Abstract
Objective:
Alcohol, tobacco, and cannabis are the three most frequently used drugs in the United States and co-use is common. Alcohol, tobacco, and cannabis use have been separately associated with altered brain structure, and alcohol and tobacco co-use results in decreases in gray matter volume. Less is known about the effect of alcohol and cannabis co-use, and alcohol, tobacco, and cannabis tri-use. Therefore, this study examined the effect of co- and tri-use on gray matter volume, a measure of brain cell density, in heavy drinkers.
Method:
Heavy drinkers (n=237; 152m/85f; age=32.52; white=111; black=28; Latino=9; American Indian=2; Pacific Islander=4; Asian=59; mixed=15; other=9) were classified into four groups based on their alcohol, tobacco, and cannabis use: alcohol only users (n=70), alcohol and tobacco co-users (n=90), alcohol and cannabis co-users (n=35), and alcohol, tobacco, and cannabis tri-users (n=42). All participants completed a structural MRI scan. Voxel-based morphometry was conducted to evaluate the effect of co-use on gray matter volume, with alcohol only users as the reference group. Age, sex, and scanner were included as covariates.
Results:
Alcohol and tobacco co-users had significantly decreased left orbitofrontal gray matter volume relative to alcohol only users (Cohen’s d=0.79). There were no differences in gray matter volume between the alcohol only and alcohol and cannabis co-users, or between the alcohol only and tri-user groups.
Conclusions:
The additive effect of tobacco co-use on gray matter volumes in heavy drinkers was limited and localized. The effect of tri-use of alcohol, tobacco, and cannabis may have interacted, such that overlapping cannabis and tobacco use masked volume differences present in separate co-using groups.
Keywords: voxel-based morphometry, alcohol, cannabis, tobacco, co-use
Introduction
Alcohol, tobacco, and cannabis are the three most commonly used substances in the United States (Substance Abuse and Mental Health Services Administration [SAMHSA], 2016). Moreover, co- and tri-use of alcohol, tobacco, and cannabis is common (Falk, Yi, & Hiller-Sturmhöfel, 2006; Moss, Chen, & Yi, 2014), and the use of alcohol, tobacco, or cannabis independently increases the probability of co-use of the two remaining substances (Roche et al., 2019). Individuals with a 12-month or lifetime diagnosis of alcohol use disorder (AUD) have an increased likelihood of tobacco dependence and an increased likelihood of having another substance use disorder (Grant et al., 2015). Similarly, co-users of cannabis and alcohol were found to be at heightened risk of having AUD and cannabis use disorder (CUD) (Blanco et al., 2016; Weinberger, Platt, & Goodwin, 2016; Winters & Lee, 2008), and cannabis and tobacco co-use is associated with greater likelihood of meeting diagnostic criteria for both cannabis and tobacco dependence (Rubinstein, Rait, & Prochaska, 2014).
A growing body of literature has highlighted the adverse consequences associated with alcohol and tobacco co-use. Heavy-drinking tobacco smokers have worse health outcomes than alcohol or tobacco smokers alone (Hart, Davey Smith, Gruer, & Watt, 2010; Xu et al., 2007), and have a faster cognitive decline relative to non-smoking moderate drinkers (Hagger-Johnson et al., 2013). Moreover, alcohol and tobacco co-users have a lower likelihood of successful smoking cessation (Kahler et al., 2010), and smoking lapses occur more frequently when individuals are drinking alcohol, especially during heavy drinking days (Kahler, Spillane, & Metrik, 2010). Similarly, co-use of alcohol and cannabis is quite common (Agrawal, Lynskey, Madden, Bucholz, & Heath, 2007; Midanik, Tam, & Weisner, 2007). Co-users of alcohol and cannabis report greater use of both substances, have a greater likelihood of an AUD, and have more negative consequences compared to single substance users (Martin, Kaczynski, Maisto, & Tarter, 1996; Midanik et al., 2007; Sokolovsky, Gunn, Micalizzi, White, & Jackson, 2020). Additionally, alcohol and cannabis co-use negatively impacts clinical outcomes, including increased risk of meeting criteria for a comorbid psychiatric disorder, heavy drinking, and poorer prognoses for AUD treatment (Metrik, Gunn, Jackson, Sokolovsky, & Borsari, 2018; Mojarrad, Samet, Cheng, Winter, & Saitz, 2014; Subbaraman, Metrik, Patterson, & Swift, 2017; Yurasek, Aston, & Metrik, 2017).
In addition to a host of health and clinical correlates, abnormalities in brain structure have been shown in alcohol, tobacco, and cannabis users compared to healthy controls. Brain volume loss is well-documented in individuals with AUD. Consistent and significant gray matter reductions are found in corticostriatal-limbic circuits, with areas including the bilateral insula, superior temporal and frontal gyri, striatum, dorsolateral prefrontal cortex (DLPFC), precentral gyrus, anterior and posterior cingulate cortices (ACC/PCC), nucleus accumbens, thalamus, and hippocampus (Grodin & Momenan, 2017; Li et al., 2019; Xiao et al., 2015; X. Yang et al., 2016). Additionally, volume reductions in these brain areas have been related to alcohol use factors, such that right striatum volume is negatively correlated with duration of alcohol dependence, and left frontal cortex and thalamus gray matter atrophy is related to lifetime alcohol consumption (Cardenas, Studholme, Gazdzinski, Durazzo, & Meyerhoff, 2007; X. Yang et al., 2016).
Current smokers show gray matter atrophy in similar regions. Meta-analyses of smoking studies showed consistent regional gray matter volume decrease in the prefrontal cortex, insula, and ACC (Pan et al., 2013; Z. Yang, Zhang, Cheng, & Zheng, 2020; Zhong et al., 2016) as well as the olfactory gyrus, with correlations between pack-years and gray matter loss in the prefrontal cortex and ACC (Fritz et al., 2014). Gray matter density was shown to be lower in the left prefrontal cortex in high pack-years smokers, and density was inversely correlated with pack-years (Zhang et al., 2011). However, some studies found the converse, with gray matter volume increase in the right lingual cortex (Zhong et al., 2016) and left occipital cortex (Z. Yang et al., 2020), and increased gray matter density in the left insular cortex (Zhang et al., 2011) of smokers compared to controls.
Cannabis use has been associated with gray matter volume reductions in the amygdala and hippocampus (Cousijn et al., 2012; Koenders et al., 2016; Weinstein, Livny, & Weizman, 2016) as well as medial temporal cortex, parahippocampal gyrus, insula, and orbitofrontal cortex (Battistella et al., 2014). These regions are functionally implicated in motivational, emotional, and affective processing, and are known to be rich in cannabinoid CB1 receptors (Burns et al., 2007). However, cannabis users also showed increased gray matter volume in basal ganglia regions including the caudate, putamen, pallidum, and nucleus accumbens, compared to controls (Moreno-Alcázar et al., 2018). Furthermore, THC/CBD ratio was shown to inversely correlate with gray matter volume in the right hippocampus, possibly indicating some protective effects of CBD and neurotoxic effects of THC (Demirakca et al., 2011). In brief, the literature on the effects of cannabis on gray matter volume is mixed.
