Abstract
Aims:
Sudden unexpected death in epilepsy (SUDEP) is a serious and underestimated public health burden. Both clinical and animal studies show that seizure-induced respiratory arrest (S-IRA) is the primary cause of death in SUDEP. Our previous studies demonstrated that atomoxetine, a norepinephrine reuptake inhibitor (NRI), suppresses S-IRA in DBA/1 mice, suggesting that noradrenergic neurotransmission modulates S-IRA. However, it remains unclear which adrenoceptors are implicated in S-IRA in DBA/1 mice.
Materials and Methods:
Naïve DBA/1 mice exhibit a low incidence of S-IRA, but after primed by acoustic stimulation, they become consistently susceptible to S-IRA. Atomoxetine, adrenoceptor agonists, antagonists or vehicle was intraperitoneally (i.p.) administered alone or in combination, and the effects of drug treatments on S-IRA incidence and seizure behaviors were examined.
Key findings:
The incidence of S-IRA in primed DBA/1 mice was significantly reduced by clonidine, an α2 adrenoceptor agonist, as compared with that of the vehicle control. However, compared with the vehicle control, S-IRA was not altered by cirazoline, an α1 agonist. Consistent with previous reports, atomoxetine reduced S-IRA in primed DBA/1 mice. The suppressing effect of atomoxetine on S-IRA was prevented by injection of an α2 adrenoceptor antagonist, yohimbine or atipamezole, but not by prazosin, an α1 antagonist. Administration of α1 or α2 antagonists alone did not promote the incidence of S-IRA in nonprimed DBA/1 mice.
Significance:
These data demonstrate that noradrenergic neurotransmission modulates S-IRA predominantly via α2 adrenoceptors in DBA/1 mice, indicating that selective activation of α2 adrenoceptors can potentially prevent SUDEP.
Keywords: SUDEP, noradrenergic neurotransmission, atomoxetine, clonidine, yohimbine, prazosin
1. Introduction
The average incidence of sudden unexpected death in epilepsy (SUDEP) was estimated to be 1.4 per 1000 patient-years in patients with epilepsy (Saetre and Abdelnoor, 2018). SUDEP rate is especially high in patients with refractory epilepsy, which is up to 9 per 1000 patient-years (Tomson et al., 2008). Given that SUDEP mostly occurs in epilepsy patients at younger ages, it imposes a significant public health burden (Thurman et al., 2014, Thurman et al., 2017). Clinical and animal studies demonstrate that seizure-induced respiratory arrest (S-IRA) is the primary cause of death in most SUDEP or near-SUDEP cases (Dhaibar et al., 2019, Faingold et al., 2010, Ryvlin et al., 2013, Zhang et al., 2016). Several neurotransmission or neuromodulation systems, including serotonergic (Feng and Faingold, 2017, Zhang et al., 2018), noradrenergic (Zhang et al., 2017, Zhao et al., 2017) and adenosinergic (Faingold et al., 2016a, Kommajosyula et al., 2016, Shen et al., 2010) signaling, have been shown to contribute to the pathogenesis of S-IRA in animal models.
Our previous studies demonstrated that atomoxetine, a norepinephrine reuptake inhibitor (NRI), suppresses S-IRA in primed DBA/1 mice (Zhang et al., 2017, Zhao et al., 2017), suggesting that noradrenergic neurotransmission is implicated in S-IRA in provoked seizure models. However, it remains unknown which adrenoceptors contribute to S-IRA in DBA/1 mice. Studies show that many SUDEP patients die in the prone position (Liebenthal et al., 2015), indicating that deficits in arousal response contribute to S-IRA. Noradrenergic neurotransmission is involved in arousal response (Berridge et al., 2012, Espana et al., 2016), in which α1 and α2 adrenoceptors play important roles (Berridge et al., 2012). Thus, we hypothesized that one or both of these adrenoceptors are involved in S-IRA. The aim of this study is to identify the adrenoceptors that contribute to the modulation of S-IRA in DBA/1 mice.
2. Materials and methods
2.1. Animals
DBA/1 mice were originally purchased from Envigo (Indianapolis, IN, USA). All mice were housed and bred in the animal facility at Massachusetts General Hospital, with ad libitum access to water and standard rodent food. The animal facility is temperature- and humidity-controlled with a 12-h light/dark cycle. All animal experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) of Massachusetts General Hospital (animal protocol # 2012N000024). Every effort was made to reduce the stress of the mice during experiments and minimize the number of mice used in the study. Animals were euthanized using carbon dioxide overdose after completion of the experiment.
Although the incidence of S-IRA is low in naïve DBA/1 mice, it can reach ~100% after 3–4 consecutive daily exposure to acoustic stimulation (priming) (Feng and Faingold, 2017). A DBA/1 mouse was placed in a plexiglass chamber situated in a sound-proof room, subjected to the acoustic stimulation by an electric bell (96 dB SPL, UC4–150, Zhejiang People’s Electronics, China) (Zhao et al., 2017) for a maximum of 1 min or until the occurrence of generalized seizures that are characterized by wild running and tonic-clonic seizures followed by S-IRA and subsequent death if not resuscitated (Zhang et al., 2016). Priming usually started from postnatal day 26–28, and once a DBA/1 mouse exhibited S-IRA, it was considered fully primed. Both nonprimed (never exposed to acoustic stimulation) and primed DBA/1 mice at about one month of age were used in this study.
