Abstract
Fibronectin (Fn) and fibrinogen (Fg) are major host proteins present in the extracellular matrix, blood, and coatings on indwelling medical devices. The ability of Staphylococcus aureus to cause infections in humans depends on favorable interactions with these host ligands. Closely related bacterial adhesins, fibronectin-binding proteins A and B (FnBPA, FnBPB) were evaluated for two key steps in pathogenesis: clumping and adhesion. Experiments utilized optical spectrophotometry, flow cytometry, and atomic force microscopy to probe FnBPA/B alone or in combination in seven different strains of S. aureus and Lactococcus lactis, a Gram-positive surrogate that naturally lacks adhesins to mammalian ligands. In the absence of soluble ligands, both FnBPA and FnBPB were capable of interacting with adjacent FnBPs from neighboring bacteria to mediate clumping. In the presence of soluble host ligands, clumping was enhanced particularly under shear stress and with Fn present in the media. FnBPB exhibited greater ability to clump compared to FnBPA. The strength of adhesion was similar for immobilized Fn to FnBPA and FnBPB. These findings suggest that these two distinct but closely related bacterial adhesins, have different functional capabilities to interact with host ligands in different settings (e.g., soluble vs. immobilized). Survival and persistence of S. aureus in a human host may depend on complementary roles of FnBPA and FnBPB as they interact with different conformations of Fn or Fg (compact in solution vs. extended on a surface) present in different physiological spaces.
Keywords: adhesion, AFM, aggregation, bacteria, clumping, fibronectin-binding proteins
1. Introduction
Fibronectin (Fn) and fibrinogen (Fg) are multidomain glycoproteins that are major protein components of blood plasma. Fn is part of the fibrous extracellular matrix supporting endothelial cells in an insoluble fibrillar form, and it circulates as a soluble form in blood plasma (Henderson, et al., 2011, Mezzenga and Mitsi, 2019, Singh, et al., 2010) at a concentration of 0.2 to 0.4 g/L (Mosher, 2006). Fg is the most abundant coagulation factor at a concentration of 1.5–4.5 g/L (Ariens, 2013) in the blood. Because Fn and Fg are found in blood, they also form coatings on devices implanted in humans (Herrmann, et al., 1988, Vaudaux, et al., 1993).
Staphylococcus aureus is commonly found living on the skin and anterior nares of humans (Krismer, et al., 2014, Lowy, 1998). When it gains entry inside a human host, S. aureus can lead to serious diseases like bacteremia and infective endocarditis. The incidence of S. aureus infections is rising (Naber, 2008, Tong, et al., 2015), and mortality can be as high as 15–50% (van Hal, et al., 2012). S. aureus is one of only 11 bacteria and fungi listed as a “Serious Threat” in the Antibiotic Resistant Threats Report by the Centers for Disease Control and Prevention (2019). Therefore, it is critical to understand the mechanisms underlying S. aureus virulence so that we can develop novel therapies for these infections. For example, clinical studies of bloodstream infections have recently found higher binding affinity for immobilized Fn in S. aureus strains collected form human patients with infected cardiovascular devices (Hos, et al., 2015, Lower, et al., 2011) and infected endocarditis (Xiong, et al., 2015).
Interaction with host proteins is a critical first step in pathogenesis of S. aureus in the body. Binding between S. aureus and Fn and Fg was first reported several decades ago (Kapral, 1966, Kuusela, 1978). Humans ligands, like Fn and Fg, often play a key role in bacterial infections (Henderson, et al., 2011, Vaudaux, et al., 1989, Vaudaux, et al., 1993). When S. aureus first enter the blood, the bacterial cells may aggregate together. This clumping is mediated by Fn and Fg, two of the most abundant host plasma proteins. Past work has primary focused on the role of Fg because it is present at 10x the blood concentration of Fn (1.5 to 4.5 g/L vs. 0.2–0.4 g/L, respectively) (Lowe, et al., 2004, Mosher, 2006). Yet, Fn-mediated cell aggregation may become predominant in areas where recruitment of Fn occurs, for instance at sites of injury wounds (Henderson, et al., 2011). Soluble Fn is also important because it mediates S. aureus internalization in host cells (Sinha, et al., 1999). In addition to clumping and internalization, adherence of S. aureus to immobilized Fn has been found to be associated with infections of implanted devices, endocarditis, and sepsis (Hos, et al., 2015, Lower, et al., 2011, Xiong, et al., 2015).
The initial molecular pathogenesis of S. aureus infections is likely dependent on cell-wall anchored adhesins of the MSCRAMM (microbial surface components recognizing adhesive matrix molecules) type, which bind to host proteins, particularly Fn and Fg (Foster, et al., 2014, Herrmann, et al., 1988). The fibronectin-binding proteins A and B (FnBPA and FnBPB) are two key members of the MSCRAMM family (Foster, 2016). FnBPA and FnBPB are multidomain adhesins meaning that they can bind several mammalian ligands common in the blood, particularly Fn and Fg.
FnBPA and FnBPB consist of ~1000 residues that contain an N-terminal signal sequence responsible for secretion, an A region comprising subdomains N1, N2, and N3, followed by the repeat region with 10 to 11 domains, and a C-terminal cell wall and membrane-spanning regions containing the cell wall anchoring motif LPETG (Figure 1). The Fg-binding site is located in the A-region near the N-terminus (Foster, 2016); whereas the Fn-binding site is located in the C-terminal repeat regions of FnBPA and FnBPB (Meenan, et al., 2007, Schwarz-Linek, et al., 2003).
Figure 1.
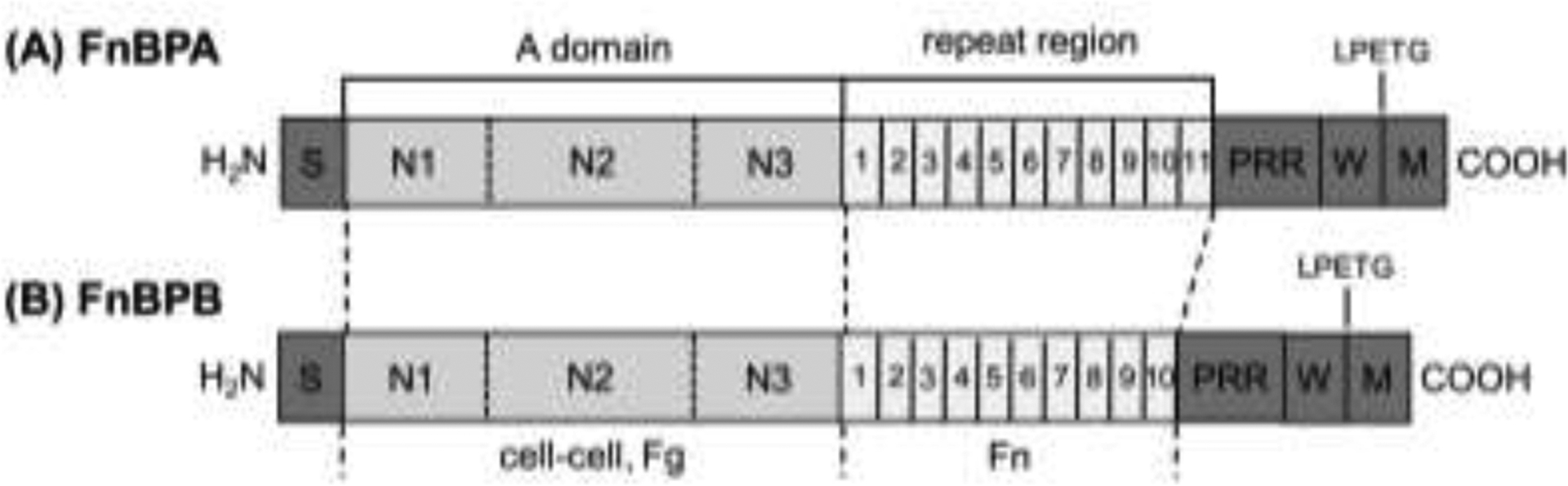
Schematic representation of fibronectin binding protein A (FnBPA) and B (FnBPB) of S. aureus 8325-4. The N-termini of FnBPA and FnBPB contain a signal sequence (S) followed by the A domain that comprises subdomains N1, N2, and N3 that are involved in cell-cell aggregation, and binding to fibrinogen (Fg) and elastin. The A-domain of FnBPB has also been shown to bind fibronectin (Fn). Following the A domains are tandemly repeated fibronectin-binding motifs (numbered). At the C-termini are proline-rich repeats (PRR), wall (W)- and membrane (M)-spanning domains, and the sortase recognition motif LPETG. Identity percentage for the A region between the two proteins is 45%, whereas the repeat region is 94% (Jonsson, et al., 1991).
Fg-binding occurs through a variant of the dock-lock-latch mechanism of the N2 and N3 subdomains (Foster, et al., 2014, Keane, et al., 2007, Ponnuraj, et al., 2003, Wann, et al., 2000). The A region has also been reported to mediate cell-cell aggregation of bacteria (Geoghegan, et al., 2013, Herman-Bausier, et al., 2015). Fn-binding takes place through a tandem β-zipper mechanism by forming anti-parallel strand along the type-I modules at the N-terminus of Fn (Bingham, et al., 2008, Schwarz-Linek, et al., 2003). Another Fn-binding site has also been identified within the N2 and N3 subdomains of the A region of FnBPB (Burke, et al., 2011).