While gray matter abnormalities have been well-studied in these three substances separately, relatively few studies have explored these structural changes in the context of substance co-use. Tobacco and alcohol co-use is the most well-studied form of substance co-use, with studies finding consistent brain volume decreases in smoking heavy drinkers compared to non-smoking heavy drinkers, in both total neocortical gray matter (Durazzo, Gazdzinski, & Meyerhoff, 2007) and specifically frontal, temporal, and parietal gray matter volume (Durazzo, Cardenas, Studholme, Weiner, & Meyerhoff, 2007). Furthermore, alcohol-dependent smokers and non-smokers both demonstrated smaller volumes than non-smoking light-drinking controls in most cortical regions of interest, with volume differences between alcohol-dependent smokers and non-smokers only seen in the putamen (Durazzo et al., 2014). Taken together, these results indicate important interaction effects of tobacco and alcohol co-use. A recent study of substance co-use found a negative correlation between the number of substances used (alcohol, tobacco, cannabis, and cocaine) and gray matter volume of the medial prefrontal cortex, indicating that alterations may not be substance-specific but may be related to the number of substances used, while other regions (including gray matter reductions in the thalamus associated with tobacco use and ventrolateral prefrontal cortex associated with cocaine) showed substance-specific effects. (Kaag et al., 2018). Another study exploring polysubstance use disorder (alcohol and at least two other substances) showed volume reductions in the thalamus compared to healthy controls, as well as polysubstance users showing volume increases in the right caudate compared to participants with alcohol dependence only (Grodin & Momenan, 2017). Tobacco smokers, cannabis users, and tobacco-cannabis co-users all exhibited increased gray matter volume in the putamen compared to healthy controls, while tobacco smokers and tobacco-cannabis co-users showed decreased gray matter volume in the thalamus (Wetherill et al., 2015). Finally, though not a study of co-use, a mega-analysis of individuals with dependence on any one of five substances (alcohol, nicotine, cannabis, cocaine, or methamphetamine) found that participants with any substance use disorder had lower subcortical volume in bilateral hippocampus, amygdala, and right nucleus accumbens. Some regions were substance-specific (i.e., alcohol-specific deficits in the right nucleus accumbens), while others, including volume reductions in the left supramarginal gyrus and insula, were substance-general, indicating that differential alterations in gray matter volume may underlie different cognitive effects associated with various substance use disorders (Mackey et al., 2019).
No studies to date have explored differences in gray matter volume between the co- and tri-use of alcohol, tobacco, and cannabis and single-substance use. Thus, in the current study, we compare gray matter volume differences between heavy drinking subjects who solely drank alcohol against those who were alcohol and tobacco co-users, alcohol and cannabis co-users, and alcohol, tobacco, and cannabis tri-users. As the literature regarding co-use and polysubstance use suggests a complex interplay between substance use and regional volume increases and decreases, we did not have a directional hypothesis for this study. Instead, we used a data-driven, voxel-based morphometry approach to localize the effect of co- and tri-substance use on gray matter volume.
Methods:
Data source and sample:
The current sample is culled from seven separate clinical and experimental psychopharmacology neuroimaging studies with similar inclusion and recruitment methods, all conducted in the Addictions Laboratory at the University of California, Los Angeles. The study sample was drawn from studies examining the neural correlates of risk-taking and alcohol administration (Courtney, Ghahremani, & Ray, 2013; Courtney & Ray, 2014), the neural correlates of alcohol prediction error (Cservenka, Courtney, Ghahremani, Hutchison, & Ray, 2017), the effect of a brief intervention on neural response to alcohol taste cues (Grodin, Ray, MacKillop, Lim, & Karno, 2019), and four pharmacotherapy trials investigating the effect of naltrexone (Lim et al., 2019), the combination of and naltrexone and varenicline (Ray et al., 2015) and (Ray et al., under review), and ibudilast (Grodin et al., under review). Although some studies involved pharmacological manipulations, all demographic and clinical characteristics analyzed herein were collected at a baseline assessment visit (prior to medication randomization or any experimental procedures). All studies recruited community samples of non-treatment-seeking drinkers from the greater Los Angeles Area. All study procedures were approved by the University of California, Los Angeles Institutional Review Board, and all participants provided written informed consent after receiving a full explanation of the study procedures.
Heavy drinking was verified through one of the following methods: (1) diagnosis of alcohol dependence (DSM-IV-Tr) or alcohol use disorder (DSM-5); (2) greater than 7 drinks per week for women and greater 14 drinks per week for men; (3) an Alcohol Use Disorder Identification Test (AUDIT; Saunders, Aasland, Babor, de la Fuente, & Grant, 1993) score of 8 of higher; or (4) drinking at binge levels (4 or more drinks per episode for women, 5 or more drinks per episode for men) 4 or more times in a month. All studies had the following exclusion criteria: (1) current involvement in treatment programs for alcohol use or have received treatment in the prior 30 days to study participation; (2) use of psychoactive drugs (excluding cannabis) or use of prescription medications for recreational purposes; (3) lifetime diagnosis of schizophrenia, bipolar disorder, or psychotic disorders; (4) current use of mood stabilizers, sedatives, anti-anxiety medications, seizure medications, or prescription painkillers; (5) self-reported history of contraindicated medical conditions (e.g., chronic liver disease, cardiac disease); (6) if female, pregnant (as verified by a urine sample), nursing, or planning to get pregnant in the next 6 months or refusal to use a reliable method of birth control; (7) breath alcohol concentration (BrAC) of greater than 0.000 g/dl; and (8) positive urine toxicology screen for any drug (other than cannabis). Additionally, all studies had the following exclusion criteria for neuroimaging: (1) history of epilepsy, seizures, or severe head trauma; (2) claustrophobia; and (3) non-removable ferromagnetic objects in body.
