Abstract
In the striatum, two main types of GABAergic medium spiny neurons (MSNs), denoted striatonigral (or direct-pathway MSNs, dMSNs) and striatopallidal neurons (indirect-pathway MSNs, iMSNs), form circuits with distinct pallidal nuclei, which sends “GO” or “NO-GO” signals through the thalamus. These striatopallidal circuits evaluate and execute reward-seeking and taking behaviors. Especially, the dorsal striatum can be further divided into the dorsomedial striatum (DMS, equivalent to caudate in primates and humans) and dorsolateral striatum (DLS, equivalent to putamen), which orchestrates goal-directed and habitual reward-seeking and taking behaviors, respectively. Using optogenetics, chemogenetics and in vivo calcium imaging technologies combined with electrophysiology and digitalized behavior phenotyping, recent studies have revealed cell-, circuit- and context-specific functions of these microcircuits in addictive behaviors. Also, region-specific astrocytes regulate the homeostatic activities of the dMSNs and iMSNs as well as the downstream circuits, which determine the net balance of cortico-striato-pallidal activities to the thalamic neurons. This review will summarize the recent progress of striatopallidal circuits focusing on astrocyte-neuron interaction and, reward- and alcohol-seeking behaviors. Our review will also discuss the translational and clinical implications of these microcircuit studies.
Keywords: striatum, globus pallidus, goal-directed, habits, alcohol use disorder, addiction
1. Introduction
Uncontrolled compulsive reward-seeking despite negative consequences is a hallmark of addiction (Luscher et al., 2020). What role do conscious thoughts or actions play in abstaining from substance (like alcohol, cocaine, nicotine, or heroin) or non-substance (like gambling or gaming) rewards? The weakened executive control over compulsive reward-seeking or consumption is often attributed to irrational or uncontrollable behaviors, including addiction. Addicted humans and animals may exhibit the addictive behavioral modes without evaluating the proper or healthy cost/benefit analysis, which results in uncontrolled desire to procure the reward despite the negative consequences such as damaged relationships, financial and legal challenges, and addiction-related illnesses.
Among several plausible explanations of addiction theories, in this review, we will focus on how controllable goal-directed reward-seeking behavior shifts to uncontrollable (or less controllable) habitual seeking or taking behavior. Upon the loss of control in drug-seeking or -taking, repeated withdrawal and craving cycles will eventually lead to compulsive drug-seeking behavior (Koob, 2020; Koob and Volkow, 2010). Of note, it is a matter of imbalance between goal-directed and habitual reward-seeking behaviors, rather than complete switching between two behavioral patterns (Graybiel, 2008; Yin and Knowlton, 2006). Likewise, imbalance of the brain regions and circuits regulating these two behaviors may result in addiction including alcohol use disorder (AUD) (O’Tousa and Grahame, 2014).
This review will concentrate on brain circuits and microcircuits between the dorsal striatum and globus pallidus, although the ventral striatum is also a critical neural substrate for the value-based behavior, including goal-directed behavior (Keiflin and Janak, 2015; Mannella et al., 2013). Two distinct neurons denoted as direct-pathway medium spiny neurons (dMSNs) and indirect-pathway medium spiny neurons (iMSNs) interact with several different types of pallidal neurons directly or through the subthalamic nucleus (STN). Thus, these striatopallidal circuits will modify the cortical inputs by integrating many other brain regions, including ventral tegmental (VTA), substantial nigra pars compacta (SNc), amygdala, and hippocampus.
Interestingly, astrocytes are known to regulate striatal MSNs’ activities in the dorsal striatum and alter animal behaviors (Yu et al., 2018). Moreover, astrocyte activation changes behaviors through astrocyte-neuron interactions (Bull et al., 2014; Chen et al., 2016; Reissner and Pletnikov, 2020). Thus, we will provide updated information on how astrocyte-neuron interaction in the striatopallidal circuits contributes to goal-directed and habitual reward-seeking behaviors.
2. Striatal MSNs-GP circuit and its implication in goal-directed and habitual behaviors
Although no anatomical marker exists in rodents, the dorsal striatum (DS) has two functionally recognizable subregions. The dorsomedial striatum (DMS, equivalent to caudate in humans) is mainly known to regulate goal-directed reward-seeking behaviors, while the dorsolateral striatum (DLS, equivalent to putamen in humans) is primarily involved in movement and habitual behaviors (Graybiel, 2008; Voorn et al., 2004; Yin and Knowlton, 2006). These two dorsal striatum areas, DMS and DLS, are part of cortico-striato-pallidal circuits (Fig. 1a and b). In rodents, pyramidal glutamatergic neurons in layer 5 of the prefrontal cortex project to the striatum, where ionotropic and metabotropic glutamate receptors mediate the excitatory function of glutamate signaling in the striatal neurons. Since most striatal neurons (> 90%) are GABAergic, upon excitation, the axon terminals of striatal neurons release GABA to the output neurons, including pallidal areas. Upon activation of striatal neurons, the inhibited pallidal neurons disinhibit the thalamic neurons to drive the excitatory glutamatergic projections to the cortical areas, which completes a cortico-striato-pallido-thalamo-cortical loop (Fig. 1a).
Fig. 1.
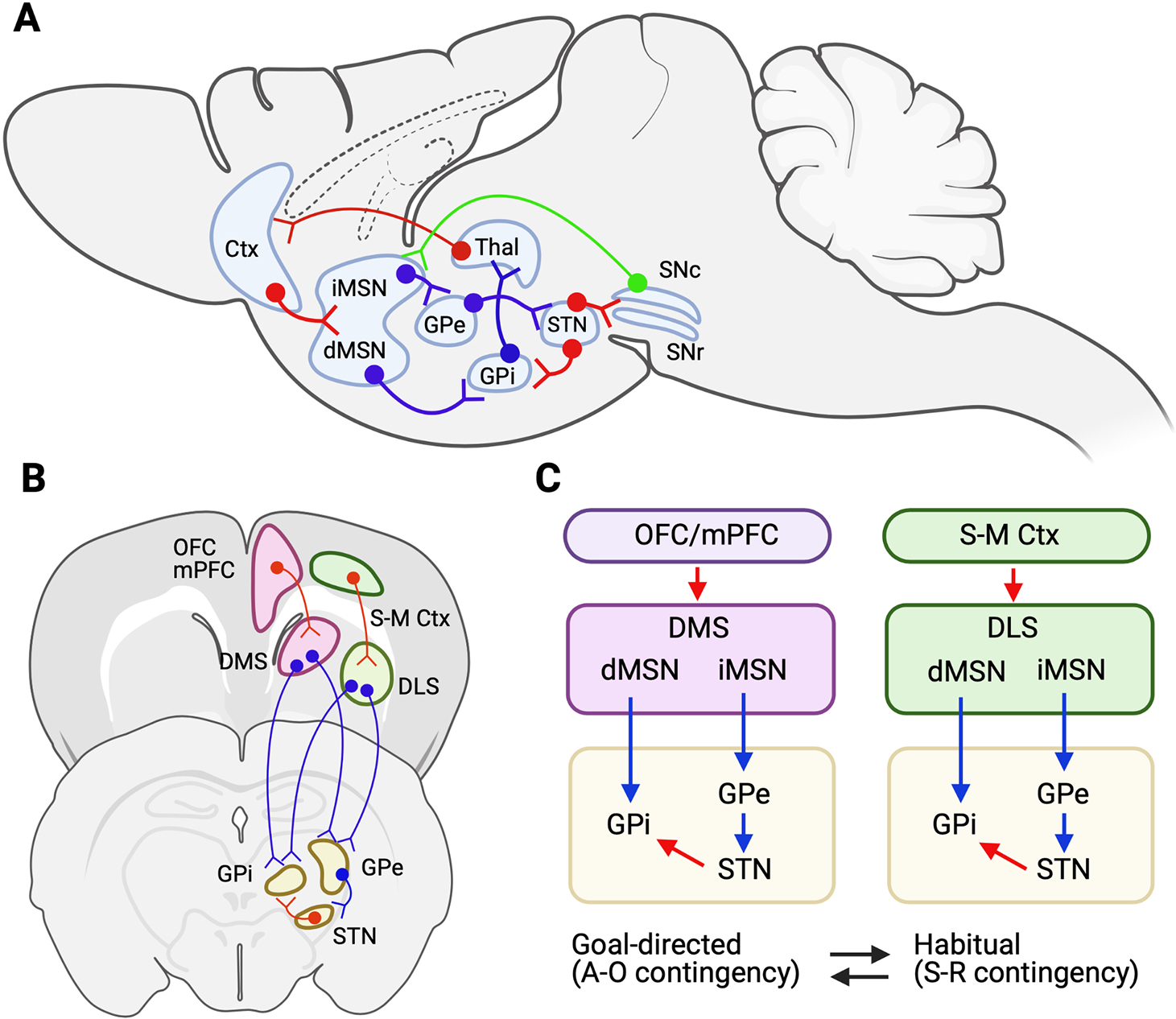
Schematic illustration of cortico-basal ganglia (striatopallidal)-thalamic circuit in mice. (A) Sagittal view of the circuits. (B) Coronal view of the circuits. (C) Illustration of the cortico-striato-pallidal circuits for goal-directed and habitual reward- or substance-seeking behaviors. OFC, orbitofrontal cortex; mPFC, medial prefrontal cortex; S-MS Ctx, sensorimotor cortex; Chol, cholinergic neurons; dMSN, direct medium spiny neurons; iMSN, indirect medium spiny neurons; GPe, external part of globus pallidus; GPi, internal part of GP; STN, subthalamic nucleus; SNc, substantia nigra pars compacta, SNr, substantia nigra pars reticulata. Red arrow, glutamatergic neurons; Blue arrow, GABAergic neurons; Green arrow, dopaminergic neurons.
