Abstract
Human Atg3 (hAtg3) is an E2-like enzyme that catalyzes the conjugation of LC3 family proteins to phosphatidylethanolamine (PE) lipids in the autophagosomal membrane during autophagy. The reaction product, LC3-PE, acts as a marker for autophagic cargo and is required for the effective construction of functional autophagosomes. However, the structural and molecular basis of this conjugation reaction remains elusive, at least in part, because of the absence of lipid bilayers in structural studies conducted to date. Here, we report a sequential resonance assignment for an hAtg3 construct both in aqueous solution and in bicelles. hAtg3 has 314 residues, and our construct lacks the unstructured region from residues 90 to 190. Our results demonstrate a structural rearrangement of hAtg3 N-terminus when it interacts with membranes.
Keywords: Human Atg3, Autophagy, NMR assignment, Protein-lipid interaction
Biological context
Autophagy (macroautophagy), triggered by stress, is a major catabolic pathway for the degradation and recycling of cellular materials in all eukaryotes (Yang and Klionsky 2009, Lamb et al. 2013). During this process, cellular contents that are targeted for degradation such as aggregated proteins, damaged organelles, and foreign organisms including viruses and bacteria, are enclosed within a double-walled membrane structure called the autophagosome, which fuses with a lysosome (or vacuole in yeast) to degrade its contents. During the biogenesis of the autophagosome, Atg3 (autophagy related 3, an E2-like enzyme) catalyzes the direct covalent conjugation of LC3 (or Atg8 in yeast) to the phosphatidylethanolamine (PE) lipid (Yamada et al. 2007, Mizushima et al. 2011). The product of this reaction, LC3-PE, is required for phagophore expansion and acts as an adaptor for sequestering cargos for breakdown (Kaufmann et al. 2014, Sawa-Makarska et al. 2014). Despite conceptual similarities, Atg3 functions quite differently from canonical E2 enzymes. Atg3 catalyzes the transfer of the ubiquitin-like LC3 to a lipid instead of a protein substrate; it preferentially binds to membranes with packing defects and selects the substrate of PE lipids; and it is able to catalyze this reaction in the absence of the E3-like Atg5-Atg12/Atg16 complex in vitro.
Human Atg3 (hAtg3) consists of 314 residues, of which one third are expected to be in unstructured regions based on two existing crystal structures, one from Saccharomyces cerevisiae (yAtg3, PDB 2DYT) (Yamada et al. 2007) and another from Arabidopsis thaliana (AtAtg3, PDB 3VX8) (Yamaguchi et al. 2012). The disordered N-terminal 25 residues presumably can form an amphipathic α-helix when interacting with highly curved membranes (Hanada et al. 2009, Nath et al. 2014, Hervas et al. 2017), and the long unstructured region (residues 90 to 190) contains elements that interact with the E1-like Atg7 protein (Yamaguchi et al. 2012) and the E3-like Atg5-Atg12/Atg16 complex (Metlagel et al. 2013). Here, we report NMR resonance assignments for an hAtg3 construct, in which the unstructured region from residues 90 to 190 has been removed (hAtg3Δ90-190), in aqueous solution and in bicelles using 15N, 13C, and 2H-labeled proteins, reverse amino acid labeling (Vuister et al. 1994, Verardi et al. 2012, Bellstedt et al. 2013, Prasanna et al. 2015), and 3D triple resonance assignment experiments. These results provide a basis for further NMR studies of how hAtg3 is activated and regulated to produce LC3-PE at the targeted membrane surface, a process that currently remains elusive.
Methods and experiments
Protein preparation
The plasmid of the full-length hAtg3 (Clone ID:3938447) that was subcloned into a pET28a expression vector at the BamHI/XhoI sites with a HiS6-tag and a T7-tag at the N-termini (His6-T7-hAtg3) was provided by Dr. Xuejun Jiang’s lab at the Memorial Sloan Kettering Cancer Center. A thrombin cleavage site was introduced between the T7 tag and hAtg3. An hAtg3 construct, in which residues 90 to 190 (hAtg3Δ90-190) have been deleted, was prepared using the Q5® Site-Directed Mutagenesis Kit (New England Biolabs) with a forward primer, GAAGATGCTATTTTGCAAACC, and a reverse primer, TTCCATCTGTTTGCACCG. The plasmid containing the target gene was transformed into E. coli Rosetta™(DE3) pLysS competent cells (Novagen) for protein expression.