Clinical Battery:
Across studies, all participants completed a phenotypic battery consisting of sociodemographic (i.e., age, sex, years of education, race and ethnicity, and estimated income) and clinical assessments capturing harmful and hazardous alcohol drinking (AUDIT; Saunders et al., 1993), alcohol use disorder severity (Alcohol Dependence Scale; ADS; Skinner, Horn, & Addiction Research Foundation of, 1984), alcohol-related problems (The Drinker Inventory of Consequences; DrInC; Miller, Longabaugh, National Institute on Alcohol, & Alcoholism, 2000), alcohol craving (Obsessive-Compulsive Dependence Scale; OCDS; Anton, Moak, & Latham, 1995 & Penn Alcohol Craving Scale; PACS; Flannery, Volpicelli, & Pettinati, 1999), tobacco dependence (Fagerstrom Test of Nicotine Dependence; FTND Heatherton, Kozlowski, Frecker, & Fagerstom, 1991), and cannabis use severity (Cannabis Use Disorder Identification Test; CUDIT; Adamson et al., 2010). Interview based assessments captured alcohol withdrawal symptoms (Clinical Institute Withdrawal Assessment for Alcohol Scale, Revised; CIWA-Ar; Sullivan, Sykora, Schneiderman, Naranjo, & Sellers, 1989) and a 30-day Timeline Followback; TLFB; Sobell & Sobell, 1992), which assessed alcohol drinking, tobacco smoking, and cannabis use over the past month. From the TLFB the following indices were calculated: total drinks, drinks per day (DPD), drinks per drinking day (DPDD), heavy drinking days (HDD; indicated by ≥5 drinks/day for men, and ≥4 drinks/day for women), total tobacco smoked, and number of days of cannabis use. The Structured Clinical Interview of DSM-IV-Tr (SCID) or DSM-5 was administered by a master’s level clinician to determine age at first drink and assess for current AUD and substance use disorder (SUD) symptoms. In order to facilitate (and streamline) the merging of data across multiple studies using both DSM-IV and DSM-5 criteria, diagnoses from the DSM-IV were transformed to the DSM-5 use disorder classification, such that participants who were diagnosed with alcohol dependence using DSM-IV terminology were considered to have an AUD
Co-Use Classification:
Participants were classified into one of four groups based on their substance co-use behavior (ascertained via questionnaires and face-to-face interviews): (1) alcohol use only (AO); (2) alcohol and tobacco co-use (AT); (3) alcohol and cannabis co-use (AC), and (4) alcohol, tobacco, and cannabis use (ATC). Individuals in the AO group had a negative urine toxicology screen and reported no tobacco or cannabis use on the 30-day TLFB. These reports were corroborated by data analyses of the FTND, CUDIT, and SCID substance use disorder module. Individuals in the AT group had a negative urine toxicology screen and reported alcohol and tobacco use on the 30-day TLFB, which was corroborated by the FTND. This group reported no cannabis use on the 30-day TLFB and reported no use on the CUDIT and SCID SUD module. Finally, individuals in the ATC group reported alcohol, tobacco, and cannabis use on the 30-day TLFB and had a positive urine toxicology screen for THC. Individuals in the ATC group also reported tobacco use on the FTND and cannabis use on the CUDIT and/or in the SCID SUD module. Of note, not all of the studies from which data was culled for the present study collected the CUDIT and/or FTND; as such, corroborating data was used when available. See Table 1 for descriptive statistics and sample size for each measure.
Table 1.
Demographic and Clinical Characteristics
| Variable Mean ± SD or n (%) | Alcohol only (n = 70) | Alcohol + Tobacco (n = 90) | Alcohol + Cannabis (n=35) | Alcohol + Tobacco + Cannabis (n=42) | Statistic | p |
|---|---|---|---|---|---|---|
|
| ||||||
| Age d, g | 30.74 ± 9.18 | 34.42 ± 10.46 | 28.11 ± 7.26 | 35.07 ± 12.59 | F = 4.89 | 0.003 |
| Sex (Male) | 36 (51.42%) | 64 (71.11%) | 24 (68.57%) | 28 (66.67%) | Χ2 = 7.23 | 0.07 |
| Education (years) e | 14.54 ± 2.30 | 14.92 ± 2.24 | 14.63 ± 2.51 | 13.55 ± 3.34 | F = 2.86 | 0.04 |
| Estimated Income | n=48 | n=76 | n=27 | n=33 | Χ 2 = 39.95 | 0.02 |
| <$15,000 | 5 (7.14%) | 17 (18.89%) | 2 (5.71%) | 14 (33.33%) | ||
| $15–000-$29,999 | 12 (17.14%) | 21 (23.33%) | 8 (22.86%) | 6 (14.29%) | ||
| $30–000-$44,999 | 3 (4.29%) | 12 (13.33%) | 5 (14.29%) | 5 (11.90%) | ||
| $45–000-$59,999 | 3 (4.29%) | 10 (11.11%) | 1 (2.86%) | 3 (7.14%) | ||
| $60–000-$74,999 | 5 (7.14%) | 4 (4.44%) | 3 (8.57%) | 2 (4.76%) | ||
| $75–000-$89,999 | 6 (8.57%) | 6 (6.67%) | 3 (8.57%) | 2 (4.76%) | ||
| $90–000-$104,999 | 2 (2.86%) | 3 (3.33%) | 1 (2.86%) | 0 (0%) | ||
| $105–000-$119,999 | 3 (4.29%) | 1 (1.11%) | 0 (0%) | 1 (2.38%) | ||
| >$120,000 | 9 (12.76%) | 2 (2.22%) | 4 (11.42%) | 0 (0%) | ||
| Race/Ethnicity | Χ2 = 27.88 | 0.14 | ||||
| White | 37 (52.76%) | 45 (50%) | 10 (28.57%) | 19 (45.23%) | ||
| Black | 5 (7.14%) | 11 (12.22%) | 5 (14.29%) | 7 (16.67%) | ||
| Latino | 1 (1.42%) | 6 (6.67%) | 2 (5.71%) | 0 (0%) | ||
| American Indian | 1 (1.42%) | 1 (11.11%) | 0 (0%) | 0 (0%) | ||
| Pacific Islander | 0 (0%) | 2 (2.22%) | 0 (0%) | 2 (4.76%) | ||
| Asian | 21 (30%) | 16 (17.78%) | 15 (42.86%) | 7 (16.67%) | ||
| Mixed | 3 (4.29%) | 6 (6.67%) | 1 (2.86%) | 5 (11.90%) | ||
| Other/Unknown | 2 (2.86%) | 3 (3.33%) | 2 (5.71%) | 2 (4.76%) | ||
| Hispanic/Latino | 25 (35.71%) | 18 (20%) | 9 (25.71%) | 15 (35.71%) | Χ2 = 6.21 | 0.10 |
| Total Drinks (30 Day TLFB) a, c, d, g | 87.27 ± 66.55 | 120.23 ± 89.99 | 72.33 ± 42.26 | 130.15 ± 94.88 | F = 5.70 | 0.001 |
| Drinking Days (30 Day TLFB) a, d | 16.61 ± 7.20 | 19.66 ± 7.13 | 15.71 ± 6.73 | 19.76 ± 7.60 | F = 4.43 | 0.005 |
| DPDD (30 Day TLFB) g | 5.26 ± 2.62 | 5.99 ± 3.02 | 4.72 ± 1.81 | 6.46 ± 3.48 | F = 3.23 | 0.02 |
| HDD (30 Day TLFB) d, g | 8.81 ± 7.38 | 11.38 ± 8.21 | 6.77 ± 5.05 | 11.93 ± 9.60 | F = 4.26 | 0.006 |
| Current AUD (%) c, f | 55 (78.57%); n = 70 | 43 (86%); n = 50 | 26 (76.47%); n = 34 | 42 (100%); n =42 | x 2 = 8.99 | 0.03 |