What determines goal-directed and habitual behaviors? The sensitivity to outcome devaluation and A-O contingency (a.k.a., A-O association) are the most commonly used behavioral measurement for goal-directed and habitual behaviors (Yin and Knowlton, 2006). Early studies suggested a functional dichotomous dissociation between DMS and DLS. While the DMS is involved in flexible goal-directed learning, the DLS is responsible for inflexible habitual response learning (Devan et al., 1999; Devan and White, 1999). Through DMS lesioning experiments (particularly the posterior DMS), the DMS’s inactivation abolished sensitivity to devaluation, which contributes to the A-O contingency degradation (Yin et al., 2005). Other studies validated the role of the DMS in the acquisition and expression of goal-directed behaviors (Ragozzino, 2003; Ragozzino et al., 2002; Yin and Knowlton, 2004). In humans, the DMS homolog region, the caudate, has a similar role in A-O contingency and goal-directed behaviors (Delgado et al., 2004; Tricomi et al., 2004; Zink et al., 2004). In contrast, DLS (or putamen) lesions impede the transition from goal-directed behavior to habit, and thereby goal-directed behavior remains through high A-O contingency (Yin et al., 2004, 2006). Additional preclinical and clinical studies further confirmed that neural connectivity from ventral to dorsal, and from caudate to putamen (DMS to DLS) transitions are correlated to severity or risk of addictive behaviors (Burton et al., 2015; Ersche et al., 2020; Everitt and Robbins, 2005).
Both the DMS and DLS containing two morphologically indistinguishable striatal MSNs, dMSNs, and iMSNs are known to project mainly to the internal part of GP (GPi) and the external part of GP (GPe), respectively. As illustrated in Fig. 1c, a majority of GPe neurons are GABAergic projections to the STN, then glutamatergic STN neurons activate the GPi, which balances the circuits between iMSNs and dMSNs in both the DMS and DLS to ultimately regulate movement and motivational behaviors. Since the DMS is receiving most cortical inputs from the medial prefrontal cortex (mPFC), including the anterior cingulate cortex (ACC), prelimbic (PL) and infralimbic (IL) regions, and orbitofrontal cortex (OFC) (de Kloet et al., 2021; Hunnicutt et al., 2016; Joel and Weiner, 2000; Lu et al., 2021), DMS → GP circuits regulate goal-directed behavior or A-O contingency, which requires the instrumental acquisition and learning to maximize the chance to acquire a reward. In contrast, the DLS forms a circuit with the sensorimotor cortex (SMC). Thus, it is known that DLS → GP circuits govern habitual behavior with stimulus-response (S-R) contingency. From the reward-seeking perspective, it is an effective brain system to shift from an energy-consuming A-O to an energy-saving S-R system in similar environments. Simply, repetition and over-training in the same context is a critical factor for habit formation (Packard, 1999; Packard and Knowlton, 2002).
Principally, animals have common patterns in procuring rewards depending on the types of conditioning they experience (a.k.a., experienced contingency). Two notable schedules are employed to shape goal-directed and habitual behaviors (Fig. 2a and b). In ratio schedule (often called random ratio, or RR), a proper and effortful response (such as a lever-press or a nose-poke; action) is associated with reward outcome with a certain probability (various probability in the case of RR). If the outcome value is high (or more pleasurable), its reinforcing effects promote a higher action rate. Thus, once the animal has sufficient motivation, the animal will establish optimal behavioral policy along with updating the probability of reward acquisition based on the action demand (the number of lever-press or nose-poke) for each reward outcome. Upon the outcome devaluation via free and unlimited access to the reward before operant tasks (mostly 1 h in animal’s home cage), the animal reduces its action rate compared to the animal in the valued state (even though the animal is still motivated to obtain the reward), showing flexible action selection. In an interval schedule (often called random interval, or RI), a response is only rewarded in a given time with a range of variable lap time regardless of response rates. The interval of uncertainty for acquiring reward upon a response is known to generate S-R habits (Derusso et al., 2010), meaning that action is not an essential factor for determining reward delivery. In contrast to RR schedules, the animal trained through RI schedules exhibits a lack of behavioral flexibility and a similar response between valued and devalued states (Fig. 2c and d).
Fig. 2.
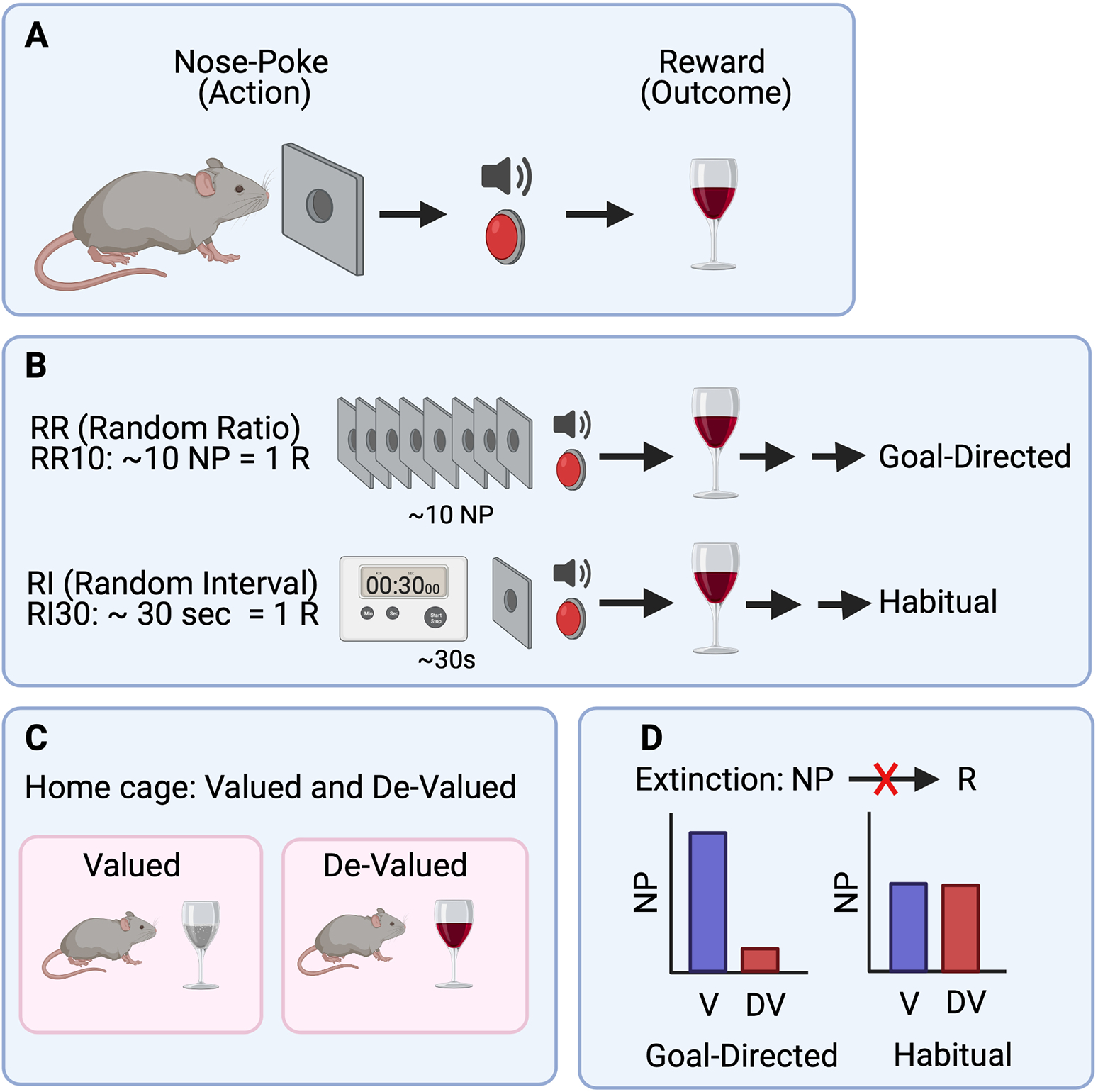
Operant conditioning schedules for goal-directed and habitual reward-seeking behaviors. (A) Mice are trained in an operant chamber to learn an association between nose-poke (NP) and reward-delivery in the magazine. Tone and sound are reinforcing this association during the fixed ratio (FR) schedule. Through the repeated FR1 (one nose-poke = one reward), mice learn that nose-poking (action) results in reward (outcome). (B) After the FR1 training, when mice are given a random ratio schedule (RR), mice learn a policy-related probability of getting the reward with a trial and error process. In the case of RR, mice have to nose-poke random numbers to get a reward in a variable requirement. Since mice need to keep updating the policy through different RR schedules (e.g., RR5, RR10, etc), this schedule is similar to model-based (MB) reinforcement learning (RL). By contrast, after the FR1 training, when mice are given random interval (RI) schedule, mice learn that the reward is delivered in a random time interval independent of nose-poke. Mice learn a dissociation between nose-poke (action) and reward (outcome). Through repeated exposure to a different RI schedule (e.g., RI 30, RI60), mice learn that a regular nose-poke is the best policy to maximally obtain the reward, equivalent to model-free (MF) RL. (C) To test whether mice exhibit goal-directed or habitual behavior after experiencing the RR and RI schedules, mice are transferred to a home cage and given excessive amounts of reward or water (or food chow, nothing). After experiencing effortless reward, mice devalued the reward and lost motivation to nose-poke (action) while non-rewarded mice in the home cage are still eager to nose-poke in an operant chamber. (D) Thus, right after reward devaluation in the home cage, when mice are exposed to extinction in an operant chamber, mice trained in RR inhibit nose-poking (action). However, mice are trained in RI to keep nose-poking due to a strong S-R association (contingency) or weak A-O association (contingency).
