Abstract
Anemia is common in patients with chronic kidney disease. Treatment with erythropoiesis-stimulating agents has decreased transfusion rates, but has not been consistently shown to improve cardiovascular outcomes or quality of life. Moreover, treatment to hemoglobin levels normal for the general population (13–14 g/dL) has resulted in increased cardiovascular morbidity and mortality versus lower hemoglobin targets, and some patients with chronic kidney disease do not reach these lower hemoglobin targets despite escalating doses of erythropoiesis-stimulating agents. The pathophysiology of anemia in patients with chronic kidney disease has been informed by the discovery of hypoxia-inducible factor and hepcidin pathways. Recent innovations in anemia treatment leverage knowledge of these pathways to effectively raise hemoglobin levels independent of erythropoiesis-stimulating agent administration. Several agents that stabilize hypoxia-inducible factor are undergoing or have completed phase 3 clinical trials. These agents appear to have equal efficacy as erythropoiesis-stimulating agents in raising hemoglobin levels and have not been associated with major safety signals to date. Because of the potential for off-target effects from non–anemia-related gene transcription by hypoxia-inducible factor stabilization, longer-term follow-up studies and registries will be needed to ensure safety. Agents that modulate hepcidin have undergone early clinical trials with mixed results regarding safety and efficacy in increasing hemoglobin levels. Sodium–glucose cotransporter 2 inhibitors, which also decrease hepcidin levels, have been associated with increased hemoglobin levels among patients with chronic kidney disease in clinical trials exploring proteinuria and kidney disease progression.
Keywords: anemia, chronic kidney disease, erythropoietin, hepcidin, hypoxia-inducible factor, iron
Anemia in CKD: Prevalence and Pathophysiology
Anemia is common in patients with chronic kidney disease (CKD)1,2 and is associated with increased risk of cardiovascular events and hospitalization,3,4 progression to end-stage kidney disease,4 death,4,5 and decreased quality of life.3,4 On the basis of data from the United States (US) National Health and Nutrition Examination Survey in 2007–2008 and 2009–2010 and the World Health Organization’s definition of anemia as hemoglobin (Hb) < 13 g/dl in men and < 12 g/dl in women, anemia is twice as prevalent in people with CKD (15.4%) as in the general population (8.4%).1 The prevalence of anemia increases with stage of CKD, with 8.4% at stage 1, 12.2% at stage 2, 17.4% at stage 3, 50.3% at stage 4, and 53.4% at stage 5.1 Among US patients aged 65 to 88 years, the incidence of anemia increases with stage of CKD: 43.9% at stage 3 CKD, 64.0% at stage 4, and 72.8% at stage 5.2 Because the kidneys are the major source of erythropoietin (EPO), the hormone responsible for the production of red blood cells (RBCs) by the bone marrow, anemia of CKD was originally thought to simply represent a condition of EPO deficiency due to the loss of EPO-producing cells.
The US Food and Drug Administration approval of recombinant human EPO in 1989 held promise that a safe and effective treatment for the anemia of CKD would universally decrease the morbidity and mortality associated with the condition. Recombinant human EPO and its derivatives, collectively known as erythropoiesis-stimulating agents (ESAs), did not live up to this promise due to safety issues and blunted efficacy in some patients.
Although a variety of agents stimulate erythropoiesis and could technically be classified as ESAs, the term ESAs in this review will be reserved for pharmacologic agents that directly interact with the EPO receptor. When used to target normal (13–14 g/dl) as opposed to subnormal (9–11 g/dl) Hb levels, treatment with ESAs was associated with increased cardiovascular events in hemodialysis (HD)10 and non–dialysis-dependent (NDD) patients with CKD.7,8 The Food and Drug Administration released an announcement in 2011 recommending more conservative dosing of ESAs in anemic patients with CKD,9 which is reflected in a revised boxed warning for the drugs.10 Anemia of CKD is now understood to be multifactorial in nature, including decreased EPO production, iron deficiency, inflammation, blood loss, and reduced RBC survival.
ESA-Hyporesponsiveness
Since randomized controlled trials targeting normal versus subnormal Hb levels with ESAs6, 7, 8 were designed to examine target Hb level versus outcomes rather than ESA dose versus outcomes, the linking of adverse outcomes with high ESA dose can be interpreted as an association, not cause-and-effect. A post hoc analysis of the Correction of Hemoglobin with Epoetin Alfa in Chronic Kidney Disease (CHOIR)7 study revealed that high-dose epoetin was associated with a significant increased risk of the primary end point (death, myocardial infarction, congestive heart failure, and stroke) irrespective of the patient’s target Hb assignment or achieved Hb level. There was no risk associated with the higher target Hb (13.5 g/dl) if that target was achieved in the CHOIR study11; nonetheless, potential harm of higher achieved Hb level cannot be excluded.
It remains unclear whether poorer outcomes among patients failing to achieve target Hb levels with ESA treatment (ESA-hyporesponsiveness) are due to the comorbidities that may be contributing to the ESA-hyporesponsiveness or due to toxicity of ESAs. The association among ESA-hyporesponsiveness, major adverse cardiovascular events (MACEs), and all-cause mortality has been reported in observational studies,12, 13, 14, 15, 16 but confounding by comorbidities and indication cannot be completely excluded.
The causes of ESA-hyporesponsiveness were reviewed by Ogawa et al.,17 the most common being iron deficiency, which can be absolute or functional. Absolute iron deficiency is defined as transferrin saturation (TSAT) < 20%, serum ferritin < 100 ng/ml in NDD-CKD and peritoneal dialysis patients and < 200 ng/ml in HD patients; it generally responds to oral or i.v. iron supplementation.18 Functional iron deficiency is associated with TSAT < 20% and serum ferritin higher than those noted above, it is more challenging to overcome, and our understanding of its physiologic basis has provided insight to the development of new approaches to treating anemia of CKD.
Hepcidin
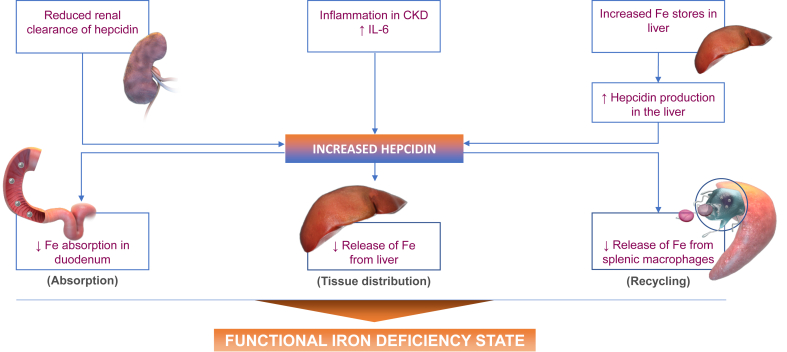
Hepcidin, a peptide produced in the liver, was discovered in 2000 and is the key regulator of iron use, recycling, and transport.19, 20, 21 Its physiologic role is to protect the organism against iron overload and to sequester iron in storage sites in the presence of infection by siderophilic pathogens. Hepcidin synthesis is stimulated by inflammation and increased storage iron and is decreased by iron deficiency and erythropoiesis. Inflammatory cytokines, especially interleukin 6 (IL-6), stimulate synthesis of hepcidin, which, by internalizing the ferroportin iron exporter on duodenal enterocytes and macrophages, leads to decreased iron absorption by the gut and decreased iron release from storage sites in the liver and spleen. This results in functional iron deficiency with less circulating iron available for erythropoiesis (represented by low TSAT) and increased storage iron (represented by high ferritin).
Because CKD is an inflammatory condition itself and may be compounded by conditions associated with inflammation, such as diabetes, heart failure, and the HD procedure, hepcidin levels are elevated in patients with CKD and rise as the CKD stage advances.22,23 Higher hepcidin levels in patients with CKD are associated with higher serum ferritin and lower Hb levels.23
Erythroferrone is produced by RBC precursors in the bone marrow in response to EPO stimulation and leads to downregulation of hepcidin synthesis.24 Although ESA treatment has been to shown to decrease hepcidin levels in patients with CKD,23 it is not clear why ESA-induced stimulation of erythroferrone does not overcome the hepcidin-induced functional iron deficiency that may lead to ESA-hyporesponsiveness in many anemic patients with CKD. The pathophysiology of hepcidin in CKD is summarized in Figure 1.
Figure 1.
Hepcidin pathway. CKD, chronic kidney disease; IL-6, interleukin 6; Fe, iron.