S. aureus interactions with Fn and Fg have been determined to be associated with infections in humans (Piroth, et al., 2008, Que, et al., 2005). For example, adhesive interactions between Fn and S. aureus have been linked to biofilm-based infections of the blood and circulatory system (Hos, et al., 2015, Lower, et al., 2011, Xiong, et al., 2015). Fn and Fg have also been linked to other aspects of S. aureus pathogenesis such as aggregation (Heilmann, et al., 2004, Henderson, et al., 2011, McAdow, et al., 2011). Cell aggregation or clumping between neighboring bacteria may occurs through FnBPA and FnBPB, or other MSCRAMM surface adhesins such as clumping factor A and B (Crosby, et al., 2016, Dastgheyb, et al., 2015, Geoghegan, et al., 2013, Herman-Bausier, et al., 2015, McAdow, et al., 2011). Immune evasion and antibiotic resistance are enhanced when S. aureus form cell aggregates in the bloodstream (Crosby, et al., 2016). Host ligand proteins like Fn and Fg may even bind to S. aureus forming a protective shield around bacteria cells (Crosby, et al., 2016, Thomas, et al., 2019).
In this study, we examine aggregation and adhesion of S. aureus in the presence of Fg and Fn, present in either a free (i.e. soluble) or immobilized form. A number of complementary techniques were used including optical spectrophotometry, flow cytometry and atomic force microscopy (AFM). Cell clumping and adhesion were evaluated under both physiological levels of shear and static conditions. Full-length FnBPA and FnBPB were individually (and collectively) expressed in S. aureus mutant strains of 8325-4 as well as Lactococcus lactis, which is a non-virulent, Gram positive surrogate that lacks adhesins for mammalian proteins including Fn and Fg.
Overall, the results demonstrate that both FnBPA and FnBPB facilitate cell-to-cell clumping through interactions with neighboring bacteria. This aggregation is enhanced by the addition of soluble Fg, soluble zinc, and especially by soluble Fn under physiological levels of shear. Normalized for the density of cell wall proteins (molecules per nm2), FnBPB presented a greater ability to clump compared to FnBPA. In contrast, adhesion to immobilized ligand was similar for both bacterial adhesins under physical stress. This finding suggests that these two distinct but closely related bacterial adhesins, have different functional capabilities to interact with host ligands in different settings (e.g., soluble vs. immobilized). Further, these results reveal that the conformation of host ligand (compact in solution vs. extended on a surface) impacts the interactions with these bacterial adhesins. This would mean that planktonic bacteria in blood interact more favorably with circulating host ligands like Fn and Fg through FnBPB. Whereas both bacterial adhesins may play a role when interacting with host ligands immobilized on a surface (e.g. part of the extracellular matrix or coating on an implant). This apparent specialization of each Fn-binding adhesin could play complementary roles in the onset and progression of infection in the human body.
2. Materials and Methods
2.1. Bacteria strains and growth conditions.
Cryopreserved S. aureus strains were grown at 37°C in triptic soy broth (TSB) supplemented with 10 μg/ml erythromycin and 0.5% dextrose. L. lactis strains were grown in M17 broth supplemented with 5 μg/ml erythromycin and 0.5% dextrose at 30 °C. Both bacteria were grown in the presence of antibiotics since these strains were constructed by insertion of DNA fragments enconding antibiotic resistance in their plasmids. (Greene, et al., 1995, Que, et al., 2000, Que, et al., 2001). For clumping assays, bacteria were grown in their respective broth and temperature conditions and then diluted to an OD600nm of 1.0 in sterile TSB or M17(Pestrak, et al., 2020). For the AFM studies, bacteria were grown to exponential phase, harvested and then washed in PBS. AFM data were aquired within two hours after haversting the cell to ensure cell viability (Boonaert, et al., 2001).
2.2. Clumping assay with soluble host ligands.
For studies under shear conditions, host proteins (Fn or Fg) were added to tubes with broth to a final concentration of 1 μg/ml. Since Fn and Fg have different molecular weights (i.e., Fn is ~440 kDa; Fg is ~340 kDa), a larger number of Fg molecules were tested compared to Fn molecules. A control tube was included with only bacteria (no host protein added). Tubes were incubating under shaking conditions at 200 rpm inducing an estimated shear ≈ 8 dyn/cm2, as estimated according to (Ley, et al., 1989). Aggregation of cells caused sedimentation of the clumps. The amount of clumping was estimated by removing 700 μl aliquot from the top of the tube and measuring the OD at 600nm according to Kwiecinski et al (Kwiecinski, et al., 2019). The percentage of clumping was calculated as the percentage decrease from the OD at time zero. Percentage difference of clumping relative to control conditions (ligand free) in the presence of soluble host proteins fibronectin, and fibrinogen was determined by subtracting the OD values from the ligand free broth minus protein-containing broth then dividing by their average and multiplying by 100. Results shown are the means ± standard deviation of at least three independent experiments. For every experiment, an independent, fresh preparation of each strain was used. p-values were calculated using t-test where p < 0.05 is indicated by *.
For studies under static conditions, washed cells were stained with SYTO9 (Invitrogen, Thermo Fisher Scientific, USA) for 10 min at room temperature and then washed three times in Ringer’s solution. Next, the cells were suspended in 500 μl of Ringer’s solution. Fn (or Fg) solution was added to a final concentration of 1 μg/ml. Then cells were incubated for 60 min at room temperature. After incubation, 100 μl of the cells were collected and transferred slowly to a 5 ml round bottom polystyrene tube.
Cells aggregates can be differentiated from single cells using flow cytometry(Ambriz-Avina, et al., 2014), so we quantified bacterial aggregation using a BD FACsCanto II flow cytometer (BD sciences), as previously described (Pestrak, et al., 2018). The forward and side scatter of the SYTO9+ population was quantified to exclude unstained protein debris and quantify only the bacterial population. Flow cytometry data were quantified using FlowJo 9.0. The single cell population was determined by gating a population of single bacterial cells in the negative control confirmed by light microscopy. The percentage of the population existing as aggregates was calculated by subtracting the single celled population from the total population. Results shown are the means ± standard deviation of at least two independent experiments.
2.3. Atomic force microscopy with immobilized ligand
Force measurements were acquired with a Bioscope AFM and NanoSCOPE IV controller (Veeco/Digital Instruments) as described in Buck et al. (Buck, et al., 2010, Oestreicher, et al., 2012). The results presented herein focus on adhesion to immobilize Fn because we recently examined molecular binding of these same S. aureus and L. lactis strains to immobilized Fg (Casillas-Ituarte, et al., 2019). For the experiments in this manuscript, an attached inverted microscope (Axiovert 200M; Zeiss) was used to position the AFM tip over bacteria cells. A total of 167 different S. aureus and L. lactis cells from 20 independent cell cultures were probed with Si3N4 probes with nominal tip radius of 20 nm. The spring constant for each AFM tip was estimated by thermal tuning method (average = 0.094 nN nm−1). The AFM tips were coated with Fn according to published protocols (Casillas-Ituarte, et al., 2012, Lower, 2011). Briefly, a clean AFM tip was coated with Fn by immersion in a 100 μg/ml Fn PBS solution for 45 min, and then rinsed in PBS. Fn was deposited through this non-specific method, to mimic the conditions in the human body where these blood proteins coat surfaces in the circulatory system.
A total of 23 different tips were used. AFM measurements were conducted in PBS, at a single retraction velocity of 5.4 μm/s generating over 100,000 force curves. From these 5,120 and 5,522 force curves were obtained from S. aureus expressing FnBPA or FnBPB, respectively. A total of 8,332 curves were collected from S. aureus expressing both FnBPA and FnBPB. Force curves for FnBPA and FnBPB present in L. lactis were 5,823 and 6,194, respectively. A total of 29,823 force curves were obtained for non-specific interactions between Fn and the surface of S. aureus DU 5883 and L. lactis pIL253 (negative controls). Other control experiments (e.g., with uncoated AFM tips) generated > 40, 000 force curves.
To ensure specificity, only the final binding peak was included in the analyses in all the studies. Specific interactions between Fn-FnBPA and Fn-FnBPB were confirmed by monitoring successive unbinding events. A peak-to-peak distance (ΔL) of ~30 nm was indicative of the unfolding distance of multiple F1 repeats in Fn (Meadows, et al., 2003). These ΔL measurements were confirmed at the beginning and the end of each experiment. Each AFM tip was used on only a few cells before being discarded when the characteristic unfolding patterns (ΔL values) were no longer observed. Negative controls included S. aureus DU5583 (fnbA fnbB double mutant) and L. lactis cells with an empty plasmid (pIL253). Force strength (or adhesion) was plotted as a histogram of force frequency to see the distribution of force values. This force frequency or frequency of binding was reported as percentage of force curves observed in a force range divided by total number of curves with adhesion events multiplied by 100.