2.2. The effect of adrenergic agents on S-IRA
Twenty four hours before a drug or vehicle treatment, the susceptibility of a primed DBA/1 mouse to S-IRA was confirmed. If the DBA/1 mouse displayed S-IRA, an α1 or α2 adrenoceptor agonist, or vehicle was intraperitoneally (i.p.) administered 30 min prior to acoustic stimulation. To identify the adrenoceptors that are involved in the effect of atomoxetine on S-IRA, an α1 or α2 adrenoceptor antagonist or vehicle was injected 20–30 min prior to atomoxetine administration, and acoustic stimulation was given 2 h after atomoxetine administration (Zhang et al., 2017).
As enhancing noradrenergic signaling reduces S-IRA (Zhang et al., 2017, Zhao et al., 2017), it is possible that curtailing noradrenergic neurotransmission promotes S-IRA. To investigate this possibility, we examined the effects of adrenoceptor antagonists on the incidence of S-IRA in nonprimed DBA/1 mice, which exhibit a low incidence of S-IRA (Feng and Faingold, 2017). A vehicle, or an α1 or α2 adrenoceptor antagonist was administered 30 min prior to induction of audiogenic seizures by acoustic stimulation in nonprimed DBA/1 mice, which were not previously subjected to acoustic stimulation.
The effects of adrenergic agents on the incidence of S-IRA and seizure behaviors in both primed and nonprimed DBA/1 mice were video taped for offline analysis.
2.3. Adrenergic agents
Clonidine hydrochloride (C7897), cirazoline hydrochloride (C223), atomoxetine hydrochloride (Y0001586), yohimbine hydrochloride (Y3125), atipamezole (A9611) and prazosin hydrochloride (P7791) were purchased from Millipore Sigma (St. Louis, MO, USA). All the adrenergic agents were dissolved in saline (vehicle).
2.4. Statistical analysis
We used Prism 5.0d software (GraphPad Software Inc., La Jolla, CA, USA) for statistical analysis. The incidence of S-IRA between the drug and vehicle control group was compared using Chi-square test. Statistical significance was inferred if p < 0.05.
3. Results
3.1. The effects of adrenoceptor agonists on the incidence of S-IRA in primed DBA/1 mice
As compared with the incidence of S-IRA in vehicle control group (100%, n = 10), administration of clonidine, an α2 agonist, significantly reduced that 30 min after injection at 0.1 mg/kg (37.5%, n = 8; p < 0.01), 0.5 mg/kg (10%, n = 10; p < 0.001), 2 mg/kg (0%, n = 8; p < 0.001) and 8 mg/kg (11.1%, n = 9; p < 0.001) (Fig. 1). For those DBA/1 mice without S-IRA after clonidine treatment, tonic seizures were blocked by clonidine at 0.1 mg/kg, 2 mg/kg and 8 mg/kg. However, in DBA/1 mice treated with 0.5 mg/kg clonidine, tonic seizures were intact in 22.2% of mice in which S-IRA was blocked, suggesting that clonidine at this dose specifically suppressed S-IRA in these mice. Wild running and/or clonic seizures were still present in all or in a proportion of mice administered with clonidine at 0.1 mg/kg (100%), 0.5 mg/kg (66.7%), 2 mg/kg (50%) and 8 mg/kg (62.5%). S-IRA was not altered 30 min after i.p. injection of cirazoline, an α1 agonist, at 0.04 mg/kg (100%, n = 8), 0.2 mg/kg (100%, n = 9) and 1 mg/kg (100%, n = 8) as compared with vehicle controls (100%, n = 8) (Fig. 2).
Figure 1. The α2 adrenoceptor agonist clonidine suppresses S-IRA in primed DBA/1 mice.