Hypoxia-Inducible Factor
A second breakthrough in our understanding of the pathophysiology of anemia in CKD was the elucidation of the hypoxia-inducible factor (HIF) pathway in the late 1990s by Kaelin and Ratcliffe25 and Semenza,26 who shared the 2019 Nobel Prize in Physiology and Medicine for this discovery. The HIF family is central to the cellular, tissue, and systemic response to decreases in oxygen availability.25,26 The spectrum of responses to hypoxia includes angiogenesis, ventilation, adenosine 5ʹ-triphosphate production, and the switch from aerobic to anaerobic metabolism. The HIF pathway also has a central role in the entirety of the erythropoietic response to hypoxia, including EPO stimulation and iron mobilization.
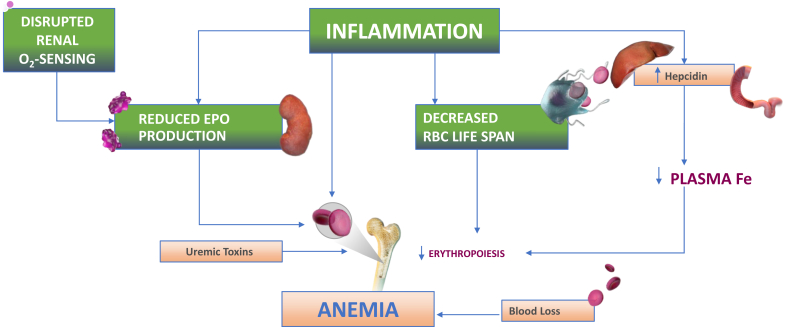
In healthy adults, peritubular cells in the corticomedullary area of the kidneys are responsible for EPO production; in patients with CKD, EPO production may be augmented by perisinusoidal hepatic cells.27 The location of renal EPO-producing cells in a zone of the kidney with relative hypoxia makes them sensitive to small decreases in oxygen content, leading to an increase in HIF, increased transcription of the EPO gene, increased levels of circulating EPO, and increased erythropoiesis.
HIF has 3 isoforms, HIF-1, HIF-2, and HIF-3, each of which contains an α-subunit unique to the isoform and a common β-subunit. The controlling isoform for EPO production is HIF-2. HIF-2 also stimulates iron absorption in the duodenum by increasing the transcription of genes that encode for proteins augmenting iron transport, including divalent metal transporter 1 and duodenal cytochrome B. HIF-1 augments the transcription of additional genes that encode proteins involved in iron mobilization, including ceruloplasmin and transferrin.28
HIF activation increases the expression of EPO and transferrin receptors on erythroblasts and decreases apoptosis of erythroid progenitors in the bone marrow.29 The pathophysiology of decreased EPO production in patients with CKD appears to be due more to abnormalities in oxygen sensing than by a loss of renal EPO-producing cells.30 Decreased renal blood flow and anemia as CKD progresses lead to diminished oxygen delivery to the kidneys. The diminished glomerular filtration rate results in a decrease in filtered sodium and decreased tubular sodium reabsorption which decreases oxygen utilization. Despite the hypoxia caused by CKD anemia, the simultaneous decreased oxygen use by the kidneys results in no change or an increase in local oxygen sensed by the renal EPO-producing cells,27,31 which is compounded by the loss of renal EPO-producing cells due to parenchymal fibrosis.32 The multiple factors contributing to anemia in CKD are illustrated in Figure 2.
Figure 2.
Pathophysiology of anemia in chronic kidney disease. EPO, erythropoietin; Fe, iron; O2, oxygen; RBC, red blood cell.
Anemia in CKD: New Approaches to Treatment
Our understanding of the roles of hepcidin and HIF in the pathogenesis of CKD anemia has led to the development of innovative approaches to anemia treatment. Despite the important role of absolute and functional iron deficiency in CKD anemia, this review will not discuss newer oral, i.v., and dialysate-delivered iron preparations and will focus on interventions that leverage the HIF and hepcidin pathways.
HIF Stabilizers
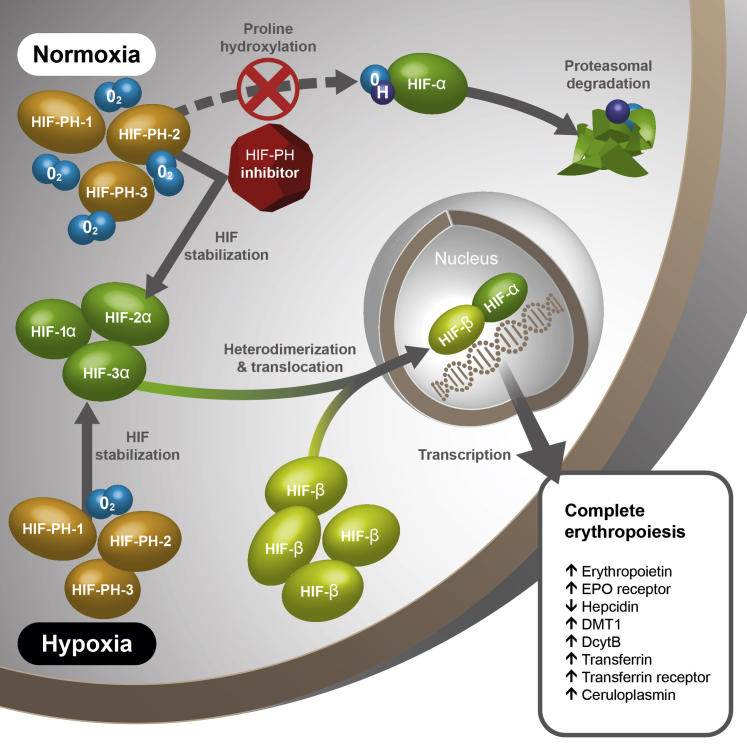
The HIF-α and HIF-β subunits form a heterodimer and travel to the cell nucleus to induce transcription of HIF-responsive element genes. In the presence of oxygen, which activates a prolyl hydroxylase (PH) enzyme, HIF-α undergoes hydroxylation of 2 proline residues that target it for degradation. In the absence of oxygen, HIF-α escapes degradation and is available to dimerize with HIF-β, which is continuously available. An essential cofactor for this PH is 2-oxaglutarate. Small-molecule orally administered analogues of 2-oxaglutarate have been shown to inactivate HIF-PH in the presence of oxygen and thus function as HIF stabilizers. On the basis of this mechanism of action, these compounds are known as HIF-PH inhibitors (HIF-PHIs).31,33, 34, 35
PH has 3 domains: PHD-1, PHD-2, and PHD-3, and the HIF-PHIs under development have differing specificities for these PHDs.35,36 Phase 3 programs with 6 HIF-PHIs are ongoing or have been completed. The pharmacologic characteristics of these agents are summarized in Table 1.34,35 As of this writing, 4 agents have been licensed in Japan: roxadustat, vadadustat, daprodustat, and enarodustat. Roxadusat is licensed in China and Chile. Because of their effects on transcription of genes related to iron absorption, mobilization, and transport, as well as their indirect suppression of hepcidin though erythroferrone, HIF-PHIs would be expected to decrease ferritin and hepcidin and increase serum iron (Figure 3).33
Table 1.
Hypoxia-inducible factor stabilizers under developmenta
| Variable | Daprodustat | Desidustat | Enarodustat | Molidusat | Roxadustat | Vadadustat |
|---|---|---|---|---|---|---|
| Investigational designation | GSK1278863 | ZYAN1 | JTZ-951 | BAY 85-3934 | FG-4592 ASP1517 AZD9941 |
AKB-6548 MT-6548 |
| PHD isoform specificity | PHD2 > PHD1 > PHD3 | Not reported | PHD3 > PHD2 > PHD1 | PHD2>PHD1>PHD3 | PHD1 > PHD3 > PHD2 | PHD2 > PHD1 > PHD3 |
| Effective half-life (h) | 2.25 | 7.0–11.4 | 8.96 | 4–10 | 12–15 | 4.9–9.1 |
| Dosing interval | Daily | Daily | Daily | Daily | 3 times weekly | Daily |
Figure 3.
Hypoxia-inducible factor (HIF) pathway. DcytB, duodenal cytochrome B; DMT, divalent metal transporter; EPO, erythropoietin; O2, oxygen; PH, prolyl hydroxylase. Reproduced from Gupta and Wish.33
A 2021 meta-analysis of 12 phase 2 and 3 randomized controlled trials of HIF stabilizers in patients with NDD-CKD with placebo and active controls37 demonstrated statistically significant changes in TSAT% (−4.51), ferritin (−47.29 ng/ml), hepcidin (−0.94 ng/ml), and total iron binding capacity (TIBC; +9.15 μg/dl), with no change in serum iron versus the comparator. Another 2021 meta-analysis of 15 phase 2 and 3 trials of these agents38 demonstrated reduction in serum ferritin (P < 0.01) among dialysis-dependent (DD-CKD) versus control participants but not among NDD-CKD patients (P = 0.11). Hepcidin reduction versus control in DD-CKD was not significant for the group of HIF-PHIs (P = 0.15) but was significant for roxadustat (P < 0.01). In NDD-CKD, hepcidin reduction versus control was significant for the group of HIF-PHIs (P < 0.05).