3. Goal-directed and habitual reward-seeking to compulsive addictive behaviors
Addiction has been viewed as maladaptive and habitual reward-seeking behavior (Everitt and Robbins, 2016). Repeated drug use shifts from positive reinforcement-based drug-taking behaviors to negative reinforcement-driven drug-taking, which generates a vicious cycle of relapse (Koob, 2020; Koob and Volkow, 2010). The negative reinforcement stage values alleviation of negative affects through drug use rather than drug itself for previous pleasure seeking, and thereby it drives compulsive and habitual-seeking behaviors (Gillan et al., 2014; Voon et al., 2015). This stage seems to overlap with the extreme S-R contingency regarding the deficit of behavioral inhibition or uncontrolled drug-seeking/taking (Luscher et al., 2020). As compulsive drug-taking is defined as “uncontrolled use despite negative consequences” (Luscher et al., 2020), habitual drug-seeking (less controllable and higher S-R contingency) is an intermediate state between goal-directed (controllable and higher A-O contingency) and compulsive drug-seeking/taking behaviors (Fig. 1 and 2). What can be utilized as negative consequences in animal studies? For alcohol, resilient drinking of quinine adulteration is commonly used (Lesscher and Vanderschuren, 2012; Vengeliene et al., 2009). Collectively, the persistent drug-seeking/taking behaviors against harmful punishment merge into the generalized two other criteria of addiction-like behavior in animal studies, consisting of deficit of behavioral inhibition, high motivation for the drug which can be measured by progressive ratio schedule (Deroche-Gamonet et al., 2004; Luscher et al., 2020). Interestingly, enhanced orbitofrontal cortex (OFC)-dorsal striatum activities (especially DMS) are likely responsible for compulsive drug-taking for stimulants (Hu et al., 2019). Since the OFC is known to regulate goal-directed behavior and value-guided decisions (Hirokawa et al., 2019), compulsive drug-taking can be viewed as excessive goal-directed drug choice under the negative effects (Hogarth, 2020). Context-dependent circuit studies will precisely illustrate the nature of addiction and AUD (Vandaele and Ahmed, 2021).
4. Distinguishable expression and roles of dopamine and adenosine receptors in the Striatal MSNs and AUD
Using bacterial artificial chromosome (BAC) transgenic mice expressing enhanced green fluorescent protein (EGFP), the gene expression profile of the two distinct striatal neurons (dMSNs and iMSNs) are now well-characterized (Gong et al., 2003; Surmeier et al., 2007). Many serial studies revealed that dMSNs express dopamine D1 receptors (D1R) or adenosine A1 receptors (A1R) while iMSNs contain dopamine D2 receptors (D2R) or adenosine A2A receptors (A2AR), which comprise approximately 90~95% of striatal neurons (Humphries and Prescott, 2010; Lobo et al., 2006) (Fig. 3). Interestingly, D1R are coupled to mainly stimulatory G-protein (Gs) and subsequently increase cAMP through adenylyl cyclase when activated, whereas A1R is coupled to inhibitory G-protein (Gi) in the dMSNs and has an opposite effect on the downstream signaling cascade. Furthermore, D1R and A1R are co-expressed in dMSNs and can form D1R-A1R heterodimers (Ferre et al., 2010; Fuxe et al., 2007; Nam et al., 2013a). Because of the opposite receptor-mediated signaling between D1R and A1R, pharmacological activation of A1R dampens the D1R function (Fuxe et al., 2007). In contrast, A1R antagonists increase D1R-mediated signaling (Fuxe et al., 2007). Similarly, D2R and A2AR which are coupled to Gi and Gs proteins respectively, form a D2R-A2AR heterodimer and have an antagonistic effect on iMSNs (Ferre et al., 2010; Ferre et al., 2008; Ferre et al., 1993). Specifically, A2AR agonists inhibit D2R activation, minimizing Gi signaling and promoting the activation of striatal neurons (Ferre et al., 1993; Stromberg et al., 2000). Conversely, D2R signaling via Gi directly counteracts A2AR-mediated increases in cAMP driven by Gs activation in the striatal cells (Hillion et al., 2002). Interestingly, a recent study demonstrated that low D2R expression leads to compulsive-like ethanol drinking (Bocarsly et al., 2019). Selective loss of D2Rs on iMSNs not only promotes ethanol-induced stimulation via D1 activation but also creates resilience to ethanol sedation. Those mice with low striatal D2R also showed more escalation of ethanol intake in an operant paradigm (Bocarsly et al., 2019). Thus, the precise phase of adenosinergic and dopaminergic signaling may be necessary for the precise response to either transmitter, leading to the changes in downstream signaling events and consequent behaviors (Morita and Kawaguchi, 2018).
Fig. 3.
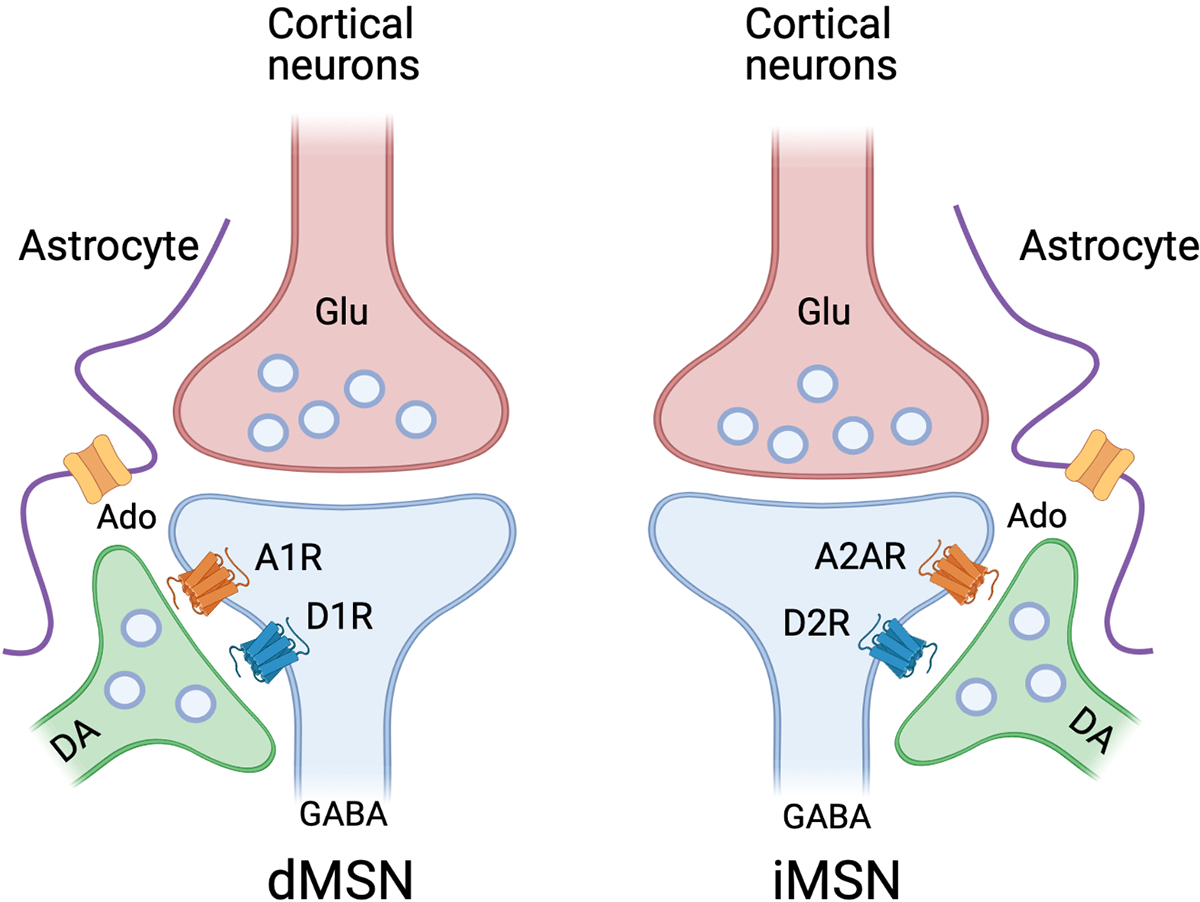
Illustration of cortico-striatal and substantia nigra-striatal circuits in the direct medium spiny neurons (dMSNs) and indirect medium spiny neurons (iMSNs). Astrocyte-neuron interactions play a critical role in fine-tuning the dMSN and iMSN and sorting out the signals. dMSNs express dopamine D1R and adenosine A1R, while iMSNs express mainly dopamine D2 and adenosine A2AR. In the two distinct MSNs, D1R and A1R, or D2R and A2AR interact through direct receptor-receptor interaction and/or downstream signaling pathways.
In a simplified perspective, activation of D1R or A1R expressing GABAergic dMSNs encodes “GO” signaling through dMSNs → GPi circuits while D2R or A2AR expressing GABAergic iMSNs has an inhibitory control (NO GO or STOP) through iMSNs → GPe → STN → GPi (Graybiel, 2008). Having considered the anatomical distinction between DMS and DLS, and the functional difference between dMSNs and iMSNs, the dysregulation or disruption of harmonized signaling of the striatopallidal circuits is critical for drug-seeking/taking behaviors.