| AUDIT c, g | 11.93 ± 9.78; n = 56 | 14.18 ± 9.09; n = 44 | 9.31 ± 7.73; n = 35 | 16.83 ± 9.44; n = 42 | F = 4.84 | 0.003 |
| ADS a, b, d, e | 21.13 ± 15.11; n = 48 | 29.55 ± 14.02; n = 66 | 10.96 ± 5.06; n = 27 | 15.63 ± 6.50; n = 19 | F = 16.36 | <0.001 |
| AUD Symptom Count | 4.69 ± 2.79; n = 52 | 5.13 ± 2.54; n =39 | 4.45 ± 1.99; n = 20 | 5.05 ± 2.42; n = 37 | F = 0.47 | 0.71 |
| CIWA-Ar | 0.73 ± 1.27; n = 70 | 0.82 ± 1.56; n = 50 | 0.31 ± .63; n = 35 | .043 ± .831; n= 42 | F = 1.80 | 0.15 |
| PACS d | 13.95 ± 7.58; n = 65 | 15.67 ± 7.63; n =90 | 10.97 ± 8.13; n=35 | 14.88 ± 6.16; n = 40 | F = 3.39 | 0.02 |
| OCDS b, d | 20.28 ± 13.56; n = 43 | 21.04 ± 13.20; n = 27 | 12.54 ± 8.18; n = 26 | 19.12 ± 10.78; n = 17 | F = 2.86 | 0.04 |
| DrInC b, d | 56.24 ± 31.04; n = 59 | 61.74 ± 34.46; n = 31 | 36.09 ± 26.33; n =23 | 57.31 ± 24.24; n = 16 | F = 3.50 | 0.02 |
| Total Tobacco (30 Day TLFB) | - | 332.24 ± 254.96; n = 84 | - | 305.40 ± 281.41; n = 42 | T = 0.12 | 0.73 |
| FTND | - | 3.76 ± 2.27; n = 88 | - | 3.93 ± 2.26; n = 40 | T = 0.001 | 0.99 |
| Total Cannabis Days (30 Day TLFB) f | - | - | 6.20 ± 7.49 | 12.43 ± 11.85 | T = 15.79 | <0.001 |
| CUDIT | - | - | 6.00 ± 3.53; n = 15 | 6.57 ± 3.26; n = 42 | T = 0.53 | 0.48 |
AO < AT
AO > AC
AO < ATC
AT > AC
AT > ATC
AT < ATC
AC < ATC
AO = alcohol only; AT = alcohol and tobacco co-user; AC = alcohol and cannabis co-user; ATC = alcohol, tobacco, and cannabis tri-user; TLFB = Timeline Followback; DPDD = drinks per drinking day; HDD = heavy drinking days; AUD = alcohol use disorder; AUDIT = Alcohol Use Disorder Identification Test; ADS = Alcohol Dependence Scale; CIWA-AR = Clinical Institute Withdrawal Assessment for Alcohol Scale, Revised; PACS = Penn Alcohol Craving Scale; OCDS = Obsessive Compulsive Drinking Scale; DrInC = Drinker Inventory of Consequences; FTND = Fagerstrom Test of Nicotine Dependence; CUDIT = Cannabis Use Disorder Identification Test
MRI Acquisition:
Scanning took place at the UCLA Center for Cognitive Neuroscience on one of two scanners: (1) a 3.0T Siemens Magnetom Trio Scanner (4 studies); or (2) a 3.0T Siemens Prisma scanner (3 studies). Regardless of study, a structural scan was acquired for registration to the study-specific functional data. Specifically, T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence (TR = 2,530 ms, TE = 1.74 ms, time to inversion = 1,260 ms, flip angle = 7°, voxel size: 1 mm3, FOV = 256 mm2, ~6.2 minutes) was collected for each participant. For all studies, at the start of the scanning visit, participants were required to have a BrAC of 0.000 g/dL and a urine toxicology screen negative for all drugs (excluding cannabis), and women were required to have a negative pregnancy test.
Data Analysis:
To compare the four groups on gray matter volume, a series of analyses of variance (ANOVAs) were conducted as omnibus tests comparing the co-use groups on continuous demographic and clinical measures. Similarly, chi-square tests were used to compare co-use groups on categorical measures. Tukey-Kramer t-tests were used to follow-up significant omnibus ANOVAs to identify the specific group differences. Group comparison analyses were conducted in SPSS 26.
Neuroimaging structural data was analyzed with FSL-VBM (Douaud et al., 2007; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM), an optimized VBM protocol (Good et al., 2001) conducted through the FSL library of analysis tools (Smith et al., 2004). First, participants’ structural images were brain-extracted and gray matter segmented before being registered to the MNI 152 standard space using non-linear registration (Andersson, Jenkinson, & Smith, 2007). Next, the registered images were averaged and flipped along the x-axis to create a left-right symmetric, study-specific gray matter template. The study-specific template was created for each comparison (i.e., 3 study specific templates were created). Second, all native gray matter images were non-linearly registered to the study-specific template and modulated to correct for local expansion or contraction due to the non-linear component of the spatial transformation. The modulated gray matter images were then smoothed with an isotropic Gaussian kernel with a sigma of 4 mm. Finally, a series of voxelwise general linear modes were applied using permutation-based non-parametric testing, correcting for multiple comparisons across space. Five thousand permutations were run for each comparison. For all VBM comparisons age, biological sex, and scanner (Magnetom Trio or Prisma) were included as covariates. Years of education was explored as a covariate but was non-significant in all comparisons. Effect size (Cohen’s d) was calculated for significant results.
Results:
Co-Use Classification:
A total of 251 individuals had structural MRI scans and completed the clinical battery. Fourteen participants were not included in the final analyses due to inconsistent reporting of alcohol, tobacco, and cannabis use (n=10) or poor MRI data quality (n=4). Therefore, the final analyzed sample consisted of a total of 237 participants. Of the final sample, 70 individuals were classified as alcohol only (AO; 29.54%), 90 individuals were classified as alcohol and tobacco co-users (AT; 37.97%), 35 individuals were classified as alcohol and cannabis co-users (AC; 14.77%), and 42 individuals were classified as alcohol, tobacco, and cannabis co-users (ATC; 17.72%). The groups differed on a host of alcohol use variables, including drinking quantity and frequency (TLFB), alcohol use severity, and alcohol craving. Generally, the AC group reported the lowest amount of drinking and lowest severity levels. The 2 tobacco smoking groups (AT and ATC) did not differ on 30-day tobacco use or on tobacco dependence. The 2 cannabis using groups (AC and ATC) differed on the number of days of cannabis use, but not on cannabis use severity (see Table 1 for full breakdown).