A 2021 meta-analysis of 19 studies of HIF-PHIs in patients with NDD-CKD39 demonstrated all 6 HIF-PHIs, except vadadustat, increased Hb levels significantly compared with placebo, and all HIF-PHIs demonstrated noninferior efficacy to ESAs. An excessive risk of all-cause mortality with HIF-PHIs was not found. Phase 3 global trials including the US have been completed for roxadustat and vadadustat as of this writing and are nearing completion for daprodustat. Efficacy (Hb increase) and safety (MACEs) have been evaluated by comparisons with placebo (in NDD-CKD patients) or ESA (in NDD- and DD-CKD patients). Robust statistical assessment of MACEs (all-cause mortality, nonfatal myocardial infarction, and nonfatal stroke) has required large numbers of participants monitored ≥ 2 years to determine whether HIF-PHIs share the risks of ESAs.6, 7, 8
Roxadustat
A phase 3 study from China of 305 DD-CKD patients randomized to roxadustat versus epoetin demonstrated noninferior efficacy and similar adverse events in the 2 arms, except for an increased incidence of hyperkalemia with roxadustat.40 A phase 3 study of 154 NDD-CKD patients randomized to roxadustat versus placebo demonstrated a significant Hb increase of ~2 g/dl greater than placebo.41 Adverse events were similar in the 2 arms, except for an increased incidence of hyperkalemia and metabolic acidosis with roxadustat.41 Earlier-phase studies of HIF-PHIs also reported hyperkalemia,42, 43, 44 the mechanism of which is not understood.
A phase 3 study from Japan45 of 99 ESA-naïve partially iron-depleted NDD-CKD patients with mean baseline Hb 9.82 g/dl treated with roxadustat demonstrated an overall response rate of 97% (Hb > 10.0 g/dl) and 94.9% (Hb > 10.5 g/dl). Nasopharyngitis and hypertension were the most common adverse events.
A 2020 meta-analysis of 6 phase 2 and 3 studies of roxadustat revealed increased transferrin levels versus placebo and epoetin, decreased ferritin and TSAT versus control in NDD-CKD but not DD-CKD patients, and decreased hepcidin versus control in all patients.46
A 2021 meta-analysis of 8 phase 2 and 3 studies of roxadustat demonstrated significant increases versus control (placebo and ESAs) in TIBC (P < 0.00001) and serum iron level (P = 0.05), and significant reductions in hepcidin (P < 0.0001) and ferritin (P < 0.00001).47
The global phase 3 program for roxadustat includes 4 studies in NDD-CKD patients: Treatment of Anemia in Chronic Kidney Disease Patients Not Requiring Dialysis (ALPS), A Study of FG-4592 for the Treatment of Anemia in Chronic Kidney Disease Patients Not Receiving Dialysis (ANDES), and Safety and Efficacy Study of Roxadustat to Treat Anemia in Patients With Chronic Kidney Disease (CKD), Not on Dialysis (OLYMPUS) using placebo control; Roxadustat in the Treatment of Anemia in Chronic Kidney Disease (CKD) Patients, Not on Dialysis, in Comparison to Darbepoetin Alfa (DOLOMITES) using ESA control; and 4 studies in DD-CKD patients: Safety and Efficacy Study for Treatment of Anemia in ESRD Newly Initiated Dialysis Patients (Himalayas), Safety and Efficacy Study of Roxadustat to Treat Anemia in Patients With Chronic Kidney Disease, on Dialysis (ROCKIES), and Evaluation of Efficacy and Safety of Roxadustat in the Treatment of Anemia in Stable Dialysis Subjects (SIERRAS] using epoetin control, and Roxadustat in the Treatment of Anemia in End Stage Renal Disease (ESRD) Patients on Stable Dialysis (PYRENEES) using epoetin or darbepoetin control.
The first 2 publications48,49 from the roxadustat global phase 3 program appeared in KI Reports in 2021, accompanied by an editorial.50 In the ANDES study of NDD-CKD patients,48 915 patients were randomized 2:1 to roxadustat or placebo. The mean Hb change from baseline over weeks 28 to 52 was significantly greater for roxadustat (mean, 2.00 g/dl) than placebo (0.16 g/dl; P < 0.0001), the portion of patients achieving an Hb response (Hb ≥ 11.0 g/dl and increase ≥ 1.0 g/dl for baseline > 8.0 g/dl or increase > 2.0 g/dl for baseline ≤ 8.0 g/dl) was 95% for roxadustat and 6.6% for placebo (P < 0.0001), and fewer patients receiving roxadustat (8.9%; placebo, 28.9%) required rescue therapy (RBC transfusion, ESA, or i.v. iron) at week 52 (hazard ratio [HR], 0.19; P < 0.0001). Treatment-emergent adverse events (TEAEs) and treatment-emergent serious adverse events (TESAEs) were comparable between the 2 groups.
In the OLYMPUS study of NDD-CKD patients,51 2781 patients were randomized 1:1 to roxadustat or placebo. The mean Hb change from baseline over weeks 28 to 52 was significantly larger for roxadustat (mean 1.75 g/dl) than placebo (0.40 g/dl; P < 0.01). Among 411 patients with elevated baseline high-sensitivity C-reactive protein, the Hb change from baseline was 1.75 g/dl with roxadustat versus 0.62 g/dl with placebo (P < 0.001). Roxadustat reduced the risk of RBC transfusion by 63% versus placebo. TEAEs were comparable between the 2 groups.
In the pooled analysis of ALPS, ANDES, and OLYMPUS, efficacy results were similar to the individual studies noted above, and the increase in Hb was not affected by iron status.52 The time to first MACE in the NDD-CKD pooled analysis was noninferior for roxadustat versus placebo (HR, 1.08; 95% CI, 0.94–1.24), and the overall rate of TEAEs and TESAEs was similar between the 2 arms. The rate of hyperkalemia was 10.9% with roxadustat versus 7.1% with placebo. The increase in Hb levels among roxadustat-treated patients was similar irrespective of baseline high-sensitivity C-reactive protein level52 and was not influenced by iron status.53 In one of these NDD-CKD studies, roxadustat-treated patients experienced a 29.9% decrease in hepcidin versus a 4% decrease among patients in the placebo group.52 In the DOLOMITES study of NDD-CKD patients using active ESA control, roxadustat demonstrated noninferiority in Hb correction and blood pressure control with superiority in low-density lipoprotein cholesterol reduction and time to first i.v. iron use during first 36 weeks of therapy (HR, 0.46; P = 0.006).54
The pooled analysis of global phase 3 studies of roxadustat in DD-CKD patients52,55 includes HIMALAYAS, ROCKIES, and SIERRAS studies with 3950 patients monitored for a mean of 1.71 years in the roxadustat-treated group and 1.92 years in the epoetin-treated group. Although the roxadustat-treated patients experienced a greater Hb increase, this may have reflected different dose titration protocols in the 2 arms. Blood transfusions were fewer in the roxadustat-treated patients (P = 0.046), and there was 11% reduction in i.v. iron treatment in the roxadustat-treated patients, which also may have reflected different iron management protocols in the 2 arms. The time to first MACEs was similar between roxadustat- and epoetin-treated patients, (HR, 1.02; 95% CI, 0.88–1.20). In a prespecified subanalysis of 1530 incident (randomized during first 4 months of dialysis) DD-CKD patients, treatment with roxadustat demonstrated noninferiority for MACEs (HR, 0.82; 95% CI, 0.60–1.11), MACEs plus hospitalization for heart failure and unstable angina (HR, 0.78; 95% CI, 0.59–1.02), and all-cause mortality (HR, 0.82; 95% CI, 0.57–1.18).49,55 Among DD-CKD patients in the pooled analysis, the rate of adverse events and hyperkalemia was similar in the 2 groups, although arteriovenous fistula thrombosis rate (5.2 vs. 3.9 per 100 patient-years), deep vein thrombosis (0.7 vs. 0.2 patient-years), and seizures (2.0% vs. 0.7%) were all higher in the roxadustat- versus epoetin-treated patients, respectively.53
In the European PYRENEES phase 3 study of 838 DD-CKD patients treated with roxadustat or ESA for 52 to 104 weeks, roxadustat demonstrated noninferiority in maintaining stable Hb levels and superiority in low-density lipoprotein cholesterol reduction from baseline and reduction in i.v. iron use. However, there were imbalances in the occurrence of TESAEs leading to patient death and withdrawal over time, with HRs favoring ESA treatment. None of these safety differences could be explained by baseline disease factors.56
A cost-effectiveness analysis of roxadustat was performed by the Institute for Clinical and Economic Review in March 2021.57 Key policy recommendations are summarized in Table 2.57
Table 2.
