Abstract
Tobacco use is now a leading cause of death in people living with HIV in the USA. Increasing cessation rates in this group is a public health priority, yet the results of clinical trials aimed at optimising tobacco treatment strategies have been largely disappointing. Combinations of behavioural and pharmacological cessation therapies in people living with HIV have yielded increases in short-term quit rates, but few have shown long-term efficacy. Even with aggressive therapy combining intensive behavioural treatment with pharmacological agents, most smokers living with HIV continue to smoke. The generalised approach to tobacco treatment that prevails in guidelines and in clinical practices might do a disservice to these individuals, who represent a sizable segment of the population of people living with HIV. Harm reduction is a sensible and needed approach for smokers living with HIV who are unable or unwilling to quit. In this Viewpoint, we take an expansive view of harm reduction to include not only cutting down on cigarette intake for persistent smokers, but also reducing smoking’s downstream health effects by increasing lung cancer screening and by controlling concurrent cardiovascular risk factors, especially hypertension and hyperlipidaemia.
Introduction
Just over 20 years ago, Raymond Niaura and colleagues authored a prescient editorial about HIV infection, AIDS, and smoking cessation.1 This editorial was the first high-profile paper to bring attention to a looming public health problem. The landscape of HIV infection was vastly different back then. It had been only 4 years since the introduction of HIV-1 protease inhibitors—the emergence of the modern antiretroviral therapy (ART) era. In 2000, the most common ART regimens still necessitated the ingestion of multiple pills with more pronounced side-effects than current treatments, and rates of HIV-related infections and neoplasms were just beginning to fall. Tobacco use, although epidemic among people living with HIV in the USA, was far down on the list of priorities in the clinical care of patients. The largest study of the health effects of tobacco use on people living with HIV at the time, published in 1997, concluded that “cigarette smoking does not have a major effect on the progression of HIV-1 infection to AIDS or death but may affect the incidence of oral thrush”2—in retrospect, a grave underestimation of the future importance of the problem. Some experts have lamented that many HIV care practitioners treated cigarette smoking as an allowable vice.3 In their editorial,1 Niaura and colleagues accurately predicted that traditional tobacco treatment approaches might be less effective in people living with HIV given the array of sociobehavioural and medical challenges that these people are faced with.
This Viewpoint addresses the limited success that strategies intended to increase smoking cessation rates in people living with HIV have yielded, and we argue that a harm reduction approach, defined by Mary Hawk and colleagues in their recent review as “reducing the negative effects of health behaviours without necessarily extinguishing the problematic health behaviours completely,”4 might yield greater net benefits on a population level.
Most of the literature used to guide our recommendations derives from research done in the USA, and, thus, care providers in the USA might be the most suitable target for this Viewpoint. However, tobacco use in people living with HIV is an international problem, and many of the opinions voiced in this Viewpoint are applicable regardless of the treatment setting.
We hope this Viewpoint will help to begin shifting the idea of tobacco treatment in the setting of comprehensive HIV care from a strictly all-or-none cessation approach, which succeeds for only a small minority of smokers living with HIV, to a harm reduction approach that might extend substantial benefit to both those who are able to quit and to the majority who continue smoking.
Tobacco use: the scope of the problem
Population-based surveys suggest that approximately half of people living with HIV in the USA are current cigarette smokers,5,6 more than triple the prevalence in all US adults,7 and 15–20% of cigarette smokers living with HIV use other tobacco products as well, most commonly cigars.8,9 Cigarette smoking is a risk factor for a range of infectious (eg, bacterial pneumonia, tuberculosis, and Pneumocystis jirovecii pneumonia)10 and non-infectious diagnoses (eg, lung cancer, myocardial infarction, stroke, and emphysema),11–13 and tobacco use has emerged as a leading killer of people living with HIV in the USA and Europe in the past decade14,15 and the most influential factor perpetuating the survival gap between those living with and without HIV.16 The overlap of tobacco use and HIV is not limited to high-income countries. Although North America and west and central Europe have the highest prevalences of smoking among people living with HIV, the absolute burden of tobacco use is highest in eastern and southern Africa, Asia, and the Pacific.17 People living with HIV in low-income and middle-income nations worldwide have significantly higher cigarette smoking prevalences than people without HIV in those regions.18 Current projections for people living with HIV in care in the USA estimate that a staggering 9·3% of them—almost 60 000 people—will die of lung cancer if patterns of tobacco use do not change.11 Unfortunately, many more are destined to die from tobacco-attributable cardiovascular disease, non-neoplastic pulmonary disease, and other malignancies.19–21
Smoking cessation: the ongoing challenge
