Abstract
Rabbit anti-thymocyte globulin (RATG) preparations are widely used in transplantation. They are developed in vivo against thymocytes and contain polyclonal antibodies specific for myriad cellular targets. The rhesus monkey is commonly used as a pre-clinical transplant model, but the fidelity of commercially available human-specific RATGs to anticipate effects of RATGs in rhesus has not been established. We therefore developed two rhesus-specific ATGs (rhATG) and compared them to human specific RATG (huATG, Thymoglobulin®) in rhesus monkeys, assessing the magnitude and phenotype of depletion peripherally and in lymph nodes. Four primates were assigned to each group and received 20mg/kg of drug. Depletion, repopulation and changes in lymphocyte subsets were evaluated in peripheral blood and lymph nodes by flow cytometry over four months. We observed similar qualitative changes in lymphocyte subsets, but a generally more profound depletion with huATG compared to either rhATG. Peripheral homeostatic proliferation rather than thymic output was the major mechanism for repopulation with all RATGs. Repopulation was slower but qualitatively similar when examining RATGs in additional animals receiving concomitant chronic immunosuppression. Depletional induction is similar with human- and rhesus-specific RATGs in rhesus macaques. Both rhesus- and human-specific agents appear appropriate for preclinical modeling of clinical RATG use.
1. Introduction
Kidney allotransplantation is a lifesaving operation with superior mortality and economic outcomes compared to dialysis1,2. In the United States, approximately 70% of kidney recipients are treated with depletional induction, typically rabbit anti-thymocyte globulin (RATG). Multiple clinical studies have shown that RATG reliably decreases the incidence of acute rejection 3,4,5. As such, depletional induction with RATG is increasingly considered as a therapeutic approach for novel immunosuppressive regimens in pre-clinical investigation. Non-human primates such as rhesus monkeys are commonly used pre-clinically to test new immunosuppressants.
RATG is a polyclonal antibody preparation that is developed by immunizing large numbers of rabbits with thymocytes from another species6. It works by a variety of mechanisms including direct depletion of lymphocytes as well as interference with cell trafficking and the cell-cell interactions needed to mount an efficient immune response7. Additionally, though it was developed as a T lymphocyte depleting agent and is produced by immunization against thymocytes, it has also been shown to contain antibodies that are specific to and act on B cells8. RATG has profound effects on immune cell subsets including an acute preferential decrease in naïve T cells9,10, an increase in CD4 Tregs 9,11, and an acute increase in senescent T cells expressing CD5712. Over time, repopulation typically occurs through a variable combination of thymic output and peripheral homeostatic proliferation 10, 12,13,14. Though some theories have been advanced, there is little consensus on which of these exact mechanisms is the most important in mediating the clinical efficacy of ATG15.
Given the myriad effects of clinical grade RATGs and their derivation from human-specific immunizations, it has not been established whether these agents, when used in rhesus monkeys, have effects that anticipate their clinical use. This distinction has become increasingly relevant as many biologic agents have presented themselves for assessment in relevant pre-clinical transplant models. As such, a truly analogous non-human primate (NHP) model to examine the effects of RATG would be of great benefit. In this study we tested a Rhesus macaque-specific form of ATG (rhATG) and compared it directly with human-specific RATG (huATG, Thymoglobulin®) in order to determine whether species specificity of the product immunogen significantly altered the in vivo effect of the product. We find that both products have similar qualitative effects, though some distinguishing features exist.
2. Methods
2.1. Drug Manufacture, Regimen and Clinical Monitoring
Twelve Rhesus macaques were selected to receive one of three drugs for a total of four NHP per group. NHP received either huATG (Thymoglobulin®, Genzyme Corporation, MA) or a rhATG that was either adjuvanted (RhATG #5) or non-adjuvanted (RhATG #6) on preparation (NIH Nonhuman Primate Reagent Resource Cat# PR-1077, RRID:AB_2716327). These rhATGs were developed and manufactured by the Nonhuman Primate Reagent Resource (NHPRR; nhpreagents.org). To produce a macaque-specific ATG, NHPRR immunized rabbits with rhesus macaque thymocytes aiming to recapitulate the immunization procedure employed in the production of Thymoglobulin®. In brief, polyclonal rabbit sera was collected post a prime and two booster immunizations of 50 million rhesus thymocytes per each dose in the presence of adjuvants (lot 5) or not (lot 6). Anti-rhesus thymocyte rabbit serum was then pooled, diluted in phosphate buffered solution, and the IgG fraction was purified via protein A affinity chromatography. The purified rabbit IgG was formulated in 20 mM Citrate 150 mM NaCl, pH 6.0, 5% Maltose, and quality control tested in binding specificity, bioburden, and endotoxin assays. The final rhATG lots were stored at 4°C until infusion.