In AUD, the DMS and DLS’s distinct roles have been elegantly examined (Corbit et al., 2012). The chemogenetic activation of dMSNs in the DMS mimics the strengthening of glutamatergic neurotransmission and promotes ethanol drinking (Cheng et al., 2017; Wang et al., 2015). Considering the opposite role of dMSNs and iMSNs, inhibition of iMSNs may increase ethanol consumption. Consistently, our previous studies demonstrated that pharmacological inhibition of DMS A2AR increases goal-directed ethanol-seeking behavior and ethanol consumption (Nam et al., 2013b). Recently, we revealed that pharmacological activation of A2AR or optogenetic activation of iMSNs in the DMS decreases ethanol-seeking behaviors (Hong et al., 2019), further validating the opposite role of dMSNs and IMSNs in the DMS. However, the correlation of behavioral transition between goal-directed and habitual ethanol-seeking and circuit changes between DMS and DLS is not fully understood in AUD and other addictions.
5. Astrocyte-neuron interaction in the striatum and AUD
Synaptic transmission is regulated by a “tripartite synapse” consisting of a presynaptic neuron, postsynaptic neuron, and perisynaptic astrocyte (Araque et al., 2014; Araque et al., 1999; Haydon and Carmignoto, 2006) (Fig. 3). Astrocytes play an essential role in energy metabolism, synaptic signaling, neurotransmitter clearance, and ionic homeostasis (Khakh, 2019). Recently, several studies have demonstrated that astrocyte dysregulation is associated with neuropsychiatric disorders including substance use disorder (SUD) (Corkrum et al., 2020; Scofield and Kalivas, 2014) and AUD (Ayers-Ringler et al., 2016; Erickson et al., 2021; Erickson et al., 2018; Lindberg et al., 2018). Interestingly, extrasynaptic neurotransmitters can trigger astrocyte Ca2+ signaling (Charles et al., 1991; Dani et al., 1992), and reciprocally astrocyte Ca2+ signals regulate the function of neural circuits (Smith, 1994), especially through gliotransmitters (Araque et al., 2014; Jiang et al., 2016; Wojtowicz et al., 2013), which illustrates the dynamic astrocyte-neuron interaction. Indeed, astrocytic Ca2+-dependent signaling in the dorsal striatum has been reported to alter the MSNs’ activities and animal behaviors (Yu et al., 2018). Several studies show that the activation of Gq-GPCRs induces calcium influx in astrocytes (Bazargani and Attwell, 2016; Chai et al., 2017; Durkee et al., 2019), thereby facilitating astrocyte-neuron interaction and resulting in significant behavioral changes (Bull et al., 2014; Chen et al., 2016; Reissner and Pletnikov, 2020). Recently, we demonstrated that chemogenetic activation of astrocytes in DMS differentially regulated MSNs’ activities and shifted from habitual to goal-directed reward-seeking behavior, which reveals the potential role of astrocyte-regulated adenosine signaling in reward-(Groman, 2020; Kang and Choi, 2021; Kang et al., 2020) and alcohol-seeking behaviors (Hong et al., 2020). Also, chemogenetic activation of prefrontal cortical neurons increases alcohol-drinking in alcohol-naïve mice, but not in mice pre-exposed to alcohol in an adenosine-dependent manner (Erickson et al., 2021; Moffat and Ron, 2021), validating the importance of astrocyte-neuron interaction and adenosinergic signaling. Despite these findings, it has not been fully answered how astrocytic activation may distinctly regulate neuronal activities in the DMS and DLS, encoding goal-directed and habitual reward/ethanol-seeking behaviors. Given the unique characters of striatal astrocytes such as the paradoxical increase of intracellular Ca2+ through even Gi signaling-driven activation (Nagai et al., 2019), a further detailed investigation is required on how DMS and DLS astrocytes regulate MSN activities.
6. Globus pallidus and astrocytes
As shown in Fig. 1, the main output of MSNs is the internal or external GP (GPi and GPe), mainly composed of GABAergic neurons (Shink and Smith, 1995). Of note, iMSNs are principally projecting to the GPe, while dMSNs form circuits with both the GPi and SNr. In movement control, the GPe is an essential nuclei of inhibitory indirect pathway (Hegeman et al., 2016). However, GPe is also critical for reward prediction (Arkadir et al., 2004), learning (Schroll et al., 2015), and sleep (Qiu et al., 2016).
Approximately two-thirds of striatal inputs of the GPe are from iMSNs and one-third from dMSNs (Kawaguchi et al., 1990). About 80% of inputs to the GPe are inhibitory GABAergic MSNs and 20% of synapses are excitatory inputs from the STN, cerebral cortex, and thalamus (Kita, 2007). Thus, the GPe does not simply project to STN and subsequently innervating the GPi, but also receives glutamatergic inputs from the STN (Kita, 2007). However, the STN → GPe circuit’s role remains unknown although the numbers of synapses of MSNs → GPe are well characterized in the rat brain (Oorschot, 1996). Furthermore, the role of cortico → STN circuit (a.k.a., hyper-direct circuit) has not been fully studied in addictive behaviors (Lovinger and Gremel, 2021; Mathai and Smith, 2011) (Fig. 4).
Fig. 4.
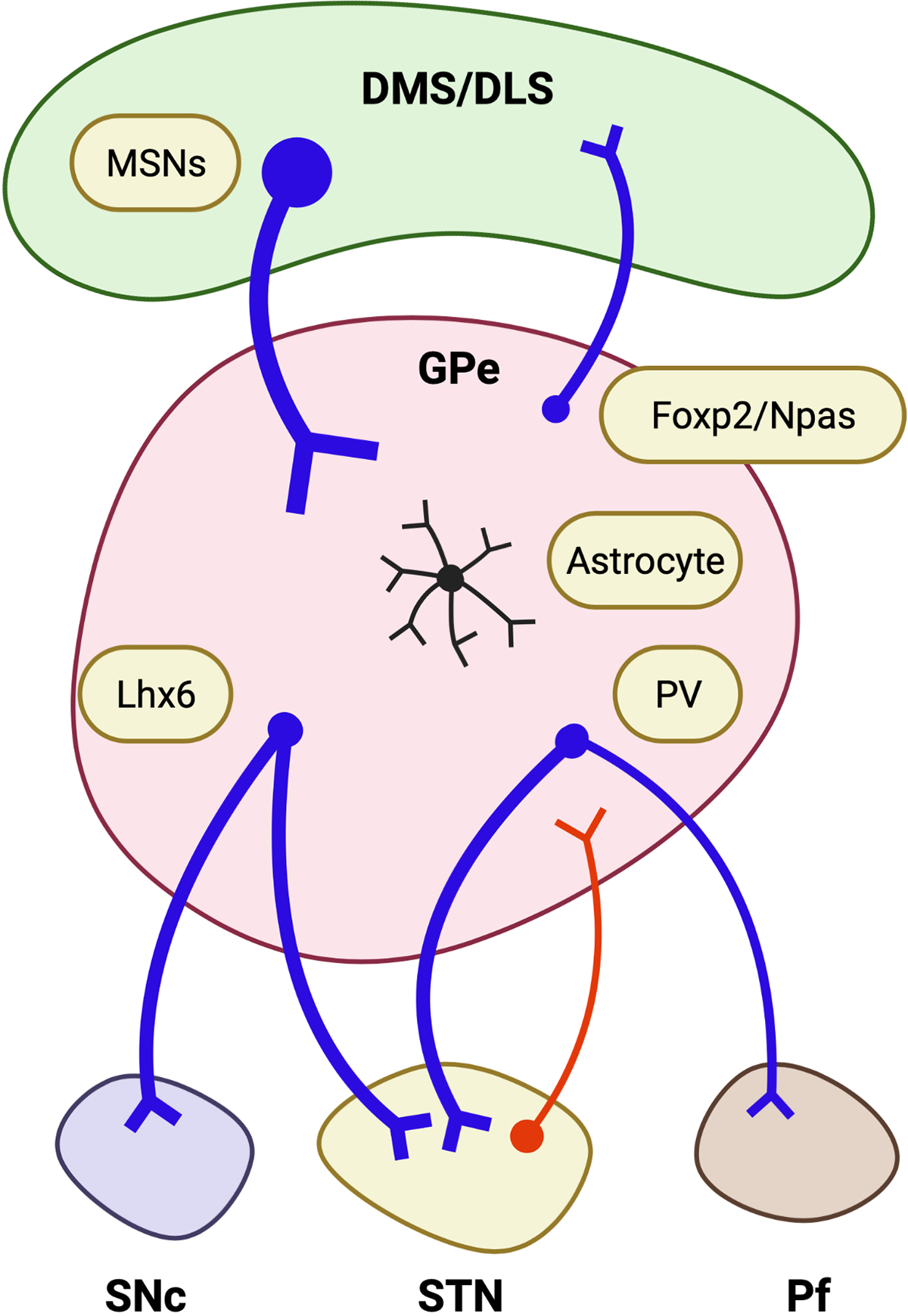
Dorsal striatum to pallidal circuits. Two main dorsal striatal regions, dorsal medial striatum (DMS) and dorsolateral striatum (DLS) project to the external part of globus pallidus (GPe), especially indirect MSNs are mainly projecting to the GPe. GPe containing three main GABAergic neurons. Foxp2 and Npas positive arkypallidal neurons project back to the dorsal striatum. Lhx6 positive prototype neurons evenly project to substantia nigra (SNc) and subthalamic nucleus (STN). PV positive neurons project to the STN and substantia nigra pars reticulata (Pf). STN glutamatergic neurons are projecting to GPe as well. Red arrow, glutamatergic neurons; Blue arrow, GABAergic neurons.