Voxel-Based Morphometry Results
Alcohol Only vs. Alcohol + Tobacco Co-Users
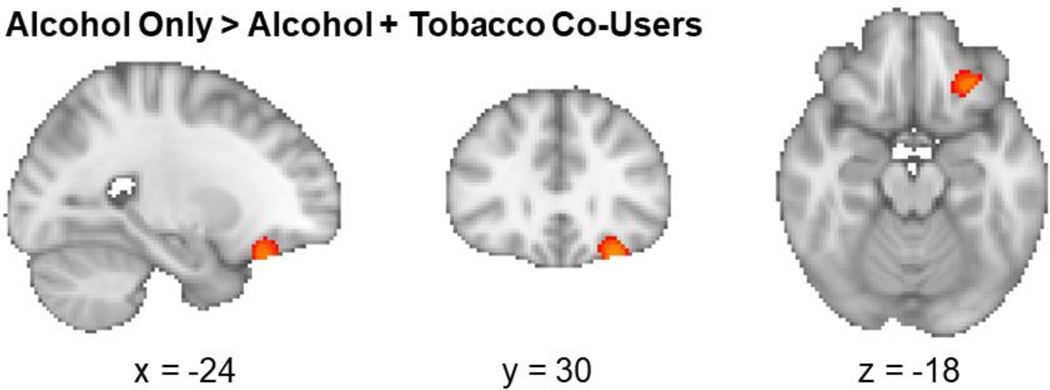
There was a significant group difference in gray matter volume in the left orbitofrontal cortex (OFC; peak coordinates: x = −28, y = 30, z = −22; 162 voxels) between the AO and AT groups, such that the AO group had significantly larger volumes compared to the AT group (p = 0.046; see Figure 1). There were no regions where AT had larger gray matter volumes compared to AO (p > 0.05). There were also no sex differences between AT and AO groups (p > 0.05).
Figure 1.
Alcohol and tobacco co-users had decreased gray matter volume in the left orbitofrontal cortex (significant voxels shown in red [dark gray]) relative to alcohol only users (p=0.046, corrected).
Alcohol Only vs. Alcohol + Cannabis Co-Users
There were no significant group differences between the AO and AC co-users in gray matter volume after controlling for age, sex, and scanner type (p > 0.05)1. There were no sex differences between AC and AO groups (p > 0.05).
Alcohol Only vs. Alcohol + Tobacco + Cannabis Tri-Users
There were no significant group differences between the AO and ATC tri-users in gray matter volume (p > 0.05). There were also no sex differences between ACT and AO groups (p > 0.05).
Discussion
This study examined the effect of co- and tri-use of alcohol, tobacco, and cannabis on gray matter volume in heavy drinkers. Despite widespread group differences on demographic and clinical measures, gray matter differences were highly localized and sparse. Specifically, the alcohol only group had larger left orbitofrontal gray matter volumes relative to alcohol and tobacco co-users. There were no significant group differences in gray matter between the alcohol only group and alcohol and cannabis co-users, or the alcohol only group and the alcohol, tobacco, and cannabis tri-users.
The decreased left orbitofrontal gray matter volume in the alcohol and tobacco co-users relative to alcohol only users is supported by previous work in this field. Durazzo et al. (2007) found that smoking heavy drinkers had smaller frontal gray matter volumes than non-smoking light drinkers, while non-smoking heavy drinkers had similar frontal gray volumes relative to non-smoking light drinkers, indicating that smoking combined with heavy drinking leads to additional frontal gray matter volume deficits. A study investigating the effect of polysubstance use in alcohol, tobacco, and cocaine users found a negative dose-response association between the number of substances used and medial orbitofrontal gray matter volume (Kaag et al., 2018). Smoking also impacts cortical thickness in the orbitofrontal cortex; tobacco smokers had thinner left medial orbitofrontal cortices than never smokers after controlling for alcohol intake (Kühn, Schubert, & Gallinat, 2010). Gray matter volume of the left OFC is also associated with interactions between smoking and the rs1137070 polymorphism of monoamine oxidase A (Shen et al., 2019). The orbitofrontal cortex is involved in many processes implicated in addictive disorders, including executive function. While not examined in the present study, previous work has found poorer executive function in smokers with AUD relative to non-smokers with an AUD (Durazzo, Rothlind, Gazdzinski, Banys, & Meyerhoff, 2006), indicating that chronic tobacco smoking worsens neurocognition in AUD (Glass et al., 2006). Regarding clinical characteristics, the alcohol only and alcohol and tobacco co-users differed on some alcohol use measures, including the ADS, where alcohol and tobacco co-users had higher alcohol dependence severity than the alcohol only group. However, the groups did not differ on heavy drinking days or drinks per drinking day in the past month, indicating that the groups were engaging in similar patterns of recent heavy drinking, and did not differ in measures of alcohol craving or drinking-related consequences. However, this study was unable to examine the effect of cumulative use, which may have differed between the alcohol only and alcohol and tobacco co-users.
There were no significant differences between the alcohol only group and the alcohol and cannabis co-users, after controlling for age, biological sex, and scanner type. The literature on the effects of cannabis use on gray matter volume is mixed, with some studies finding increased gray matter volume in cannabis users (Moreno-Alcázar et al., 2018) and others finding reductions Weinstein, Livny, & Weizman, 2016). Interestingly, a group difference was originally seen, such that the alcohol only group had larger gray matter volume in the left lateral occipital cortex than the alcohol and cannabis co-use group; however, this difference did not remain significant when controlling for sex. Therefore, we recommend that future studies examine potential sex interactions with alcohol and cannabis co-use. The alcohol only and alcohol and cannabis co-using groups did not differ significantly on alcohol frequency or quantity measures (e.g., TLFB indices), or on alcohol use severity measures (e.g., AUD diagnosis, AUDIT and ADS scores). The groups differed on alcohol craving and drinking consequences, such that the alcohol only group reported higher alcohol craving on the OCDS and higher total DrInC scores. This result adds to the mixed literature on cannabis and alcohol co-use, which largely suggests that co-use of alcohol and cannabis results in an additive effect of poorer mental health and substance use outcomes (Agrawal et al., 2007; Blanco et al., 2016; Midanik et al., 2007; Weinberger et al., 2016; Yurasek et al., 2017). However, these effects have not been consistently represented within this body of literature (Mallett, Turrisi, Trager, Sell, & Linden-Carmichael, 2019). It is also important to note that participants from the studies from which these data were culled generally represent primary alcohol users, as these studies specifically recruited heavy drinkers. As such, it is possible that the alcohol-only group displayed higher levels of craving and increased alcohol-related problems as a result of a ceiling effect, such that the effects of drinking-related consequences were more pronounced in the alcohol only group.
There were no significant group differences in gray matter volume between the alcohol only users and the alcohol, tobacco, and cannabis tri-users. The lack of volume deficits in the tri-use group may be due to an interaction between cannabis and tobacco. While gray matter volume decreases are well-established in alcohol and tobacco co-users (Durazzo et al., 2007; Durazzo et al., 2007; Durazzo et al., 2014), tobacco and cannabis co-use has been associated with gray matter volume increases (Wetherill et al., 2015), and cannabis users alone have shown increased subcortical gray matter volumes (Moreno-Alcázar et al., 2018). Therefore, the interacting effects of tobacco and cannabis on gray matter volume may have resulted in the present finding of no group differences between the alcohol only and the tri-using groups. Importantly, the tri-using group reported greater cannabis use than the alcohol and cannabis co-using group, while reporting similar tobacco smoking and nicotine dependence severity as the alcohol and tobacco co-using groups. Further complicating this interaction is the evidence for a potential neuroprotective effect of cannabidiol (CBD) and a neurotoxic effect of tetrahydrocannabinol (THC) (Demirakca et al., 2011). In the present study, cannabis use was recorded as a binary variable at each day (i.e., yes/no use on a given day) and therefore, we were unable to evaluate the THC/CBD ratio in the cannabis co-using groups. Additionally, this study did not include individuals with a cannabis use disorder (CUD). The effects cannabis and the interactive effects of alcohol, tobacco, and cannabis on gray matter volume may only become present in individuals with higher severity of use.