All animals were administered 4mg/kg of drug for 5 days for a total of 20mg/kg. One dose of 10mg/kg methylprednisolone was given prior to the first infusion to prevent possible infusion reactions, but no additional immunosuppression was given thereafter. Animals were clinically monitored for adverse events. They underwent peripheral blood draws on day 0 (just prior to infusion) and days 5, 14, 28, 56, 84, and 112. Additionally, all animals underwent peripheral lymph node (LN) sampling (either axilla or groin) on days 0, 5, and 84. Splenic and thymic tissue were obtained at the time of sacrifice. Cytomegalovirus titers were monitored at each sedation day by polymerase chain reaction. The experimental timeline is summarized in Figure 1.
Figure 1: Experimental timeline.
Animals received 20mg/kg total of ATG over 5 days (4mg/kg per dose). Lymph node biopsies were performed at Day 0, Day 5, 3 Months, and 4 months. Sacrifice was performed at 4 months.
2. 2. Flow Cytometry Assays
Lymphocyte subsets were monitored throughout the study period by flow cytometry of ficoll isolated peripheral blood mononuclear cells and single cell suspensions of peripheral lymph nodes and spleens at the above listed time points. We stained for various lymphocyte population markers as listed in Supplemental Table 1. Our gating strategy for all lymphocyte subsets is shown in Supplemental Figure 1. Flow cytometry was performed on a BD LSRFortessa™ (BD Biosciences, San Jose, CA, USA) and analyzed using FlowJo software version 10 (Tree Star, Ashland, OR, USA).
2.3. Histology
Peripheral LN, splenic, and thymic tissue were evaluated by histology. Standard H&E staining was performed on all tissue. On LN tissue, immunofluoresence (IF) using anti-CD3 and anti-CD20 (Dako, Carpinteria, CA) staining was performed in order to evaluate germinal center formation. We also examined the thymus for evidence of binding of RATG using a biotinylated goat-anti-rabbit polyclonal antibody (BA-1000-1.5; Vector Laboratories, Burlingame, CA) to detect RATG. A primary mouse anti-cytokeratin Ab (MS-611-P0; Neomarkers, Fremont, CA) followed by a secondary biotinylated horse anti-mouse Ab (BA200, Vector Laboratories, Burlingame, CA) as a positive control. Samples were developed using the Vector Elite Kit (PK-6100; Vector Laboratories, Burlingame, CA).
2.3. In Vitro Binding Assay
In order to test the ability of the various RATGs to bind to NHP cells, we obtained PBMC from n=3 NHP prior to any treatment. We first blocked the cells using purified polyclonal goat antibody (Jackson Immunoresearch, West Grove, PA). We next incubated the cells with the various ATGs at 1ug/mL. We then stained the cells using a cocktail that included various lymphocyte markers as described in Supplemental Table 1 as well as a polyclonal goat-anti-rabbit secondary antibody (Thermofisher, Waltham, MA) to detect the various RATGs. The samples were then analyzed on our custom BD LSRFortessa as above.
2.4. Statistical Analysis
Statistical analyses were performed using GraphPad Prism software version 8.0 (GraphPad Software, San Diego, CA, USA). In order to determine differences in lymphocyte subsets over time, mixed effect models were postulated with treatment (huATG, rhATG#5, and rhATG#6) and time (days since first infusion) and the interaction of treatment and time as independent variables. For models where any of the main effects were significant, a post-test comparing the “control” group (huATG) to the two “treatment” groups (rhATG#5 and rhAT#6) was performed using Dunnett’s post-test. In some analyses, T cell subsets was used as a main effect instead of treatment group. In these models, post-tests were utilized to compare day 0 values with all subsequent days to compare trends over time, again using Dunnett’s post-test. This method was chosen as opposed to traditional ANOVA due to missingness of data at late time points. Simple linear regression was used for some comparisons. For the in vitro binding studies, an ANOVA with a Tukey’s post-test was used to compare all groups. Two-sided p-values <0.05 were considered significant.
3. Results
3. 1. No treatment-specific adverse events with huATG or rhATG were observed
The treatments with all preparations of RATG was well tolerated. We observed a total of three adverse events in the study (two in the huATG group and one in the rhATG#5 group). In the huATG group, one NHP experienced an infusion reaction with transient fever and one primate had breakdown of a wound after lymph node harvest. In the rhATG#5 group, one NHP had a seizure like event, which was determined to be secondary to sedation. All animals were monitored for rhesus cytomegalovirus viremia. Peak drug levels were similar between all groups (Supplemental Figure 2A). Peak viral load was numerically, but not statistically higher in the huATG group with all 4 animals experiencing at least transient viremia in the absence of CMV prophylaxis (Supplemental Figure 2B).