The GPe contains several subtypes of GABAergic neurons categorized by its localization (medial and lateral), unique gene expression (PV, Lhx6, Npas1, or Foxp2), and firing patterns (slow or fast-spiking, or low or high-frequency) (Abrahao and Lovinger, 2018; Hernandez et al., 2015; Mastro et al., 2014). Although multiple inhibitory neuronal subtypes in the GPe are innervated from the dorsal striatum (Hernandez et al., 2015), it is unknown how the individual GPe cell types respond to iMSNs’ activities of the DMS and DLS and how GPe cellular activities change during operant alcohol-seeking behaviors (Lovinger and Gremel, 2021).
Two largely different prototypical GPe cell types are classified as Lhx6-positive slow-firing and PV-positive fast-spiking neurons (Abrahao and Lovinger, 2018). Although overlapping, each subpopulation of GPe GABAergic neurons forms a distinct circuit with different brain regions (Fig. 4). For example, while the major output pathways of the GPe neurons are STN and GPi (Mastro et al., 2014), the Lhx6- and PV-positive GPe neurons distinctly project to SNc and the parafascicular nucleus of the thalamus (Pf), respectively (Mastro et al., 2014). Also, slow-firing arkypallidal neurons in the GPe are known to express Foxp2 (Abrahao and Lovinger, 2018) or Npas (Glajch et al., 2016; Hernandez et al., 2015). Noteworthy, slow-firing arkypallidal neurons in the GPe project back to the dorsal striatum (DS) (Glajch et al., 2016; Hernandez et al., 2015). These feedback circuits (GPe → DMS or DLS) potentiate the “NO-GO” signal to the striatum (Mallet et al., 2016). Interestingly, a clinical neuroimaging study suggests that alcohol dampens GPe neuronal activity (Nikolaou et al., 2013). Recently, in mice, alcohol decreases the firing of low-frequency GPe neurons, specifically Lhx6 and Npas expressing neurons, without altering the firing of the high-frequency neurons (Abrahao et al., 2017). Thus, alcohol-induced dampening of the Npas neurons may result in decreasing “NO-GO” or increase “GO” signal at least transiently with low-moderate doses of alcohol (Abrahao et al., 2017). Interestingly, astrocytes are highly enriched in the GPe (Cui et al., 2016; Dervan et al., 2004; Lange et al., 1976; Salvesen et al., 2015). Considering the feedforward and feedback circuits of GPe neurons, the role of GPe astrocytes may be critical for fine regulation of behavioral flexibility. However, further studies are warranted to investigate how two distinct striatal regions (DMS and DLS)-specific circuits to the GPe, synergistically with the GPe astrocytes, affects the GPe neuronal activities, leading to the changes in alcohol-seeking behaviors.
7. Computation modeling of goal-directed and habitual behaviors and striatopallidal circuits in addictive behaviors
Considering the complexity of neural networks and information processing, computational modeling for reward-seeking and addictive behaviors becomes a critical tool (Ferrante et al., 2019; Friston et al., 2017; Redish, 2004; Redish et al., 2016). In both preclinical and clinical studies, DMS-based goal-directed and DLS-habitual circuits are emerging topics in the field of computational neuroscience (Baladron and Hamker, 2020; Keramati et al., 2016). To employ a computational approach, we have to surmise that subjects (humans and animals) are comprised of hierarchic circuits for information processing (Kamali Sarvestani et al., 2011). Especially, it is noteworthy that the “learning” process is a critical component to maximize the chance to procure the reward (desired outcome) in the context of behavioral economics (internal cost-benefit analysis). Thus, in a “reinforcement learning (RL)” model (Dasgupta et al., 2014), the striatopallidal circuits are the subject for computational analysis, especially focusing on the role of dopamine signaling (Dabney et al., 2020; Keiflin and Janak, 2015). However, computational neuroscience approaches should consider beyond dopamine-centered neurobiology since other neurotransmitters contribute to reinforcement learning (Morita and Kawaguchi, 2018).
In an actor-critic model (Fig. 5a and b), goal-directed processes adjust actions based on updated reward prediction to obtain the goal (outcome), which is applicable with the “model-based (MB)” strategy while the habitual processes (learning) use generalized responses with a previously rewarded stimulus without flexible modification of the action, or “model-free (MF)” strategy (Daw et al., 2005; Groman et al., 2019; van Steenbergen et al., 2017) (Fig. 5a). Several recent studies indicate that dorsal striatum-GP circuits are functioning as the actor while ventral striatum-VP circuits estimate the value as the critic in an actor-critic model (Dunovan and Verstynen, 2016; Takahashi et al., 2008) (Fig. 5b). This dichotomic approach has been elegantly used for fMRI-based goal-directed (MB) and habitual (MF) decisions, especially in human studies (Daw et al., 2011; Glascher et al., 2010; Huang et al., 2020; Lee et al., 2014). In addition to the existing MB model, two MF models, motor MF and stimulus MF models have been suggested to distinguish between compulsive and impulsive-related disorders (Deserno and Hauser, 2020). In particular, cell-specific molecular dynamics with high-resolution of temporal neural recording for a state (or an activity)-dependent manner will be useful to understand neural-based goal-directed and habitual behaviors rather than region-specific simplified denotation (e.g., iMSNs = inhibitory GABAergic neurons receiving cortical glutamatergic inputs, or GPe = inhibitory GABAergic system receiving iMSNs GABAergic inputs). Recently, in the OpAL (opponent actor learning) model, the actor has two components D1R/A1R-expressing dMSNs and D2R/A2AR expressing iMSNs (Morita and Kawaguchi, 2018). Several studies showed the differential regulation of dMSNs and iMSNs in reward-seeking (Shin et al., 2018) and action selection (Markowitz et al., 2018). As we described in section 4, in the OpAL model, the antagonistic and synergistic relationship between dopamine and adenosine receptors between dMSNs and iMSNs or between DMS and DLS will provide comprehensive features if proper mathematical and computational modeling can be employed. Furthermore, we revealed that DMS astrocyte activation differentially regulates postsynaptic currents of iMSNs in an adenosine signaling-dependent manner (Kang et al., 2020). Considering the fast responsiveness of astrocytes to various stimulation or environmental changes (Khakh, 2019; Khakh and Deneen, 2019), astrocyte activity will be a critical modifier in the OpAL model (Kang and Choi, 2021). With the advent of innovative neuro-modulation and recording methods, including optogenetics, chemogenetics, fiber-photometry and endo-microscopy, and real-time and digitalized behavioral monitoring systems (Baker et al., 2020; Geuther et al., 2019; Markowitz et al., 2018), additional studies will shed light on the full-scope of reward-seeking/taking behaviors (Mujica-Parodi and Strey, 2020).
Fig. 5.
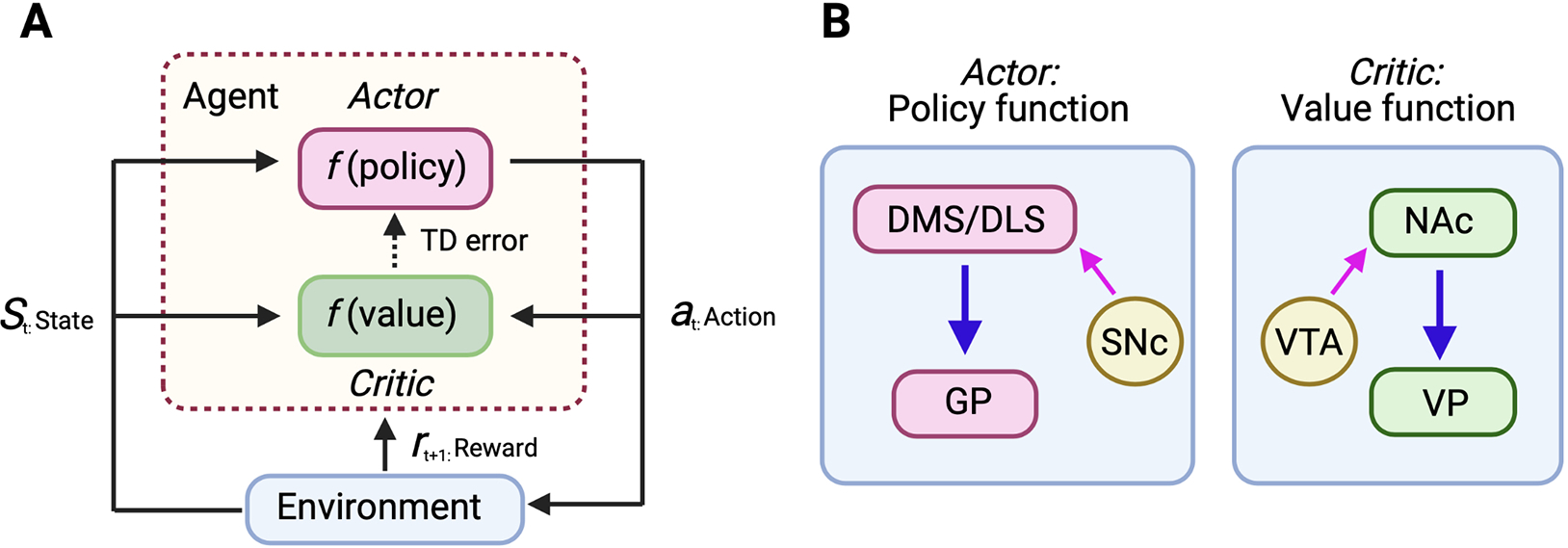
Computational modeling of striatopallidal circuits for addictive behaviors. (A) Actor-critic model of reinforcement learning in computation. The agent selects action based on policy and value information, which will be revised and optimized depending on the environment and state. (B) Dorsal striatum (DMS/DLS)-pallidal circuits with dopaminergic inputs from SNc function as an actor while nucleus accumbal (NAc)-ventral pallidum (VP) circuits with dopaminergic inputs from VTA act like a critic.
