incongruities may be related to abnormal ocular biomechanical properties.5, 6 Understanding the differences in biomechanical properties between glaucoma patients and healthy subjects may provide a better understanding of glaucoma pathogenesis and reveal potential targets for novel treatment modalities.
Biomechanical properties of the eye affect the degree of optic nerve deformation that occurs with IOP changes. Elevation of IOP can result in distension of the lamina cribrosa,7 which may lead to strain and damage of the axons of the optic nerve.8 Finite element models have suggested that eyes with abnormal biomechanical properties can have greater distension of the lamina cribrosa, predisposing to the development of glaucoma and disease progression.9 However, the nature of these changes and the tissues involved in glaucoma are not known.
Our group previously described a novel method for non-invasive in vivo measurement of wave propagation speed and estimation of Young’s modulus of elasticity in corneas of human subjects by using ultrasound surface wave elastography (USWE).10 This method has also been used to measure biomechanical properties of other tissues in vivo, such as skin,11 lung,12 abdominal muscles,13 and tendons.14 Ocular rigidity is another biomechanical parameter of the eye which describes the relationship between pressure and volume changes in the eye.15 It is an ocular biomechanical measurement that can be affected by tissues throughout the eye, particularly the elasticity of the cornea and sclera. However, in vivo measurements of biomechanical properties that can be directly related to modulus of elasticity have not previously been compared between the eyes of glaucoma patients and normal controls.
In this study, we used USWE to determine wave speed propagation as a measure of tissue elasticity in the cornea, and compared the results from normal and glaucomatous eyes. We also determined the ocular rigidity coefficient as a global measure of ocular biomechanics, and evaluated differences between normal and glaucomatous eyes.
Materials and Methods
This prospective experimental study was approved by the Institutional Review Board at Mayo Clinic. The study followed the tenets of the Declaration of Helsinki and was in accordance with HIPAA regulations. All subjects provided written informed consent to participate after discussion of the nature and possible risks of the study.
Study Subjects
Ten patients with mild to moderate primary open angle glaucoma (POAG) on medical therapy with prostaglandin analogues (PGAs), were recruited from the Department of Ophthalmology, Mayo Clinic, Rochester, Minnesota. Thirteen healthy subjects matched for age, IOP and axial length were recruited from employees and patients of Mayo Clinic, or local area residents. All participants underwent a comprehensive ophthalmologic examination including visual acuity, IOP with pneumatonometry, biomicroscopy of the anterior segment, gonioscopy and dilated fundoscopy. Both males and females were included and both eyes were studied. Subjects were excluded if they had a history or evidence of any clinically significant ocular pathology other than glaucoma, previous intraocular or corneal refractive surgery, ocular trauma in the last 6 months, laser treatment for glaucoma, narrow angles, or retinal pathologies that could predispose to a retinal detachment. In addition, healthy control subjects were excluded if they had a vertical cup-to-disc ratio ≥ 0.6 or an asymmetry of the vertical cup-to-disc ratio ≥ 0.2.
POAG was defined according to the American Academy of Ophthalmology Preferred Practice Pattern16 criteria as the presence of open angles along with evidence of optic nerve damage characterized by either or both: (1) optic nerve or retinal nerve fiber layer (RNFL) damage consistent with glaucoma (diffuse thinning, focal narrowing, or notching of the optic disc rim; progressive narrowing of the neuroretinal rim; diffuse or localized abnormalities of the parapapillary RNFL; disc hemorrhages; or neural rim asymmetry); (2) visual field (VF) damage consistent with RNFL damage (nasal step, arcuate field defect, or paracentral depression in clusters of test sites); hemifield loss; absence of other explanations). Visual fields (Humphrey Field Analyzer with SITA standard 24-2 test strategy) were considered reliable if fixation losses were less than 20% and false positive and negative rates were less than 33%.
Measurements
Intraocular Pressure (IOP)
IOP was measured in both eyes in the sitting position by using a pneumatonometer (Model 30 Classic, Reichert Inc., Buffalo, NY) (Figure 1). Subjects were then placed in the supine position and IOP was re-measured after 5 minutes. Calibration of the tonometer was verified according to the manufacturer’s instruction and the tip was cleaned before each set of measurement. Topical proparacaine 0.5% was instilled before each IOP measurement. The right eye was always measured first.
Figure 1.
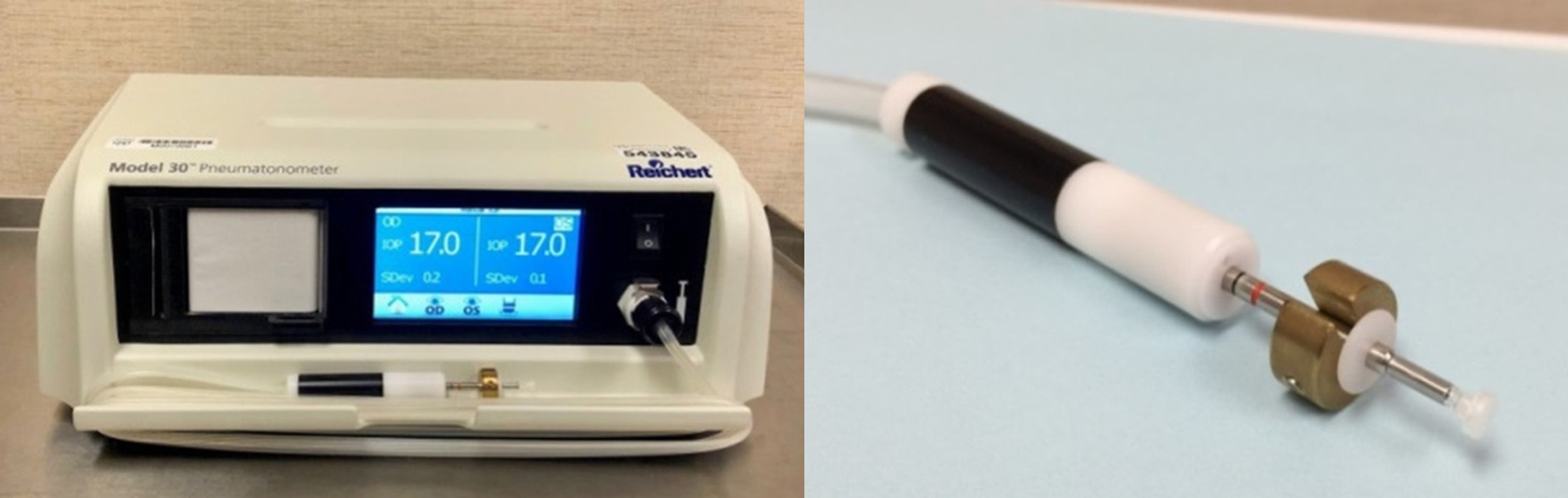
Pneumatonometer and its probe with a 10-g weight. Pneumatonometer was used to measure intraocular pressure in the sitting and supine positions and calculate ocular rigidity coefficient after adding a 10-g weight to the probe in the supine position measurement.
Ocular Rigidity
After IOP was measured by pneumatonometry in the supine position, a 10-gram weight was added to the pneumatonometer probe (Figure 1) and the supine IOP measurement was repeated. The ocular rigidity coefficient was calculated based on Friedenwald’s equation15 and by using Langham’s tables17, 18:
where P0 is the supine IOP before placing the weight, P1 is the supine IOP with the added 10-g weight, and V1 − V0 is the change in ocular volume after placing the pneumatonometer probe with the 10-g weight.
Ultrasound Surface Wave Elastography (USWE)
A detailed description of USWE has been previously published.10, 19 In brief, a handheld electromagnetic shaker (Model: FG-142, Labworks Inc., Costa Mesa, CA) with a 3-mm diameter probe was gently placed on the closed eyelid and a gentle harmonic vibration at 100 Hz for 0.1 seconds was produced by a function generator (Model 33120A, Agilent, Santa Clara, CA). The excitation signal was amplified by an audio amplifier (Model D150A, Crown Audio Inc., Elkhart, IN). The resulting wave propagation through the ocular tissues was recorded by using a linear array ultrasound probe with a central frequency of 6.4 MHz and elevation focus of 18 mm (L11-5V, Verasonics Inc., Kirkland, WA) (Figure 2). The ultrasound probe L11-5v had 120 elements with a pitch of 0.3 mm. The stimulus signal was synchronized to the ultrasound system to enable detection of wave phase based on tissue displacement in the ocular tissues.
Figure 2.