2.4. Western Ligan Blots
Surface expression of FnBPs in L. lactis and S. aureus were determined by ligand affinity blotting by incubation with pure Fn as described in detail by Que et al., and Bisognano et al., (Bisognano, et al., 2000, Que, et al., 2000) and summarized in Casillas et al. (Casillas-Ituarte, et al., 2012, Casillas-Ituarte, et al., 2019).
3. Results
Bacterial cell aggregation and adhesion interactions were examined for full-length FnBPA and FnBPB expressed individually (and collectively) in S. aureus mutant strains and surrogate host L. lactis. These reference strains are described in Buck et al. (Buck, et al., 2010). A total of four different S. aureus strains were tested for the experiments presented herein: (1) FnBPA+ FnBPB−, (2) FnBPA− FnBPB+, (3) wild type strain expressing both FnBPA and FnBPB and (4) FnBPA− FnBPB−, a fnbA fnbB double mutant called DU5883. Three strains were tested in the L. lactis envelop: (1) FnBPA+ FnBPB−, (2) FnBPA− FnBPB+ and (3) pIL253, an empty vector as negative control.
3.1. Aggregation with soluble ligand under physiological shear stress
Clumping experiments were performed with bacteria in moving solution to simulate physiological levels of shear stress (~8 dyn/cm2). In the absence of host ligands, S. aureus formed clumps (see ligand free experiment in Figure 2A). Clumping was limited to only ~12% in the DU5883 mutant strain of S. aureus, which does not express FnBPA nor FnBPB (see Figure 2A). These observations demonstrate that cell-cell aggregation depends on the presence of FnBPA and FnBPB.
Figure 2.
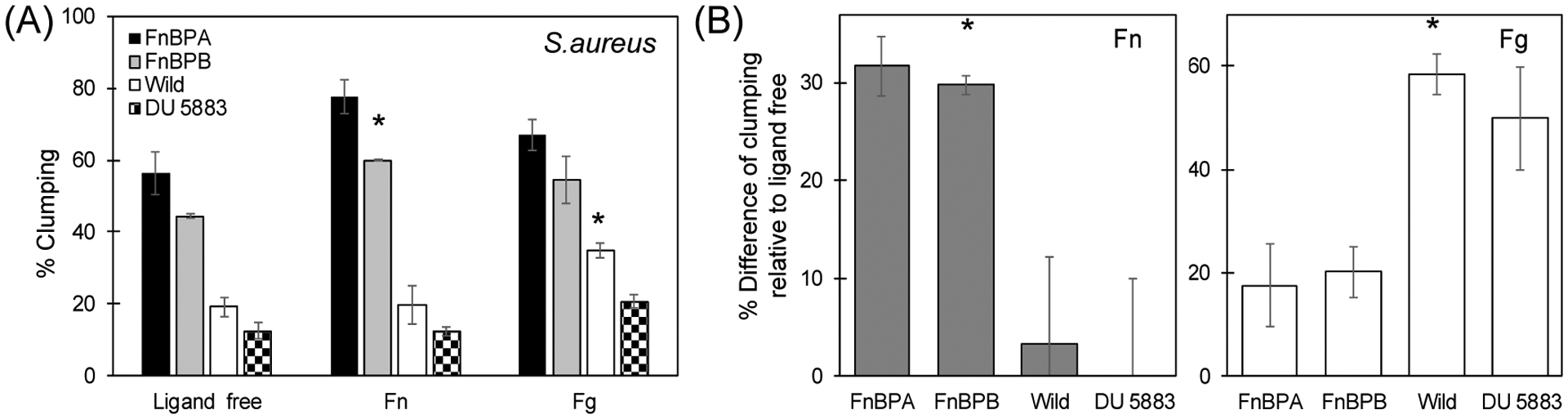
(A) Aggregation of S. aureus strains after 90 min incubation with host ligands under shear conditions of ≈ 8 dyn/cm2. (B) Percentage difference of clumping relative to control conditions (ligand free) in the presence of soluble host proteins fibronectin (Fn) and fibrinogen (Fg). Wild type S. aureus strain 8325 expresses both FnBPA and FnBPB. Mutants of 8325 express exclusively FnBPA or FnBPB. Mutant DU 5883 does not express FnBPA nor FnBPB. Results shown are the means ± standard deviation of at least three independent experiments. For every experiment, an independent, fresh preparation of each strain was used. p-values were calculated using t-test where p < 0.05 is indicated by *.
Clumping was indeed observed in the two mutant strains of S. aureus that produced only FnBPA or FnBPB (Figure 2A) in the ligand free conditions. These findings suggest that aggregation can occur as a result of FnBPA-FnBPA and FnBPB-FnBPB interactions. Aggregation with these two strains was significantly greater than the wild-type strain. The relatively smaller aggregation in the wild-type strain is attributed to a lower level of expression of both FnBPA/B (see Western blots in Figure 5C). There was a slightly greater aggregation for S. aureus that produced only FnBPA compared to S. aureus that produced only FnBPB. This could be due to differences in the number of protein present in the surface of the bacteria and/or to variation in the binding affinities between FnBPA-FnBPA and FnBPB-FnBPB. Semi-quantification of these proteins with Western blots (Figure 5C) shows slightly greater concentration of FnBPA (~30%) suggesting that number density could be the reason for greater clumping.
Figure 5.
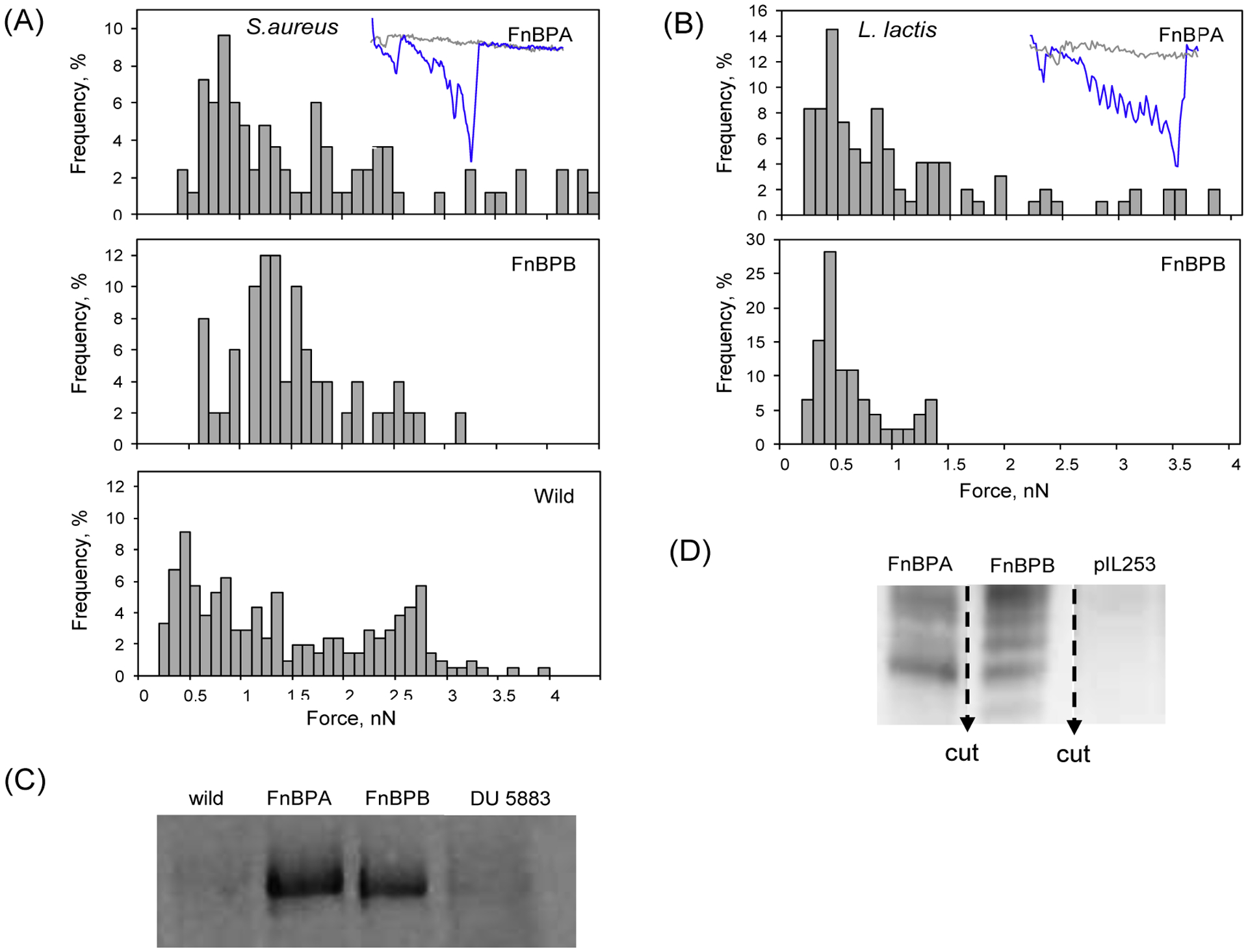
Binding forces to immobilized Fn as determined by AFM for (A) wild type S. aureus strain 8325 (bottom panel) and its mutants (top and middle panels) and (B) L lactis mutants. Western blots were used to quantify FnBPs in the S. aureus (C) and L. lactis (D) strains. In the insets of top panels, representative spectra from specific interactions Fn-FnBPA and Fn-FNBPB are shown in blue, whereas examples of non-specific interactions are shown gray. S. aureus wild expresses both fnbA and fnbB. Mutants of this wild-type strain express either FnBPA or FnBPB. DU5883 does not express either Fn-binding protein. L. lactis strains express exclusively FnBPA or FnBPB from S. aureus 8325. pIL253 carries an empty vector and therefore does not express FnBPA nor FnBPB.