8. Discussion and Conclusion
We reviewed the essential role of striatopallidal circuits focusing on two different MSNs in the DMS and DLS on goal-directed and habitual reward (alcohol)-seeking/taking behaviors. Furthermore, astrocytes in this circuit play a critical role in regulating neural activities or possibly a specific circuit. Emerging computation modeling with an activity-dependent neural or astrocyte recording approach will elucidate precise mapping and circuits associated with behaviors (Markowitz and Datta, 2020; Markowitz et al., 2018). With the aid of machine-learning and prediction modeling, additional studies will improve our understanding of context- and cell-specific AUD-related behaviors.
From a neuropsychiatric perspective, AUD is in part attributed to circuitry dysfunction. Restoring the dysfunctional circuits associated with compulsive and severe alcohol-seeking behaviors may be a daunting task (Madeo and Bonci, 2018). However, harnessed with advanced brain activity monitoring and high-resolution brain imaging technologies such as fMRI and PET, several brain stimulation or modulation approaches are now being considered as electroceuticals for reverse-engineering brain dysfunction, including AUD (Diana et al., 2017; Ekhtiari et al., 2019; Famm et al., 2013; Javitt et al., 2020). Besides, human-based reverse translational studies (Venniro et al., 2020) will guide us toward clinically useful research.
Highlights.
Striatopallidal circuits are critical for goal-directed and habitual reward-seeking behaviors
Direct- and indirect-pathway medium spiny neurons regulate reward-seeking behaviors.
Astrocyte-neuron interaction in the dorsal striatum is essential for alcohol use disorder.
Acknowledgments
We thank all the Choi laboratory members for their discussion and proofreading the manuscript. This work was supported by the Samuel C. Johnson for Genomics of Addiction Program at Mayo Clinic, the Ulm Foundation, and National Institute on Alcohol Abuse and Alcoholism (AA018779, AA027486, AG072898 to DSC, AA027773 to SK). All the figures were created with BioRender.com.
Footnotes
Conflict of Interest
DSC is a scientific advisory board member to Peptron Inc. and the Peptron had no role in preparation, review, or approval of the manuscript; nor the decision to submit the manuscript for publication. All the other authors declare no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahao KP, Chancey JH, Chan CS, Lovinger DM, 2017. Ethanol-Sensitive Pacemaker Neurons in the Mouse External Globus Pallidus. Neuropsychopharmacology 42, 1070–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahao KP, Lovinger DM, 2018. Classification of GABAergic neuron subtypes from the globus pallidus using wild-type and transgenic mice. J Physiol 596, 4219–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A, 2014. Gliotransmitters travel in time and space. Neuron 81, 728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG, 1999. Astrocyte-induced modulation of synaptic transmission. Can J Physiol Pharmacol 77, 699–706. [PubMed] [Google Scholar]
- Arkadir D, Morris G, Vaadia E, Bergman H, 2004. Independent coding of movement direction and reward prediction by single pallidal neurons. J Neurosci 24, 10047–10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers-Ringler JR, Jia YF, Qiu YY, Choi DS, 2016. Role of astrocytic glutamate transporter in alcohol use disorder. World J Psychiatry 6, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Hong SI, Kang S, Choi DS, 2020. Rodent models for psychiatric disorders: problems and promises. Lab Anim Res 36, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladron J, Hamker FH, 2020. Habit learning in hierarchical cortex - basal ganglia loops. Eur J Neurosci. [DOI] [PubMed] [Google Scholar]
- Bazargani N, Attwell D, 2016. Astrocyte calcium signaling: the third wave. Nat Neurosci 19, 182–189. [DOI] [PubMed] [Google Scholar]
- Bocarsly ME, da Silva ESD, Kolb V, Luderman KD, Shashikiran S, Rubinstein M, Sibley DR, Dobbs LK, Alvarez VA, 2019. A Mechanism Linking Two Known Vulnerability Factors for Alcohol Abuse: Heightened Alcohol Stimulation and Low Striatal Dopamine D2 Receptors. Cell Rep 29, 1147–1163 e1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C, Freitas KC, Zou S, Poland RS, Syed WA, Urban DJ, Minter SC, Shelton KL, Hauser KF, Negus SS, Knapp PE, Bowers MS, 2014. Rat nucleus accumbens core astrocytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology 39, 2835–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton AC, Nakamura K, Roesch MR, 2015. From ventral-medial to dorsal-lateral striatum: neural correlates of reward-guided decision-making. Neurobiol Learn Mem 117, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS, Vondriska TM, Whitelegge JP, Coppola G, Khakh BS, 2017. Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 95, 531–549 e539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ, 1991. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron 6, 983–992. [DOI] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Kim J, Fu Z, Barak B, Sur M, Feng G, Han W, 2016. Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Huang CCY, Ma T, Wei X, Wang X, Lu J, Wang J, 2017. Distinct Synaptic Strengthening of the Striatal Direct and Indirect Pathways Drives Alcohol Consumption. Biol Psychiatry 81, 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH, 2012. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol Psychiatry 72, 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkrum M, Covelo A, Lines J, Bellocchio L, Pisansky M, Loke K, Quintana R, Rothwell PE, Lujan R, Marsicano G, Martin ED, Thomas MJ, Kofuji P, Araque A, 2020. Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity. Neuron 105, 1036–1047 e1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Pitt JE, Pamukcu A, Poulin JF, Mabrouk OS, Fiske MP, Fan IB, Augustine EC, Young KA, Kennedy RT, Awatramani R, Chan CS, 2016. Blunted mGluR Activation Disinhibits Striatopallidal Transmission in Parkinsonian Mice. Cell Rep 17, 2431–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabney W, Kurth-Nelson Z, Uchida N, Starkweather CK, Hassabis D, Munos R, Botvinick M, 2020. A distributional code for value in dopamine-based reinforcement learning. Nature 577, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JW, Chernjavsky A, Smith SJ, 1992. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron 8, 429–440. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Worgotter F, Manoonpong P, 2014. Neuromodulatory adaptive combination of correlation-based learning in cerebellum and reward-based learning in basal ganglia for goal-directed behavior control. Front Neural Circuits 8, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ, 2011. Model-based influences on humans’ choices and striatal prediction errors. Neuron 69, 1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Niv Y, Dayan P, 2005. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci 8, 1704–1711. [DOI] [PubMed] [Google Scholar]
- de Kloet SF, Bruinsma B, Terra H, Heistek TS, Passchier EMJ, van den Berg AR, Luchicchi A, Min R, Pattij T, Mansvelder HD, 2021. Bi-directional regulation of cognitive control by distinct prefrontal cortical output neurons to thalamus and striatum. Nat Commun 12, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Stenger VA, Fiez JA, 2004. Motivation-dependent responses in the human caudate nucleus. Cereb Cortex 14, 1022–1030. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV, 2004. Evidence for addiction-like behavior in the rat. Science 305, 1014–1017. [DOI] [PubMed] [Google Scholar]
- Derusso AL, Fan D, Gupta J, Shelest O, Costa RM, Yin HH, 2010. Instrumental uncertainty as a determinant of behavior under interval schedules of reinforcement. Front Integr Neurosci 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervan AG, Meshul CK, Beales M, McBean GJ, Moore C, Totterdell S, Snyder AK, Meredith GE, 2004. Astroglial plasticity and glutamate function in a chronic mouse model of Parkinson’s disease. Exp Neurol 190, 145–156. [DOI] [PubMed] [Google Scholar]
- Deserno L, Hauser TU, 2020. Beyond a Cognitive Dichotomy: Can Multiple Decision Systems Prove Useful to Distinguish Compulsive and Impulsive Symptom Dimensions? Biol Psychiatry 88, e49–e51. [DOI] [PubMed] [Google Scholar]
- Devan BD, McDonald RJ, White NM, 1999. Effects of medial and lateral caudate-putamen lesions on place- and cue-guided behaviors in the water maze: relation to thigmotaxis. Behav Brain Res 100, 5–14. [DOI] [PubMed] [Google Scholar]
- Devan BD, White NM, 1999. Parallel information processing in the dorsal striatum: relation to hippocampal function. J Neurosci 19, 2789–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Raij T, Melis M, Nummenmaa A, Leggio L, Bonci A, 2017. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat Rev Neurosci 18, 685–693. [DOI] [PubMed] [Google Scholar]