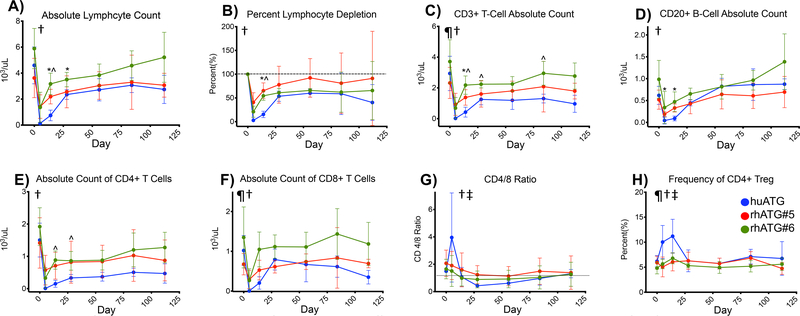
3. 2. huATG depletes lymphocytes more effectively than rhATG
Generally, huATG produced more profound lymphopenia when examined by both absolute counts and percent depletion (Figure 2 A&B). Absolute CD3+ T cell counts were lower with huATG than the rhATG preparations at multiple time points, but most pronounced at 14 days (Figure 2C). CD20+ B cell counts were significantly lower with huATG compared to rhATG#5 at days 5 and 14 (Figure 2D). Of note, the percent depletion of T cells (Median 88%, IQR 68–99%) was similar to that of B cells (74%, IQR 57–98%, p=0.34 by Wilcoxon-Sign Rank Test).
Figure 2:
All ATGs cause depletion but huATG causes more efficient depletion, greater changes in the CD4/8 Ratio and greater increases in Treg. A) Absolute and B) percent lymphocytes stratified by ATG preparation. Absolute counts of C) CD3+ T Cells, D) CD20+ B Cells, E) CD4+ T cells and F) CD8+ T cells stratified by ATG preparation. G) CD4/8 ratio as stratified by ATG preparation. H) Frequency of CD4+ Treg stratified by ATG preparation. All comparisons by mixed effects model comparing for the main effects of treatment, time and interaction of treatment and time with Dunnett’s post-test for comparison of huATG vs. rhATG#5 or rhATG#6. ¶= p<0.05 for treatment, †=p<0.05 for time, and ‡=p<0.05 for interaction of treatment and time. *=p<0.05 for huATG vs. rhATG#5, ^=p<0.05 for huATG vs. rhATG#6.
We also examined CD4 and CD8 T cell counts. Both decreased with time, however there were no significant differences in depletion by RATG agent (Figure 2 E&F). We also examined the CD4:8 ratio. All RATGs followed a similar pattern with a decrease in the ratio from baseline, with a nadir at 28 days and then a slow rebound back close to baseline by day 84 with no significant difference between groups. Finally, we examined the frequency of CD4+ Tregs and saw that these varied with time, RATG-treatment, and the interaction of time and RATG-treatment in our main model though there were no pairwise differences between treatment groups at individual timepoints. However, huATG had a statistically significant increase in Treg frequency at day 14 vs. day 0 (p=0.01 by Dunnett’s post-test).
3.3. RATG leads to an increase in memory type T cells with little effect on B cell subsets
We next examined the effect of RATG on T cell subsets. We saw that the frequency of naïve CD4+ T cells (Tnaïve) cells decreased with time and was lower with huATG depletion than with rhATG#6 from 28 days onwards. Central memory CD4+ (Tcm) increased with time and was significantly higher at day 84 among huATG vs. rhATG#6. Finally, though effector memory CD4 (Tem) increased with time there were no significant differences between RATG treatments (Figure 3 A–C). There were no significant differences between treatment groups among CD8+ T cell subsets, though CD8+ Tnaïve generally decreased and CD8+ Tem generally increased (Figure 3 D–F). B cell phenotypes were also interrogated but showed little variation except for a transient increase in exhausted memory B cells at early time points (Supplemental Figure 3).
Figure 3:
huATG causes more profound phenotypic changes in T cells than rhATGs. Frequency (%) of A) CD4+ Tnaive, B) CD4+ Tcm C) CD4+ Tem, D) CD8+ Tnaive, E) CD8+ Tcm, and F) CD8+ Tem stratified by ATG preparation. All comparisons by mixed effects model comparing for the main effects of treatment, time and interaction of treatment and time with Dunnett’s post-test for comparison of huATG vs. rhATG#5 or rhATG#6. ¶= p<0.05 for treatment, †=p<0.05 for time, and ‡=p<0.05 for interaction of treatment and time. *=p<0.05 for huATG vs. rhATG#5, ^=p<0.05 for huATG vs. rhATG#6.
3.4. Secondary Lymphoid Tissues are Moderately, and Similarly Affected by Different RATG Treatments
We next examined the effect of RATG on peripheral lymph nodes and the spleen by flow cytometry. We noted that both CD3+ T cell and CD20+ B cell frequency varied in the LN with time with a decrease in T cell and concomitant increase in B cell frequency after RATG treatment (Figure 4 A&B). At sacrifice, flow cytometry of cellular suspensions from splenic samples from animals treated with huATG had a significantly lower percentage of CD3+ T cells than controls (Supplemental Figure 4). There were no clear trends in CD4 and CD8 T cells over time or between treatment groups in the spleen (Figure 4 C&D, Supplemental Figure 4). Interestingly, T cell phenotypes in the peripheral LN follow a similar trend to peripheral T cells with an increase in memory phenotype after induction. Splenic samples from time of sacrifice had a generally high proportion of memory T cell phenotypes that was similar to controls (Supplemental Figure 5).