Key policy recommendations from the institute for clinical and economic review regarding roxadustat57
|
ESAs, erythropoiesis-stimulating agents.
Vadadustat
A 52-week phase 3 study of vadadustat versus darbepoetin among 323 DD-CKD patients in Japan revealed efficacy noninferiority for vadadustat and no safety concerns.58 In an open-label phase 3, 24-week study of 42 Japanese peritoneal dialysis patients, vadadustat was well-tolerated and maintained target Hb levels.59 The global phase 3 program for vadadustat includes Efficacy and Safety Study to Evaluate Vadadustat for the Maintenance Treatment of Anemia in Subjects With Dialysis-dependent Chronic Kidney Disease (DD-CKD) (INNO2VATE) for DD-CKD patients60 and Efficacy and Safety Study to Evaluate Vadadustat for the Correction of Anemia in Subjects With Non-dialysis-dependent Chronic Kidney Disease (NDD-CKD) (PRO2TECT) for NDD-CKD patients.61
The INNO2VATE program includes 2 studies, 1 in incident and 1 in prevalent DD-CKD patients using darbepoetin comparator, totaling 3923 individuals.62 The Hb level in both studies was lower with vadadustat at 24 to 36 and 40 to 52 weeks, but this may have reflected different dose titration protocols in the 2 arms. In the pooled INNO2VATE analysis of cardiovascular safety, vadadustat was noninferior for a first MACE event (HR, 0.96; 95% CI, 0.83–0.11) and time to expanded MACE (MACE plus hospitalization for heart failure or thromboembolic event excluding vascular access thrombosis; HR, 0.96; 95% CI, 0.84–1.10). There were no clinically meaningful differences in TEAEs or TESAEs between vadadustat- and darbepoetin-treated individuals.
The PRO2TECT program includes 2 studies with darbepoetin comparator, 1 in ESA-naïve patients and 1 in ESA-treated patients, with a total of 3476 NDD-CKD patients.63 The Hb target was 10 to 11 g/dl among US subjects and 10 to 12 g/dl among non-US subjects. Vadadustat demonstrated noninferior efficacy compared with darbepoetin. The primary safety end point was MACEs (time frame from baseline visit to end of study, event driven, minimum 1 year). A first pooled MACE occurred in 22% of vadadustat-treated patients and in 19.9% of darbepoetin-treated patients (HR, 1.17; 95% CI, 1.01–1.36), which did not meet the prespecified margin for safety noninferiority. All increases in MACE risk occurred in the non-US study population. Whether this discrepancy was related to the difference in Hb targets between US patients and non-US patients or other factors is unclear.
Daprodustat
A meta-analysis of 8 phase 2 daprodustat studies in NDD- and DD-CKD patients versus placebo or ESA revealed daprodustat to be superior in efficacy to placebo and noninferior to ESA. Iron metabolism indices were improved significantly with daprodustat versus placebo or ESA. TESAEs were comparable to placebo and lower than ESAs. There was no trend of increasing plasma vascular endothelial growth factor among daprodustat-treated individuals.64 Despite its relatively short half-life (Table 1), a 29-day phase 2 study of daprodustat in 103 HD patients demonstrated efficacy of daprodustat when administered 3-times weekly at approximately twice the daily dose.65
In a phase 3 study in Japan, 271 HD patients were randomized to continue darbepoetin or conversion to daprodustat with a 52-week follow-up.66 Hb was maintained equally with daprodustat versus darbepoetin. Iron treatment (i.v.) was required in 32% of patients receiving daprodustat versus 43% receiving darbepoetin. In a 52-week phase 3 study in Japan of 299 NDD-CKD patients with anemia randomized to daprodustat or epoetin beta pegol, daprodustat demonstrated noninferiority in maintaining target Hb levels and no meaningful difference in frequency of TEAEs.67
The global phase 3 program for daprodustat includes 5 Anemia Studies in Chronic Kidney Disease (CKD): Erythropoiesis Via a Novel Prolyl Hydroxylase Inhibitor (PHI) Daprodustat (ASCEND) studies68: ASCEND in Incident Dialysis (ASCEND-ID) (330 incident dialysis patients, daprodustat administered daily, comparator darbepoetin), ASCEND-Three-times Weekly Dosing in Dialysis (ASCEND-TD) (407 HD patients, daprodustat administered 3-times weekly, comparator epoetin or placebo), ASCEND in Non-Dialysis Subjects Evaluating Hemoglobin (Hgb) and Quality of Life (ASCEND-NHQ) (600 NDD-CKD patients, daprodustat administered daily, comparator placebo), ASCEND-dialysis (ASCEND-D) (2964 HD patients, daprodustat administered daily, comparator epoetin), and ASCEND-Non-Dialysis (ASCEND-ND) (4000 NDD-CKD patients, daprodustat administered daily, comparator darbepoetin).
Molidustat
The 16-week phase 2 DIALOGUE (DaIly orAL treatment increasing endOGenoUs Erythropoietin) studies of molidustat69 revealed efficacy in raising Hb levels to target range and tolerance using placebo (NDD-CKD) or ESA comparators (NDD- and DD-CKD). In anemia treatment–naïve patients receiving molidustat, concentrations of TSAT, hepcidin, and iron decreased with molidustat, whereas TIBC increased. In previously ESA-treated HD patients, hepcidin and TIBC remained stable with molidustat, whereas TSAT, ferritin, and iron increased.70 Three 36-month extension studies showed molidustat was well tolerated and of comparable efficacy to ESAs.71
The phase 3 MIYABI (MolIdustat once dailY improves renal Anaemia By Inducing erythropoietin) program for molidustat includes 3 studies in DD-CKD patients: Hb correction in ESA-naïve patients (MIYABI-C), Hb maintenance in ESA-treated patients (MYABI-M), and peritoneal dialysis patients (MYABI-PD)72; and 2 studies in NDD-CKD patients: Hb correction in ESA-naïve patients (MIYABI ND-C), and Hb maintenance in ESA-treated patients (MIYABI ND-M).73 In the MYABI-M study, 229 patients were randomized to 2:1 to convert to molidustat or continue darbepoetin and monitored for 52 weeks. Efficacy noninferiority was established for molidustat, and there were no apparent between-group differences in TEAEs, TESAEs, or adverse events of special interest.74
Enarodustat
Phase 2 studies of enarodustat in Japan demonstrated a dose-dependent increase in Hb level, tolerability, decreased hepcidin and ferritin levels, and increased TIBC in NDD-CKD75 and DD-CKD76 patients. The Phase 3 Study of Enarodustat in Anemic Patients with CKD not Requiring Dialysis (SYMPHONY) program in Japan includes studies of HD and NDD-CKD patients. In the 24-week study of HD patients, 173 patients were randomized 1:1 to enarodustat or darbepoetin. Enarodustat was noninferior in efficacy (Hb level in target range), produced an increase in TIBC and a decrease in hepcidin after switching from ESA, and had comparable TEAEs and TESAEs to control.77 In the 24-week study of NDD-CKD patients, 216 individuals were randomized 1:1 to enarodustat or darbepoetin. Enarodustat was noninferior in efficacy (Hb level in target range), produced an increase in TIBC and a decrease in hepcidin versus darbepoetin, and had comparable TEAEs, including cardiovascular and hypertension-related events to control.78
Desidustat
A phase 2 study of desidustat in 117 anemic NDD-CKD patients79 demonstrated dose-related increases in Hb levels. There were no TESAEs. Two 24-week phase 3 clinical trials are underway in India: a 392-participant open-label randomized trial versus epoetin in DD-CKD patients (Desidustat in the Treatment of Anemia in CKD on Dialysis Patients [DREAM-D]),80 and a 588-participant open-label randomized trial versus darbepoetin in anemic NDD-CKD patients (DREAM-ND).81
Risks of HIF Stabilizers
Because of their mechanism of action to stimulate the transcription of hypoxia-responsive genes, concerns remain regarding the potential for HIF stabilizers to produce off-target effects, including angiogenesis, thrombotic events, pulmonary hypertension, kidney fibrosis, and acceleration of cyst growth in patients with polycystic kidney disease. Furthermore, approximately 60 human 2-oxaglutarate oxygenases other than HIF-PH are involved in a variety of cellular functions, including collagen biosynthesis, nucleic acid repair, protein biosynthesis, and fatty acid metabolism. Off-target inhibition by PHD inhibitors of these 2-oxagulatarate oxygenases may also be undesirable.36
Phase 3 clinical trials of sufficient duration and statistical robustness to assess MACEs and other cardiovascular end points may not be adequate to address some of these other potential complications of HIF-stabilizer therapy, which may be less frequent and take longer to manifest. With regards to angiogenesis, studies of vadadustat and daprodustat examined vascular endothelial growth factor levels and reported no consistent increases,34,64 data from phase 3 trials do not show acceleration of diabetic retinopathy,66,82 and studies to date have not demonstrated any increase in malignancies.83
Thrombotic events were increased in DD-CKD patients treated with roxadustat versus epoetin,52 but similar findings have not been reported in other phase 3 studies of HIF stabilizers. Pulmonary arterial hypertension is common in patients on dialysis, and phase 3 trials may not offer certainty regarding the role of HIF stabilizers due to the dedicated examinations needed. However, there has been no significant increase in the incidence of heart failure-related TEAEs and TESAEs, which could be due to worsening of pulmonary arterial hypertension.