Encouraging smokers living with HIV to quit for life, keeping ex-smokers from relapsing, and preventing youth with and at risk for HIV from initiating tobacco use, are currently, and should remain, among the main goals of comprehensive HIV care. Getting people living with HIV who smoke cigarettes to quit is difficult, and most attempts end in failure. Interested individuals are encouraged to review the excellent qualitative research published by Nancy Reynolds and colleagues,22 to hear the voices and opinions of those with lived experience as they reflect on smoking and quitting in the context of the life experiences of people living with HIV. In this Viewpoint, we do not intend to minimise the importance of smoking cessation or to disparage the efforts of the many clinicians, public health agencies, and researchers who are making abstinence from tobacco products their priority. Indeed, we count ourselves among them. Nonetheless, it would be unrealistic to ignore the facts. Although the scientific literature regarding tobacco treatment for people living with HIV might be limited by the heterogeneity of populations and treatment and control conditions, multiple randomised controlled trials of evidence-based tobacco treatments have been completed in people living with HIV. However, only two trials have shown efficacy in promoting abstinence at their definitive endpoints (ie, at 6 months or more)23,24 and one other trial showed a cessation advantage in recipients receiving intervention at a late timepoint (at least 1 year after initial enrolment).25 Two of these trials compared an intensive, social cognitive theory-based, multisession behavioural intervention plus an offer of nicotine replacement therapy with control conditions that included an offer of nicotine replacement therapy,23,25 and the other trial compared varenicline with placebo in a cohort of smokers living with HIV, all of whom were offered multisession, one-on-one, face-to-face cessation counseling.24 Notably, abstinence rates in these three successful studies were at best modest, at 15·0%,23 14·6%,24 and 12·7%.25 The dominant theme in these studies is that intensive interventions sometimes yield short-term increases in quit rates (at ≤3 months) but that long-term cessation is a largely elusive goal.26 The Infectious Diseases Society of America’s primary care guidance for people living with HIV, updated in November, 2020, advises providers that “[a]ll patients who smoke should be strongly encouraged to stop smoking and offered smoking cessation assistance. Screening for smoking should be done at every healthcare encounter”.27 These recommendations are sensible, but they do not offer guidance on how to achieve the cessation goal. Without citing any evidence-based, efficacious treatments, the practical value of these recommendations to providers and patients is limited. Barring a dramatic advance in tobacco treatment and control, most people living with HIV who are smokers are going to continue smoking into the future.
Harm reduction: a different approach
The term harm reduction entered the mainstream medical literature in the late 1980s and focused on controlling the risk of HIV acquisition by modifying behaviours associated with substance use.28,29 Since that time, the application of harm reduction has expanded to include behaviours associated with sexual risk, alcohol use, and tobacco use.30–32 One attraction of the harm reduction approach is that this method is already deeply embedded in many aspects of HIV care. The typical HIV clinic posts many signs, flyers, and pamphlets encouraging the mitigation of sex-related and drug use-related behaviours associated with risk. The harm reduction message is thoroughly familiar to most, if not all, people living with HIV in the USA. The proven effectiveness of this message at reducing sex-related and drug use-related behaviours that confer risk for HIV transmission offers hope that analogous strategies might be helpful in controlling tobacco use.33
In the context of tobacco treatment, harm reduction is largely focused on reducing average daily cigarette intake (cigarettes per day [CPD]) or cutting down, or transitioning to an alternative tobacco or nicotine product believed to confer less harm. In this Viewpoint, we also discuss strategies, consistent with the expansive definition of harm reduction, that are likely to reduce tobacco-related morbidity and mortality in smokers living with HIV, such as intensified screening for lung cancer, and mitigation of other cardiovascular risk factors that might act in concert with tobacco use to cause harm.
Cutting down
Smokers living with HIV who do not quit should be encouraged to cut down, a goal that is realistic for most smokers (figure). Even though the literature regarding tobacco treatment is dominated by studies with cessation as their primary outcomes, reduction in cigarette intake is a worthy objective for at least two reasons. First, although there is no low-level threshold of cigarette use that is considered safe,34 smoking less decreases tobacco-related morbidity and mortality. Large, population-based surveys in the USA (>500 000 participants) show a definitive survival advantage for lighter smokers versus heavier smokers.35,36 Based on modelling analyses, our group has estimated a 34·9% reduction in lung cancer mortality among light smokers living with HIV versus heavy smokers living with HIV.11 Large cohort studies have also shown an unequivocal dose–response association between CPD and cardiovascular disease risk and mortality.37,38 These studies did not do longitudinal analyses of smokers who changed their CPD over time, so the benefits that accrue to a population of cigarette smokers who cut down, the timing of such risk reduction, and the stability of risk reduction in the face of a CPD dynamic that might include periods of higher smoking rates are not well defined. Moreover, a clinician’s or a researcher’s ability to precisely quantitate CPD relies on self-reporting, with its attendant potential of unreliability. However, based on what is known, it is very probable that a transition from heavier smoking to lighter smoking will decrease the risk of lung cancer and cardiovascular disease over time. Second, the so-called reduce-to-quit approach is an important and underused method for tobacco treatment that yields cessation rates similar to traditional interventions involving the abrupt quitting of smoking.39 Robust literature suggests that reducing CPD predicts future cessation and is a frequent action towards that goal.40 We are not aware of any previous reduce-to-quit research in people living with HIV, and we believe this research is a worthy area of future scientific enquiry.
Figure : Change in CPD from baseline to 24 weeks among participants who did not quit smoking.