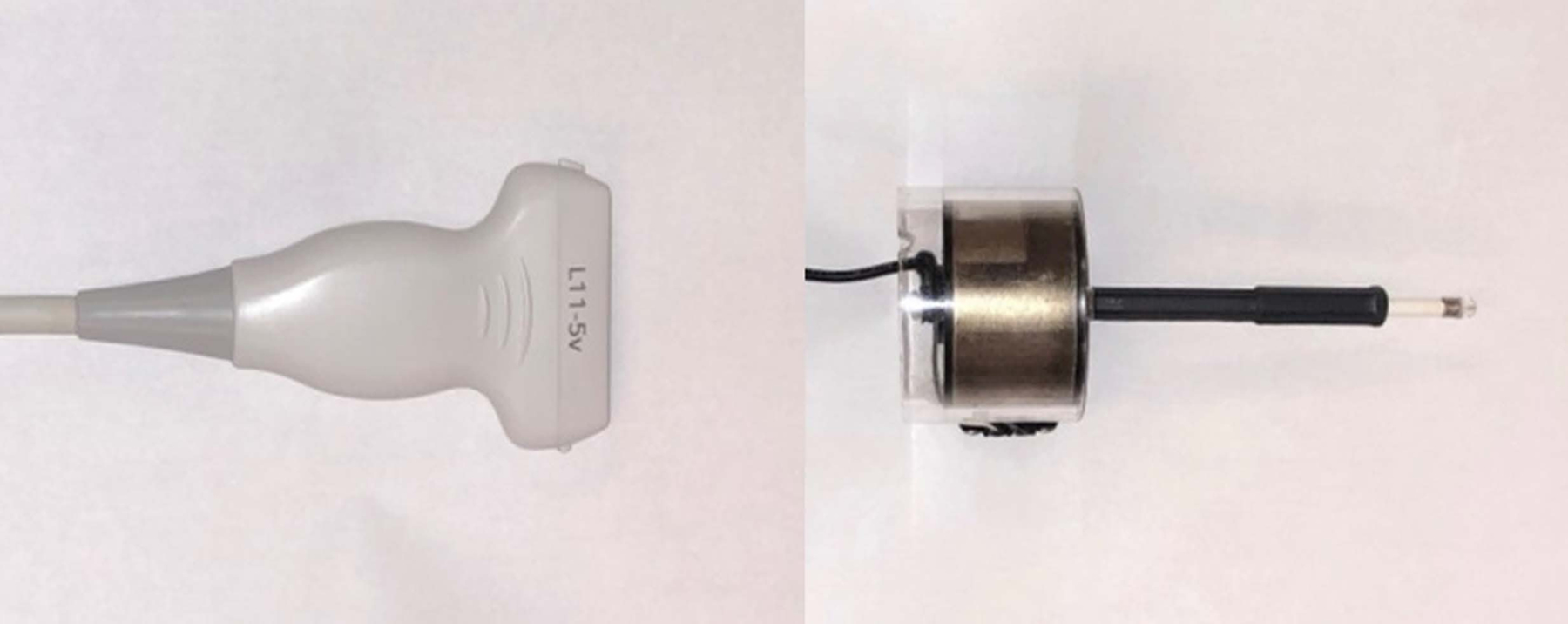
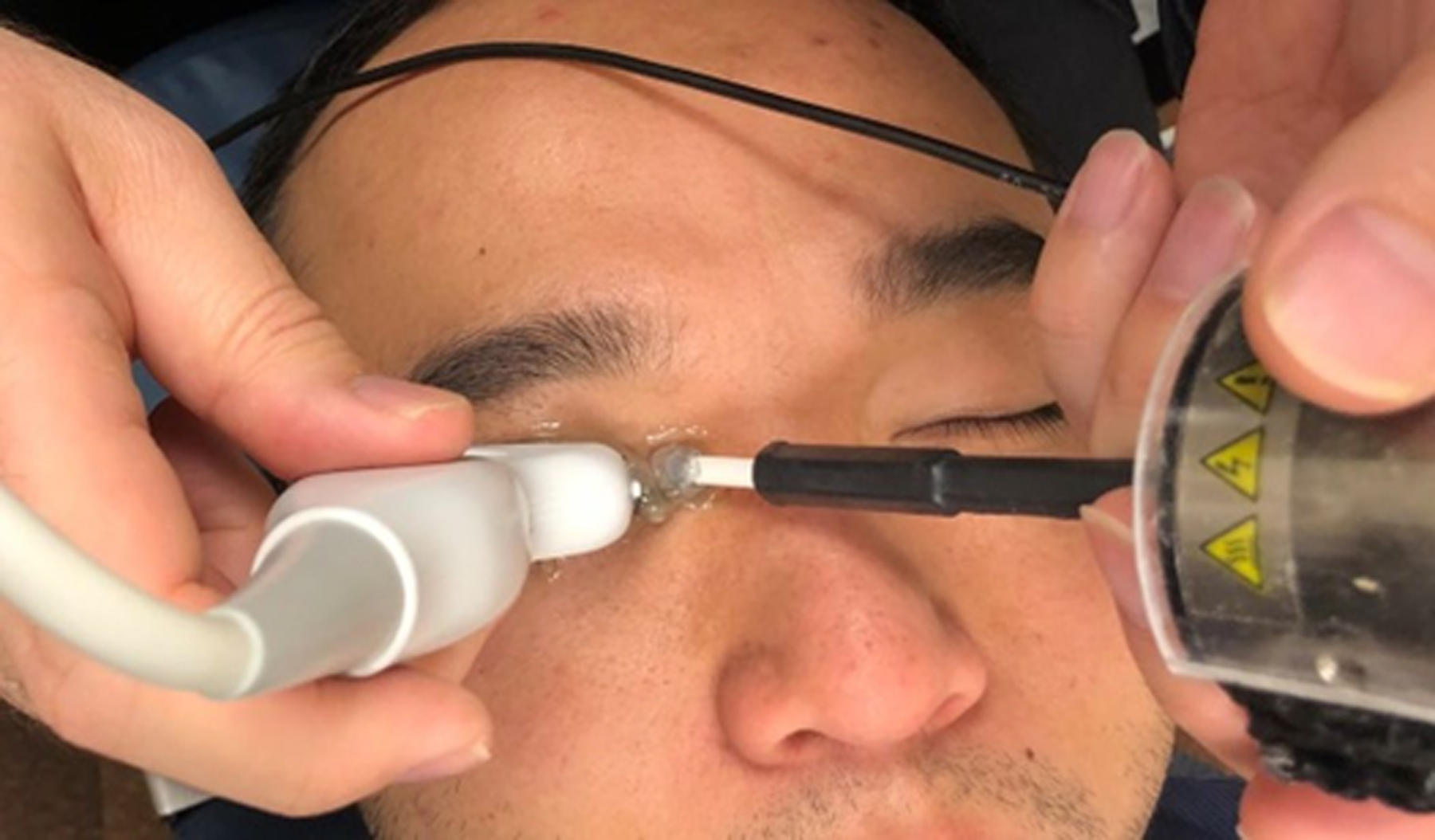
Ultrasound surface wave elastography system. A, Function generator (left) and audio amplifier (right). B, 6.4 MHz linear array ultrasound probe (left) and handheld mechanical shaker (right) of the ultrasound surface wave elastography system. C, Using ultrasound probe and handheld shaker for ultrasound surface wave elastography.
The wave speed, cs(f), was determined from the change in phase with distance and was measured by the phase gradient method20:
| (1) |
where f is the frequency, Δr is the radial distance between 2 detection locations and Δϕ is the wave phase change over that distance.
To improve the estimation of wave speed over the measurement region, the parameters used in Equation 1 were estimated by linear regression analysis of the phase change measured over multiple locations:
| (2) |
where denotes the regression value for phase change at distance Δr, α is the slope, and β is the regression constant.
USWE was performed with the subjects in the supine position with eyes in primary position, while both eyes closed (Figure 2). A layer of non-irritating ultrasound gel (Aquasonic 100, Parker Laboratories, Inc., Fairfield, NJ) was placed over the gently closed eyelid. Imaging was performed by placing the vertically oriented ultrasound probe in contact with the eyelid. The probe contacted only the gel, and did not indent or displace the globe. The tip of the shaker was then gently placed on the eyelid adjacent to the probe, near the medial canthus, without adding pressure to the eye, and images were captured. Five measurements were performed in each eye and then repeated in the contralateral eye.
Analysis of wave propagation in the cornea was guided by ultrasound imaging. The plane wave technique20, 21 was used for transmitting and receiving unfocused ultrasound beams. All 128 ultrasound beams were measured for the given depth of ultrasound imaging. Ultrasound images were recorded during a continuous 0.1-second vibration at 100 Hz frequency with a frame rate of 2000 images/sec. The cornea was then identified in the ultrasound images and 10–12 positions in the central 6 mm of the mid-stroma cornea were selected (Figure 3). The tissue motion at each pixel of the ultrasound image was analyzed. Tissue motion at the selected locations in the cornea was analyzed by cross-correlation analysis of ultrasound tracking beams.22 In one testing, two hundred images were synchronized with the excitation of the shaker to measure the wave propagation in duration of 0.1 second. Wave speed was then measured by determining the change in wave phase at each location relative to the first location by using several ultrasound tracking beams.23–25 The quality of wave speed measurement was considered acceptable if the square of correlation coefficient (R2) of the linear regression was > 0.8. The mean of 3 acceptable measurements was used for data analysis.
Figure 3.