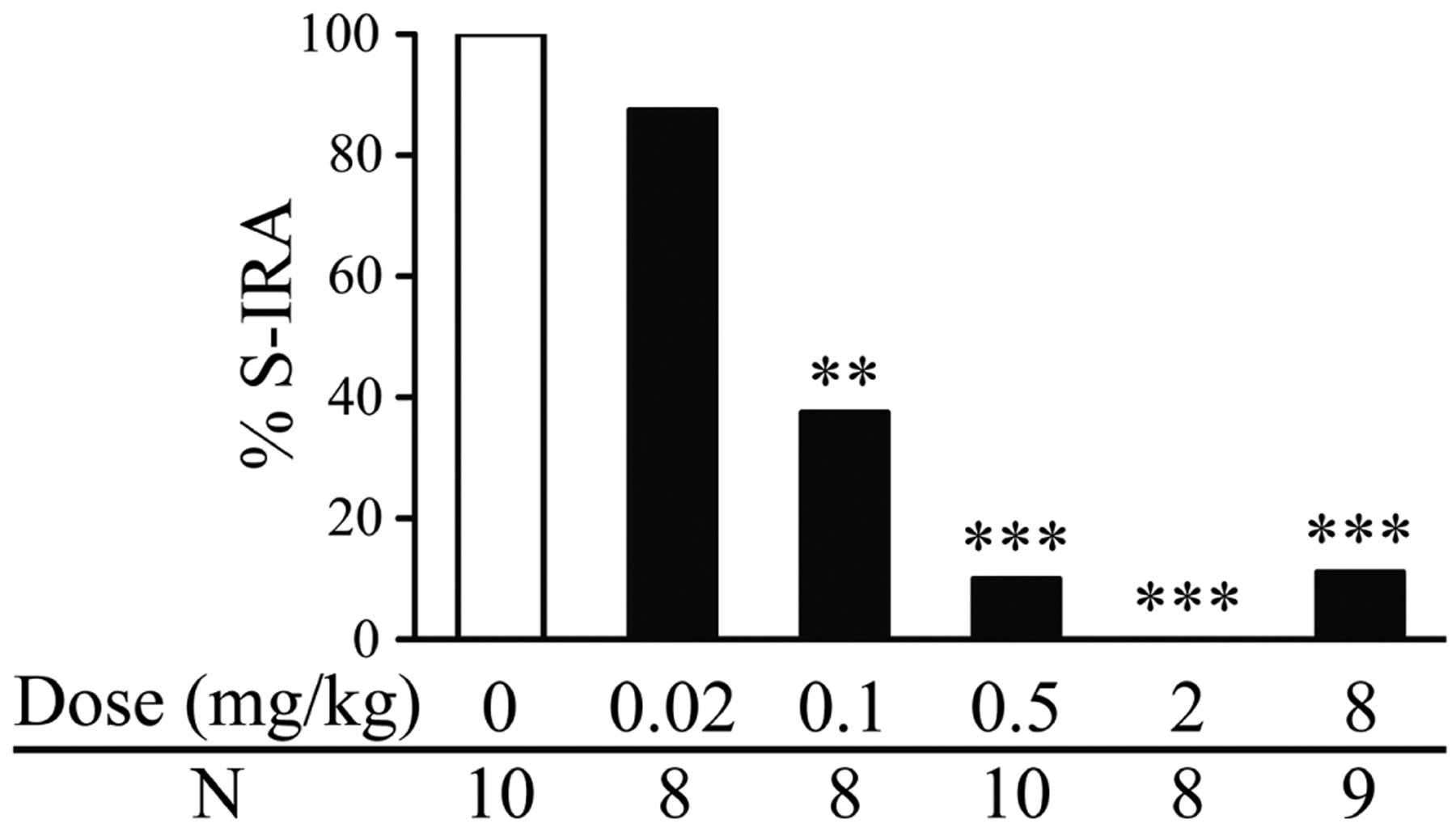
Intraperitoneal (i.p.) administration of clonidine at 0.1–8 mg/kg significantly reduced the incidence of S-IRA as compared with that in vehicle controls. Compared with the vehicle control group, clonidine at 0.02 mg/kg did not significantly suppress S-IRA.
**, p < 0.01; ***, p < 0.001: Significantly different from the vehicle control group (dose zero)
Figure 2. The α1 adrenoceptor agonist cirazoline exerts no effect on S-IRA in primed DBA/1 mice.
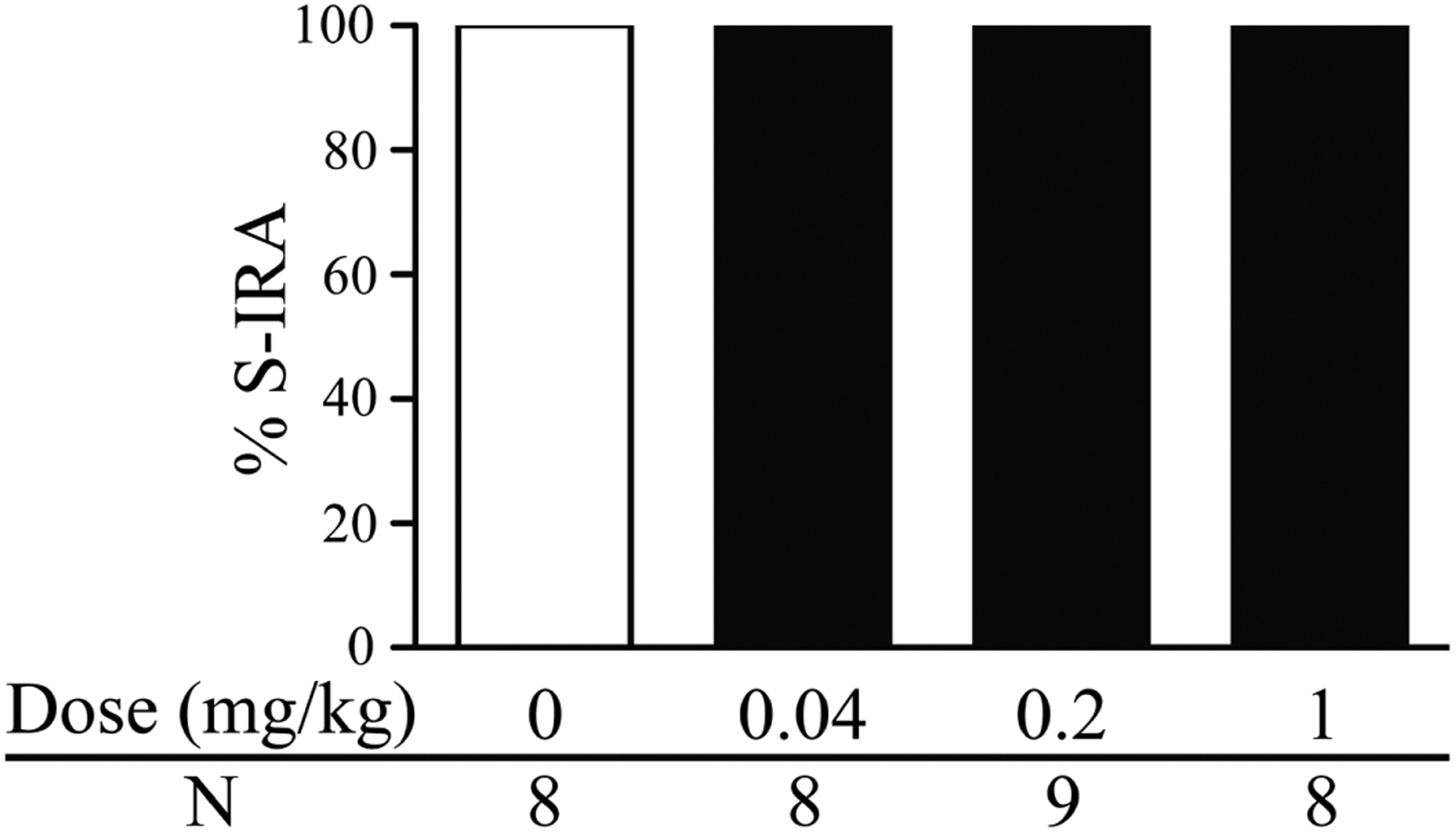
Intraperitoneal (i.p.) administration of cirazoline at 0.04–1 mg/kg did not alter the incidence of S-IRA as compared with that in vehicle controls.
3.2. Adrenoceptors involved in atomoxetine effect on S-IRA in primed DBA/1 mice
S-IRA was reduced 2 h after i.p. administration of atomoxetine at 20 mg/kg (12.5%, n = 8) in primed DBA/1 mice. However, administration of yohimbine, an α2 antagonist, 30 min prior to atomoxetine injection significantly reversed the suppressing effect of atomoxetine on S-IRA (90%, n = 10; p < 0.01) (Fig. 3A). We used another α2 