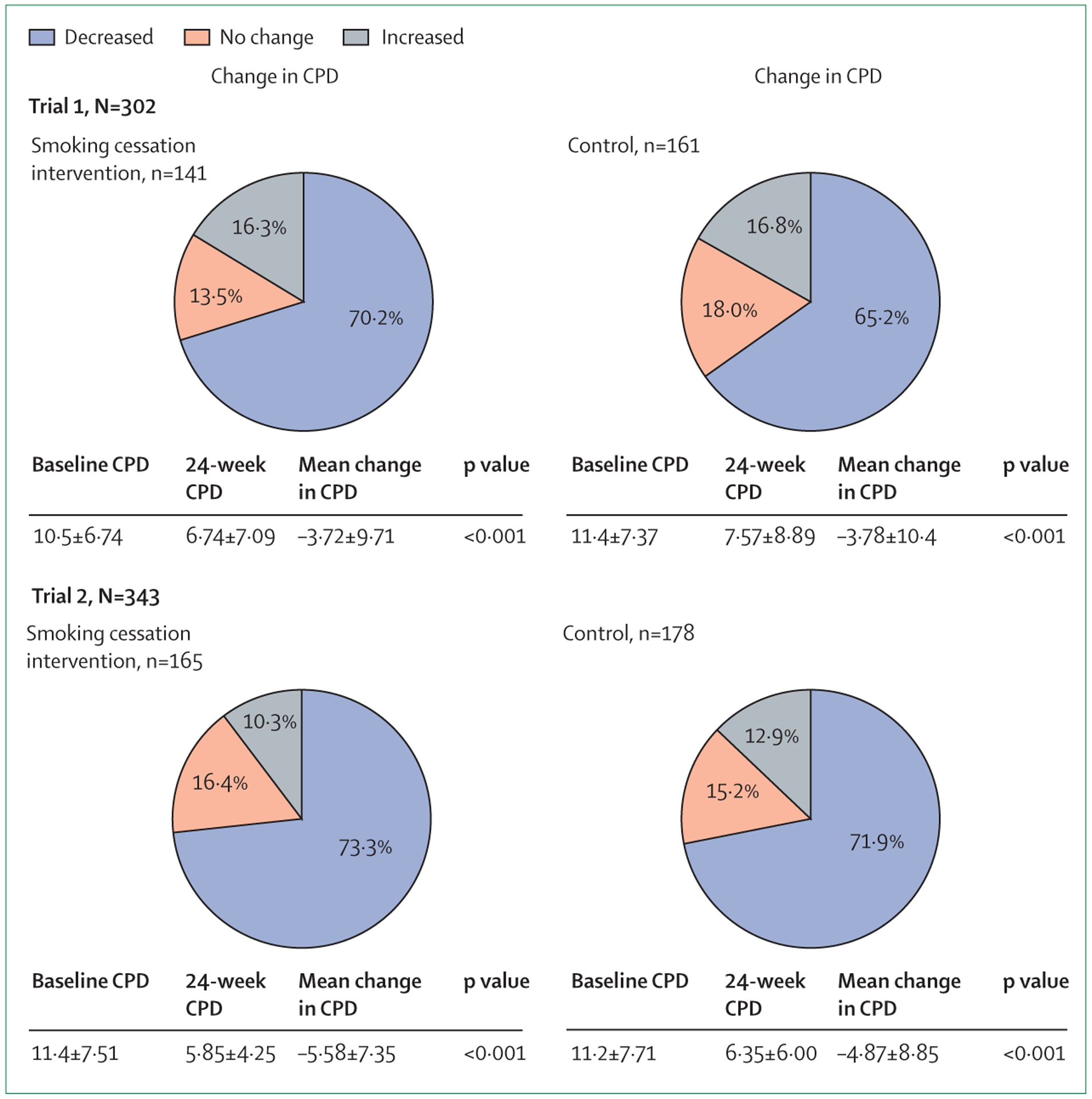
Trial 1 refers to the 2020 randomised controlled trial8 and trial 2 refers to the 2021 randomised controlled trial.23 CPD=cigarettes per day.
A meta-analysis in 2016 evaluated the evidence base for various harm reduction strategies for cigarette smokers.41 The authors concluded that there is adequate evidence that nicotine replacement therapy can reduce CPD in those who do not wish to quit. Other treatments that have been tried include behavioural therapy, brief advice, electronic cigarettes, snus, bupropion, and varenicline, but the few available studies were insufficient to reach any conclusions regarding their efficacy.
We have shown in two separate randomised controlled trials8,23 that most smokers living with HIV can reduce their CPD over 24 weeks of follow-up. In both of these studies, done in three different cities in the USA, participants were randomly assigned 1:1 to either an intensive cessation programme designed specifically for people living with HIV or to a control condition with minimal cessation counselling. All participants were offered a 12-week course of nicotine replacement therapy. Although assignment to intensive counselling conferred a cessation advantage, when successful quitters were excluded from the analyses most of the participants in the intervention and control groups had significantly reduced their CPD at 24 weeks compared with baseline (p<0·001 in all comparisons), and the magnitude of reduction was similar in both groups for both trials (figure).8,23 In the aggregate sample of over 600 smokers living with HIV followed up for 24 weeks (excluding those who quit), 70·2% reduced, 15·8% did not change, and 14·0% increased their CPD. Notably, the control participants, who received minimal cessation counselling, reduced their CPD at similar rates to those allocated to intensive tobacco treatment. Nicotine replacement therapy probably had a role in reducing CPD rates, but the 12-week supply had finished in almost all participants by the 24-week timepoint. Our data suggest that most smokers living with HIV can succeed in cutting down their tobacco use at 24 weeks, which is a far greater proportion than those who can quit completely. These findings have important implications for the risk of tobacco-associated morbidity and mortality among continued smokers living with HIV, and highlight the potential positive effect that even brief cessation counselling can offer.