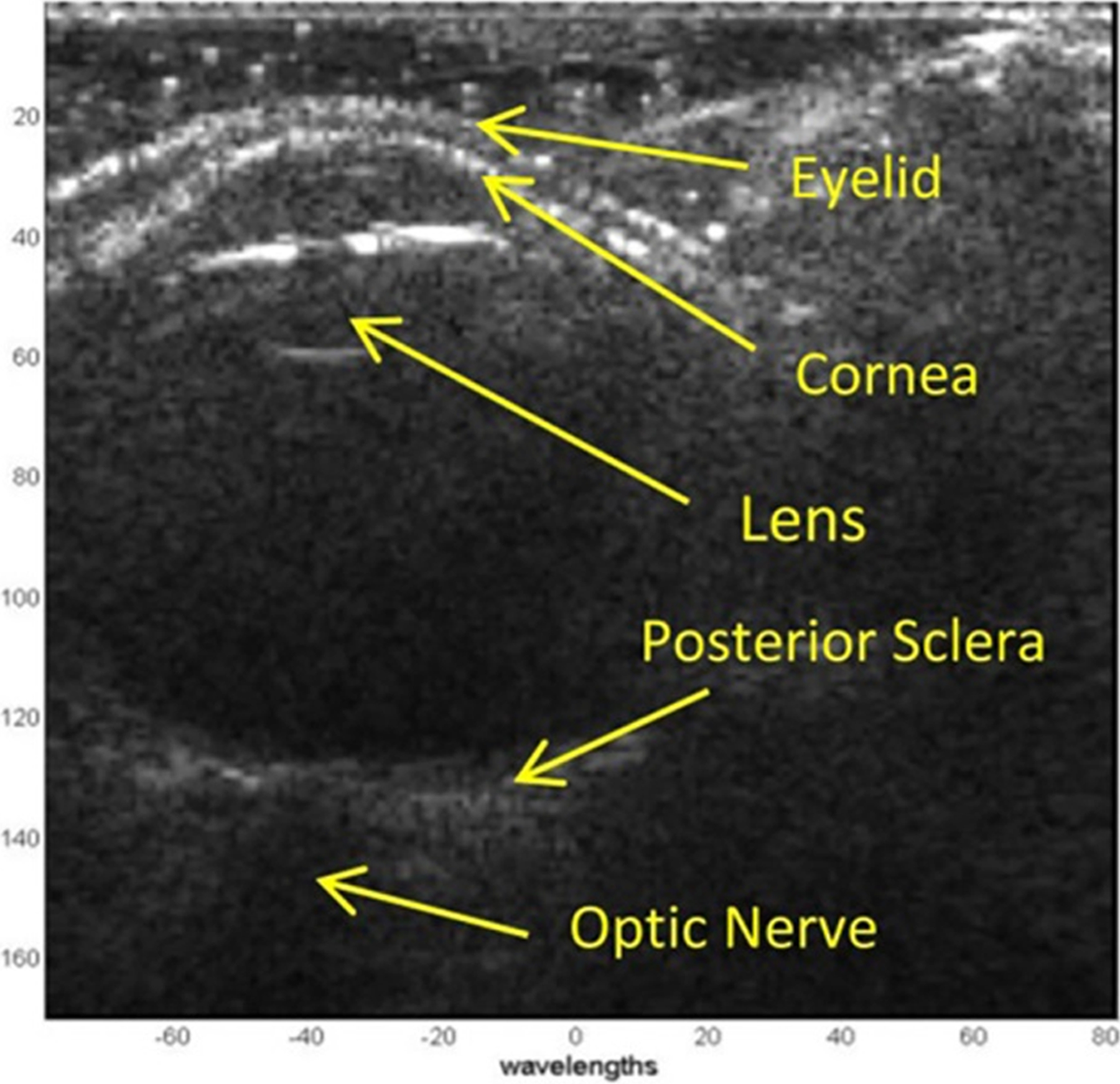
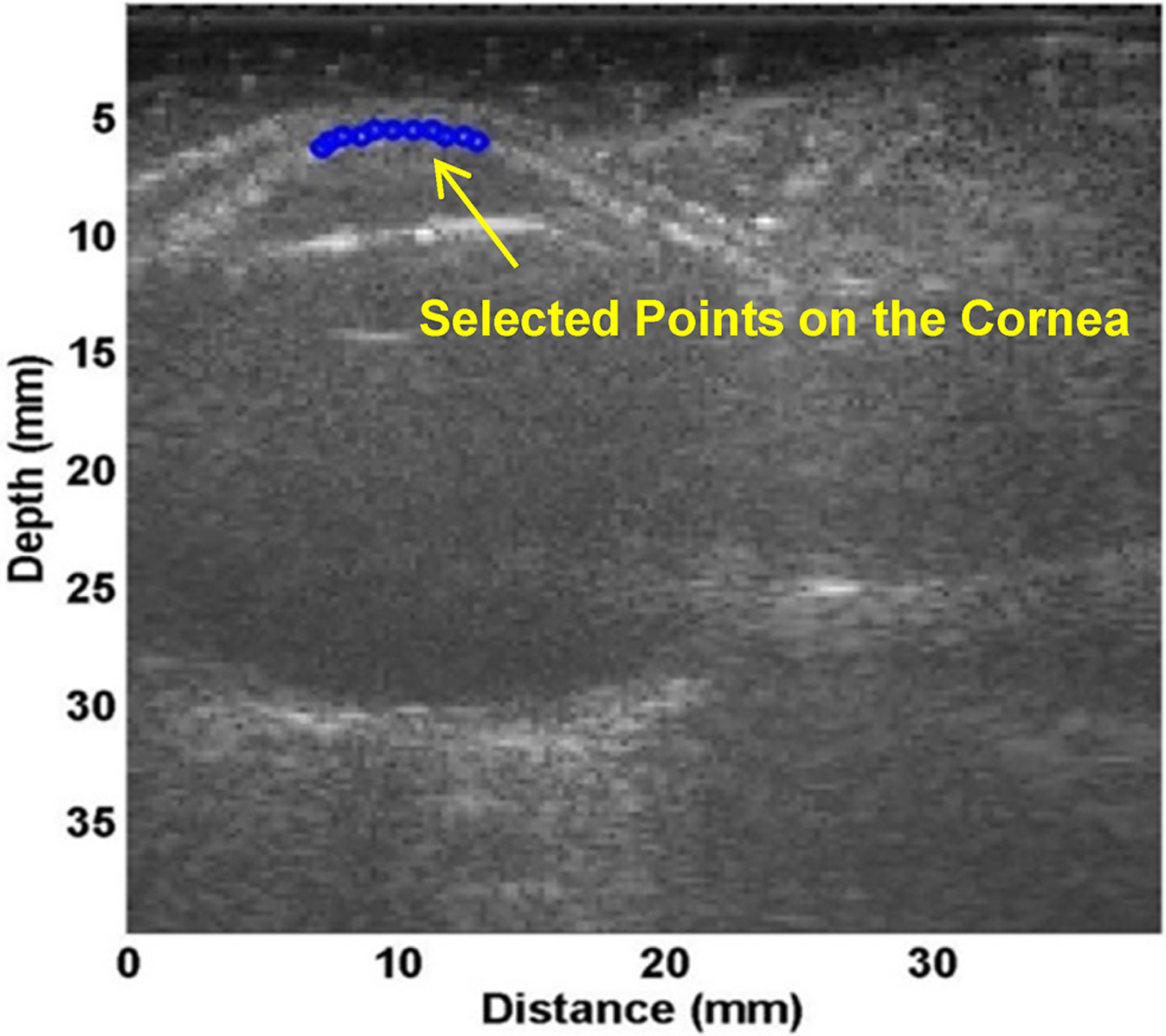
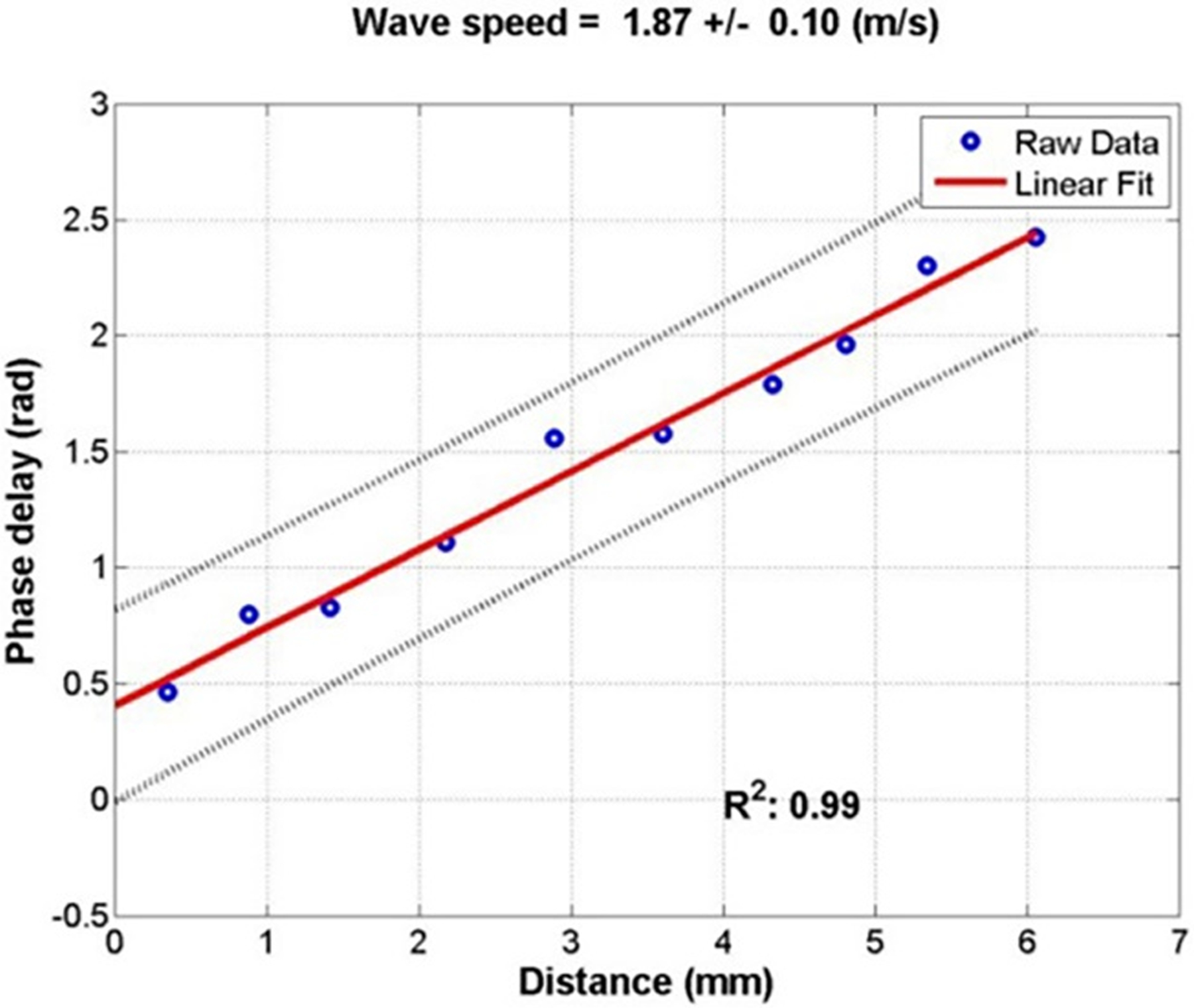
Measurement of wave speed using ultrasound surface wave elastography. A, Ultrasound B-mode image of the eye. B, Several points in the central 6 mm of the cornea were selected (blue dots) to measure wave propagation by using ultrasound tracking. C, The wave phase change with position, in response to a 0.1 second excitation at 100 Hz, was used to measure the wave speed.
Axial Length and Central Corneal Thickness
Axial length (AL) was measured by A-scan ultrasonography (Sonomed; New Hyde Park, NY) and central corneal thickness (CCT) was measured by using Scheimpflug imaging (Pentacam; Oculus, Wetzlar, Germany).
Statistical Analysis
Wave speed, ocular rigidity coefficient, IOP, CCT, and axial length were compared between normal and glaucomatous eyes by using generalized estimating equation (GEE) models to account for possible correlation between fellow eyes of the same subject. Correlations between wave speed and ocular rigidity coefficient with other parameters including age, IOP, CCT, and axial length were determined by linear regression analysis, with statistical significance determined by GEE models. All statistical tests were two-sided and were calculated by using SAS software (version 9.4; SAS Institute, Cary, NC, USA). Differences were considered significant if P was less than 0.05.
Repeatability Analysis
Measurement of wave speed requires the selection of analysis points within the tissue of interest. Interrater variability was assessed to determine the repeatability of USWE measurement when different sets of analysis points are selected. The first and second analyses of wave speed in all 40 eyes were compared. Interrater repeatability was evaluated by using intraclass correlations (ICC). ICC was analyzed by the Pearson correlation of the 2 data sets. Repeatability was considered as good for 0.60 ≤ ICC ≤ 0.74, and excellent for 0.75 ≤ ICC ≤ 1.00.
Test-to-test variability was assessed by comparing the wave speed of the first measurement with the wave speed from the second measurement at the same session in all eyes studied. Pearson correlation coefficient between the two measurements was calculated, and statistical significance was determined using GEE models.