antagonist atipamezole to confirm this result. Injection of atipamezole (1 mg/kg) 20 min prior to atomoxetine (20 mg/kg) administration (66.7%, n = 12) significantly prevented the suppressing effect of atomoxetine on S-IRA (p < 0.05) (Fig. 3B). In contrast, administration of prazosin (1 mg/kg), an α1 antagonist, 30 min prior to atomoxetine (20 mg/kg) injection (42.9%, n = 14) did not significantly block the suppressing effect of atomoxetine on S-IRA (11.1%, n = 9) (Fig. 4).
Figure 3. The α2 adrenoceptor antagonists reverse the suppressing effect of atomoxetine on S-IRA in primed DBA/1 mice.
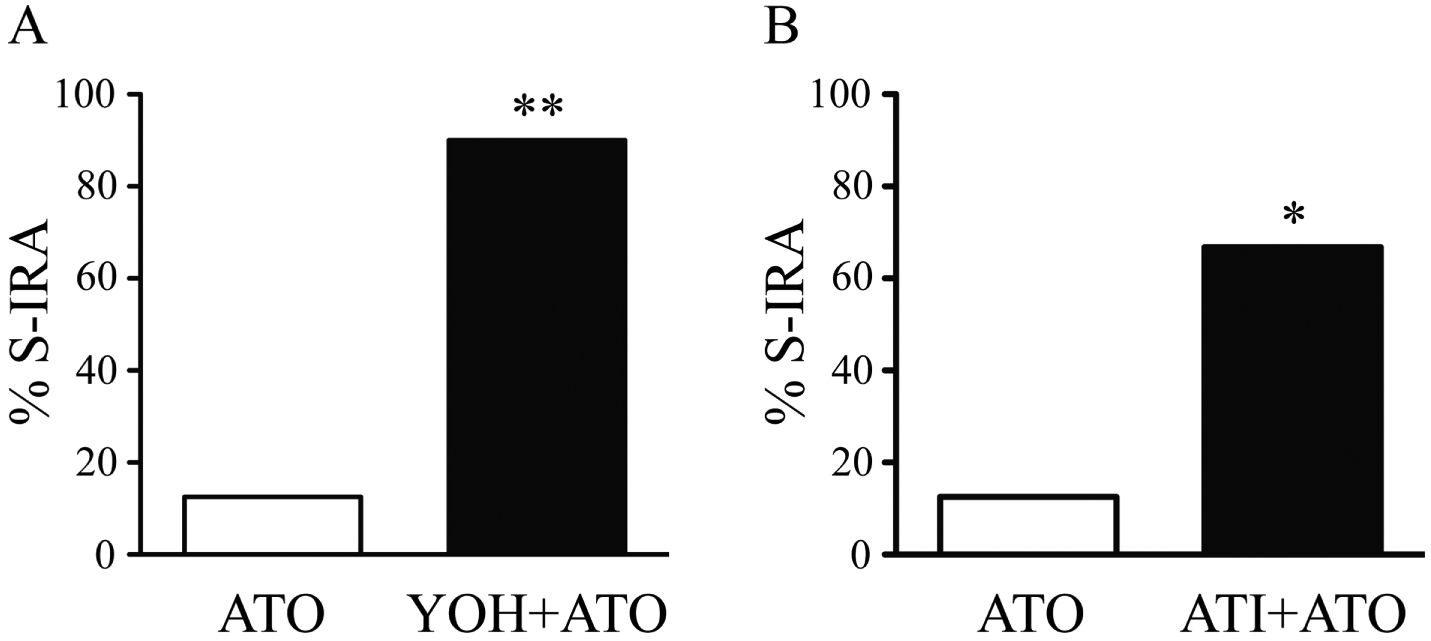
A, compared with the incidence of S-IRA by atomoxetine (ATO, 20 mg/kg) alone, pre-treatment with yohimbine (YOH, 5 mg/kg), an α2 antagonist, 30 min prior to atomoxetine (20 mg/kg) administration significantly reversed the suppressing effect of atomoxetine on S-IRA. B, pre-treatment with atipamezole (ATI, 1 mg/kg), another α2 antagonist, 20 min prior to atomoxetine (20 mg/kg) injection significantly prevented the suppressing effect of atomoxetine on S-IRA.