- Dunovan K, Verstynen T, 2016. Believer-Skeptic Meets Actor-Critic: Rethinking the Role of Basal Ganglia Pathways during Decision-Making and Reinforcement Learning. Front Neurosci 10, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkee CA, Covelo A, Lines J, Kofuji P, Aguilar J, Araque A, 2019. Gi/o protein-coupled receptors inhibit neurons but activate astrocytes and stimulate gliotransmission. Glia 67, 1076–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekhtiari H, Tavakoli H, Addolorato G, Baeken C, Bonci A, Campanella S, Castelo-Branco L, Challet-Bouju G, Clark VP, Claus E, Dannon PN, Del Felice A, den Uyl T, Diana M, di Giannantonio M, Fedota JR, Fitzgerald P, Gallimberti L, Grall-Bronnec M, Herremans SC, Herrmann MJ, Jamil A, Khedr E, Kouimtsidis C, Kozak K, Krupitsky E, Lamm C, Lechner WV, Madeo G, Malmir N, Martinotti G, McDonald WM, Montemitro C, Nakamura-Palacios EM, Nasehi M, Noel X, Nosratabadi M, Paulus M, Pettorruso M, Pradhan B, Praharaj SK, Rafferty H, Sahlem G, Salmeron BJ, Sauvaget A, Schluter RS, Sergiou C, Shahbabaie A, Sheffer C, Spagnolo PA, Steele VR, Yuan TF, van Dongen JDM, Van Waes V, Venkatasubramanian G, Verdejo-Garcia A, Verveer I, Welsh JW, Wesley MJ, Witkiewitz K, Yavari F, Zarrindast MR, Zawertailo L, Zhang X, Cha YH, George TP, Frohlich F, Goudriaan AE, Fecteau S, Daughters SB, Stein EA, Fregni F, Nitsche MA, Zangen A, Bikson M, Hanlon CA, 2019. Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: A consensus paper on the present state of the science and the road ahead. Neurosci Biobehav Rev 104, 118–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson EK, DaCosta AJ, Mason SC, Blednov YA, Mayfield RD, Harris RA, 2021. Cortical astrocytes regulate ethanol consumption and intoxication in mice. Neuropsychopharmacology 46, 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson EK, Farris SP, Blednov YA, Mayfield RD, Harris RA, 2018. Astrocyte-specific transcriptome responses to chronic ethanol consumption. Pharmacogenomics J 18, 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Meng C, Ziauddeen H, Stochl J, Williams GB, Bullmore ET, Robbins TW, 2020. Brain networks underlying vulnerability and resilience to drug addiction. Proc Natl Acad Sci U S A 117, 15253–15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW, 2005. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 8, 1481–1489. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW, 2016. Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol 67, 23–50. [DOI] [PubMed] [Google Scholar]
- Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M, 2013. Drug discovery: a jump-start for electroceuticals. Nature 496, 159–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante M, Redish AD, Oquendo MA, Averbeck BB, Kinnane ME, Gordon JA, 2019. Computational psychiatry: a report from the 2017 NIMH workshop on opportunities and challenges. Mol Psychiatry 24, 479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Navarro G, Casado V, Cortes A, Mallol J, Canela EI, Lluis C, Franco R, 2010. G protein-coupled receptor heteromers as new targets for drug development. Prog Mol Biol Transl Sci 91, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Quiroz C, Woods AS, Cunha R, Popoli P, Ciruela F, Lluis C, Franco R, Azdad K, Schiffmann SN, 2008. An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr Pharm Des 14, 1468–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Snaprud P, Fuxe K, 1993. Opposing actions of an adenosine A2 receptor agonist and a GTP analogue on the regulation of dopamine D2 receptors in rat neostriatal membranes. Eur J Pharmacol 244, 311–315. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Redish AD, Gordon JA, 2017. Computational Nosology and Precision Psychiatry. Comput Psychiatr 1, 2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Genedani S, Franco R, Agnati LF, 2007. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav 92, 210–217. [DOI] [PubMed] [Google Scholar]
- Geuther BQ, Deats SP, Fox KJ, Murray SA, Braun RE, White JK, Chesler EJ, Lutz CM, Kumar V, 2019. Robust mouse tracking in complex environments using neural networks. Commun Biol 2, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan CM, Morein-Zamir S, Urcelay GP, Sule A, Voon V, Apergis-Schoute AM, Fineberg NA, Sahakian BJ, Robbins TW, 2014. Enhanced avoidance habits in obsessive-compulsive disorder. Biol Psychiatry 75, 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glajch KE, Kelver DA, Hegeman DJ, Cui Q, Xenias HS, Augustine EC, Hernandez VM, Verma N, Huang TY, Luo M, Justice NJ, Chan CS, 2016. Npas1+ Pallidal Neurons Target Striatal Projection Neurons. J Neurosci 36, 5472–5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Daw N, Dayan P, O’Doherty JP, 2010. States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron 66, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N, 2003. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, 2008. Habits, rituals, and the evaluative brain. Annu Rev Neurosci 31, 359–387. [DOI] [PubMed] [Google Scholar]
- Groman SM, 2020. Astrocytic Activation to Restore Goal-Directed Behaviors. Biol Psychiatry 88, 744–745. [DOI] [PubMed] [Google Scholar]
- Groman SM, Massi B, Mathias SR, Lee D, Taylor JR, 2019. Model-Free and Model-Based Influences in Addiction-Related Behaviors. Biol Psychiatry 85, 936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G, 2006. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86, 1009–1031. [DOI] [PubMed] [Google Scholar]
- Hegeman DJ, Hong ES, Hernandez VM, Chan CS, 2016. The external globus pallidus: progress and perspectives. Eur J Neurosci 43, 1239–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez VM, Hegeman DJ, Cui Q, Kelver DA, Fiske MP, Glajch KE, Pitt JE, Huang TY, Justice NJ, Chan CS, 2015. Parvalbumin+ Neurons and Npas1+ Neurons Are Distinct Neuron Classes in the Mouse External Globus Pallidus. J Neurosci 35, 11830–11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Mallol J, Canela EI, Zoli M, Agnati LF, Ibanez CF, Lluis C, Franco R, Ferre S, Fuxe K, 2002. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem 277, 18091–18097. [DOI] [PubMed] [Google Scholar]
- Hirokawa J, Vaughan A, Masset P, Ott T, Kepecs A, 2019. Frontal cortex neuron types categorically encode single decision variables. Nature 576, 446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L, 2020. Addiction is driven by excessive goal-directed drug choice under negative affect: translational critique of habit and compulsion theory. Neuropsychopharmacology 45, 720–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SI, Bullert A, Baker M, Choi DS, 2020. Astrocytic equilibrative nucleoside transporter type 1 upregulations in the dorsomedial and dorsolateral striatum distinctly coordinate goal-directed and habitual ethanol-seeking behaviors in mice. Eur J Neurosci In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SI, Kang S, Chen JF, Choi DS, 2019. Indirect Medium Spiny Neurons in the Dorsomedial Striatum Regulate Ethanol-Containing Conditioned Reward Seeking. J Neurosci 39, 7206–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Salmeron BJ, Krasnova IN, Gu H, Lu H, Bonci A, Cadet JL, Stein EA, Yang Y, 2019. Compulsive drug use is associated with imbalance of orbitofrontal- and prelimbic-striatal circuits in punishment-resistant individuals. Proc Natl Acad Sci U S A 116, 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yaple ZA, Yu R, 2020. Goal-oriented and habitual decisions: Neural signatures of model-based and model-free learning. Neuroimage 215, 116834. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Prescott TJ, 2010. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Progress in neurobiology 90, 385–417. [DOI] [PubMed] [Google Scholar]
- Hunnicutt BJ, Jongbloets BC, Birdsong WT, Gertz KJ, Zhong H, Mao T, 2016. A comprehensive excitatory input map of the striatum reveals novel functional organization. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Siegel SJ, Spencer KM, Mathalon DH, Hong LE, Martinez A, Ehlers CL, Abbas AI, Teichert T, Lakatos P, Womelsdorf T, 2020. A roadmap for development of neuro-oscillations as translational biomarkers for treatment development in neuropsychopharmacology. Neuropsychopharmacology 45, 1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Diaz-Castro B, Looger LL, Khakh BS, 2016. Dysfunctional Calcium and Glutamate Signaling in Striatal Astrocytes from Huntington’s Disease Model Mice. J Neurosci 36, 3453–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Weiner I, 2000. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience 96, 451–474. [DOI] [PubMed] [Google Scholar]
- Kamali Sarvestani I, Lindahl M, Hellgren-Kotaleski J, Ekeberg O, 2011. The arbitration-extension hypothesis: a hierarchical interpretation of the functional organization of the Basal Ganglia. Front Syst Neurosci 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Choi DS, 2021. Astrocyte adenosine signaling and neural mechanisms of goal-directed and habitual reward-seeking behaviors. Neuropsychopharmacology 46, 227–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SW, Hong SI, Lee J, Peyton L, Baker M, Choi S, Kim H, Chang SY, Choi DS, 2020. Activation of Astrocytes in the Dorsomedial Striatum Facilitates Transition from Habitual to Goal-Directed Reward-Seeking Behavior Biol Psychiatry In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC, 1990. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci 10, 3421–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiflin R, Janak PH, 2015. Dopamine Prediction Errors in Reward Learning and Addiction: From Theory to Neural Circuitry. Neuron 88, 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramati M, Smittenaar P, Dolan RJ, Dayan P, 2016. Adaptive integration of habits into depth-limited planning defines a habitual-goal-directed spectrum. Proc Natl Acad Sci U S A 113, 12868–12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, 2019. Astrocyte-Neuron Interactions in the Striatum: Insights on Identity, Form, and Function. Trends Neurosci 42, 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Deneen B, 2019. The Emerging Nature of Astrocyte Diversity. Annu Rev Neurosci 42, 187–207. [DOI] [PubMed] [Google Scholar]
- Kita H, 2007. Globus pallidus external segment. Prog Brain Res 160, 111–133. [DOI] [PubMed] [Google Scholar]
- Koob GF, 2020. Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biol Psychiatry 87, 44–53. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange H, Thorner G, Hopf A, Schroder KF, 1976. Morphometric studies of the neuropathological changes in choreatic diseases. J Neurol Sci 28, 401–425. [DOI] [PubMed] [Google Scholar]
- Lee SW, Shimojo S, O’Doherty JP, 2014. Neural computations underlying arbitration between model-based and model-free learning. Neuron 81, 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, Vanderschuren LJ, 2012. Compulsive drug use and its neural substrates. Rev Neurosci 23, 731–745. [DOI] [PubMed] [Google Scholar]