Although we believe that encouraging smokers living with HIV who cannot or will not quit to cut down is a worthwhile endeavour, shortcomings of this approach require mention. Smokers who reduce their daily cigarette consumption might offset some or all of the potential benefit by compensatory smoking behaviours (eg, inhaling more deeply).42 Smokers might also under-report their daily cigarette consumption,43 and there is no readily available biomarker to verify their estimates.
Using alternative products
The use of electronic nicotine delivery systems (also known as ENDS, and referred to as e-cigarettes henceforth) as a means of decreasing combustible tobacco intake is a subject of intense interest and substantial controversy.44 Little is known about the effect of e-cigarettes on tobacco use-related behaviours in people living with HIV, and research has been slowed by the emergence of e-cigarette, or vaping, product use-associated lung injury in 2019. One small pilot study of e-cigarettes reported a statistically significant decrease in CPD and an increase in motivation to quit in a group of 19 cigarette smokers living with HIV (p<0·001).45 Notably, high-quality research does support the efficacy of e-cigarettes in promoting reduced CPD in the general population.46 Despite the potential promise of switching from combustible tobacco to e-cigarettes, research is necessary to determine whether the risk of e-cigarette-associated lung injury is amplified in people living with HIV, whether these devices would be accessible and acceptable to this population, and, if so, what the optimal mode of usage is to maximise their effect. A search of the ClinicalTrials.gov website shows that at least two prospective studies of the role of e-cigarettes as a harm reduction device for smokers living with HIV are in their preparatory stages (NCT03862924 and NCT04218708).
Substantial literature exists regarding the use of smokeless tobacco products, such as chewing tobacco and snus, as a means of reducing the risk of cancer, cardiovascular disease, and lung disease among cigarette smokers.47 Given the particular susceptibility of people living with HIV to head and neck cancer,48 more research on these products, which are associated with head and neck cancers, is needed before transition from cigarettes to smokeless tobacco can be considered as a recommended option for smokers living with HIV.
Lung cancer screening
Based on the mortality benefit shown in a landmark study published in 2011,49 the American Cancer Society recommends annual low-dose computed tomography (LDCT) screening for lung cancer for smokers who are at high risk. A 2020 meta-analysis reported that LDCT almost tripled the odds of detecting lung cancer at an early (resectable) stage, which decreased lung cancer mortality by 16%.50 Smokers living with HIV acquire lung cancer at younger ages and with lower lifetime cigarette exposures than smokers without HIV,51–53 leading some experts to recommend LDCT screening at a younger age and at a lower pack-year threshold (ie, current smoker at ≥45 years old with ≥20 pack-years of smoking history54 vs standard LDCT at ≥55 years old with ≥30 pack-years of smoking history). Notably, the US Preventive Services Task Force recently published updated LDCT screening recommendations for the general population,55 lowering the standard age cutoff to 50 years and the lifetime cigarette exposure to 20 pack-years. One study in France of LDCT screening of 442 smokers living with HIV aged 40 years or older detected ten lung cancers, six of which were in early stages.56 The available data show that LDCT screening might be substantially underused in people living with HIV.57 Additionally, LDCT screening might offer other benefits; in an early trial of CT screening for lung cancer in people without HIV, a 23% tobacco quit rate was observed during follow-up, and 58% of these successful quitters used a reduce-to-quit strategy.58
Reducing risk of cardiovascular disease
Although tobacco use remains the most important cause of preventable death in the USA and worldwide,59,60 improving the management of cardiovascular risk factors can save more lives in the USA than any other clinical intervention.61 In addition to smoking cessation, this management requires control of hypertension and hyperlipidaemia. On a national level, every 10% increase in the number of people effectively treated for hypertension would prevent 14 000 additional deaths.61,62 Hypertension is over-represented in people living with HIV, with a US national prevalence of more than 42% versus 29% in the general population.63 People living with HIV who have hypertension have inferior blood pressure control and higher rates of hypertension-associated morbidities compared with the general population.64,65
An enormous body of evidence has established hyperlipidaemia as a key contributor to cardiovascular events and mortality, and the 3-hydroxy-3-methyl-glutaryl co-enzyme A reductase inhibitors (also known as statins) are effective at lowering both of these outcomes.66 Hyperlipidaemia, sometimes related to ART, is more prevalent in people living with HIV (affecting more than 60%) than in the general population and is an important contributor to the excess risk of cardiovascular events and mortality in this population.67,68 The management of hyperlipidaemia in people living with HIV is complicated by the many interactions of statin medications with commonly prescribed ART regimens.69
Our proposal for a feasible harm reduction approach
In this Viewpoint, we focus on lung cancer screening and improved management of hypertension and lipids for two main reasons. First, the evidence base is robust in support of these strategies to reduce morbidity and mortality. Aside from smoking cessation, increased use of LDCT screening in people living with HIV who are smokers is the most realistic route to reducing lung cancer mortality, the leading cause of cancer-related deaths in people living with HIV in the USA.11,70 Control of hypertension and hyperlipidaemia is within the purview of most HIV care providers and can be accomplished with relatively simple interventions because the medications available are very effective and well tolerated. Aside from tobacco use, hypertension and hyperlipidaemia are the only modifiable factors in the American College of Cardiology and American Heart Association (ACA/AHA) Risk Calculator, the most accurate risk assessment instrument for people living with HIV.71 Even a busy HIV care provider can refer a patient for LDCT screening and manage hypertension and hyperlipidaemia, or refer a patient to a primary care provider or for specialty care to reduce cardiovascular risk. Hypertension and lipid management are core tasks done by primary care providers. In our experience, the additional step of referring patients at high risk to subspecialists with expertise in cardiovascular risk reduction results in an intensified approach towards blood pressure and lipid management. The role and value of such consultation or of more comprehensive training of primary HIV care providers in the treatment of hypertension and lipid disorders are worthy topics for future research and policy discussions.