*, p < 0.05; **, p < 0.01: Significantly different from atomoxetine treatment alone
Figure 4. The α1 adrenoceptor antagonist fails to reverse the suppressing effect of atomoxetine on S-IRA in primed DBA/1 mice.
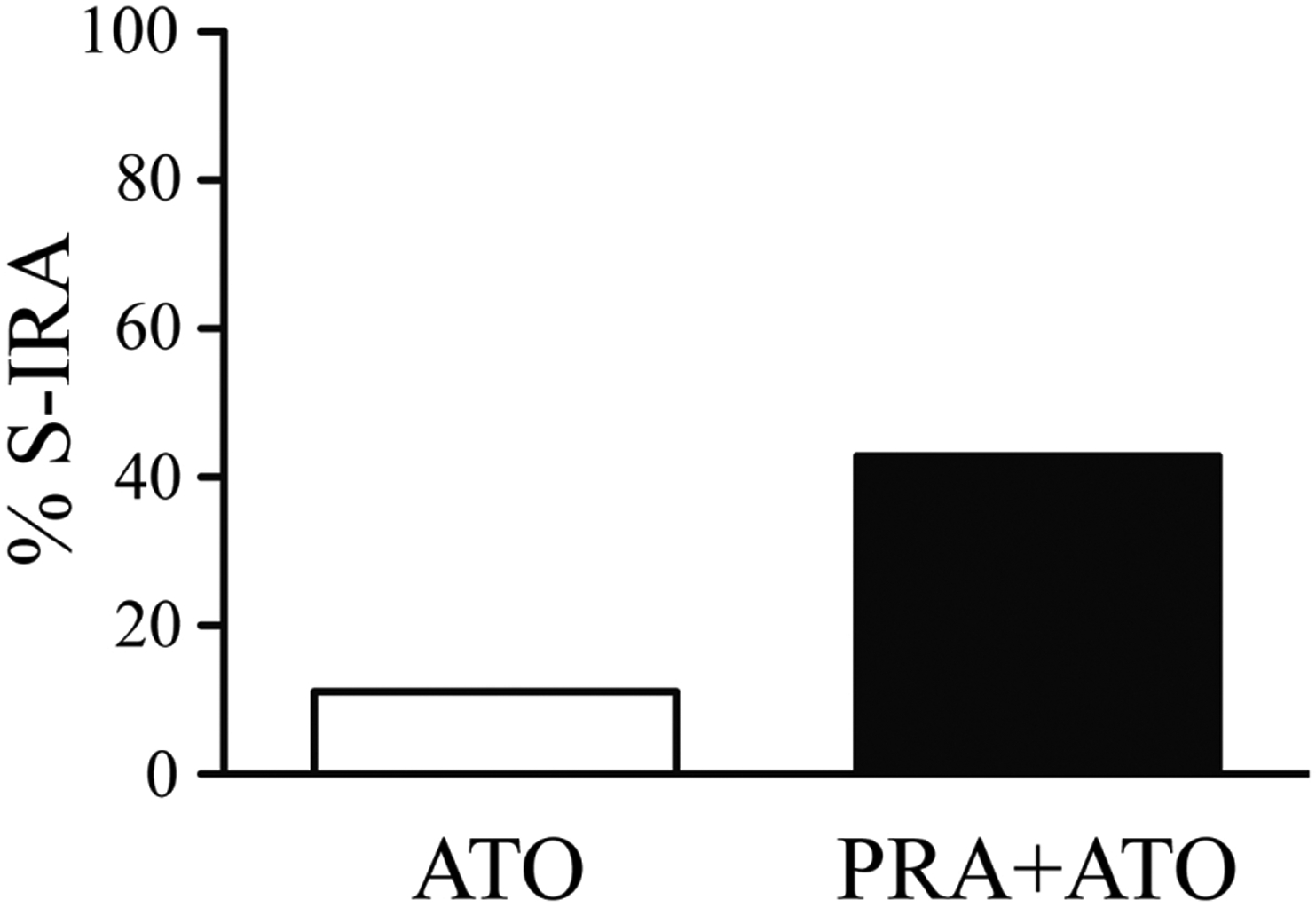
Pre-treatment with prazosin (PRA, 1 mg/kg), an α1 antagonist, 30 min prior to atomoxetine (ATO, 20 mg/kg) administration did not prevent the suppressing effect of atomoxetine on S-IRA.