Clumping was also evaluated in the presence of host proteins Fn and Fg. Addition of Fn increased cell aggregation relative to the control experiments, which lacked host proteins (Figure 2A and 2B). Relative to the ligand free conditions, the addition of soluble Fn increase clumping in ~30% in both the FnBPA and FnBPB strains whereas minimum increase was observed in the wild type and DU 5883 strain (< 4%). In the presence of Fg, there was an increase in clumping in all the strains. Yet, this increase was more pronounced in the wild type and DU 5883 strains (~50 % to 60 %) (Figure 2B).
Clumping in the wild-type and DU 5883 cells was not substantially enhanced in the presence of Fn compared to the control. The exception was in the presence of Fg, where an increment in clumping was observed. This clumping increase is attributed to the presence of the other surface adhesins on S. aureus (e.g., clumping factor A and B), which are known to bind to Fg (Ganesh, et al., 2008).
To address this confounding issue (i.e., S. aureus proteins other than FnBPA and FnBPB that participate in clumping), we tested aggregation in a L. lactis model. This surrogate is Gram positive like S. aureus but lacks all known mammalian adhesins (Que, et al., 2000). Clumping in the model surrogate L. lactis expressing FnBPA or FnBPB is shown in Figure 3. Aggregation was significantly 90 minutes) slower in L. lactis compared to that in S. aureus (Figure 2A and 2B). Longer incubation times (150 vs. were needed to observed clumping in both FnBPA and FnBPB cells of L. lactis. A smaller number of proteins expressed in L. lactis compared to S. aureus could be the reason for this slower rate (see results for adhesion studies below). In the absence of FnBPA and FnBPB, no clumping was observed in L. lactis.
Figure 3.
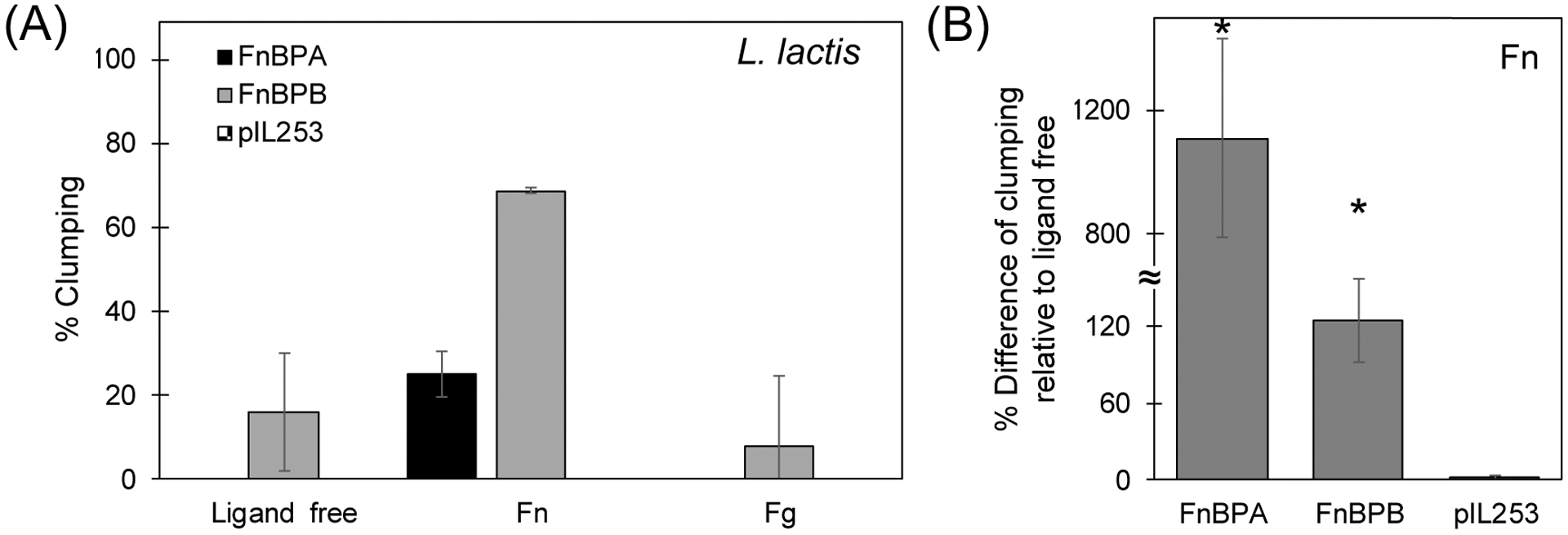
(A) Aggregation of L. lactis strains after 2.5 hrs incubation with host ligands under shear conditions of ≈ 8 dyn/cm2. (B) Percentage difference of clumping relative to control conditions (ligand free) in the presence of soluble host protein fibronectin (Fn). L. lactis has been transformed with genes from S. aureus to express exclusively FnBPA or FnBPB. pIL253 carries an empty vector and therefore does not express FnBPA nor FnBPB. Results shown are the means ± standard deviation of at least three independent experiments. For every experiment, an independent, fresh preparation of each strain was used. p-values were calculated using t-test where p < 0.05 is indicated by *.
Consistent with the S. aureus clumping experiments, FnBPB-FnBPB interactions under physiological levels of shear stress appear to promote clumping in the FnBPB L. lactis cells. Yet, clumping in the FnBPA variant was not detected under the incubation time shown here (150 minutes). The FnBPA-variant of L. lactis finally clumped after 24 hrs of incubation under shear conditions without the additional of a bridging ligand (data not shown).
Similar to S. aureus aggregation, L. lactis also demonstrate similar aggregation enhancement in the presence of Fn in both FnBPA and FnBPB. Clumping in the presence of Fg was similar to that of the control, but only for the FnBPB variant. In all cases, FnBPB presented a greater ability to clump compared to FnBPA, which is an interesting difference from the experiments with S. aureus (e.g., compare Figure 2A with 2B). Western blots (Figure 5D) showed comparable levels of expression of these bacterial proteins (< 11% greater for FnBPB). These findings suggest higher FnBPB-mediated intercellular clumping compared to FnBPA molecules. L. lactis expressing only fnbB also showed greater clumping for soluble Fg compared to FnBPA (Figure 3A).
3.2. Aggregation with soluble ligand under static conditions
Flow cytometry was used to assess FnBPA and FnBPB role in aggregate formation under static conditions in both S. aureus and L. lactis (Figure 4A and 4B). Cell clumping was observed in the presence and absence of host ligands. In S. aureus, clumping was almost largely absent in the wild type due to the low levels of expression of FnBPA and FnBPB as described above (Figure 5C).
Figure 4.
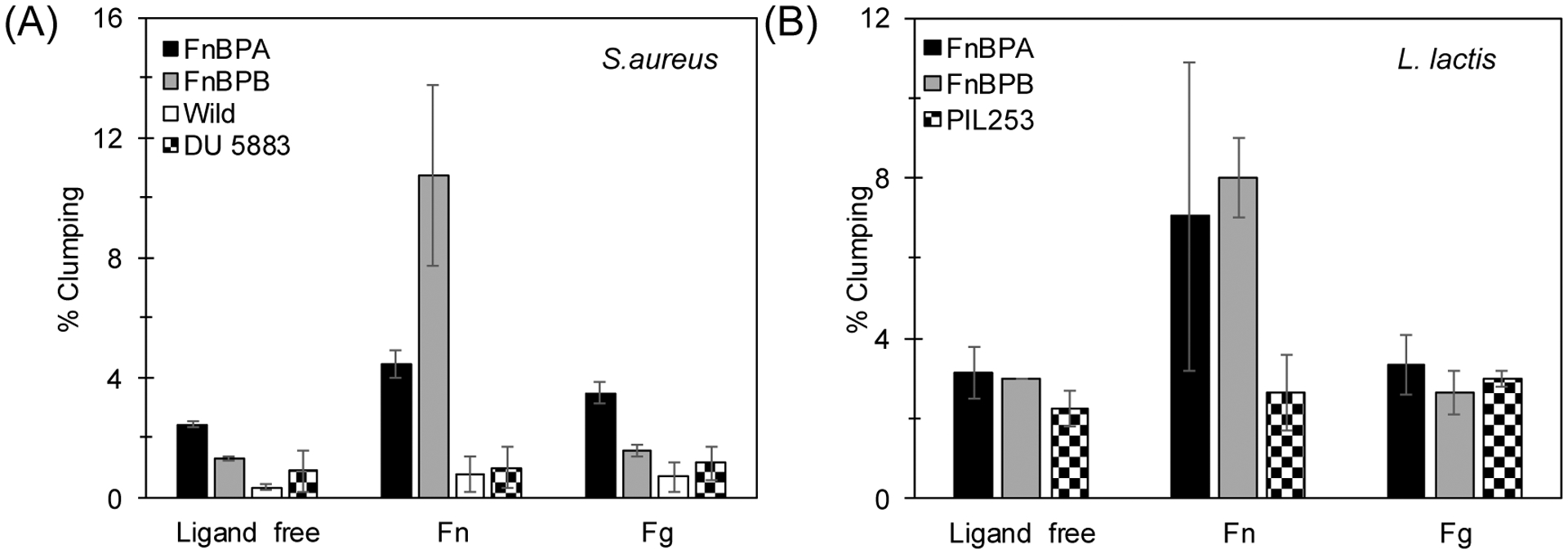
Aggregation of (A) S. aureus and (B) L. lactis after 1 hr incubation under static conditions determined by flow cytometry. Results shown are the means ± standard deviation of at least three independent experiments. For every experiment, an independent, fresh preparation of each strain was used. p-values were calculated using t-test where p < 0.05 is indicated by *.