For the expression of isotope-labeled hAtg3Δ90-190 protein, a single colony was first grown in 10 mL LB medium prepared with 50 μg/mL kanamycin at 37 °C for 7 h. Cell cultures were then spun down at 2000 g and resuspended in 250 mL M9 medium (6.78 g/L Na2HPO4, 3 g/L KH2PO4, 0.5 g/L NaCl, 1 g/L NH4Cl, 3 g/L D-glucose, 0.1 mM CaCl2, 2 mM MgSO4) containing 50 μg/mL kanamycin. 1 g/L 15NH4Cl and 3 g/L 13C-enriched D-glucose (U-13C6, 99%, Cambridge Isotope Laboratories, Andover, MA, USA) was used for 15N-enrichment and 13C-enrichment, respectively. For 2H-labeled proteins, 0.5 mL cell cultures in LB were centrifuged at 2000 g and resuspended in 500 mL M9 medium prepared in D2O. When the OD600 of starting cell cultures in M9 reached 0.7-0.8, the temperature was decreased to 25 °C and protein expression was induced with 0.5 mM IPTG (isopropyl β-d-1-thiogalactopyranoside). For Lys-, His/Met-, or Cys/Arg-unlabeled samples, 1 g/L (except for Cys at 0.1 g/L) of the respective unlabeled amino acid (Sigma Aldrich) was added to the M9 medium 15 min prior to induction (Bellstedt et al. 2013). Cells were harvested after 16 h of protein expression by centrifugation at 7000 rpm for 10 min and stored at −80 °C until required.
For protein purification, cells were suspended in a lysis buffer containing 20 mM sodium phosphate, pH 7.5, 300 mM NaCl, 2 mM BME, 1 mM MgCl2, 100 U of Benzonase® nuclease, and cOmplete™ protease inhibitor. The cell suspension was sonicated (2s on, 7s off) on ice for 100 cycles and then centrifuged (Sorvall RC5B Plus Refrigerated Centrifuge) at 26,900 g at 10 °C for 30 min. The supernatant was loaded onto a Ni-NTA column. The column was washed first with a high salt buffer of 20 mM sodium phosphate, pH 7.5, 1 M NaCl, 2 mM BME and then with a buffer containing 30 mM imidazole, 20 mM sodium phosphate, pH 7.5, 300 mM NaCl, and 2 mM BME. The column was subsequently eluated with a buffer containing 20 mM sodium phosphate, pH 7.5, 300 mM NaCl, 500 mM imidazole, and 2 mM BME. The elute was exchanged into a buffer containing 50 mM Hepes, pH 7.5, 150 mM NaCl, and 2 mM BME using an Amicon®Ultra centrifugal filter, and cleaved with the addition of 100 Unit of thrombin and 0.1% Tween20 for 16 h at 4 °C. The mixture was concentrated and diluted with an anion exchange buffer of 20 mM Tris, pH 8.0, 2 mM BME, and applied to a HiTrap Capto Q anion exchange column (GE Healthcare). The target protein was eluted using a high salt buffer containing 20 mM Tris buffer, 1 M NaCl, pH 8.0, and 2 mM BME. The elute was concentrated and further purified with a S75 column (HiLoad 16/60 Superdex75) with a running buffer of 50 mM Hepes, pH 7.5, 1 M NaCl, and 1 mM DTT. Fractions containing targeted protein were pooled, concentrated and exchanged into a buffer of 50 mM HEPES, pH 6.8 (or pH 7.5 for bicelles), 150 mM NaCl, and 2 mM TCEP (tris(2-carboxyethyl)phosphine). Protein concentration was measured using a Nanodrop (Thermo Scientific (Waltham, MA)).
NMR Spectroscopy
The NMR samples used for resonance assignment contained 0.3 to 0.5 mM labeled protein in a buffer of 25-50 mM HEPES, pH 6.8, 150 mM NaCl, 2 mM TCEP, 0.02% NaN3, and 4% D2O in a 4 mm O.D. thin wall NMR tube. For the stability of samples with 12% bicelles (DMPC/DMPG/DHPC=4:1:20, molar ratio, q=0.25) the pH was increased to 7.5. All NMR data were collected at 25 °C on Bruker 600MHz and 850 MHz spectrometers equipped with cryoprobes. Sequential backbone resonance assignments were obtained using TROSY-based triple resonance experiments including HNCO, HN(CA)CO, HNCA, HN(CO)CA, HNCACB, and HN(CO)CACB. All data were processed with NMRPipe (Delaglio et al. 1995) and analyzed with NMRView (Johnson and Blevins 1994).