The focus on these is not meant to minimise other important harm reduction strategies that should be addressed in the ongoing comprehensive care of people living with HIV. These strategies would include suppression of HIV-1 viral load below the limits of detection, avoidance of antiretroviral medications that might increase cardiovascular risk, management of obesity, controlling diabetes and prediabetes, increasing physical activity, improving dental health, and addressing alcohol use disorder and other substance use disorders. Any or all of these measures will probably reduce morbidity and mortality among smokers living with HIV.
Practical advice for the clinical management of smokers living with HIV
A generic recommendation to take measures to mitigate cancer and cardiovascular risk in smokers living with HIV is unlikely to affect the day-to-day behaviours of HIV care providers or to benefit their patients. We suggest various steps that are easily implementable in most health-care settings. First, discuss tobacco use with all people living with HIV. Explain to patients that, after controlling the virus, the next most important thing that they can do to improve the duration and quality of their lives is to quit, or at least to cut down on, tobacco use, and to manage their risk for lung cancer mortality, myocardial infarction, and stroke. Strongly encourage smokers living with HIV to quit and offer them assistance in the quitting process (eg, referral to a quitline and pharmacotherapy). For individuals who demur or who are unsuccessful, emphasise that many health benefits can be achieved by cutting down tobacco use. Although not approved for this indication, most of the available tobacco treatment pharmacotherapies have been used to help smokers reduce their cigarette intake.41 Second, familiarise the HIV care providers with current LDCT screening eligibility criteria, and refer eligible patients for LDCT screening. Third, familiarise the HIV care providers with blood pressure and lipid targets that are appropriate for smokers living with HIV, most of whom are probably in a high-risk category, and use pharmacotherapy and dietary intervention aggressively to reach goals (ie, systolic blood pressure <130 mm Hg, LDL cholesterol <70 mg/dL).72,73 Finally, use the online ACA/AHA pooled cohort equation score as a clinical teaching tool with patients.71 Calculate the current cardiovascular risk (ie, risk of a major cardiac event in the next 10 years) together with the patient, and then recalculate the risk to show the salutary effects of quitting smoking, controlling blood pressure, and improving the lipid profile.
Conclusions
In tobacco research, there are far more trials of cessation strategies than trials of harm reduction. However, the current data regarding tobacco treatment for people living with HIV suggest that we have few effective strategies, and that the outlook for smokers living with HIV, in the aggregate, is not optimistic. The treatment options that are available are underused,74 and the medical community must do more to ensure that pharmacotherapy and behavioural cessation therapies reach the population that can benefit from them. Substantial research is ongoing, with the goal of offering more and better choices for cigarette smokers living with HIV who are trying to quit. Although providers and patients should retain lifelong abstinence as the ideal goal, the importance of efforts to reduce smoking and its attendant health risks should not be overlooked or minimised. We suggest that practitioners, patients, researchers, and policy makers should take a more expansive view of tobacco treatment in smokers living with HIV, to encourage reduced cigarette intake in those who are unable or unwilling to quit completely, to increase screening of eligible individuals for lung cancer, and to aggressively control other cardiovascular risk factors, especially hypertension and hyperlipidaemia.
Acknowledgments
This work was supported by the National Institute on Drug Abuse at the National Institutes of Health (grant number 1R01DA036445 to JS and grant number K01DA042687 to KPR), by the National Cancer Institute at the National Institutes of Health (grant number 1R01CA192954 to JS), by the National Heart, Lung, and Blood Institute (grant number K01HL123349 to EPH), and by the Einstein-Rockefeller-CUNY Center for AIDS Research, National Institutes of Health (grant number P30AI124414 to JS). None of these sources were involved in the design, analysis, data interpretation, writing, or decision to publish the completed manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Declaration of interests
NAR has served as a consultant to Achieve Life Sciences regarding an investigational smoking cessation medication (cytisinicline). She is the principal investigator of a multicentre trial of the medication that is sponsored by Achieve Life Sciences, and her institution, the Massachusetts General Hospital (Boston, MA, USA), receives research funding to serve as a site for the trial. NAR also receives royalties from UpToDate for authoring the section on smoking cessation. All other authors declare no competing interests.