Kidney fibrosis may be enhanced by 2-oxaglutarate oxygenases, but studies to date have not demonstrated acceleration of renal function decline among patients treated with HIF stabilizers. Because cyst growth and progression of polycystic kidney disease may be affected by the HIF pathway, ongoing and completed phase 3 trials of HIF stabilizers that include monitoring for cyst growth should offer useful information. Dedicated studies of patients with polycystic kidney disease may be necessary.
Hyperkalemia is an unexpected finding in some phase 3 clinical trials40,41,52 and requires further exploration. In their KI Reports commentary, Winkelmayer and Walther50 raise the question whether an assumption of class homogeneity will be met for HIF stabilizers as further phase 3 and follow-up studies are published. Because HIF stabilizers have differing PH specificity and pharmacokinetics (Table 1), a class effect cannot be assumed.
After the approval of HIF stabilizers in Japan and China, the Asian-Pacific Society of Nephrology published recommendations on the appropriate use of these agents.84 Key recommendations are summarized in Table 3.84
Table 3.
Key recommendations of the Asian-Pacific Society of Nephrology regarding the use of hypoxia-inducible factor prolyl hydroxylase inhibitors84
|
CKD, chronic kidney disease; DD, dialysis dependent; ESA, erythropoiesis-stimulating agent; HIF-PH, hypoxia-inducible factor prolyl hydroxylase; NDD, non–dialysis dependent; TSAT, transferrin saturation.
Hepcidin Modulators
Inhibitors of Hepcidin, Its Synthetic Pathway, and Its Action
Phase 1 trial data have been reported on several agents that block the activity of hepcidin, thereby improving iron mobilization and potentially treating anemia in patients with CKD. An anti–hepcidin l-oligribonucleotide (lexaptepid) suppressed hepcidin activity in healthy volunteers85 and produced dose-dependent increases in serum iron, ferritin, and TSAT.86 A study of lexaptepid in 33 ESA-hyporesponsive HD patients was completed in 201587 but not published. Monoclonal antibody LY2928052 targets ferroportin, and monoclonal antibody LY3113593 targets bone morphogenic protein (BMP) 6, a key regulator of hepcidin expression. Both agents have been shown to increase serum iron and TSAT in healthy volunteers. In phase 1 studies of patients with CKD, both agents increased serum iron and decreased serum ferritin relative to placebo, and LY3113593 decreased hepcidin levels.88 These phase 1 studies were conducted in 2015, and no later-stage clinical trials are reported on ClinicalTrials.gov.
Vitamin D directly represses transcription of the hepcidin antimicrobial peptide (HAMP) gene, which encodes hepcidin. Because vitamin D deficiency is common in patients with CKD, trials have been undertaken to determine whether vitamin D supplementation improves iron parameters, with mixed results. In a study of healthy volunteers, a single 100,000 IU oral dose of vitamin D2 decreased hepcidin levels by one-third.89 However, a randomized trial of 50,000 IU oral vitamin D2 weekly in HD patients produced no changes in serum ferritin, TSAT, or ESA dose at 3 or 6 months (hepcidin levels were not reported),90 and a study of calcitriol supplementation in patients with stage 3 or 4 CKD produced no changes in hepcidin, ferritin, TSAT, or Hb compared with placebo.91
Because IL-6 is one of the major mediators by which inflammation stimulates hepcidin synthesis, the effect of an anti–IL-6 ligand antibody, ziltivekimab, on anemia of inflammation in HD patients was explored in a phase 1/2 placebo-controlled trial.92 The researchers randomized 68 HD patients with elevated IL-6 to placebo or 1 of 3 ziltivekimab doses administered i.v. during HD every 2 weeks for 12 weeks. Compared with patients receiving placebo, those receiving the active drug had greater reductions in high-sensitivity C-reactive protein, serum amyloid A, and fibrinogen, as well as a decreased ESA resistance index and increased serum iron, TIBC, TSAT, and serum albumin. However, 17.6% of patients receiving the highest dose of the study drug had adverse events leading to study discontinuation (compared with 0% of patients receiving placebo or lower doses of the study drug). TEAEs were significantly higher at all doses of study drug compared with placebo, and 4 patients died during the study, all of whom received the 2 higher doses of the study drug.92
SGLT2 Inhibitors
SGLT2 inhibitors have been demonstrated to decrease proteinuria and slow the rate of progression of CKD in patients with93, 94, 95 and without95 type 2 diabetes mellitus (T2DM). In a study of 52 patients with T2DM randomized 1:1 to dapagliflozin or placebo for 12 weeks, dapagliflozin significantly reduced hepcidin and ferritin while increasing transferrin levels. The increase in Hb levels versus placebo was 0.5 g/dl (P = 0.02).96 A 104-week double-blind placebo-controlled trial of dapagliflozin (2.5, 5, or 10 mg) demonstrated an increase in hematocrit among patients with T2DM receiving the active drug. Mean increase in hematocrit from baseline at 104 weeks was 2.32, 2.95, and 3.06, respectively.97 Data from 5235 patients in 14 placebo-controlled dapagliflozin studies of at least 24 weeks’ duration were pooled, revealing correction of baseline anemia in 52% patients treated with dapagliflozin and in 26% patients treated with placebo. The placebo-adjusted mean Hb increase from baseline to week 24 among patients with baseline anemia treated with dapagliflozin was 0.53 g/dl and was 0.76 g/dl among patients without baseline anemia. New-onset anemia was also lower in dapagliflozin-treated patients.98
A post hoc analysis of the Canagliflozin and Renal Events in Diabetes With Established Nephropathy Clinical Evaluation (CREDENCE) trial of 4401 patients with T2DM randomized to 100 mg daily canagliflozin or placebo for a mean follow-up of 2.6 years was performed to determine an investigator-reported anemia event or treatment for anemia (ESAs, iron preparations, or RBC transfusions). Canagliflozin-treated patients had a lower incidence of composite anemia events (HR, 0.65; 95% CI, 0.55–0.77; P < 0.0001), anemia events alone (HR, 0.58; 95% CI, 0.47–0.72; P < 0.0001), initiation of iron therapy (HR, 0.64; 95% CI 0.52–0.80; P < 0.001), and need for ESAs (HR, 0.65; 95% CI, 0.46–0.91; P = 0.012).99 Nine patients with T2DM administered canagliflozin demonstrated an increase in serum EPO levels between 2 and 4 weeks, increased Hb by 12 weeks, and decreased ferritin levels.100 The benefits of SGLT2 inhibitors are likely a “class effect” that can be extrapolated from studies of specific agents.101 Additional large-scale clinical trials of SGLT2 inhibitors with anemia parameters as the primary outcome are needed to determine their role in anemia treatment, particularly with the advent of HIF stabilizer therapy.
Summary
The discoveries of hepcidin and the HIF pathway, as well of our understanding of the complex pathophysiology of anemia in patients with CKD, have led to the development of innovative pharmaceuticals for anemia treatment unimaginable 2 decades ago. HIF stabilizers are completing large phase 3 development programs to assess efficacy, cardiovascular safety, and TEAEs. However, because of the possibility of off-target effects, longer-term follow-up studies and registries will be needed. Their oral route of administration makes HIF stabilizers attractive in NDD-CKD and home dialysis patients, and their effects on iron mobilization offer promise of efficacy in ESA-hyporesponsive patients. Because endogenous EPO production stimulated by HIF stabilizers leads to lower blood EPO levels than pharmacologic doses of ESAs, HIF stabilizers held the promise of being associated with fewer cardiovascular events than ESAs. However, HIF stabilizers have demonstrated only noninferiority to ESAs with regards to cardiovascular outcomes in most studies reported to date.
Hepcidin modulators received considerable attention with phase 1 and 2 studies of several agents, but these development programs have waned, possibly due to the advent of HIF stabilizers that also decrease hepcidin levels.