In the presence of Fn, S. aureus expressing exclusively FnBPA or FnBPB presented an increased in clumping at least 50% (ligand-free vs. addition of Fn; see Figure 4A). Clumping in the wild type and the negative control (DU 5883) cells was not significantly enhanced in the presence of Fn. These results are consistent with the studies conducted under shear stress (Figure 2A and 2B), that is, aggregation is affected by the presence of FnBPA and FnBPB. Yet a clear difference between the results from the studies conducted under static and shear stress, is the remarkable clumping enhancement observed in the S. aureus variant that produced only FnBPB (~150% difference relative to ligand free conditions). Addition of Fg to the different S. aureus variants, (Figure 4A) produced a slight increase in aggregation in the FnBPA and wild type cells.
Clumping in L. lactis, was enhanced in the presence of soluble Fn in both FnBPA and FnBPB variants relative to free ligand control (Fig. 4B). Addition of soluble Fg addition did not play a significant contribution in cell aggregation (Fig. 4B).
3.2. Adhesion to immobilized Fn under physical stress.
Atomic force microscopy (AFM) was used to measure adhesion or binding forces associated with bacterial adhesion to immobilized Fn. AFM data for immobilized Fg was the focus on a recent paper by Casillas et al. (Casillas-Ituarte, et al., 2019), and will be presented in the Discussion section. For the AFM experiments presented here, full length FnBPA and FnBPB was expressed in the surface of S. aureus and L. lactis. AFM was performed as described in prior work (Buck, et al., 2010, Casillas-Ituarte, et al., 2019). Unlike traditional binding assays (static adhesion studies, e.g., microtiter), AFM allows direct measurement of bond strength on ligand-receptor pairs through a dynamic process of pushing and pulling the linkages. Example binding force spectra are shown as insets in Figures 5A and 5B. Binding events are represented as a series of sawteeth (Evans, 2001) where the final sawtooth represents the rupture or unbinding force between Fn on the AFM tip and Fn-binding receptors on a bacterium.
Force histograms for Fn binding to FnBPA or FnBPB in S. aureus and L. lactis are shown in Figure 5A and 5B, respectively. For comparison, a Fn force histogram for wild-type S. aureus expressing both FnBPA and FnBPB is also shown (bottom panel in Figure 5A). Fn-FnBPA and Fn-FnBPB interactions exhibited a median of ~40% binding frequency (i.e., retraction curves that exhibit an adhesion event since not all the molecular interactions result in the formation of a bond) in both S. aureus mutant strains; whereas the wild-type generated a frequency of binding of 22%. There was lower frequency of binding observed for L. lactis with median values of 16% and 8%, respectively for FnBPA and FnBPB. Non-specific binding between Fn and the surface of S. aureus DU 5883 and L. lactis pIL253 (negative controls) exhibited binding frequencies of <10% and <4%, respectively. Differences in binding frequency of Fn-FnBPA or FnBPB expressed in S. aureus and L. lactis can be attributed to the different levels of protein expression in each type of bacterium (compare Figure 5C to 5D).
The force spectra obtained from the different bacterial systems and summarized in Figure 5A and 5B, were further analyzed by the worm-like chain model to estimate the number of Fn-FnBP pairs according to prior work (Casillas-Ituarte, et al., 2012). For L. lactis, three or fewer pairs were involved in the measured interactions. For AFM experiments with S. aureus <10 pairs were estimated. This is consistent with the Western blot and binding frequency analyses described above. Western blots showed smaller amounts of FnBPs in L. lactis. Binding frequency (or frequency of observing curves with adhesion events) was also lower for L. lactis.
Fn-FnBPA and Fn-FnBPB interactions presented large adhesion force peaks from ~300 pN to ~4 nN in the S. aureus envelop. Binding forces for S. aureus mutants were centered around ~800 pN, and ~1.3 nN for the Fn-FNBPA, and Fn-FnBPB, respectively. Forces from the wild-type S. aureus (expressing both FnBPA and FnBPB) presented a bimodal distribution with one population centered at ~670 pN and other at ~2.5 nN. Interacting forces between FnBPA or FnBPB with Fn in L. lactis were centered around ~460 pN and ~410 pN, respectively. These forces were significantly weaker compared to those in S. aureus. This is attributed to a smaller number of proteins present in L. lactis as described above.
4. Discussion
Here, we examined how FnBPA and FnBPB on the outer cell wall of S. aureus impact clumping and adhesion, keys steps in molecular pathogenesis, as described above. Despite their name, FnBPs have sites that bind to Fn as well as Fg (see Figure 1), which allowed us to test the roles of both human ligands. Experiments were performed with S. aureus as well as L. lactis to unravel potentially confounding attributes of FnBPA vs. FnBPB. Furthermore, the use of L. lactis allowed us to overcome the problem of redundancy since a single adhesin could be expressed alone in a surrogate gram-positive bacteria host lacking other receptors for mammalian ligands like Fn and Fg (Que, et al., 2001, Que, et al., 2005)
FnBP–mediated intercellular adhesion or clumping was tested under both static and shear conditions. Table 1 summarizes the results presented in Figures 2 through 4. One of the most striking observations is the influence of flow on cell aggregation. Under stagnant conditions, the maximum observed clumping was 11% (S. aureus FnBPB with addition of Fn ligand). Under dynamic conditions mimicking 8 dyn/cm2 of shear, cell aggregation was consistently higher reaching a maximum observed value of up to 78% (S. aureus FnBPA with addition of Fn ligand). Shear stress clearly enhances clumping suggesting that conformational changes in Fn and/or FnBPA/B take place under these conditions. These mechanical deformations would result in an increased binding affinity and, hence clumping. That is, the forces created by the shear stress could partially unfold Fn and/or FnBPB/A exposing previously sequestered regions and thus increase the likelihood of these molecules to interact productively to form a bond. Protein unfolding under shear stress and consequent enhanced aggregation have been described in other proteins (Dobson, et al., 2017).
Table 1.
Percent increase in the clumping of S. aureus and L. lactis cells under shear (and static) conditions. Shown are results of strains that produce only one particular FnBP protein as well as mutants that produce neither FnBPs (labelled “others”). Percent clumping is highlighted in different intensities of pink for visualization.
| bacteria | cell-wall proteins | ligand-free | fibronectin (Fn) | fibrinogen (Fg) |
|---|---|---|---|---|
| S. aureus | FnBPA | 56%† (2%) | 78% (4%) | 67% (4%) |
| FnBPB | 44%† (1%) | 60% (11%) | 55% (2%) | |
| others | 12% (1%) | 12% (1%) | 21% (1%) | |
| L lactis | FnBPA | 0%*,† (3%) | 25% (7%) | 0% (3%) |
| FnBPB | 16%† (3%) | 69% (8%) | 8% (3%) | |
| others | 0% (2%) | 0% (3%) | 0% (3%) |
clumping detected after 24 hours;
soluble Zn enhanced homo-aggregation under shear conditions.
Homophilic aggregation between molecules from adjacent cells (ligand-free clumping between bacterial cells) of up to 56% was observed for both S. aureus and L. lactis expressing FnBP’s on their outer envelope. Lesser homo-aggregation for L. lactis expressing FnBPs (Table 1) can be explained by the lower density of cell-wall proteins as confirmed by the Western blots (Fig. 5C vs 5D). This type of cell-cell clumping was also observed for S. aureus in the absence of FnBPs (see data for DU5883 in Table 1), indicating that other cell wall proteins on S. aureus (e.g., clumping factors A or B, ClfA/B; serine-aspartate repeat proteins D or E, SdrD/E; von Willebrand factor) may play a role in homo-aggregation. But, as shown in Table 1, FnBPA and/or FnBPB clearly play the major role in enhancing cell-cell aggregation, particularly under shear conditions. Cell-cell aggregation through FnBPA/B was also enhanced through the addition of soluble zinc; whereas removal of zinc with the divalent cation chelator EDTA decreased cell aggregation (see Supplemental Fig 1). This finding is consistent with previous studies with zinc (Geoghegan, et al., 2013, Herman-Bausier, et al., 2015).