Footnotes
For the American College of Cardiology and American Heart Association (ACA/AHA) Risk Calculator see https://reference.medscape.com/calculator/37/acc-aha-cv-risk-calculator-2013?src=ppc_google_rlsa-traf_mscp_ref-hdle-cohort_md_us
For the ACA/AHA cardiovascular risk calculator see https://reference.medscape.com/calculator/37/acc-aha-cv-risk-calculator-2013?src=ppc_google_rlsa-traf_mscp_ref-hdle-cohort_md_us
References
- 1.Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, Abrams DB. Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clin Infect Dis 2000; 31: 808–12. [DOI] [PubMed] [Google Scholar]
- 2.Galai N, Park LP, Wesch J, Visscher B, Riddler S, Margolick JB. Effect of smoking on the clinical progression of HIV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 14: 451–58. [DOI] [PubMed] [Google Scholar]
- 3.Chaisson RE. Smoking cessation in patients with HIV. JAMA 1994; 272: 564. [PubMed] [Google Scholar]
- 4.Hawk M, Coulter RWS, Egan JE, et al. Harm reduction principles for healthcare settings. Harm Reduct J 2017; 14: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015; 162: 335–44. [DOI] [PubMed] [Google Scholar]
- 6.Asfar T, Perez A, Shipman P, et al. National estimates of prevalence, time-trend, and correlates of smoking in US people living with HIV (NHANES 1999–2016). Nicotine Tob Res 2021; published onlineApril15. 10.1093/ntr/ntaa277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States: current smoking among adults in 2019. 2019https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm (accessed Jan 5, 2021).
- 8.Stanton CA, Kumar PN, Moadel AB, et al. A multicenter randomized controlled trial of intensive group therapy for tobacco treatment in HIV-infected cigarette smokers. J Acquir Immune Defic Syndr 2020; 83: 405–14. [DOI] [PubMed] [Google Scholar]
- 9.Tamí-Maury I, Vidrine DJ, Fletcher FE, Danysh H, Arduino R, Gritz ER. Poly-tobacco use among HIV-positive smokers: implications for smoking cessation efforts. Nicotine Tob Res 2013; 15: 2100–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossouw TM, Anderson R, Feldman C. Impact of HIV infection and smoking on lung immunity and related disorders. Eur Respir J 2015; 46: 1781–95. [DOI] [PubMed] [Google Scholar]
- 11.Reddy KP, Kong CY, Hyle EP, et al. Lung cancer mortality associated with smoking and smoking cessation among people living with HIV in the United States. JAMA Intern Med 2017; 177: 1613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation 2019; 140: e98–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris A, George MP, Crothers K, et al. HIV and chronic obstructive pulmonary disease: is it worse and why? Proc Am Thorac Soc 2011; 8: 320–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helleberg M, May MT, Ingle SM, et al. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS 2015; 29: 221–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy KP, Parker RA, Losina E, et al. Impact of cigarette smoking and smoking cessation on life expectancy among people with HIV: a US-based modeling study. J Infect Dis 2016; 214: 1672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcus JL, Chao CR, Leyden WA, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016; 73: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ale BM, Amahowe F, Nganda MM, et al. Global burden of active smoking among people living with HIV on antiretroviral therapy: a systematic review and meta-analysis. Infect Dis Poverty 2021; 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mdege ND, Shah S, Ayo-Yusuf OA, Hakim J, Siddiqi K. Tobacco use among people living with HIV: analysis of data from Demographic and Health Surveys from 28 low-income and middle-income countries. Lancet Glob Health 2017; 5: e578–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandra D, Gupta A, Fitzpatrick M, et al. Lung function, coronary artery disease, and mortality in HIV. Ann Am Thorac Soc 2019; 16: 687–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert AA, Crothers K. Abnormal lung function in HIV-infected adults: an under-recognized risk factor for early mortality. Ann Am Thorac Soc 2018; 15: 160–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smilowitz NR, Gupta N, Guo Y, Coppola JT, Bangalore S. Influence of human immunodeficiency virus seropositive status on the in-hospital management and outcomes of patients presenting with acute myocardial infarction. J Invasive Cardiol 2016; 28: 403–09. [PubMed] [Google Scholar]
- 22.Reynolds NR, Neidig JL, Wewers ME. Illness representation and smoking behavior: a focus group study of HIV-positive men. J Assoc Nurses AIDS Care 2004; 15: 37–47. [DOI] [PubMed] [Google Scholar]
- 23.Stanton CA, Kim RS, Chander G, Shuter J. Multicenter RCT of a web-based cessation program plus online social network for HIV+ smokers. Society for Research on Nicotine and Tobacco 2021 annual meeting; online; Feb 24–27, 2021 (abstr POD41–3). [Google Scholar]
- 24.Mercié P, Arsandaux J, Katlama C, et al. Efficacy and safety of varenicline for smoking cessation in people living with HIV in France (ANRS 144 Inter-ACTIV): a randomised controlled phase 3 clinical trial. Lancet HIV 2018; 5: e126–35. [DOI] [PubMed] [Google Scholar]