SGLT2 inhibitors, which have been shown to decrease proteinuria and slow CKD progression in patients with T2DM and other proteinuric disorders, appear to offer the additional benefit of anemia reduction. Studies specifically addressing the role of these agents in anemia management of patients with stages 3 and 4 CKD are needed.
Disclosure
JBW has served on advisory boards for AstraZeneca, Akebia, Otsuka, Vifor, and Rockwell Medical. He is on speakers bureaus for AstraZeneca and Akebia.
References
- 1.Stauffer M.E., Fan T. Prevalence of anemia in chronic kidney disease in the United States. PloS One. 2014;9 doi: 10.1371/journal.pone.0084943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.St Peter W.L., Guo H., Kabadi S. Prevalence, treatment patters, and healthcare resource utilization in Medicare and commercially insured non-dialysis-dependent chronic kidney disease patients with and without anemia in the United States. BMC Nephrol. 2018;19:67. doi: 10.1186/s12882-018-0861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Covic A., Jackson J., Hadfield A. Real-world impact of cardiovascular disease and anemia on quality of life and productivity in patients with non-dialysis-dependent chronic kidney disease. Adv Ther. 2017;34:1662–1672. doi: 10.1007/s12325-017-0566-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorp M.L., Johnson E.S., Yang X. Effect of anemia on mortality, cardiovascular hospitalizations, and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton) 2009;14:240–246. doi: 10.1111/j.1440-1797.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 5.Sato Y., Fugimoto S., Konta T. Anemia as a risk factor for all-cause mortality: obscure synergistic effect of chronic kidney disease. Clin Exp Nephrol. 2018;22:388–394. doi: 10.1007/s10157-017-1468-8. [DOI] [PubMed] [Google Scholar]
- 6.Besarab A., Bolton W.K., Browne J.K. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 7.Singh A.K., Szczech L., Tang K.L. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer M.A., Burdmann E.A., Chen C.Y. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration FDA Drug Safety Communication: Modified dosing recommendations to improve the safe use of Erythropoiesis-Stimulating Agents (ESAs) in chronic kidney disease. Table of Key Trials. June 24, 2011. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-modified-dosing-recommendations-improve-safe-use-erythropoiesis#table
- 10.Amgen Highlights of prescribing information for EPOGEN, revised May 2012. https://www.pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/epogen/epogen_pi_hcp_english.pdf
- 11.Szczech L.A., Bernhart H.X., Inrig J.K. Secondary analysis of the CHOIR trial epoetin-α dose and achieved hemoglobin outcomes. Kidney Int. 2008;74:791–798. doi: 10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo J., Jensen D.E., Maroni B.J. Spectrum and burden of erythropoiesis-stimulating agent hyporesponsiveness among contemporary hemodialysis patients. Am J Kidney Dis. 2016;68:763–771. doi: 10.1053/j.ajkd.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Nisho A., Chatkuli B.P., Ma J.Z. Higher doses of erythropoietin-stimulating agents and hyporesponsiveness to their effect are associated with increased mortality among prevalent hemodialysis patients. Blood Purif. 2013;36:29–36. doi: 10.1159/000350583. [DOI] [PubMed] [Google Scholar]
- 14.Bae M.N., Kim S.H., Kim Y.O. Association of erythropoietin-stimulating agent responsiveness with mortality in hemodialysis and peritoneal dialysis patients. PloS One. 2015;10:e0143348. doi: 10.1371/journal.pone.0143348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cizman B., Smith H.T., Camejo R.R. Clinical and economic outcomes of erythropoiesis-stimulating agent hyporesponsiveness in the post-bundling era. Kidney Med. 2020;2:589–599. doi: 10.1016/j.xkme.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sibbel S.P., Koro C.E., Brunelli S.M. Characterization of chronic and acute ESA hyporesponse: a retrospective cohort study of hemodialysis patients. BMC Nephrol. 2015;16:144. doi: 10.1186/s12882-015-0138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa T., Nitta K. Erythropoiesis-stimulating hyporesponsiveness in end-stage renal disease patients. Contrib Nephrol. 2015;185:76–86. doi: 10.1159/000380972. [DOI] [PubMed] [Google Scholar]
- 18.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 19.Agarwal A.K., Yee J. Hepcidin. Adv Chronic Kidney Dis. 2019;26:298–305. doi: 10.1053/j.ackd.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Gaweda A.E. Markers or iron status in chronic kidney disease. Hemodialysis Int. 2017;21(suppl 1):S21–S27. doi: 10.1111/hdi.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal A.K. Iron metabolism and management: focus on chronic kidney disease. Kidney Int Suppl. 2021;11:46–58. doi: 10.1016/j.kisu.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babitt J.L., Lin H.Y. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–1634. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashby D.R., Gale D.P., Busbridge M. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009;75:976–981. doi: 10.1038/ki.2009.21. [DOI] [PubMed] [Google Scholar]
- 24.Kim A., Nemeth E. New insights into iron regulation and erythropoiesis. Curr Opin Hematol. 2015;22:199–205. doi: 10.1097/MOH.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaelin W.G., Jr., Ratcliffe P.J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Semenza G.L. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 27.de Seigneux S., Lundby A.K., Berchtold L. Increased synthesis of liver erythropoietin with CKD. J Am Soc Nephrol. 2016;27:2265–2269. doi: 10.1681/ASN.2015050508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukhopadhyay C.K., Mazumder B., Fox P.L. Role of hypoxia-inducible factor-1 in transcriptional activation of ceruloplasmin by iron deficiency. J Biol Chem. 2000;275:21048–21054. doi: 10.1074/jbc.M000636200. [DOI] [PubMed] [Google Scholar]
- 29.Haase V.H. Hypoxic regulation of erythropoiesis and iron metabolism. Am J Physiol Renal Physiol. 2010;299:F1–F13. doi: 10.1152/ajprenal.00174.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckardt K.U., Kurtz A., Bauer C. Regulation of erythropoietin production is related to proximal tubular function. Am J Physiol. 1989;256:F942–F947. doi: 10.1152/ajprenal.1989.256.5.F942. [DOI] [PubMed] [Google Scholar]
- 31.Locatelli F., Fishbane S., Block G.A. Targeting hypoxia-inducible factors for the treatment of anemia in chronic kidney disease patients. Am J Nephrol. 2017;45:187–199. doi: 10.1159/000455166. [DOI] [PubMed] [Google Scholar]
- 32.Maxwell P.H., Osmond M.K., Pugh C.W. Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int. 1993;44:1149–1162. doi: 10.1038/ki.1993.362. [DOI] [PubMed] [Google Scholar]
- 33.Gupta N., Wish J.B. Hypoxia-inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am J Kidney Dis. 2017;69:815–826. doi: 10.1053/j.ajkd.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Sanghani N.S., Haase V.H. Hypoxia-inducible factor activators in renal anemia: current clinical experience. Adv Chronic Kidney Dis. 2019;26:253–266. doi: 10.1053/j.ackd.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haase V.H. Hypoxia-inducible factor-prolyl hydroxylase inhibitors in the treatment of anemia of chronic kidney disease. Kidney Int Suppl. 2021;11:18–25. doi: 10.1016/j.kisu.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeh T.-Z., Leissing T.M., Abboud M.I. Molecular and cellular mechanisms of HIF prolyl hydroxylase inhibitors in clinical trials. Chem Sci. 2017;8:7651–7668. doi: 10.1039/c7sc02103h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J., Xie Q.-H., You L. Effects of hypoxia-inducible prolyl hydroxylase inhibitors on iron regulation in non-dialysis-dependent chronic kidney disease patient with anemia: a systemic review and meta-analysis. Pharmacol Res. 2021;163:105256. doi: 10.1016/j.phrs.2020.105256. [DOI] [PubMed] [Google Scholar]
- 38.Li M., Lan J., Dong F. Effectiveness of hypoxia-induced factor prolyl hydroxylase inhibitor for managing anemia in chronic kidney disease patients: a systemic review and meta-analysis. Eur J Clin Pharmacol. 2021;77:491–507. doi: 10.1007/s00228-020-03037-1. [DOI] [PubMed] [Google Scholar]