Figure 4:
Effects of RATGs in the LN are similar to the peripheral blood without long term changes in the spleen. Frequency (%) of A) CD3+ T cells, B) CD20+ B Cells C) CD4+ T Cells, and D) CD8+ T cells stratified by ATG preparation. E) Representative IF staining of LN using anti-CD3 (green) and anti CD20(red) over time (4x magnification). F) Spleen H&E staining (sac, Day 112) from 3 representative animals. All comparisons by mixed effects model comparing for the main effects of treatment, time and interaction of treatment and time with Dunnett’s post-test for comparison of huATG vs. rhATG#5 or rhATG#6. ¶= p<0.05 for treatment, †=p<0.05 for time, and ‡=p<0.05 for interaction of treatment and time. *=p<0.05 for huATG vs. rhATG#5, ^=p<0.05 for huATG vs. rhATG#6.
On histologic examination, we saw a drastic decrease in CD3+ cells in lymph nodes and a concomitant decrease in germinal center organization immediately after RATG (Day 5) administration that was resolved by three months (Figure 4 E). This effect appeared most pronounced in the huATG group. There were no observed differences in splenic architecture between groups (Figure 4 F).
3.5. Peripheral proliferation is directly correlated with degree of depletion whereas RTE frequency is inversely associated with degree of peripheral proliferation
We next examined the relative proliferation of different T cell subsets. Examining bulk CD4+ and CD8+ T cells, we saw an increase in proliferation with all treatments and that this increase was greatest with huATG, with statistically significant differences early in the time course for CD8 and more sustained differences for CD4 T cells (Figure 5 A&B). We also examined the frequency of RTEs to determine the contribution of thymic output on repopulation. Interestingly, RTE frequency declined for all treatments among both CD4+ and CD8+ T cells and there was a significantly greater decline with huATG compared to rhATG#6 treatment at multiple time points (Figure 5 C&D). Similar trends that were more attenuated were seen in lymph node samples (data not shown).
Figure 5:
Proliferation after RATG is proportional to depletion. Frequency (%) of A) Ki-67+ CD4+ T cells, B) Ki-67+ CD8 T cells C) CD4+ RTEs (CD31+ Tnaive), and D) CD8+ RTEs (CD103+ Tnaive). E) Correlation of maximum depletion with maximum proliferation. F) Correlation of maximum proliferation with minium frequency RTE. G) Representative histology of thymus with staining using polyclonal anti-rabbit Ab (brown). All comparisons in panels A-D by Mixed effects model comparing for the main effects of treatment, time and interaction of treatment and time with Dunnett’s post-test for comparison of huATG vs. rhATG#5 or rhATG#6. ¶= p<0.05 for treatment, †=p<0.05 for time, and ‡=p<0.05 for interaction of treatment and time. *=p<0.05 for huATG vs. rhATG#5, ^=p<0.05 for huATG vs. rhATG#6. Panels E and F with simple linear regression. P-value and R2 reported. P<0.05 significant.
We correlated the maximum degree of proliferation with maximum percent depletion and saw a significant positive correlation among both CD4+ (p=0.0047, R2=−.56) and CD8+ (p=0.0002, R2=0.75) T cells (Figure 5E). That is, the lower the nadir lymphocyte count, the higher the maximum proliferative fraction of CD4+ or CD8+ T cells as determined by Ki-67 positivity. Conversely, we saw a significant negative correlation between RTE frequency and maximum proliferation for both CD4+ (p=0.022, r2=0.42) and CD8+ (p=0.013, R2=0.47; Figure 5F). We also examined the phenotype of proliferating cells combining all RATG types (as patterns were similar, data not shown) and saw that the majority of proliferating cells were Tcm among CD4+ T cells and Tem among CD8 T Cells (Supplemental Figure 6). Of note, we were able to observe persistence of RATG on some thymocytes up to 107 days after last administration in all treatment groups (Figure 5G).
We examined the relative depletion of different T cell memory cells at maximal depletion. Given that some of the samples contained very low cell counts, making flow cytometric analysis of specific memory subsets difficult, we chose to exclude all samples for which the absolute lymphocyte count at Day 5 (maximum depletion, prior to repopulation) was less than 100 cells/uL. We calculated the percent depletion as 1-(T cell count at Day 5/T cell count at Day 0) for Tem, Tcm, and Tnaïve. In this analysis, no significant differences were seen between T cell subsets (Supplemental Figure 7).