3.3. The effects of adrenoceptor antagonists on S-IRA in nonprimed DBA/1 mice
As compared with the incidence of S-IRA in vehicle control group (11.1%, n = 9), administration of prazosin at 1 mg/kg (12.5%, n = 8) or 10 mg/kg (0%, n = 10), or yohimbine at 5 mg/kg (15.8%, n = 19) did not significantly alter that in nonprimed DBA/1 mice (Tab. 1).
Table 1.
The effects of adrenoceptor antagonists on the incidence of S-IRA in nonprimed DBA/1 mice.
| Treatment | Dose (mg/kg) | N | % S-IRA |
|---|---|---|---|
| Saline | 0 | 9 | 11.1 |
| Yohimbine | 5 | 19 | 15.8 |
| Prazosin | 1 | 8 | 12.5 |
| 10 | 10 | 0 |
4. Discussion
In the current study, we demonstrate that an α2 adrenoceptor agonist but not an α1 agonist suppresses S-IRA in primed DBA/1 mice. In line with a previous report (Zhang et al., 2017), atomoxetine reduces S-IRA in primed DBA/1 mice, and an α2 antagonist but not an α1 antagonist reverses the suppressing effect of atomoxetine on S-IRA. These data support the idea that α2 adrenoceptors play an important role in modulating S-IRA in DBA/1 mice.
Although multiple mechanisms underlying SUDEP have been proposed, cardiorespiratory dysfunction and arousal deficit receive considerable attention (Feng and Faingold, 2017, Friedman et al., 2018, Lhatoo et al., 2015, Manolis et al., 2019, Sowers et al., 2013). It was reported that more than 70% of SUDEP patients die in the prone position (Liebenthal et al., 2015), indicating that a deficit in arousal response may contribute to SUDEP. Our recent studies demonstrated that both systematic and intracerebroventricular injection of the NRI atomoxetine reduces S-IRA in primed DBA/1 mice (Zhang et al., 2017, Zhao et al., 2017). Another study using an electroshock seizure model also observed that the NRIs including atomoxetine suppress S-IRA in C57BL/6 mice (Kruse et al., 2019). These studies suggest that abnormal noradrenergic neurotransmission contributes to the pathogenesis of S-IRA. It is well known that noradrenergic signaling plays an important role in modulating arousal response (Berridge et al., 2012, Hansen, 2017). Previous studies show that α1 and α2 adrenoceptors are involved in arousal response (Berridge et al., 2003, Berridge et al., 1993, Berridge et al., 2005, Mohan Kumar et al., 1984, Sood et al., 1997). Thus, we examined if these adrenoceptors are implicated in S-IRA. Our data demonstrate that an α2 adrenoceptor agonist reduces S-IRA, and an α2 antagonist blocks the suppressing effect of atomoxetine on S-IRA in primed DBA/1 mice. These findings indicate that α2 adrenoceptors play an important role in modulating S-IRA in DBA/1 mice. The α2 adrenoceptor expresses both presynaptically and postsynaptically in the brain (Hussain and Maani, 2019). Our data do not support the involvement of presynaptic α2 adrenoceptors in S-IRA. Both the α2 agonist clonidine and the NRI atomoxetine suppress S-IRA in DBA/1 mice, suggesting that they exert similar effect on noradrenergic neurotransmission to achieve this effect. However, presynaptic α2 adrenoceptors are autoreceptors, whose activation by its agonists reduces norepinephrine release (Hussain and Maani, 2019), in contrast to atomoxetine that enhances norepinephrine availability by inhibiting its reuptake. In addition, atomoxetine reduces S-IRA likely via stimulating postsynaptic receptors to activate the downstream effector pathways. The suppressing effect of atomoxetine on S-IRA can be specifically blocked by α2 antagonists, which cannot be solely explained by antagonizing presynaptic receptors.
We observed that activation of α1 adrenoceptors exerts no effect on S-IRA, and an α1 antagonist cannot block the suppressing effect of atomoxetine on S-IRA in primed DBA/1 mice. These data indicate that α1 adrenoceptors are not critically involved in the pathogenesis of S-IRA in DBA/1 mice. It was reported that α1 adrenoceptors contribute to S-IRA in another provoked seizure model (Kruse et al., 2019). The reasons underlying these discrepancies are unknown. However, in that study, the dosage of prazosin (10 mg/kg) is much higher than that used in other CNS studies (Dhingra and Chhillar, 2012, Salako et al., 2019, Srinivasan et al., 2015, Zhu et al., 2017). It is possible that prazosin at high doses produces off-target effects (Zhang et al., 2012). Additionally, it is possible that adrenoceptor subtypes implicated in S-IRA are dependent on animal models.