Results
Twenty eyes of 10 glaucoma patients (age 45–78; 65.7 ± 11.6 years, mean ± SD) and 20 eyes of 13 healthy subjects (age 41–72; 60.8 ± 7.9 years) matched for age, IOP, and axial length were included in the study (Table 1). Mean IOP in the eyes with glaucoma was 17.5 ± 2.5 mmHg (95% confidence interval [CI] 16.2–18.9) and was not different from normal eyes (17.4 ± 2.1 mmHg, 95% CI 16.0–18.4, P= 0.81). Mean corneal wave speed at 100 Hz was 2.16 ± 0.25 m/s (95% CI 2.05–2.27) in glaucomatous eyes and 2.07 ± 0.16 m/s (95% CI 2.00–2.12) in normal eyes and there was not a significant difference between two groups (P= 0.17) (Table 2, Figure 4). Ocular rigidity coefficient was significantly lower in glaucomatous eyes compared to normal eyes (0.0218 ± 0.0033 μL−1, 95% CI 0.0204–0.0233, vs 0.0252 ± 0.0050 μL−1, 95% CI 0.0230–0.0280, P= 0.01; Figure 5).
Table 1.
Study Population Characteristics
| Glaucoma | Normal | P | |
|---|---|---|---|
| mean ± SD (95% CI) | mean ± SD (95% CI) | ||
| Age (yrs) | 65.7 ± 11.6 (58.7–72.7) | 60.8 ± 7.9 (54.7–63.6) | 0.11 |
| IOP - sitting (mmHg) | 17.5 ± 2.5 (16.2–18.9) | 17.4 ± 2.1 (16.0–18.4) | 0.81 |
| IOP – supine (mmHg) | 21.6 ± 2.5 (20.2–23.0) | 22.4 ± 2.2 (21.2–23.4) | 0.53 |
| CCT (μm) | 552 ± 28 (535–569) | 544 ± 40 (523–565) | 0.68 |
| Axial Length (mm) | 24.01 ± 0.63 (23.64–24.39) | 23.79 ± 0.77 (23.26–24.10) | 0.19 |
CI: Confidence interval
CCT: Central corneal thickness
Table 2.
Comparison of Wave Speed and Ocular Rigidity Coefficient between Glaucoma and Normal Eyes
| Glaucoma | Normal | P | |
|---|---|---|---|
| mean ± SD (95% CI) | mean ± SD (95% CI) | ||
| Wave Speed (m/s) | 2.16 ± 0.25 (2.05–2.27) | 2.07 ± 0.16 (2.00–2.12) | 0.17 |
| Ocular Rigidity (μL−1) | 0.0218 ± 0.0033 (0.0201–0.0235) | 0.0252 ± 0.0050 (0.0230–0.0280) | 0.01 |
Figure 4.
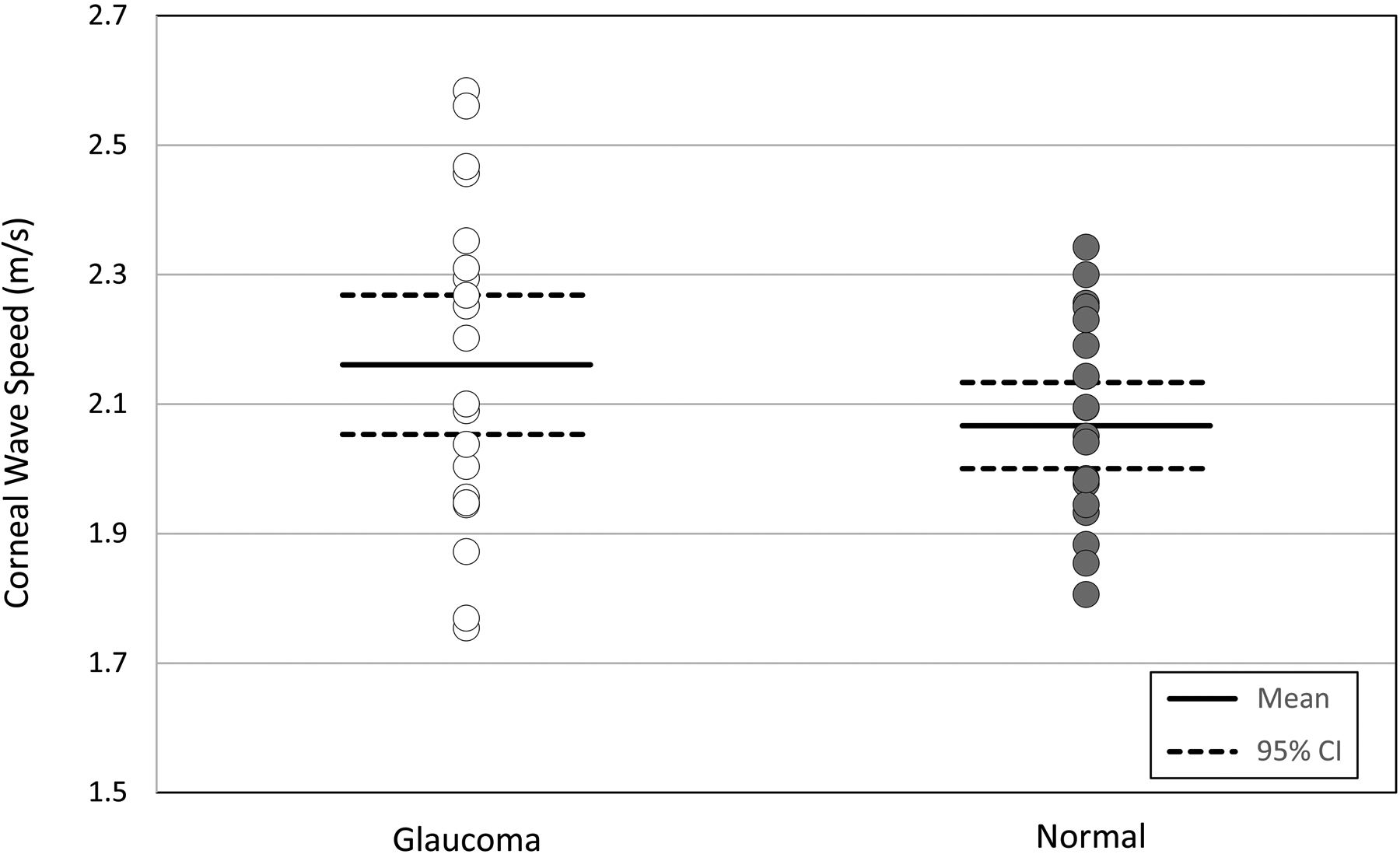
Comparison of corneal wave speed between normal and glaucoma subjects. Individual eye data are presented against mean and 95% confidence intervals for each cohort.