Homo-aggregation was also impacted by the different sequences of amino acids making up FnBPA vs. FnBPB. This was evident in the ligand-free experiments with L. lactis. For instance, FnBPB mutants show enhanced clumping compared to FnBPA variants (Figure 3A and 4B). FnBPA and FnBPB have a relatively low (~45%) sequence identity in the A region which has been previously identified as a possible site for cell-cell interactions (Geoghegan, et al., 2013, Herman-Bausier, et al., 2015, Jonsson, et al., 1991). Our aggregation experiments suggest that the A-domain of FnBPB has a greater affinity for A-domains of FnBPB from adjacent cells compared to that observed for pairs of A-domains in FnBPA (see Figure 3A). This might explain the reason clumping was observed at a similar, low level (~3%) for all L. lactis under static conditions (Fig. 4A); whereas clumping was observed for only FnBPB variants of L. lactis under shear conditions (Fig. 3A). It seems that interactions between adjacent A-domains on FnBPB molecules are more resilient than those between FnBPA molecules.
In terms of bridging host ligands, the addition of soluble Fn significantly enhanced clumping for both S. aureus and L. lactis strains expressing FnBPA and/or FnBPB (Table 1). Relative to ligand-free conditions, clumping increased by >100% for L. lactis expressing solely FnBPA or FnBPB on the cell wall (Fig. 3B). In S. aureus, this increase was more modest (30%; Fig. 2B) likely due to the confounding impact of other cell-wall adhesins able to bind to mammalian ligands like Fn.
As shown in Figure 2B, the addition of soluble Fg significantly enhanced clumping for S. aureus lacking FnBPs on their cell wall (DU5883 mutant). Fg likely served as a bridging ligand between cell-wall MSCRAMMS like ClfA/B and SdrD/E, which are well-known to bind to this ligand (Foster, et al., 2014). Fg-enhanced clumping was also observed for L. lactis and S. aureus expressing FnBPA/B (Table 1). This form of ligand-assisted clumping could be important in vivo since Fg is about ten times more abundant than Fn in the blood.
S. aureus clumping in the presence of Fg likely involves interactions with the A region, which is the active Fg-binding site in FnBPA/B (Foster, et al., 2014, Keane, et al., 2007). Differences in the clumping affinity between FnBPA and FnBPB (see Figures 2A, 2B, 3A, 4A and 4B) are attributed to the low sequence identity in this A region, as discussed previously for the cell-cell interactions. Studies of the diversity of the A domain of FnBPA and FnBPB from S. aureus strains, have shown that there are at least seven distinct isoforms with 60 to 85% sequence identify. Each distinct isoform binds to the same site in Fg although with a different affinity (Burke, et al., 2010, Loughman, et al., 2008).
Comparing the two host, blood proteins, significantly more clumping was observed in the presence of Fn compared to Fg (Table 1). This is likely due to the multivalent binding capacity towards Fn for both FnBPA and FnBPB. Each of these bacterial proteins are able to bind up to nine Fn molecules through the FnBR region (Bingham, et al., 2008); whereas the A-region of FnBPA/B binds to a single Fg molecule (Foster, et al., 2014). Therefore, Fn dimers could more readily act as a bridging molecule between FnBPs molecules on adjacent bacterium. It is also possible that a conformational change in Fn upon adhesion to one bacterial adhesin (Liang, et al., 2016) could favor an attractive interaction with an adjacent adhesin.
Aggregation of bacterial cells is one of at least two key processes that governor the initiation of S. aureus pathogenesis. Binding of S. aureus to solid substrates such as internal tissue or implanted materials is the other key initiation step for infection. These binding reactions are often mediated through interactions between bacterial MSCRAMMs (e.g., FnBPA/B) and host ligands that are immobilized on surfaces.
Microtiter is commonly used to measure adhesion reactions involving bacterial cells, including S. aureus binding to human ligands (Casillas-Ituarte, et al., 2019, Peacock, et al., 2000). While this is a straight-forward technique it offers only an indirect measure of adhesion because it detects changes in the optical density of (dead) labelled cells on well plates. AFM, on the other hand, provides a means of directly probing biophysical forces and/or the mechanical stability of ligand-receptor bonds (on live cells). Furthermore, AFM is a more dynamic technique capable of pulling or tugging on ligand-receptor pairs. This is important for ligand interactions with MSCRAMMs as demonstrated above for the clumping experiments under static vs. shear conditions.
AFM adhesion data provided herein shows a bond strength centered at ~430 pN for immobilized Fn with FnBPA or FnBPB on L. lactis (Figure 5B). Strength of binding to Fn was slightly stronger for FnBPA compared to Fn-FnBPB; ~460 pN vs. ~410 pN, respectively. Because similar number of proteins contributed to the interaction, this difference in bond strength could be traced to the extra repeat of ~40 amino acids in FnBPA (see Fig. 1). Even single amino acid changes in FnBPA have been reported to change the binding affinity towards immobilized Fn in clinical isolates of S. aureus (Hos, et al., 2015, Lower, et al., 2011).
The range of adhesion data shown in Figure 5B is consistent with Fn-binding data that was reported in another publication for this same strain of L. lactis expressing FnBPA with up to three amino acid substitutions in the repeat region (Casillas-Ituarte, et al., 2019). Figure 5B shows a narrow force distribution and small adhesion frequency (<20%). This, along with an analysis with the worm-like chain model, indicate single ligand-receptor pairs for the AFM experiments with L. lactis.
Stronger adhesion forces were acquired in S. aureus (800–1300 pN, Fig. 5A). This is expected given the higher level of FnBPs in this species of bacteria (compare Fig. 5C vs. 5D). The magnitude of adhesion shown in Fig. 5A is consistent with previous AFM studies for immobilized Fn on S. aureus expressing FnBPs (Buck, et al., 2010). Stronger adhesion for S. aureus likely originates through multivalent interactions (Casillas-Ituarte, et al., 2012) with the repeat region (see Fig 1) that can bind up to nine molecules of Fn (Bingham, et al., 2008).
Focusing on the simpler, ligand-receptor interaction in the L. lactis surrogate, the ~430 pN adhesion force on Fn is stronger than the 241 pN adhesion force (median value) for Fg binding to FnBPs on the same L. lactis surrogate (Casillas-Ituarte, et al., 2012). A different strength of binding for the two host ligands is not surprising since there is a different mode or mechanism of binding for each ligand (Fn vs. Fg). Binding to Fg is expected to take place through interactions with the A-domain of FnBPA/B (see Figure 1).
Interestingly, these two host ligands respond quite differently to tensile loading on the ligand-FnBP bond. Under conditions comparable to physiological load, binding between single pairs of Fg and FnBPA reach strengths of greater than 1300 pN (Casillas-Ituarte, et al., 2019, Milles, et al., 2018). Furthermore, the bond strength between Fg-FnBPA was found to be dependent on amino acid substitutions in the repeat region, a part of FnBPA that does not directly interact with Fg. Casillas-Ituarte et al. (Casillas-Ituarte, et al., 2019) attribute this to catch-bond behavior of Fg when it binds to FnBPs under high tensile force.
In summary, FnBP adhesins in S. aureus adhesins are capable of homophilic interactions with neighboring bacteria that leads to clumps. This aggregation is enhanced by soluble Fg, and particularly soluble Fn under physiological levels of shear (Table 1). In general, FnBPB presented a greater ability to clump in the presence of solution host ligands compared to FnBPA. Interestingly, when the host ligand was immobilized on a surface, both adhesins FnBPA and FnBPB presented similar strength of adhesion. This indicates a critical condition for the interaction of these bacterial adhesins is the conformation of the host ligand (e.g., soluble vs. immobilized). In previous studies, we found that small variations in the amino acid sequences of the bacterial receptor alter the strength of adhesion to immobilized Fn and Fg (Casillas-Ituarte, et al., 2012, Casillas-Ituarte, et al., 2019, Lower, et al., 2011). In this study, we demonstrate that host protein configuration also plays a role in the initial molecular pathogenesis of S. aureus.
5. Conclusions
S. aureus has evolved to interact with multiple components of the host to avoid immune response and to facilitate adhesion to surfaces of indwelling medical devices. We have shown that two closely related S. aureus adhesins, FnBPA and FnBPB promote clumping by intercellular adhesion, in addition to their well-known ability to adhere to Fn. FnBP-mediated clumping is affected by the different physiological conditions (static vs. shear) and by the presence of soluble host proteins, particularly Fn. We found that these bacterial adhesins have different capabilities to interact with soluble ligand. This might explain the reason that most clinical and reference strains of S. aureus express both of these two adhesins despite the fact that they bind to similar target ligands (Burke, et al., 2011, Loughman, et al., 2008). Perhaps FnBPB plays a role when S. aureus are in the bloodstream exposed to soluble ligand, whereas both FnBPs are important when S. aureus interact with immobilized ligands on a surface (e.g., extracellular matrix or foreign medical device). The specialization of FnBPB could also explain the reason that both adhesins cooperate in the induction of severe infections by S. aureus (Shinji, et al., 2011). This could also mean that there are different regulatory mechanisms for these two genes allowing expression under different conditions.
Supplementary Material
Highlights.
Staphylococcus aureus aggregates form through attractive interactions between wall adhesins
Bacterial aggregation is enhanced in the presence of soluble host proteins
These same cell wall adhesins exhibit a strong affinity for immobilized ligands.