- 39.Zheng Q., Yang H., Sun L. Efficacy and safety of HIF prolyl-hydroxylase inhibitor vs epoetin and darbepoetin for anemia in chronic kidney disease patients not undergoing dialysis: a network meta-analysis. Pharmacol Res. 2020;59:105020. doi: 10.1016/j.phrs.2020.105020. [DOI] [PubMed] [Google Scholar]
- 40.Chen N., Hao C., Liu B.C. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med. 2019;381:1011–1022. doi: 10.1056/NEJMoa1901713. [DOI] [PubMed] [Google Scholar]
- 41.Chen N., Hao C., Peng X. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med. 2019;381:1001–1010. doi: 10.1056/NEJMoa1813599. [DOI] [PubMed] [Google Scholar]
- 42.Besarab A., Provenzano R., Hertel J. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant. 2015;30:1665–1673. doi: 10.1093/ndt/gfv302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meadowcroft A.M., Cizman B., Holdstock L. Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants on hemodialysis. Clin Kidney J. 2019;12:139–148. doi: 10.1093/ckj/sfy014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pergola P.E., Spinowitz B.S., Hartman C.S. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int. 2016;90:1115–1122. doi: 10.1016/j.kint.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Akizawa T., Yamaguchi Y., Otsuka T. A phase 3, multicenter, randomized, two-arm, open-label study of intermittent oral dosing of roxadustat for the treatment of anemia in Japanese erythropoiesis-stimulating agent-naïve chronic kidney patients not on dialysis. Nephron. 2020;144:372–382. doi: 10.1159/000508100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng Q, Yang H, Fu X, et al. The efficacy and safety of roxadustat for anemia in patients with chronic kidney disease: a meta-analysis. Nephrol Dial Transplant. Published online October 14, 2020. https://doi.org/10.1093/ndt/gfaa110. Accessed June 18, 2021. [DOI] [PubMed]
- 47.Qie S., Jiao N., Duan K. The efficacy of roxadustat treatment for anemia in patients with kidney disease: a meta-analysis and systemic review. Int Urol Nephrol. 2021;53:985–997. doi: 10.1007/s11255-020-02693-7. [DOI] [PubMed] [Google Scholar]
- 48.Coyne D.W., Roger S.D., Shin S.K. Roxadustat for CKD-related anemia in non-dialysis patients. Kidney Int Rep. 2021;6:624–635. doi: 10.1016/j.ekir.2020.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Provenzano R., Fishbane S., Szczech L. Pooled analysis of roxadustat for anemia in patients with kidney failure incident to dialysis. Kidney Int Rep. 2021;6:613–623. doi: 10.1016/j.ekir.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Winkelmayer W.C., Walther C.P. Roxadustat for CKD anemia—starting the jigsaw puzzle, what will the finished picture show? Kidney Int Rep. 2021;6:559–561. doi: 10.1016/j.ekir.2021.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Fishbane S., El-Shahawy M.A., Pecoits-Filho R. Roxadustat for treating anemia in patients with CKD not on dialysis: results from a randomized phase 3 study. J Am Soc Nephrol. 2021;32:37–755. doi: 10.1681/ASN.2020081150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Provenzano R., Fishbane S., Wei L.-J. Pooled Efficacy and Cardiovascular Safety Results of Roxadustat in the Treatment of Anemia in Chronic Kidney Disease Patients On and Not On Dialysis. Abstract FR-OR131. Presented at American Society of Nephrology 2019 Kidney Week November 5–10, 2019, Washington, DC. https://www.astrazeneca.com/content/dam/az/Investor_Relations/events/AZN%20Investor%20science%20conference%20call%20ASN%202019%20Presentation.pdfhttps://fibrogen.gcs-web.com/news-releases/news-release-details/fibrogen-announces-positive-phase-3-pooled-roxadustat-safety-and
- 53.Provenzano R., Fishbane S., Coyne D. Roxadustat Treatment of Anemia in Non-Dialysis-Dependent CKD Is Not Influenced by Iron Status. Oral presentation TH-OR03. Presented at American Society of Nephrology Kidney Week 2020 Reimagined, October 22–25, 2020. https://asn.scientificposters.com/epsSearchASN.cfm
- 54.Barratt J., Andrić B., Tataradze A. Roxadustat for the treatment of anaemia in chronic kidney disease patients non on dialysis: a phase 3 randomised, open-label, active-controlled study. Nephrol Dial Transplant. 2020;35(suppl 3) doi: 10.1093/ndt/gfab349. https://asn.scientificposters.com/epsSearchASN.cfm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.FibroGen FibroGen Provides Additional Information on Roxadustat. April 6, 2021. https://fibrogen.gcs-web.com/news-releases/news-release-details/fibrogen-provides-additional-information-roxadustat
- 56.Astellas. Clinical Trial Data Disclosure. Clinical Study Result, PYRENEES. https://astellasclinicalstudyresults.com/study.aspx?ID=364
- 57.Institute for Clinical and Economic Review Treatments for Anemia in Chronic Kidney Disease: Final Policy Recommendations. March 5, 2021. https://icer.org/wp-content/uploads/2020/10/ICER_CKD_Policy_Recommendations_030521.pdf
- 58.Nangaku M, Kondo K, Ueta K, et al. Efficacy and safety of vadadustat compared with darbepoetin alfa in Japanese anemia patients on hemodialysis: a phase 3, multicenter, randomized double-blind study. Nephrol Dial Transplant. Published online February 26, 2021. https://doi.org/10.1093/ndt/gfab055. Accessed June 18, 2021. [DOI] [PMC free article] [PubMed]
- 59.Nangaku M, Kondo K, Takabe S. Vadadustat for anemia in chronic kidney disease patients on peritoneal dialysis: a phase 3 open-label study in Japan. Ther Apher Dial. Published online December 7, 2020. https://doi.org/10.1111/1744-9987.13611. Accessed June 18, 2021. [DOI] [PMC free article] [PubMed]
- 60.Eckardt KU, Agarwal R, Farag YM, et al. Global phase 3 programme of vadadustat for treatment of anemia of chronic kidney disease: rationale, study design and baseline characteristics of dialysis-dependent patients in the INNO2VATE trials. Nephrol Dial Transplant. Published online November 14, 2020. https://doi.org/10.1093/ndt/gfaa204. Accessed June 18, 2021. [DOI] [PMC free article] [PubMed]
- 61.Chertow G.M., Pergola P.E., Agarwal R. Cardiovascular safety and efficacy of vadadustat for the treatment of anemia in non-dialysis-dependent CKD: design and baseline characteristics. Am Heart J. 2021;235:1–11. doi: 10.1016/j.ahj.2020.10.068. [DOI] [PubMed] [Google Scholar]
- 62.Eckardt K.U., Agarwal R., Aswad A. Safety and efficacy of vadadustat for anemia in patients undergoing dialysis. N Engl J Med. 2021;384:1601–1612. doi: 10.1056/NEJMoa2025956. [DOI] [PubMed] [Google Scholar]
- 63.Chertow G.M., Pergola P.E., Farag Y.M.K. Vadadustat in patients with anemia and non-dialysis-dependent CKD. N Engl J Med. 2021;384:1589–1600. doi: 10.1056/NEJMoa2035938. [DOI] [PubMed] [Google Scholar]
- 64.Zheng Q., Want Y., Yang H. Efficacy and safety of daprodustat for anemia therapy in chronic kidney disease patients: a systemic review and meta-analysis. Front Pharmacol. 2021;11:573645. doi: 10.3389/fphar.2020.573645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bailey C.K., Caltabiano S., Cobitz A.R. A randomized, 29-day, dose-ranging, efficacy and safety study of daprodustat, administered three times weekly in patients with anemia on hemodialysis. BMC Nephrol. 2019;20:372. doi: 10.1186/s12882-019-1547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akizawa T., Nangaku M., Yonekawa T. Efficacy and safety of daprodustat compared with darbepoetin alfa in Japanese hemodialysis patients with anemia: a randomized, double-blind, phase 3 trial. Clin J Am Soc Nephrol. 2020;15:1155–1165. doi: 10.2215/CJN.16011219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nangaku M., Hamano T., Akizawa T. Daprodustat compared with epoetin beta pegol for anemia in Japanese patients not on dialysis: a 52-week randomized open-label phase 3 trial. Am J Nephrol. 2021;52:26–35. doi: 10.1159/000513103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ishii T., Tanaka T., Nangaku M. Profile of daprodustat in the treatment of renal anemia due to chronic kidney disease. Ther Clin Risk Manag. 2021;17:155–163. doi: 10.2147/TCRM.S293879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Macdougall I.C., Akizawa T., Berns J.S. Effects of molidustat in the treatment of anemia in CKD. Clin J Am Soc Nephrol. 2019;14:28–39. doi: 10.2215/CJN.02510218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akizawa T., Macdougall I.C., Berns J.S. Iron regulation by molidustat, a daily oral hypoxia-inducible factor prolyl hydroxylase inhibitor, in patients with chronic kidney disease. Nephron Clin Pract. 2019;143:243–254. doi: 10.1159/000502012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akizawa T., Macdougall I.C., Berns J.S. Long-term efficacy and safety of molidustat for anemia in chronic kidney disease: DIALOGUE extension studies. Am J Nephrol. 2019;49:271–280. doi: 10.1159/000499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akizawa T., Taguchi M., Matsuda Y. Molidustat for the treatment of renal anaemia in patients with dialysis-dependent chronic kidney disease. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamamoto H., Taguchi M., Matsuda Y. Molidustat for the treatment of renal anemia in patients with non-dialysis dependent chronic kidney disease: design and rationale of two phase III studies. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akizawa T., Yamada T., Nobon K. Results from a Phase 3 Study Comparing the Efficacy and Safety of Molidustat vs. Darbepoetin Alfa in Patients Receiving Hemodialysis and Treated With Erythropoiesis-Stimulating Agents (ESAs). Poster PO2623. Presented at American Society of Nephrology Kidney Week 2020 Reimagined, October 22–25, 2020. https://asn.scientificposters.com/epsAbstractASN.cfm?id=1