Assignments and data deposition
The well-resolved TROSY spectrum of hAtg3Δ90-190 in aqueous solution is shown in Fig. 1a. We have assigned 181 out of 199 non-proline residues in this construct, and 92% of Cα, 85% of Cβ and 91% of CO. Only two NH peaks in the TROSY spectrum are not assigned. 9 out of 18 unassigned residues are located in the loop (residues 261 to 270) containing the active site C264. These residues are likely involved in millisecond motions since their NH peaks were not observed due to exchange broadening. Interestingly, similar dynamics at the active site of yAtg3 were reported in a recent study of yAtg3 (Zheng et al. 2019). In addition, the N-terminal 25 residues of Atg3 are predicted to be unstructured by TALOS-N (Shen and Bax 2013) (Fig. 1c). These residues are known to selectively interact with highly curved membranes that are essential for LC3-PE conjugation (Nath et al. 2014). We have recently demonstrated that isotropic bicelles are a valid membrane model for studying the membrane curvature-sensitive binding of hAtg3 by high-resolution NMR (Ye et al. 2021). Fig. 1b shows the TROSY spectrum of hAtg3Δ90-190 in bicelles (DMPC/DMPG/DHPC=4:1:20, molar ratio, q=0.25). Clearly, significant spectral perturbations occur when hAtg3 interacts with bicelles. Despite degraded spectral resolution as a result of increased size and exchange broadening, we were able to assign 155 out of 199 non-proline residues using traditional triple resonance experiments in conjunction with reverse amino acid labeling. 84% of Cα, 70% of Cβ and 75% of CO were assigned. 29 out of 44 unassigned residues are within the sequence from Q238 to V297 (including the active site C264). Resonances from these residues shift significantly from their positions in aqueous solution and display pronounced exchange broadening. This indicates that the binding of hAtg3 to membrane mimicking bicelles induces profound structural and dynamic changes around the active site. In addition, we were able to assign most of the N-terminal first 25 residues, and a TALOS-N analysis of their secondary structural propensity indicates a conformational transition from a random coil to an α-helix upon interacting with bicelles, as expected (Fig. 1d).
Fig. 1:

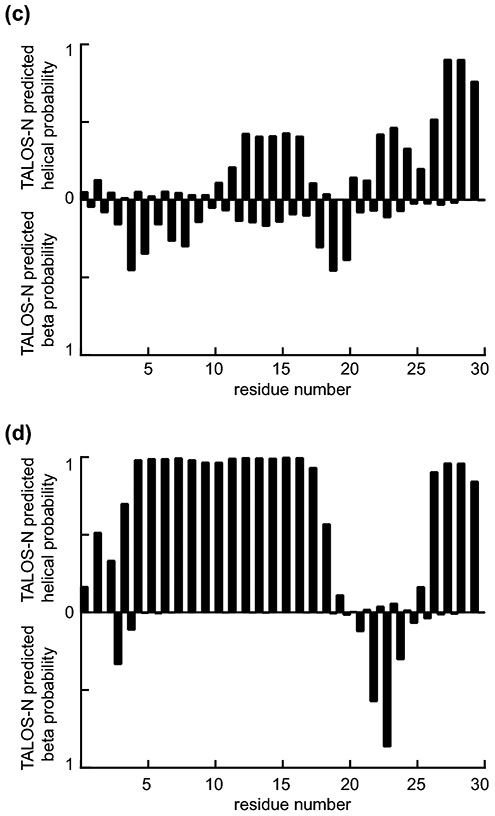
2H, 15N, 13C-labeled hAtg3Δ90-190 TROSY spectra in (a) aqueous solution and (b) bicelles with resonance assignments. Residue number corresponding to the wildtype protein is used (i.e. 1-89 and 191-314). Several peaks are not visible at the displayed contour level due to either incomplete amide 2H/1H back-exchange or exchange broadening. (c) and (d) show the secondary structural propensity for residues 1 to 30 of hAtg3Δ90-190 using TALOS-N in aqueous solution and bicelles, respectively. The N-terminus of hAtg3Δ90-190 rearranges to an α-helical conformation upon interacting with the membrane.
In summary, we have obtained sequential resonance assignments for the hAtg3Δ90-190 construct in aqueous solution and in bicelles. These assignments provide a starting point for examining hAtg3 interaction with the membrane, a critical component of hAtg3 activation and the regulation of LC3-PE conjugation during autophagy.
Acknowledgements
We gratefully acknowledge Dr. Xuejun Jiang’s lab for providing the expression vector. We are thankful for financial support from the National Institutes of Health NIGMS (1R01GM127730 and 1R01GM127954) and the Four Diamonds Fund.
Footnotes
Availability of data and material
The assignments of hAtg3Δ90-190 have been deposited to the BMRB under the accession codes: 50479 (in aqueous) and 50480 (in bicelles).