FnBPA and FnBPB present complementary capabilities to aggregate and adhere
Acknowledgments:
We thank Dr. Yok-Ai Que for kindly providing S. aureus 8325-4 and L. lactis subsp. cremoris 1363. We acknowledge the support of P.En and J. Tak.
Funding:
This research was funded by National Institutes of Health (NIH) Grant R01HL119648 (SKL) and R01 GM124436-01 (PS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- CDC. Antibiotic Resistance Threats in the United States,. (Department of Health and Human Services, CDC, Atlanta, GA, USA, 2019). [Google Scholar]
- Ambriz-Avina V, Contreras-Garduno JA Pedraza-Reyes M, 2014. Applications of flow cytometry to characterize bacterial physiological responses. Biomed Res. Int, 2014 doi: 10.1155/2014/461941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariens RAS, 2013. Fibrin(ogen) and thrombotic disease. J. Thromb. Haemost, 11, 294–305 doi: 10.1111/jth.12229. [DOI] [PubMed] [Google Scholar]
- Bingham RJ, Rudino-Pinera E, Meenan NAG, Schwarz-Linek U, Turkenburg JP, Hook M, Garman EF Potts JR, 2008. Crystal structures of fibronectin-binding sites from Staphylococcus aureus FnBPA in complex with fibronectin domains. Proc. Natl. Acad. Sci. U. S. A, 105, 12254–12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisognano C, Vaudaux P, Rohner P, Lew DP Hooper DC, 2000. Induction of fibronectin-binding proteins and increased adhesion of quinolone-resistant Staphylococcus aureus by subinhibitory levels of ciprofloxacin. Antimicrob. Agents Ch, 44, 1428–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonaert CJP, Dufrene YF, Derclaye SR Rouxhet PG, 2001. Adhesion of Lactococcus lactis to model substrata: direct study of the interface. Colloid Surf. B-Biointerfaces, 22, 171–182 doi: 10.1016/s0927-7765(01)00196-5. [DOI] [Google Scholar]
- Buck AW, Fowler VG, Yongsunthon R, Liu J, DiBartola AC, Que YA, Moreillon P Lower SK, 2010. Bonds between fibronectin and fibronectin-binding proteins on Staphylococcus aureus and Lactococcus lactis. Langmuir, 26, 10764–10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke FM, McCormack N, Rindi S, Speziale P Foster TJ, 2010. Fibronectin-binding protein B variation in Staphylococcus aureus. Bmc Microbiology, 10 doi: 10.1186/1471-2180-10-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke FM, Di Poto A, Speziale P Foster TJ, 2011. The A domain of fibronectin-binding protein B of Staphylococcus aureus contains a novel fibronectin binding site. Febs J, 278, 2359–2371 doi: 10.1111/j.1742-4658.2011.08159.x. [DOI] [PubMed] [Google Scholar]
- Casillas-Ituarte NN, Lower BH, Fowler VG Jr., Lamlertthon S Lower SK, 2012. Dissociation rate constants of human fibronectin binding to fibronectin-binding proteins on living Staphylococcus aureus isolated from clinical patients. J. Biol. Chem, 287, 6693–6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casillas-Ituarte NN, DiBartola AC, Broughton MJ, Perez-Guzman L, Wheeler RM, Ibaraki M, Lower BA, Dunn JA, Lower BH, Fowler VG Jr., Hook M, McIntyre LM, Lower SK Sharma-Kuinkel BK, 2019. Fibrinogen binding is affected by amino acid substitutions in C-terminal repeat region of fibronectin binding protein A. Scientific Reports, 9 doi: 10.1038/s41598-019-48031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby HA, Kwiecinski J Horswill AR in Advances in Applied Microbiology, Vol 96 Vol. 96 Advances in Applied Microbiology (eds Sariaslani S & Gadd GM) 1–41 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastgheyb S, Parvizi J, Shapiro IM, Hickok NJ Otto M, 2015. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. J. Infect. Dis, 211, 641–650 doi: 10.1093/infdis/jiu514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson J, Kumar A, Willis LF, Tuma R, Higazi DR, Turner R, Lowe DC, Ashcroft AE, Radford SE, Kapur N Brockwell DJ, 2017. Inducing protein aggregation by extensional flow. Proc. Natl. Acad. Sci. U. S. A, 114, 4673–4678 doi: 10.1073/pnas.1702724114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, 2001. Probing the relation between force - lifetime - and chemistry in single molecular bonds. Annu. Rev. Biophys. Biom, 30, 105–128. [DOI] [PubMed] [Google Scholar]
- Foster T, 2016. The remarkably multifunctional fibronectin binding proteins of Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis, 35, 1923–1931 doi: 10.1007/s10096-016-2763-0. [DOI] [PubMed] [Google Scholar]
- Foster TJ, Geoghegan JA, Ganesh VK Hook M, 2014. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol, 12, 49–62 doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh VK, Rivera JJ, Smeds E, Ko Y-P, Bowden MG, Wann ER, Gurusiddappa S, Fitzgerald JR Hoeoek M, 2008. A structural model of the staphylococcus aureus ClfA-fibrinogen interaction opens new avenues for the design of anti-staphylococcal therapeutics. PLoS Pathog., 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan JA, Monk IR, O’Gara JP Fostera TJ, 2013. Subdomains N2N3 of fibronectin binding protein a mediate staphylococcus aureus biofilm formation and adherence to fibrinogen using distinct mechanisms. J. Bacteriol, 195, 2675–2683 doi: 10.1128/jb.02128-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene C, McDevitt D, Francois P, Vaudaux PE, Lew DP Foster TJ, 1995. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol. Microbiol, 17, 1143–1152. [DOI] [PubMed] [Google Scholar]
- Heilmann C, Niemann S, Sinha B, Herrmann M, Kehrel BE Peters G, 2004. Staphylococcus aureus fibronectin-binding protein (FnBP)-mediated adherence to platelets, and aggregation of platelets induced by FnBPA but not by FnBPB. J. Infect. Dis, 190, 321–329 doi: 10.1086/421914. [DOI] [PubMed] [Google Scholar]
- Henderson B, Nair S, Pallas J Williams MA, 2011. Fibronectin: a multidomain host adhesin targeted by bacterial fibronectin-binding proteins. Fems Microbiol. Rev, 35, 147–200 doi: 10.1111/j.1574-6976.2010.00243.x. [DOI] [PubMed] [Google Scholar]
- Herman-Bausier P, El-Kirat-Chatel S, Foster TJ, Geoghegan JA Dufrene YF, 2015. Staphylococcus aureus fibronectin-binding protein A mediates cell-cell adhesion through low-affinity homophilic bonds. mBio, 6, 10 doi: 10.1128/mBio.00413-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M, Vaudaux PE, Pittet D, Auckenthaler R, Lew PD, Schumacherperdreau F, Peters G Waldvogel FA, 1988. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J. Infect. Dis, 158, 693–701. [DOI] [PubMed] [Google Scholar]
- Hos NJ, Rieg S, Kern WV, Jonas D, Fowler VG, Higgins PG, Seifert H Kaasch AJ, 2015. Amino acid alterations in fibronectin binding protein A (FnBPA) and bacterial genotype are associated with cardiac device related infection in Staphylococcus aureus bacteraemia. J. Infect, 70, 153–159 doi: 10.1016/j.jinf.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Jonsson K, Signas C, Muller HP Lindberg M, 1991. 2 Different genes encode fibronectin binding-proteins in Staphylococcus-aureus - the complete nucleotide-sequence and characterization of the 2nd gene. Eur. J. Biochem, 202, 1041–1048 doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- Kapral FA, 1966. Clumping of Staphylococcus aureus in peritoneal cavity of mice. J. Bacteriol, 92, 1188-& doi: 10.1128/jb.92.4.1188-1195.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane FM, Loughman A, Valtulina V, Brennan M, Speziale P Foster TJ, 2007. Fibrinogen and elastin bind to the same region within the A domain of fibronectin binding protein A, an MSCRAMM of Staphylococcus aureus. Mol. Microbiol, 63, 711–723. [DOI] [PubMed] [Google Scholar]
- Krismer B, Liebeke M, Janek D, Nega M, Rautenberg M, Hornig G, Unger C, Weidenmaier C, Lalk M Peschel A, 2014. Nutrient limitation governs Staphylococcus aureus metabolism and niche adaptation in the human nose. PLoS Pathog, 10 doi: 10.1371/journal.ppat.1003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusela P, 1978. Fibronectin binds to Staphylococcus-aureus. Nature, 276, 718–720 doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Kwiecinski JM, Crosby HA, Valotteau C, Hippensteel JA, Nayak MK, Chauhan AK, Schmidt EP, Dufrene YF Horswill AR, 2019. Staphylococcus aureus adhesion in endovascular infections is controlled by the ArlRS-MgrA signaling cascade. PLoS Pathog., 15 doi: 10.1371/journal.ppat.1007800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Lundgren E, Berger E Arfors KE, 1989. Shear-dependent inhibition of granulocyte adhesion to cultured endothelium by dextran sulfate. Blood, 73, 1324–1330. [PubMed] [Google Scholar]