Figure 5.
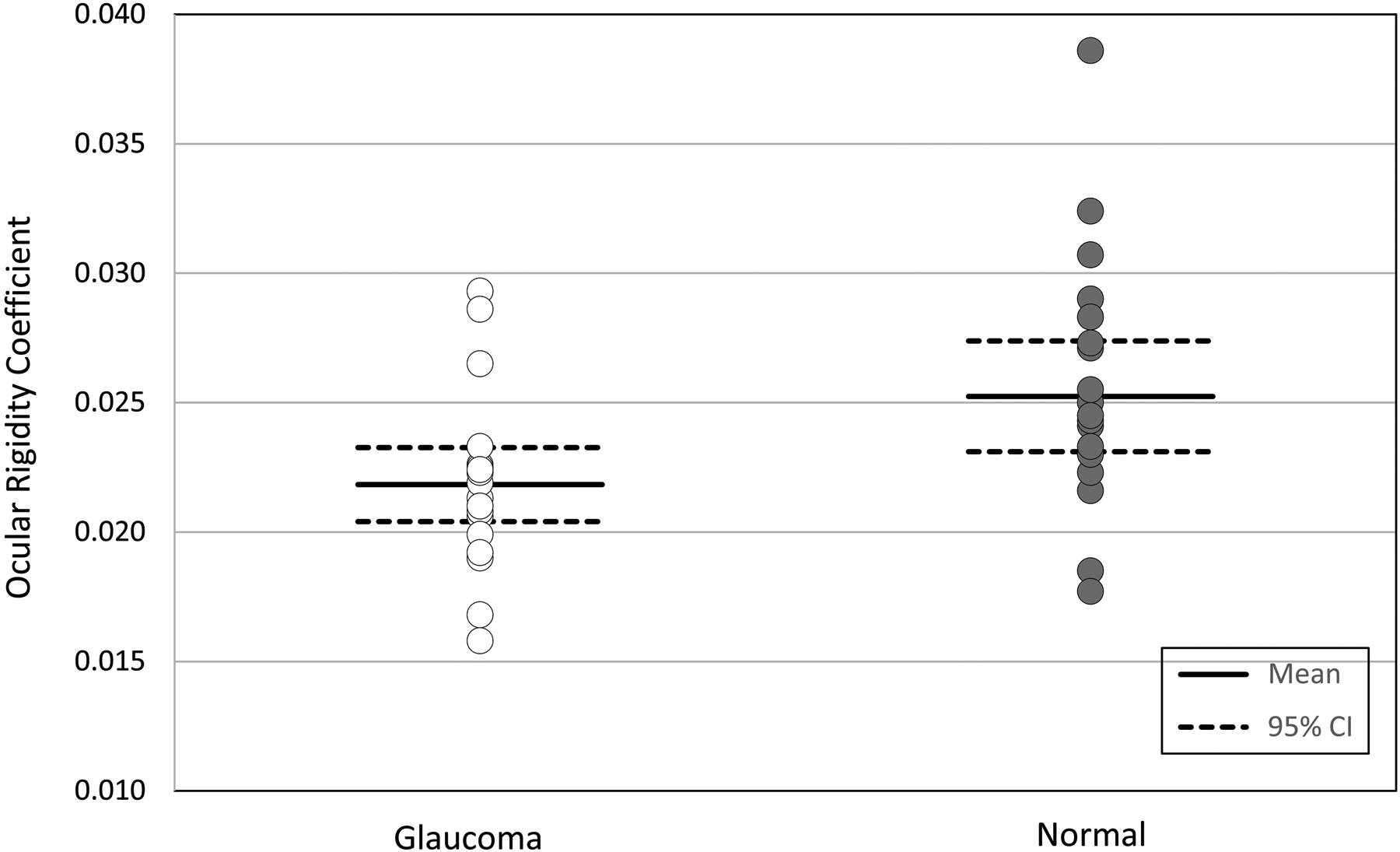
Comparison of ocular rigidity coefficient between normal and glaucoma subjects. Individual eye data are presented against mean and 95% confidence intervals for each cohort.
Mean central corneal thickness was 552 ± 28 μm (95% CI 535–569) in glaucoma eyes and 544 ± 40 μm (95% CI 523–565) in normal eyes (P= 0.68). Axial length in glaucoma eyes was not significantly different from normal eyes (24.01 ± 0.63 mm, 95% CI 23.64–24.39 vs 23.79 ± 0.77 mm, 95% CI 23.26–24.10, P= 0.19).
Corneal wave speed was not significantly correlated with age, IOP, and CCT in either glaucoma or normal subjects (P ≥ 0.13) but was correlated with ocular rigidity (R= 0.48, P= 0.02) and inversely correlated with axial length (R= −0.53, P= 0.01) in glaucoma subjects. (Table 3). Ocular rigidity was not significantly correlated with IOP and CCT in either group (P≥ 0.17) but was correlated inversely with age (R= −0.54, P= 0.02) and axial length (R= −0.45, P= 0.02) in normal subjects (Table 4).
Table 3.
Relationship between Wave Speed and Ocular Parameters
| Glaucoma | r (Pearson Correlation) | P | Normal | r (Pearson Correlation) | P | |
|---|---|---|---|---|---|---|
| mean ± SD | mean ± SD | |||||
| Wave Speed (m/s) | 2.16 ± 0.25 | 2.05 ± 0.16 | ||||
| Ocular Rigidity (μL−1) | 0.0218 ± 0.0033 | 0.48 | 0.02 | 0.0252 ± 0.0050 | −0.08 | 0.48 |
| IOP - sitting (mmHg) | 17.5 ± 2.5 | −0.05 | 0.62 | 17.4 ± 2.1 | −0.11 | 0.38 |
| IOP – supine (mmHg) | 21.6 ± 2.5 | −0.20 | 0.25 | 22.4 ± 2.2 | −0.05 | 0.76 |
| Age (yrs) | 65.7 ± 11.6 | −0.14 | 0.66 | 60.8 ± 8.0 | −0.22 | 0.23 |
| CCT (μm) | 552 ± 28 | −0.18 | 0.43 | 544 ± 40 | −0.21 | 0.13 |
| Axial Length (mm) | 24.01 ± 0.63 | −0.53 | 0.01 | 23.79 ± 0.77 | −0.22 | 0.31 |
Table 4.
Relationship between Ocular Rigidity and Ocular Parameters
| Glaucoma | r (Pearson Correlation) | P | Normal | r (Pearson Correlation) | P | |
|---|---|---|---|---|---|---|
| mean ± SD | mean ± SD | |||||
| Ocular Rigidity (μL−1) | 0.0218 ± 0.0033 | * | 0.0252 ± 0.0050 | * | ||
| IOP - sitting (mmHg) | 17.5 ± 2.5 | 0.11 | 0.63 | 17.4 ± 2.1 | 0.21 | 0.53 |
| IOP – supine (mmHg) | 21.6 ± 2.5 | 0.07 | 0.62 | 22.4 ± 2.2 | 0.22 | 0.68 |
| Age (yrs) | 65.7 ± 11.6 | −0.23 | 0.23 | 60.8 ± 7.9 | −0.54 | 0.02 |
| CCT (μm) | 552 ± 28 | −0.02 | 0.80 | 544 ± 40 | 0.29 | 0.17 |
| Axial Length (mm) | 24.01 ± 0.63 | −0.19 | 0.50 | 23.79 ± 0.77 | −0.45 | 0.02 |
Interrater repeatability for wave speed measurements was excellent with an ICC of 0.97. As well, there was a strong correlation between the first and the second wave speed measurements (R= 0.70, P < 0.001; Figure 6).