The present study must be evaluated based on its strengths and weaknesses. Study strengths include the sample sizes of the single, co-, and tri-use groups and the use of a data-driven, voxel-based morphometry approach to investigate gray matter deficits. Among the study limitations are the lack of a non-heavy drinking control group, the lack of detailed collection on cannabis use, including cannabis strain, potency of THC, and modality of delivery, and the relatively young age of the sample. Of note, this study was unable to examine the effect of years of alcohol use on gray matter volume due to missing data. Moreover, this was a cross-sectional study and cannot identify if gray matter volume differences are a cause or a consequence of use or co-use. Detailed longitudinal studies will be needed to interrogate the temporal precedence of structural brain changes. Relatedly, this study and many other structural neuroimaging studies did not control for socioeconomic factors, as this information was not collected from all participants, which may affect brain development. Finally, this study did not explicitly exclude individuals with depressive or anxiety disorders, which may also impact gray matter volume.
In conclusion, this study found only small, localized differences in gray matter between alcohol only users and alcohol and tobacco co-users. There were no significant group differences in gray matter volume between alcohol only users and alcohol and cannabis co-users, or between alcohol only users and alcohol, tobacco, and cannabis tri-users. Given that the effects of substance co-use, particularly cannabis, is poorly understood, this study adds to the body of literature by providing a well-phenotyped sample with high-quality voxel-based morphometry assessments. The pattern of findings does not suggest a detriment in gray matter volume for alcohol and cannabis co-users in this sample; however, additional research with more severe samples is required to fully elucidate these effects and investigate the potential neuroprotective properties of cannabis.
Public Health Significance:
This study highlights the importance of investigating co- and tri-use of tobacco and cannabis in heavy alcohol users.
This study indicates that the effect of co-use of alcohol and tobacco on the brain in heavy drinkers is localized when compared to heavy alcohol users alone.
This study indicates that the effect of cannabis and alcohol co-use did not affect brain volume when compared to heavy alcohol users alone.
Acknowledgments
Funding: This work was supported by the National Institute on Alcohol Abuse and Alcoholism grants to ENG (F32AA027699), LAR (K24AA025704), EB (F31AA028976), and AV (3R01AA026190-02S1).
Footnotes
When sex was not included as a covariate, there was a significant group difference in gray matter volume in the left lateral occipital cortex (peak coordinates: x = −28, y = 30, z = −22; 235 voxels) between the AO and AC groups, such that the AO group had significantly larger volumes compared to the AC group (p = 0.03).
Data Dissemination: The data in this manuscript represent a combination of structural neuroimaging data collected by our lab. Some of the functional neuroimaging data, which use the structural images as a reference, have been previously reported (Courtney, Ghahremani, & Ray, 2013; Courtney & Ray, 2014; Cservenka, Courtney, Ghahremani, Hutchison, & Ray, 2017; Grodin, Ray, MacKillop, Lim, & Karno, 2019; Lim et al., 2019; Ray et al., 2015). However, none of the aforementioned studies examined structural brain changes, and this study represents a unique combination of these data sets.
References
- Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, & Sellman JD (2010). An improved brief measure of cannabis misuse: the Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug and Alcohol Dependence, 110(1–2), 137–143. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT, Madden PA, Bucholz KK, & Heath AC (2007). A latent class analysis of illicit drug abuse/dependence: results from the National Epidemiological Survey on Alcohol and Related Conditions. Addiction, 102(1), 94–104. doi: 10.1111/j.1360-0443.2006.01630.x [DOI] [PubMed] [Google Scholar]
- Andersson JL, Jenkinson M, & Smith S. (2007). Non-linear registration aka Spatial normalisation FMRIB Technial Report TR07JA2. FMRIB Analysis Group of the University of Oxford, 1–22. [Google Scholar]
- Anton RF, Moak DH, & Latham P. (1995). The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res, 19(1), 92–99. doi: 10.1111/j.1530-0277.1995.tb01475.x [DOI] [PubMed] [Google Scholar]
- Battistella G, Fornari E, Annoni J-M, Chtioui H, Dao K, Fabritius M, … Giroud C. (2014). Long-term effects of cannabis on brain structure. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 39(9), 2041–2048. doi: 10.1038/npp.2014.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Hasin DS, Wall MM, Flórez-Salamanca L, Hoertel N, Wang S, … Olfson M. (2016). Cannabis use and risk of psychiatric disorders: prospective evidence from a US national longitudinal study. JAMA Psychiatry, 73(4), 388–395. [DOI] [PubMed] [Google Scholar]
- Burns HD, Van Laere K, Sanabria-Bohórquez S, Hamill TG, Bormans G, Eng W. s., … Hargreaves RJ. (2007). [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proceedings of the National Academy of Sciences of the United States of America, 104(23), 9800–9805. doi: 10.1073/pnas.0703472104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, & Meyerhoff DJ (2007). Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage, 34(3), 879–887. doi: 10.1016/j.neuroimage.2006.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, & Ray LA (2013). Fronto-striatal functional connectivity during response inhibition in alcohol dependence. Addiction biology, 18(3), 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, & Ray LA (2014). Subjective responses to alcohol in the lab predict neural responses to alcohol cues. Journal of studies on alcohol and drugs, 75(1), 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, & Goudriaan AE (2012). Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage, 59(4), 3845–3851. doi: 10.1016/j.neuroimage.2011.09.046 [DOI] [PubMed] [Google Scholar]