- Liang XW, Garcia BL, Visai L, Prabhakaran S, Meenan NAG, Potts JR, Humphries MJ Hook M, 2016. Allosteric Regulation of fibronectin/alpha(5)beta(1) interaction by fibronectin-binding MSCRAMMs. PLoS One, 11 doi: 10.1371/journal.pone.0159118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughman A, Sweeney T, Keane FM, Pietrocola G, Speziale P Foster TJ, 2008. Sequence diversity in the A domain of Staphylococcus aureus fibronectin-binding protein A. BMC Microbiol, 8:74, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe GDO, Rumley A Mackie IJ, 2004. Plasma fibrinogen. Ann. Clin. Biochem, 41, 430–440 doi: 10.1258/0004563042466884. [DOI] [PubMed] [Google Scholar]
- Lower SK, 2011. Atomic force microscopy to study intermolecular forces and bonds associated with bacteria. Ad. Exp. Med. Biol, 715, 285–299. [DOI] [PubMed] [Google Scholar]
- Lower SK, Lamlertthon S, Casillas-Ituarte NN, Lins RD, Yongsunthon R, Taylor ES, DiBartola AC, Edmonson C, McIntyre LM, Reller LB, Que YA, Ros R, Lower BH Fowler VG, 2011. Polymorphisms in fibronectin binding protein A of Staphylococcus aureus are associated with infection of cardiovascular devices. Proc. Natl. Acad. Sci. U. S. A, 108, 18372–18377 doi: 10.1073/pnas.1109071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy FD, 1998. Staphylococcus aureus infections. New Engl. J. Med, 339, 520–532. [DOI] [PubMed] [Google Scholar]
- McAdow M, Kim HK, DeDent AC, Hendrickx APA, Schneewind O Missiakas DM, 2011. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog, 7 doi: 10.1371/journal.ppat.1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows PY, Bemis JE Walker GC, 2003. Single-molecule force spectroscopy of isolated and aggregated fibronectin proteins on negatively charged surfaces in aqueous liquids. Langmuir, 19, 9566–9572. [Google Scholar]
- Meenan NAG, Visai L, Valtulina V, Schwarz-Linek U, Norris NC, Gurusiddappa S, Höök M, Speziale P Potts JR, 2007. The tandem β-zipper model defines high affinity fibronectin-binding repeats within Staphylococcus aureus FnBPA. J. Biol. Chem, 282, 25893–25902. [DOI] [PubMed] [Google Scholar]
- Mezzenga R Mitsi M, 2019. The molecular dance of fibronectin: conformational flexibility leads to functional versatility. Biomacromolecules, 20, 55–72 doi: 10.1021/acs.biomac.8b01258. [DOI] [PubMed] [Google Scholar]
- Milles LF, Schulten K, Gaub HE Bernardi RC, 2018. Molecular mechanism of extreme mechanostability in a pathogen adhesin. Science, 359, 1527–1532 doi: 10.1126/science.aar2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher DF, 2006. Plasma fibronectin concentration - A risk factor for arterial thrombosis? Arterioscler. Thromb. Vasc. Biol, 26, 1193–1195 doi: 10.1161/01.ATV.0000223342.15969.7a. [DOI] [PubMed] [Google Scholar]
- Naber CK, 2008. Future strategies for treating Staphylococcus aureus bloodstream infections. Clin. Microbiol. Infec, 14, 26–34 doi: 10.1111/j.1469-0691.2008.01924.x. [DOI] [PubMed] [Google Scholar]
- Oestreicher Z, Valverde-Tercedor C, Chen L, Jimenez-Lopez C, Bazylinski DA, Casillas-Ituarte NN, Lower SK Lower BH, 2012. Magnetosomes and magnetite crystals produced by magnetotactic bacteria as resolved by atomic force microscopy and transmission electron microscopy. Micron, 43, 1331–1335 doi: 10.1016/j.micron.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Peacock SJ, Day NPJ, Thomas MG, Berendt AR Foster TJ, 2000. Clinical isolates of Staphylococcus aureus exhibit diversity in fnb genes and adhesion to human fibronectin. J. Infect, 41, 23–31. [DOI] [PubMed] [Google Scholar]
- Pestrak MJ, Chaney SB, Eggleston HC, Dellos-Nolan S, Dixit S, Mathew-Steiner SS, Roy S, Parsek MR, Sen CK Wozniak DJ, 2018. Pseudomonas aeruginosa rugose small-colony variants evade host clearance, are hyper-inflammatory, and persist in multiple host environments. PLoS Pathog., 14 doi: 10.1371/journal.ppat.1006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestrak MJ, Gupta TT, Dusane DH, Guzior DV, Staats A, Harro J, Horswill AR Stoodley P, 2020. Investigation of synovial fluid induced Staphylococcus aureus aggregate development and its impact on surface attachment and biofilm formation. PLoS One, 15 doi: 10.1371/journal.pone.0231791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piroth L, Que Y-A, Widmer E, Panchaud A, Piu S, Entenza JM Moreillon P, 2008. The fibrinogen- and fibronectin-binding domains of Staphylococcus aureus fibronectin-binding protein a synergistically promote endothelial invasion and experimental endocarditis. Infect. Immun, 76, 3824–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnuraj K, Bowden MG, Davis S, Gurusiddappa S, Moore D, Choe D, Xu Y, Hook M Narayana SVL, 2003. A “dock, lock, and latch” structural model for a staphylococcal adhesin binding to fibrinogen. Cell, 115, 217–228. [DOI] [PubMed] [Google Scholar]
- Que YA, Haefliger JA, Francioli P Moreillon P, 2000. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp cremoris using a new shuttle vector. Infect. Immun, 68, 3516–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que YA, Francois P, Haefliger JA, Entenza JM, Vaudaux P Moreillon P, 2001. Reassessing the role of Staphylococcus aureus clumping factor and fibronectin-binding protein by expression in Lactococcus lactis. Infect. Immun, 69, 6296–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que YA, Haefliger JA, Piroth L, Francois P, Widmer E, Entenza JM, Sinha B, Herrmann M, Francioli P, Vaudaux P Moreillon P, 2005. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J. Exp. Med, 201, 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Linek U, Werner JM, Pickford AR, Gurusiddappa S, Kim JH, Pilka ES, Briggs JAG, Gough TS, Hook M, Campbell ID Potts JR, 2003. Pathogenic bacteria attach to human fibronectin through a tandem beta-zipper. Nature, 423, 177–181. [DOI] [PubMed] [Google Scholar]
- Shinji H, Yosizawa Y, Tajima A, Iwase T, Sugimoto S, Seki K Mizunoe Y, 2011. Role of fibronectin-binding proteins A and B in in vitro cellular infections and in vivo septic infections by Staphylococcus aureus. Infect. Immun, 79, 2215–2223 doi: 10.1128/iai.00133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Carraher C Schwarzbauer JE in Annual Review of Cell and Developmental Biology, Vol 26 Vol. 26 Annual Review of Cell and Developmental Biology (eds Schekman R, Goldstein L, & Lehmann R) 397–419 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B, Francois PP, Nusse O, Foti M, Hartford OM, Vaudaux P, Foster TJ, Lew DP, Herrmann M Krause KH, 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin alpha(5)beta(1). Cell Microbiol, 1, 101–117 doi: 10.1046/j.1462-5822.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- Thomas S, Liu W, Arora S, Ganesh V, Ko Y-P Hook M, 2019. The Complex fibrinogen interactions of the Staphylococcus aureus coagulases. Front. Cell. Infect. Mi, 9 doi: 10.3389/fcimb.2019.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong SYC, Davis JS, Eichenberger E, Holland TL Fowler VG, 2015. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev, 28, 603–661 doi: 10.1128/cmr.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL Gosbell IB, 2012. Predictors of mortality in Staphylococcus aureus bacteremia. Clin. Microbiol. Rev, 25, 362–386 doi: 10.1128/cmr.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudaux P, Pittet D, Haeberli A, Huggler E, Nydegger UE, Lew DP Waldvogel FA, 1989. Host factors selectively increase staphylococcal adherence on inserted catheters - a role for fibronectin and fibrinogen or fibrin. J. Infect. Dis, 160, 865–875. [DOI] [PubMed] [Google Scholar]
- Vaudaux P, Pittet D, Haeberli A, Lerch PG, Morgenthaler JJ, Proctor RA, Waldvogel FA Lew DP, 1993. Fibronectin is more active than fibrin or fibrinogen in promoting Staphylococcus-aureus adherence to inserted intravascular catheters. J. Infect. Dis, 167, 633–641. [DOI] [PubMed] [Google Scholar]
- Wann ER, Gurusiddappa S Hook M, 2000. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J. Biol. Chem, 275, 13863–13871. [DOI] [PubMed] [Google Scholar]
- Xiong YQ, Sharma-Kuinkel BK, Casillas-Ituarte NN, Fowler VG, Rude T, DiBartola AC, Lins RD, Abdel-Hady W, Lower SK Bayer AS, 2015. Endovascular infections caused by methicillin-resistant Staphylococcus aureus are linked to clonal complex-specific alterations in binding and invasion domains of fibronectin-binding protein a as well as the occurrence of fnbB. Infect. Immun, 83, 4772–4780 doi: 10.1128/iai.01074-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