Figure 6.
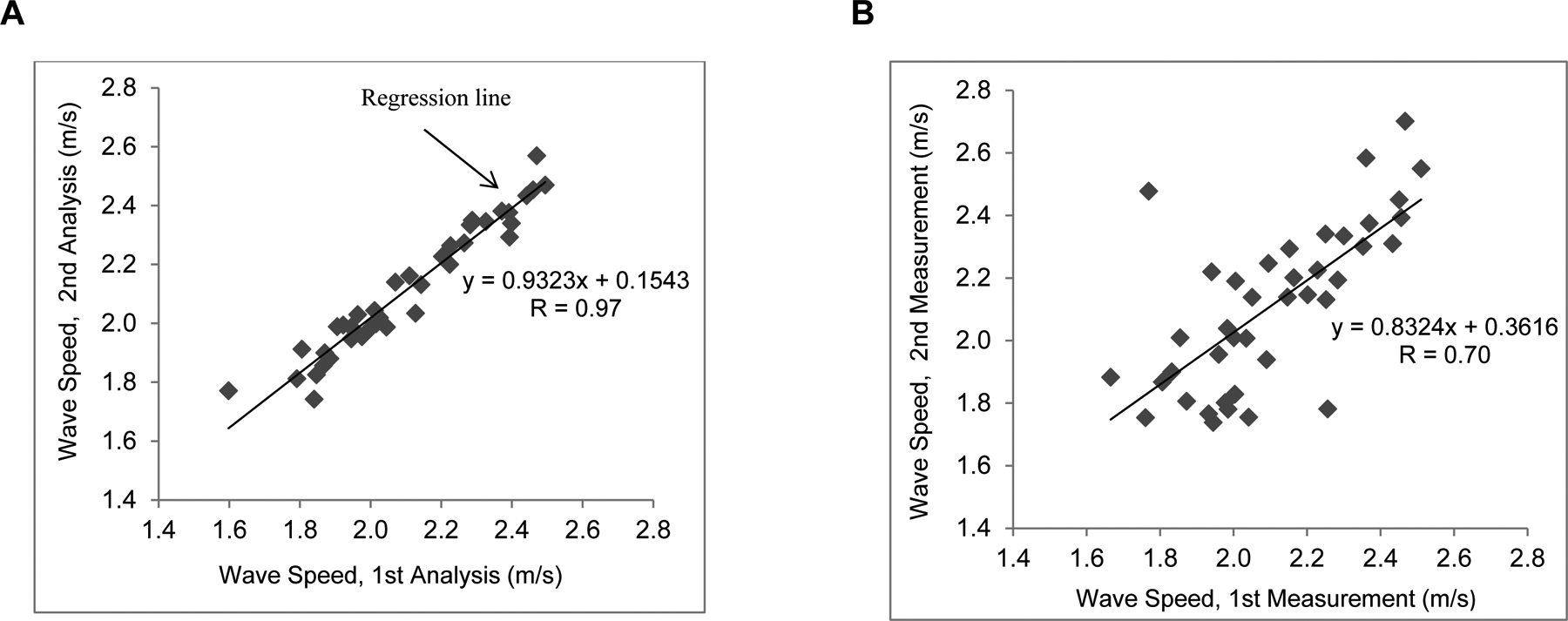
Repeatablity analysis of wave speed. A, Interrater repeatability. The first and second analyses of wave speed were compared. B, Intrasession (test-to-test) repeatability. The wave speed of the first measurement compared with the wave speed from the second measurement at the same session.
Eight of the 10 patients in the glaucoma cohort were on latanoprost monotherapy while 2 were on latanoprost plus brimonidine in both eyes. The duration of latanoprost therapy ranged from 6 weeks to 12 years (mean: 148 ± 222 months). There was no correlation between duration of latanoprost treatment with either ocular rigidity (R= 0.10, P= 0.69) or corneal wave speed (R= 0.19, P= 0.42).
Discussion
Abnormal biomechanical properties of the eye may be an important risk factor for glaucoma development and progression. However, there are no commercially available devices that can non-invasively measure tissue specific biomechanical properties in the human eye. We have previously used the technique of USWE in the other tissues of the body to determine changes in disease states.13, 19, 26, 27 We have also used USWE in the corneas of normal subjects to determine Young’s modulus of elasticity in the cornea.10 In the current study, we measured corneal wave speed using USWE to investigate the differences in ocular biomechanical properties between normal and glaucomatous eyes. Also, we compared ocular rigidity between these two groups.
Our study found no significant difference in corneal wave speed at 100 Hz between medically treated glaucomatous eyes and normal eyes. Since wave speed is an indicator of tissue elasticity, and can be used to calculate a Young’s modulus of elasticity,10 our results suggest that corneal elasticity is not altered in glaucomatous eyes. This suggests that, if ocular biomechanical properties in glaucomatous eyes are abnormal, the differences occur in tissues other than cornea, or affect viscosity instead of elasticity.
When assessing ocular tissue elasticity (or surrogate measures of elasticity), a potentially complicating factor is the well-described dependence of elasticity on IOP. In one study, Elsheikh et al28 mounted cadaver human and porcine corneas on a pressure chamber, measured the displacement of the cornea as IOP was varied, and investigated the stress-strain behavior of the intact cornea. They found that Young’s modulus of elasticity was related to the pressure and age of the donor. In our pilot study of normal subjects, we also found a relationship between IOP and Young’s modulus.10 Our current study design therefore matched glaucoma eyes with controls matched for age, IOP, and axial length. However, an apparent effect of this was the lack of a correlation between wave speed and IOP either in sitting or supine position in either glaucoma patients or controls. This was likely due to the limited IOP range in both normal subjects and medically treated glaucoma patients (12.7–21.3 mmHg in glaucoma eyes and 12.5–19.8 mmHg in normal eyes).
Although corneal wave speed was similar between normal subjects and glaucoma patients, ocular rigidity was found to be significantly less in glaucomatous eyes compared with normal controls. Ocular rigidity is assumed to describe the combined structural stiffness of the cornea, sclera, choroid, Bruch’s membrane, and retina, but scleral stiffness is assumed to be the principal component. Because of possible effects of IOP on ocular rigidity, our subjects were IOP matched. Ocular rigidity coefficient in our cohort of normal eyes was 0.0252 μL−1 which was consistent with a previous study17 in normal subjects (0.0276 μL−1). In the literature, the mean calculated ocular rigidity coefficient in normal eyes ranges from 0.0126 to 0.0280 μL−1.17, 29–35 While there is currently no gold standard for ocular rigidity measurement, and different measurement techniques can produce different results, comparison of results using a single technique can still provide useful information. Lower ocular rigidity and a more deformable ocular coat may result in greater optic nerve head deformation (and more axonal stress) from raised IOP, potentially predisposing to glaucomatous optic neuropathy.36, 37 In support of this theory, experimental murine models have shown that strains with stiffer sclera and greater resistance to deformation are less susceptible to glaucomatous damage.38 We did not find a correlation between corneal wave speed and ocular rigidity in normal subjects, although there was a weak correlation in the glaucoma cohort. This suggests that corneal biomechanical properties may not be representative of biomechanical properties of the whole globe, and may be a poor indicator or optic nerve head susceptibility.
The results of this study support the idea that a more compliant ocular coat may predispose the optic nerve head to IOP-related damage.37 However, results from other studies comparing ocular rigidity in normal and glaucomatous subjects have been inconclusive. Wang et al39 estimated ocular rigidity by using ocular pulse amplitude and pulsatile choroidal blood flow in several groups. There were no differences in IOP and axial length between normal group and glaucoma group, but glaucoma patients were older. Ocular rigidity was significantly lower in the glaucoma group compared to the normal group (0.188 ± 0.14 μL−1 vs 0.230 ± 0.12 μL−1), consistent with our results. However, Dastiridou et al29 calculated ocular rigidity with an invasive manometric technique and matched the glaucoma group and control group for axial length. IOP and age were not different between the two groups. They reported that the OR coefficient was 0.0220 ± 0.0053 μL−1 in the medically treated OAG patients and 0.0222 ± 0.0039 μL−1 in the control group, which was not different from glaucoma group. Beaton et al40 used optical coherence tomography imaging and choroidal segmentation to determine pulsatile ocular volume change from pulsatile sub-macular choroidal thickness change and calculate ocular rigidity. The mean ocular rigidity was 0.028 ± 0.022 μL−1 with their method. Later, the same group proposed a new mathematical model of choroidal thickness measurement over the entire choroid and determined ocular rigidity of 0.0248 ± 0.013 μL−1 in their 260 subjects.41 Ocular rigidity coefficient in our cohort of normal eyes was 0.0252 μL−1 which is very close to their results.