Conflicts of interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher's embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature's terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
References
- Bellstedt P, Seiboth T, Hafner S, Kutscha H, Ramachandran R and Gorlach M (2013) Resonance assignment for a particularly challenging protein based on systematic unlabeling of amino acids to complement incomplete NMR data sets. J Biomol NMR 57: 65–72. [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J and Bax A (1995) NMRPIPE - a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6: 277–293. [DOI] [PubMed] [Google Scholar]
- Hanada T, Satomi Y, Takao T and Ohsumi Y (2009) The amino-terminal region of Atg3 is essential for association with phosphatidylethanolamine in Atg8 lipidation. FEBS Letters 583: 1078–1083. [DOI] [PubMed] [Google Scholar]
- Hervas JH, Landajuela A, Anton Z, Shnyrova AV, Goni FM and Alonso A (2017) Human ATG3 binding to lipid bilayers: role of lipid geometry, and electric charge. Sci Rep 7: 15614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA and Blevins RA (1994) NMR View: A computer program for the visualization and analysis of NMR data. J Biomol NMR 4: 603–614. [DOI] [PubMed] [Google Scholar]
- Kaufmann A, Beier V, Franquelim HG and Wollert T (2014) Molecular mechanism of autophagic membrane-scaffold assembly and disassembly. Cell 156: 469–481. [DOI] [PubMed] [Google Scholar]
- Lamb CA, Yoshimori T and Tooze SA (2013) The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Bio 14: 759–774. [DOI] [PubMed] [Google Scholar]
- Metlagel Z, Otomo C, Takaesu G and Otomo T (2013) Structural basis of ATG3 recognition by the autophagic ubiquitin-like protein ATG12. Proc Natl Acad Sci U S A 110: 18844–18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T and Ohsumi Y (2011) The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 27: 107–132. [DOI] [PubMed] [Google Scholar]
- Nath S, Dancourt J, Shteyn V, Puente G, Fong WM, Nag S, Bewersdorf J, Yamamoto A, Antonny B and Melia TJ (2014) Lipidation of the LC3/GABARAP family of autophagy proteins relies on a membrane-curvature-sensing domain in Atg3. Nat Cell Biol 16: 821–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanna C, Dubey A and Atreya HS (2015) Amino acid selective unlabeling in protein NMR spectroscopy. Isotope Labeling of Biomolecules - Labeling Methods 565: 167–189. [DOI] [PubMed] [Google Scholar]
- Sawa-Makarska J, Abert C, Romanov J, Zens B, Ibiricu I and Martens S (2014) Cargo binding to Atg19 unmasks additional Atg8 binding sites to mediate membrane–cargo apposition during selective autophagy. Nat Cell Biol 16: 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y and Bax A (2013) Protein backbone and sidechain torsion angles predicted from NMR chemical shifts using artificial neural networks. J Bio NMR 56: 227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verardi R, Traaseth NJ, Masterson LR, Vostrikov VV and Veglia G (2012) Isotope labeling for solution and solid-state NMR spectroscopy of membrane proteins. Isotope Labeling in Biomolecular NMR 992: 35–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuister GW, Kim SJ, Wu C and Bax A (1994) 2D and 3D NMR-study of phenylalanine residues in proteins by reverse isotopic labeling. J Am Chem Soc 116: 9206–9210. [Google Scholar]
- Yamada Y, Suzuki NN, Hanada T, Ichimura Y, Kumeta H, Fujioka Y, Ohsumi Y and Inagaki F (2007) The crystal structure of Atg3, an autophagy-related ubiquitin carrier protein (E2) enzyme that mediates Atg8 lipidation. J Biol Chem 282: 8036–8043. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Matoba K, Sawada R, Fujioka Y, Nakatogawa H, Yamamoto H, Kobashigawa Y, Hoshida H, Akada R, Ohsumi Y, Noda NN and Inagaki F (2012) Noncanonical recognition and UBL loading of distinct E2s by autophagy-essential Atg7. Nat Struct Mol Biol 19: 1250–1256. [DOI] [PubMed] [Google Scholar]
- Yang Z and Klionsky DJ (2009) An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol 335: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye YS, Tyndall ER, Bui V, Tang ZY, Shen Y, Jiang XJ, Flanagan JM, Wang HG and Tian F (2021) An N-terminal conserved region in human Atg3 couples membrane curvature sensitivity to conjugase activity during autophagy. Nat Commun 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YM, Qiu Y, Grace CRR, Liu X, Klionsky DJ and Schulman BA (2019) A switch element in the autophagy E2 Atg3 mediates allosteric regulation across the lipidation cascade. Nat Commun 10: 3600. [DOI] [PMC free article] [PubMed] [Google Scholar]