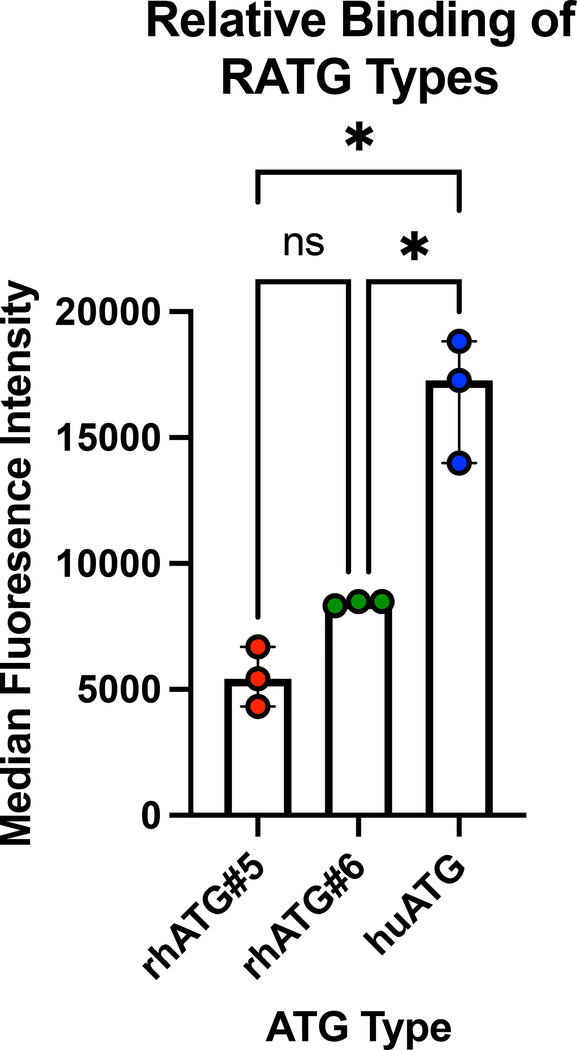
Finally, we examined the relative binding of the various RATG types. As shown in Figure 6, cells incubated with huATG and then a secondary detection antibody had a higher delta median fluorescence intensity (the fluorescence intensity after subtracting background MFI of staining with the secondary antibody alone), indicating more binding of the huATG relative to rhATG#5 and rhATG#6.
Figure 6:
Relative binding of various RATGs. Median Fluorescence Intensity (MFI) of a goat-anti-rabbit APC antibody shown. Comparison by ANOVA with Tukey’s post-test. *=p<0.05
3.6. Repopulation under immunosuppression is slower than without immunosuppression
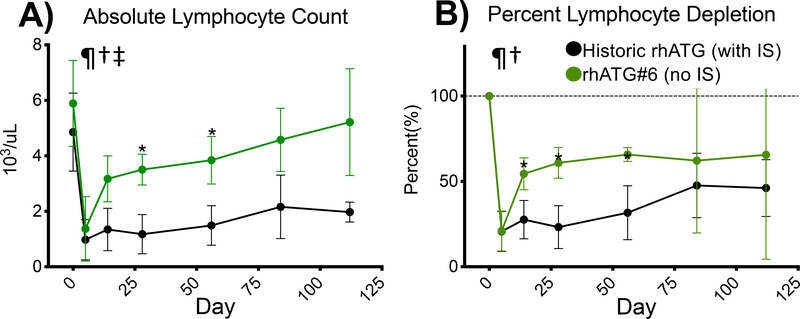
As this set of experiments was without the influence of immunosuppression, we next sought to examine how our results may vary in the context of immunosuppression. Utilizing a historical cohort of NHP that was induced with rhATG with maintenance immunosuppression consisting of belatacept and rapamycin, we compared the effects of rhATG under immunosuppression to rhATG#6. When examining absolute lymphocyte count, the historic controls had lower lymphocyte counts at Days 28 and 56 indicating slower lymphocyte repopulation in the presence of immunosuppression (Figure 7A). These differences, and a difference at Day 14 were also observed when examining the percent lymphocyte depletion (Figure 7B).
Figure 7:
Immunosuppression slows the rate of lymphocyte repopulation. A) Absolute lymphocyte count and B) percent lymphocyte depletion stratified by RATG preparation. All data are plotted as Mean±SD. Comparisons are by mixed effects model comparing for the main effects of treatment, time and interaction of treatment and time with Dunnett’s post-test for comparison of rhATG#6 with historic ATG with subsequent IS. ¶= p<0.05 for treatment, †=p<0.05 for time, and ‡=p<0.05 for interaction of treatment and time. *=p<0.05 for rhATG#6 vs. historic ATG. IS=immunosuppression.
4. Discussion
In the present study, we characterized the effects of RATG in an NHP model. We demonstrated that rhATG and huATG have similar depletional profiles, with a more pronounced change in cellular phenotype with huATG. We also demonstrated evidence that a major mechanism of repopulation after depletional induction is homeostatic proliferation. We believe that this has implications for regimens that attempt to modify the repopulating immune system as the therapeutics selected should target memory T cell types. Overall, all tested RATG formulations provided adequate lymphocyte depletion and can be used for modeling RATG depletion in preclinical NHP models.