One potentially complicating factor in our study was that all of our glaucoma subjects were treated with PGAs. PGAs are known to upregulate matrix metalloproteinases and reduce collagen in the cornea42 and sclera43, with the potential to alter biomechanical properties. However, in a different study, our group examined the effect of 6 weeks of latanoprost treatment on corneal biomechanical properties and found no changes in corneal wave speed or ocular rigidity compared to baseline in glaucoma patients (Kazemi A, Zhou BR, Zhang XM, et al. Effect of IOP Reduction by Latanoprost on Corneal Biomechanical Properties in Glaucomatous Eyes Using Ultrasound Surface Wave Elastography. Invest Ophth Vis Sci 2019;60). As well, we did not find an association between duration of PGA treatment and ocular rigidity or corneal wave speed in our study subjects.
Aging has been shown to be associated with increased stiffness of the trabecular meshwork, lamina cribrosa, sclera, and cornea.44 However, unlike previous studies of cadaver eyes,28, 45 we did not find a relationship between corneal elasticity (as indicated by corneal wave speed) and age, likely because of our small sample size and clustering of our subjects in a fairly narrow age range (range 41 to 72 years in normal group and 45 to 78 years in glaucoma group). However, there was a weak association between corneal wave speed and ocular rigidity in the glaucoma cohort, suggesting that these measurements may be at least partially influenced by the same tissue parameters. As well, lower wave speed was associated with greater axial length in the glaucoma cohort, consistent with other investigators who have found myopia to be associated with lower ocular tissue modulus.46 We did not find a relationship between the corneal wave speed and central corneal thickness in either group, consistent with our pilot study.10 This suggests that, in our subject population, tissue dimensions (corneal thickness) are not correlated to the intrinsic elastic properties of the tissue. However, it is also possible that our sample size was not large enough to detect such a relationship.
Our study has several potential limitations. The small sample size of our study was driven by the requirement of finding normal and glaucoma subjects with matched age, pressure and axial length. It is possible that some correlations between wave speed and ocular parameters were not detected because of the limited sample size. However, the strength of association was weak for most variables, suggesting a minimal contribution of these parameters to wave speed variation. One potential concern about USWE is that measurements are performed through closed eyelids, and overlying tissues may interfere with the measurement of wave speed. However, since wave propagation can be directly visualized in different tissues and layers by using the ultrasound probe, the overlying tissues would not have any direct effect on wave propagation. Our current USWE implementation is also limited by the measurement of wave propagation along one direction, whereas ocular tissues demonstrate significant anisotropy.47 As well, ocular tissues, such as the cornea, do not have uniform properties throughout the tissue,47 and we are assuming that corneal wave speed is a bulk property. To limit these potential sources of variability, all ocular measurements and analyses were performed by one experienced investigator (AK), who maintained consistent placement of the shaker and ultrasound probe for each eye. However, it is possible that differences in elasticity between the two cohorts only occur in specific layers or regions of the cornea and were not detected by our measurements.
Another potential limitation was our use of Friedenwald’s equation for ocular rigidity calculation. Estimates of the volume change with a 10 gram weight on the pneumatonometer are based on empiric studies and a fixed volume change is assumed for each level of IOP regardless of the individual eye size.18 As a result, axial length can be a confounding factor since larger eyes will have a larger relative volume for a given pressure change than smaller eyes. To control for this confounding factor, we matched axial length between glaucoma patients and normal controls. Another issue with ocular rigidity measurement is that the specific tissue differences between the normal and glaucoma cohorts cannot be determined. While changes in the sclera are certainly possible, the volume change with the 10 gram weight is relatively small (26.5 μL at 15 mmHg), so that focal tissue changes could potentially account for the difference in rigidity. For example, a highly distensible lamina cribrosa or parapapillary sclera could be causing the differences in rigidity measurements while the remainder of the globe is unaltered. Changes in choroidal volume can also complicate the measurement as a change in blood volume could potentially occur in conjunction with distention of the ocular coat.
Finally, it is important to note that our results show differences in ocular rigidity between a cohort of glaucoma patients and normal controls but cannot infer causality. Some studies in non-human primates with experimental glaucoma suggest that the sclera changes in response to elevated IOP, first becoming hypercompliant48 followed by stiffening as the disease progresses.49 However, experimental animal models have glaucoma induced by causing an elevation of IOP. In contrast, a large proportion of human glaucoma patients develop disease with IOP in the statistically normal range. As well, studies in human patients using the Ocular Response Analyzer (Reichert Inc., Depew, NY) to measure corneal hysteresis (CH) have reported altered biomechanics prior to the development of glaucoma. Susanna et al. found that glaucoma suspects with lower CH were more likely to progress to POAG than patients with higher CH and similar IOP.6 However, CH cannot be directly related to ocular rigidity, wave speed (which can be used to determine Young’s modulus – the elasticity due to tensile stress) or other measures of elasticity. Instead, CH is a behavior of the eye in response to sudden deformation and will reflect both elasticity and viscosity. The mechanical properties of ocular tissues that predispose to glaucoma (if any) remain to be elucidated.
Conclusions:
In summary, our study found that corneal wave speed, a measure of corneal elasticity, was not different between normal and glaucomatous eyes. However, ocular rigidity coefficient, a parameter that is potentially influenced by biomechanical properties of the whole globe, was lower in glaucomatous eyes compared to normal eyes. Whether or not lower ocular rigidity in glaucomatous eyes is a predisposing factor or a response to glaucoma is unknown. Future USWE studies are needed to measure and compare scleral elasticity between glaucoma and normal subjects. As well, future studies will need to assess tissue viscosity in addition to elasticity to determine the contribution of this property to glaucoma pathogenesis.
Funding/Support:
This study was supported by National Institutes of Health/National Eye Institute (Grant R21 EY026095) (Co-PIs Sit/Zhang), and Mayo Foundation for Medical Education and Research - Leonard and Mary Lou Hoeft Career Development Award (Sit). Xiaoming Zhang is a co-inventor of ultrasound surface wave elastography (Patent number 9044192) but no royalties have been paid. The funding organizations had no role in study design; collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. All authors have approved the final article. The authors alone are responsible for the content and writing of the paper.
Financial Disclosures:
Arthur Sit: Aerie Pharmaceuticals, Inc. (Consultant), Allergan, Inc. (Consultant), Bausch & Lomb, Inc. (Consultant), Injectsense, Inc. (Consultant), PolyActiva, Pty (Consultant), Qlaris Bio, Inc. (Research support). Xiaoming Zhang: Patent (Number 9044192). Arash Kazemi: No financial disclosures. Boran Zhou: No financial disclosures.