- 25.Shuter J, Kim RS, Durant S, Stanton CA. Brief report: long-term follow-up of smokers living with HIV after an intensive behavioral tobacco treatment intervention. J Acquir Immune Defic Syndr 2020; 84: 208–12. [DOI] [PubMed] [Google Scholar]
- 26.Pool ER, Dogar O, Lindsay RP, Weatherburn P, Siddiqi K. Interventions for tobacco use cessation in people living with HIV and AIDS. 2016. https://core.ac.uk/reader/77600011?utm_source=linkout (accessed Aug 9, 2021). [DOI] [PMC free article] [PubMed]
- 27.Thompson MA, Horberg MA, Agwu AL, et al. Primary care guidance for persons with human immunodeficiency virus: 2020 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2020; published onlineNovember6. 10.1093/cid/ciaa1391. [DOI] [PubMed] [Google Scholar]
- 28.Drummond C, Edwards G, Glanz A, et al. Rethinking drug policies in the context of the acquired immunodeficiency syndrome. Bull Narc 1987; 39: 29–35. [PubMed] [Google Scholar]
- 29.Sorge R Drug policy in the age of AIDS: the philosophy of ‘harm reduction’. Health PAC Bull 1990; 20: 4–10. [PubMed] [Google Scholar]
- 30.Ashcroft RE, Langley T. Ethics and harm reduction approaches in tobacco control. Nicotine Tob Res 2021; 23: 1–2. [DOI] [PubMed] [Google Scholar]
- 31.Ivsins A, Pauly B, Brown M, et al. On the outside looking in: finding a place for managed alcohol programs in the harm reduction movement. Int J Drug Policy 2019; 67: 58–62. [DOI] [PubMed] [Google Scholar]
- 32.Parsons JT, Schrimshaw EW, Wolitski RJ, et al. Sexual harm reduction practices of HIV-seropositive gay and bisexual men: serosorting, strategic positioning, and withdrawal before ejaculation. AIDS 2005; 19 (suppl 1): S13–25. [DOI] [PubMed] [Google Scholar]
- 33.Gilchrist G, Swan D, Widyaratna K, et al. A systematic review and meta-analysis of psychosocial interventions to reduce drug and sexual blood borne virus risk behaviours among people who inject drugs. AIDS Behav 2017; 21: 1791–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hackshaw A, Morris JK, Boniface S, Tang JL, Milenković D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ 2018; 360: j5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue-Choi M, Christensen CH, Rostron BL, et al. Dose-response association of low-intensity and nondaily smoking with mortality in the United States. JAMA Netw Open 2020; 3: e206436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med 2013; 368: 351–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banks E, Joshy G, Korda RJ, et al. Tobacco smoking and risk of 36 cardiovascular disease subtypes: fatal and non-fatal outcomes in a large prospective Australian study. BMC Med 2019; 17: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lubin JH, Couper D, Lutsey PL, Woodward M, Yatsuya H, Huxley RR. Risk of cardiovascular disease from cumulative cigarette use and the impact of smoking intensity. Epidemiology 2016; 27: 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindson N, Klemperer E, Hong B, Ordóñez-Mena JM, Aveyard P. Smoking reduction interventions for smoking cessation. Cochrane Database Syst Rev 2019; 9: CD013183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Begh R, Lindson-Hawley N, Aveyard P. Does reduced smoking if you can’t stop make any difference? BMC Med 2015; 13: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindson-Hawley N, Hartmann-Boyce J, Fanshawe TR, Begh R, Farley A, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst Rev 2016; 10: CD005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Restrepo BJ. Nicotine intake per cigarette smoked among smokers nationally and in New York City. Am J Prev Med 2017; 53: e77–78. [DOI] [PubMed] [Google Scholar]
- 43.Blank MD, Breland AB, Enlow PT, Duncan C, Metzger A, Cobb CO. Measurement of smoking behavior: comparison of self-reports, returned cigarette butts, and toxicant levels. Exp Clin Psychopharmacol 2016; 24: 348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas R, Parker LS, Shiffman S. The ethics of tobacco harm reduction: an analysis of e-cigarette availability from the perspectives of utilitarianism, bioethics, and public health ethics. Nicotine Tob Res 2021; 23: 3–8. [DOI] [PubMed] [Google Scholar]
- 45.Cioe PA, Mercurio AN, Lechner W, et al. A pilot study to examine the acceptability and health effects of electronic cigarettes in HIV-positive smokers. Drug Alcohol Depend 2020; 206: 107678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cobb CO, Foulds J, Yen MS, et al. Effect of an electronic nicotine delivery system with 0, 8, or 36 mg/mL liquid nicotine versus a cigarette substitute on tobacco-related toxicant exposure: a four-arm, parallel-group, randomised, controlled trial. Lancet Respir Med 2021; 9: 840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fisher MT, Tan-Torres SM, Gaworski CL, Black RA, Sarkar MA. Smokeless tobacco mortality risks: an analysis of two contemporary nationally representative longitudinal mortality studies. Harm Reduct J 2019; 16: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gleber-Netto FO, Zhao M, Trivedi S, et al. Distinct pattern of TP53 mutations in human immunodeficiency virus-related head and neck squamous cell carcinoma. Cancer 2018; 124: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365: 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffman RM, Atallah RP, Struble RD, Badgett RG. Lung cancer screening with low-dose CT: a meta-analysis. J Gen Intern Med 2020; 35: 3015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shcherba M, Shuter J, Haigentz M Jr. Current questions in HIV-associated lung cancer. Curr Opin Oncol 2013; 25: 511–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiels MS, Cole SR, Mehta SH, Kirk GD. Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. J Acquir Immune Defic Syndr 2010; 55: 510–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winstone TA, Man SFP, Hull M, Montaner JS, Sin DD. Epidemic of lung cancer in patients with HIV infection. Chest 2013; 143: 305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kong CY, Sigel K, Criss SD, et al. Benefits and harms of lung cancer screening in HIV-infected individuals with CD4+ cell count at least 500 cells/μL. AIDS 2018; 32: 1333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA 2021; 325: 962–70. [DOI] [PubMed] [Google Scholar]
- 56.Makinson A, Eymard-Duvernay S, Raffi F, et al. Feasibility and efficacy of early lung cancer diagnosis with chest computed tomography in HIV-infected smokers. AIDS 2016; 30: 573–82. [DOI] [PubMed] [Google Scholar]
- 57.Galeas JNGR, Serrano M, Shmukler A, et al. Improving lung cancer screening in the HIV population. Proc Am Soc Clin Oncol 2019; 37: 69(abstr). [Google Scholar]
- 58.Ostroff JS, Buckshee N, Mancuso CA, Yankelevitz DF, Henschke CI. Smoking cessation following CT screening for early detection of lung cancer. Prev Med 2001; 33: 613–21. [DOI] [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention. Smoking & tobacco use: health effects of cigarette smoking. 2020. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/index.htm (accessed Jan 5, 2021).
- 60.Centers for Disease Control and Prevention. Smoking & tobacco use: fast facts. 2021. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm#:~:text=Smoking%20is%20the%20leading%20cause,7%20million%20deaths%20per%20year.&text=If%20the%20pattern%20of%20smoking,to%20tobacco%20use%20by%202030 (accessed Jan 5, 2021).
- 61.Frieden TR. Shattuck lecture: the future of public health. N Engl J Med 2015; 373: 1748–54. [DOI] [PubMed] [Google Scholar]
- 62.Farley TA, Dalal MA, Mostashari F, Frieden TR. Deaths preventable in the U.S. by improvements in use of clinical preventive services. Am J Prev Med 2010; 38: 600–09. [DOI] [PubMed] [Google Scholar]
- 63.Olaiya O, Weiser J, Zhou W, Patel P, Bradley H. Hypertension among persons living with HIV in medical care in the United States—Medical Monitoring Project, 2013–2014. Open Forum Infect Dis 2018; 5: ofy028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nüesch R, Wang Q, Elzi L, et al. Risk of cardiovascular events and blood pressure control in hypertensive HIV-infected patients: Swiss HIV Cohort Study (SHCS). J Acquir Immune Defic Syndr 2013; 62: 396–404. [DOI] [PubMed] [Google Scholar]
- 65.Armah KA, Chang CC, Baker JV, et al. Prehypertension, hypertension, and the risk of acute myocardial infarction in HIV-infected and -uninfected veterans. Clin Infect Dis 2014; 58: 121–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 388: 2532–36. [DOI] [PubMed] [Google Scholar]
- 67.Samaras K The burden of diabetes and hyperlipidemia in treated HIV infection and approaches for cardiometabolic care. Curr HIV/AIDS Rep 2012; 9: 206–17. [DOI] [PubMed] [Google Scholar]
- 68.Serrão R, Piñero C, Velez J, et al. Non-AIDS-related comorbidities in people living with HIV-1 aged 50 years and older: The AGING POSITIVE study. Int J Infect Dis 2019; 79: 94–100. [DOI] [PubMed] [Google Scholar]
- 69.Chastain DB, Stover KR, Riche DM. Evidence-based review of statin use in patients with HIV on antiretroviral therapy. J Clin Transl Endocrinol 2017; 8: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Engels EA, Yanik EL, Wheeler W, et al. Cancer-attributable mortality among people with treated human immunodeficiency virus infection in North America. Clin Infect Dis 2017; 65: 636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feinstein MJ, Nance RM, Drozd DR, et al. Assessing and refining myocardial infarction risk estimation among patients with human immunodeficiency virus: a study by the Centers for AIDS Research Network of Integrated Clinical Systems. JAMA Cardiol 2017; 2: 155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2019; 139: e1046–81. [DOI] [PubMed] [Google Scholar]
- 73.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension 2018; 71: e13–115. [DOI] [PubMed] [Google Scholar]
- 74.Shuter J, Salmo LN, Shuter AD, Nivasch EC, Fazzari M, Moadel AB. Provider beliefs and practices relating to tobacco use in patients living with HIV/AIDS: a national survey. AIDS Behav 2012; 16: 288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