We showed that huATG caused significantly greater depletion than the rhesus specific preparations used in this study. Indeed, in vitro studies showed less binding of the rhATGs relative to huATG. Though the rhATGs used in this study were manufactured by an experienced team with assistance from industry partners, it is possible that the exact preparations are different in multiple ways. First, the specificities of the two preparations may be different due to the polyclonal nature of RATGs. Additionally, while the doses of the different preparations were matched, the concentration of effective antibody could be different between reagents as previously described in differences between Thymoglobulin and ATG-Fresenius, though peak levels were similar between groups16. One known difference between the preparation of the rhATGs and huATG is the number of rabbits used for preparation as the two rhATG lots used in this study were prepared from n=10 (rhATG#5) and n=40 (rhATG#6) animals whereas commercial huATG is prepared from greater than 2000 rabbits 6.
We also noted depletion of both B and T cells in our study that was proportional to overall depletion. That is, huATG caused more overall depletion as well as more depletion of both T and B cells. Though some authors have noted no B cell depletion under ATG9,17, others have observed this effect in NHP, especially at high doses of ATG as we administered18. Additionally, it has been reported that some preparations of ATG contain anti-CD20 and CD19 antibodies6 as these proteins are indeed expressed on some thymic cells, including B cells involved in antigen presentation 19,20. Data from the LN showed that there is a relatively greater depletion of T than B cell, based on both flow cytometric and histological analysis. Overall, our data suggest that depletion of B cells by ATG may be dose dependent but does not appear to depend on the species (rhesus vs. human) of thymocytes utilized in manufacturing the ATG.
Similar to previous groups we observed the ability of huATG to induce Tregs that was transient 9. The ability of ATGs to induce Treg has been observed in vitro for some time 21;however, the functional significance of it has been questioned due to multiple conflicting results 22,23. In our study, the rise in frequency of FoxP3+ CD25+ cells was very transient (observed within the first 2 weeks) and most pronounced in the huATG group which experienced the most depletion. This adds credence to mechanistic data that shows that the induction of CD25 and FoxP3 occurs with activation of T cells via antibodies to CD3 and CD28 in ATG preparations 16. In the prior study it was noted that these activated T cells were also able to suppress activity in vitro, likely in order to prevent an over-exuberant immune response.
We next examined the effects of the different RATGs on naïve/memory T cell subsets. We and others have previously reported that RATG causes a preferential depletion of naïve T lymphocytes 9,10, we did not observe such an effect in our cohort. Mechanistic experiments examining this phenomenon have been performed in mice but not in NHP or humans 24. Additionally, it has been described that T cells proliferating in the context of lymphopenia acquire markers of memory T cells, at least transiently 25–27,28. Indeed we observe that very few of our recently proliferated cells (as examined by Ki-67 positivity) are a naïve phenotype. Therefore, though we did not directly observe the preferential depletion of naïve cells, it may occur and the increase in memory markers seen post depletion may also be a function of homeostatic proliferation. Interestingly, this effect of depletion may be altered by subsequent immunosuppression, which can shift the immune system to a more naïve repertoire, as we have previously shown with a regimen utilizing alemtuzumab induction, belatacept and rapamycin 29. Finally, ATG has previously been associated with a long-term inversion of the CD4:8 ratio30. However, we observe a return to baseline within our short (4 month) study period, suggesting that it is subsequent immunosuppression, and not ATG itself, which causes this lasting inversion.
We were also interested in the mechanisms of repopulation induced by the different RATGs. In all groups, we saw an increase in Ki-67+ cells and a decrease in RTEs for both CD4+ and CD8+ T cells after depletion. We showed that whereas maximum proliferation was correlated with maximum depletion it was inversely correlated with minimum RTE frequency. That is, while proliferation increased with greater depletion, the frequency of RTEs declined with greater proliferation. This suggests that homeostatic proliferation, not thymopoesis, is the major contributor to lymphocyte repopulation. In support of this, ATG has previously been observed to decrease thymic output in humans; this was thought to be due to a decrease in thymic inputs from lymphoid progenitor cells in the bone marrow12. In the present study, we also found residual RATG in the thymus up to 107 days after administration, perhaps inhibiting thymic output. Finally, RTEs may be an important mediator of outcomes after depletional induction as pre-transplant level of RTEs has been correlated with acute rejection in patients undergoing thymoglobulin induction15.
An important feature of our NHP model is the ability to sample more tissue compartments in an animal physiologically similar to humans. In the present experiment, we saw trends within the LN that mirrored those in the peripheral immune system though they were somewhat more attenuated, perhaps secondary to decreased trafficking in the LN compared to peripheral blood due to retention molecules like S1P31. Additionally, there were profound changes in the lymphoid architecture, with disruption of germinal centers, early after depletion that resolved by approximately 12 weeks after depletion.