- Cservenka A, Courtney KE, Ghahremani DG, Hutchison KE, & Ray LA (2017). Development, initial testing and challenges of an ecologically valid reward prediction error FMRI task for alcoholism. Alcohol and Alcoholism, 52(5), 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirakca T, Sartorius A, Ende G, Meyer N, Welzel H, Skopp G, … Hermann D (2011). Diminished gray matter in the hippocampus of cannabis users: possible protective effects of cannabidiol. Drug and Alcohol Dependence, 114(2–3), 242–245. doi: 10.1016/j.drugalcdep.2010.09.020 [DOI] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, … Matthews PM (2007). Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain, 130(9), 2375–2386. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Cardenas VA, Studholme C, Weiner MW, & Meyerhoff DJ (2007). Non-treatment-seeking heavy drinkers: effects of chronic cigarette smoking on brain structure. Drug Alcohol Depend, 87(1), 76–82. doi: 10.1016/j.drugalcdep.2006.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, & Meyerhoff DJ (2007). The neurobiological and neurocognitive consequences of chronic cigarette smoking in alcohol use disorders. Alcohol and Alcoholism, 42(3), 174–185. doi: 10.1093/alcalc/agm020 [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Pennington D, Abé C, Gazdzinski S, & Meyerhoff DJ (2014). Interactive effects of chronic cigarette smoking and age on brain volumes in controls and alcohol-dependent individuals in early abstinence. Addict Biol, 19(1), 132–143. doi: 10.1111/j.1369-1600.2012.00492.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Gazdzinski S, Banys P, & Meyerhoff DJ (2006). A comparison of neurocognitive function in nonsmoking and chronically smoking short-term abstinent alcoholics. Alcohol, 39(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi H. y., & Hiller-Sturmhöfel S. (2006). An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Research & Health, 29(3), 162. [PMC free article] [PubMed] [Google Scholar]
- Flannery BA, Volpicelli JR, & Pettinati HM (1999). Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res, 23(8), 1289–1295. [PubMed] [Google Scholar]
- Fritz H-C, Wittfeld K, Schmidt CO, Domin M, Grabe HJ, Hegenscheid K, … Lotze M (2014). Current smoking and reduced gray matter volume-a voxel-based morphometry study. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 39(11), 2594–2600. doi: 10.1038/npp.2014.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JM, Adams KM, Nigg JT, Wong MM, Puttler LI, Buu A, … Zucker RA(2006). Smoking is associated with neurocognitive deficits in alcoholism. Drug and Alcohol Dependence, 82(2), 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, & Frackowiak RS (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage, 14(1), 21–36. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, … Hasin DS (2015). Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry, 72(8), 757–766. doi: 10.1001/jamapsychiatry.2015.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, & Momenan R. (2017). Decreased subcortical volumes in alcohol dependent individuals: effect of polysubstance use disorder. Addiction biology, 22(5), 1426–1437. doi: 10.1111/adb.12421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Ray LA, MacKillop J, Lim AC, & Karno MP (2019). Elucidating the Effect of a Brief Drinking Intervention Using Neuroimaging: A Preliminary Study. Alcoholism: Clinical and Experimental Research, 43(2), 367–377. doi: 10.1111/acer.13941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger-Johnson G, Sabia S, Brunner EJ, Shipley M, Bobak M, Marmot M, … Singh-Manoux A (2013). Combined impact of smoking and heavy alcohol use on cognitive decline in early old age: Whitehall II prospective cohort study. British Journal of Psychiatry, 203(2), 120–125. doi: 10.1192/bjp.bp.112.122960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Davey Smith G, Gruer L, & Watt GCM (2010). The combined effect of smoking tobacco and drinking alcohol on cause-specific mortality: a 30 year cohort study. BMC Public Health, 10(1), 789. doi: 10.1186/1471-2458-10-789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstom K-O (1991). The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Infante MA, Nguyen-Louie TT, Worley M, Courtney KE, Coronado C, & Jacobus J. (2020). Neuropsychological trajectories associated with adolescent alcohol and cannabis use: A prospective 14-year study. Journal of the International Neuropsychological Society, 26(5), 480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaag AM, Schulte MHJ, Jansen JM, van Wingen G, Homberg J, van den Brink W, … Reneman L. (2018). The relation between gray matter volume and the use of alcohol, tobacco, cocaine and cannabis in male polysubstance users. Drug and Alcohol Dependence, 187, 186–194. doi: 10.1016/j.drugalcdep.2018.03.010 [DOI] [PubMed] [Google Scholar]
- Kahler CW, Borland R, Hyland A, McKee SA, O’Connor RJ, Fong GT, & Cummings KM (2010). Quitting smoking and change in alcohol consumption in the International Tobacco Control (ITC) Four Country Survey. Drug and Alcohol Dependence, 110(1–2), 101–107. doi: 10.1016/j.drugalcdep.2010.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, & Metrik J. (2010). Alcohol use and initial smoking lapses among heavy drinkers in smoking cessation treatment. Nicotine Tob Res, 12(7), 781–785. doi: 10.1093/ntr/ntq083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenders L, Cousijn J, Vingerhoets WAM, van den Brink W, Wiers RW, Meijer CJ, … de Haan L. (2016). Grey Matter Changes Associated with Heavy Cannabis Use: A Longitudinal sMRI Study. PloS One, 11(5), e0152482. doi: 10.1371/journal.pone.0152482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Schubert F, & Gallinat J. (2010). Reduced Thickness of Medial Orbitofrontal Cortex in Smokers. Biological Psychiatry, 68(11), 1061–1065. doi: 10.1016/j.biopsych.2010.08.004 [DOI] [PubMed] [Google Scholar]
- Lalanne L, Ferrand-Devouge E, Kirchherr S, Rauch L, Koning E, Speeg C, … Giersch A. (2017). Impaired contrast sensitivity at low spatial frequency in cannabis users with early onset. European Neuropsychopharmacology, 27(12), 1289–1297. [DOI] [PubMed] [Google Scholar]
- Li J, Wang Y, Xu Z, Liu T, Zang X, Li M, & Ma L. (2019). Whole-brain morphometric studies in alcohol addicts by voxel-based morphometry. Annals of Translational Medicine, 7(22), 635. doi: 10.21037/atm.2019.10.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AC, Ghahremani DG, Grodin EN, Green R, Bujarski S, Hartwell EE, … Ray LA. (2019). Neuroimaging findings from an experimental pharmacology trial of naltrexone in heavy drinkers of East Asian descent. Drug and Alcohol Dependence, 200, 181–190. doi: 10.1016/j.drugalcdep.2019.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S, Allgaier N, Chaarani B, Spechler P, Orr C, Bunn J, … Garavan H. (2019). Mega-Analysis of Gray Matter Volume in Substance Dependence: General and Substance-Specific Regional Effects. Am J Psychiatry, 176(2), 119–128. doi: 10.1176/appi.ajp.2018.17040415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallett KA, Turrisi R, Trager BM, Sell N, & Linden-Carmichael AN (2019). An examination of consequences among college student drinkers on occasions involving alcohol-only, marijuana-only, or combined alcohol and marijuana use. Psychology of addictive behaviors, 33(3), 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Kaczynski NA, Maisto SA, & Tarter RE (1996). Polydrug Use in Adolescent Drinkers with and without DSM-IV Alcohol Abuse and Dependence. Alcoholism: Clinical and Experimental Research, 20(6), 1099–1108. doi: 10.1111/j.1530-0277.1996.tb01953.x [DOI] [PubMed] [Google Scholar]
- Metrik J, Gunn RL, Jackson KM, Sokolovsky AW, & Borsari B. (2018). Daily patterns of marijuana and alcohol co-use among individuals with alcohol and cannabis use disorders. Alcoholism: Clinical and Experimental Research, 42(6), 1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midanik LT, Tam TW, & Weisner C. (2007). Concurrent and simultaneous drug and alcohol use: Results of the 2000 national alcohol survey. Drug and Alcohol Dependence, 90(1), 72–80. doi: 10.1016/j.drugalcdep.2007.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulskaya E, & Martin F. (2018). Visual attention to motion stimuli and its neural correlates in cannabis users. European journal of neuroscience, 47(3), 269–276. [DOI] [PubMed] [Google Scholar]