Adenosine regulates respiration (Tescarollo et al., 2020), and enhancing adenosine function facilitates seizure-induced death in provoked seizure models (Kommajosyula et al., 2016, Shen et al., 2010). Thus, administration of caffeine, a nonselective adenosine receptor antagonist, prevents seizure-induced death (Faingold et al., 2016a, Shen et al., 2010). Application of a selective adenosine A2A receptor antagonist reduces S-IRA in DBA/2 mouse model of SUDEP (Faingold et al., 2016a), indicating that adenosine A2A receptors are involved in S-IRA. Serotonin (5-HT) neurons in the brainstem control both respiration and arousal in response to the fluctuation of blood CO2 (Sowers et al., 2013). Augmenting 5-HT function alleviates S-IRA (Feng and Faingold, 2017). Defective expression of 5-HT2B, 5-HT2C, 5-HT3 or 5-HT4 receptors was observed in DBA/1 and DBA/2 mice (Faingold et al., 2011, Uteshev et al., 2010). It was reported that 5-HT3 receptors play an important role in modulating S-IRA in DBA/1 mice (Faingold et al., 2016b, Zhang et al., 2018). Interestingly, administration of a nonselective 5-HT receptor antagonist facilitates the occurrence of S-IRA in nonprimed DBA/1 mice (Faingold et al., 2014). This observation suggests that abnormal 5-HT receptor expression may be a major instigator leading to S-IRA in DBA/1 mice. However, in the current study, injection of adrenoceptor antagonists does not increase the incidence of S-IRA in nonprimed DBA/1 mice. It is possible that the machinery of noradrenergic neurotransmission other than the adrenoceptor is the major contributor to S-IRA in DBA/1 mice. Further studies are needed to explore this possibility in the future.
5. Conclusion
Our data demonstrate that a selective α2 agonist reduces S-IRA, and the suppressing effect of atomoxetine on S-IRA can be blocked by a specific α2 antagonist. These findings substantiate the idea that α2 adrenoceptors are importantly implicated in the pathogenesis of S-IRA in DBA/1 mice.
Acknowledgements
This work was supported by NIH R21 NS101311 and funds from Massachusetts General Hospital Department of Anesthesia, Critical Care and Pain Medicine to HJF. Rui Zhang is a recipient of postdoctoral fellowship (CSC 201708640002), and Zheren Tan is a recipient of graduate fellowship (CSC 201806370116) from China Scholarship Council.
Abbreviations:
- i.p.
Intraperitoneal(ly)
- NRI
Norepinephrine reuptake inhibitor
- S-IRA
Seizure-induced respiratory arrest
- SUDEP
Sudden unexpected death in epilepsy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Berridge CW, Isaac SO, Espana RA. Additive wake-promoting actions of medial basal forebrain noradrenergic alpha1- and beta-receptor stimulation. Behav Neurosci. 2003;117:350–9. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55:381–93. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Schmeichel BE, Espana RA. Noradrenergic modulation of wakefulness/arousal. Sleep medicine reviews. 2012;16:187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Stellick RL, Schmeichel BE. Wake-promoting actions of medial basal forebrain beta2 receptor stimulation. Behav Neurosci. 2005;119:743–51. [DOI] [PubMed] [Google Scholar]
- Dhaibar H, Gautier NM, Chernyshev OY, Dominic P, Glasscock E. Cardiorespiratory profiling reveals primary breathing dysfunction in Kcna1-null mice: Implications for sudden unexpected death in epilepsy. Neurobiology of disease. 2019;127:502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra D, Chhillar R. Antidepressant-like activity of ellagic acid in unstressed and acute immobilization-induced stressed mice. Pharmacological reports : PR. 2012;64:796–807. [DOI] [PubMed] [Google Scholar]
- Espana RA, Schmeichel BE, Berridge CW. Norepinephrine at the nexus of arousal, motivation and relapse. Brain Res. 2016;1641:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL, Kommajosyula SP, Long X, Plath K, Randall M. Serotonin and sudden death: Differential effects of serotonergic drugs on seizure-induced respiratory arrest in DBA/1 mice. Epilepsy & behavior : E&B. 2014;37:198–203. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Randall M, Kommajosyula SP. Susceptibility to seizure-induced sudden death in DBA/2 mice is altered by adenosine. Epilepsy research. 2016a;124:49–54. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Randall M, Mhaskar Y, Uteshev VV. Differences in serotonin receptor expression in the brainstem may explain the differential ability of a serotonin agonist to block seizure-induced sudden death in DBA/2 vs. DBA/1 mice. Brain Res. 2011;1418:104–10. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Randall M, Tupal S. DBA/1 mice exhibit chronic susceptibility to audiogenic seizures followed by sudden death associated with respiratory arrest. Epilepsy & behavior : E&B. 2010;17:436–40. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Randall M, Zeng C, Peng S, Long X, Feng HJ. Serotonergic agents act on 5-HT3 receptors in the brain to block seizure-induced respiratory arrest in the DBA/1 mouse model of SUDEP. Epilepsy & behavior : E&B. 2016b;64:166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HJ, Faingold CL. Abnormalities of serotonergic neurotransmission in animal models of SUDEP. Epilepsy & behavior : E&B. 2017;71:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Kannan K, Faustin A, Shroff S, Thomas C, Heguy A, et al. Cardiac arrhythmia and neuroexcitability gene variants in resected brain tissue from patients with sudden unexpected death in epilepsy (SUDEP). NPJ Genom Med. 2018;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen N The Longevity of Hippocampus-Dependent Memory Is Orchestrated by the Locus Coeruleus-Noradrenergic System. Neural Plast. 2017;2017:2727602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain LS, Maani CV. Physiology, Noradrenergic Synapse. StatPearls. Treasure Island (FL) 2019. [PubMed] [Google Scholar]