- 75.Akizawa T., Nangaku M., Yamaguchi T. A placebo-controlled, randomized trial of enarodustat in patients with chronic kidney disease followed by long term trial. Am J Nephrol. 2019;49:165–174. doi: 10.1159/000496929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akizawa T., Nangaku M., Yamaguchi T. Enarodustat, conversion and maintenance therapy for anemia in hemodialysis patients: a randomized, placebo-controlled phase 2b trial followed by long-term trial. Nephron. 2019;142:77–85. doi: 10.1159/000500487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akizawa T., Maeda K., Miyazawa Y. Phase 3 Study to Compare Efficacy and Safety on Enarodustat (JTZ-951), an Oral HIF-PH Inhibitor, With Darbepoetin Alfa in Patients With CKD Receiving Maintenance Hemodialysis. Poster TH-PO1186. Presented at American Society of Nephrology 2019 Kidney Week, November 5–10, 2019, Washington, DC. https://asn.scientificposters.com/epsSearchASN.cfm
- 78.Akizawa T., Matsu A., Miyazawa Y. Phase 3 Study to Compare the Efficacy and Safety on Enarodustat (JTZ-951), an Oral HIF-PH Inhibitor, With Darbepoetin Alfa in Anemia Patients With CKD Not Requiring Dialysis. Poster TH-PO1185. Presented at American Society of Nephrology 2019 Kidney Week, November 5–10, 2019, Washington, DC. https://asn.scientificposters.com/epsSearchASN.cfm
- 79.Parmar D.V., Kansagra K.A., Patel J.C. Outcomes of desidustat treatment in people with anemia and chronic kidney disease: a phase 2 study. Am J Nephrol. 2019;49:470–478. doi: 10.1159/000500232. [DOI] [PubMed] [Google Scholar]
- 80.U.S. National Library of Medicine ClinicalTrials.gov. Desidustat in the Treatment of Anemia in CKD on Dialysis Patients (DREAM-D) https://clinicaltrials.gov/ct2/show/NCT04215120?term=desidustat&draw=2&rank=2
- 81.U.S. National Library of Medicine ClinicalTrials.gov. Desidustat in the Treatment of Anemia in CKD (DREAM-ND) https://clinicaltrials.gov/ct2/show/NCT04012957?term=desidustat&draw=2&rank=3
- 82.Nguyen Q.D., Sepah Y.J., Yamaguchi Yu. Ophthalmological effects of roxadustat in the treatment of anemia in dialysis-dependent and non-dialysis-dependent CKD patients: findings from two phase 3 studies. Poster PO0267. Presented at American Society of Nephrology Kidney Week 2020 Reimagined, October 22–25, 2020. https://asn.scientificposters.com/epsSearchASN.cfm
- 83.Coyne D.W., Fishbane S., Pergola P.E. Roxadustat Is Not Associated With an Increased Risk of Neoplasm in Patients With CKD and Anemia. Poster TH-OR04. Presented at American Society of Nephrology Kidney Week 2020 Reimagined, October 22–25, 2020. https://asn.scientificposters.com/epsSearchASN.cfm
- 84.Yap D.Y.H., McMahon L.P., Hao C.M. Recommendations by the Asian Pacific Society of Nephrology (ASPN) on the appropriate use of HIF-PH inhibitors. Nephrology. 2021;26:105–118. doi: 10.1111/nep.13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Eijk L.T., John A.S.E., Schwoebel F. Effect of the antihepcidin Spiegelmer lexaptepid on inflammation-induced decrease in serum iron in humans. Blood. 2014;124:2643–2646. doi: 10.1182/blood-2014-03-559484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boyce M., Warrington S., Cotezi B. Safety, pharmacokinetics and pharmacodynamics of the anti-hepcidin Spiegelmer lexaptepid pegol in healthy subjects. B J Pharmacol. 2016;173:1580–1588. doi: 10.1111/bph.13433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.U.S. National Library of Medicine ClinicalTrials.gov. Lexaptepid Pegol (NOX-H94) in ESA-Hyporesponsive Anemia in Dialysis Patients. https://clinicaltrials.gov/ct2/show/NCT02079896?term=lexaptepid&draw=2&rank=1
- 88.Sheetz M., Barrington P., Callies S. Targeting the hepcidin-ferroportin pathway in anemia of chronic kidney disease. Br J Pharmacol. 2019;85:935–948. doi: 10.1111/bcp.13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bacchetta J., Zaritsky J.J., Sea J.L. Suppression of iron-regulatory hepcidin by vitamin D. J Am Soc Nephrol. 2014;25:564–572. doi: 10.1681/ASN.2013040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miskulin D.C., Majchrzak K., Tighiouart H. Ergocalciferol supplementation in hemodialysis patients with vitamin D deficiency: a randomized clinical trial. J Am Soc Nephrol. 2016;27:1801–1810. doi: 10.1681/ASN.2015040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Panwar B., McCann D., Olbina G. Effect of calcitriol on serum hepcidin in individuals with chronic kidney disease: a randomized controlled trial. BMC Nephrol. 2018;19:35. doi: 10.1186/s12882-018-0823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pergola P.E., Devalaraja M., Fishbane S. Ziltivekimab for treatment of anemia of inflammation in patients on hemodialysis: results from a phase 1/2 multicenter, randomized, double-blind, placebo-controlled trial. J Am Soc Nephrol. 2021;32:212–222. doi: 10.1681/ASN.2020050595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kelly M.S., Lewis J., Huntsberry A.M. Efficacy and renal outcomes of SGLT2 inhibitors in patients with type 2 diabetes and chronic kidney disease. Postgrad Med. 2019;131:31–42. doi: 10.1080/00325481.2019.1549459. [DOI] [PubMed] [Google Scholar]
- 94.Yu B., Dong C.X., Hu Z.J. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on renal outcomes in patients with type 2 diabetes mellitus and chronic kidney disease. A protocol for systemic review and meta-analysis. Medicine (Baltimore) 2021;100 doi: 10.1097/MD.0000000000024655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wheeler D.C., Stefánsson B.V., Jongs N. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9:22–31. doi: 10.1016/S2213-8587(20)30369-7. [DOI] [PubMed] [Google Scholar]
- 96.Ghanim H., Abuaysheh S., Hejna J. Dapagliflozin suppresses hepcidin and increases erythropoiesis. J Clin Endocrinol Metab. 2020;105:e1056–e1063. doi: 10.1210/clinem/dgaa057. [DOI] [PubMed] [Google Scholar]
- 97.Aberle J., Menzen M., Schmid S.M. Dapagliflozin effects on haematocrit, red blood cell count and reticulocytes in insulin treated patients with type 2 diabetes. Sci Rep. 2020;10:22396. doi: 10.1038/s41598-020-78734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stefánsson B.V., Heerspink H.J.L., Wheeler D.C. Correction of anemia by dapagliflozin in patients with type 2 diabetes. J Diabetes Complications. 2020;34:107729. doi: 10.1016/j.jdiacomp.2020.107729. [DOI] [PubMed] [Google Scholar]
- 99.Oshima M., Neuen B.L., Jardine M.J. Effects of canagliflozin on anemia in patients with type 2 diabetes and chronic kidney disease: a post-hoc analysis from the CREDENCE trial. Lancet Diabetes Endocrinol. 2020;8:903–914. doi: 10.1016/S2213-8587(20)30300-4. [DOI] [PubMed] [Google Scholar]
- 100.Maruyanma T., Takashima H., Oguma H. Canagliflozin improves erythropoiesis in diabetes patients with anemia of chronic kidney disease. Diabetes Technol Ther. 2019;21:7132–7720. doi: 10.1089/dia.2019.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schmidt D.W., Argyropoulos C., Singh N. Are the protective effects of SGLT2 inhibitors a “class effect” or are there differences between agents? Kidney360. 2021;2:881–885. doi: 10.34067/KID.0000622021. [DOI] [PMC free article] [PubMed] [Google Scholar]