- Lindberg D, Andres-Beck L, Jia YF, Kang S, Choi DS, 2018. Purinergic Signaling in Neuron-Astrocyte Interactions, Circadian Rhythms, and Alcohol Use Disorder. Front Physiol 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW, 2006. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci 9, 443–452. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Gremel CM, 2021. A Circuit-Based Information Approach to Substance Abuse Research. Trends Neurosci 44, 122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Cheng Y, Xie X, Woodson K, Bonifacio J, Disney E, Barbee B, Wang X, Zaidi M, Wang J, 2021. Whole-Brain Mapping of Direct Inputs to Dopamine D1 and D2 Receptor-Expressing Medium Spiny Neurons in the Posterior Dorsomedial Striatum. eNeuro 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Robbins TW, Everitt BJ, 2020. The transition to compulsion in addiction. Nat Rev Neurosci 21, 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo G, Bonci A, 2018. Rewiring the Addicted Brain: Circuits-Based Treatment for Addiction. Cold Spring Harb Symp Quant Biol 83, 173–184. [DOI] [PubMed] [Google Scholar]
- Mallet N, Schmidt R, Leventhal D, Chen F, Amer N, Boraud T, Berke JD, 2016. Arkypallidal Cells Send a Stop Signal to Striatum. Neuron 89, 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella F, Gurney K, Baldassarre G, 2013. The nucleus accumbens as a nexus between values and goals in goal-directed behavior: a review and a new hypothesis. Front Behav Neurosci 7, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz JE, Datta SR, 2020. The striatum specifies the statistics of behavior. Neuropsychopharmacology 45, 222–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz JE, Gillis WF, Beron CC, Neufeld SQ, Robertson K, Bhagat ND, Peterson RE, Peterson E, Hyun M, Linderman SW, Sabatini BL, Datta SR, 2018. The Striatum Organizes 3D Behavior via Moment-to-Moment Action Selection. Cell 174, 44–58 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastro KJ, Bouchard RS, Holt HA, Gittis AH, 2014. Transgenic mouse lines subdivide external segment of the globus pallidus (GPe) neurons and reveal distinct GPe output pathways. J Neurosci 34, 2087–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai A, Smith Y, 2011. The corticostriatal and corticosubthalamic pathways: two entries, one target. So what? Front Syst Neurosci 5, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat JJ, Ron D, 2021. Astrocytes and alcohol: cortical astrocytes regulate alcohol consumption and intoxication. Neuropsychopharmacology 46, 487–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Kawaguchi Y, 2018. A Dual Role Hypothesis of the Cortico-Basal-Ganglia Pathways: Opponency and Temporal Difference Through Dopamine and Adenosine. Front Neural Circuits 12, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujica-Parodi LR, Strey HH, 2020. Making Sense of Computational Psychiatry. Int J Neuropsychopharmacol 23, 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai J, Rajbhandari AK, Gangwani MR, Hachisuka A, Coppola G, Masmanidis SC, Fanselow MS, Khakh BS, 2019. Hyperactivity with Disrupted Attention by Activation of an Astrocyte Synaptogenic Cue. Cell 177, 1280–1292 e1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HW, Bruner RC, Choi DS, 2013a. Adenosine signaling in striatal circuits and alcohol use disorders. Mol Cells 36, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HW, Hinton DJ, Kang NY, Kim T, Lee MR, Oliveros A, Adams C, Ruby CL, Choi DS, 2013b. Adenosine Transporter ENT1 Regulates the Acquisition of Goal-Directed Behavior and Ethanol Drinking through A2A Receptor in the Dorsomedial Striatum. J. Neurosci 33, 4329–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaou K, Critchley H, Duka T, 2013. Alcohol affects neuronal substrates of response inhibition but not of perceptual processing of stimuli signalling a stop response. PLoS One 8, e76649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Tousa D, Grahame N, 2014. Habit formation: implications for alcoholism research. Alcohol 48, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oorschot DE, 1996. Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: a stereological study using the cavalieri and optical disector methods. J Comp Neurol 366, 580–599. [DOI] [PubMed] [Google Scholar]
- Packard MG, 1999. Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proc Natl Acad Sci U S A 96, 12881–12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ, 2002. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci 25, 563–593. [DOI] [PubMed] [Google Scholar]
- Qiu MH, Chen MC, Wu J, Nelson D, Lu J, 2016. Deep brain stimulation in the globus pallidus externa promotes sleep. Neuroscience 322, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, 2003. Acetylcholine actions in the dorsomedial striatum support the flexible shifting of response patterns. Neurobiol Learn Mem 80, 257–267. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Jih J, Tzavos A, 2002. Involvement of the dorsomedial striatum in behavioral flexibility: role of muscarinic cholinergic receptors. Brain Res 953, 205–214. [DOI] [PubMed] [Google Scholar]
- Redish AD, 2004. Addiction as a computational process gone awry. Science 306, 1944–1947. [DOI] [PubMed] [Google Scholar]
- Redish AD, Schultheiss NW, Carter EC, 2016. The Computational Complexity of Valuation and Motivational Forces in Decision-Making Processes. Curr Top Behav Neurosci 27, 313–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Pletnikov MV, 2020. Contributions of nonneuronal brain cells in substance use disorders. Neuropsychopharmacology 45, 224–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen L, Ullerup BH, Sunay FB, Brudek T, Lokkegaard A, Agander TK, Winge K, Pakkenberg B, 2015. Changes in total cell numbers of the basal ganglia in patients with multiple system atrophy - A stereological study. Neurobiol Dis 74, 104–113. [DOI] [PubMed] [Google Scholar]
- Schroll H, Horn A, Groschel C, Brucke C, Lutjens G, Schneider GH, Krauss JK, Kuhn AA, Hamker FH, 2015. Differential contributions of the globus pallidus and ventral thalamus to stimulus-response learning in humans. Neuroimage 122, 233–245. [DOI] [PubMed] [Google Scholar]
- Scofield MD, Kalivas PW, 2014. Astrocytic dysfunction and addiction: consequences of impaired glutamate homeostasis. Neuroscientist 20, 610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Kim D, Jung MW, 2018. Differential coding of reward and movement information in the dorsomedial striatal direct and indirect pathways. Nat Commun 9, 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shink E, Smith Y, 1995. Differential synaptic innervation of neurons in the internal and external segments of the globus pallidus by the GABA- and glutamate-containing terminals in the squirrel monkey. J Comp Neurol 358, 119–141. [DOI] [PubMed] [Google Scholar]
- Smith SJ, 1994. Neural signalling. Neuromodulatory astrocytes. Curr Biol 4, 807–810. [DOI] [PubMed] [Google Scholar]
- Stromberg I, Popoli P, Muller CE, Ferre S, Fuxe K, 2000. Electrophysiological and behavioural evidence for an antagonistic modulatory role of adenosine A2A receptors in dopamine D2 receptor regulation in the rat dopamine-denervated striatum. Eur J Neurosci 12, 4033–4037. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W, 2007. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends in neurosciences 30, 228–235. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Schoenbaum G, Niv Y, 2008. Silencing the critics: understanding the effects of cocaine sensitization on dorsolateral and ventral striatum in the context of an actor/critic model. Front Neurosci 2, 86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA, 2004. Modulation of caudate activity by action contingency. Neuron 41, 281–292. [DOI] [PubMed] [Google Scholar]
- van Steenbergen H, Watson P, Wiers RW, Hommel B, de Wit S, 2017. Dissociable corticostriatal circuits underlie goal-directed vs. cue-elicited habitual food seeking after satiation: evidence from a multimodal MRI study. Eur J Neurosci 46, 1815–1827. [DOI] [PubMed] [Google Scholar]
- Vandaele Y, Ahmed SH, 2021. Habit, choice, and addiction. Neuropsychopharmacology 46, 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Celerier E, Chaskiel L, Penzo F, Spanagel R, 2009. Compulsive alcohol drinking in rodents. Addict Biol 14, 384–396. [DOI] [PubMed] [Google Scholar]
- Venniro M, Banks ML, Heilig M, Epstein DH, Shaham Y, 2020. Improving translation of animal models of addiction and relapse by reverse translation. Nat Rev Neurosci 21, 625–643. [DOI] [PubMed] [Google Scholar]
- Voon V, Derbyshire K, Ruck C, Irvine MA, Worbe Y, Enander J, Schreiber LR, Gillan C, Fineberg NA, Sahakian BJ, Robbins TW, Harrison NA, Wood J, Daw ND, Dayan P, Grant JE, Bullmore ET, 2015. Disorders of compulsivity: a common bias towards learning habits. Mol Psychiatry 20, 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM, 2004. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 27, 468–474. [DOI] [PubMed] [Google Scholar]
- Wang J, Cheng Y, Wang X, Roltsch Hellard E, Ma T, Gil H, Ben Hamida S, Ron D, 2015. Alcohol Elicits Functional and Structural Plasticity Selectively in Dopamine D1 Receptor-Expressing Neurons of the Dorsomedial Striatum. J Neurosci 35, 11634–11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz AM, Dvorzhak A, Semtner M, Grantyn R, 2013. Reduced tonic inhibition in striatal output neurons from Huntington mice due to loss of astrocytic GABA release through GAT-3. Front Neural Circuits 7, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, 2004. Contributions of striatal subregions to place and response learning. Learn Mem 11, 459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, 2006. The role of the basal ganglia in habit formation. Nat Rev Neurosci 7, 464–476. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW, 2004. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci 19, 181–189. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW, 2006. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav Brain Res 166, 189–196. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW, 2005. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci 22, 513–523. [DOI] [PubMed] [Google Scholar]
- Yu X, Taylor AMW, Nagai J, Golshani P, Evans CJ, Coppola G, Khakh BS, 2018. Reducing Astrocyte Calcium Signaling In Vivo Alters Striatal Microcircuits and Causes Repetitive Behavior. Neuron 99, 1170–1187 e1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS, 2004. Human striatal responses to monetary reward depend on saliency. Neuron 42, 509–517. [DOI] [PubMed] [Google Scholar]