- Miller WR, Longabaugh R, National Institute on Alcohol, A., & Alcoholism. (2000). The Drinker Inventory of Consequences (DrInC): An Instrument for Assessing Adverse Consequences of Alcohol Abuse : Test Manual: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- Mojarrad M, Samet JH, Cheng DM, Winter MR, & Saitz R. (2014). Marijuana use and achievement of abstinence from alcohol and other drugs among people with substance dependence: a prospective cohort study. Drug Alcohol Depend, 142, 91–97. doi: 10.1016/j.drugalcdep.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Alcázar A, Gonzalvo B, Canales-Rodríguez EJ, Blanco L, Bachiller D, Romaguera A, … Pomarol-Clotet E. (2018). Larger Gray Matter Volume in the Basal Ganglia of Heavy Cannabis Users Detected by Voxel-Based Morphometry and Subcortical Volumetric Analysis. Frontiers in Psychiatry, 9, 175. doi: 10.3389/fpsyt.2018.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Chen CM, & Yi H.-y. (2014). Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug and Alcohol Dependence, 136, 51–62. [DOI] [PubMed] [Google Scholar]
- Pan P, Shi H, Zhong J, Xiao P, Shen Y, Wu L, … He G. (2013). Chronic smoking and brain gray matter changes: evidence from meta-analysis of voxel-based morphometry studies. Neurological Sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 34(6), 813–817. doi: 10.1007/s10072-012-1256-x [DOI] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, & London ED (2015). Varenicline, naltrexone, and their combination for heavy-drinking smokers: preliminary neuroimaging findings. The American journal of drug and alcohol abuse, 41(1), 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJO, Bujarski S, Green R, Hartwell EE, Leventhal AM, & Ray LA (2019). Alcohol, tobacco, and marijuana consumption is associated with increased odds of same-day substance co- and tri-use. Drug and Alcohol Dependence, 200, 40–49. doi: 10.1016/j.drugalcdep.2019.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein ML, Rait MA, & Prochaska JJ (2014). Frequent marijuana use is associated with greater nicotine addiction in adolescent smokers. Drug and Alcohol Dependence, 141, 159–162. doi: 10.1016/j.drugalcdep.2014.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, & Grant M. (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction, 88(6), 791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- Shen Z, Huang P, Wang C, Qian W, Luo X, Gu Q, … Zhang M. (2019). Interactions between monoamine oxidase A rs1137070 and smoking on brain structure and function in male smokers. Eur J Neurosci, 50(3), 2201–2210. doi: 10.1111/ejn.14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Horn JL, & Addiction Research Foundation of, O. (1984). Alcohol Dependence Scale (ADS) user’s guide. Toronto: Addiction Research Foundation. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, … Flitney DE (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline Follow-Back. In Litten RZ & Allen JP (Eds.), Measuring Alcohol Consumption: Psychosocial and Biochemical Methods (pp. 41–72). Totowa, NJ: Humana Press. [Google Scholar]
- Sokolovsky AW, Gunn RL, Micalizzi L, White HR, & Jackson KM (2020). Alcohol and marijuana co-use: Consequences, subjective intoxication, and the operationalization of simultaneous use. Drug and Alcohol Dependence, 212, 107986. doi: 10.1016/j.drugalcdep.2020.107986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaraman MS, Metrik J, Patterson D, & Swift R. (2017). Cannabis use during treatment for alcohol use disorders predicts alcohol treatment outcomes. Addiction, 112(4), 685–694. doi: 10.1111/add.13693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Servies Administration (2016). 2015 National Survey on Drug Use and Health. U.S. Department of Health and Human Services. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, & Sellers EM (1989). Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). British journal of addiction, 84(11), 1353–1357. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Platt J, & Goodwin RD (2016). Is cannabis use associated with an increased risk of onset and persistence of alcohol use disorders? A three-year prospective study among adults in the United States. Drug and Alcohol Dependence, 161, 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein A, Livny A, & Weizman A. (2016). Brain Imaging Studies on the Cognitive, Pharmacological and Neurobiological Effects of Cannabis in Humans: Evidence from Studies of Adult Users. Current Pharmaceutical Design, 22(42), 6366–6379. doi: 10.2174/1381612822666160822151323 [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Jagannathan K, Hager N, Childress AR, Rao H, & Franklin TR (2015). Cannabis, Cigarettes, and Their Co-Occurring Use: Disentangling Differences in Gray Matter Volume. The International Journal of Neuropsychopharmacology, 18(10), pyv061-pyv061. doi: 10.1093/ijnp/pyv061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters KC, & Lee C-YS (2008). Likelihood of developing an alcohol and cannabis use disorder during youth: association with recent use and age. Drug and Alcohol Dependence, 92(1–3), 239–247. doi: 10.1016/j.drugalcdep.2007.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao P, Dai Z, Zhong J, Zhu Y, Shi H, & Pan P. (2015). Regional gray matter deficits in alcohol dependence: A meta-analysis of voxel-based morphometry studies. Drug and Alcohol Dependence, 153, 22–28. doi: 10.1016/j.drugalcdep.2015.05.030 [DOI] [PubMed] [Google Scholar]
- Xu W-H, Zhang X-L, Gao Y-T, Xiang Y-B, Gao L-F, Zheng W, & Shu X-O (2007). Joint effect of cigarette smoking and alcohol consumption on mortality. Preventive medicine, 45(4), 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Tian F, Zhang H, Zeng J, Chen T, Wang S, … Gong Q. (2016). Cortical and subcortical gray matter shrinkage in alcohol-use disorders: a voxel-based meta-analysis. Neuroscience and Biobehavioral Reviews, 66, 92–103. doi: 10.1016/j.neubiorev.2016.03.034 [DOI] [PubMed] [Google Scholar]
- Yang Z, Zhang Y, Cheng J, & Zheng R. (2020). Meta-analysis of brain gray matter changes in chronic smokers. European Journal of Radiology, 132, 109300. doi: 10.1016/j.ejrad.2020.109300 [DOI] [PubMed] [Google Scholar]
- Yurasek AM, Aston ER, & Metrik J. (2017). Co-use of alcohol and cannabis: a review. Current Addiction Reports, 4(2), 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, & Stein EA (2011). Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage, 54(1), 42–48. doi: 10.1016/j.neuroimage.2010.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Shi H, Shen Y, Dai Z, Zhu Y, Ma H, & Sheng L. (2016). Voxelwise meta-analysis of gray matter anomalies in chronic cigarette smokers. Behavioural Brain Research, 311, 39–45. doi: 10.1016/j.bbr.2016.05.016 [DOI] [PubMed] [Google Scholar]