References
- 1.Quigley HA, Vitale S. Models of open-angle glaucoma prevalence and incidence in the United States. Invest Ophthalmol Vis Sci 1997;38:83–91. [PubMed] [Google Scholar]
- 2.Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. Jama 1991;266:369–374. [PubMed] [Google Scholar]
- 3.Investigators TA. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS Investigators. Am J Ophthalmol 2000;130:429–440. [DOI] [PubMed] [Google Scholar]
- 4.Brandt JD, Beiser JA, Kass MA, Gordon MO. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS). Ophthalmology 2001;108:1779–1788. [DOI] [PubMed] [Google Scholar]
- 5.Medeiros FA, Meira-Freitas D, Lisboa R, Kuang TM, Zangwill LM, Weinreb RN. Corneal Hysteresis as a Risk Factor for Glaucoma Progression: A Prospective Longitudinal Study. Ophthalmology 2013;120:1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Susanna CN, Diniz A, Daga FB, et al. A Prospective Longitudinal Study to Investigate Corneal Hysteresis as a Risk Factor for Predicting Development of Glaucoma. Am J Ophthalmol 2018;187:148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sigal IA, Yang H, Roberts MD, Burgoyne CF, Downs JC. IOP-induced lamina cribrosa displacement and scleral canal expansion: an analysis of factor interactions using parameterized eye-specific models. Invest Ophthalmol Vis Sci 2011;52:1896–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downs JC, Suh JK, Thomas KA, Bellezza AJ, Hart RT, Burgoyne CF. Viscoelastic material properties of the peripapillary sclera in normal and early-glaucoma monkey eyes. Invest Ophthalmol Vis Sci 2005;46:540–546. [DOI] [PubMed] [Google Scholar]
- 9.Downs JC. Optic nerve head biomechanics in aging and disease. Exp Eye Res 2015;133:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sit AJ, Lin SC, Kazemi A, McLaren JW, Pruet CM, Zhang X. In Vivo Noninvasive Measurement of Young’s Modulus of Elasticity in Human Eyes: A Feasibility Study. J Glaucoma 2017;26:967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Zhou B, Kalra S, Bartholmai B, Greenleaf J, Osborn T. An Ultrasound Surface Wave Technique for Assessing Skin and Lung Diseases. Ultrasound Med Biol 2018;44:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Osborn T, Zhou B, et al. Lung Ultrasound Surface Wave Elastography: A Pilot Clinical Study. IEEE Trans Ultrason Ferroelectr Freq Control 2017;64:1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zielinski MD, Kuntz M, Zhang X, et al. Botulinum toxin A-induced paralysis of the lateral abdominal wall after damage-control laparotomy: A multi-institutional, prospective, randomized, placebo-controlled pilot study. J Trauma Acute Care Surg 2016;80:237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubo K, Zhou BR, Cheng YS, et al. Ultrasound elastography for carpal tunnel pressure measurement: A cadaveric validation study. J Orthop Res 2018;36:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedenwald JS. Contribution to the theory and practice of tonometry. Am J Ophthalmol 1937;20:985–1024. [Google Scholar]
- 16.Gedde SJ, Vinod K, Wright MM, et al. Primary Open-Angle Glaucoma Preferred Practice Pattern (R). Ophthalmology 2021;128:P71–P150. [DOI] [PubMed] [Google Scholar]
- 17.Kazemi A, McLaren JW, Lin SC, et al. Comparison of Aqueous Outflow Facility Measurement by Pneumatonography and Digital Schiotz Tonography. Invest Ophthalmol Vis Sci 2017;58:204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langham ME, Leydhecker W, Krieglstein G, Waller W. Pneumatonographic studies on normal and glaucomatus eyes. Adv Ophthalmol 1976;32:108–133. [PubMed] [Google Scholar]
- 19.Zhang X, Osborn TG, Pittelkow MR, Qiang B, Kinnick RR, Greenleaf JF. Quantitative assessment of scleroderma by surface wave technique. Med Eng Phys 2011;33:31–37. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Osborn T, Kalra S. A noninvasive ultrasound elastography technique for measuring surface waves on the lung. Ultrasonics 2016;71:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou B, Sit AJ, Zhang X. Noninvasive measurement of wave speed of porcine cornea in ex vivo porcine eyes for various intraocular pressures. Ultrasonics 2017;81:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa H, Kanai H. Improving accuracy in estimation of artery-wall displacement by referring to center frequency of RF echo. IEEE Trans Ultrason Ferroelectr Freq Control 2006;53:52–63. [DOI] [PubMed] [Google Scholar]
- 23.Zhang XM, Zhou BR, Miranda AF, Trost LW. A Novel Noninvasive Ultrasound Vibro-elastography Technique for Assessing Patients With Erectile Dysfunction and Peyronie Disease. Urology 2018;116:99–105. [DOI] [PubMed] [Google Scholar]
- 24.Zhang XM, Zhou BR, Osborn T, Bartholmai B, Kalra S. Lung Ultrasound Surface Wave Elastography for Assessing Interstitial Lung Disease. Ieee T Bio-Med Eng 2019;66:1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clay R, Bartholmai BJ, Zhou BR, et al. Assessment of Interstitial Lung Disease Using Lung Ultrasound Surface Wave Elastography A Novel Technique With Clinicoradiologic Correlates. J Thorac Imag 2019;34:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubo K, Cheng YS, Zhou B, et al. The quantitative evaluation of the relationship between the forces applied to the palm and carpal tunnel pressure. J Biomech 2018;66:170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Zhou B, VanBuren WM, Burnett TL, Knudsen JM. Transvaginal Ultrasound Vibro-elastography for Measuring Uterine Viscoelasticity: A Phantom Study. Ultrasound Med Biol 2019;45:617–622. [DOI] [PubMed] [Google Scholar]
- 28.Elsheikh A, Alhasso D, Rama P. Biomechanical properties of human and porcine corneas. Exp Eye Res 2008;86:783–790. [DOI] [PubMed] [Google Scholar]
- 29.Dastiridou AI, Tsironi EE, Tsilimbaris MK, et al. Ocular rigidity, outflow facility, ocular pulse amplitude, and pulsatile ocular blood flow in open-angle glaucoma: a manometric study. Invest Ophthalmol Vis Sci 2013;54:4571–4577. [DOI] [PubMed] [Google Scholar]
- 30.Dastiridou AI, Ginis H, Tsilimbaris M, et al. Ocular rigidity, ocular pulse amplitude, and pulsatile ocular blood flow: the effect of axial length. Invest Ophthalmol Vis Sci 2013;54:2087–2092. [DOI] [PubMed] [Google Scholar]
- 31.Pallikaris IG, Kymionis GD, Ginis HS, Kounis GA, Christodoulakis E, Tsilimbaris MK. Ocular rigidity in patients with age-related macular degeneration. Am J Ophthalmol 2006;141:611–615. [DOI] [PubMed] [Google Scholar]
- 32.Dastiridou AI, Ginis HS, De Brouwere D, Tsilimbaris MK, Pallikaris IG. Ocular rigidity, ocular pulse amplitude, and pulsatile ocular blood flow: the effect of intraocular pressure. Invest Ophthalmol Vis Sci 2009;50:5718–5722. [DOI] [PubMed] [Google Scholar]
- 33.Detorakis ET, Tsaglioti E, Kymionis G. Non-Invasive Ocular Rigidity Measurement: A Differential Tonometry Approach. Acta Medica (Hradec Kralove) 2015;58:92–97. [DOI] [PubMed] [Google Scholar]
- 34.Pallikaris IG, Kymionis GD, Ginis HS, Kounis GA, Tsilimbaris MK. Ocular rigidity in living human eyes. Invest Ophthalmol Vis Sci 2005;46:409–414. [DOI] [PubMed] [Google Scholar]
- 35.Drance SM. The coefficient of scleral rigidity in normal and glaucomatous eyes. Arch Ophthalmol 1960;63:668–674. [DOI] [PubMed] [Google Scholar]
- 36.Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res 2005;24:39–73. [DOI] [PubMed] [Google Scholar]
- 37.Sigal IA, Flanagan JG, Ethier CR. Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci 2005;46:4189–4199. [DOI] [PubMed] [Google Scholar]
- 38.Cone FE, Gelman SE, Son JL, Pease ME, Quigley HA. Differential susceptibility to experimental glaucoma among 3 mouse strains using bead and viscoelastic injection. Exp Eye Res 2010;91:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Freeman EE, Descovich D, et al. Estimation of ocular rigidity in glaucoma using ocular pulse amplitude and pulsatile choroidal blood flow. Invest Ophthalmol Vis Sci 2013;54:1706–1711. [DOI] [PubMed] [Google Scholar]
- 40.Beaton L, Mazzaferri J, Lalonde F, et al. Non-invasive measurement of choroidal volume change and ocular rigidity through automated segmentation of high-speed OCT imaging. Biomed Opt Express 2015;6:1694–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sayah DN, Mazzaferri J, Ghesquiere P, et al. Non-invasive in vivo measurement of ocular rigidity: Clinical validation, repeatability and method improvement. Exp Eye Res 2020;190. [DOI] [PubMed] [Google Scholar]
- 42.Lopilly Park HY, Kim JH, Lee KM, Park CK. Effect of prostaglandin analogues on tear proteomics and expression of cytokines and matrix metalloproteinases in the conjunctiva and cornea. Exp Eye Res 2012;94:13–21. [DOI] [PubMed] [Google Scholar]
- 43.Sagara T, Gaton DD, Lindsey JD, Gabelt BT, Kaufman PL, Weinreb RN. Topical prostaglandin F2alpha treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Arch Ophthalmol 1999;117:794–801. [DOI] [PubMed] [Google Scholar]
- 44.Liu B, McNally S, Kilpatrick JI, Jarvis SP, O’Brien CJ. Aging and ocular tissue stiffness in glaucoma. Surv Ophthalmol 2018;63:56–74. [DOI] [PubMed] [Google Scholar]
- 45.Geraghty B, Jones SW, Rama P, Akhtar R, Elsheikh A. Age-related variations in the biomechanical properties of human sclera. J Mech Behav Biomed Mater 2012;16:181–191. [DOI] [PubMed] [Google Scholar]
- 46.Hon Y, Chen GZ, Lu SH, Lam DCC, Lam AKC. High myopes have lower normalised corneal tangent moduli (less ‘stiff’ corneas) than low myopes. Ophthal Physl Opt 2017;37:42–50. [DOI] [PubMed] [Google Scholar]
- 47.Labate C, Lombardo M, De Santo MP, Dias J, Ziebarth NM, Lombardo G. Multiscale Investigation of the Depth-Dependent Mechanical Anisotropy of the Human Corneal Stroma. Invest Ophthalmol Vis Sci 2015;56:4053–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ivers KM, Yang HL, Gardiner SK, et al. In Vivo Detection of Laminar and Peripapillary Scleral Hypercompliance in Early Monkey Experimental Glaucoma. Invest Ophthalmol Vis Sci 2016;57:Oct388–Oct403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Girard MJA, Suh JKF, Bottlang M, Burgoyne CF, Downs JC. Biomechanical Changes in the Sclera of Monkey Eyes Exposed to Chronic IOP Elevations. Invest Ophthalmol Vis Sci 2011;52:5656–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]