Our study has several limitations. First, though we describe in great detail the T cell phenotypes we observed, we do not present any data on the clinical effect of those changes as this is a model system without any intervention such as an organ transplant. However, this was indeed the intent of our study. The specific description of the effects of depletional induction in a relevant NHP model provides important data for the rational design of immunosuppressive regimens in order to synergize with the effects of depletion. Additionally, we only monitored these animals for up to 112 days. Though a relatively short period of time with regards to an organ transplant, the NHP in our cohort generally showed reconstitution of lymphocyte counts to near baseline by this timepoint. Therefore, we believe we adequately captured both depletion and repopulation. We focused exclusively on cellular phenotype and do not capture the effector function of these cells. Further assays to describe the functional significance of the differentially affected phenotypes in this study (e.g. early expansion of FoxP3+ CD4+ Treg) would be helpful. Additionally, we do not use maintenance immunosuppression in this study. However, given the myriad different available regimens, studying repopulation in the absence of immunosuppression yields some generalizable data. Finally, though we do see differences in depletion we are unable to comment on the exact mechanisms driving these differences. Continued examination of the antibody components of the three RATGs would be useful to understand what drives these differences.
Conclusion
Depletional induction using human or rhesus specific ATGs in rhesus macaques is similar, with human specific forms causing more profound depletion. Further work should be performed to elucidate how the transition to memory T cells that occurs with homeostatic proliferation after depletion can be manipulated in order to maximize the long-term benefits of induction.
Supplementary Material
Acknowledgements/Funding
We would like to thank the NIH for funding this project through a supplemental grant to NIH Grant U19AI131471 to Allan Kirk’s laboratory and the U24AI126683 to the Nonhuman Primate Reagent Resource. We would also like to thank the staff of Duke’s Division of Laboratory Animal Resources (DLAR) and the Department of Surgery’s Large Animal Resources Division of Animal Research and Training Core (SLACR) for helping to complete these experiments.
Abbreviations
- (ATG)
Anti-thymocyte globulin
- (Tcm)
Central memory T cell
- (Tem)
Effector memory T cell
- (huATG)
Human specific antithymocyte globulin
- (IF)
Immunofluoresence
- (LN)
Lymph Node
- (Tnaïve)
Naïve T cell
- (NHP)
Nonhuman primate
- (RTE)
Recent Thymic Emigrant
- (RATG)
Rabbit anti-thymocyte globulin
- (rhATG)
Rhesus specific anti-thymocyte globulin
- (SD)
Standard Deviation
Footnotes
Disclosures
The authors have no relevant disclosures.
Data Availability Statement
Primary data are available upon request from the corresponding author.
References
- 1.Axelrod DA, Schnitzler MA, Xiao H, et al. An economic assessment of contemporary kidney transplant practice. Am J Transplant. 2018;18(5):1168–1176. doi: 10.1111/ajt.14702. [DOI] [PubMed] [Google Scholar]
- 2.USRDS. 2017USRDS Annual Data Report: Executive Summary. January 2018:1–8. [Google Scholar]
- 3.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D, Grp TIS. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355(19):1967–1977. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 4.Alloway RR, Woodle ES, Abramowicz D, et al. Rabbit anti-thymocyte globulin for the prevention of acute rejection in kidney transplantation. Am J Transplant. 2019;19(8):2252–2261. doi: 10.1111/ajt.15342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirk AD, Guasch A, Xu H, et al. Renal transplantation using belatacept without maintenance steroids or calcineurin inhibitors. Am J Transplant. 2014;14(5):1142–1151. doi: 10.1111/ajt.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller TF. Mechanisms of Action of Thymoglobulin. Transplantation. 2007;84(Supplement):S5–S10. doi: 10.1097/01.tp.0000295420.49063.b1. [DOI] [Google Scholar]
- 7.Mohty M Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21(7):1387–1394. doi: 10.1038/sj.leu.2404683. [DOI] [PubMed] [Google Scholar]
- 8.Zand MS, Vo T, Huggins J, et al. Polyclonal rabbit antithymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. 2005;79(11):1507–1515. doi: 10.1097/01.tp.0000164159.20075.16. [DOI] [PubMed] [Google Scholar]
- 9.Gurkan S, Luan Y, Dhillon N, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 2010;10(9):2132–2141. doi: 10.1111/j.1600-6143.2010.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearl JP, Parris J, Hale DA, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5(3):465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 11.Tang Q, Leung J, Melli K, et al. Altered balance between effector T cells and FOXP3+ HELIOS+ regulatory T cells after thymoglobulin induction in kidney transplant recipients. Transpl Int. 2012;25(12):1257–1267. doi: 10.1111/j.1432-2277.2012.01565.x. [DOI] [PubMed] [Google Scholar]