- Kommajosyula SP, Randall ME, Faingold CL. Inhibition of adenosine metabolism induces changes in post-ictal depression, respiration, and mortality in genetically epilepsy prone rats. Epilepsy research. 2016;119:13–9. [DOI] [PubMed] [Google Scholar]
- Kruse SW, Dayton KG, Purnell BS, Rosner JI, Buchanan GF. Effect of monoamine reuptake inhibition and alpha1 blockade on respiratory arrest and death following electroshock-induced seizures in mice. Epilepsia. 2019;60:495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhatoo S, Noebels J, Whittemore V, Research NCfS. Sudden unexpected death in epilepsy: Identifying risk and preventing mortality. Epilepsia. 2015;56:1700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebenthal JA, Wu S, Rose S, Ebersole JS, Tao JX. Association of prone position with sudden unexpected death in epilepsy. Neurology. 2015;84:703–9. [DOI] [PubMed] [Google Scholar]
- Manolis TA, Manolis AA, Melita H, Manolis AS. Sudden unexpected death in epilepsy: The neuro-cardio-respiratory connection. Seizure : the journal of the British Epilepsy Association. 2019;64:65–73. [DOI] [PubMed] [Google Scholar]
- Mohan Kumar V, Datta S, Chhina GS, Gandhi N, Singh B. Sleep-awake responses elicited from medial preoptic area on application of norepinephrine and phenoxybenzamine in free moving rats. Brain Res. 1984;322:322–5. [DOI] [PubMed] [Google Scholar]
- Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet neurology. 2013;12:966–77. [DOI] [PubMed] [Google Scholar]
- Saetre E, Abdelnoor M. Incidence rate of sudden death in epilepsy: A systematic review and meta-analysis. Epilepsy & behavior : E&B. 2018;86:193–9. [DOI] [PubMed] [Google Scholar]
- Salako OA, Akindele AJ, Balogun AO, Adeyemi OO. Investigation of Antidepressant, Anxiolytic and Sedative Activities of the Aqueous Leaf Extract of Musa sapientum Linn. (Banana; Musaceae). Drug Res (Stuttg). 2019;69:136–43. [DOI] [PubMed] [Google Scholar]
- Shen HY, Li T, Boison D. A novel mouse model for sudden unexpected death in epilepsy (SUDEP): role of impaired adenosine clearance. Epilepsia. 2010;51:465–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood S, Dhawan JK, Ramesh V, John J, Gopinath G, Kumar VM. Role of medial preoptic area beta adrenoceptors in the regulation of sleep-wakefulness. Pharmacology, biochemistry, and behavior. 1997;57:1–5. [DOI] [PubMed] [Google Scholar]
- Sowers LP, Massey CA, Gehlbach BK, Granner MA, Richerson GB. Sudden unexpected death in epilepsy: fatal post-ictal respiratory and arousal mechanisms. Respiratory physiology & neurobiology. 2013;189:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng H, et al. Ca(2+) signaling in astrocytes from Ip3r2(−/−) mice in brain slices and during startle responses in vivo. Nat Neurosci. 2015;18:708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tescarollo FC, Rombo DM, DeLiberto LK, Fedele DE, Alharfoush E, Tome AR, et al. Role of Adenosine in Epilepsy and Seizures. J Caffeine Adenosine Res. 2020;10:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman DJ, Hesdorffer DC, French JA. Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia. 2014;55:1479–85. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Logroscino G, Beghi E, Hauser WA, Hesdorffer DC, Newton CR, et al. The burden of premature mortality of epilepsy in high-income countries: A systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia. 2017;58:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet neurology. 2008;7:1021–31. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Tupal S, Mhaskar Y, Faingold CL. Abnormal serotonin receptor expression in DBA/2 mice associated with susceptibility to sudden death due to respiratory arrest. Epilepsy research. 2010;88:183–8. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhao H, Feng HJ. Atomoxetine, a norepinephrine reuptake inhibitor, reduces seizure-induced respiratory arrest. Epilepsy & behavior : E&B. 2017;73:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhao H, Yang X, Xue Q, Cotten JF, Feng HJ. 5-Hydroxytryptophan, a precursor for serotonin synthesis, reduces seizure-induced respiratory arrest. Epilepsia. 2016;57:1228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhao H, Zeng C, Van Dort C, Faingold CL, Taylor NE, et al. Optogenetic activation of 5-HT neurons in the dorsal raphe suppresses seizure-induced respiratory arrest and produces anticonvulsant effect in the DBA/1 mouse SUDEP model. Neurobiology of disease. 2018;110:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang W, Bedigian AV, Coughlin ML, Mitchison TJ, Eggert US. Dopamine receptor D3 regulates endocytic sorting by a Prazosin-sensitive interaction with the coatomer COPI. Proc Natl Acad Sci U S A. 2012;109:12485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Cotten JF, Long X, Feng HJ. The effect of atomoxetine, a selective norepinephrine reuptake inhibitor, on respiratory arrest and cardiorespiratory function in the DBA/1 mouse model of SUDEP. Epilepsy research. 2017;137:139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Wu Q, Li J, Grycel K, Liu B, Sun X, et al. A single dose of cocaine potentiates glutamatergic synaptic transmission onto locus coeruleus neurons. Cell calcium. 2017;67:11–20. [DOI] [PubMed] [Google Scholar]