- 12.Crepin T, Carron C, Roubiou C, et al. ATG-Induced Accelerated Immune Senescence: Clinical Implications in Renal Transplant Recipients. Am J Transplant. 2015;15(4):1028–1038. doi: 10.1111/ajt.13092. [DOI] [PubMed] [Google Scholar]
- 13.Na IK, Wittenbecher F, Dziubianau M, et al. Rabbit antithymocyte globulin (Thymoglobulin(R)) impairs the thymic output of both conventional and regulatory CD4+ T cells after allogeneic hematopoietic stem cell transplantation in adult patients. Haematologica. 2012;98(1):23–30. doi: 10.3324/haematol.2012.067611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havenith SHC, Remmerswaal EBM, Bemelman FJ, et al. Rapid T cell repopulation after rabbit anti-thymocyte globulin (rATG) treatment is driven mainly by cytomegalovirus. Clin Exp Immunol. 2012;169(3):292–301. doi: 10.1111/j.1365-2249.2012.04622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bamoulid J, Courivaud C, Crepin T, et al. Pretransplant thymic function predicts acute rejection in antithymocyte globulin-treated renal transplant recipients. Kidney Int. 2016;89(5):1136–1143. doi: 10.1016/j.kint.2015.12.044. [DOI] [PubMed] [Google Scholar]
- 16.Popow I, Leitner J, Grabmeier-Pfistershammer K, et al. A comprehensive and quantitative analysis of the major specificities in rabbit antithymocyte globulin preparations. Am J Transplant. 2013;13(12):3103–3113. doi: 10.1111/ajt.12514. [DOI] [PubMed] [Google Scholar]
- 17.Todeschini M, Cortinovis M, Perico N, et al. In kidney transplant patients, alemtuzumab but not basiliximab/low-dose rabbit anti-thymocyte globulin induces B cell depletion and regeneration, which associates with a high incidence of de novo donor-specific anti-HLA antibody development. J Immunol. 2013;191(5):2818–2828. doi: 10.4049/jimmunol.1203261. [DOI] [PubMed] [Google Scholar]
- 18.Préville X, Flacher M, LeMauff B, et al. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation. 2001;71(3):460–468. doi: 10.1097/00007890-200102150-00021. [DOI] [PubMed] [Google Scholar]
- 19.Rother MB, Schreurs MWJ, Kroek R, Bartol SJW, van Dongen JJM, van Zelm MC. The Human Thymus Is Enriched for Autoreactive B Cells. J Immunol. 2016;197(2):441–448. doi: 10.4049/jimmunol.1501992. [DOI] [PubMed] [Google Scholar]
- 20.Schuh E, Berer K, Mulazzani M, et al. Features of Human CD3+CD20+ T Cells. J Immunol. 2016;197(4):1111–1117. doi: 10.4049/jimmunol.1600089. [DOI] [PubMed] [Google Scholar]
- 21.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006;17(10):2844–2853. doi: 10.1681/ASN.2006050422. [DOI] [PubMed] [Google Scholar]
- 22.Broady R, Yu J, Levings MK. ATG-induced expression of FOXP3 in human CD4(+) T cells in vitro is associated with T-cell activation and not the induction of FOXP3(+) T regulatory cells. Blood. 2009;114(24):5003–5006. doi: 10.1182/blood-2009-04-214437. [DOI] [PubMed] [Google Scholar]
- 23.Feng X, Kajigaya S, Solomou EE, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008;111(7):3675–3683. doi: 10.1182/blood-2008-01-130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruzek MC, Neff KS, Luong M, et al. In vivo characterization of rabbit anti-mouse thymocyte globulin: a surrogate for rabbit anti-human thymocyte globulin. Transplantation; 2009;88(2):170–179. doi: 10.1097/TP.0b013e3181abc061. [DOI] [PubMed] [Google Scholar]
- 25.Murali-Krishna K, Ahmed R. Cutting Edge: Naive T Cells Masquerading as Memory Cells. J Immunol. 2000;165(4):1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 26.Cho BK, Rao VP, Ge Q, Eisen HN, Chen JZ. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192(4):549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gudmundsdottir H, Turka LA. A Closer Look at Homeostatic Proliferation of CD4 +T Cells: Costimulatory Requirements and Role in Memory Formation. J Immunol. 2001;167(7):3699–3707. doi: 10.4049/jimmunol.167.7.3699. [DOI] [PubMed] [Google Scholar]
- 28.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Samy KP, Guasch A, et al. Postdepletion Lymphocyte Reconstitution During Belatacept and Rapamycin Treatment in Kidney Transplant Recipients. Am J Transplant. 2016;16(2):550–564. doi: 10.1111/ajt.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller TF, Grebe SO, Neumann MC, et al. Persistent long-term changes in lymphocyte subsets induced by polyclonal antibodies. Transplantation. 1997; 64(10), 1432–1437. [DOI] [PubMed] [Google Scholar]
- 31.Hunter MC, Teijeira A, Halin C. T Cell Trafficking through Lymphatic Vessels. Front Immunol. 2016;7:613. doi: 10.3389/fimmu.2016.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.